Abstract
Purpose
Chemicals with hormonelike activity, such as estrogenic isoflavones, may perturb human development. Infants exclusively fed soy-based formula are highly exposed to isoflavones, but their physiologic responses remain uncharacterized. Estrogen-responsive postnatal development was compared in infants exclusively fed soy formula, cow-milk formula, and breast milk.
Methods
We enrolled 410 infants born in Philadelphia-area hospitals between 2010 and 2014; 283 were exclusively fed soy formula (n = 102), cow-milk formula (n = 111), or breast milk (n = 70) throughout the study (birth to 28 or 36 weeks for boys and girls, respectively). We repeatedly measured maturation index (MI) in vaginal and urethral epithelial cells using standard cytological methods, uterine volume and breast-bud diameter using ultrasound, and serum estradiol and follicle-stimulating hormone levels. We estimated MI, organ-growth, and hormone trajectories by diet using mixed-effects regression splines.
Results
Maternal demographics did not differ between cow-milk–fed and soy-fed infants but did differ between formula-fed and breastfed infants. Vaginal-cell MI trended higher (P = 0.01) and uterine volume decreased more slowly (P = 0.01) in soy-fed girls compared with cow-milk–fed girls; however, their trajectories of breast-bud diameter and hormone concentrations did not differ. We observed no significant differences between boys fed cow-milk vs soy formula; estradiol was not detectable. Breastfed infants differed from soy-formula–fed infants in vaginal-cell MI, uterine volume, and girls’ estradiol and boys’ breast-bud diameter trajectories.
Conclusions
Relative to girls fed cow-milk formula, those fed soy formula demonstrated tissue- and organ-level developmental trajectories consistent with response to exogenous estrogen exposure. Studies are needed to further evaluate the effects of soy on child development.
In a longitudinal study of infant diet and estrogen-sensitive development, trajectories of uterine involution and vaginal cell maturation differed between infants fed soy formula and cow-milk formula.
Healthy development of estrogen-responsive tissues in early life is critical for normal reproductive function and dependent, in part, on steroid-hormone signaling (1). Early-life exposure to endocrine-disrupting compounds (EDCs) may perturb reproductive development in ways that affect later disease risk. The association of prenatal exposure to diethylstilbestrol (a potent synthetic estrogen) with the subsequent development of cancer, reproductive tract malformations, and infertility supports this concept (2). Epidemiologic evidence for associations of other purported EDCs with adverse reproductive development is limited.
Presently, the greatest source of human exposure to estrogenic EDCs, apart from pharmaceuticals such as oral contraceptives, comes from dietary phytoestrogens (3). Genistein, a phytoestrogen found in soy protein, can bind to and activate estrogen receptors (ERs), particularly ER-β, albeit with much lower affinity than endogenous estradiol (4). Soy formula, in particular, provides a large, well-characterized amount of genistein (5). Combining all such dietary exposures into “estrogen equivalents,” infants fed soy formula have approximately 1000-fold greater total estrogen exposure (6, 7) and urinary genistein concentrations 500 times higher than other infants (5). These higher genistein concentrations overlap genistein concentrations that induce, for example, changes in reproductive tract histopathology, estrous cyclicity, and fertility in rodent models (serum levels of genistein of ∼500 to 1800 ng/mL ) (8). Several epidemiologic studies suggest an association between soy formula use and adult reproductive health (9–12), but little is known about reproductive system changes that may occur during infancy, near the time of soy formula exposure (13–15).
Estrogen-responsive development begins in utero and continues into the postnatal period. It is closely tied to exposure to maternal estrogen during pregnancy, withdrawal from maternal estrogen after birth, and, subsequently, in female infants, transient and variable estrogen production for approximately 2 years, often termed “minipuberty” (16, 17). Fetuses and infants of both sexes have estrogen-responsive tissue. Maternal estrogen concentration increases steeply through gestation, and male and female newborns exhibit increasing estrogenization of breast and external genitalia with advancing gestational age (18). Then, in the early postnatal period, concurrent with the loss of maternally sourced estrogen, infants exhibit declining estrogenization, evidenced by decreasing size of the breast (15) and uterus (19), and loss of superficial cells in the urogenital epithelium (15, 20).
We hypothesized that early postnatal exposure to soy formula prolongs the physiologic estrogenization of the newborn. This study’s primary aim was to characterize the postnatal development of estrogen-responsive tissues (i.e., uterus, breast bud, and urogenital epithelium), serum estradiol concentrations, and follicle-stimulating hormone (FSH) concentrations according to infant-feeding practices, with an emphasis on comparison between infants fed soy formula and those fed cow-milk formula. Evidence of a contemporaneous physiologic response in soy formula–fed infants, who would be predicted to respond on the basis of the laboratory evidence, would support the concept that endocrine disrupters detected in the laboratory are active in humans. Such evidence would also contribute to our understanding of early-life disruption of steroid-hormone signaling in humans, not only by soy but also by estrogenic EDCs more generally.
Materials and Methods
Study design
The Infant Feeding and Early Development (IFED) Study was an observational study that assessed estrogen-responsive tissues and organs longitudinally during the postnatal period. Between August 2010 and November 2013, IFED staff enrolled mothers and their neonates from eight Philadelphia, Pennsylvania, regional hospitals (21) (Supplemental Methods). Eligible mothers had decided before contact by study staff to feed their infants soy formula, cow-milk formula, or breast milk exclusively from birth, spoke English, were 18 years of age or older, and had no endocrine disorder (including polycystic ovary syndrome), diabetes (including gestational diabetes), or thyroid conditions, and no use of steroid medication (except inhaled or topical glucocorticoids), immunosuppressants, or hormones to maintain pregnancy during the pregnancy. Eligible infants were healthy singletons of 37 to 42 weeks’ gestation who weighed between 2500 and 4500 g at birth. Male infants with hypospadias or nonpalpable testes, and infants with ambiguous genitalia or serious illness, including major congenital malformations, chromosomal anomalies, sustained respiratory distress, or other condition known to affect growth and development, were excluded. No siblings were enrolled. We compensated families for their time and inconvenience. In addition, incentives such as formula and supplemental diapers were given (Supplemental Methods).
Abstraction from 26,383 medical charts at delivery identified 3946 potentially eligible mother-infant dyads who were approached at birth. Of these, 524 mothers granted consent to undergo screening for the study and 340 enrolled. An additional 94 mothers were recruited from prenatal clinics before giving birth, 70 of whom met birth eligibility criteria and enrolled following delivery. Mothers provided informed written consent. Institutional review boards at the Children’s Hospital of Philadelphia, Virtua Hospitals, Abington Memorial Hospital, the National Institute of Environmental Health Sciences, and Copernicus Group approved the protocol.
