Abstract
Context
Thyroid nodules are increasingly recognized in children and are associated with a greater risk for thyroid cancer compared with adults. Thyroid ultrasound is the favored tool for evaluation of thyroid nodules; however, there are limited data regarding the accuracy of thyroid ultrasound to confirm features associated with a low risk of thyroid cancer in children.
Objectives
We examined whether thyroid ultrasound is capable of accurately identifying thyroid nodules at a low risk of malignancy in children.
Design and Setting
Using a retrospective cohort study design, we identified children age ≤18 years with thyroid nodules and adequate follow-up. Ultrasound images were reviewed independently by two blinded expert radiologists, and ultrasound characteristics were analyzed to determine optimal predictive value and reliability.
Patients and Results
A total of 417 subjects were found to have thyroid nodules, and 152 subjects had adequate follow-up; 59 (38.8%) of these were diagnosed with thyroid cancer. We evaluated 236 individual nodules. Features most consistent with benign nodules included small size, isoechoic echogenicity, partially cystic structure, sharp or noninfiltrative margins, absent Doppler flow, and absent calcifications. Significant variability was found between expert interpretations of ultrasound features. Thyroid nodule composition appears to be the most sensitive and reliable feature for stratifying the risk of thyroid cancer. Ultrasound accurately identified benign thyroid nodules in 80.9% of subjects (95% confidence interval, 74–86.6).
Conclusions
Ultrasonography is useful for the evaluation of thyroid nodules, but we found no combination of ultrasound features sufficient to exclude thyroid cancer without a biopsy.
Ultrasound is useful to stratify risk for thyroid cancer, but the variability in interpretation and high prevalence of thyroid cancer make exclusion of thyroid cancer in children difficult.
The incidence of thyroid nodules and cancers in children and adolescents has increased over the past 30 years (1–3). The annual incidence of thyroid cancer has increased by 3.74% per year in all children, with the majority of cases being identified in adolescent female patients (4). Although thyroid nodules are generally associated with a low risk of malignancy in adults (∼5% to 10%), the risk of malignancy is significantly higher in children (22% to 26%) (5, 6). Nonetheless, most thyroid nodules in children are benign.
Thyroid ultrasound is the principal tool for risk stratification of thyroid nodules. Although ultrasound features that suggest malignancy are well recognized, there are sparse data to confirm which nodules have a low risk of representing thyroid cancer for which biopsy and surgery could be reasonably avoided. Large studies in adults have demonstrated a low risk for thyroid cancer in nodules with partially cystic composition and isoechoic echogenicity combined with the absence of high-risk features (e.g., calcifications, infiltrative borders, taller-than-wide shape, and extrathyroidal extension). The American Thyroid Association (ATA) guidelines for evaluation of thyroid nodules in adults recognize combinations of ultrasound features associated with low (<10%) risk of thyroid cancer and support withholding thyroid nodule biopsy until the nodule exceeds 1.5 cm in maximal dimension (7, 8). No such risk stratification strategy has been identified in children, so fine needle aspiration (FNA) biopsy is routinely recommended for children with solid or partially cystic thyroid nodules ≥1 cm in maximal diameter or any size nodule with any suspicious features (1).
Current data regarding ultrasound evaluation of thyroid nodules in children are limited by small numbers, variability in ultrasound protocols and technologies, referral bias, potential bias from nonblinded evaluation of ultrasounds, and a lack of assessment of reliability of ultrasound interpretation between radiologists (5, 9–14). These limitations undermine confidence in the predictive ability of thyroid ultrasonography in children.
This study was designed to examine the diagnostic accuracy of thyroid ultrasonography with color flow Doppler in a large cohort of children analyzed by experienced radiologists blinded to the subjects’ clinical information. Our primary objective was to determine whether any ultrasound features alone or in combination could be associated with a low enough risk of malignancy (<10% risk) to allow for the same conservative approach currently practiced in adults. Additionally, we sought to determine the reliability of thyroid ultrasound features between expert radiologists.
Materials and Methods
We performed a retrospective cohort study including all children (defined as age ≤18 years) diagnosed with a thyroid nodule in the Children's Hospital of Philadelphia medical system between January 2009 and March 2013 who underwent adequate clinical follow-up to determine whether the nodule was malignant. The protocol was reviewed and approved by the Children's Hospital of Philadelphia institutional review board.
