Abstract
Purpose
Rehabilitation can improve health outcomes in candidates for lung transplantation. The purpose of this study was to retrospectively evaluate the effect of a one-month physical therapy (PT)-based outpatient program on exercise capacity, symptoms, quality of life and examine predictors of functional outcome changes in adults awaiting lung transplantation.
Methods
Participants (n=141) completed a 23-session exercise and educational program over one month. Outcomes included 6-minute walk distance (6MWD), San Diego Shortness of Breath Questionnaire (SOBQ), Center for Epidemiological Studies-Depression Scale (CESD), and Ferrans and Powers Quality of Life Index Pulmonary Version III (QOL).
Results
Participants were older (median age 63) with restrictive (59%) or obstructive (24%) disease. Moderate-to-large improvements in 6MWD were observed (69 m, p < 0.001, d = 0.72), independent of demographics, symptoms, and QOL. Lower initial 6MWD and lower oxygen utilization were associated with greater 6MWD improvements, with largest gains occurring in initial 6MWD < 305 m. Small-to-moderate improvements were observed on CESD (p < 0.001, d = 0.26) and in overall QOL (p < 0.001, d = 0.27), with a non-significant improvement observed on SOBQ (p = 0.248, d = 0.13).
Conclusions
Completion of a one-month PT-based outpatient rehabilitation program was associated with improved exercise capacity, depressive symptoms and QOL.
Keywords: exercise, rehabilitation, six-minute walk distance
Introduction and Purpose
Lung transplantation is the only remaining treatment option to extend survival and improve quality of life (QOL) for select individuals with end-stage lung disease.1 Candidates for transplantation who live with significant disease burden and who cope with the arduous process of transplant qualification and wait for donor lungs experience a range of physical and psychological stressors. Common among these are symptoms of dyspnea, depression and limitations in exercise capacity, all of which contribute to decrements in QOL.2–4 Participation in pre-transplant rehabilitation is intended to optimize both the candidate’s physical and psychological readiness for surgery.
A recent systematic review and an expert consensus statement concluded that pre-transplant rehabilitation is associated with improvements in exercise capacity and overall QOL in lung transplant candidates.5,6 However, the total number of studies are limited (e.g., 6 studies included in the systematic review) and, of those, wide variation in exercise modes, exercise parameters (intensity, duration), and program lengths (range 6 to 16 weeks) exist.5–12 Despite the variations, 5 of the 6 studies reported enhanced QOL and 5 reported improved exercise capacity using six-minute walk distance (6MWD).7–10,12 Rehabilitation programs that ranged between 6 and 12 weeks resulted in exercise capacity improvement7–9,12 while the longer 15 to 16 week program did not.11 The authors of the latter study, Li et al., postulated that disease progression was likely responsible for the lack of improvement in exercise capacity. To that end, shorter duration rehabilitation could be important for lung transplant candidates given concern for disease progression and mortality risk prior to surgery. To our knowledge, there are no reports that examined the effects of outpatient pre-transplant rehabilitation provided in less than 6 weeks. Furthermore, predictors associated with improved exercise capacity and QOL are not well-defined.
The purpose of this retrospective report is to evaluate the effect of a short, one-month physical therapy (PT)-based outpatient rehabilitation program on exercise capacity, dyspnea, depressive symptoms, and QOL in patients with very advanced lung disease awaiting lung transplantation. Exercise capacity, measured by 6MWD, is a strong predictor of health outcomes in candidates for lung transplant; greater 6MWD is associated with lower waitlist mortality and one-year post-transplant mortality.13–15 Given these associations, 6MWD is an integral component of the United Network for Organ Sharing (UNOS) Lung Allocation Score. A primary objective of this report is to examine the effect of the month long program on outcomes, including the 6MWD. In addition, understanding predictors for improving 6MWD as well as symptoms and QOL can have important implications for optimizing pre-transplant management, and therefore, a secondary objective of our report is to examine individual-level predictors of outcome improvements.
Methods
Study Design and Participants
This study was approved by the academic medical center’s Institutional Review Board (Pro00072212). Participants who completed the Pre-Transplant Rehabilitation Program (PTRP) between January 2013 and October 2014 and subsequently received a lung transplant were considered for inclusion. Those who received multi-organ transplant or a re-transplant were excluded. Criteria to be considered a transplant candidate at our center follow the absolute contraindications for lung transplant set by the International Society for Heart and Lung Transplantation (ISHLT)16, such as no active tobacco use, limited co-morbidities and consistent caregiver support. Body mass index criteria, which is more restrictive than the ISHLT 30–34 relative contraindication guideline, is > 18 and ≤ 27. Also, all candidates for lung transplantation at our center are required to attend the PTRP, except for a few select patients under the age of 65 who are not medically stable to be outpatients and are transplanted from the inpatient setting. Given the association of a greater 6MWD distance with improved pre- and post-transplant mortality outcomes, an additional recommendation is a minimal 6MWD of 305 meters.13–15
Rehabilitation Program
The PTRP consisted of structured and progressive multi-domain (aerobic, resistance, balance, flexibility, breathing) exercise and educational sessions. Participants typically attended 5 exercises sessions per week until they reached a total of 23 sessions. If a session was missed due to illness, hospitalization, or another medical appointment, the participant continued in the program until all 23 sessions were completed. The participants also attended 4–5 educational lectures per week regarding the complex transplant process and strategies for self-management of their health. Example lecture topics included developing skills for coping with life as a transplant candidate and recipient, for understanding spirometry and results, and for managing nutrition, diabetes, medications, and fitness and exercise. Upon completion of the DPTRP, participants transitioned to a 5-day per week maintenance program at the facility until the time of transplant.