Continued participation in the IFED Study required that the infant continue to meet the health criteria specified at enrollment and also that the mother adhere to one of the study’s three simple feeding regimens (i.e., breast milk, cow-milk formula, or soy formula), allowing for only limited excursions. At the week-2 visit, we assessed feeding history since birth and placed infants into feeding groups, using the following criteria: breastfed infants received 90% to 100% of calories from breast milk and ≤1% of calories from soy formula; infants fed cow-milk formula received 90% to 100% of calories from cow-milk formula and ≤1% of calories from soy formula; and soy formula–fed infants received 90% to 100% of calories from soy formula. If infants did not meet these criteria at the week-2 visit, they were withdrawn from the study. At each subsequent visit, we administered the feeding-history questionnaire to assess such adherence since the previous visit. The threshold for feeding-regimen adherence lowered from 90% to 100% of calories to 85% at 13 weeks of age and then 80% at 25 weeks of age to accommodate mothers who were supplementing, though the strict 1% upper limit on calories from soy formula for breastfed and cow-milk formula–fed infants was retained. Nonadherence resulted in withdrawal. To validate feeding adherence assessed by questionnaire, we measured isoflavones in urine samples from a randomly selected subset of infants fed cow-milk formula (n = 19) and those fed soy formula (n = 20) at age 12 weeks (Supplemental Methods).
Birth visits were completed within 72 hours of delivery. For girls, subsequent visits were scheduled at 2 and 4 weeks (±4 days); 8 and 12 weeks (±10 days); 16, 20, 24, 28, and 32 weeks (±14 days); and 36 weeks (±30 days). Before June 2012, subjects were also seen at week 6. Boys’ follow-up visits were scheduled similarly and were designed to conclude at 28 weeks (±30 days) based on pilot data that suggested more prolonged response in girls than boys (15) (Supplemental Table 1). At each visit, we verified adherence to feeding regimen by structured questionnaire, performed anthropometry, and collected urogenital epithelial cells; blood collection began at week 2. Also at week 2, mothers completed questionnaires and provided information on reasons for feeding choices. We obtained ultrasound images of breast buds and uterus at birth and weeks 4, 16, 24, and 32. Our protocol allowed ultrasound measures to be performed at the visit immediately before or after the designated visit to accommodate the scheduling needs of our study subjects. Before June 2012, we also obtained ultrasound images at week 8.
Optimal participation allowed for many of these measures (e.g., anthropometry, urogenital cells, blood) to be collected at every visit, but at minimum, mothers and infants were required to contribute a core set of three (boys) or four (girls) blood samples. For boys, required blood-sample collections were set at 2 or 4 weeks; 8, 12, or 16 weeks; and 24 or 28 weeks. For girls, required blood-sample collections were set at 2 or 4 weeks; 8 or 12 weeks; 20 or 24 weeks; and 32 or 36 weeks. Follow-up visits ended in March 2014.
Ultrasound imaging
All sonographers were trained and certified according to the IFED Study protocol. For uterus and breast buds, we obtained three or four images each in the transverse (TRV) and sagittal (SAG) views (21). Each dimension—SAG, TRV, and anterior-posterior (AP)—was measured three or four times from separate images according to protocol. The geometric mean of the multiple measurements was used as a dimension-specific diameter. We calculated uterine volume, approximated as a cylinder, from the diameters as follows [Eq. (1)]:
Breast-bud diameter was calculated as the geometric mean of the TRV and SAG diameters from both left and right breasts. Additional details are described in the Supplemental Methods.
Urogenital cytology
Urogenital epithelial cells were collected via swab of the vaginal introitus (girls) or urethral meatus (boys) at every study visit and were processed according to previously described methods (Tricore Reference Laboratories, Albuquerque, NM) (20). In brief, samples were stained using standard procedures (GYN SurePath and SurePath PrepStain; BD, Franklin Lakes, NJ). Applying established criteria (22), blinded cytotechnologists scored 100 cells per sample and classified each cell as either basal/parabasal, intermediate, or superficial. In 6% of boys and 1% of girls, samples included fewer than 100 cells, so 50 cells were counted and doubled. Superficial cells are indicative of estrogenization (23–25), a phenomenon that is widely established in adults and infants (26–28) and has been measured in studies of dietary soy exposure in postmenopausal women (29, 30). We quantified estrogenization using a Maturation Index (MI) equal to half the percentage of intermediate cells plus the percentage of superficial cells (15, 20, 31).
Serum hormones
Serum 17-β estradiol (E2) was analyzed using an isotope-dilution liquid chromatography–tandem mass spectrometry method (US Centers for Disease Control and Prevention, Atlanta, GA) (32). E2 was dissociated from binding proteins, extracted from serum using liquid-liquid extractions, and analyzed by liquid chromatography–tandem mass spectrometry using negative electrospray ionization. The E2 method interassay coefficient of variation was < 4.6% over 20 analytical runs and the limit of detection was 2.99 pg/mL. To monitor accuracy, each run included the reference material BCR576, at 31.1 pg/mL, from the Institute for Reference Materials and Measurement; the mean bias was −0.7% [95% confidence interval (CI), −2.0% to 0.5%]. FSH in girls’ serum was analyzed using an electrochemiluminescence immunoassay (Quest Diagnostics Nichols Institute, San Juan Capistrano, CA) optimized for pediatric use (limit of quantitation: 0.05 mIU/mL; limit of detection: 0.007 mIU/mL; intrassay coefficient of variation, 9.75%; and interassay coefficient of variation, 11%, at an approximate FSH concentration of 0.5 mIU/mL).
Statistical analysis
Analyses were limited to infants who contributed a minimum of three (boys) to four (girls) blood samples (Supplemental Methods). We tested feeding-group differences in maternal and infant characteristics with χ2 tests or t tests. We examined feeding-group differences in age trajectories using mixed-effects regression splines under normality assumptions. Our strategy was similar for all outcomes, though some details differed. Trajectories were represented as natural cubic splines with four knots, common to all feeding groups and equally spaced in the square root of age (days) to accommodate closer visit spacing in early weeks. The mixed-effects model included subject-specific random effects for each regression coefficient, allowing each study subject to have a separate trajectory that deviated from the average. Uterine volume, breast-bud diameter, and hormone concentrations were log2 transformed for analyses; MI was untransformed. We report comparisons between all feeding groups, but emphasize cow-milk formula as the most appropriate comparison diet for soy formula, because women who breastfeed tend to differ from women who formula feed in multiple and often unmeasurable ways, and because breast milk may contain estrogenic pollutants or hormones (33), albeit at low concentrations, that were not measured in this study.
Under the assumption that any differences in MI or organ size present at birth are unrelated to postnatal feeding regimen, we compared postnatal trajectories between the feeding groups for those outcomes, using contrasts among spline coefficients, excluding the intercept. To display these postnatal trajectory comparisons (denoted “relative trajectories”), we shifted the fitted feeding-group–specific trajectories (denoted “absolute trajectories”) vertically to have a common intercept. In sensitivity analyses, we adjusted all models for neonatal weight and gestational age. SAS, version 9.3 (SAS Institute, Cary, NC) was used for statistical analysis.
Results
Of 410 mother-infant dyads enrolled, 283 (69%) completed the study, comprising 111 infants fed cow-milk formula (73% of enrolled), 102 fed soy formula (68%), and 70 breastfed (64%). Infants who did not complete the study did not differ demographically or by feeding group from those who completed the study (Supplemental Table 2). Common reasons for exiting the study included feeding-method change (38%) and loss of contact or missed appointments (39%); 69% of exits occurred before age 8 weeks. Of those who completed the study (Table 1; Supplemental Table 2), 70% of mothers were black and 57% had a high school education or less. Mothers of breastfed infants had higher educational attainment and were more likely to be white. At the birth visit, breastfed boys were heavier than boys fed cow-milk formula or soy formula, whereas girls fed soy formula were heavier than those fed cow-milk formula. Weight gain between birth and the 12-week visits did not differ between feeding groups for boys (P = 0.22) or girls (P = 0.58). Common reasons for choosing soy formula were successful use with a previous child, perceived healthfulness, and anticipated lactose intolerance in the infant based on family history (Table 2).