Subjects were identified through a system-wide search based on completion of a neck ultrasound (CPT 76536) for any reason and were considered for inclusion if chart review confirmed the presence of a thyroid nodule. Review of the ultrasound images and medical record was performed to confirm that subjects had a thyroid nodule ≥0.5 cm. Clinical follow-up was considered adequate if any of the following were achieved: (1) surgical pathology; (2) FNA biopsy consistent with a benign lesion plus follow-up including ultrasound imaging without evidence of substantial growth (>20% increase in maximal dimension) or new suspicious features for ≥12 months; or (3) clinical follow-up including ultrasound imaging without evidence of substantial growth (>20% increase in maximal dimension), no new suspicious sonographic features, and no clinical suspicion of malignancy for ≥12 months.
Study data were collected and managed using REDCap electronic capture tools hosted at the University of Pennsylvania. Ultrasound image sets (including dynamic images when available) were manually extracted and coded (ShowCase 5.0; Trilium Technology, Inc., Ann Arbor, MI) to remove identifiers. Two blinded radiologists with expertise in adult and pediatric thyroid ultrasonography evaluated the image sets independently. One radiologist reviewed all images for the study, and the acquired data were used for the primary analysis. Reliability of ultrasound interpretation was determined by duplicating a random selection of images (30%) for review by the primary radiologist and the second radiologist. Consistent with real-world experience, disagreements between reports were not reconciled.
Statistical analysis
Data analysis was performed using Stata 12.1 (StataCorp, College Station, TX). A P value of <0.05 was considered statistically significant, and two-sided tests were used. Group differences in subjects with thyroid carcinoma and subjects with benign lesions were determined using Wilcoxon rank sum or Kruskall-Wallis as indicated. Differences in proportions were assessed using Fisher’s exact test or χ2 test as indicated. Reliability was assessed through use of Cohen’s κ. Based on our power calculations and on the expectation of a prevalence of malignancy of 30%, 149 subjects would be required to provide 90% power to detect an inferior negative predictive value of 90% with α = 0.05.
Data were analyzed in a bivariate logistic regression model with adjustment for clustering within individuals. Variables that were clinically important or found to be statistically significant in bivariate modeling were included in a multivariable logistic regression model with adjustment for clustering; backward deletion was used to determine the variables that affected the final model. The final model was validated by a sensitivity analysis. Variables were further analyzed in isolation and in combination to determine their sensitivity, specificity, negative predictive value, and positive predictive value with 95% confidence intervals (CIs). Predictive modeling was accomplished with multivariable logistic regression modeling and receiver operator curve analysis.
Results