Aerobic exercise consisted of ambulation on the facility’s indoor, oval track (80.5 to 122 m) with participants either pulling their oxygen apparatus or pushing it in a rollator. Exercise duration was gradually increased to a goal of 20 continuous minutes, 3 days per week, and 30 continuous minutes, 2 days per week at a pace participants could sustain without resting. Also included was stationary cycling using either an upright or recumbent cycle (TRS 4000 recumbent stepper, NuStep, Inc; R70, Vision Fitness, Inc; EC-3500, Cat Eye Co, Ltd.; or U70, Vision Fitness, Inc.) Once the participant could cycle 20 minutes continuously, the pedal resistance was gradually increased to maintain a moderately intense workload, which equated to a rating of 4–6 on a 0 to 10 rate of perceived exertion visual analog scale.17–19
Resistance exercise consisted of alternating days of upper body and lower body exercises. The initial weight was set to elicit muscle fatigue at 15 to 20 repetitions. Once the patient performed one set of 20 or more repetitions for 2 consecutive visits, the weight was increased by 5 pounds on weight machines and 1 pound for free weights. Also, balance and flexibility exercises were performed on an alternating schedule.
In addition to individualized exercise, all participants attended a 30-minute group exercise class, which varied daily. The class included generalized strengthening, flexibility, and breathing exercises using arm weights, ankle weights, and resistance bands. See supplemental digital content Table E1 and E2 for details of the resistance, flexibility, and balance exercises and the group exercise class.
Participants were evaluated by a physical therapist before starting the PTRP. Therapists (n = 18) experienced in treating patients with lung disease supervised all sessions and adjusted the exercise prescription based on the participant’s symptoms, heart rate, oxygen saturation of hemoglobin via pulse oximetry (SpO2), and ratings of perceived exertion. Supplemental oxygen was titrated either by the physical therapist or the collaborating respiratory therapist, who is available for the exercise sessions, to maintain SpO2 ≥ 88%. Oxygen levels were monitored and tanks were changed by staff, as needed, to minimize interruptions in participant activity. Participants were in the facility approximately 3 to 4 hours per day and total exercise time equated to approximately 2 to 2.5 hours of that total time. This schedule allowed for rest breaks between exercise bouts and at least 5-minute transition periods between aerobic, resistance and group exercises.
Outcome Measurements
At the start and end of the PTRP, exercise capacity was measured via 6MWD. The assessment was administered on the facility’s indoor, oval track by the treating physical therapist as part of their routine care examination. 6MWD was assessed prior to performing other exercises for that day. Participants transported their own supplemental oxygen in a pull cart or rollator. Full oxygen tanks eliminated the need to change tanks during the test. Supplemental oxygen was administered based on each participant’s exercise oxygen need, with the goal of maintaining SpO2 ≥ 88% as per finger or forehead probe (Masimo Rad-5v, Masimo Corporation, Irvine CA). Oxygen use may have differed from initial to final 6MWD assessment. Field test recommendations allow for increasing oxygen to account for worsening gas exchange.20,21 To that end, the assessment served to measure the participant’s exercise capacity, not extent of lung disease progression.
The participants also completed the following self-administered questionnaires: Ferrans and Powers Quality of Life Index Pulmonary Version III22 (QLI), the Center for Epidemiological Studies-Depression Scale23 (CESD), and the San Diego Shortness of Breath Questionnaire24 (SOBQ). The QLI assesses QOL as an overall score and is partitioned into 4 subscales: health and functioning, psychological/spiritual, social and economic, and family. The QLI scores range from 0 to 30 with a score of 30 indicating the highest possible QOL. The QLI was used because it has a pulmonary specific version that is applicable to candidates for transplantation. The CESD is a 20-item questionnaire designed and validated to assess major aspects of depression. Scores on the CESD range 0–60, and scores ≥ 16 points are suggestive of clinically elevated depressive symptoms. The SOBQ is a 24-question assessment that considers dyspnea with activities of daily life. Scores range 0–120, with 0 indicating no shortness of breath and 120 indicating maximal shortness of breath for all activities.
Demographic data for participants were electronically extracted from our center’s secure Pulmonary Transplant Program database, and 6MWD was extracted from our center’s electronic health record. All other outcomes were inputted into the database by one research assistant and then electronically extracted. All extracted data was then compiled into a single excel file, which was then uploaded into the statistical program.
Statistical Analysis
All statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC) and R 3.3.1 (https://cran.r-project.org). Standard descriptive statistics were performed to characterize the cohort and P < 0.05 was used to determine significance in all analyses. In order to examine changes in CESD, SOBQ, QLI, and 6MWD we conducted separate, unadjusted, repeated measures models in which the pre- and post-rehabilitation CESD, SOBQ, QLI scores and 6MWD were modeled as the outcomes. In order to examine the association between pre-rehabilitation characteristics and changes in 6MWD, a linear model was fit with a priori selected pre-rehabilitation covariates, including age, diagnostic category of lung disease, ethnicity, gender, pre-rehabilitation exercise oxygen use (liters/min), SOBQ, CESD, QLI, initial 6MWD, with change in 6MWD as outcome variable. Our 6MWD model also included the total number of days in rehabilitation as a predictor and we evaluated the extent to which any of the observed associations were non-linear using Harrell’s restricted cubic spline function within R. Changes from pre-to-post rehabilitation were assessed using repeated measures, general linear models.
