Abstract
Selective serotonin reuptake inhibitors (SSRIs) have been linked to osteopenia and fracture risk; however, their long-term impact on bone health is not well understood. SSRIs are widely prescribed to pregnant and breastfeeding women who might be at particular risk of bone pathology because lactation is associated with considerable maternal bone loss. We used microCT and molecular approaches to test whether the SSRI fluoxetine, administered to C57BL/6 mice from conception through the end of lactation, causes persistent maternal bone loss. We found that peripartum fluoxetine increases serum calcium and reduces circulating markers of bone formation during lactation but does not affect osteoclastic resorption. Peripartum fluoxetine exposure also enhances mammary gland endocrine function during lactation by increasing synthesis of serotonin and PTHrP, a hormone that liberates calcium for milk synthesis and reduces bone mineral volume. Peripartum fluoxetine exposure reduces the trabecular bone volume fraction at 3 months after weaning. These findings raise new questions about the long-term consequences of peripartum SSRI use on maternal health.
Peripartum dams were dosed with saline or fluoxetine and aged for 3 or 9 months. Fluoxetine elevated mammary gland PTHrP during lactation and reduced trabecular bone volume after weaning.
It has been estimated that 53 million people in the United States have osteoporosis (1), and a disproportionate number of those afflicted are women. Osteoporosis of the femur neck or lumbar spine affects 5.4% of men aged >65 years compared with a staggering 24.5% of women of the same age (2). The peak bone mass, achieved in the mid-20s, is an important predictor of postmenopausal bone health (3). Most US women bear their first child and breastfeed, mobilizing substantial bone to support milk synthesis, at approximately the same age as when peak maternal bone mass is achieved (4, 5). Women experience a decline of 6% to 10% of bone mineral density across 6 months of exclusive breastfeeding (6). A longstanding paradigm has been that bone mineral lost during lactation is recovered after weaning (6, 7). However, recent evidence has suggested that women who breastfeed might be at risk of osteoporosis and fracture later in life, depending on the duration of lactation and site of bone resorption (8–10). Also, excessive bone turnover during the peripartum period, when the peak bone mass is achieved, might be a risk factor for bone pathology.
The drivers of clinically substantial lactational bone loss are poorly understood, especially the role of preventable influences such as medications. Selective serotonin reuptake inhibitors (SSRIs) are the most commonly used antidepressants for pregnant and breastfeeding women (11, 12) and elevate serotonin activity throughout the body. SSRIs such as fluoxetine reduce bone mineral density in animal models (13–15), and their use has been associated with low bone mass in the general human population (16, 17). The long-term effects of SSRI use during the peripartum period are unknown because SSRIs only became popular in the 1980s and 1990s.
It is possible that SSRI use during pregnancy and lactation could reduce bone density at a critical life stage when women reach their peak bone mass, placing them at risk of bone complications. SSRIs could affect bone health through mechanisms involving both central and peripheral serotonin (18). A serotonin microenvironment exists within bone and includes multiple serotonin receptors and the serotonin reuptake transporter, which is the target of SSRIs (19). As such, SSRIs might act on bone tissue to directly reduce maternal bone mass, potentially through inhibited osteoblast proliferation and bone formation (20–22). Additionally, prolonged SSRI use causes a brain-serotonin–dependent increase in sympathetic output, which increases bone resorption, resulting in net bone loss (15). We tested another mechanism by which SSRIs affect mammary-derived endocrine signals that drive bone turnover. During lactation, mammary epithelial cells synthesize serotonin, which, in turn, stimulates synthesis and secretion of PTHrP into the circulation (23–25). PTHrP binds its receptor on bone tissue to drive osteoclastic resorption and osteocytic osteolysis, liberating calcium for milk (26–28).
Lactational bone loss to support milk synthesis is conserved across species, with mice resorbing 25% to 35% of their bone mineral during a 21-day lactation period (29–31). As such, we used a C57BL/6 mouse model to evaluate the effects of peripartum fluoxetine exposure on postweaning bone health. Our experiments have demonstrated that peripartum fluoxetine exposure elevates mammary gland serotonin and Pthrp synthesis during lactation and causes a persistent reduction in bone mineral volume ≥3 months after weaning. The sustained maternal bone deficits as a result of peripartum fluoxetine exposure in mice raise new concerns about long-term bone health among women taking an SSRI during pregnancy and lactation.
Methods
Experiments to examine postweaning bone loss
Animals
All experiments were performed under protocol number A01473 and approved by the Research Animal Care and Use Committee at the University of Wisconsin-Madison. Female C57BL/6 mice were individually housed in a controlled environmental facility for biological research in the Animal Science Department vivarium at the University of Wisconsin-Madison. The mice were either obtained through our mating colony or ordered from Jackson Laboratories when they were aged 7 to 9 weeks ±3 days (stock no. 000664; Jackson Laboratories, Bar Harbor, ME). The mice were maintained at a temperature of 25°C and humidity of 50% to 60%, with a 12-hour light:12-hour dark cycle and free access to food (2019 Teklad Global Diet, 19% protein extruded; Envigo, Indianapolis, IN) and water. Beginning at 7 weeks of age, female mice were bred overnight with a male of approximately the same age. Wherever possible, littermates were used. Pregnancy was determined via visualization of the vaginal plug, at which time the mice were housed individually. The dams were randomly assigned to 2 treatments: saline (n = 16) or the SSRI fluoxetine (n = 14) injections. Beginning on the plug date, the dams received daily intraperitoneal injections of either sterile saline or 20 mg/kg bodyweight fluoxetine hydrochloride (F312; Sigma-Aldrich, St. Louis, MO) reconstituted in sterile saline. This dose produces plasma fluoxetine concentrations in mice that correspond to humans prescribed 20 to 80 mg/d (32). The final volume injected into each dam was 0.12 mL of either saline or fluoxetine reconstituted in saline. Injections were performed daily between 7:00 and 8:00 am daily from the plug date through day 21 of lactation. The litters were not standardized. On day 21 of lactation, the pups were weaned from the dams. After weaning, the dams were aged for an additional 3 months (n = 8 for saline; n = 7 for fluoxetine) or 9 months (n = 8 for saline; n = 7 for fluoxetine) after weaning. The mice aged to 3 months were ~20 ± 2 weeks old (4.5 months), and the mice aged to 9 months were ~48 ± 2 weeks old (11 months).
Sample collection
Milk yield was determined daily throughout lactation using the weigh-suckle-weigh (WSW) method (24, 25). The pups were removed from their mothers at 8:00 am. After being separated for 4 hours, each litter was weighed, and the number of pups was counted. The litters were then returned to their dam at 12:00 pm and allowed to nurse for 45 minutes. After 45 minutes of suckling, the litters were weighed again to estimate the milk yield. The milk yields were standardized per pup by dividing the total milk yield for the litter by the number of pups in each litter on each day of lactation. After a 7- to 8-hour fast during the day, blood was collected into capillary blood collection tubes (Terumo T-MG, Somerset, NJ) from the maxillary vein on the plug date, day 1 of lactation, day 21 of lactation, and at euthanasia at either 3 or 9 months after weaning. Blood was allowed to sit at 4°C overnight to allow the platelets to disrupt. The blood was spun down at 3000 rpm for 20 minutes to isolate serum, which was stored at −80°C until the time of assay.
At 3 or 9 months after weaning, the dams were euthanized via carbon dioxide inhalation, followed by cervical dislocation. One femur was collected for microCT evaluation by allowing it to fix overnight in a histological cassette at 4°C in 4% paraformaldehyde and then placed in 70% alcohol until microCT analysis.
microCT analysis
A Scanco Medical microCT 35 system with an isotropic voxel size of 7 mm was used to image the femurs. The scans were conducted in 70% alcohol using an X-ray tube potential of 55 kVp, an X-ray intensity of 0.145 mA, and an integration time of 600 ms. For the scans of the femur, a region beginning 0.28 mm proximal to the growth plate and extending 1.05 mm proximally was selected for trabecular bone analysis. A second region, 0.6 mm in length and centered at the midpoint of the femur, was used to calculate the diaphyseal parameters. A semiautomated contouring approach was used to distinguish cortical from trabecular bone. The region of interest was thresholded using a global threshold that set the bone/marrow cutoff at 600 mg HA/cm3 for trabecular bone and 786 mg HA/cm3 for cortical bone. The three-dimensional microstructural properties of bone were calculated using software supplied by the manufacturer and reported according to consensus guidelines on rodent microCT. One mouse in the saline 3-month postweaning group was omitted from the trabecular analyses because her femur proximal to the growth plate became damaged during bone harvesting.