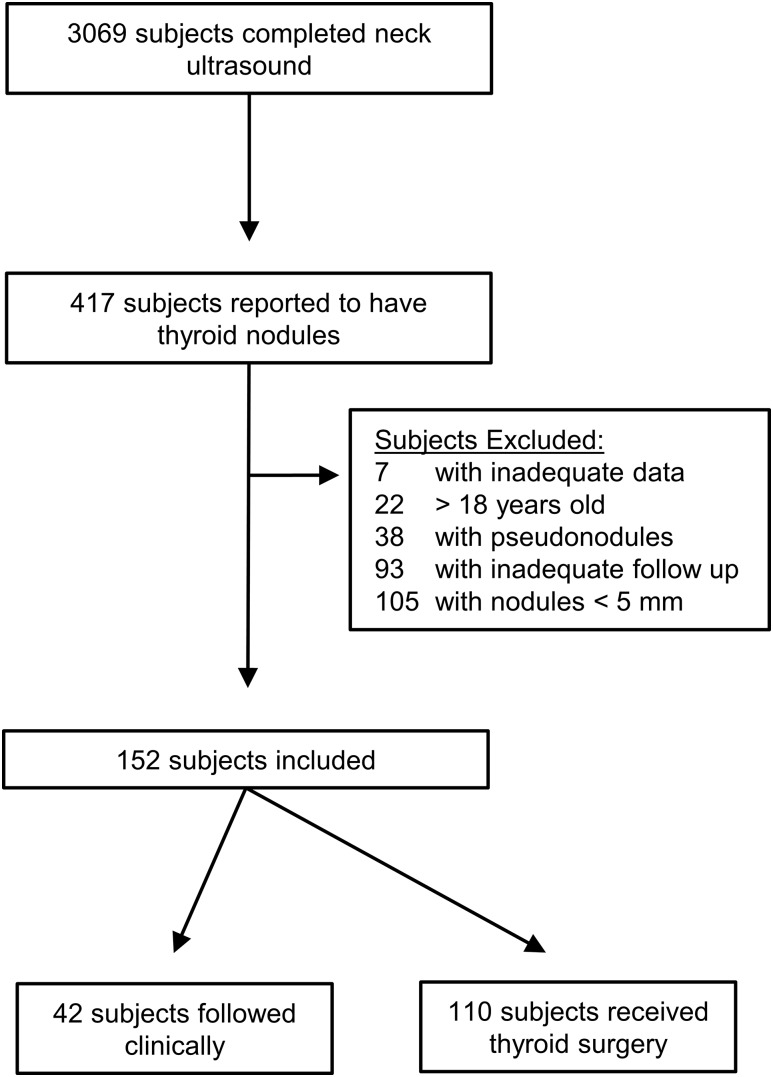
A total of 3069 children received neck ultrasounds during the study period. The most common indications were cervical lymphadenopathy, cellulitis, salivary gland enlargement, vascular imaging, and neck masses. A total of 417 (13.6%) subjects were reported to have thyroid nodules, and 152 subjects with 241 unique thyroid nodules met criteria for inclusion in the study (Fig. 1). Of these subjects, 93 (61.2%) were diagnosed with benign thyroid nodules, and 59 (38.8%) were diagnosed with thyroid cancer (Table 1). Eighty-five subjects (55.9%) were diagnosed with a thyroid nodule in our hospital system, and the remainder of subjects were referred externally. The median age was 14.2 ± 3.8 years, and 121 (79%) were female. The majority (72.7%) of subjects were white and were asymptomatic at the time of diagnosis. Seventeen subjects (11.3%) had a history of cancer, and 16 (10.5%) had received total body irradiation or craniospinal or mediastinal radiation therapy prior to being diagnosed with thyroid nodules; no increased prevalence of thyroid cancer was seen in these subjects. Two subjects had received radioactive iodine ablation therapy for hyperthyroidism, and neither had thyroid cancer. The majority (59.2%) of subjects had a positive family history of thyroid disease, 18 (12.8%) had a positive family history of thyroid cancer, and 55 (39.3%) had a positive family history of nonthyroidal cancer; none of these historical features was significantly associated with final diagnosis of thyroid cancer in the subjects.
Figure 1.
Consort diagram for subject selection and inclusion into the study.
Table 1.
Baseline Characteristics of Subjects at Time of Diagnosis of a Thyroid Nodule
| Feature | All Subjects (n = 152) | Benign Nodules (n = 93) | Malignant Nodules (n = 59) | P Value a |
|---|---|---|---|---|
| Historical findings | ||||
| Age, y (SD) | 14.2 (3.8) | 14.1 (3.8) | 14.4 (4.1) | 0.52 |
| Female | 121 (79.6) | 74 (79.6) | 47 (79.7) | 0.99 |
| White | 104 (72.7) | 70 (78.7) | 34 (62.9) | 0.04 |
| Prepubertal | 12 (9.2) | 10 (12.5) | 2 (3.9) | 0.10 |
| Incidental finding | 54 (35.5) | 36 (38.7) | 18 (30.5) | 0.30 |
| Symptoms of tracheoesophageal pressureb | 12 (7.9) | 7 (11.9) | 5 (5.38) | 0.15 |
| History of malignancy | 17 (11.3) | 12 (12.9) | 5 (8.6) | 0.59 |
| History of radiation therapyc | 16 (10.5) | 11 (11.8) | 5 (8.5) | 0.60 |
| Family history of thyroid diseased | 84 (59.2) | 52 (60.5) | 32 (57.1) | 0.70 |
| Family history of thyroid nodules or thyroid cancer | 45 (32.1) | 29 (34.1) | 15 (29.1) | |
| Family history of thyroid cancer | 18 (12.8) | 11 (12.9) | 7 (12.5) | |
| Family history of nonthyroid malignancy | 55 (39.3) | 34 (40) | 21 (38.2) | 0.83 |
| Clinical findings | ||||
| Diffuse goiter | 60 (39.2) | 35 (37.6) | 25 (42.4) | 0.56 |
| Palpable nodule | 91 (59.9) | 50 (53.8) | 41 (69.5) | 0.05 |
| Palpable cervical lymphadenopathy | 31 (20.3) | 13 (14) | 18 (30.5) | 0.01 |
| TSH, mIU/L (SD) | 1.25 (1.55) | 1.14 (1.49) | 1.31 (1.71) | 0.30 |
| Positive antithyroid antibodies | 36 (35.3) | 20 (32.3) | 16 (40) | 0.42 |
Values are n (%) unless noted otherwise.