In order to provide clinically meaningful parameter estimates, initial 6MWD was also considered as a categorical predictor based on 6MWD distribution (≤ 305, 306–396, and ≥ 397 meters). In order to examine predictors of post-rehabilitation CESD, SOBQ, and QLI, separate linear models were examined using changes in each outcome with the same covariates as our 6MWD model, as well as changes in 6MWD, and changes in the other psychometric outcomes. Because the psychometric outcomes were only modestly correlated (r’s < 0.42), we did not aggregate outcomes. In addition, we conducted a series of secondary, explanatory models for QLI to determine the magnitude of change within individual subscales (health, psychological/spiritual, socioeconomic, and family).
Missing data was managed using multiple imputation with Markov chain Monte Carlo methods available in SAS (PROC MI) and 50 imputations. Few participants were missing data in CESD and SOBQ (<2%) and missing data had no impact on the observed pattern of findings. In order to provide clinically-relevant data on the magnitude of change over time, changes from pre- to post-rehabilitation are also reported using standardized effect sizes using Cohen’s d, which can be interpreted as small (d = 0.2), moderate (d = 0.5), or large (0.8). We also characterized the magnitude of change in each metric using the minimally important difference (MID) using published MIDs when available or the distribution-based MID using Cohen’s effect size (0.5*SDΔ).25,26
Results
Sample Description
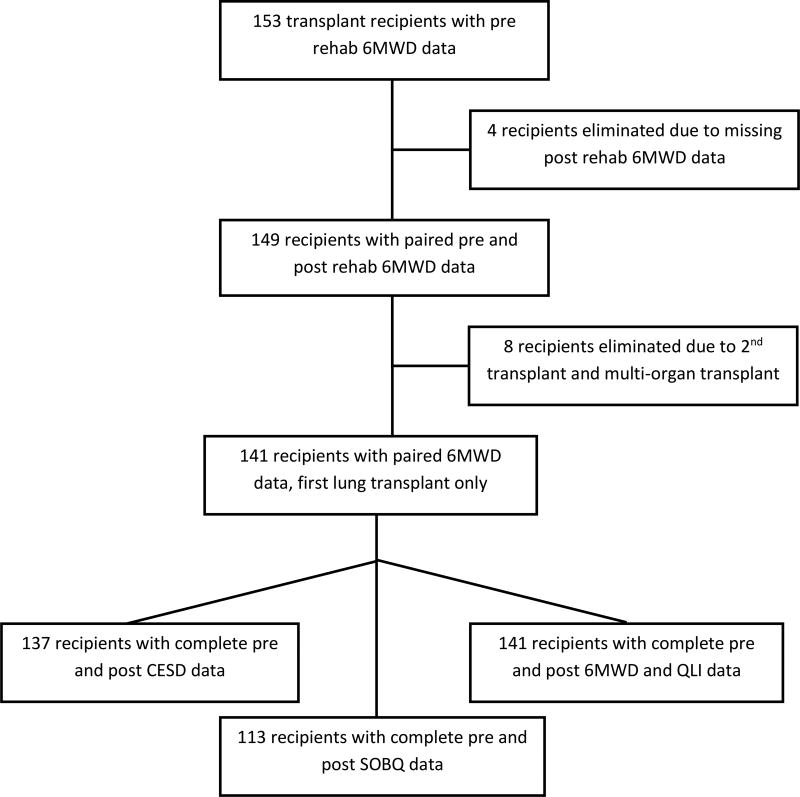
One hundred forty-one participants had complete 6MWD and QLI data (Figure 1). The median age of the sample was 63 years, comprised primarily of Caucasian male patients with restrictive lung disease, reflecting our center referral pattern (Table 1). Oxygen utilization was substantial with the majority of the sample utilizing 10 or more liters/minute during the initial assessment of 6MWD. Oxygen utilization increased during 6MWD assessment by the end of rehabilitation (median 15 liters/minute [IQR = 6, 25]), with the majority of patients using 15 liters/minute or more. Pre- and post-rehabilitation assessments were generally conducted 5 weeks apart (median 34 days [IQR = 28, 42 days]).
Figure 1.
Flowchart of patient inclusion
Table 1.
Background and demographic characteristics
| Variable | n=141 |
|---|---|
|
| |
| Lung Disease | |
| Restrictive, n (%) | 83 (59%) |
| Obstructive, n (%) | 34 (24%) |
| Cystic Fibrosis, n (%) | 21 (15%) |
| Pulmonary Vascular, n (%) | 3 (2%) |
|
| |
| Age, years mean (SD) | 58.5 (14.9) |
| median (IQR) | 63 (54, 69) |
|
| |
| Male Gender, n (%) | 85 (60%) |
|
| |
| Caucasian, n (%) | 130 (92%) |
|
| |
| Bilateral Transplant, n (%) | 108 (77%) |
|
| |
| Baseline Exercise Oxygen Prescription, liters/minute median (IQR) | 10 (6, 15) |
|
| |
| Baseline Exercise Oxygen Saturation (SD) | 92.90 (3.46) |
|
| |
| Baseline Exercise Heart Rate (SD) | 118 (18.23) |
|
| |
| Post-rehab Exercise Oxygen Saturation (SD) | 92.89 (4.05) |
|
| |
| Post-rehab Exercise Heart Rate (SD) | 120 (17.81) |
|
| |
| Days Between 6MWD Assessments, median days (IQR) | 34 (28, 42) |
Changes in 6MWD with Rehabilitation
Examination of initial 6MWD demonstrated an average distance of 386 meters (SD = 96), with 24 individuals (17%) exhibiting 6MWD less than the minimum level for listing (305 meters) at our facility. Participants exhibited moderate-to-large improvements in 6MWD following rehabilitation (d = 0.72; p < 0.001), with an average improvement of 69 meters (SD = 74). Participants initially ambulated 68% of predicted 6MWD27 (SD = 18) and finished at about 80% predicted distance (SD = 20).