Assays
Serum serotonin concentrations were determined using a Beckman Coulter Enzyme Immunoassay Kit (catalog no. IM1749; Beckman Coulter, Vršovice, Czech Republic) per the manufacturer’s instructions. Serum samples were diluted 1:100 to fit within the standard curve. Serum procollagen I intact N-terminal (P1NP) concentrations were determined using the Immunodiagnostics Systems enzyme immunoassay (catalog no. AC-33F1; Immunodiagnostics Systems, Tyne and Wear, United Kingdom) per the manufacturer’s instructions. Samples were diluted 1:10 to fit within the standard curve. Serum collagen type 1 cross-linked C-telopeptide (CTX) concentrations were determined by the RatLaps™ (CTX-I) Immunodiagnostics Systems enzyme immunoassay (catalog no. AC-06F1) per the manufacturer’s instructions. Total serum calcium concentrations were determined using the Cayman Chemicals Calcium Assay Kit (catalog no. 701220; Cayman Chemicals, Ann Arbor, MI) per the manufacturer’s instructions. Samples were diluted 1:2 to fit within the standard curve.
Porsolt forced swim test
The Porsolt forced swim test (FST) was administered to eight saline mice and seven fluoxetine mice on day 10 of lactation (peak lactation). The FST was administered between 8:00 and 10:00 am. The mice were allowed to acclimate to the testing room for ≥15 minutes before the FST. For the FST, the mice were placed in a clear tank containing 25°C water at a height of 20 cm for a total of 6 minutes. The session was recorded by video, and the total immobility time was scored by two independent observers who were unaware of the treatments given. The first 2 minutes were not used in the determination of the total immobility time to allow the mice to adjust to the test (33). Our definition of mobility was any movement other than those necessary to balance the body and keep the head above water. The tank was emptied and rinsed clean after each mouse.
Methods for experiments to examine lactational contribution to bone loss
Animals
To determine the site and timing of fluoxetine action, a separate cohort of mice was exposed to saline or 20 mg/kg/d fluoxetine from embryonic day 13 (E13) of pregnancy through day 10 (peak) lactation, a timeline previously used in lactation studies (24, 25). All experiments were performed under protocol number V01426 and approved by the Research Animal Care and Use Committee at the University of Wisconsin-Madison. Female C57BL/6 mice were individually housed in a controlled environmental facility for biological research in the Hanson Laboratories vivarium at the University of Wisconsin-Madison. The mice were housed in polysulfone cages containing corn cob bedding and maintained on a 12-hour light/dark cycle at 25°C and 20% to 50% relative humidity with free access to food and water. Beginning at 7 weeks of age, female mice were harem mated with 1 male overnight. Pregnancy was determined via visualization of the vaginal plug, at which time the mice were housed individually. The dams were randomly assigned to two treatments: saline injections (n = 8) or fluoxetine injections (n = 8). Beginning on E13, dams received daily intraperitoneal injections of either sterile saline or 20 mg/kg bodyweight fluoxetine hydrochloride (F312; Sigma-Aldrich) reconstituted in sterile saline. The final volume injected into each dam was 0.12 mL of either saline or fluoxetine reconstituted in saline. The injections occurred daily between 7:00 and 8:00 am daily from E13 through day 10 of lactation, at which time the dams were euthanized. The litters were not standardized.
Sample collection
The WSW method was used to determine the milk yield daily from day 1 through day 10 of lactation, as described. Blood was collected using the same methods as described on E13 and day 1 and day 10 of lactation. The mice were not fasted before blood sample collection. On day 10 of lactation, the dams were euthanized via carbon dioxide inhalation, followed by cervical dislocation. The pups were euthanized via decapitation. The number four mammary gland was collected for histological examination, fixed overnight in 4% paraformaldehyde, and stored in 70% ethanol until paraffin embedding. The duodenum, one femur, and all other mammary glands were rapidly extracted and immediately snap frozen in liquid nitrogen to preserve tissue integrity and stored at −80°C until analysis.
Mammary gland, duodenum, and femur RNA and protein extraction and RT-PCR
Protein was extracted from the mammary gland and duodenum using radioimmunoprecipitation assay buffer plus 10 μL/mL of Halt Protease and Phosphatase Inhibitors Cocktail (catalog no. 78441; Thermo Fisher Scientific, Waltham, MA). Protein concentrations were determined using the bicinchoninic acid assay (catalog no. 20831001-1; Bioworld, Dublin, OH). RNA was extracted from the mammary gland, femur, and duodenum using TRI-Reagent (Molecular Research, Cincinnati, OH). RNA was reverse transcribed (1 μg) to cDNA using the Applied Biosystems High Capacity cDNA Reverse Transcription Kit (catalog no. 4368814; Applied Biosystems, Foster City, CA). Quantitative RT-PCR was conducted with the CFX96 Touch Real-Time PCR Detection System (Bio-Rad Laboratories, Rodeo, CA). Reaction mixtures and cycling conditions were performed as previously described (24). All primers were designed to span exon–exon junctions and an optimal annealing temperature of 60°C. Amplification efficiencies of primers were accepted within a range of 95% to 105% efficiency and primer specificity was assessed by the presence of a single temperature dissociation peak, eliminating any primers with indication of secondary structures. The primer sequences are listed in Table 1. In the mammary gland, the housekeeping parameter was the geometric mean of Hprt1, Rps15, and Rps9. In the duodenum, the housekeeping parameter was Rps15. In the femur, the housekeeping parameter was the geometric mean of Hprt1 and Rps15. Analysis was conducted using the 2−ΔΔCT method (34).
Table 1.
Primer Sequences Used for Quantitative Real-Time PCR
| Gene | Forward Primer 5′ → 3′ | Reverse Primer 3′ → 5′ |
|---|---|---|
| Alac | CTGCCTCTGAGCCTTGTACC | GTAAAACCCCCATCGAGACC |
| Bcas | GGTGAATCTCATGGGACAGC | GAGATGGTTTGAGCCTGAGC |
| Bglap | TTCTGCTCACTCTGCTGACC | ACCTTATTGCCCTCCTGCTT |
| Calbindin D9K | CCCGAAGAAATGAAGAGCATTTT | TTCTCCATCACCGTTCTTATCCA |
| Casr | TCTTGTGGAGTGGGTTCTCC | AAAACAGCAGGTGGGCTCT |
| Hprt1 | CTGGTGAAAAGGACCTCTCG | AACTTGCGCTCATCTTAGGC |
| Opg | AAGCTGGAACCCCAGAGC | GTGCTGCACTTCGTGTGTTT |
| Pmca1 | AACGACTGGAGCAAGGAGAA | CCGTACTTCACTTGGGCAAT |
| Pthrp | TTCCTGCTCAGCTACTCCGT | GATGGACTTGCCCTTGTCAT |
| Rankl | GGCCACAGCGCTTCTCAG | GAGTGACTTTATGGGAACCCC |
| Rps9 | GGAGACCCTTCGAGAAGTCG | GGGGATCCTTCTCGTCTAGC |
| Rps15 | TTGAGAAAGGCCAAAAAGGA | GTTGAAGGTCTTGCCGTTGT |
| Tph1 | TTCACCATGATTGAAGACAAC | TCCGACTTCATTCTCCAAGG |
Abbreviations: Alac, α-lactalbumin; Bcas, β-casein, Bglap, bone γ-carboxyglutamic acid–containing protein; Casr, calcium sensing receptor; Hprt1, hypoxanthine phosphoribosyltransferase 1; Opg, osteoprotegerin; Pmca1, plasma membrane Ca2+ ATPase 1; Pthrp, PTHrP, Rankl, receptor activator of nuclear factor κΒ ligand; Rps9, 40S ribosomal protein S9; Rps15, 40S ribosomal protein S15; Tph1, tryptophan hydroxylase 1.
Methylated DNA immunoprecipitation
Genomic DNA was isolated using the Qiagen DNeasy Blood and Tissue Kit (catalog no. 69056; Qiagen, Germantown, MD) according to the manufacturer’s instructions. Methylated DNA immunoprecipitation was performed on 4 μg of genomic DNA extracted from the mammary gland as previously described (25, 35). The enriched methylated DNA was quantified using RT-PCR using the methods described. The primer for the Pthrp promoter was forward: 5′-TGAGAAGCTGAAGCAAATGG-3′ and reverse: 3′-TACTGTTTGGGAAGGGTTGG-5′. The results were evaluated as enrichment over IgG and analyzed using the 2−ΔΔCT method (34).