Abbreviations: SD, standard deviation; TSH, thyroid-stimulating hormone.
P values refer to statistical significance of the difference between subjects with benign and malignant nodules.
Symptoms of dysphagia, dysphonia, or globus.
Radiation therapy included total body, craniospinal, and/or mediastinal radiation therapy.
Any disease causing abnormal thyroid function or thyroid injury, including thyroiditis, Graves disease, hypothyroidism, and hyperthyroidism.
Thyroid nodules were most commonly (64.5%) identified on palpation by the physician, a family member, or the subject; the remainder (35.5%) were found incidentally during unrelated imaging studies. Physical examination by a physician in our center identified a palpable nodule in 91 subjects (59.9%) and palpable cervical lymphadenopathy in 31 subjects (20.3%). The median thyroid-stimulating hormone value was 1.25 mIU/L (interquartile range, 1.55), and 36 subjects (35.3%) had positive antithyroid autoantibodies. There were no significant baseline differences in the characteristics between patients with benign and malignant thyroid nodules except for the distribution of race (P = 0.04) and the presence of palpable cervical lymphadenopathy (P = 0.01).
The endpoint of the study was achieved based on surgical pathology in 110 (72.4%) of subjects, by FNA biopsy followed by observation in 28 (12.3%) of subjects, and by observation without biopsy in 24 (15.3%) subjects.
Ultrasound findings
Ultrasound images identified 241 unique thyroid nodules. Data were complete for 236 nodules; 80 (33.8%) of these nodules were confirmed to be thyroid cancer (Table 2). A single nodule was identified in 94 (61.8%) subjects, 27 (17.8%) subjects had two nodules, and 31 (20.4%) subjects had three or more nodules. There was no association between the number of nodules and the risk of malignancy. The median maximal dimension of thyroid nodules was 1.6 ± 1.7 cm; benign thyroid nodules were smaller (1.5 ± 1.4 cm) than malignant thyroid nodules (2.0 ± 1.7 cm). Thyroid nodule size ≥1 cm was associated with an increased odds ratio (OR) for thyroid cancer (OR, 1.78; 95% CI, 1.15 to 2.75).
Table 2.
