Abstract
It has been reported that class I histone deacetylase (HDAC) inhibition increases thermogenesis in fat, but adipocyte-specific Hdac3 deletions have presented inconsistent results. In this study, we observed that HDAC3 protein levels were lower in brown fat compared with inguinal subcutaneous adipose tissue, and they decreased in both fat depots upon cold exposure. PR domain–containing 16 (PRDM16) physically interacted with HDAC3, and treatment with HDAC3-selective inhibitor RGFP966 induced thermogenic gene expression in murine and human fat cultures. This induction was blunted in the absence of PRDM16. Our results provide evidence that HDAC3 is involved in thermogenesis, suggesting selective inhibition of HDAC3 in brown and beige fat might hold therapeutic potential for counteracting human obesity and metabolic disorders.
HDAC3 protein levels decreased in brown and beige fat after cold exposure. HDAC3 inhibitor induced thermogenesis in murine and human fat cultures, and this induction is mediated through PRDM16.
Epigenetic dysregulation has been shown to be involved in the pathogenesis of many diseases, including metabolic disorders. Histone deacetylases (HDACs) are enzymes that remove acetyl group from lysine residues within histone tails, leading to transcriptional repression by allowing chromatin compaction (1). There are four classes of HDACs with different structures and enzymatic functions. Class I HDACs consist of HDAC1, HDAC2, HDAC3, and HDAC8, which are ubiquitously expressed and located predominantly in the nucleus (1).
Recent studies have revealed that thermogenic fat, including both brown adipocytes and inducible beige adipocytes that reside in subcutaneous white adipose tissue, play an important role in maintaining metabolic homeostasis (2). The function of brown and beige fat is closely modulated through genetic and epigenetic control. It has been reported that inhibition of class I HDACs leads to increased oxidative metabolism in both fat and skeletal muscle in mice (3). Genetic deletion of Hdac3 in adipocytes revealed inconsistent results in brown and beige fat (4, 5). In the interscapular brown adipose tissue (BAT), deletion of Hdac3 leads to lower basal levels of thermogenic gene expression and a defective response to acute cold exposure (4). It was proposed that this uncanonical coactivation through HDAC3 is mediated through mechanisms involving ERRα and PGC-1α (4). On the other hand, in the subcutaneous inguinal white adipose tissue (iWAT) where beige adipocytes reside, adipocyte-specific deletion of Hdac3 promotes thermogenic activation (5). In our investigation of how HDAC3 may influence brown and beige fat function, we found that HDAC3 protein levels are lower in BAT than in iWAT. After cold exposure, the protein levels of HDAC3 are decreased in both depots. A selective pharmacological inhibitor of HDAC3, RGFP966 (RGFP), induces the thermogenic program in multiple types of adipocytes, including primary human subcutaneous fat cells. HDAC3 physically interacts with PR-domain–containing 16 (PRDM16), one of the key regulators of brown and beige fat function (6). In Prdm16 knockout or knockdown fat cells, the RGFP-induced thermogenic response is blunted. These data collectively support a reconciled model for how acute inhibition of HDAC3 in both brown and beige fat leads to thermogenic activation through a mechanism involving PRDM16.
Materials and Methods
Reagents
DMEM/F-12 GlutaMax (ILT10565042), DMEM (ILT11995073), and MesenPRO RS medium (12746012) were purchased from Life Technologies. Fetal bovine serum (FBS) (F2442), dexamethasone (D4902), insulin (I5500), 3-isobutyl-1-methylxanthine (IBMX) (I7018), biotin (B4639), and d-pantothenic acid hemicalcium salt (P5155) were purchased from Sigma-Aldrich. Rosiglitazone (71740) was purchased from Cayman Chemicals. RGFP966 (S7229) was purchased from Selleck Chemicals. T247 (A2897) was purchased from Tokyo Chemical Industry Co. Ltd. Collagenase D (11088882001), collagenase B (11088831001), dispase II (04942078001), and protease inhibitor cocktail (11836153001) were purchased from Roche.
Animals
All animal experiments were approved by the University of Michigan Institutional Animal Care and Use Committee and conducted in conformity with the Public Health Service Policy for Care and Use of Laboratory Animals. Multiple inbred strains of wild type mice were used in this study, and similar results were observed, including C57BL/6J mice (JAX 000664; the Jackson Laboratory) and 129SVE and BALB/c mice (Taconic Farms Inc.). PRDM16f/f mice (JAX 024992), adiponectin-CRE (AQcre) mice (JAX 028020), and Myf5-CRE (Myf5cre) mice (JAX 007893) were obtained from the Jackson Laboratory. All animals were housed in forced ventilation racks with ad libitum access to food and maintained on a 12-hour light:12-hour dark cycle (6:00 am to 6:00 pm). Mice of both sexes were used in this study, and similar results were observed.