Table 1.
Maternal and Infant Characteristics by Feeding Group and Infant Sex
| Girls | Boys | |||||||
|---|---|---|---|---|---|---|---|---|
| Soy Milk | Cow Milk | Breast Milk | Total | Soy Milk | Cow Milk | Breast Milk | Total | |
| No. of dyads | 48 | 56 | 32 | 136 | 54 | 55 | 38 | 147 |
| Maternal race | ||||||||
| Black | 38 (79) | 45 (80) | 15 (47) | 98 (72) | 42 (78) | 45 (82) | 14 (37) | 101 (69) |
| White | 7 (15) | 8 (14) | 14 (44) | 29 (21) | 11 (20) | 7 (13) | 16 (42) | 34 (23) |
| Other/multiracial/unknown | 3 (6) | 3 (5) | 3 (9) | 9 (7) | 1 (2) | 3 (5) | 8 (21) | 12 (8) |
| χ2P valuea | 1.00 | <0.01 | 0.38 | <0.0001 | ||||
| Maternal education | ||||||||
| Less than high school | 14 (29) | 8 (14) | 0 | 22 (16) | 11 (20) | 19 (35) | 0 (0) | 30 (20) |
| High school or GED | 18 (37) | 34 (61) | 8 (25) | 60 (44) | 26 (48) | 16 (29) | 8 (21) | 50 (34) |
| Some college or Associates degree | 13 (27) | 12 (21) | 10 (31) | 35 (26) | 14 (26) | 15 (27) | 10 (26) | 39 (27) |
| College or postgraduate | 3 (6) | 2 (4) | 14 (44) | 19 (14) | 3 (6) | 5 (9) | 20 (53) | 28 (19) |
| χ2P valuea | 0.14 | <0.0001 | 0.19 | <0.0001 | ||||
| Maternal age (y) | ||||||||
| Mean (SD) | 26.1 (5.0) | 25.2 (5.5) | 26.9 (5.2) | 25.9 (5.3) | 25.9 (5.5) | 25.7 (6.6) | 28.0 (5.4) | 26.4 (5.9) |
| Median | 26 | 24 | 27.5 | 26 | 25 | 25 | 28 | 26 |
| Minimum, maximum | 18, 40 | 18, 40 | 18, 37 | 18, 40 | 18, 43 | 18, 42 | 19, 38 | 18, 43 |
| P valueb | 0.38 | 0.27 | 0.86 | 0.05 | ||||
| Neonatal weight,c kg | ||||||||
| Mean (SD) | 3.24 (0.4) | 3.06 (0.4) | 3.13 (0.4) | 3.14 (0.4) | 3.21 (0.4) | 3.24 (0.4) | 3.42 (0.4) | 3.27 (0.4) |
| Median | 3.20 | 3.03 | 3.12 | 3.09 | 3.27 | 3.21 | 3.41 | 3.28 |
| Minimum, maximum | 2.40, 4.09 | 2.40, 3.87 | 2.51, 3.99 | 2.40, 4.09 | 2.47, 4.12 | 2.36, 4.28 | 2.51, 4.20 | 2.36, 4.28 |
| P valueb | 0.02 | 0.88 | 0.77 | 0.01 | ||||
| Gestational age, wk | ||||||||
| 37 | 3 (6) | 6 (11) | 3 (9) | 12 (9) | 5 (9) | 5 (9) | 1 (3) | 11 (7) |
| 38 | 6 (13) | 16 (29) | 7 (22) | 29 (21) | 9 (17) | 9 (16) | 4 (10) | 22 (15) |
| 39 | 23 (48) | 19 (34) | 9 (28) | 51 (37) | 27 (50) | 17 (31) | 11 (29) | 55 (37) |
| 40 | 13 (27) | 8 (14) | 10 (31) | 31 (23) | 10 (18) | 19 (35) | 9 (24) | 38 (26) |
| 41 | 3 (6) | 7 (12) | 3 (9) | 13 (10) | 3 (6) | 5 (9) | 13 (34) | 21 (14) |
| χ2P valuea | 0.09 | 0.71 | 0.25 | <0.01 | ||||
Data given as no. (%) unless otherwise indicated.
Abbreviations: GED, general equivalency diploma; SD, standard deviation.
χ2P values are from exact tests based on likelihood ratio statistics. The P value straddling Cow Milk and Soy Milk columns compares those two feeding groups; the P value under Breast Milk compares the breast-fed group with the cow- and soy-formula–fed groups combined.
P values from t tests of contrasts in a one-way analysis of variance. The P value straddling Cow Milk and Soy Milk columns compares those two feeding groups; the P value under Breast Milk compares the breast-fed group with the cow- and soy-formula–fed groups combined.
Neonatal weight is based on birth-visit weight measurement, conducted within first 72 hours after birth.
Table 2.
Maternal Report of Reasons for Soy Formula Use
| Reasona | No.b | Major Reason, (%) | Minor Reason (%) | Not a Reason (%) |
|---|---|---|---|---|
| I fed my other child(ren) soy formula. | 102 | 54 | 6 | 40 |
| I think soy is healthier than other types of formula. | 88 | 54 | 15 | 31 |
| I suspect my baby had milk intolerance or my family has trouble digesting cow’s milk. | 88 | 52 | 14 | 34 |
| My family or friends recommended it. | 88 | 27 | 7 | 66 |
| I chose soy formula for religious reasons. | 102 | 17 | 4 | 79 |
| Health care provider recommended soy. | 102 | 15 | 10 | 75 |
| I prefer a dairy-free diet. | 102 | 12 | 14 | 74 |
| My child had colic or other digestive problem. | 102 | 7 | 5 | 88 |
| I suspected my baby had a food allergy or intolerance (other than milk). | 102 | 7 | 13 | 80 |
| Other | 102 | 4 | 1 | 95 |
| Family follows a vegan diet. | 102 | 1 | 3 | 96 |
Mothers evaluated each reason independently as a major reason, minor reason, or not a reason for choosing soy formula. More than one major or minor reason could be selected.
Owing to questionnaire version changes, some responses were only captured for 88 of 102 soy formula–feeding mothers.
Our urinary isoflavone validation substudy provided evidence of high feeding-regimen adherence among those who completed the study. Median (minimum, maximum) urinary genistein concentrations, measured in the feeding-group validation subset, were 9,460 ng/mL (52, 102,000 ng/mL) and 7 ng/mL (1, 102 ng/mL) for the 20 soy formula–fed and 19 cow-milk formula–fed infants, respectively. Using a 200 ng/mL cut point as evidence of a soy diet (5), our feeding assessment questionnaire had specificity 0.95 and sensitivity 1.0.