Bivariable Logistic Regression Analysis of the Odds of Thyroid Cancer and Reliability of Ultrasound Features
| All Nodules (n = 236) | Benign Nodules (n = 156) | Malignant Nodules (n = 80) | OR for Thyroid Cancer Diagnosis (95% CI) | Intrarater Variability, κ (P Value) | Interrater Variability, κ (P Value) | |
|---|---|---|---|---|---|---|
| No. of nodules | ||||||
| One | 94 | 57 | 37 | 0.77 (0.53–1.13) | ||
| Two | 54 | 37 | 17 | |||
| Three | 88 | 62 | 26 | |||
| Size (greatest dimension), cm | ||||||
| <1 | 65 | 50 | 15 | 1.78 (1.15–2.75) | 1.0 (<0.001) | 0.96 (<0.001) |
| 1–4 | 155 | 97 | 58 | |||
| >4 | 16 | 9 | 7 | |||
| Nodule composition | ||||||
| >25% cystic | 84 | 72 | 12 | 3.46 (1.96–6.09) | 0.8 (<0.001) | 0.79 (<0.001) |
| ≤25% cystic | 152 | 84 | 68 | |||
| Echogenicity | ||||||
| Isoechoic | 93 | 80 | 13 | 1.0 | 0.57 (<0.001) | 0.19 (0.003) |
| Hypoechoic | 87 | 47 | 40 | 3.69 (1.97–6.93) | ||
| Hyperechoic | 35 | 19 | 16 | 3.99 (1.79–8.86) | ||
| Mixed hypoechoic and hyperechoic | 16 | 6 | 10 | 4.91 (1.98–12.18) | ||
| Margins | ||||||
| Sharply defined/noninfiltrative | 177 | 139 | 38 | 5.58 (2.79–11.18) | 0.57 (<0.001) | 0.34 (0.001) |
| Infiltrative/microlobular | 58 | 17 | 41 | |||
| Calcifications | ||||||
| Absent | 165 | 133 | 32 | 6.85 (3.47–13.49) | 0.86 (<0.001) | 0.39 (0.002) |
| Present | 70 | 22 | 48 | |||
| Doppler flow | ||||||
| Absent | 45 | 38 | 7 | 3.52 (1.24–10.01) | 0.58 (<0.001) | 0.77 (<0.001) |
| Increased | 179 | 109 | 70 | |||
| Taller than wide | ||||||
| Absent | 203 | 140 | 63 | 2.22 (1.27–3.88) | 0.58 (<0.001) | 0.39 (0.002) |
| Present | 33 | 16 | 17 | |||
| Extrathyroidal extension | ||||||
| Absent | 220 | 153 | 67 | 6.15 (2.39–15.86) | −0.03 (0.61) | 0.4 (<0.001) |
| Present | 16 | 3 | 13 |
Logistic regression was performed with adjustment for clustering of features within individuals.
Thyroid nodule composition was ≤25% cystic in 152 (64%) nodules, and 68 (44.7%) of these nodules were malignant. Conversely, >25% cystic composition was observed in 84 nodules, and 12 (14.3%) of these nodules were malignant. Thyroid nodules with ≤25% cystic composition were found to harbor 91.4% of observations of nonisoechoic echogenicity, 96.7% of observations of infiltrative/microlobulated margins, and 91.5% of observations of calcifications in the population. Altogether, ≤25% cystic composition identified 85% of thyroid cancer cases in the study and was associated with a significant increase in the OR for thyroid cancer (OR, 3.46; 95% CI, 1.96 to 6.09).
Thyroid nodule echogenicity was isoechoic in 93 (39.4%) nodules, and 13 (13.9%) of these nodules were malignant. Hypoechoic echogenicity was observed in 87 (36.9%) nodules, and 40 (45.9%) of these were malignant. Thirty-five nodules demonstrated hyperechoic echogenicity, and 16 nodules demonstrated mixed hypoechoic/hyperechoic echogenicity; 16 (45.7%) and 10 (62.5%) of these nodules, respectively, were malignant. The majority of high-risk ultrasound features were seen in nodules with nonisoechoic echogenicity, including 94.9% of infiltrative/microlobulated margin observations, 94.1% of extrathyroidal extension observations, and 84.5% of calcification observations. The presence of nonisoechoic echogenicity identified 83.8% of thyroid cancer cases in the study and was associated with a significantly increased OR for thyroid cancer (OR, 3.91; 95% CI, 2.18 to 7.01).
Thyroid nodule margins were sharply defined or noninfiltrative in 177 (75%) nodules, and 38 (21.4%) of these nodules were malignant. Infiltrative or microlobulated margins were seen in 58 (24.7%) nodules, and 41 (70.7%) of these were malignant. The presence of infiltrative or microlobulated margins was associated with a significantly increased OR for malignancy (OR, 5.58; 95% CI, 2.79 to 11.18).
Taller-than-wide structure on transverse imaging was seen in 33 (13.9%) nodules, and 16 (51.5%) of these nodules were malignant. Calcifications were present in 70 (29.8%) nodules, and 48 (68.6%) of these nodules were malignant. Extrathyroidal extension was described in 16 (6.8%) nodules, and 13 (81.3%) of these nodules were malignant. Each of these features was associated with a marked increase in OR for thyroid carcinoma (Table 2).