Cell culture and differentiation
Primary murine preadipocytes were isolated from the interscapular brown and inguinal fat depots of mice and differentiated as previously described (7). Briefly, interscapular brown or inguinal subcutaneous fat depots were isolated from mice, minced, and digested by collagenase and dispase II (collagenase B for BAT and collagenase D for iWAT). The stromal vascular fraction formed a pellet, which was filtered, centrifuged, resuspended in DMEM/F12 + GlutaMAX supplemented with 10% FBS and 1% penicillin-streptomycin, and plated on a collagen-coated plate. After one round of subculture, the preadipocytes were plated on collagen-coated 12-well plates for differentiation. To induce differentiation, confluent cells were stimulated in DMEM/F12 + GlutaMAX containing 10% FBS, 1% penicillin-streptomycin, dexamethasone (5 μM), insulin (0.5 μg/mL), IBMX (0.5 mM), and rosiglitazone (1 μM). After 2 days of stimulation, cells were maintained in DMEM/F12 + GlutaMAX containing 10% FBS, 1% penicillin-streptomycin, and insulin (0.5 μg/mL). Mature adipocytes were used for analysis 6 to 7 days after stimulation. C3H-10T1/2 (ATCC CCL-226) cells, a pluripotent cell line derived from mouse embryos, display fibroblastic morphology and are functionally similar to mesenchymal stem cells (8). C3H-10T1/2 cells were included in this study as a commonly used generic adipocyte cell line model and were cultured similarly to primary fat cells except that the base medium DMEM was used instead of DMEM/F12 + GlutaMAX. Immortalized murine brown preadipocytes were maintained in DMEM supplemented with 10% FBS and 1% penicillin-streptomycin and subjected to adipocyte differentiation as reported (9). Briefly, confluent brown preadipocytes were cultured in differentiation medium (DMEM, 10% FBS, 20 nM insulin, and 1 nM T3) for 2 days, then switched to differentiation medium containing 0.5 mM IBMX and 2 μM dexamethasone for 2 days. The cells were subsequently maintained in differentiation medium for ≤6 days. Human adipose stem cells (hASCs) were isolated from lipoaspirates from the subcutaneous adipose tissue of healthy donors undergoing voluntary surgery (a gift from Dr. Jeffrey M. Gimble, Tulane University, New Orleans, LA). All specimens were collected and handled under the protocols reviewed and approved by the Western Institutional Review Board (Puyallup, WA) and the University of Michigan Medical School Institutional Review Board. The demographics of the donors are presented in Supplemental Table 1. hASCs were cultured in MesenPRO RS media and grown on collagen-coated 10-cm plates. To differentiate, cells were seeded onto 12-well collagen-coated plates and stimulated in DMEM/F12 + GlutaMAX supplemented with 10% FBS, dexamethasone (5 μM), insulin (0.5 μg/mL), IBMX (0.5 mM), rosiglitazone (5 μM), biotin (33 μM), and pantothenic acid (17 μM). After 4 days of stimulation, cells were maintained in DMEM/F12 + GlutaMAX containing 10% FBS, 1% penicillin-streptomycin, insulin (0.5 μg/mL), dexamethasone (5 μM), biotin (33 μM), and pantothenic acid (17 μM) until they were fully differentiated (10 to 12 days).
RNA isolation and real-time quantitative PCR analysis
RNA was extracted from cells and tissues with TRI Reagent (T9424; Sigma-Aldrich). RNA was reverse transcribed to cDNA with M-MLV (28025021; Life Technologies), and real-time quantitative PCR (qPCR) was performed with SYBR Green (4368708; Life Technologies) on a 7900HT Fast Real-Time PCR System (Applied Biosystems). Fold change was determined via the ΔΔCt method, with samples normalized to the reference gene 36B4 (RPLP0). All qPCR primer sequences are listed in the Supplemental Table 2.
