Abstract
Cell fate transitions involve rapid changes in gene expression patterns, yet the role of post-translational modifications in these processes remain underexplored. A recent study identifies SUMOylation as a guardian of cell identity that acts during differentiation and reprogramming by reinforcing active enhancers and maintaining silenced heterochromatin in a context-specific manner.
Main Text
Post-translational modifications (PTMs) expand the diversity and functionality of the cell’s proteome, enabling a single protein to contribute to different processes depending on the cellular and developmental context [1]. SUMOylation has recently emerged as a dynamic PTM that controls protein activity across a wide range of cellular processes, including DNA damage repair, immune responses, cancer progression, transcription and chromatin organization [2]. Similar to ubiquitination, dedicated enzymes termed E1, E2 and E3 catalyze the step-wise, covalent attachment of small ubiquitin-related modifier (SUMO) proteins to target proteins to affect their function, stability or localization. While SUMO-2 is the predominant isoform, the highly similar SUMO-3 isoform (97% sequence identity) and the more divergent SUMO-1 isoform (47% sequence identity) are also present for conjugation to target proteins in mammalian cells. Although SUMOylation was previously shown to be essential for development and to resist reprogramming of somatic cells into induced pluripotent stem cells (iPSCs) [3, 4], its broader role in controlling cellular plasticity and the underlying molecular mechanisms remain essentially unexplored.
A recent study by Cossec et al. in Cell Stem Cell [5] analyzed the functional role of SUMOylation across different cell fate transitions. The authors initially explored the role of SUMOylation during the reprogramming of mouse embryonic fibroblasts (MEFs) into iPSCs, given that SUMO-2 as well as the SUMO E2 conjugase UBC9 (Ube2i) scored prominently in previous shRNA screens for barriers to reprogramming [4, 6]. After confirming that depletion of Ube2i strongly enhanced the generation of iPSCs, the authors performed ChIP-seq for SUMO-1 and SUMO-2 (abbreviated as SUMO) in MEFs and embryonic stem cells (ESCs) to map the location of chromatin-bound SUMOylated proteins. Strikingly, SUMO-binding patterns differed significantly between the two cell types. While SUMO was largely present at enhancer elements in MEFs, it was enriched at heterochromatic regions, such as endogenous retroviral elements (ERVs) and a rare subset of pluripotency-associated super-enhancers, in ESCs. This unexpected contrast in binding patterns suggested that SUMO contributes to gene repression in pluripotent cells but to gene activation in somatic cells. Consistent with this notion, fibroblast-associated genes were downregulated more effectively in Ube2i-depleted cells undergoing reprogramming compared to control cells. However, pluripotency-associated genes were not upregulated more efficiently in these cells. In further agreement with the role of SUMO in stabilizing the somatic program, Ube2i knockdown during reprogramming facilitated the decommissioning of MEF enhancers by reducing the binding of somatic TFs such as C/EBPα and C/EBPβ. Thus, SUMOylation appears to primarily stabilize the somatic program, but has little impact on the reactivation of the ESC-specific program during the induction of pluripotency.
The authors next investigated whether hyposumoylation also facilitates transcription factor-induced cell fate transitions that do not involve a pluripotent state. Indeed, Ube2i suppression, or pharmacological inhibition of the SUMO pathway, facilitated the conversion of MEFs into neurons and B cells into macrophages. Perturbing SUMO also enhanced the differentiation of hematopoietic progenitors into mature cells. Collectively, these functional experiments strengthen the hypothesis that SUMOylation acts a general stabilizer of cell identity, while its suppression facilitates both differentiation and dedifferentiation using different model systems. It remains to be determined how hyposumoylation facilitates these alternative cell fate transitions and whether this involves similar mechanisms as the reprogramming of MEFs to iPSCs (i.e. more efficient extinction of the somatic program). One observation that argues against a role for SUMOylation in preserving the somatic program in these alternative systems is the finding that B cells undergoing transdifferentiation to macrophages following C/EBPα expression and Ube2i suppression show delayed rather than accelerated downregulation of the B cell marker CD19. A more trivial explanation for the effect of Ube2i suppression on transdifferentiation is that hyposumoylation may simply promote the stability of the overexpressed transcription factors, leading to more efficient fate conversion. Indeed, a previous study showed that C/EBPα is directly targeted by SUMOylation, which promotes its degradation [7].