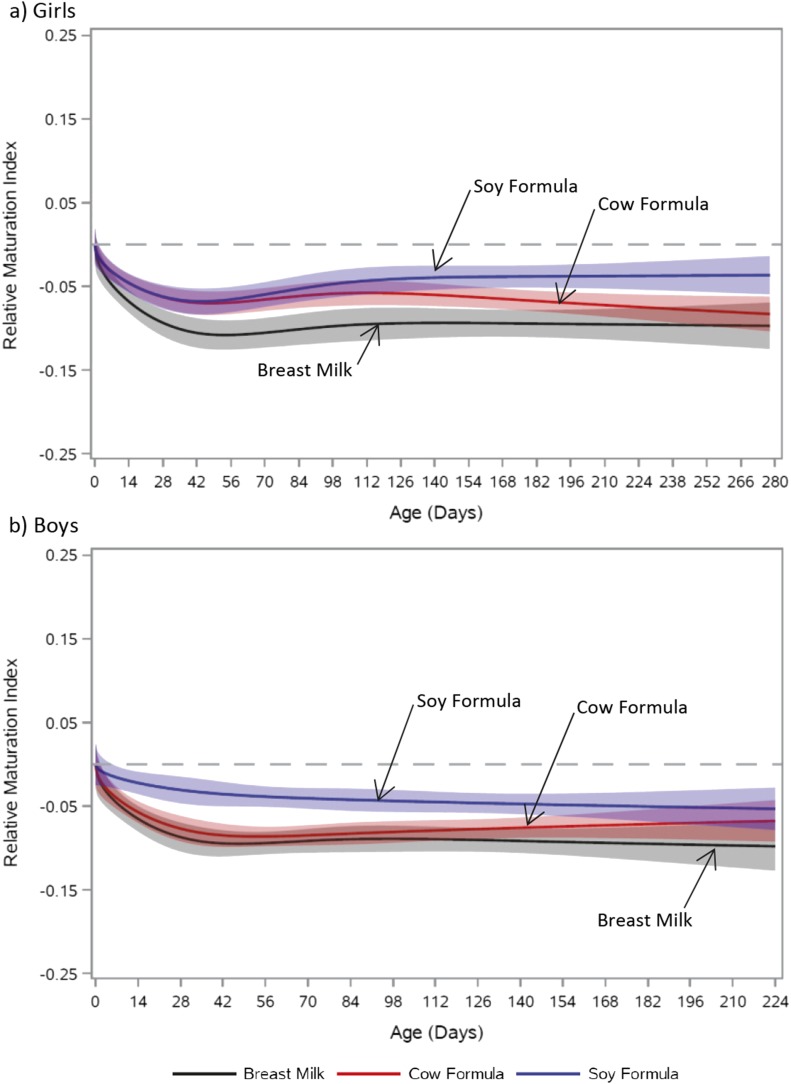
The relative MI trajectory of soy formula–fed girls differed significantly from those of girls fed cow-milk formula (P = 0.01) and those who were breastfed (P = 0.01; Fig. 1a; Supplemental Fig. 1a). Trajectories for each of the girls’ feeding groups demonstrated an initial decline in MI; however, with increasing age, the MI trajectory of girls fed soy formula diverged upward relative to that of the other feeding groups. Breastfed and cow-milk formula–fed boys also had MI trajectories with an initial decline. This decline was absent in soy formula–fed boys (Fig. 1b; Supplemental Fig. 1b). Ultimately, these feeding-group MI trajectory differences in boys were not statistically significant (cow-milk formula vs soy formula P = 0.08; breast milk vs soy formula P = 0.10; breast milk vs cow-milk formula P = 0.67).
Figure 1.
Relative trajectory of MI vs age by feeding group. (a) Girls: soy formula vs cow-milk formula, P = 0.01; soy formula vs breast milk, P = 0.01; breast milk vs cow-milk formula, P = 0.23. (b) Boys: soy formula vs cow-milk formula, P = 0.08; soy formula vs breast milk, P = 0.10; breast milk vs cow-milk formula, P = 0.67. Feeding-group–specific trajectories fitted to data were shifted vertically to a common intercept at zero (dashed line) to better display postnatal changes (i.e., fitted MI at a given age minus fitted MI at birth). Thus, the vertical axis represents change in MI since birth. Shaded bands represent 95% pointwise confidence limits for each feeding-group–specific trajectory. Absolute trajectories (i.e., those without vertical shift to common intercept) are shown in Supplemental Fig. 2.
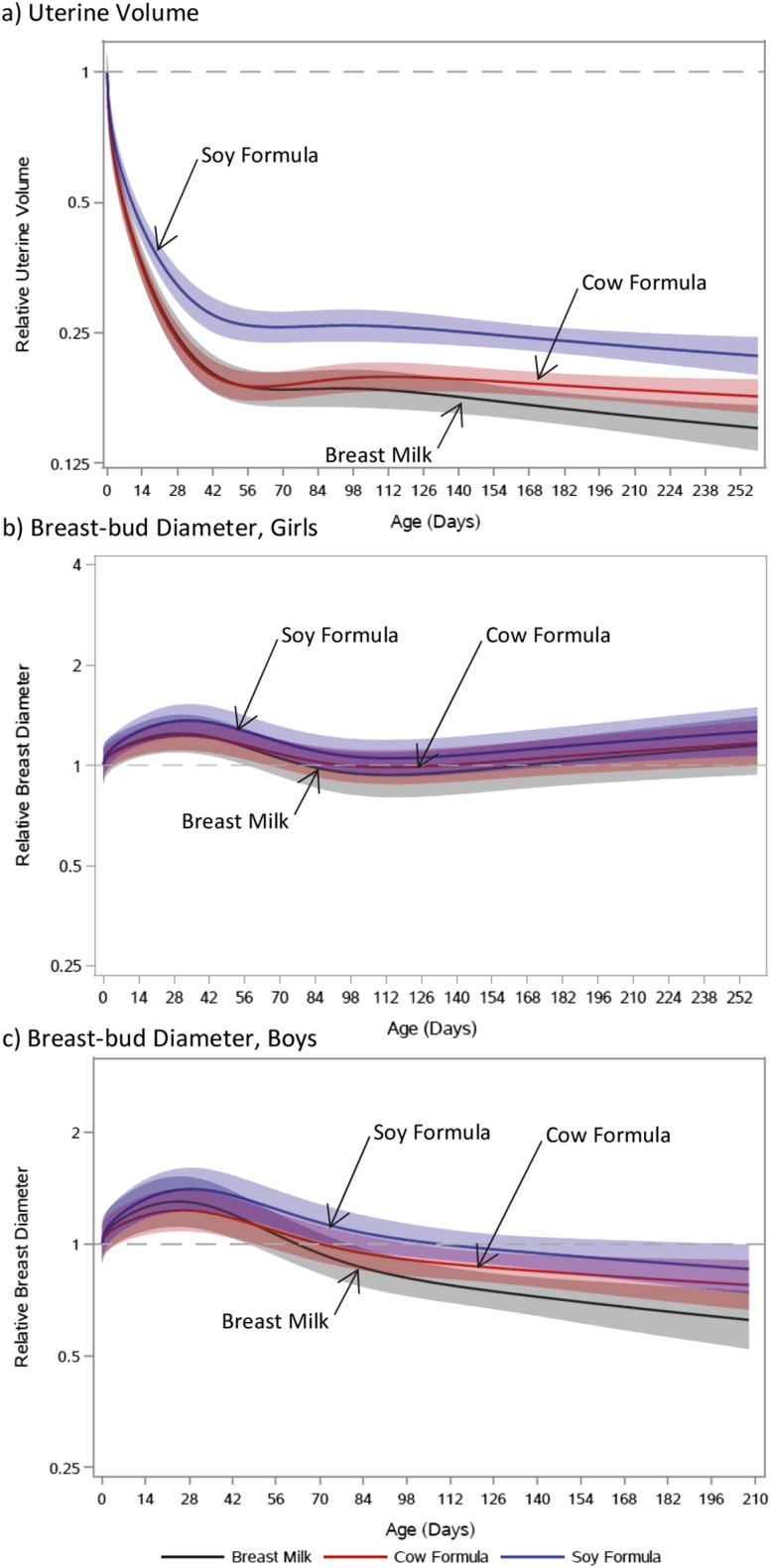
The uteri of soy formula–fed girls were smaller at the near-birth measurement (11.2 cm3) than those of cow-milk formula–fed (14.0 cm3) or breastfed girls (13.5 cm3; Supplemental Fig. 2a). Trajectories of relative uterine volume showed that involution was slower among soy formula–fed girls than among cow-milk formula–fed (P = 0.01) and breastfed girls (P < 0.01; Fig. 2a). Though trajectories for cow-milk formula–fed and breastfed girls were similar, breastfed girls had lower uterine volumes later in infancy (P = 0.07).