Coimmunoprecipitation and immunoblotting
Murine BAT and iWAT were homogenized with a handheld homogenizer (Cole Parmer) in radioimmunoprecipitation assay buffer supplemented with protease inhibitor (11836153001; Sigma-Aldrich) and phosphatase inhibitors (10 mM NaF, 60 mM β-glycerolphosphate, pH 7.5, 2 mM sodium orthovanadate, 10 mM sodium pyrophosphate) and lysed on ice for 15 minutes with occasional vortexing. Lysates were cleared by centrifugation at 4°C for 30 minutes to remove fatty acid. Protein extracts were quantified with DC Protein Assay Reagents (5000116; Bio-Rad), and 20 µg proteins from fat tissue were separated by SDS-PAGE gel electrophoresis. Polyvinylidene difluoride membranes were used for transfer. Cells were lysed with radioimmunoprecipitation assay buffer supplemented with protease inhibitor (11836153001; Sigma-Aldrich) and phosphatase inhibitors (10 mM NaF, 60 mM β-glycerolphosphate, pH 7.5, 2 mM sodium orthovanadate, 10 mM sodium pyrophosphate). Cell lysates were quantified similarly as the tissue lysates and resolved by SDS-PAGE gel electrophoresis. Coimmunoprecipitation was done on differentiated immortalized brown adipocytes, infected with adenoviral vectors expressing HDAC3, or both HDAC3 and PRDM16, with GFP as a control. After 48 hours, these cells were lysed in ice-cold immunoprecipitation (IP) lysis buffer (20 mM HEPES, 150 mM NaCl, 0.2% NP40) supplemented with protease inhibitor. Cell lysates were cleared by centrifugation at 4°C for 15 minutes. Protein was precleared with protein G agarose beads (sc-2002; Santa Cruz Biotechnology) for 2 hours at 4°C with rotation. Ten percent of total lysate was saved for input. We incubated 500 μg of protein with FLAG antibody (F1804-50UG; Sigma-Aldrich) or mouse IgG (AF007; R&D Systems) at 4°C with rotation overnight, followed by incubation with protein G agarose beads at 4°C for 3 hours with rotation. Beads were gently washed four times with IP lysis buffer plus inhibitors. Immunoprecipitated proteins were eluted in 2× loading buffer by boiling at 98°C for 5 minutes, and supernatant was isolated from beads. Eluted proteins were resolved by immunoblotting as described above. The membranes were probed with following primary antibodies: from Cell Signaling Technology, HDAC3 (3949S; RRID: AB_2118371), acetylated lysine (9441S; RRID: AB_331805), GAPDH (5174S; RRID: AB_10622025), and HSP90 (4874S; RRID: AB_2121214); from Abcam, uncoupling protein 1 (UCP1; ab10983; RRID: AB_2241462); from R&D Systems, PRDM16 (AF6295-SP; RRID: AB_10717965); and from Sigma-Aldrich, FLAG (F1804-50UG; RRID: AB_262044). The membranes were incubated with horseradish peroxidase–conjugated secondary antibodies as needed for certain primary antibodies: goat anti-mouse IgG (115-035-003; Jackson ImmunoResearch Laboratories; RRID: AB_10015289), goat anti-rabbit IgG (7074S; Cell Signaling Technology; RRID: AB_2099233), rabbit anti-sheep IgG (12-342; MilliporeSigma; RRID: AB_11213530), and VeriBlot (ab131366; Abcam) for IP detection reagent was used for HDAC3 after co-IP. The blots were developed with enhanced chemiluminescence detection reagents.
Construction of adenovirus