Finally, the authors revisited the puzzling observation that SUMOylation is concentrated proximal to repressed ERV elements in ESCs but not in MEFs. ERVs are typically expressed at the 2C-like state, which corresponds to the 2-cell blastomeres of the preimplantation embryo. Experimentally, ESCs can be converted to the 2C-like state by manipulating certain factors that control cell identity, such as microRNAs and chromatin regulators. As a result, Cossec and colleagues explored whether hyposumoylation might be sufficient to reactivate ERVs and induce a 2C-like state. Indeed, Ube2i suppression in ESCs led to the transcriptional induction of multiple ERV families as well as transcriptional regulators that were shown to be important for acquisition of a 2C-like state such as Dux and Zscan4b/c/d/f. Mechanistically, the authors demonstrate that SUMO suppresses the 2C program in part by stabilizing the binding of components of the alternative PRC1.6 complex (e.g., Ring1b, L3mbtl2, Pcgf6) to the Dux locus in ESCs and maintaining it in a repressed state. These results thus extend the importance of SUMOylation as a safeguard mechanism from somatic to pluripotent cells where it resists acquisition of a more primitive state by reinforcing the silencing of the Dux gene.
The discovery that hyposymoylation increases cellular plasticity across diverse developmental contexts contributes to our basic understanding of how cell identities are preserved at a molecular level. Moreover, the observation that SUMO safeguards cell identity in a context-specific manner by either reinforcing active enhancers in MEFs or silenced heterochromatin in ESCs reveals the unexpected versatility of this PTM in positively or negatively modulating gene expression. However, while this study provides a number of exciting new insights, it also raises several key questions. For instance, it remains unclear which chromatin-associated proteins are SUMOylated and which are functionally relevant during the examined cell fate transitions. The availability of large-scale analysis tools for SUMOylated proteins [8] should help to establish unbiased lists of targets in different cell types that can be validated experimentally. In parallel, functional analyses of SUMOylation sites for known targets could be informative in the context of defined cell fate transitions, such as reprogramming to iPSCs. Indeed, a recent study showed that SUMOylation of the transcription factor Gatad2a blocks reprogramming to iPSCs by favoring assembly and stability of the repressive NURD complex [9], which could partially explain the higher reprogramming efficiency observed in Ube2i-depleted cells.
Related fundamental questions are (1) why is heterochromatin SUMOylated in ESCs but not in MEFs and (2) what distinguishes SUMOylated from non-SUMOylated super-enhancers in ESCs? It is possible that additional epigenetic silencing mechanisms, such as DNA methylation, keep heterochromatic regions in a repressed state in MEFs, thus rendering SUMO dispensable for efficient silencing. Alternatively, SUMOylation of heterochromatin-associated factors, such as HP1a, may contribute to stable chromatin silencing by facilitating phase-separated droplets in ESCs. The advent of nuclease-deficient CAS9 (dCas9) technology to pulldown specific loci may help to address some of these questions in an unbiased way [10]. For example, affinity-tagged dCas9 coupled with ChIP-seq could be employed to understand biochemical differences between silenced heterochromatin in ESCs (SUMOylated) and MEFs (non-SUMOylated), as well as between SUMOylated and non-SUMOylated ESC-specific super-enhancers.
Future studies will undoubtedly focus on these and related questions in order to obtain a more complete picture of the role of SUMOylation in chromatin regulation and cellular plasticity. Resolving these questions may also be relevant in a potential therapeutic setting, as pharmacological modulation of SUMOylation could be exploited to manipulate cell fate in a regenerative or cancer setting.
