Abstract
Context
A subset of thyroid carcinomas expresses an oncogenic paired box 8 (PAX8) and peroxisome proliferator activated receptor γ (PPARγ) fusion protein (PPFP). The PPARγ/PPFP ligand pioglitazone is highly therapeutic in a transgenic mouse model of PPFP thyroid carcinoma, but whether pioglitazone is therapeutic in patients with PPFP thyroid carcinoma is unknown.
Case Description
Tumor blocks from 40 patients with progressive thyroid cancer despite standard-of-care therapy were screened for PPFP, and the tumor from only one patient (2.5%) was positive. The patient had a 6.0-cm acetabular soft tissue metastasis from Hürthle cell carcinoma that caused severe pain on weight bearing and had a serum thyroglobulin level of 1974 ng/mL. After 24 weeks of therapy with pioglitazone, the metastatic lesion was 3.9 cm, the thyroglobulin level was 49.4 ng/mL, and the patient was pain-free. Thirteen months after discontinuation of pioglitazone, the metastatic lesion was 3.6 cm, the thyroglobulin level was 4.7 ng/mL, and the patient remained pain-free.
Conclusions
Pioglitazone may be therapeutic in patients with PPFP thyroid cancer. However, thyroid cancers that are progressive despite standard-of-care therapy appear to only rarely express PPFP.
A patient with PPFP-positive thyroid cancer had a major clinical improvement, as well as radiological and biochemical improvements, after therapy with pioglitazone was begun.
PAX8-PPARγ fusion protein (PPFP) is the product of a gene fusion between paired box 8 (PAX8) and peroxisome proliferator activated receptor γ (PPARG) (1). PPFP contains all but the carboxyl terminal region of the PAX8 protein fused to full-length PPARγ1. PAX8 is a transcription factor required for thyroid development (2), and in the mature gland it drives the expression of thyroid-specific genes (3). PPARγ is the master regulator of adipogenesis but is expressed at extremely low levels in the normal thyroid and has no known role in normal thyroid biology (4).
PPFP is a driver mutation in ∼30% of follicular thyroid carcinomas (FTCs) and ∼5% of follicular variant papillary carcinomas (FVPTCs) (5, 6). PPFP also is found in small fractions of poorly differentiated thyroid carcinomas (7, 8), Hürthle cell carcinomas (7, 9), and benign follicular adenomas (6).
PPARγ ligands, such as pioglitazone, are insulin sensitizers and are used to treat type 2 diabetes (10). Pioglitazone, which also is a ligand for PPFP, was remarkably therapeutic in a transgenic mouse model of PPFP thyroid carcinoma, greatly shrinking thyroid size and preventing metastatic disease (11). On the basis of these findings, a clinical trial of pioglitazone in advanced PPFP thyroid carcinomas was instituted.
Clinical Trial and Case Report
After patients provided informed consent, tumor blocks from patients with progressive, radioiodine-refractory thyroid cancer despite standard-of-care therapy were screened for PPFP, with a primary focus on FTCs and FVPTCs. Inclusion and exclusion criteria are available at ClinicalTrials.gov (identifier NCT01655719). Patients with PPFP-positive tumors were eligible for therapy with pioglitazone. The primary endpoint was objective response rate of target lesions based on RECIST (Response Evaluation Criteria In Solid Tumors) 1.1 criteria. Change in serum thyroglobulin was a secondary endpoint. Fifteen patients with PPFP-positive tumors were to be accrued, to yield 14 patients evaluable for response; this would provide 87% power to detect a 40% response rate compared with the expected 5% or less at a 95% two-sided significance level. The institutional review boards of all participating sites approved the protocol.
Mutation testing was performed initially by Asuragen (Austin, TX) and then subsequently by Interpace Diagnostics (Parsippany, NJ) after it acquired the Asuragen assay, for point mutations in BRAF, HRAS, KRAS, and NRAS and for gene rearrangements/translocations RET/PTC1, RET/PTC3, and PPFP. Asuragen used the polymerase chain reaction followed by hybridization on probes conjugated to microspheres and detection by flow cytometry. Interpace Diagnostics used next-generation sequencing. Four samples were tested at the University of Michigan by using fluorescent in situ hybridization with probes near the 5′ and 3′ ends of PPARG to detect a split PPARG allele (Poseidon PPARg [3p25] Break probes, catalog #KBI-10707; Veridex, Raritan, NY). These samples were not tested for other mutations. In the one treated patient, thyroid-stimulating hormone (TSH) and thyroglobulin were measured in the University of Michigan Clinical Pathology Laboratory by chemiluminescent dual antibody sandwich immunoassays on a Siemens (Tarrytown, NY) Centaur analyzer and a Siemens Immulite 2000 analyzer, respectively.