Full-length mouse complementary DNA sequence for Hdac3 was cloned into the pAdTrack-CMV adenoviral vector, tagged with an N-terminal FLAG. Adenoviral-mHDAC3 was generated and amplified as described (10). Adenoviruses expressing GFP or PRDM16 and adenoviruses expressing a short hairpin RNA targeting Prdm16 or scrambled as the control were described previously (11).
Statistical analysis
Data are expressed as mean ± SEM, and P < 0.05 was considered statistically significant. Two-tailed unpaired Student t test was used for two-group comparison, and statistical significance was noted as *P < 0.05, **P < 0.01, and ***P < 0.001. A one-way ANOVA with post hoc Tukey honest significant difference test was used for multiple comparisons. Data were graphed in GraphPad Prism 6 (GraphPad Software).
Results
HDAC3 protein levels are decreased in brown and beige fat after chronic cold exposure
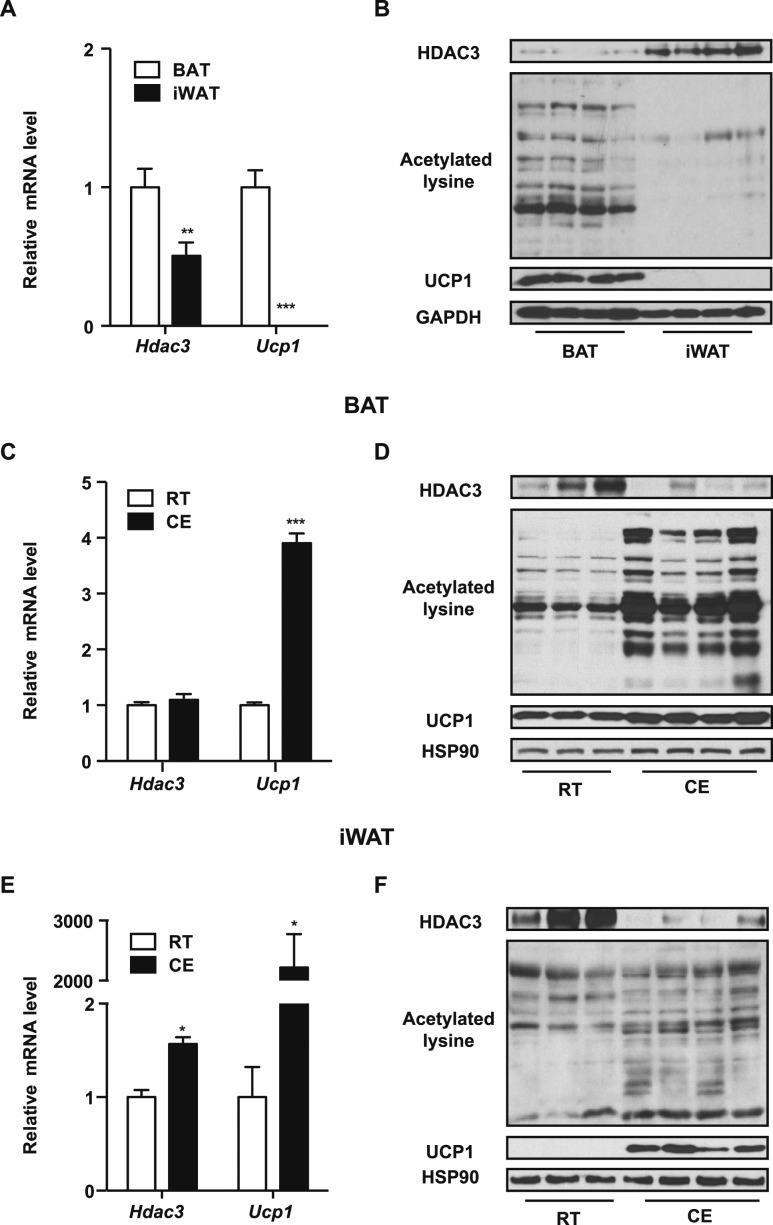
To investigate how HDAC3 functions in thermogenic fat tissue, we began by testing its expression level in different fat depots. At ambient temperature, expression of the thermogenic marker Ucp1, at both the mRNA and protein levels were much higher in BAT than in iWAT (Fig. 1A and 1B), consistent with the notion that brown fat is the major thermogenic organ. The mRNA levels of Hdac3 were slightly higher in BAT than in iWAT (Fig. 1A), but HDAC3 protein levels in BAT were much lower than in iWAT (Fig. 1B). A similar pattern was seen in multiple strains of inbred wild type mice (Fig. 1A and 1B; Supplemental Fig. 1). Consistent with the role of HDACs in mediating deacetylation, acetylated lysine levels were higher in BAT than in iWAT (Fig. 1B). We challenged the mice with chronic cold exposure (10°C for 7 days) to activate thermogenesis and observed that UCP1 was significantly upregulated in both brown and inguinal fat, as expected (Fig. 1C, 1D, 1E, and 1F). Even though only subtle changes were observed in the mRNA levels of Hdac3 in fat tissues after cold exposure (Fig. 1C and 1E), protein levels of HDAC3 were significantly decreased in both BAT and iWAT, together with an increase in acetylated lysine levels, after cold exposure in both fat depots (Fig. 1D and 1F).
Figure 1.
HDAC3 protein levels are lower in BAT than in iWAT and are reduced in both thermogenic fat depots after cold exposure. (A, B) Real-time qPCR analyses of (A) Hdac3 and Ucp1 (n = 3 per group) and (B) representative immunoblots of HDAC3, acetylated lysine, UCP1, and GAPDH as a loading control in BAT and iWAT isolated from C57BL/6J mice housed at room temperature. (C–F) Real-time qPCR analyses of Hdac3 and Ucp1 in (C) BAT (n = 3) and (E) iWAT (n = 3) and representative immunoblots of HDAC3, acetylated lysine, UCP1 and HSP90 as a loading control in (D) BAT and (F) iWAT isolated from C57BL/6J mice housed at room temperature or exposed to 10°C for 7 days. Data are presented as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001. CE, cold exposure; RT, room temperature.
Pharmacological inhibition of HDAC3 promotes thermogenesis in adipocytes
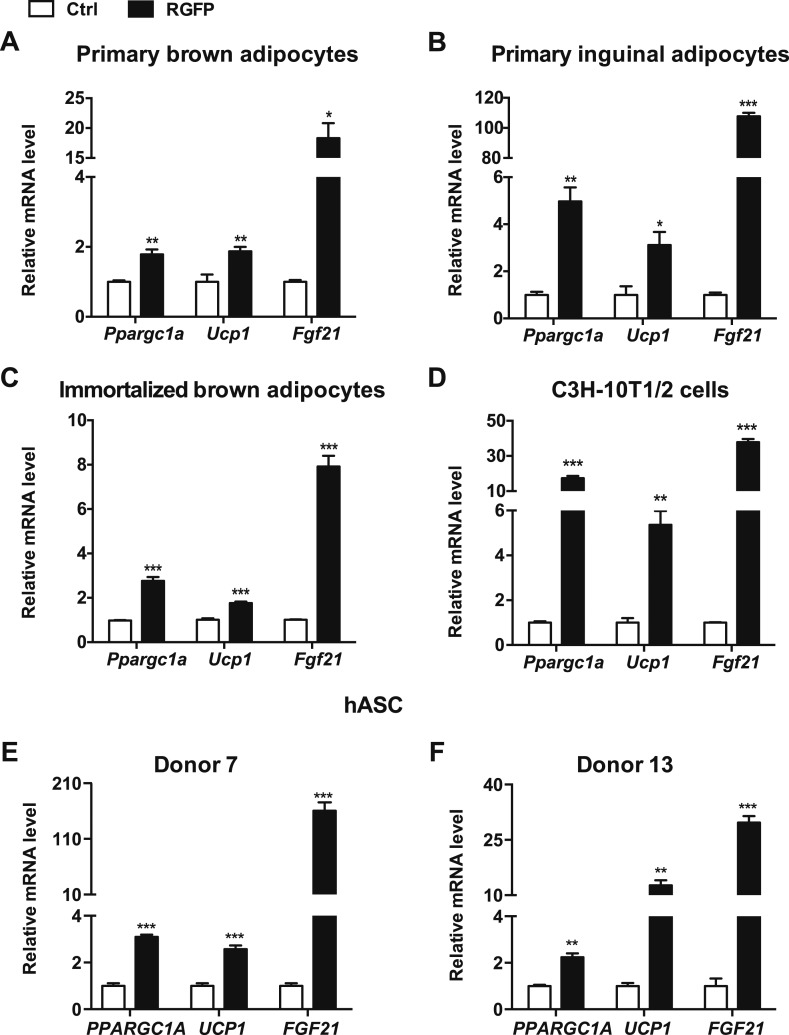