Intrarater reliability was found to be excellent for thyroid nodule size (κ = 1.0; P < 0.001), nodule composition (κ = 0.8; P < 0.001), and calcifications (κ = 0.86; P < 0.001), whereas it was moderate for nodule echogenicity (κ = 0.57; P < 0.001), nodule margins (κ = 0.57; P < 0.001), and Doppler flow (κ = 0.58; P < 0.001). Interrater reliability was variable, with excellent agreement for nodule size (κ = 0.96; P < 0.001), good agreement for nodule composition (κ = 0.79; P < 0.001) and Doppler flow (κ = 0.77; P < 0.001), moderate agreement for extrathyroidal extension (κ = 0.4, P < 0.001), fair agreement for nodule margins (κ = 0.34; P < 0.001) and calcifications (κ = 0.39; P = 0.002), and poor agreement for nodule echogenicity (κ = 0.19; P = 0.003).
Using a multivariable logistic regression model including all ultrasound features and accounting for clustering within subjects and statistical interaction between calcifications and Doppler flow patterns, we found that nonisoechoic echogenicity (P = 0.019) and extrathyroidal extension (P = 0.01) remained statistically significant predictors of thyroid cancer in children. A prespecified sensitivity analysis revealed that these features were affected by clinical features such as subject age, pubertal status, and race/ethnicity but not by personal history of cancer or radiation exposure.
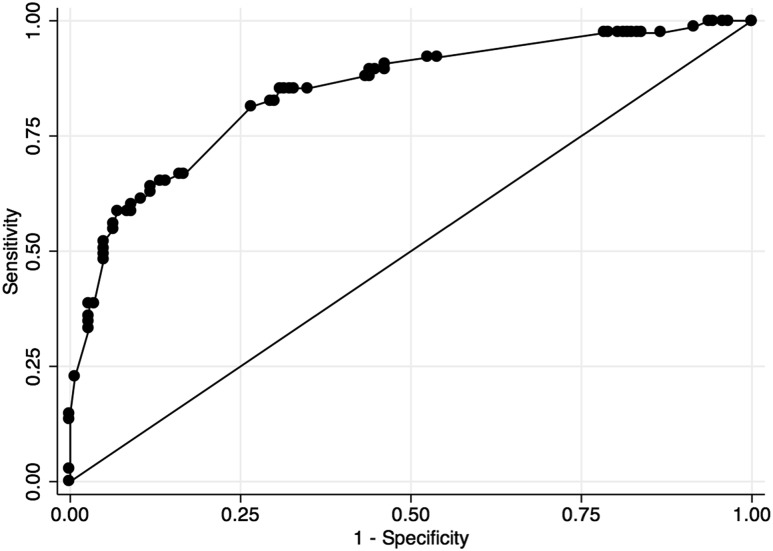
Receiver operator curve analysis
The clinical utility of ultrasound features for prediction of thyroid cancer was evaluated in bivariable and multivariable models (Figure 2; Table 3). Numerous patterns of features were observed in the sample, and this significantly affected the predictive value of each feature. Optimal sensitivity was found in ≤25% cystic thyroid nodule composition (85%; 95% CI, 75.3 to 92), increased Doppler flow (90.9%; 95% CI, 82.2 to 96.3), and nonisoechoic echogenicity (83.5%; 95% CI, 73.5 to 90.9). Optimal specificity was found in extrathyroidal extension (98.1%; 94.5 to 99.6), taller-than-wide shape (89.7%; 95% CI, 83.9 to 94), and infiltrative/microlobulated margins (89.1%; 95% CI, 83.1 to 93.5). The most powerful predictive values for thyroid cancer were extrathyroidal extension (81.2%; 95% CI, 54.4 to 96), infiltrative/microlobulated margins (70.7%; 95% CI, 57.3 to 81.9), and calcifications (68.6%; 95% CI, 56.4 to 79.1). The most powerful predictive values for benign thyroid nodules were isoechoic echogenicity (86%; 95% CI, 77.3 to 92.3), >25% cystic composition (85.7%; 95% CI, 76.4 to 92.4), and absence of Doppler flow (84.4%; 95% CI, 70.5 to 93.5).
Figure 2.