Forty patients had their tumors screened for PPFP, and only one was positive (Table 1). The patient had undergone a hemithyroidectomy on 13 April 2009 at age 84 years for a 7.5-cm follicular Hürthle cell carcinoma. The neck and upper mediastinum then received external-beam radiotherapy, 60 to 64 Gy at 2 to 1.8 Gy/fraction, completed on 6 July 2009. He was maintained on TSH-suppressive therapy with levothyroxine. On 13 June 2011, the thyroglobulin level was 1.3 ng/mL, with a TSH level of 0.13 mIU/L. Thyroglobulin antibodies were negative throughout the patient's course. In 2013, he developed severe right hip pain on weight bearing that resulted in his need to use a wheelchair. Computed tomography (CT) identified a lytic lesion of the right acetabulum with a soft tissue component that measured 3.1 cm on 11 June and 4.3 cm on 14 August 2013. External-beam radiation therapy totaling 30 Gy in 10 fractions was administered; this therapy was completed on 28 August 2013. However, this had no acute effect on the patient’s pain, and he still had to use a wheelchair and was unable to bear weight, even with the support of a walker, during the 2 months after radiation therapy. On CT scan, the acetabulum metastatic lesion measured 6.0 cm on 18 October 2013. The patient received infusions of 4 mg zoledronic acid on 19 August and 10 December 2013 and on 4 November 2014.
Table 1.
Tumor Histologic Types, Sites of Disease, and Mutations Identified in Thyroid Cancers
| Patient a | Sample ID b | Diagnosis | Mutation c | Sites of Disease | Mutation Testing Location |
|---|---|---|---|---|---|
| 1 | 01-007 | FTC | HRAS Q61R | Lung, mediastinum | Asuragen |
| 2 | 02-008 | FTC | HRAS Q61R | Bone, lung | Asuragen |
| 3 | 01-002 | FTC | NRAS Q61K | Bone, lung | Asuragen |
| 4 | 01-012 | FTC | NRAS Q61R | Lung | Interpace Diagnostics |
| 5 | 02-015 | FTC | NRAS Q61R | Bone, lung | Interpace Diagnostics |
| 6 | 03-002 | FTC | NRAS Q61R | Bone, brain, liver, lung, pleura | Asuragen |
| 7 | 03-004 | FTC | NRAS Q61R | Lung, mediastinum | Asuragen |
| 8 | 04-002 | FTC | NRAS Q61R | Bone, lung | Interpace Diagnostics |
| 9 | 04-003 | FTC | NRAS Q61R | Lung | Interpace Diagnostics |
| 10 | 02-005 | FTC | None | Bone, lung, mediastinum | Asuragen |
| 11 | 02-006 | FTC | None | Brain, lung, neck, skin | Asuragen |
| 12 | 03-003 | FTC | None | Lung, mediastinum | Asuragen |
| 13 | 04-001 | FTC | None | Lung | Interpace Diagnostics |
| 14 | 04-004 | FTC | None | Lung, neck | Interpace Diagnostics |
| 15 | 04-005 | FTC | None | Neck | Interpace Diagnostics |
| 16 | 01-003 | FTC | no PPFP | Bone, lung | University of Michigan |
| 17 | 01-004 | FTC | no PPFP | Bone, liver | University of Michigan |
| 18 | 02-012 | FVPTC | BRAF V600E | Bone, lung | Asuragen |
| 19 | 02-013 | FVPTC | BRAF V600E | Lung, mediastinum | Interpace Diagnostics |
| 20 | 02-014 | FVPTC | BRAF V600E | Lung, mediastinum | Interpace Diagnostics |
| 21 | 02-002 | FVPTC | HRAS Q61K | Lung, mediastinum | Asuragen |
| 22 | 01-013 | FVPTC | HRAS Q61R | Lung | Interpace Diagnostics |
| 23 | 02-003 | FVPTC | NRAS Q61K | Lung | Asuragen |
| 24 | 01-009 | FVPTC | NRAS Q61R | Lung | Asuragen |
| 25 | 01-011 | FVPTC | NRAS Q61R | Adrenal, bone, brain, lung, mediastinum, neck | Interpace Diagnostics |
| 26 | 04-006 | FVPTC | None | Lung, mediastinum | Interpace Diagnostics |
| 27 | 01-001 | FVPTC | No PPFP | Bone, liver, lung, mediastinum, neck | University of Michigan |
| 28 | 01-005 | FVPTC | No PPFP | Lung | University of Michigan |
| 29 | 01-006 | Hürthle | PPFP | Bone | Asuragen |
| 30 | 01-010 | Hürthle | None | Bone, lung | Asuragen |
| 31 | 01-008 | Hürthle | None | Lung, mediastinum, neck | Asuragen |
| 32 | 02-001 | Hürthle | None | Bone, lung, mediastinum | Asuragen |
| 33 | 02-009 | Hürthle | None | Lung, neck | Asuragen |
| 34 | 02-010 | Hürthle | None | Bone | Interpace Diagnostics |
| 35 | 02-011 | Hürthle | None | Abdomen lymph node, liver | Asuragen |
| 36 | 02-004 | Insular | NRAS Q61K | Bone, lung | Asuragen |
| 37 | 01-014 | Poorly differentiated and PTC | HRAS Q61K | Bone, lung | Interpace Diagnostics |
| 38 | 02-007 | PTC | NRAS Q61K | Lung, mediastinum | Asuragen |
| 39 | 03-001 | PTC | NRAS Q61R | Adrenal, bone, lung, neck | Asuragen |
| 40 | 01-015 | PTC | None | Lung | Interpace Diagnostics |
Patients are sorted by histologic diagnosis and mutation.