Our data indicate that HDAC3 protein is decreased in both BAT and iWAT after chronic cold exposure, and therefore we hypothesized that repression of HDAC3 activity may correlate with the activation of thermogenesis in fat. Specific inhibitors have been developed to target individual HDACs, such as RGFP966 (RGFP), which is highly selective for HDAC3 (12). We treated primary brown fat cells with RGFP for 6 hours and observed elevated expression of thermogenic genes such as peroxisome proliferator–activated receptor γ coactivator 1-α (Ppargc1a), Ucp1, and fibroblast growth factor 21 (Fgf21) (Fig. 2A). A similar pattern of thermogenic gene induction by RGFP was observed in primary brown and inguinal fat cells isolated from different strains of inbred mice (Fig. 2B; Supplemental Fig. 2 and Supplemental Fig. 3A, and 3B), indicating that inhibition of HDAC3 activity increases thermogenic activity in both brown and beige fat cells regardless of genetic background. RGFP treatments also increased thermogenic gene expression in an immortalized brown fat cell line and immortalized C3H-10T1/2 cells (Fig. 2C and Fig. 2D; Supplemental Fig. 3C), suggesting that this response observed in primary fat cells is mediated through an adipocyte cell–autonomous mechanism. As expected, the acetylated lysine levels were also increased in these adipocytes when treated with RGFP (Supplemental Fig. 3D). To further ensure that the observed response is the result of HDAC3 inhibition, we treated adipocytes with T247, another highly selective and potent inhibitor for HDAC3 (13). A similar pattern of increased thermogenic gene expression and acetylated lysine was observed in these cells in response to T247 treatment (Supplemental Fig. 4). It has been reported that stromal cells isolated from the human subcutaneous fat depot respond to thermogenic stimuli, indicating that human thermogenic adipocytes may exist in other depots besides the neck area (14–16). Indeed, when treated with RGFP, thermogenic genes (PPARGC1A,UCP1, and FGF21) were elevated in differentiated hASCs isolated from multiple donors (Fig. 2E and 2F), indicating that this signaling pathway is conserved in human adipocytes.
Figure 2.
Selective HDAC3 inhibitor treatment increases thermogenic gene expression in adipocytes. (A–D) Real-time qPCR analyses of thermogenic genes after treatment with vehicle control or 50 μM RGFP for 6 h in (A) primary brown adipocytes and (B) primary inguinal adipocytes isolated from C57BL/6J mice, (C) immortalized brown adipocytes, and (D) C3H-10T1/2 cells. (E, F) Real-time qPCR analyses of thermogenic genes in differentiated hASCs treated with vehicle control or 50 μM RGFP for 24 h. Data are presented as mean ± SEM; n = 3. *P < 0.05; **P < 0.01; ***P < 0.001. Ctrl, control.
HDAC3 inhibition mediates thermogenesis through PRDM16
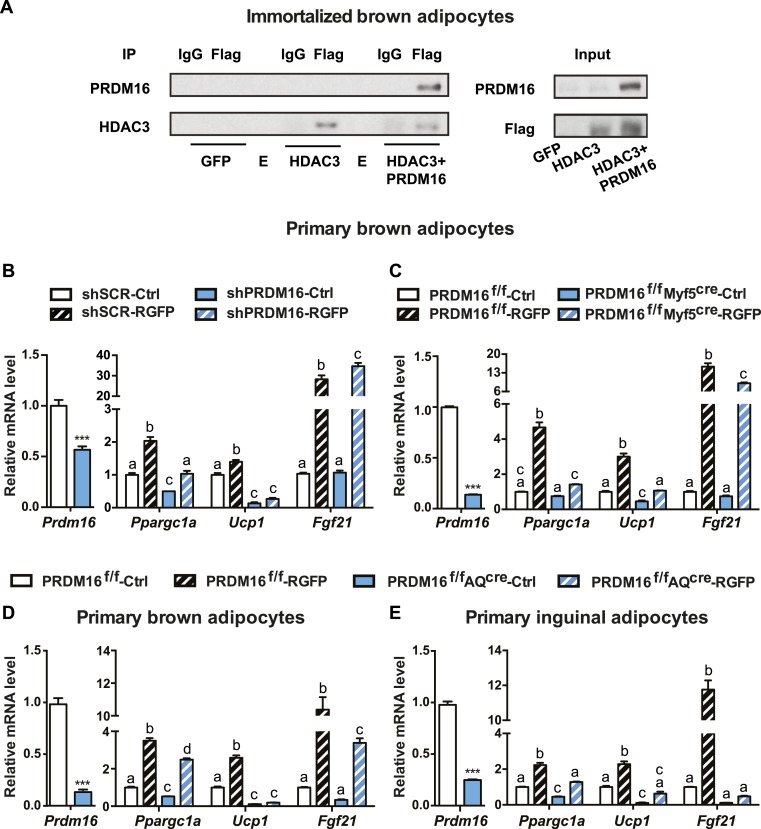
HDAC3 has been reported to function as part of multiprotein complexes (1). PRDM16 plays key regulatory functions in both brown and beige fat through interactions with other regulatory proteins (6). We found that these two proteins physically interacted with one another in adipocytes, as shown by coimmunoprecipitation assays (Fig. 3A). These data suggest that PRDM16 may participate in HDAC3 regulatory signaling in thermogenic fat.