Figure 2.
Relative trajectories of organ size via ultrasound vs age by feeding group. (a) Uterine volume in girls (soy formula vs cow-milk formula, P = 0.01; soy formula vs breast milk, P < 0.01; breast milk vs cow-milk formula, P = 0.07). (b) Breast-bud diameter in girls (soy formula vs cow-milk formula, P = 0.72; soy formula vs breast milk, P = 0.73; breast milk vs cow-milk formula, P = 0.92). (c) Breast-bud diameter in boys (soy formula vs cow-milk formula, P = 0.37; soy formula vs breast milk, P = 0.02; breast milk vs cow-milk formula, P = 0.05). Feeding-group–specific trajectories fitted to data were shifted vertically to a common intercept at a log2-transformed value of zero (dashed line) to better display postnatal changes (i.e., fitted log2-transformed organ size at a given age minus fitted log2-transformed organ size at birth). Thus, the vertical axis represents the ratio of current size to size at birth. Shaded bands represent 95% pointwise confidence limits for each feeding-group–specific trajectory. Absolute trajectories (i.e., those without vertical shift to common intercept) are shown in Supplemental Fig. 3.
Girls demonstrated a transient peak in breast-bud diameter at 4 weeks of age, then stable diameters, with no evident feeding-group differences (Fig. 2b; Supplemental Fig. 2b). Boys demonstrated a transient peak at 4 weeks of age, then falling diameters (Fig. 2c; Supplemental Fig. 2c). After the transient peak, there was a larger decrease in breast-bud diameter in breastfed boys than boys fed soy formula (P = 0.02) or cow-milk formula (P = 0.05).
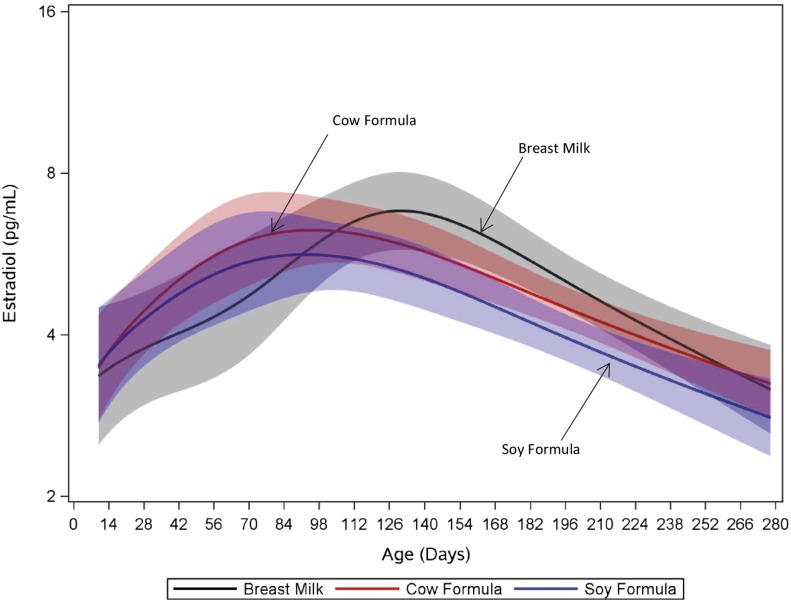
Approximately 33% of girls had detectible concentrations of E2 at week 2, increasing to 85% at week 16 and decreasing to 50% by week 36 (Supplemental Fig. 3). The proportion of samples having detectible E2 did not differ by feeding group (P = 0.69). Median (25th, 75th percentile) E2 concentrations were 6.0 pg/mL (2.1, 10.7 pg/mL) and 6.4 pg/mL (3.9, 10.0 pg/mL) at 12 and 16 weeks, respectively. E2 trajectories did not differ between soy formula–fed and cow-milk formula–fed girls (P = 0.44) or between cow-milk formula–fed and breastfed girls (P = 0.17) but did differ between breastfed and soy formula–fed girls (P = 0.02) (Fig. 3). We saw no differences in girls’ FSH concentration trajectories among feeding groups (Supplemental Fig. 4). Among boys, we detected E2 in only 10 of 1150 samples (0.9%) from 10 subjects, all at week 2 or 4.
Figure 3.
Fitted E2 trajectory vs age by feeding group in girls. Colors code feeding groups: black, breast milk; red, cow-milk formula; blue, soy formula. Values below the limit of detection (2.99 pg/mL) are modeled at the limit of detection divided by the square root of 2. Shaded bands represent 95% pointwise confidence limits for each feeding-group–specific trajectory. Each subject contributed up to 11 measurements through time. Soy formula vs cow-milk formula, P = 0.44; soy formula vs breast milk, P = 0.02; breast milk vs cow-milk formula, P = 0.17.
Results for MI, organ size, and E2 and FSH levels were insensitive to neonatal weight and gestational age adjustment, with minor exceptions for borderline associations: Differences between MI trajectories of soy-fed boys and both cow-milk formula–fed and breastfed boys more closely approached significance after gestational age adjustment (P = 0.05 for both comparisons); differences between uterine volume trajectories for cow-milk formula–fed and breastfed girls reached statistical significance after adjustment for neonatal weight (P = 0.04) and gestational age (0.03).
Discussion
Compared with infants fed cow-milk formula, infants fed soy formula from birth demonstrated evidence of estrogen response in the urogenital epithelium and uterus but did not demonstrate differences in breast or serum E2 concentrations, or, in girls, FSH concentrations. Cells from the vaginal introitus had an increasingly higher MI in soy formula–fed girls than in cow-milk formula–fed girls through 36 weeks. Cells from the distal urethra had a higher MI in soy formula–fed boys than cow-milk formula–fed boys through approximately 6 weeks, after which the difference diminished and then disappeared by approximately age 28 weeks. In addition, uterine volumes involuted more slowly in soy formula–fed girls. These effects are accepted as evidence of estrogen exposure (34, 35).