Receiver operator curve for performance of ultrasound features in correctly diagnosing thyroid cancer in children.
Table 3.
Analysis of Predictive Statistics and Receiver Operator Curve for Ultrasound Features for Thyroid Carcinoma in Children
| Variable | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | ROC (95% CI) |
|---|---|---|---|---|---|
| Nodule size >1 cm | 81% (71–89.1) | 32.1% (24.8–40) | 38% (30.7–45.7) | 76.9% (64.8–86.5) | 0.57 (0.51–0.62) |
| ≤25% solid nodule composition | 85% (75.3–92) | 46.2% (38.2–54.3) | 44.7% (36.7–53) | 85.7% (76.4–92.4) | 0.66 (0.6–0.71) |
| Nonisoechoic echogenicity | 83.5% (73.5–90.9) | 52.6% (44.4–60.8) | 47.8% (39.3–56.5) | 86% (77.3–92.3) | 0.68 (0.62–0.74) |
| Taller-than-wide | 21.2% (12.9–31.8) | 89.7% (83.9–94.0) | 51.5% (33.5–69.2) | 69% (62.1–75.3) | 0.56 (0.5–0.61) |
| Increased Doppler flow | 90.9% (82.2–96.3) | 25.9% (19–33.7) | 39.1% (31.9–46.7) | 84.4% (70.5–93.5) | 0.58 (0.54–0.63) |
| Presence of calcifications | 60% (48.4–70.8) | 85.8% (79.3–90.9) | 68.6% (56.4–79.1) | 80.6% (73.7–86.3) | 0.73 (0.67–0.79) |
| Infiltrative/microlobular margin | 51.9% (40.4–63.3) | 89.1% (83.1–93.5) | 70.7% (57.3–81.9) | 78.5% (71.7–84.3) | 0.71 (0.64–0.77) |
| Extrathyroidal extension | 16.2% (8.9–26.2) | 98.1% (94.5–99.6) | 81.2% (54.4–96.0) | 69.5% (63.0–75.6) | 0.57 (0.53–0.61) |
| All features combined | 58.7% (46.7–69.9) | 91.6% (85.8–95.6) | 78.6% (65.6–88.4) | 80.9% (74–86.6) | 0.8449 |
Abbreviations: NPV, negative predictive value; PPV, positive predictive value; ROC, receiver operator curve.
Multivariable analyses of these ultrasound features in combination demonstrated combined sensitivity of 58.7% (95% CI, 46.7 to 69.9), specificity of 91.6% (95% CI, 85.8 to 95.6), positive predictive value of 78.6% (95% CI, 65.6 to 88.4), and negative predictive value of 80.9% (95% CI, 74 to 86.6) for thyroid cancer.
Discussion
The diagnostic evaluation of thyroid nodules in children has thus far been limited to cohorts of children at increased risk of thyroid cancer due to exposure to radiation (10) or associated with referral to tertiary care centers for care (5, 9). Analysis of these data has been limited by small numbers of observations, a lack of a rigorous review of primary imaging data (9), a lack of assessment of reliability of radiology interpretation (5), and reconciliation of disagreements between radiologists (13, 14). This study was designed to address these limitations, and the principle question we sought to answer was whether thyroid ultrasound could reliably identify thyroid nodules in children associated with a low (<10%) risk of thyroid cancer so that biopsy may be avoided.
This study contains the largest and most rigorous primary evaluation of thyroid nodules in children thus far reported. By using a broad case-finding strategy, we recruited most of our subjects from the local population of the tertiary care center, and the majority of subjects had no known risk factors for thyroid nodules or thyroid cancer. We believe that this strategy was effective because of the diversity of subjects recruited and because the study met the prespecified recruitment goal by evaluating images obtained over the course of 4.25 years, compared with >10 years required by other large pediatric studies (5, 9). This short recruitment phase also allowed for greater consistency in ultrasound protocol and technology.
Once children were identified to have thyroid nodules, they received further evaluation at the direction of a dedicated pediatric thyroid center within the hospital system, which included either observation or diagnostic procedures. Although this was considered a strength during the design of the study, it introduced an important limitation in that children with low-risk ultrasound features were given appropriate reassurance and were less likely to complete follow-up, making them ineligible for inclusion in this study. Conversely, children with more concerning ultrasound features were more likely to complete the recommended follow-up and could be included in the study. This ascertainment bias resulted in a relatively high prevalence of thyroid cancer in our population, although the prevalence is similar to what has been reported by other groups (1, 5, 10, 15).