Western blotting
Mammary gland total protein extracts (n = 5 per treatment; 25 μg) were separated by electrophoresis on a 7.5% SDS-polyacrylamide gel and transferred for 1 hour at 100 V onto polyvinylidene difluoride membrane (catalog no. IPVH00010; Millipore, Burlington, MA). Membranes were blocked with 5% BSA for 2 hours and probed overnight at 4°C with 1:500 calcium sensing receptor (CaSR; catalog no. MA1-934; Thermo Fisher Scientific; RRID: AB_325374) (36) and 1:5000 β-actin (catalog no. A5441; Sigma-Aldrich). The next day, the membranes were incubated for 1 hour at room temperature with horseradish peroxidase–conjugated secondary antibodies [donkey anti-mouse; catalog no. sc-2314; RRID: AB_641170 (37) at 1:10,000 for β-actin and 1:500 for CaSR; Santa Cruz Biotechnology, Dallas, TX] and StrepTactin-HRP conjugate (catalog no. 161-0381; Bio-Rad Laboratories) for chemiluminescent detection. Protein bands were detected at 5 to 10 seconds of exposure time using Immun-Star™ WesternC™ Kit (catalog no. 170-5070; Bio-Rad Laboratories) and visualized using the Omega Lum™ G Imaging System (catalog no. 81-12100-00; Aplegen, San Francisco, CA). Image processing and protein band quantification were performed using Image Studio™ Lite software (Li-Cor Biosciences, Lincoln, NE).
Mammary gland histological findings
Sectioned mammary glands (n = 4 per treatment) were deparaffinized and stained with hematoxylin and eosin. Three nonoverlapping images were taken of each section, and alveoli were quantified and measured for each image using ImageJ software, version 1.8.0 (National Institutes of Health, Bethesda, MD).
Assays
Serum serotonin concentrations were determined using a Beckman Coulter Enzyme Immunoassay Kit (catalog no. IM1749) per the manufacturer’s instructions. Serum samples were diluted 1:100 to fit within the standard curve. The mammary gland and duodenum content of serotonin was also determined using the Beckman Coulter kit by loading 50 μg of protein per sample. Mammary gland cAMP activity was determined by loading 50 μg protein into the Cell Signaling Technology cAMP XP Assay Kit (catalog no. 4339S; Cell Signaling Technology, Danvers, MA) in accordance with the manufacturer’s instructions.
Statistical analysis
All statistical analyses were conducted using SAS software, version 9.3 (SAS Institute Inc., Cary, NC). Analyses between the saline and fluoxetine mice without the effect of time were performed using a Student unpaired two-sided t test using PROC TTEST. When the data were not normally distributed, a Kruskal-Wallis test was performed using PROC NPAR1WAY for nonparametric data. Analyses with multiple time points [bone volume/tissue volume (BV/TV), serum P1NP, serum CTX, total serum calcium, and serum serotonin] were conducted using a PROC MIXED model with time, treatment, and the interaction of treatment by time. For WSW data, repeated measures were included. The residuals from each model were analyzed with a normality test; when the normality assumption failed, the ranks of the data were analyzed in a nonparametric manner. Each parameter was evaluated for extreme influential data points and outliers, which were removed when identified. For serum calcium and serotonin, the data points were corrected to a baseline serum sample (the day of conception or E13, respectively) taken from each mouse before any treatment was applied, and ANOVAs were then run using the baseline-corrected values. For all analyses, differences between the mean were considered significant at P < 0.05. All values are reported as means ± SEM.
Results
Peripartum fluoxetine causes a sustained reduction in maternal trabecular bone volume and increases serum calcium concentrations
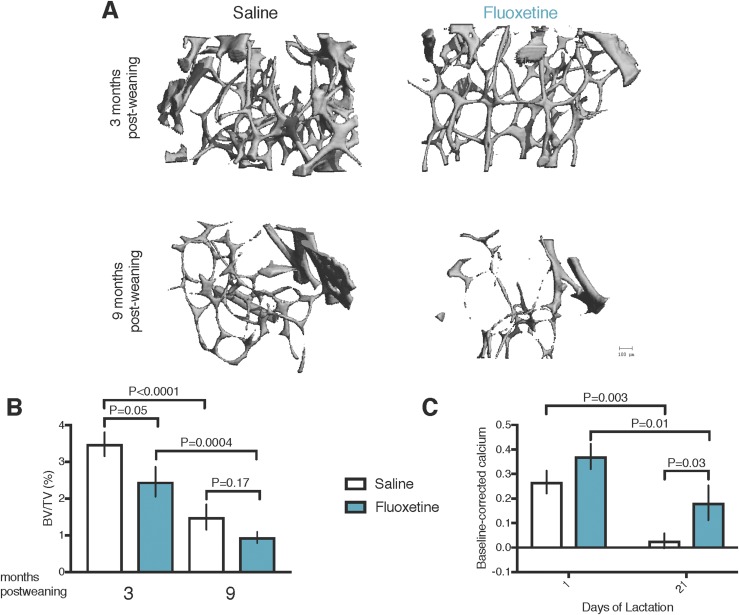
MicroCT analysis was used to examine the effect of peripartum fluoxetine exposure on postweaning maternal bone. Peripartum fluoxetine exposure reduced the femoral BV/TV at 3 months after weaning compared with saline exposure (Fig. 1A and 1B). Although a similar trend was observed at 9 months after weaning (Fig. 1B), age-related trabecular deficits characteristic of female C57BL/6 mice diminished the differences between the groups (38). No effect was found from fluoxetine treatment on trabecular (Table 2) or cortical (Table 3) parameters after weaning. All mice experienced age-associated skeletal changes from 3 to 9 months after weaning, including fewer and more spaced trabeculae and greater cortical porosity and less cortical thickness (Tables 2 and 3). Peripartum fluoxetine exposure also increased the serum total calcium concentrations on day 21 of lactation compared with mice exposed to saline (Fig. 1C).
Figure 1.
Peripartum fluoxetine exposure to C57BL/6 dams causes sustained reductions in femoral bone volume after weaning. Saline (n = 15) or 20 mg/kg fluoxetine (n = 14) was administered from the day of conception through day 21 of lactation. Mice were then aged to either 3 mo (n = 7, saline group; n = 7, fluoxetine group) or 9 mo (n = 8, saline group; n = 7, fluoxetine group) after weaning, at which time, the femurs were collected for microCT analysis. (A) Three-dimensional reconstruction images of distal femur trabecular bone. Scale bar, 100 µm. (B) BV/TV. (C) Blood samples were collected from all mice before any treatment (baseline), on the day of conception, and on days 1 and 21 of lactation, and serum was isolated. Serum calcium concentrations (mM) were generated by subtracting each mouse’s baseline serum calcium concentration from her serum calcium concentration on days 1 or 21 of lactation, respectively, to correct for variation within each dam. Data presented as mean ± SEM and analyzed using an SAS mixed linear model for treatment and time.
Table 2.
Femoral Trabecular Parameters as Evaluated by MicroCT
| Treatment Group |
P Value |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3 mo |
9 mo |
Time | Tx | 3 mo vs 9 mo |
Saline vs Fluoxetine |
|||||
| Saline (n = 7) | Fluoxetine (n = 7) | Saline (n = 8) | Fluoxetine (n = 7) | Saline | Fluoxetine | 3 mo | 9 mo | |||
| BV/TV, % | 3.5 ± 0.4 | 2.4 ± 0.4 | 1.4 ± 0.3 | 0.9 ± 0.2 | < 0.0001 | 0.02 | < 0.0001 | 0.0004 | 0.05 | 0.17 |
| Tb.N, 1/mm | 2.61 ± 0.08 | 2.54 ± 0.14 | 1.72 ± 0.07 | 1.74 ± 0.10 | < 0.0001 | 0.77 | < 0.0001 | < 0.0001 | 0.62 | 0.92 |
| Tb.S, μm | 0.38 ± 0.01 | 0.40 ± 0.03 | 0.58 ± 0.03 | 0.59 ± 0.04 | < 0.0001 | 0.87 | < 0.0001 | < 0.0001 | 0.80 | 0.98 |
| Tb.Th, μm | 0.044 ± 0.002 | 0.038 ± 0.003 | 0.046 ± 0.002 | 0.047 ± 0.004 | 0.05 | 0.39 | 0.53 | 0.04 | 0.17 | 0.85 |
| Tiss.BMD, mg Hg/cm3 | 905 ± 17.06 | 904 ± 17.28 | 908 ± 8.09 | 903 ± 15.96 | 0.99 | 0.81 | 0.95 | 0.96 | 0.91 | 0.82 |
Abbreviations: Tb.N, trabecular number; Tb.S, trabecular spacing; Tb.Th, trabecular thickness; Tiss.BMD, tissue bone mineral density; Tx, treatment.
Table 3.