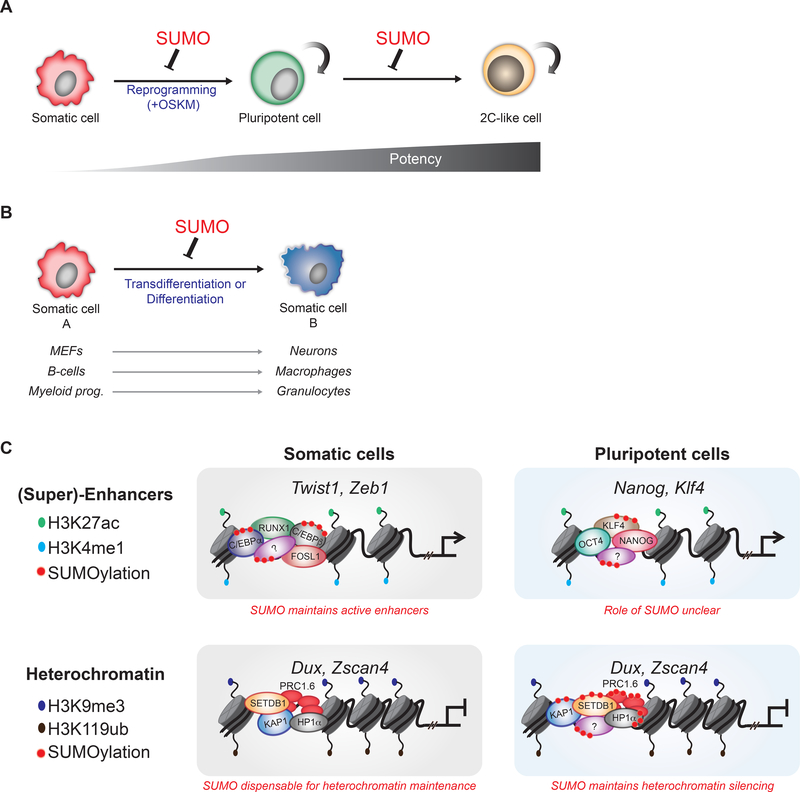
Figure 1. Roles of SUMOylation in controlling cellular and chromatin plasticity.
(A) Hyposumoylation facilitates the reprogramming of mouse embryonic fibroblasts (MEFs) into iPSCs and the conversion of ESCs to 2C-like cells. (B) SUMO suppression facilitates cell fate change in different cellular systems with or without ectopic expression of transcription factors. (C) SUMO influences enhancer activity and heterochromatin levels. In MEFs, SUMO facilitates the cooperative binding of somatic TFs at enhancers. In ESCs, SUMOylation of components of the PRC1.6 complex and of heterochromatic factors such as SETDB1, HP1α and KAP1 contributes to the silencing of ERV-associated genes.
References
- 1.Deribe YL et al. (2010) Post-translational modifications in signal integration. Nat Struct Mol Biol 17 (6), 666–72. [DOI] [PubMed] [Google Scholar]
- 2.Flotho A and Melchior F (2013) Sumoylation: a regulatory protein modification in health and disease. Annu Rev Biochem 82, 357–85. [DOI] [PubMed] [Google Scholar]
- 3.Wang L et al. (2014) SUMO2 is essential while SUMO3 is dispensable for mouse embryonic development. EMBO Rep 15 (8), 878–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borkent M et al. (2016) A Serial shRNA Screen for Roadblocks to Reprogramming Identifies the Protein Modifier SUMO2. Stem Cell Reports 6 (5), 704–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cossec J et al. (2018) SUMO safeguards somatic and pluripotent cell identities by enforcing distinct chromatin states. Cell Stem Cell. [DOI] [PubMed] [Google Scholar]
- 6.Cheloufi S et al. (2015) The histone chaperone CAF-1 safeguards somatic cell identity. Nature 528 (7581), 218–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pabst T and Mueller BU (2009) Complexity of CEBPA dysregulation in human acute myeloid leukemia. Clin Cancer Res 15 (17), 5303–7. [DOI] [PubMed] [Google Scholar]
- 8.Becker J et al. (2013) Detecting endogenous SUMO targets in mammalian cells and tissues. Nat Struct Mol Biol 20 (4), 525–31. [DOI] [PubMed] [Google Scholar]
- 9.Mor N et al. (2018) Neutralizing Gatad2a-Chd4-Mbd3/NuRD Complex Facilitates Deterministic Induction of Naive Pluripotency. Cell Stem Cell 23 (3), 412–425 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang H et al. (2016) CRISPR/Cas9 in Genome Editing and Beyond. Annu Rev Biochem 85, 227–64. [DOI] [PubMed] [Google Scholar]