Figure 3.
PRDM16 physically interacts with HDAC3 in adipocytes, and RGFP-induced thermogenesis in primary adipocytes is blunted in the absence of PRDM16. (A) Coimmunoprecipitation of PRDM16 with HDAC3. Immortalized brown adipocytes were infected with adenovirus overexpressing GFP, FLAG-tagged HDAC3, or FLAG-tagged HDAC3 and PRDM16 as indicated. Total lysates were subjected to IP with beads specific for FLAG tag or IgG as a control. Both input and precipitates were analyzed by immunoblotting as indicated. (B–E) Real-time qPCR analyses of Prdm16 and thermogenic markers after treatment with vehicle control or 50 μM RGFP for 6 h in (B) primary brown adipocytes infected with adenovirus expressing either short hairpin RNA targeting Prdm16 (shPRDM16) or a scramble control (shSCR) (n = 3), (C) in primary brown adipocytes isolated from PRDM16f/f mice or PRDM16f/fMyf5cre mice (n = 4), (D) in primary brown adipocytes isolated from PRDM16f/f mice or PRDM16f/fAQcre mice (n = 3 to 4), or (E) in primary inguinal adipocytes isolated from PRDM16f/f mice or PRDM16f/fAQcre mice (n = 3 to 4). Data are presented as mean ± SEM; a different letter denotes a significant difference between groups at P < 0.05; ***P < 0.001. Ctrl, control; E, empty lane.
We next asked whether PRDM16 was necessary for RGFP-induced thermogenic activation. Adenoviral vectors expressing short hairpin RNA targeting Prdm16 or a control scramble sequence were used to transduce primary preadipocytes isolated from BAT, where basal expression levels of Prdm16 are considerably higher than those of primary inguinal adipocytes. As shown in Fig. 3B, knockdown of Prdm16 led to a blunted response to RGFP treatment. Classic brown adipocytes arise from a common developmental lineage as the skeletal muscle (17); therefore, CRE driven by the skeletal muscle marker Myf5 can delete Prdm16 expression in brown fat cells in PRDM16f/fMyf5cre mice. Alternatively, Prdm16 deletion was achieved through adiponectin (a mature adipocyte marker)-mediated CRE expression in both primary BAT and iWAT cultures isolated from PRDM16f/fAQcre mice. Similar to what we observed in Prdm16 knockdown brown fat cells, in these models of genetic loss of function of PRDM16, RGFP-induced thermogenic gene expression is much less profound than in the cells isolated from littermate wild-type control mice (Fig. 3C, 3D, and 3E). It is worth noting that the ablation of Prdm16 had different impact on different genes’ response to RGFP. The increase of Ucp1 by RGFP treatment was almost completely abolished in the absence of PRDM16. Ppargc1a was clearly less induced by RGFP treatment in Prdm16-null cells compared with the controls, but a statistically significant upregulation was still observed, suggesting that other factors in addition to PRDM16 also participate in the HDAC3-mediated regulation.
Discussion
The HDAC family consists of four classes and 18 members, and many food-derived or synthetic HDAC inhibitors have been shown to be promising therapeutic candidates for many disorders (1). Much is still unknown about the tissue specific function of each individual HDAC under physiological and pathological conditions. Global deletion of Hdac3 leads to embryonic lethality (18–20), and cardiac and liver-specific deletion of Hdac3 both cause tissue hypertrophy and metabolic imbalance (18, 20). Recently the potential involvement of HDAC3 in regulating brown and beige fat was investigated with adipocyte-specific Hdac3 knockout mouse models. In one study, ablation of Hdac3 in adipocytes results in a lower expression of UCP1 in BAT than in controls when mice were housed at thermoneutrality, at room temperature, and after acute cold exposure (4°C for 3 hours). In contrast, no statistically significant difference was observed in iWAT between the mutant and control mice, suggesting that beige fat is not affected (4). However, in an independent study a globally activated thermogenic program, including increased Ucp1 gene expression, was observed in the iWAT of Hdac3 fat-specific knockout mice compared with that of the littermate control, whereas surprisingly no drastic reprograming was observed in Hdac3-deleted BAT, either under basal conditions or after housed at 4°C for 24 hours (5). It is of note that different strains of HDAC3loxp/loxp mice were used in these two studies. Future side-by-side investigations using these two lines of mice under the same conditions may provide an explanation for these discrepancies.