Femoral Cortical Parameters Evaluated by MicroCT
| Treatment Group |
P Value |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3 mo |
9 mo |
Time | Tx | 3 mo vs 9 mo |
Saline vs Fluoxetine |
|||||
| Saline (n = 8) | Fluoxetine (n = 7) | Saline (n = 8) | Fluoxetine (n = 7) | Saline | Fluoxetine | 3 mo | 9 mo | |||
| Periosteal area, mm2 | 0.54 ± 0.06 | 0.41 ± 0.06 | 0.70 ± 0.06 | 0.69 ± 0.06 | 0.001 | 0.25 | 0.07 | 0.004 | 0.14 | 0.90 |
| Periosteal circumference, mm | 3.09 ± 0.03 | 3.01 ± 0.04 | 3.31 ± 0.04 | 3.29 ± 0.03 | < 0.0001 | 0.19 | < 0.0001 | < 0.0001 | 0.14 | 0.73 |
| C.Po, % | 0.089 ± 0.001 | 0.090 ± 0.002 | 0.097 ± 0.003 | 0.098 ± 0.001 | 0.003 | 0.85 | 0.02 | 0.05 | 0.74 | 0.95 |
| C.Th, mm | 0.19 ± 0.003 | 0.19 ± 0.004 | 0.18 ± 0.006 | 0.18 ± 0.003 | 0.006 | 0.49 | 0.05 | 0.03 | 0.76 | 0.50 |
| C.BMD, mg/cm3 HA | 1273 ± 9.18 | 1265 ± 7.83 | 1255 ± 11.66 | 1267 ± 8.28 | 0.42 | 0.87 | 0.18 | 0.86 | 0.53 | 0.38 |
Abbreviations: C.Po, cortical porosity; C.Th, cortical thickness; C.BMD, cortical bone mineral density; Tx, treatment.
Maternal lactational circulating calcium is elevated and bone formation is repressed by peripartum fluoxetine administration
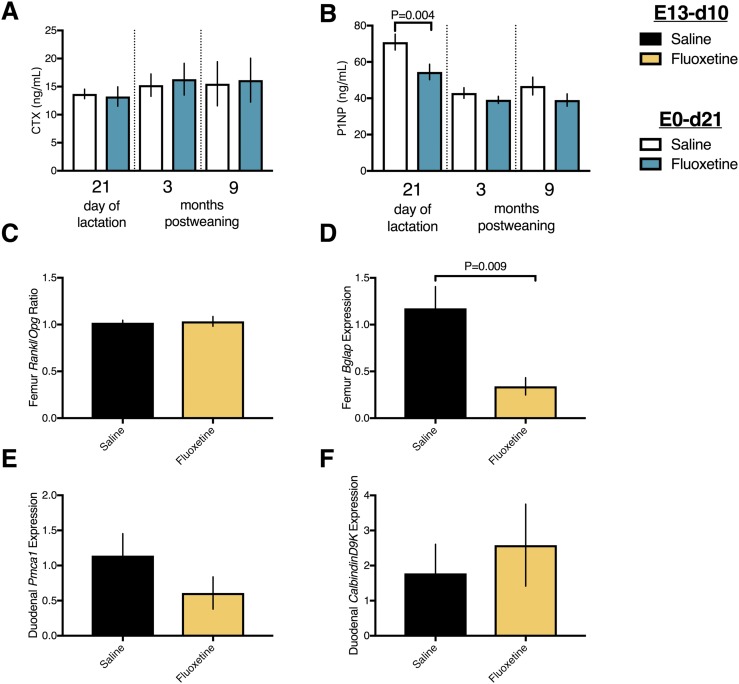
Given the fluoxetine-driven perturbations in serum calcium during lactation, it was pertinent to examine the circulating markers of bone turnover. The concentrations of serum CTX, a circulating marker of bone resorption (Fig. 2A), were unaffected by fluoxetine exposure during lactation or after weaning. In contrast, the serum concentrations of the bone formation marker P1NP (39, 40) were reduced in mice exposed to fluoxetine on day 21 of lactation (Fig. 2B). The repressive action of fluoxetine on bone formation was transient and restricted to the peripartum period, with no evidence found of postweaning perturbations in the bone turnover markers. To collect further information on bone turnover, a separate cohort of mice was administered either saline or 20 mg/kg fluoxetine from E13 through day 10 of lactation, at which time tissues were collected. The receptor activator of nuclear factor-κB ligand/osteoprotegerin mRNA ratio in the femur (Fig. 2C) was unchanged, and osteocalcin (bone γ-carboxyglutamic acid–containing protein) mRNA expression was reduced by fluoxetine in the femurs of mice euthanized on day 10 of lactation (Fig. 2D). As the maternal calcium pool is influenced by dietary calcium intake, we determined that fluoxetine exposure did not alter duodenal mRNA expression of Pmca1 (Fig. 2E) or CalbindinD9k (Fig. 2F) on day 10 of lactation.
Figure 2.
Peripartum fluoxetine exposure to C57BL/6 dams represses bone formation but does not affect bone resorption or gut calcium transport during lactation. (A and B) Saline (n = 16) or 20 mg/kg fluoxetine (n = 14) was administered from the day of conception through day 21 of lactation. Mice were then aged to either 3 mo (n = 8, saline group; n = 7, fluoxetine group) or 9 mo (n = 8, saline group; n = 7, fluoxetine group) after weaning. Blood samples were collected from all mice before any treatment on the day of conception and on days 1 and 21 of lactation and the euthanasia, and serum was isolated. Serum (A) CTX and (B) P1NP concentrations are shown. (C–F) Saline (n = 8) or 20 mg/kg fluoxetine (n = 8) was administered from E13 through day 10 of lactation, at which time the mice were euthanized. RNA samples were prepared from femur, and (C) the ratio of receptor activator of nuclear factor-κB ligand/osteoprotegerin. (D) Bone γ-carboxyglutamic acid–containing protein was measured using quantitative RT-PCR. RNA samples were also prepared from duodenum. (E) Plasma membrane Ca2+ ATPase and (F) Calbindin D9K mRNA expression was measured using quantitative RT-PCR. Results are shown as mean ± SEM. Data were analyzed using a Student t test or an SAS mixed linear model for treatment and time.
Peripartum fluoxetine exposure increases mammary gland Pthrp and Casr synthesis and mammary gland serotonin content
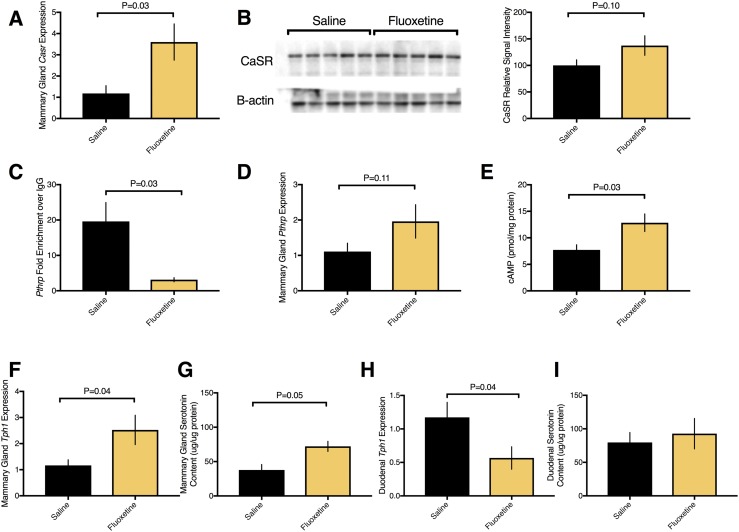
The mammary gland acts as an accessory parathyroid organ during lactation to regulate calcium homeostasis. Fluoxetine increased mammary gland Casr mRNA expression (Fig. 3A) and tended to increase CaSR protein (Fig. 3B) on day 10 of lactation. Because we found changes in CaSR expression, we next examined PTHrP, as these two signaling factors work in a loop in the lactating mammary gland to regulate calcium homeostasis. Fluoxetine decreased DNA methylation at the Pthrp promoter (Fig. 3C), tended to elevate Pthrp mRNA (Fig. 3D), and elevated cAMP (Fig. 3E), which is a functional readout of PTHrP activity in the lactating mammary gland (41). PTHrP expression is driven by serotonin in the lactating mammary gland (23). Thus, we next examined components of the serotonergic pathway. Peripartum fluoxetine exposure elevated mRNA expression of tryptophan hydroxylase 1 (Tph1), the rate-limiting enzyme in serotonin synthesis on day 10 of lactation (Fig. 3F), and elevated mammary gland serotonin content (Fig. 3G). In contrast, fluoxetine exposure reduced duodenal Tph1 mRNA expression (Fig. 3H) without changing the duodenal serotonin content (Fig. 3I).