Changes in CESD, SOBQ, and QLI with Rehabilitation
Changes in psychometric tests are shown in Table 2. Examination of initial CESD scores revealed that 31 participants (22%) exhibited clinically elevated depressive symptoms (≥ 16). As shown, CESD scores improved following rehabilitation (p < 0.001) with a small-to-moderate reduction of 1.7 points (SD=5.4) (d = 0.26) and only 15% of participants exhibiting significant depressive symptoms after completing rehabilitation. Using a distribution-based MID of 2.72, 43% of participants experienced a change of at least one MID. Similarly, small-to-moderate improvements in QLI were observed following rehabilitation (mean increase = 1.0 point [SD = 2.7], p < 0.001; d = 0.27). Using a distribution-based MID of 1.35, 40% of participants experienced a change of at least one MID.
Table 2.
Changes in SOBQ, CESD, QLI, and 6MWD from pre- to post-rehabilitation.
| Variable | Pre-Rehab | Post-Rehab | Cohen’s d | P-value |
|---|---|---|---|---|
| SOBQ (n=113) | 74.1 (19.7) | 71.5 (20.4) | 0.13 | 0.248 |
| CESD (n=137) | 10.7 (7.6) | 8.9 (6.2) | 0.26 | < 0.001 |
| CESD ≥ 16 (n = 31, 22%) | 21.3 (7.8) | 14.8 (6.3) | 0.95 | < 0.001 |
| QLI (n=141) | 18.8 (3.7) | 19.8 (3.4) | 0.27 | < 0.001 |
| 6MWD, meters (n=141) | 386 (96) | 455 (96) | 0.72 | < 0.001 |
| 6MWD % predicted (n=141) | 67.6 (18.0) | 80.4 (19.6) | 0.67 | < 0.001 |
SOBQ= San Diego Shortness of Breath Questionnaire, CESD= Center for Epidemiological Studies-Depression Scale, QLI= Ferrans and Powers Quality of Life Index Pulmonary Version III, 6MWD=six-minute walk distance
Examination of individual QLI subscales demonstrated that the largest improvements were observed on the health, psychological/spiritual, and socioeconomic subscales, with no changes observed on the family subscale (Table 3). Although SOBQ scores tended to decline following rehabilitation (−2.1 [SD = 18.8], d = 0.13) suggesting improvements in symptomatology, these improvements did not achieve statistical significance (p = 0.248).
Table 3.
Changes in Ferrans and Powers Quality of Life Index Pulmonary Version III subscales from pre- to post-rehabilitation.
| Ferrans and Powers Subscale | Pre-Rehab | Post-Rehab | Cohen’s d | P-value |
|---|---|---|---|---|
| Health and function | 12.7 (4.8) | 14.0 (4.6) | 0.28 | < 0.001 |
| Psychological/Spiritual | 23.0 (4.8) | 23.7 (4.5) | 0.15 | 0.005 |
| Family | 26.0 (4.7) | 26.2 (4.3) | 0.04 | 0.453 |
| Socioeconomic | 23.7 (4.2) | 24.5 (3.8) | 0.20 | 0.015 |
Predictors of Improvements in 6MWD
The 6MWD improved in 86% of participants, with 97% completing rehabilitation with a 6MWD ≥ 305 meters. Examination of our multivariate model (Table 4) revealed that participants with lower pre-rehabilitation 6MWD and lower exercise oxygen utilization showed the greatest gains in 6MWD. Age, gender, lung disease, pre-rehabilitation SOBQ, and CESD scores were not associated with changes in 6MWD.
Table 4.
Predictors of change in 6-minute walk distance with rehabilitation.
| Predictor | Parameter Estimate (95% CI) | P-value |
|---|---|---|
| Age, decades | 4.58 (−10.21,19.37) | 0.543 |
| Ethnicity | −12.48 (−60.05,35.08) | 0.606 |
| Gender | −2.04 (−29.98,25.89) | 0.886 |
| Days Between 6MWD Assessments | 0.40 (−0.37,1.18) | 0.310 |
| Lung Disease Type | −45.60 (−130.00,34.00) | 0.252 |
| Baseline Exercise Oxygen Use (liters/min) | −1.68 (−3.23, −0.12) | 0.035 |
| Pre-Rehab SOBQ (continuous predictor) | −0.24 (−1.07, 0.59) | 0.569 |
| Pre-Rehab CESD (≥ to 16) | 1.88 (−31.86,35.62) | 0.913 |
| Pre-Rehab QLI (continuous predictor) | −1.85 (−5.91,2.22) | 0.371 |
| Pre-Rehab 6MWD (continuous predictor) | −0.35 (−0.50, −0.21) | < 0.001 |
SOBQ= San Diego Shortness of Breath Questionnaire, CESD= Center for Epidemiological Studies-Depression Scale, QLI= Ferrans and Powers Quality of Life Index Pulmonary Version III, 6MWD=six-minute walk distance
We considered 6MWD as a categorical variable using the distance of < 305 meters as the lower boundary, as it the minimum distance for listing at our facility. The upper boundary was ≥ 397 meters, consistent with the natural break in the data. Initial 6MWD of ≤ 305 meters was as associated with the greatest improvement in 6MWD (141 meters [111, 171]). Improvements in 6MWD were more modest among individuals with higher pre-rehabilitation 6MWD levels, with improvements of 53 meters (35, 71) and 44 meters (26, 62) observed among individuals with pre-rehabilitation 6MWD levels of 305–396 meters and ≥ 397 meters, respectively. Consistent with this pattern, the test for a non-linear relationship between initial 6MWD and change in 6MWD was significant (p < 0.001). Examination of this association demonstrated a curvilinear, threshold effect, such that participants with a pre-rehabilitation 6MWD of ≥ 427 meters were less likely to experience significant 6MWD improvements (Figure 2). Similarly, participants at 75% predicted 6MWD or greater pre-rehabilitation showed less improvements than those below 75%, with improvements of 17% (12, 22) among those with < 75% of predicted 6MWD at baseline compared to an 8% (5, 12) improvement among those with ≥ 75% predicted 6MWD.