Our study revealed that PRDM16, the transcriptional cofactor that mediates both determination of brown fat cell fate and activation of beige fat function, is involved in HDAC3-mediated thermogenic regulation in fat. The complexity we observed in HDAC3 function in brown and beige fat is reminiscent of the intricate role of PRDM16 in these cells. Although the observation that knocking down of Prdm16 in primary brown adipocytes led to the cell type switch to myotubes has since revolutionized the field (17), neither deletion nor ectopic expression of Prdm16 drastically affects brown fat development in mice (21–23). On the contrary, even though cell fate mapping demonstrated that beige adipocytes arise from an independent lineage from the one shared by brown fat and skeletal muscle, in which PRDM16 serves as the molecular switch (17), gain- and loss-of-function studies of PRDM16 in adipocytes have revealed an irrefutable regulatory role of this molecule in controlling beige fat function (21, 22). The interest in brown and beige fat biology has intensified with the accumulating evidence that activation of these cells leads to improved metabolic health in humans (24). However, we are still far from understanding the molecular control of these metabolically active cells. In our study, we observed a more drastic decrease of HDAC3 protein levels in iWAT compared with the changes observed in BAT upon cold exposure, whereas the increase in acetylated lysine levels was more profound in BAT than in iWAT. A potential confounding factor here is that iWAT is a heterogenous depot, consisting of both white and beige adipocytes, and this pathway may be regulated differently in distinct cell types. It is also conceivable that other factors besides HDAC3 (e.g., other members of the HDAC superfamily) may be involved in this process. Future studies will elucidate the entangled network that mediates the function of brown and beige adipocytes, including the molecular context for the role of HDACs in activating and repressing functions in these cells. These studies should introduce and broaden the clinical applications of HDAC inhibitors in metabolic disorders.
Supplementary Material
Acknowledgments
We thank Dr. Jiandie Lin (Life Sciences Institute, University of Michigan) for providing immortalized brown adipocyte cell line and all the colleagues in J.W.’s laboratory for helpful discussion.
Financial Support: This research was supported by a Human Frontier Science Program Young Investigator grant (RGY0082/2014) to J.W.; the Mallinckrodt grant from the Edward Mallinckrodt Jr. Foundation; grants from the National Institutes of Health (R01DK107583), the National Institute of Diabetes and Digestive and Kidney Diseases (dk107583) to J.W., and the American Diabetes Association (1-18-IBS-281) to J.W.; and a postdoctoral fellowship from the American Heart Association (17POST33060001) to D.K.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- AQcre
adiponectin-CRE
- BAT
brown adipose tissue
- FBS
fetal bovine serum
- Fgf21
fibroblast growth factor 21
- hASC
human adipose stem cell
- HDAC
histone deacetylase
- IBMX
3-isobutyl-1-methylxanthine
- IP
immunoprecipitation
- iWAT
inguinal white adipose tissue
- Myf5cre
Myf5-CRE
- Ppargc1a
peroxisome proliferator–activated receptor γ coactivator 1-α
- PRDM16
PR domain–containing 16
- qPCR
quantitative PCR
- RGFP
RGFP966
- UCP1
uncoupling protein 1
References
- 1. Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet. 2009;10(1):32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kajimura S, Spiegelman BM, Seale P. Brown and beige fat: physiological roles beyond heat generation. Cell Metab. 2015;22(4):546–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Galmozzi A, Mitro N, Ferrari A, Gers E, Gilardi F, Godio C, Cermenati G, Gualerzi A, Donetti E, Rotili D, Valente S, Guerrini U, Caruso D, Mai A, Saez E, De Fabiani E, Crestani M. Inhibition of class I histone deacetylases unveils a mitochondrial signature and enhances oxidative metabolism in skeletal muscle and adipose tissue. Diabetes. 2013;62(3):732–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Emmett MJ, Lim HW, Jager J, Richter HJ, Adlanmerini M, Peed LC, Briggs ER, Steger DJ, Ma T, Sims CA, Baur JA, Pei L, Won KJ, Seale P, Gerhart-Hines Z, Lazar MA. Histone deacetylase 3 prepares brown adipose tissue for acute thermogenic challenge. Nature. 2017;546(7659):544–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ferrari A, Longo R, Fiorino E, Silva R, Mitro N, Cermenati G, Gilardi F, Desvergne B, Andolfo A, Magagnotti C, Caruso D, Fabiani E, Hiebert SW, Crestani M. HDAC3 is a molecular brake of the metabolic switch supporting white adipose tissue browning. Nat Commun. 2017;8(1):93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chi J, Cohen P. The multifaceted roles of PRDM16: adipose biology and beyond. Trends Endocrinol Metab. 2016;27(1):11–23. [DOI] [PubMed] [Google Scholar]