E2 is known to be detectable at highly variable concentrations among infant girls (16, 17). At 12 weeks of age, median E2 concentrations have been estimated at approximately 8 to 9 pg/mL (30 to 31 pmol/L) (36, 37); FSH concentrations have been estimated to be 3.8 mIU/mL (95% CI, 1.2 to 18.8 mIU/mL) at this age (36). However, many previous studies of E2 in infancy relied on methods such as radioimmunoassay, which may lack appropriate sensitivity to detect E2, particularly at low concentrations (32). We used a state-of-the-art mass spectrometry method to measure E2; our E2 results at 12 weeks were slightly lower than those in some previous reports and this potentially is attributable to improved accuracy. Notably, the most dynamic period with respect to organ and tissue trajectories were in the immediate postpartum period during withdrawal from maternal estrogen; later responses to endogenous estrogen during proposed minipuberty were not noted, although the different responses to soy formula between girls’ and boys’ MI may be due, in part, to the girls’ concurrent E2 production.
Our study was not a randomized trial, and our results may be influenced by differences in families or mothers choosing the different feeding regimens, differences between infants, or other potentially confounding factors. Postnatal urogenital cytological changes and uterine size in infants have not been much studied, and there are no established confounding factors related to these measures. The two formula groups did not differ significantly in maternal race, age, or education. The soy-fed girls were heavier at the birth visit, but adjustment for this did not change our findings. In general, few mothers in the United States choose soy formula as their first formula. In our study, mothers’ stated reasons for choosing to start with soy formula included successful previous use, perceived healthfulness, and family history, or suspicion of future onset of, lactose intolerance in their infants. Confounding by indication could produce our findings if these reasons are associated with an estrogen effect on urogenital cytology and uterus independently of actual soy formula use. Because we did not study infants whose mothers preferred soy formula but who did not use it, we cannot rule out such confounding, but we suggest it to be unlikely. An additional source of confounding may be exposure to other estrogenic compounds from the home environment or elsewhere, particularly if substantial exposure was more likely to occur among soy-fed infants. Conversely, if these exposures were more likely to occur in nonsoy-feeding groups, we may be underestimating the effects of soy. However, the current study was not designed to characterize such exposures.
Using an ultrasound protocol similar to ours (19), Gilchrist et al. (13) found no difference in uterine or breast volume among 4-month-old girls (soy formula fed, n = 20; cow-milk formula fed, n = 23; or breastfed, n = 20). Some of these children were evaluated again at age 5 years (38) and showed no feeding-group differences. The inconsistency between our findings and theirs may be due to the smaller sample size of their cohort, the later introduction of soy formula in their cohort, or to the absence of repeated measures in their study design; they could not characterize differences in organ growth trajectories nor allow adjustment for feeding-group differences present at birth. An Israeli study found greater prevalence of palpable breast tissue in 2-year-old children fed soy formula as infants (14), and premature thelarche was noted in another study among children fed soy formula (among other exposures) (39). Although we did not observe any difference in breast-tissue growth in infant girls, we did observe that those fed soy formula tended to have larger breast diameter. The transient peak in breast diameter we describe in all feeding groups has been previously reported (40), although our ultrasound-based measurements yielded slightly larger diameters than those determined by palpation in previous studies (37). We know of no other study besides our small pilot (15) that used urogenital cytology as an outcome in relation to soy-formula exposure. Results from that pilot are consistent with those described here. In addition, we have reported altered DNA methylation in vaginal cells of some girls fed soy formula in this current study and provided experimental evidence that such changes are associated with decreased gene expression (41).
Several studies have examined soy-formula use in infancy in relation to conditions that occur later in life (9–12, 42). The only longitudinal study focused on soy formula and adult health included young adults (ages 20 to 34 years) who were fed soy formula or cow-milk formula as infants (12). Women fed soy formula as infants (n = 128) reported longer menstrual cycles and more menstrual pain than women fed cow-milk formula (n = 268); no significant differences were seen in men. Notably, adverse menstrual cycle characteristics were also associated with reported soy formula use among black women (10). Reported soy-formula use has been associated with endometriosis (9), with the size of uterine fibroids (11), and with early and late age at menarche (43, 44).
Administration of pharmaceutical estrogen to premature infants produces results qualitatively similar to our findings with soy formula in term infants. In a randomized trial (35), girls born prematurely who were given estradiol and progesterone had vulvar cell samples exhibiting more estrogenization, larger uteri, and wider breast-bud diameter than those given placebo. These estrogen-mediated changes in urogenital cytology and uterus size are consistent with our findings and support our conclusions that soy formula exerts estrogenic effects at these sites. In contrast to this trial and our expectations, however, we did not observe effects on breast tissue.
Two North American advisory groups have considered the safety of soy infant formula. The Committee on Nutrition, American Academy of Pediatrics, recommends soy formula only for infants with galactosemia or hereditary lactase deficiency and mentions that soy formula might be useful for families wishing to avoid formula containing animal products (45). A US National Toxicology Program expert panel concluded that there was “minimal concern” for adverse developmental effects in infants who consume soy formula, based on data available up to 2009 (8). However, it also stated:
The existing epidemiological literature on soy infant formula exposure is insufficient to reach a conclusion on whether soy infant formula does or does not cause adverse effects on development in humans. There is ‘clear evidence’ for adverse effects of genistein on reproductive development and function in female rats and mice manifested as accelerated puberty (i.e., decreased age at vaginal opening), abnormal estrous cyclicity, cellular changes to the female reproductive tract, and decreased fecundity (i.e., decreased fertility, implants, and litter size). Also, infants fed soy infant formula can have blood levels of total genistein that exceed those measured in neonatal or weanling rodents following treatment with genistein at dose levels that induced adverse effects in the animals (8).
Public concern about bisphenol A, a chemical used in plastics that has documented estrogen activity, centers on exposures to infants and young children (46). Concern about the risks of early-life exposure to xenoestrogens dates to the experience with diethylstilbestrol, a potent estrogen given in the 1950s to prevent miscarriage (2). It resulted in the occurrence of vaginal adenocarcinoma in some young women born of such pregnancies, establishing the possibility of delayed toxicity from perinatal exposure to an estrogen.
Genistein is a weaker estrogen than diethylstilbestrol and endogenous estradiol, but is stronger than chemicals such as bisphenol A (4); here, we offer evidence that soy formula, a known source of genistein, provokes an estrogenlike response in infants. In neonatal mice, genistein exposure can produce substantial reproductive tract toxicity (47, 48). Whether our findings presage long-term effects in infants similar to those of genistein exposure in rodents is yet to be established. Epigenetic alterations in the vaginal cells of soy formula–fed girls (41) provide a possible mechanism for such delayed effects.
In summary, we show that infants who consume soy formula present with changes to tissue consistent with those seen with exogenous estrogen. Genistein is the major isoflavone in soy formula and is capable of ER binding. Experimentally, genistein has increased murine uterine wet weight and produces downstream transcriptional activation of estrogen responsive genes (34). Animal evidence predicts that genistein can induce developmental and reproductive toxicity at doses relevant to soy formula–fed infants (49). Our findings are consistent with those in the animal literature in that we detected several developmental perturbations of estrogen-responsive tissue. Our methods targeting subclinical metrics of tissue growth and response may be useful tools for identifying early responses to suspected estrogenic agents.