Figure 2.
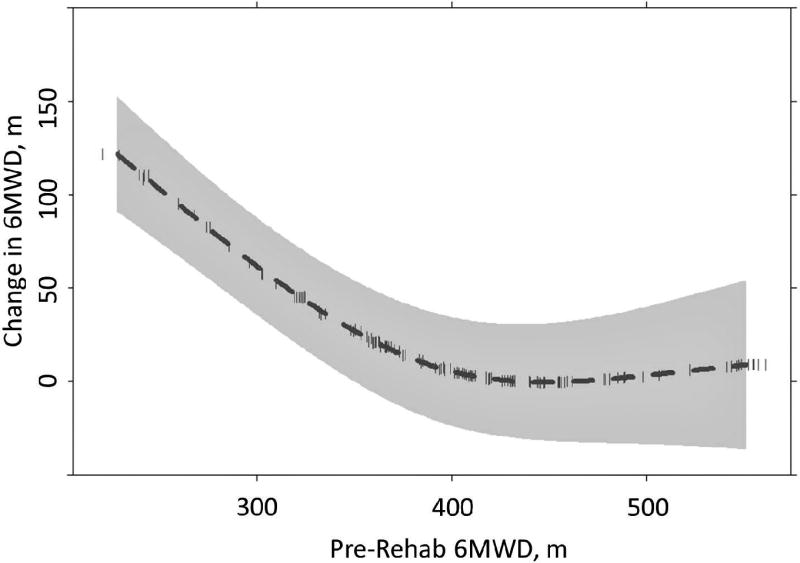
Non-linear relationship between initial 6MWD and change in 6MWD.
Predictors of Improvements in CESD, SOBQ, and QLI
Examination of changes in CESD scores from pre- to post-rehabilitation (mean change = 1.7 points [SD = 5.4]) demonstrated that greater pre-rehabilitation depressive symptoms (b = 0.5 [0.4, 0.6], p < 0.001) and lower QLI (0.6 [0.3, 0.8], p < 0.001), and improvements in QLI (b = 0.5 [0.2, 0.9], p = 0.004) demonstrated the strongest associations with CESD improvement. For changes in SOBQ scores (mean change = 2.1 points [SD = 18.8]), greater pre-rehabilitation SOBQ (b = 0.4 [0.2, 0.6], p < 0.001), improvements in QLI (2.0 [0.7, 3.4], p = 0.003), and improvements in 6MWD (0.06 [0.05, 0.11], p = .020) were all associated with SOBQ improvements. For changes in QLI scores (mean change = 0.9 points [SD = 2.7]), lower pre-rehabilitation QLI (b = −0.4 [−0.6, −0.3], p < 0.001) and SOBQ (b = −0.03 [−0.06, −0.01], p = 0.044), as well as improvements in CESD (b = 0.1 [0.02, 0.2], p = 0.012) and SOBQ (b = 0.04 [0.01, 0.07], p = 0.004) were all associated with greater improvements in QLI. In addition, older age (b = 0.5 [−0.02, 1.0], p = 0.061) and female gender (b = −0.8 [−1.8, 0.1], p = 0.076) tended to be associated with greater QLI improvements. Taken together, these findings suggest that improvements in QLI, shortness of breath, and depressive symptoms tended to be associated, and that participants exhibiting greater improvements in 6MWD also experienced reduced shortness of breath following rehabilitation. In addition, greater depressive symptoms, shortness of breath, or lower QLI at the beginning of rehabilitation were associated with improvements in those same domains.
Discussion
To our knowledge, this is the first report to detail the specific components of and outcomes associated with a short, one-month PT-based outpatient rehabilitation program for candidates for lung transplantation. Even in the setting of severe and progressive lung disease, participants in the PTRP experienced moderate to large improvements in exercise capacity. Improvements were independent of age, gender, lung disease, symptomatology, and QOL. Candidates also experienced small to moderate improvements in QOL and depressive symptoms.
6MWD is a significant predictor of mortality in candidates for lung transplantation.14,15,28 Using UNOS data, Castleberry et al. found that increasing 6MWD was associated with a lower overall hazard of death and that increases in walk distance through 366–427 meters conferred an incremental survival advantage.15 In our report 6MWD increased by an average of 69 meters and the average post-rehabilitation total distance was 455 meters. This increase far exceeds the MID reported in exercise tests for those with chronic respiratory disease (30 m)29 and the total distance surpasses the upper survival advantage distance noted by Castleberry et al.