Sample IDs are numbered sequentially by order of screening at each site [(01) University of Michigan Comprehensive Cancer Center (02) University of Colorado Comprehensive Cancer Center (03) Ohio State University Comprehensive Cancer Center (04) University of Texas MD Anderson Cancer Center].
"None" signifies no mutation detected in BRAF, HRAS, KRAS, NRAS, RET/PTC1, RET/PTC3, or PPFP. Testing at the University of Michigan was only for PPFP. See "Clinical Trial and Case Report" section for additional information.
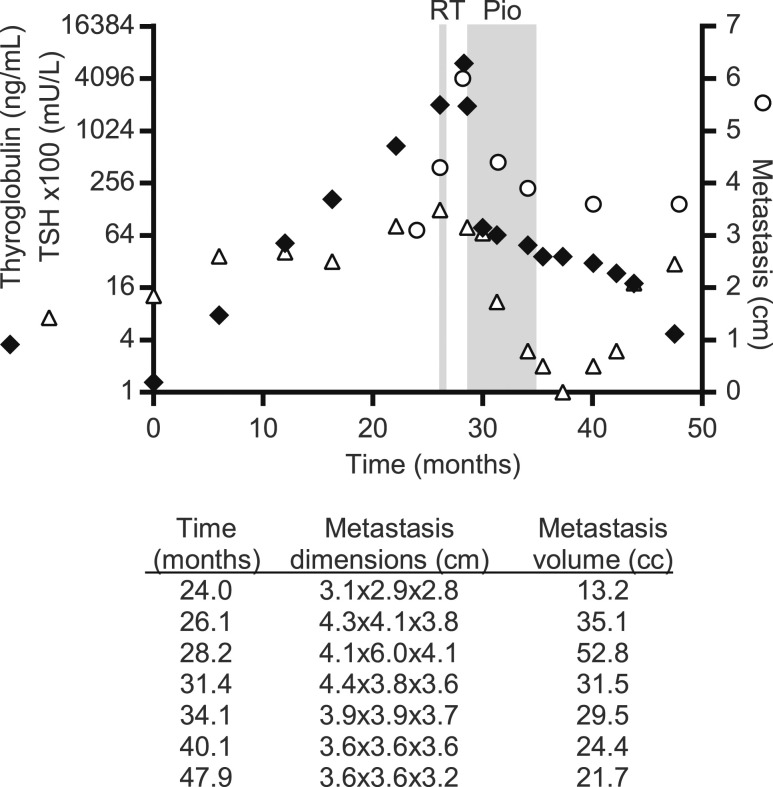
The primary thyroid tumor block tested positive for PPFP, and in consideration of the unremitting pain, the patient was treated with pioglitazone. The right acetabulum soft tissue metastasis was the only target lesion. Pioglitazone was begun on 29 October 2013 at 15 mg/d; the dose was increased to 30 mg after 1 week and to 45 mg after another 3 weeks, and this treatment continued through 24 weeks. There were no drug-associated toxicities. The serum thyroglobulin level was 6081 ng/mL 9 days before the start of pioglitazone and 1974 ng/mL the day pioglitazone was begun. At a 12-week follow-up visit on 21 January 2014, the pain was greatly improved and the patient was able to walk with a walker. The serum thyroglobulin level was 64.5 ng/mL, and the acetabulum metastatic lesion was 4.4 cm. At a 24-week follow-up visit on 15 April 2014, the hip pain had totally resolved and the patient could ambulate without a walker or cane. The serum thyroglobulin level was 49.4 ng/mL and the metastatic lesion was 3.9 cm. Pioglitazone was discontinued on 30 April 2014. The last date of follow-up was 26 May 2015, at which time the patient remained pain-free and the serum thyroglobulin level was 4.7 ng/mL; as of 9 June 2015, the hip metastatic lesion was 3.6 cm. Thyroglobulin levels, TSH levels, and dimensions of metastatic lesion over time are presented in Fig. 1. CT scans of the metastatic lesion are presented in Fig. 2.