- 7. Kim DI, Liao J, Emont MP, Park MJ, Jun H, Ramakrishnan SK, Lin JD, Shah YM, Omary MB, Wu J. An OLTAM system for analysis of brown/beige fat thermogenic activity [published online ahead of print January 23, 2018] Int J Obes. [DOI] [PMC free article] [PubMed]
- 8. Tang QQ, Otto TC, Lane MD. Commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc Natl Acad Sci USA. 2004;101(26):9607–9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang GX, Cho KW, Uhm M, Hu CR, Li S, Cozacov Z, Xu AE, Cheng JX, Saltiel AR, Lumeng CN, Lin JD. Otopetrin 1 protects mice from obesity-associated metabolic dysfunction through attenuating adipose tissue inflammation. Diabetes. 2014;63(4):1340–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Luo J, Deng ZL, Luo X, Tang N, Song WX, Chen J, Sharff KA, Luu HH, Haydon RC, Kinzler KW, Vogelstein B, He TC. A protocol for rapid generation of recombinant adenoviruses using the AdEasy system. Nat Protoc. 2007;2(5):1236–1247. [DOI] [PubMed] [Google Scholar]
- 11. Seale P, Kajimura S, Yang W, Chin S, Rohas LM, Uldry M, Tavernier G, Langin D, Spiegelman BM. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 2007;6(1):38–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Leus NG, van der Wouden PE, van den Bosch T, Hooghiemstra WT, Ourailidou ME, Kistemaker LE, Bischoff R, Gosens R, Haisma HJ, Dekker FJ. HDAC 3-selective inhibitor RGFP966 demonstrates anti-inflammatory properties in RAW 264.7 macrophages and mouse precision-cut lung slices by attenuating NF-κB p65 transcriptional activity. Biochem Pharmacol. 2016;108:58–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Suzuki T, Kasuya Y, Itoh Y, Ota Y, Zhan P, Asamitsu K, Nakagawa H, Okamoto T, Miyata N. Identification of highly selective and potent histone deacetylase 3 inhibitors using click chemistry-based combinatorial fragment assembly. PLoS One. 2013;8(7):e68669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Elsen M, Raschke S, Tennagels N, Schwahn U, Jelenik T, Roden M, Romacho T, Eckel J. BMP4 and BMP7 induce the white-to-brown transition of primary human adipose stem cells. Am J Physiol Cell Physiol. 2014;306(5):C431–C440. [DOI] [PubMed] [Google Scholar]
- 15. Bartesaghi S, Hallen S, Huang L, Svensson PA, Momo RA, Wallin S, Carlsson EK, Forslöw A, Seale P, Peng XR. Thermogenic activity of UCP1 in human white fat-derived beige adipocytes. Mol Endocrinol. 2015;29(1):130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jiang J, Emont MP, Jun H, Qiao X, Liao J, Kim DI, Wu J. Cinnamaldehyde induces fat cell-autonomous thermogenesis and metabolic reprogramming. Metabolism. 2017;77:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scimè A, Devarakonda S, Conroe HM, Erdjument-Bromage H, Tempst P, Rudnicki MA, Beier DR, Spiegelman BM. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454(7207):961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Montgomery RL, Potthoff MJ, Haberland M, Qi X, Matsuzaki S, Humphries KM, Richardson JA, Bassel-Duby R, Olson EN. Maintenance of cardiac energy metabolism by histone deacetylase 3 in mice. J Clin Invest. 2008;118(11):3588–3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bhaskara S, Chyla BJ, Amann JM, Knutson SK, Cortez D, Sun ZW, Hiebert SW. Deletion of histone deacetylase 3 reveals critical roles in S phase progression and DNA damage control. Mol Cell. 2008;30(1):61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Knutson SK, Chyla BJ, Amann JM, Bhaskara S, Huppert SS, Hiebert SW. Liver-specific deletion of histone deacetylase 3 disrupts metabolic transcriptional networks. EMBO J. 2008;27(7):1017–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cohen P, Levy JD, Zhang Y, Frontini A, Kolodin DP, Svensson KJ, Lo JC, Zeng X, Ye L, Khandekar MJ, Wu J, Gunawardana SC, Banks AS, Camporez JP, Jurczak MJ, Kajimura S, Piston DW, Mathis D, Cinti S, Shulman GI, Seale P, Spiegelman BM. Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell. 2014;156(1-2):304–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J, Cohen P, Cinti S, Spiegelman BM. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest. 2011;121(1):96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harms MJ, Ishibashi J, Wang W, Lim HW, Goyama S, Sato T, Kurokawa M, Won KJ, Seale P. Prdm16 is required for the maintenance of brown adipocyte identity and function in adult mice. Cell Metab. 2014;19(4):593–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sidossis L, Kajimura S. Brown and beige fat in humans: thermogenic adipocytes that control energy and glucose homeostasis. J Clin Invest. 2015;125(2):478–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.