The improvement in 6MWD is consistent with reports from other studies of lung transplant candidates, but exercise was performed at lesser frequencies, 2–3 times per week, and over longer periods of time, 6 to 12 weeks7–9 compared to our 5 times per week, 4.5-week program. Kenn et al. examined the same frequency of rehabilitation as in our study, 5 times per week, but it was conducted in the inpatient setting in Germany and included only resistance training, stationary cycling, and breathing exercises.12 Our program differed as it included multi-domain individualized aerobic, resistance, and balance training along with a group exercise class. The participant demographics also varied; Kenn et al. included fewer who had restrictive lung disease (24% vs. 59%) and more who were younger (≤ 54 vs. 63 years).12 Despite the differences, similar 6MWD were reported, 56 meters in Kenn et al.12 and 65 meters in this report. However, direct comparison of changes in 6MWD is challenging given the variation in 6MWD assessment procedures. Our study used an oval track while other studies administered the test along a hallway. The use of an oval track for 6MWD assessment may explain why initial 6MWDs were also higher in our study compared to other studies. Another factor could be our center’s emphasis on exercise capacity as a criteria for transplantation; our participants were motivated to participate in rehabilitation to meet the criteria. Importantly, all our participants, except for 4 (2%), achieved the minimum distance recommended for lung transplantation listing at our center, 305 meters, by the end of rehabilitation.
We examined predictors for improvement in 6MWD. Unlike Kenn et al. who did not find any relevant predictors, we found that participants with lower initial 6MWD or lower oxygen requirements had greater improvements in final 6MWD. Those with lower initial 6MWD may be more deconditioned, and therefore, had the most to gain in exercise capacity. Those with lower oxygen requirements may have been more clinically stable, and therefore, could have the potential to experience greater gains in exercise capacity. In contrast, age was not associated with 6MWD improvements. This is surprising given that the age of participants in the present study (median 63 years) is substantially higher compared with other studies,7–12 which likely reflects the growing trend in the United States of transplanting older patients.30 Our study demonstrated that participants over 65 were able to improve exercise capacity at a level similar to younger participants. Further research is needed to determine how exercise capacity and age relate to hospital length of stay and survival following lung transplantation.
Following rehabilitation, participants experienced small-to-moderate improvements in both depression and QOL, particularly in health and functional domains. This is not surprising since our intervention program primarily targeted these aspects of QOL, and our results mirror other studies that demonstrated improvement in the physical functioning component of QOL following pre-transplant rehabilitation.7,9,10,12 QLI was not predictive of change in 6MWD demonstrating differences between perceived QOL and exercise capacity. Other rehabilitation studies, not specific to transplant, demonstrated improvements in both QOL and exercise capacity without correlation between the two outcomes.31–33 It is likely that QOL among pre-transplant candidates is impacted both by factors independent of functional or disease status, including coping abilities and self-efficacy.3,34 Therefore, including assessments of both QOL and exercise capacity could be beneficial in order to obtain a holistic impression of patient changes with rehabilitation.
Overall, depressive symptoms improved following rehabilitation, and participants with the highest depressive symptoms had the largest improvements. Previous studies in individuals with COPD found reductions in depressive symptoms with rehabilitation,35,36 and that 6MWD, physical activity levels, and depression are all predictive of subsequent clinical events in this population.37 It is unclear whether the exercise itself, education about the transplant process, or social support derived from group participation contributed to these improvements. Factors outside of rehabilitation such as optimization of medications or referrals for psychological support could also have impacted the observed pattern of findings. Further research is needed to determine if improving pre-transplant QOL and depressive symptoms can improve patient outcomes after transplant.38
In this study, dyspnea improved following rehabilitation, but the improvements did not reach statistical significance. The effect of rehabilitation on dyspnea in candidates for lung transplantation is varied. Jastrezbeski et al. did not find any change in dyspnea following a 12-week Nordic walking intervention.7 On the other hand, Florian et al. demonstrated reductions in shortness of breath after a 36-session rehabilitation program.9 Florian et al. only examined dyspnea related to 6MWD and did not use an assessment of dyspnea during daily activities.9 The SOBQ used in this study evaluates dyspnea across a variety of activities of daily living from tooth brushing to walking up a hill. It is possible that people with advance lung disease may not experience the same improvements in dyspnea as people with more mild disease. It is also possible that the type of intervention performed may determine the extent of changes in dyspnea, since factors contributing to symptoms of dyspnea are multifactorial.39 The intervention in this study aimed to address the most common pre-lung transplant impairments, but focused most heavily on strength and endurance. Further research is needed to determine the most effective interventions to reduce dyspnea in candidates for lung transplantation.
Limitations
This report is a retrospective analysis of routine care in a single center. A non-exercise control group for comparison is not available in our setting because all candidates for lung transplantation are required to attend rehabilitation. Another limitation was the select nature of the patient cohort. Only patients who met our center’s listing criteria, completed the rehabilitation program and received a lung transplant were included. Therefore, results may not be generalizable to all people with advanced lung disease. Only one 6MWD trial was performed at each time point; thus, the learning effect could explain some of the increase in 6MWD. However, the learning effect may be mitigated given that our participants have performed 6MWD at prior routine clinical care visits and are familiar with the assessment. Also, 6MWD was assessed on an oval track, which complicates comparisons with other studies. The treating therapist in the PTRP administered the 6MWD; thus, another factor that could impact the increase in 6MWD is the lack of blinded assessment. Our prediction model could have been strengthened by including peripheral muscle strength and frailty assessments, but that data was not available pre- and post-rehabilitation. This program may be difficult for other transplant centers to replicate due to the scope and frequency of the program. It also requires rehabilitation facilities to have the capacity to provide patients with high amounts of oxygen with exercise. Despite these challenges, the exercise component is designed to be performed in a traditional outpatient setting using standard gym equipment and the condensed program duration may potentially provide favorable economic and health status returns on investment across the health system, insurers, and patients. Future studies will explore the potential impact of the program on post-transplant healthcare utilization and outcomes (i.e., mortality, morbidity, functional capacity).