Figure 1.
Time course of serum thyroglobulin, TSH, and maximum diameter of the soft tissue metastatic lesion in a patient with PPFP thyroid carcinoma. Radiation therapy (RT) and pioglitazone (Pio) administrations are indicated. To simplify the y-axis, TSH values are given ×100; for example, y-axis 16 = TSH of 0.16. The table below the graph shows, for each time point, all three metastasis dimensions in centimeters (craniocaudal × transverse × anteroposterior) and the metastasis volume, calculated by using the formula of a prolate ellipse.
Figure 2.
CT scans showing the maximum diameter of the soft tissue metastatic lesion in the right acetabulum before and after 24 weeks of pioglitazone.
Discussion
The patient had an apparent life-changing clinical response after initiation of pioglitazone with complete relief of previously debilitating pain. There were a partial tumor response in a skeletal metastatic lesion and a marked decrease in thyroglobulin level. These improvements persisted 13 months after discontinuation of pioglitazone.
However, the exact role of pioglitazone in this response is uncertain. The patient was treated with external-beam radiotherapy 2 months prior to initiation of pioglitazone. Although pain did not improve during those 2 months and the metastatic lesion continued to enlarge, the radiotherapy might have sensitized the tumor to pioglitazone by unknown mechanisms and may have independently contributed to an apparent decline in thyroglobulin in the 9 days preceding pioglitazone. In addition, zoledronic acid has been associated with improved skeletal progression-free survival in other cancers (12, 13) and has been associated with decreased skeletal-related events in thyroid cancer (14). However, we are unaware of an association between zoledronic acid and radiographic response of metastases.
Why might the response have persisted during the 13 months after discontinuation of pioglitazone? In mice with PPFP thyroid cancer, pioglitazone acts as a prodifferentiation agent, converting the thyroid cancer cells into adipocyte-like cells (11). In our patient, pioglitazone might have started the tumor cells on a path of terminal differentiation into adipocyte-like cells, thus resulting in a durable response. This would be consistent with the fact that the serum thyroglobulin decreased by three orders of magnitude, whereas the decrease in tumor size was much more modest.
Identifying patients with rare mutations to pair them with appropriately targeted therapies is a challenge of personalized medicine. PPFP appears to be a rare mutation in the tumors of patients with advanced thyroid cancer. A particularly attractive aspect of pioglitazone is that it has extremely low toxicity. Pioglitazone is US Food and Drug Administration approved for the management of diabetes and does not require special expertise to administer. However, any response of PPFP carcinomas to pioglitazone is likely unique to that driver mutation because the drug target is PPFP itself (11).
If pioglitazone is in fact active against metastatic PPFP carcinoma, we speculate that the drug also could have a role in the nonsurgical management of PPFP carcinomas confined to the thyroid. Low-grade thyroid carcinomas may not all require surgical management, but determining prospectively which tumors fall into that category is extremely difficult. Most PPFP tumors probably are low grade and might be treatable with pioglitazone instead of surgery. However, to more securely demonstrate the efficacy of pioglitazone in PPFP thyroid carcinomas, identification of patients with PPFP-positive tumors would need to be broadly performed to recruit sufficient numbers of patients to such trials.
Acknowledgments
We thank Timothy Muth, Carole Ramm, Gina Tamsen de Rosa, Bev McLaughlin, and Mamdouh Beshara for serving as clinical trial coordinators; Michelle Vinco for pathology support; and Dafydd Thomas for performing the fluorescence in situ hybridizations.
Financial Support: This work was supported by National Cancer Institute grant R01CA166033 (to R.J.K and the Michigan Institute for Clinical and Health Research National Institutes of Health grant UL1TR000433).