Supplementary Material
Acknowledgments
We thank all patients and families for their participation; the care providers and staff at our main study site, Children’s Hospital of Philadelphia (CHOP), as well as our birth/recruitment hospital sites: the University of Pennsylvania Hospital, Pennsylvania Hospital and Virtua Voorhees Hospital, Virtua Memorial Hospital, Abington Memorial Hospital, Cooper Memorial Hospital, and Holy Redeemer Hospital. We thank Els Nijs; Adeka McIntosh; Laura Poznick, RDMS; Trudy Morgan, RDMS; Marcy Hutchinson, RDMS, RVT; Danielle Drigo, Infant Feeding and Early Development (IFED) team of research assistants; and the Department of Radiology at CHOP for their work with study design, quality assurance, data procedures, and collection. We thank our collaborators at the Department of Radiology at Virtua Voorhees; Elizabeth Fong-deLeon, Department of Pediatrics at Virtua Voorhees Hospital; and Steven Shapiro, Department of Pediatrics at Abington Memorial Hospital. We thank Dr. Michael Rybak and Patrick Simon of the Centers for Disease Control and Prevention for their work on urinary isoflavone analysis; Donna Baird and Retha Newbold, MS, at National Institute of Environmental Health Sciences; and Stacy Patchel for her careful data management and statistical programming.
Formula for study participants was a gift from Nestlé and Mead Johnson Nutrition. The IFED advisory board included Sheri Berenbaum, Russ Hauser, Michael DiPietro, Linda Adair, Laurie Moyer-Mileur, RD, and our late colleagues Judson Van Wyk, Laurence Finberg, and Melvin Grumbach. The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official views or positions of the US Centers for Disease Control and Prevention/Agency for Toxic Substances and Disease Registry. Use of trade names and commercial sources is for identification only and does not constitute endorsement by the US Department of Health and Human Services and the US Centers for Disease Control and Prevention.
Financial Support: This research was supported in part by the Intramural Research Program of the National Institutes of Health (NIH), National Institute of Environmental Health Sciences (Project No. Z01-ES044006 to W.J.R.). Data collection at the Children’s Hospital of Philadelphia (CHOP) was supported through Subcontract PHR-SUPS2-S-09-00196 under Contract HHSN291200555546C between the National Institute of Environmental Health Sciences and Social & Scientific Systems Inc. This project was supported by the Nutrition Center at the CHOP and by the National Center for Research Resources, Grant UL1TR000003 (National Center for Advancing Translational Sciences).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- E2
17-β estradiol
- EDC
endocrine-disrupting compound
- ER
estrogen receptor
- FSH
follicle-stimulating hormone
- IFED
Infant Feeding and Early Development
- MI
maturation index
- SAG
sagittal
- TRV
transverse
References
- 1. Ho SM, Cheong A, Adgent MA, Veevers J, Suen AA, Tam NN, Leung YK, Jefferson WN, Williams CJ. Environmental factors, epigenetics, and developmental origin of reproductive disorders. Reprod Toxicol. 2017;68:85–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Swan SH. Intrauterine exposure to diethylstilbestrol: long-term effects in humans. APMIS. 2000;108(12):793–804. [DOI] [PubMed] [Google Scholar]
- 3. Behr M, Oehlmann J, Wagner M. Estrogens in the daily diet: in vitro analysis indicates that estrogenic activity is omnipresent in foodstuff and infant formula. Food Chem Toxicol. 2011;49(10):2681–2688. [DOI] [PubMed] [Google Scholar]
- 4. Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139(10):4252–4263. [DOI] [PubMed] [Google Scholar]
- 5. Cao Y, Calafat AM, Doerge DR, Umbach DM, Bernbaum JC, Twaddle NC, Ye X, Rogan WJ. Isoflavones in urine, saliva, and blood of infants: data from a pilot study on the estrogenic activity of soy formula. J Expo Sci Environ Epidemiol. 2009;19(2):223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Riu A, Balaguer P, Perdu E, Pandelova M, Piccinelli R, Gustafsson JA, Leclercq C, Schramm KW, Dagnino S, Debrauwer L, Cravedi JP, Zalko D. Characterisation of bioactive compounds in infant formulas using immobilised recombinant estrogen receptor-alpha affinity columns. Food Chem Toxicol. 2008;46(10):3268–3278. [DOI] [PubMed] [Google Scholar]
- 7. Stanford BD, Weinberg HS. Evaluation of on-site wastewater treatment technology to remove estrogens, nonylphenols, and estrogenic activity from wastewater. Environ Sci Technol. 2010;44(8):2994–3001. [DOI] [PubMed] [Google Scholar]
- 8. National Toxicology Program NTP-CERHR monograph on soy infant formula. NTP CERHR MON. 2010. Sep;(23):i-661. [PubMed]
- 9. Upson K, Sathyanarayana S, Scholes D, Holt VL. Early-life factors and endometriosis risk. Fertil Steril. 2015;104(4):964–971.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Upson K, Harmon QE, Laughlin-Tommaso SK, Umbach DM, Baird DD. Soy-based infant formula feeding and heavy menstrual bleeding among young African American women. Epidemiology. 2016;27(5):716–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Upson K, Harmon QE, Baird DD. Soy-based infant formula feeding and ultrasound-detected uterine fibroids among young African-American women with no prior clinical diagnosis of fibroids. Environ Health Perspect. 2016;124(6):769–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Strom BL, Schinnar R, Ziegler EE, Barnhart KT, Sammel MD, Macones GA, Stallings VA, Drulis JM, Nelson SE, Hanson SA. Exposure to soy-based formula in infancy and endocrinological and reproductive outcomes in young adulthood. JAMA. 2001;286(7):807–814. [DOI] [PubMed] [Google Scholar]
- 13. Gilchrist JM, Moore MB, Andres A, Estroff JA, Badger TM. Ultrasonographic patterns of reproductive organs in infants fed soy formula: comparisons to infants fed breast milk and milk formula. J Pediatr. 2010;156(2):215–220. [DOI] [PubMed] [Google Scholar]
- 14. Zung A, Glaser T, Kerem Z, Zadik Z. Breast development in the first 2 years of life: an association with soy-based infant formulas. J Pediatr Gastroenterol Nutr. 2008;46(2):191–195. [DOI] [PubMed] [Google Scholar]
- 15. Bernbaum JC, Umbach DM, Ragan NB, Ballard JL, Archer JI, Schmidt-Davis H, Rogan WJ. Pilot studies of estrogen-related physical findings in infants. Environ Health Perspect. 2008;116(3):416–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grumbach MM. A window of opportunity: the diagnosis of gonadotropin deficiency in the male infant. J Clin Endocrinol Metab. 2005;90(5):3122–3127. [DOI] [PubMed] [Google Scholar]
- 17. Kuiri-Hänninen T, Sankilampi U, Dunkel L. Activation of the hypothalamic-pituitary-gonadal axis in infancy: minipuberty. Horm Res Paediatr. 2014;82(2):73–80. [DOI] [PubMed] [Google Scholar]
- 18. Ballard JL, Khoury JC, Wedig K, Wang L, Eilers-Walsman BL, Lipp R. New Ballard Score, expanded to include extremely premature infants. J Pediatr. 1991;119(3):417–423. [DOI] [PubMed] [Google Scholar]