Conclusions
Despite having severe and progressive lung disease, candidates for lung transplantation who participated in our one-month PT-based rehabilitation program improved their exercise capacity, depressive symptoms, and QOL. Participants who were the most deconditioned experienced the greatest increases in exercise capacity following the rehabilitation program. Age was not predictive of improvements in 6MWD. This report adds to the body of evidence describing and supporting rehabilitation programs for lung transplant candidates. Future research should determine exercise components and parameters that optimize successful pre- and post-transplant outcomes.
Supplementary Material
Acknowledgments
The study was supported by the Duke University Claude D. Pepper Older American Independence Center Grant P30AG028716.
Footnotes
Authors’ Contributions
RB: Conceptualization and design; data extraction and interpretation; manuscript writing; and reviewed/edited manuscript. PS statistical analyses, manuscript writing, and reviewed/edited manuscript. OM data extraction and reviewed/edited manuscript. LDS and AMP conceptualization and design; data interpretation; and reviewed/edited manuscript. LDS and AMP both contributed equally as senior authors.
Conflict of Interest
The authors have no relevant financial relationships with industry to disclose.
Supplemental Digital Content material is included Supplemental Digital Content.doc
References
- 1.Hartert M, Senbaklavacin O, Gohrbandt B, Fischer BM, Buhl R, Vahld CF. Lung transplantation: a treatment option in end-stage lung disease. Dtsch Arztebl Int. 2014;111(7):107–116. doi: 10.3238/arztebl.2014.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen L, Huang D, Mou X, Chen Y, Gong Y, He J. Investigation of quality of life and relevant influence factors in patients awaiting lung transplantation. J Thorac Dis. 2011;3(4):244–248. doi: 10.3978/j.issn.2072-1439.2010.08.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singer JP, Singer LG. Quality of life in lung transplantation. Semin Respir Crit Care Med. 2013;34(3):421–430. doi: 10.1055/s-0033-1348470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenberger EM, Dew MA, DiMartini AF, DeVito Dabbs AJ, Yusen RD. Psychosocial issues facing lung transplant candidates, recipients and family caregivers. Thorac Surg Clin. 2012;22(4):517–529. doi: 10.1016/j.thorsurg.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffman M, Chaves G, Ribeiro-Samora GA, Britto RR, Parreira VF. Effects of pulmonary rehabilitation in lung transplant candidates: a systematic review. BMJ Open. 2017;7(2):e013445. doi: 10.1136/bmjopen-2016-013445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wickerson L, Rozenberg D, Janaudis-Ferreira T, et al. Physical rehabilitation for lung transplant candidates and recipients: An evidence-informed clinical approach. World J Transplant. 2016;6(3):517–531. doi: 10.5500/wjt.v6.i3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jastrzebski D, Ochman M, Ziora D, et al. Pulmonary rehabilitation in patients referred for lung transplantation. Adv Exp Med Biol. 2013;755:19–25. doi: 10.1007/978-94-007-4546-9_3. [DOI] [PubMed] [Google Scholar]
- 8.Manzetti JD, Hoffman LA, Sereika SM, Sciurba FC, Griffith BP. Exercise, education, and quality of life in lung transplant candidates. J Heart Lung Transplant. 1994;13(2):297–305. [PubMed] [Google Scholar]
- 9.Florian J, Rubin A, Mattiello R, Fontoura FF, Camargo Jde J, Teixeira PJ. Impact of pulmonary rehabilitation on quality of life and functional capacity in patients on waiting lists for lung transplantation. J Bras Pneumol. 2013;39(3):349–356. doi: 10.1590/S1806-37132013000300012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gloeckl R, Halle M, Kenn K. Interval versus continuous training in lung transplant candidates: a randomized trial. J Heart Lung Transplant. 2012;31(9):934–941. doi: 10.1016/j.healun.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Li M, Mathur S, Chowdhury NA, Helm D, Singer LG. Pulmonary rehabilitation in lung transplant candidates. J Heart Lung Transplant. 2013;32(6):626–632. doi: 10.1016/j.healun.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Kenn K, Gloeckl R, Soennichsen A, et al. Predictors of success for pulmonary rehabilitation in patients awaiting lung transplantation. Transplantation. 2015;99(5):1072–1077. doi: 10.1097/TP.0000000000000472. [DOI] [PubMed] [Google Scholar]
- 13.Kawut SM, O'Shea MK, Bartels MN, Wilt JS, Sonett JR, Arcasoy SM. Exercise testing determines survival in patients with diffuse parenchymal lung disease evaluated for lung transplantation. Respir Med. 2005;99(11):1431–1439. doi: 10.1016/j.rmed.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Martinu T, Babyak MA, O'Connell CF, et al. Baseline 6-min walk distance predicts survival in lung transplant candidates. Am J Transplant. 2008;8(7):1498–1505. doi: 10.1111/j.1600-6143.2008.02264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castleberry AW, Englum BR, Snyder LD, et al. The Utility of Preoperative Six-Minute-Walk Distance in Lung Transplantation. Am J Respir Crit Care Med. 2015;192(7):843–852. doi: 10.1164/rccm.201409-1698OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weill D, Benden C, Corris PA, et al. A consensus document for the selection of lung transplant candidates: 2014--an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2015;34(1):1–15. doi: 10.1016/j.healun.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 17.Gift AG. Validation of a vertical visual analogue scale as a measure of clinical dyspnea. Rehabil Nurs. 1989;14(6):323–325. doi: 10.1002/j.2048-7940.1989.tb01129.x. [DOI] [PubMed] [Google Scholar]