Figure 3.
Peripartum fluoxetine exposure to C57BL/6 dams increased mammary gland Casr, Pthrp, and serotonin synthesis during lactation. Saline (n = 8) or 20 mg/kg fluoxetine (n = 8) was administered from E13 through day 10 of lactation, at which time mice were euthanized. RNA and protein were extracted from the mammary gland and duodenum for quantitative RT-PCR in mRNA and Western blot and assays in protein. (A) Mammary gland mRNA expression of Casr. (B) Western blot and quantification of CaSR. (C) Genomic DNA was extracted, and DNA methylation was measured using methylated DNA immunoprecipitation at the Pthrp promoter. (D) Mammary gland mRNA expression of Pthrp. (E) Mammary gland protein was assayed for cAMP activity. (F) Mammary gland mRNA expression of tryptophan hydroxylase 1 (Tph1). (G) Mammary gland protein was assayed for content of serotonin, expressed as micrograms of serotonin per microgram of protein. (H) Duodenal mRNA expression of Tph1. (I) Duodenal protein was assayed for serotonin content, expressed as micrograms of serotonin per microgram of protein. Data presented as mean ± SEM and analyzed using a Student t test.
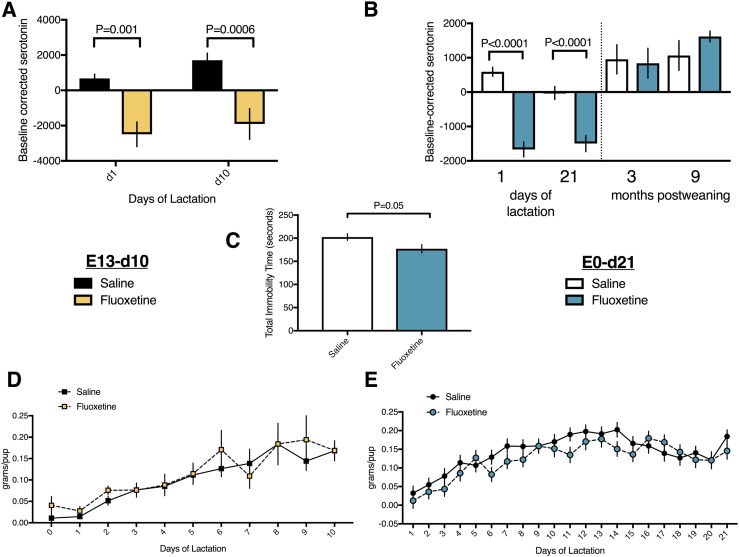
Dams administered peripartum fluoxetine have reduced circulating serotonin, characteristic behavioral changes of SSRIs, and lactate normally
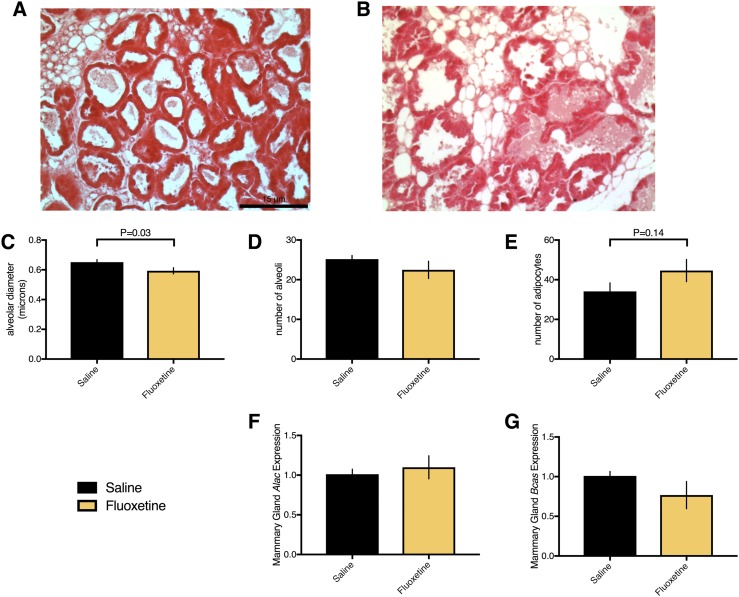
Peripartum fluoxetine exposure decreased serum serotonin concentrations (Fig. 4A and 4B) on days 1, 10, and 21 of lactation in both experiments, characteristic of SSRIs. To evaluate whether fluoxetine was also sufficient to change behavior, we administered the Porsolt FST on day 10 of lactation. Fluoxetine exposure reduced the total immobility time in the FST, suggesting that fluoxetine was effective in modifying depression-like behavior (Fig. 4C) (33). Given that lactating women prescribed SSRIs for depression or anxiety are still able to breastfeed, we did not expect any effect of fluoxetine exposure on milk synthesis. Fluoxetine exposure did not alter the milk yield (Fig. 4D and 4E) in either experiment. Although mice exposed to fluoxetine through day 10 of lactation had mammary gland alveoli that were smaller than saline-exposed mice, this did not appear to affect their ability to lactate (Fig. 5A–5C). No difference was found between saline and fluoxetine exposure with respect to mammary gland alveolar number (Fig. 5D) or adipocyte number (Fig. 5E). α-Lactalbumin (Fig. 5F) and β-casein (Fig. 5G) mRNA expression in the mammary gland was unaffected by fluoxetine exposure.
Figure 4.
Peripartum fluoxetine exposure to C57BL/6 dams reduced serum serotonin and improved depression-like behavior but had no effect on milk yield. (A) Saline (n = 16) or 20 mg/kg fluoxetine (n = 14) was administered from the day of conception through day 21 of lactation. Mice were then aged to either 3 mo (n = 8, saline group; n = 7, fluoxetine group) or 9 mo (n = 8, saline group; n = 7, fluoxetine group) after weaning. Blood samples were collected from all mice before any treatment on the day of conception, on days 1 and 21 of lactation, and at euthanasia, and serum was isolated. Serum serotonin (ng/mL) is shown, corrected to each mouse’s pre-exposure baseline sample. (B) Saline (n = 8) or 20 mg/kg fluoxetine (n = 8) was administered from E13 through day 10 of lactation. Blood samples were taken before any treatment on E13 and on days 1 and 10 of lactation, and serum was isolated. Serum serotonin (ng/mL) is shown, corrected to each mouse’s pre-exposure baseline sample. (C) Mice dosed from the day of conception through day 21 of lactation were administered Porsolt FST on day 10 of lactation, and total immobility time was recorded. (D and E) Each day of lactation, pups were removed from the dam and fasted for 4 h. After the fast, the pups were weighed, returned to the dam for 45 minutes, and weighed again. The difference in weight represented the milk yield for each litter, which was then divided by the number of pups in the litter on any given day to estimate the milk yield per pup. (D) Mice dosed from conception through day 21 of lactation. (E) Mice dosed from E13 to day 10 of lactation. Data presented as mean ± SEM and analyzed using an SAS mixed linear model for treatment and time, including repeated measures for milk yield data, or a Student t test.
Figure 5.
Peripartum fluoxetine exposure did not affect the dam’s lactation. Saline (n = 4) or 20 mg/kg fluoxetine (n = 4) was administered from E13 through day 10 of lactation, at which time, the mice were euthanized. (A and B) The number four mammary gland was collected and histological hematoxylin and eosin staining was performed. Images were taken at ×20 magnification; scale bar, 15 µm. (C–E) The number of alveoli, diameter of alveoli, and number of adipocytes were quantified from three nonoverlapping sections for each mouse. (F and G) RNA samples were prepared from mammary gland tissues and subjected to quantitative RT-PCR for expression of α-lactalbumin and β-casein. Data presented as mean ± SEM and analyzed using a Student t test.
Discussion
These results have provided evidence of postweaning reductions in maternal bone volume after peripartum fluoxetine exposure. The longstanding paradigm that bone mineral loss during lactation is fully recovered after weaning (6, 7) does not account for additional factors, such as SSRI use, that place women at risk of bone loss during the formative age when the bone mass usually peaks. Lactation (8–10) and SSRIs (16, 17) have been independently associated with persistent areal bone density deficits in humans. To the best of our knowledge, the consequence of SSRI use during lactation had not been previously examined. We have shown a sustained, negative effect of SSRI use during pregnancy and lactation on maternal trabecular bone. Additionally, we have provided evidence that mice lose significant trabecular bone volume by the age of ~11 months, or 9 months after weaning. Previous work has shown that virgin mice lose trabecular BV/TV from 4 to 11 months of age, suggesting age-associated bone loss (38). However, the trabecular microarchitecture at the tibia and femur do not fully recover in mice that have experienced lactation compared with nulliparous mice (30), possibly explaining the relatively low BV/TV of only 3.5% of the saline-exposed dams in the present experiment. More detailed experiments at various different skeletal sites and across parities are warranted to gather a time course of precisely how and when bone recovers from lactation.