Clinical Trial Information: ClinicalTrials.gov no. NCT01655719 (registered on July 25, 2012).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- CT
computed tomography
- FTC
follicular thyroid carcinoma
- FVPTC
follicular variant papillary carcinoma
- PAX8
paired box 8
- PPARG
peroxisome proliferator activated receptor γ
- PPFP
paired box 8 and peroxisome proliferator activated receptor γ fusion protein
- TSH
thyroid-stimulating hormone
References
- 1. Kroll TG, Sarraf P, Pecciarini L, Chen CJ, Mueller E, Spiegelman BM, Fletcher JA. PAX8-PPARgamma1 fusion oncogene in human thyroid carcinoma [corrected]. Science. 2000;289(5483):1357–1360. [DOI] [PubMed] [Google Scholar]
- 2. Macchia PE, Lapi P, Krude H, Pirro MT, Missero C, Chiovato L, Souabni A, Baserga M, Tassi V, Pinchera A, Fenzi G, Grüters A, Busslinger M, Di Lauro R. PAX8 mutations associated with congenital hypothyroidism caused by thyroid dysgenesis. Nat Genet. 1998;19(1):83–86. [DOI] [PubMed] [Google Scholar]
- 3. Esposito C, Miccadei S, Saiardi A, Civitareale D. PAX 8 activates the enhancer of the human thyroperoxidase gene. Biochem J. 1998;331(Pt 1):37–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, Spiegelman BM, Mortensen RM. PPAR γ is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell. 1999;4(4):611–617. [DOI] [PubMed] [Google Scholar]
- 5. Cancer Genome Atlas Research Network Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014;159(3):676–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nikiforova MN, Lynch RA, Biddinger PW, Alexander EK, Dorn GW II, Tallini G, Kroll TG, Nikiforov YE. RAS point mutations and PAX8-PPAR γ rearrangement in thyroid tumors: evidence for distinct molecular pathways in thyroid follicular carcinoma. J Clin Endocrinol Metab. 2003;88(5):2318–2326. [DOI] [PubMed] [Google Scholar]
- 7. Boos LA, Dettmer M, Schmitt A, Rudolph T, Steinert H, Moch H, Sobrinho-Simões M, Komminoth P, Perren A. Diagnostic and prognostic implications of the PAX8-PPARγ translocation in thyroid carcinomas-a TMA-based study of 226 cases. Histopathology. 2013;63(2):234–241. [DOI] [PubMed] [Google Scholar]
- 8. Landa I, Ibrahimpasic T, Boucai L, Sinha R, Knauf JA, Shah RH, Dogan S, Ricarte-Filho JC, Krishnamoorthy GP, Xu B, Schultz N, Berger MF, Sander C, Taylor BS, Ghossein R, Ganly I, Fagin JA. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J Clin Invest. 2016;126(3):1052–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. French CA, Alexander EK, Cibas ES, Nose V, Laguette J, Faquin W, Garber J, Moore F Jr, Fletcher JA, Larsen PR, Kroll TG. Genetic and biological subgroups of low-stage follicular thyroid cancer. Am J Pathol. 2003;162(4):1053–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor γ (PPAR γ). J Biol Chem. 1995;270(22):12953–12956. [DOI] [PubMed] [Google Scholar]
- 11. Dobson ME, Diallo-Krou E, Grachtchouk V, Yu J, Colby LA, Wilkinson JE, Giordano TJ, Koenig RJ. Pioglitazone induces a proadipogenic antitumor response in mice with PAX8-PPARgamma fusion protein thyroid carcinoma. Endocrinology. 2011;152(11):4455–4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gnant M, Mlineritsch B, Schippinger W, Luschin-Ebengreuth G, Pöstlberger S, Menzel C, Jakesz R, Seifert M, Hubalek M, Bjelic-Radisic V, Samonigg H, Tausch C, Eidtmann H, Steger G, Kwasny W, Dubsky P, Fridrik M, Fitzal F, Stierer M, Rücklinger E, Greil R, Marth C; ABCSG-12 Trial Investigators . Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med. 2009;360(7):679–691. [DOI] [PubMed] [Google Scholar]
- 13. Lipton A, Colombo-Berra A, Bukowski RM, Rosen L, Zheng M, Urbanowitz G. Skeletal complications in patients with bone metastases from renal cell carcinoma and therapeutic benefits of zoledronic acid. Clin Cancer Res. 2004;10(18 Pt 2):6397S–6403S. [DOI] [PubMed] [Google Scholar]
- 14. Orita Y, Sugitani I, Toda K, Manabe J, Fujimoto Y. Zoledronic acid in the treatment of bone metastases from differentiated thyroid carcinoma. Thyroid. 2011;21(1):31–35. [DOI] [PubMed] [Google Scholar]