- 18.Grant S, Aitchison T, Henderson E, et al. A comparison of the reproducibility and the sensitivity to change of visual analogue scales, Borg scales, and Likert scales in normal subjects during submaximal exercise. Chest. 1999;116(5):1208–1217. doi: 10.1378/chest.116.5.1208. [DOI] [PubMed] [Google Scholar]
- 19.Wilson RC, Jones PW. A comparison of the visual analogue scale and modified Borg scale for the measurement of dyspnoea during exercise. Clin Sci (Lond) 1989;76(3):277–282. doi: 10.1042/cs0760277. [DOI] [PubMed] [Google Scholar]
- 20.Laboratories ATSCoPSfCPF. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 21.Holland AE, Spruit MA, Singh SJ. How to carry out a field walking test in chronic respiratory disease. Breathe (Sheff) 2015;11(2):128–139. doi: 10.1183/20734735.021314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrans CE, Powers MJ. Quality of life index: development and psychometric properties. ANS Adv Nurs Sci. 1985;8(1):15–24. doi: 10.1097/00012272-198510000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Lewinsohn PM, Seeley JR, Roberts RE, Allen NB. Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychol Aging. 1997;12(2):277–287. doi: 10.1037//0882-7974.12.2.277. [DOI] [PubMed] [Google Scholar]
- 24.Eakin EG, Sassi-Dambron DE, Ries AL, Kaplan RM. Reliability and validity of dyspnea measures in patients with obstructive lung disease. Int J Behav Med. 1995;2(2):118–134. doi: 10.1207/s15327558ijbm0202_3. [DOI] [PubMed] [Google Scholar]
- 25.Demeyer H, Burtin C, Hornikx M, et al. The Minimal Important Difference in Physical Activity in Patients with COPD. PLoS One. 2016;11(4):e0154587. doi: 10.1371/journal.pone.0154587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Puhan MA, Chandra D, Mosenifar Z, et al. The minimal important difference of exercise tests in severe COPD. Eur Respir J. 2011;37(4):784–790. doi: 10.1183/09031936.00063810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med. 1998;158(5 Pt 1):1384–1387. doi: 10.1164/ajrccm.158.5.9710086. [DOI] [PubMed] [Google Scholar]
- 28.Tuppin MP, Paratz JD, Chang AT, et al. Predictive utility of the 6-minute walk distance on survival in patients awaiting lung transplantation. J Heart Lung Transplant. 2008;27(7):729–734. doi: 10.1016/j.healun.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 29.Holland AE, Spruit MA, Troosters T, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44(6):1428–1446. doi: 10.1183/09031936.00150314. [DOI] [PubMed] [Google Scholar]
- 30.Yusen RD, Christie JD, Edwards LB, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirtieth Adult Lung and Heart-Lung Transplant Report--2013; focus theme: age. J Heart Lung Transplant. 2013;32(10):965–978. doi: 10.1016/j.healun.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 31.Bailey SP, Brown L, Bailey EK. Lack of relationship between functional and perceived quality of life outcomes following pulmonary rehabilitation. Cardiopulm Phys Ther J. 2008;19(1):3–10. [PMC free article] [PubMed] [Google Scholar]
- 32.Boueri FM, Bucher-Bartelson BL, Glenn KA, Make BJ. Quality of life measured with a generic instrument (Short Form-36) improves following pulmonary rehabilitation in patients with COPD. Chest. 2001;119(1):77–84. doi: 10.1378/chest.119.1.77. [DOI] [PubMed] [Google Scholar]
- 33.de Torres JP, Pinto-Plata V, Ingenito E, et al. Power of outcome measurements to detect clinically significant changes in pulmonary rehabilitation of patients with COPD. Chest. 2002;121(4):1092–1098. doi: 10.1378/chest.121.4.1092. [DOI] [PubMed] [Google Scholar]
- 34.Blumenthal JA, Babyak MA, Keefe FJ, et al. Telephone-based coping skills training for patients awaiting lung transplantation. J Consult Clin Psychol. 2006;74(3):535–544. doi: 10.1037/0022-006X.74.3.535. [DOI] [PubMed] [Google Scholar]
- 35.Emery CF, Schein RL, Hauck ER, MacIntyre NR. Psychological and cognitive outcomes of a randomized trial of exercise among patients with chronic obstructive pulmonary disease. Health Psychol. 1998;17(3):232–240. doi: 10.1037//0278-6133.17.3.232. [DOI] [PubMed] [Google Scholar]
- 36.Tselebis A, Bratis D, Pachi A, et al. A pulmonary rehabilitation program reduces levels of anxiety and depression in COPD patients. Multidiscip Respir Med. 2013;8(1):41. doi: 10.1186/2049-6958-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blumenthal JA, Smith PJ, Durheim M, et al. Biobehavioral Prognostic Factors in Chronic Obstructive Pulmonary Disease: Results From the INSPIRE-II Trial. Psychosom Med. 2016;78(2):153–162. doi: 10.1097/PSY.0000000000000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith PJ, Blumenthal JA, Carney RM, et al. Neurobehavioral functioning and survival following lung transplantation. Chest. 2014;145(3):604–611. doi: 10.1378/chest.12-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parshall MB, Schwartzstein RM, Adams L, et al. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185(4):435–452. doi: 10.1164/rccm.201111-2042ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.