The reductions in postweaning BV/TV that were observed in dams treated with fluoxetine might derive from events during lactation, because mammals undergo rapid lactational bone turnover, favoring resorption, to liberate calcium for milk synthesis (6, 26–31). Peripartum fluoxetine exposure increased circulating concentrations of calcium during lactation, when absorption of calcium from the diet and bone demineralization both contribute to the maternal calcium pool (6). Because fluoxetine exposure did not modulate mRNA expression of calcium transporters in the intestine during lactation, which are known to be elevated during pregnancy and lactation (42), dietary intestinal absorption does not appear to be a substantial contributor to fluoxetine-dependent changes in serum calcium. This finding supports information suggesting that the skeleton is a more substantial contributor to the maternal calcium pool during lactation than the intestine (6). Fluoxetine-exposed mice had reduced markers of bone formation in their serum and bone mRNA during lactation, with no change in markers of bone resorption in the serum or bone. Various studies have examined the relationship between SSRIs and bone turnover. In nonpregnant, nonlactating mice, fluoxetine has been associated with net bone loss owing to reduced bone formation (15, 19). In other studies, fluoxetine directly inhibited osteoblast proliferation (20) and mineralization during fracture healing (21). Less evidence that fluoxetine directly affects osteoclasts or bone resorption is available (15, 22). Reduced bone formation, and not osteoclastic bone resorption, appears to be responsible for the fluoxetine-mediated reductions in postweaning maternal bone volume.
Fluoxetine exposure during the peripartum period was associated with reduced postweaning maternal bone trabecular volume; however, no indication was found of enhanced osteoclastic resorption during lactation. It is possible that fluoxetine induces lactational bone demineralization through a mechanism other than osteoclastic resorption, such as osteocytic osteolysis. Osteocytes undergo PTHrP-dependent perilacunar and canalicular matrix remodeling to liberate skeletal calcium stores during lactation (28, 43). In previous work, administering PTHrP 1-36 to virgin mice increased osteocyte lacunar size, and this response was potently blocked by conditional disruption of the PTHrP receptor in osteocytes (28). Continuous PTHrP infusion to human patients also reduced circulating markers of bone formation (40), consistent with the peripartum fluoxetine actions we found in the present study.
Because the mammary gland is the main source of circulating PTHrP during lactation (5), we hypothesized and confirmed that fluoxetine elevates mammary gland Pthrp synthesis. Previous work from our laboratory has shown that serotonin modulates the synthesis and secretion of mammary gland Pthrp through a mechanism involving DNA methylation (25). In the present study, dams exposed to fluoxetine had reduced methylation of the Pthrp promoter in their mammary glands at midlactation. Because reduced DNA methylation in the promoter region is typically associated with elevated transcription, it was surprising that Pthrp mRNA expression was only modestly elevated. Previous work on serotonin-driven Pthrp methylation was performed in Tph1 knockout mice, with findings suggesting that genetic manipulation of serotonin has effects distinct from that of SSRIs (25). Methylation at the Pthrp promoter has previously been associated with Pthrp expression in breast cancer cell lines, with evidence that specific CpG dinucleotide methylation is the dominant mechanism regulating Pthrp expression, rather than overall methylation of the CpG island (44). As such, future work should focus on using more sophisticated techniques to understand the precise mechanism by which fluoxetine regulates Pthrp methylation and synthesis in the lactating mammary gland. cAMP, a functional readout of PTHrP, was elevated in fluoxetine-exposed mammary glands. Urinary cAMP has previously been associated with plasma PTHrP levels during lactation (5), and exposing primary mammary epithelial cells to PTHrP increased cAMP expression (41). As the lactating mammary gland is a heterogeneous tissue, some caution should be taken in directly correlating an elevation in mammary gland PTHrP with cAMP activity. However, in combination with reduced DNA methylation at the Pthrp promoter and a tendency toward elevated Pthrp mRNA, we hypothesized that Pthrp synthesized and presumably secreted from the fluoxetine-exposed mammary gland might increase osteocytic osteolysis at the bone, especially because no perturbation was found in osteoclastic resorption in mice exposed to fluoxetine. With simultaneous repression of bone formation, mammary-derived PTHrP signaling during lactation might compromise bone through a process not yet determined, such that maternal trabecular bone volume cannot recover after weaning.
Mammary gland CaSR coordinates a feedback loop with PTHrP between the bone and breast (45). In normal lactating breast cells, extracellular calcium stimulation of CaSR suppresses PTHrP secretion. However, in breast cancer cells, CaSR upregulates PTHrP production, producing a forward-feeding cycle that promotes osteolysis (46). As expression of both Pthrp and Casr was elevated in the mammary glands of mice exposed to fluoxetine, it is possible that CaSR creates a forward-feeding loop with PTHrP to stimulate osteolysis at the bone and increase serum calcium concentrations. Although it was not the focus of the present study, fluoxetine might modulate calcium flux at the level of the mammary gland, potentially altering the amount of milk calcium available to the pups.
Fluoxetine-driven perturbations in CaSR and PTHrP signaling might be controlled by mammary gland serotonin. During lactation, non-neuronal serotonin regulates mammary Pthrp and Casr expression (23–25, 47). SSRIs such as fluoxetine inhibit the reuptake of serotonin into the cell for degradation, thereby increasing cellular exposure to serotonin both centrally and peripherally (48). We have confirmed that peripartum fluoxetine exposure elevates mammary gland serotonin content (Fig. 3F) during lactation but does not affect the duodenal serotonin content. In a nonlactating state, the gut is the main source of non-neuronal serotonin (49). Studies examining whether gut-derived serotonin regulates bone turnover have yielded mixed results (50, 51). A recent report has demonstrated that, during lactation, genetic disruption of Tph1 in the mammary gland during late pregnancy and lactation reduces mammary gland, but not duodenal, serotonin content, and serum serotonin concentrations are reduced by one half (52). During lactation, fluoxetine appears to selectively elevate the mammary gland but not duodenal serotonin content. Collectively, these data suggest that the mammary gland assumes partial control of serotonin homeostasis during lactation, including the coordination of breast-to-bone PTHrP signaling that regulates maternal bone volume.
Given the detrimental effects of fluoxetine on postweaning maternal trabecular bone, we were also interested in examining nonskeletal physiology. Fluoxetine administration to mice mimics both physiological and behavioral effects of SSRIs observed in humans. In lactating dams, fluoxetine exposure decreased serum serotonin concentrations (Fig. 4A and 4B), as previously reported in women (53) and mice (54). SSRIs inhibit the reuptake of serotonin into platelets, which store ~95% of serotonin in the circulation. Because platelets lack the TPH1 enzyme and therefore cannot synthesize serotonin, circulating stores of serotonin eventually become depleted with SSRI use (53, 54). SSRIs are typically prescribed to pregnant and lactating women to improve feelings of depression and anxiety. We have confirmed that fluoxetine exposure similarly reduces depression-like behavior in mice, because dams exposed to fluoxetine had reduced immobility time in the Porsolt FST (33). These data suggest that SSRIs administered to lactating mice are efficacious in the brain, matching previous work showing that lactating mice are more responsive to SSRIs compared with nonlactating postpartum or virgin females (54). Recently, fluoxetine was shown to modulate bone mass through a brain-serotonin–dependent mechanism (15). It is therefore possible that fluoxetine has redundant effects in lactating dams, potentially modulating the bone mass through the central nervous system, at the level of the bone directly, and through mammary-derived signaling cascades. Finally, although some studies have demonstrated that SSRIs delay lactogenesis (54), women taking SSRIs during lactation are still able to support their infants. Although it would be an overstatement to directly extrapolate the mammary gland histological findings of a mouse mammary gland to a clinical human population, lactating dams exposed to fluoxetine had normal mammary gland histological features and produced the same amount of milk as did the control dams. Taken together, our data have shown that peripartum administration of fluoxetine to mice causes nonskeletal physiological responses, in addition to affecting the mammary gland and bone directly.