- 19. Nguyen RH, Umbach DM, Parad RB, Stroehla B, Rogan WJ, Estroff JA. US assessment of estrogen-responsive organ growth among healthy term infants: piloting methods for assessing estrogenic activity. Pediatr Radiol. 2011;41(5):633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Adgent MA, Flake GP, Umbach DM, Stallings VA, Bernbaum JC, Rogan WJ. Urogenital epithelial cells as simple markers of estrogen response in infants: methods and applications. PLoS One. 2013;8(10):e77061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kaplan SL, Edgar JC, Ford EG, Adgent MA, Schall JI, Kelly A, Umbach DM, Rogan WJ, Stallings VA, Darge K. Size of testes, ovaries, uterus and breast buds by ultrasound in healthy full-term neonates ages 0-3 days. Pediatr Radiol. 2016;46(13):1837–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Naib ZM. Exfoliative Cytopathology, 2nd ed.Boston, MA: Little, Brown and Company; 1976:56. [Google Scholar]
- 23. DeMay RM. Practical Principles of Cytopathology. Chicago, IL: ASCP Press; 1999:1–3. [Google Scholar]
- 24. Cibas ES, Cibas ES, Ducatman BS, eds. Cytology: Diagnostic Principles and Clinical Correlates. Philadelphia, PA: W.B. Saunders Company; 1996:1–42. [Google Scholar]
- 25. Wied GL, Bibbo M, Bibbo M, ed. Comprehensive Cytopathology. Philadelphia, PA: W.B. Saunders Company; 1991:85–114. [Google Scholar]
- 26. McEndree B. Clinical application of the vaginal maturation index. The Nurse Pract. 1999;24(9):48, 51–52, 55–56. [PubMed] [Google Scholar]
- 27. Collett-Solberg PR, Grumbach MM. A simplified procedure for evaluating estrogenic effects and the sex chromatin pattern in exfoliated cells in urine: studies in premature thelarche and gynecomastia of adolescence. J Pediatrs. 1965;66:883–890. [DOI] [PubMed] [Google Scholar]
- 28. Elstein M. Vaginal cytology of the newborn. J Obstet Gynaecol Br Commonw. 1963;70:1050–1055. [DOI] [PubMed] [Google Scholar]
- 29. Chiechi LM, Putignano G, Guerra V, Schiavelli MP, Cisternino AM, Carriero C. The effect of a soy rich diet on the vaginal epithelium in postmenopause: a randomized double blind trial. Maturitas. 2003;45(4):241–246. [DOI] [PubMed] [Google Scholar]
- 30. Baird DD, Umbach DM, Lansdell L, Hughes CL, Setchell KD, Weinberg CR, Haney AF, Wilcox AJ, Mclachlan JA. Dietary intervention study to assess estrogenicity of dietary soy among postmenopausal women. J Clin Endocrinol Metab. 1995;80(5):1685–1690. [DOI] [PubMed] [Google Scholar]
- 31. van der Linden MC, Gerretsen G, Brandhorst MS, Ooms EC, Kremer CM, Doesburg WH. The effect of estriol on the cytology of urethra and vagina in postmenopausal women with genito-urinary symptoms. Eur J Obstet Gynecol Reprod Biol. 1993;51(1):29–33. [DOI] [PubMed] [Google Scholar]
- 32. Zhou H, Wang Y, Gatcombe M, Farris J, Botelho JC, Caudill SP, Vesper HW. Simultaneous measurement of total estradiol and testosterone in human serum by isotope dilution liquid chromatography tandem mass spectrometry. Anal Bioanal Chem. 2017;409(25):5943–5954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Borgert CJ, LaKind JS, Witorsch RJ. A critical review of methods for comparing estrogenic activity of endogenous and exogenous chemicals in human milk and infant formula. Environ Health Perspect. 2003;111(8):1020–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jefferson WN, Padilla-Banks E, Clark G, Newbold RR. Assessing estrogenic activity of phytochemicals using transcriptional activation and immature mouse uterotrophic responses. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;777(1-2):179–189. [DOI] [PubMed] [Google Scholar]
- 35. Trotter A, Maier L, Kohn T, Böhm W, Pohlandt F. Growth of the uterus and mammary glands and vaginal cytologic features in extremely premature infants with postnatal replacement of estradiol and progesterone. Am J Obstet Gynecol. 2002;186(2):184–188. [DOI] [PubMed] [Google Scholar]
- 36. Chellakooty M, Schmidt IM, Haavisto AM, Boisen KA, Damgaard IN, Mau C, Petersen JH, Juul A, Skakkebaek NE, Main KM. Inhibin A, inhibin B, follicle-stimulating hormone, luteinizing hormone, estradiol, and sex hormone-binding globulin levels in 473 healthy infant girls. J Clin Endocrinol Metab. 2003;88(8):3515–3520. [DOI] [PubMed] [Google Scholar]
- 37. Schmidt IM, Chellakooty M, Haavisto AM, Boisen KA, Damgaard IN, Steendahl U, Toppari J, Skakkebaek NE, Main KM. Gender difference in breast tissue size in infancy: correlation with serum estradiol. Pediatr Res. 2002;52(5):682–686. [DOI] [PubMed] [Google Scholar]
- 38. Andres A, Moore MB, Linam LE, Casey PH, Cleves MA, Badger TM. Compared with feeding infants breast milk or cow-milk formula, soy formula feeding does not affect subsequent reproductive organ size at 5 years of age. J Nutr. 2015;145(5):871–875. [DOI] [PubMed] [Google Scholar]
- 39. Freni-Titulaer LW, Cordero JF, Haddock L, Lebrón G, Martínez R, Mills JL. Premature thelarche in Puerto Rico. A search for environmental factors. Am J Dis Child. 1986;140(12):1263–1267. [DOI] [PubMed] [Google Scholar]
- 40. McKiernan JF, Hull D. Breast development in the newborn. Arch Dis Child. 1981;56(7):525–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Harlid S, Adgent M, Jefferson WN, Panduri V, Umbach DM, Xu Z, Stallings VA, Williams CJ, Rogan WJ, Taylor JA. Soy formula and epigenetic modifications: analysis of vaginal epithelial cells from infant girls in the IFED Study. Environ Health Perspect. 2016;125(3):447–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Boucher BA, Cotterchio M, Kreiger N, Thompson LU. Soy formula and breast cancer risk. Epidemiology. 2008;19(1):165–166. [DOI] [PubMed] [Google Scholar]
- 43. D’Aloisio AA, DeRoo LA, Baird DD, Weinberg CR, Sandler DP. Prenatal and infant exposures and age at menarche. Epidemiology. 2013;24(2):277–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Adgent MA, Daniels JL, Rogan WJ, Adair L, Edwards LJ, Westreich D, Maisonet M, Marcus M. Early-life soy exposure and age at menarche. Paediatr Perinat Epidemiol. 2012;26(2):163–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bhatia J, Greer F; American Academy of Pediatrics Committee on Nutrition . Use of soy protein-based formulas in infant feeding. Pediatrics. 2008;121(5):1062–1068. [DOI] [PubMed] [Google Scholar]
- 46. Sartain CV, Hunt PA. An old culprit but a new story: bisphenol A and “NextGen” bisphenols. Fertil Steril. 2016;106(4):820–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jefferson WN, Williams CJ. Circulating levels of genistein in the neonate, apart from dose and route, predict future adverse female reproductive outcomes. Reprod Toxicol. 2011;31(3):272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jefferson WN, Patisaul HB, Williams CJ. Reproductive consequences of developmental phytoestrogen exposure. Reproduction. 2012;143(3):247–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jefferson WN, Doerge D, Padilla-Banks E, Woodling KA, Kissling GE, Newbold R. Oral exposure to genistin, the glycosylated form of genistein, during neonatal life adversely affects the female reproductive system. Environ Health Perspect. 2009;117(12):1883–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.