To the best of our knowledge, our results are the first to demonstrate that fluoxetine exposure during the critical window of pregnancy and lactation reduces maternal postweaning BV/TV. Fluoxetine increases mammary serotonin content, elevates Pthrp and Casr mRNA synthesis and serum calcium concentrations, and reduces markers of bone formation during lactation. Postweaning reductions in trabecular bone might be exacerbated by lactational mammary gland signals known to regulate bone mass in mice administered SSRIs. Alternatively, SSRIs could have a direct effect on bone during lactation or work through a brain-serotonin–dependent mechanism. SSRIs are considered the safest antidepressant for peripartum women with respect to pregnancy outcomes (11, 12); however, their effect on long-term maternal health has been less well studied. Because SSRIs only became popular during the late 1980s and early 1990s, the first cohorts of pregnant and breastfeeding women using SSRIs are only now reaching menopause. Because an estimated 9.2% of US women aged 18 to 39 years are taking an antidepressant medication (56), a large population is potentially vulnerable to sustained reductions in BV/TV, possibly their increasing risk of osteoporosis and fracture. Preclinical and clinical trials are warranted to address the potential risk to maternal bone for a substantial number of aging women who take SSRIs during pregnancy and lactation.
Acknowledgments
We thank Dr. Robert Aiello for his significant financial and conceptual contributions. We are also grateful to the animal care staff at the University of Wisconsin-Madison.
Financial Support: This work was supported by Karos Pharmaceuticals, USDA-HATCH Grant WIS01732 (to L.L.H.); National Institutes of Health Grants AG046257 and AR062590 (to J.F.C.), Grant R01DK099328 (to C.M.V.), and Grant R03DE027162 (to R.J.L.); and the Department of Orthopedics at Brigham and Women’s Hospital. This material is based on work supported by the National Science Foundation Graduate Research Fellowship Program (Grant DGE-1747503). The opinions, findings, and conclusions or recommendations expressed are those of the authors and do not necessarily reflect the views of the National Science Foundation. Support was also provided by the Graduate School and the Office of the Vice Chancellor for Research and Graduate Education at the University of Wisconsin‐Madison with funding from the Wisconsin Alumni Research Foundation.
Author Contributions: S.R.W., C.M.V., R.J.L., J.F.C., and L.L.H. designed the experiment. S.R.W. and H.P.F. conducted the experiments and acquired data. C.X. and J.F.C. performed all bone analyses. S.R.W. analyzed data and wrote the manuscript. All authors read and approved the manuscript before publication.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- BV/TV
bone volume/tissue volume
- CaSR
calcium sensing receptor
- CTX
collagen type 1 cross-linked C-telopeptide
- E13
embryonic day 13
- FST
forced swim test
- P1NP
procollagen I intact N-terminal
- SSRI
selective serotonin reuptake inhibitor
- Tph1
tryptophan hydroxylase 1
- WSW
weigh-suckle-weigh
References
- 1. National Institutes of Health Handout on Health Osteoporosis. Osteoporosis Information Web Site. Available at: https://www.bones.nih.gov/health-info/bone/osteoporosis/osteoporosis-hoh. Updated February, 2016. Accessed 29 January 2018.
- 2. Looker AC, Frenk SM. Percentage of adults aged 65 and over with osteoporosis of low bone mass at the femur neck or lumbar spine: United States, 2005-2010. Centers for Disease Control and Prevention Web Site. Available at: https://www.cdc.gov/nchs/data/hestat/osteoporsis/osteoporosis2005_2010.htm. Updated 13 August 2015. Accessed 29 January 2018.
- 3. Osteoporosis: Peak Bone Mass in Women. National Institutes of Health Osteoporosis and Related Bone Disease National Resource Center Web Site. Available at: https://www.bones.nih.gov/health-info/bone/osteoporosis/bone-mass. Updated June, 2015. Accessed 29 January 2018.
- 4. Mathews TJ, Hamilton BE. Mean Age of Mothers Is on the Rise: United States, 2000-2014. NCHS Data Brief. Hyattsville, MD: National Center for Health Statistics; 2016. [PubMed] [Google Scholar]
- 5. VanHouten JN, Dann P, Stewart AF, Watson CJ, Pollak M, Karaplis AC, Wysolmerski JJ. Mammary-specific deletion of parathyroid hormone-related protein preserves bone mass during lactation. J Clin Invest. 2003;112(9):1429–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kovacs CS. Maternal mineral and bone metabolism during pregnancy, lactation, and post-weaning recovery. Physiol Rev. 2016;96(2):449–547. [DOI] [PubMed] [Google Scholar]
- 7. Wysolmerski JJ. Interactions between breast, bone, and brain regulate mineral and skeletal metabolism during lactation. Ann N Y Acad Sci. 2010;1192(1):161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mgodi NM, Kelly C, Gati B, Greenspan S, Dai JY, Bragg V, Livant E, Piper JM, Nakabiito C, Magure T, Marrazzo JM, Chirenje ZM, Riddler SA; MTN-003B Protocol Team . Factors associated with bone mineral density in healthy African women. Arch Osteoporos. 2015;10(1):206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hwang IR, Choi YK, Lee WK, Kim JG, Lee IK, Kim SW, Park KG. Association between prolonged breastfeeding and bone mineral density and osteoporosis in postmenopausal women: KNHANES 2010-2011. Osteoporos Int. 2016;27(1):257–265. [DOI] [PubMed] [Google Scholar]
- 10. Bjørnerem Å, Ghasem-Zadeh A, Wang X, Bui M, Walker SP, Zebaze R, Seeman E. Irreversible deterioration of cortical and trabecular microstructure associated with breastfeeding. J Bone Miner Res. 2017;32(4):681–687. [DOI] [PubMed] [Google Scholar]
- 11. Davanzo R, Copertino M, De Cunto A, Minen F, Amaddeo A. Antidepressant drugs and breastfeeding: a review of the literature. Breastfeed Med. 2011;6(2):89–98. [DOI] [PubMed] [Google Scholar]
- 12. Tran H, Robb AS. SSRI use during pregnancy. Semin Perinatol. 2015;39(7):545–547. [DOI] [PubMed] [Google Scholar]
- 13. Bonnet N, Bernard P, Beaupied H, Bizot JC, Trovero F, Courteix D, Benhamou CL. Various effects of antidepressant drugs on bone microarchitectecture, mechanical properties and bone remodeling. Toxicol Appl Pharmacol. 2007;221(1):111–118. [DOI] [PubMed] [Google Scholar]
- 14. Warden SJ, Nelson IR, Fuchs RK, Bliziotes MM, Turner CH. Serotonin (5-hydroxytryptamine) transporter inhibition causes bone loss in adult mice independently of estrogen deficiency. Menopause. 2008;15(6):1176–1183. [DOI] [PubMed] [Google Scholar]
- 15. Ortuño MJ, Robinson ST, Subramanyam P, Paone R, Huang YY, Guo XE, Colecraft HM, Mann JJ, Ducy P. Serotonin-reuptake inhibitors act centrally to cause bone loss in mice by counteracting a local anti-resorptive effect. Nat Med. 2016;22(10):1170–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rabenda V, Nicolet D, Beaudart C, Bruyère O, Reginster JY. Relationship between use of antidepressants and risk of fractures: a meta-analysis. Osteoporos Int. 2013;24(1):121–137. [DOI] [PubMed] [Google Scholar]
- 17. Rauma PH, Honkanen RJ, Williams LJ, Tuppurainen MT, Kröger HP, Koivumaa-Honkanen H. Effects of antidepressants on postmenopausal bone loss—a 5-year longitudinal study from the OSTPRE cohort. Bone. 2016;89:25–31. [DOI] [PubMed] [Google Scholar]
- 18. Ducy P, Karsenty G. The two faces of serotonin in bone biology. J Cell Biol. 2010;191(1):7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Warden SJ, Robling AG, Sanders MS, Bliziotes MM, Turner CH. Inhibition of the serotonin (5-hydroxytryptamine) transporter reduces bone accrual during growth. Endocrinology. 2005;146(2):685–693. [DOI] [PubMed] [Google Scholar]
- 20. Kode A, Mosialou I, Silva BC, Rached MT, Zhou B, Wang J, Townes TM, Hen R, DePinho RA, Guo XE, Kousteni S. FOXO1 orchestrates the bone-suppressing function of gut-derived serotonin. J Clin Invest. 2012;122(10):3490–3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bradaschia-Correa V, Josephson AM, Mehta D, Mizrahi M, Neibart SS, Liu C, Kennedy OD, Castillo AB, Egol KA, Leucht P. The selective serotonin reuptake inhibitor fluoxetine directly inhibits osteoblast differentiation and mineralization during fracture healing in mice. J Bone Miner Res. 2017;32(4):821–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hodge JM, Wang Y, Berk M, Collier FM, Fernandes TJ, Constable MJ, Pasco JA, Dodd S, Nicholson GC, Kennedy RL, Williams LJ. Selective serotonin reuptake inhibitors inhibit human osteoclast and osteoblast formation and function. Biol Psychiatry. 2013;74(1):32–39. [DOI] [PubMed] [Google Scholar]
- 23. Hernandez LL, Gregerson KA, Horseman ND. Mammary gland serotonin regulates parathyroid hormone-related protein and other bone-related signals. Am J Physiol Endocrinol Metab. 2012;302(8):E1009–E1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Laporta J, Peters TL, Weaver SR, Merriman KE, Hernandez LL. Feeding 5-hydroxy-L-tryptophan during the transition from pregnancy to lactation increases calcium mobilization from bone in rats. Domest Anim Endocrinol. 2013;44(4):176–184. [DOI] [PubMed] [Google Scholar]
- 25. Laporta J, Keil KP, Weaver SR, Cronick CM, Prichard AP, Crenshaw TD, Heyne GW, Vezina CM, Lipinski RJ, Hernandez LL. Serotonin regulates calcium homeostasis in lactation by epigenetic activation of hedgehog signaling. Mol Endocrinol. 2014;28(11):1866–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. VanHouten JN, Wysolmerski JJ. Low estrogen and high parathyroid hormone-related peptide levels contribute to accelerated bone resorption and bone loss in lactating mice. Endocrinology. 2003;144(12):5521–5529. [DOI] [PubMed] [Google Scholar]
- 27. Wysolmerski JJ. Parathyroid hormone-related protein: an update. J Clin Endocrinol Metab. 2012;97(9):2947–2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Qing H, Ardeshirpour L, Pajevic PD, Dusevich V, Jähn K, Kato S, Wysolmerski J, Bonewald LF. Demonstration of osteocytic perilacunar/canalicular remodeling in mice during lactation. J Bone Miner Res. 2012;27(5):1018–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ardeshirpour L, Brian S, Dann P, VanHouten J, Wysolmerski J. Increased PTHrP and decreased estrogens alter bone turnover but do not reproduce the full effects of lactation on the skeleton. Endocrinology. 2010;151(12):5591–5601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu XS, Ardeshirpour L, VanHouten JN, Shane E, Wysolmerski JJ. Site-specific changes in bone microarchitecture, mineralization, and stiffness during lactation and after weaning in mice. J Bone Miner Res. 2012;27(4):865–875. [DOI] [PubMed] [Google Scholar]
- 31. Wendelboe MH, Thomsen JS, Henriksen K, Vegger JB, Brüel A. Zoledronate prevents lactation induced bone loss and results in additional post-lactation bone mass in mice. Bone. 2016;87:27–36. [DOI] [PubMed] [Google Scholar]
- 32. Dulawa SC, Holick KA, Gundersen B, Hen R. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology. 2004;29(7):1321–1330. [DOI] [PubMed] [Google Scholar]
- 33. Can A, Dao DT, Arad M, Terrillion CE, Piantadosi SC, Gould TD. The mouse forced swim test. J Vis Exp. 2012;59(59):e3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25(4):402–408. [DOI] [PubMed] [Google Scholar]
- 35. Keil KP, Abler LL, Mehta V, Altmann HM, Laporta J, Plisch EH, Suresh M, Hernandez LL, Vezina CM. DNA methylation of E-cadherin is a priming mechanism for prostate development. Dev Biol. 2014;387(2):142–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. RRID:AB_325374.
- 37. RRID:AB_641170.
- 38. Glatt V, Canalis E, Stadmeyer L, Bouxsein ML. Age-related changes in trabecular architecture differ in female and male C57BL/6J mice. J Bone Miner Res. 2007;22(8):1197–1207. [DOI] [PubMed] [Google Scholar]
- 39. Bornstein S, Brown SA, Le PT, Wang X, DeMambro V, Horowitz MC, MacDougald O, Baron R, Lotinun S, Karsenty G, Wei W, Ferron M, Kovacs CS, Clemmons D, Wan Y, Rosen CJ. FGF-21 and skeletal remodeling during and after lactation in C57BL/6J mice. Endocrinology. 2014;155(9):3516–3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Horwitz MJ, Tedesco MB, Sereika SM, Prebehala L, Gundberg CM, Hollis BW, Bisello A, Garcia-Ocaña A, Carneiro RM, Stewart AF. A 7-day continuous infusion of PTH or PTHrP suppresses bone formation and uncouples bone turnover. J Bone Miner Res. 2011;26(9):2287–2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ferrari S, Rizzoli R, Chaponnier C, Gabbiani G, Bonjour JP. Parathyroid hormone-related protein increases cAMP production in mammary epithelial cells. Am J Physiol. 1993;264(3 Pt 1):E471–E475. [DOI] [PubMed] [Google Scholar]
- 42. Zhu Y, Goff JP, Reinhardt TA, Horst RL. Pregnancy and lactation increase vitamin D-dependent intestinal membrane calcium adenosine triphosphatase and calcium binding protein messenger ribonucleic acid expression. Endocrinology. 1998;139(8):3520–3524. [DOI] [PubMed] [Google Scholar]
- 43. Tang SY, Herber RP, Ho SP, Alliston T. Matrix metalloproteinase-13 is required for osteocytic perilacunar remodeling and maintains bone fracture resistance. J Bone Miner Res. 2012;27(9):1936–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tost J, Hamzaoui H, Busato F, Neyret A, Mourah S, Dupont JM, Bouizar Z. Methylation of specific CpG sites in the P2 promoter of parathyroid hormone-related protein determines the invasive potential of breast cancer cell lines. Epigenetics. 2011;6(8):1035–1046. [DOI] [PubMed] [Google Scholar]
- 45. Mamillapalli R, VanHouten J, Dann P, Bikle D, Chang W, Brown E, Wysolmerski J. Mammary-specific ablation of the calcium-sensing receptor during lactation alters maternal calcium metabolism, milk calcium transport, and neonatal calcium accrual. Endocrinology. 2013;154(9):3031–3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mamillapalli R, VanHouten J, Zawalich W, Wysolmerski J. Switching of G-protein usage by the calcium-sensing receptor reverses its effect on parathyroid hormone-related protein secretion in normal versus malignant breast cells. J Biol Chem. 2008;283(36):24435–24447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Laporta J, Keil KP, Vezina CM, Hernandez LL. Peripheral serotonin regulates maternal calcium trafficking in mammary epithelial cells during lactation in mice. PLoS One. 2014;9(10):e110190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Anderson GM. Peripheral and central neurochemical effects of the selective serotonin reuptake inhibitors (SSRIs) in humans and nonhuman primates: assessing bioeffect and mechanisms of action. Int J Dev Neurosci. 2004;22(5-6):397–404. [DOI] [PubMed] [Google Scholar]
- 49. Mawe GM, Hoffman JM. Serotonin signalling in the gut—functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2013;10(8):473–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yadav VK, Ryu JH, Suda N, Tanaka KF, Gingrich JA, Schütz G, Glorieux FH, Chiang CY, Zajac JD, Insogna KL, Mann JJ, Hen R, Ducy P, Karsenty G. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell. 2008;135(5):825–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cui Y, Niziolek PJ, MacDonald BT, Zylstra CR, Alenina N, Robinson DR, Zhong Z, Matthes S, Jacobsen CM, Conlon RA, Brommage R, Liu Q, Mseeh F, Powell DR, Yang QM, Zambrowicz B, Gerrits H, Gossen JA, He X, Bader M, Williams BO, Warman ML, Robling AG. Lrp5 functions in bone to regulate bone mass. Nat Med. 2011;17(6):684–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Weaver SR, Jury NJ, Gregerson KA, Horseman ND, Hernandez LL. Characterization of mammary-specific disruptions for Tph1 and Lrp5 during murine lactation. Sci Rep. 2017;7(1):15155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Epperson CN, Jatlow PI, Czarkowski K, Anderson GM. Maternal fluoxetine treatment in the postpartum period: effects on platelet serotonin and plasma drug levels in breastfeeding mother-infant pairs. Pediatrics. 2003;112(5):e425. [DOI] [PubMed] [Google Scholar]
- 54. Jury NJ, McCormick BA, Horseman ND, Benoit SC, Gregerson KA. Enhanced responsiveness to selective serotonin reuptake inhibitors during lactation. PLoS One. 2015;10(2):e0117339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Marshall AM, Nommsen-Rivers LA, Hernandez LL, Dewey KG, Chantry CJ, Gregerson KA, Horseman ND. Serotonin transport and metabolism in the mammary gland modulates secretory activation and involution. J Clin Endocrinol Metab. 2010;95(2):837–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pratt LA, Brody DJ, Gu Q. Antidepressant Use in Persons Aged 12 and Over: United States, 2005-2008. NCHS Data Brief, No. 76. Hyattsville, MD: National Center for Health Statistics; 2011. [PubMed] [Google Scholar]