The greatest strength of this study is the rigorous evaluation of ultrasound image data in every subject. Although ultrasound features were evaluated by two expert radiologists, the analysis was based on features reported by a single radiologist, as would occur in a practical clinical setting. This strategy prevented a bias toward overestimation of the reliability and accuracy of the ultrasound when taken out of the research environment. Consistent with the findings of other groups, we observed substantial variation in the interpretation of ultrasound features between reviewers (6, 12, 13). In particular, we found fair-to-poor agreement in features such as nodule echogenicity, nodule margins, taller-than-wide shape, calcifications, and extrathyroidal extension. The reliability of imaging reporting was not assessed in other large studies of pediatric thyroid nodules, and this represents a practical challenge when relying on written thyroid ultrasound reports for research or patient care. These challenges are further compounded considering the variation in the completeness of thyroid and neck ultrasound image acquisition and reports that are produced outside of dedicated thyroid centers.
Ultrasound findings such as infiltrative/microlobulated margins, calcifications, taller-than-wide appearance, and extrathyroidal extension were highly predictive of thyroid cancer, as has been reported (5, 6, 10, 11, 13). Although these features were quite specific for thyroid cancer, their utility was limited by a low number of observations and resultant poor sensitivity. Conversely, nodule size ≥1 cm, ≤25% cystic composition, nonisoechoic echogenicity, and increased Doppler flow were very sensitive for thyroid cancer but were also nonspecific.
Evaluation of combinations of features in a multivariable logistic regression model confirmed that all of the ultrasound features were important. However, we also found that the high degree of correlation between ultrasound features limited the predictive value of individual features. Combinations of ultrasound features, however, were quite useful in stratifying the odds of thyroid cancer in children. Thyroid nodule composition and echogenicity were closely associated with the presence of high-risk ultrasound features and were found to be favorable targets for risk stratification.
Thyroid nodule composition has been shown to be closely associated with risk of malignancy in adults (16, 17), but most studies in children have not explored this. Gupta et al. (5) reported an increased prevalence of thyroid cancer in thyroid nodules with entirely solid composition and <25% cystic composition (57% and 21%, respectively). In their study, ≤25% cystic composition identified 89.3% of thyroid cancers; this is in agreement with our findings. Thyroid nodule echogenicity has been closely associated with the risk of thyroid cancer in adults, but this pattern has not been consistently observed in pediatric studies. Lyshchik et al. (10) reported the prevalence of thyroid cancer to be 21% in children with isoechoic nodules, whereas the prevalence was 35% in children with nonisoechoic nodules. However, Mussa et al. (9) reported a 19% prevalence of thyroid cancer in nodules with isoechoic echogenicity, compared with 18.9% prevalence in nodules with nonisoechoic echogenicity. Our findings are in agreement with those of Lyshchik et al. (10), and it is unclear why the findings of Mussa et al. (9) differ, although we speculate that the lack of primary ultrasound image review in the study by Mussa et al. could limit the reliability of their findings.
Although we found that both thyroid nodule composition and nonisoechoic echogenicity were associated with thyroid cancer, we found that radiologist report of thyroid nodule composition was substantially more reliable than echogenicity; this finding was recently corroborated (12). Therefore, we conclude that thyroid nodule composition is the most important feature for initial risk stratification for a thyroid nodule in children.
The ATA guideline for evaluation of thyroid nodules in children recommends biopsy for thyroid nodules of any size with high-risk ultrasound features and in any solid or partially cystic thyroid nodule ≥1 cm in maximal dimension (1). This strategy is quite different from the ATA guideline for adults, which establishes size thresholds for biopsy based on the ultrasound features with an emphasis on three major components: the presence of high-risk ultrasound features (e.g., irregular margins, microcalcifications, taller-than-wide shape, and evidence of extrathyroidal extension), thyroid nodule composition, and echogenicity (8). With this approach, nodules are assigned an estimated risk of malignancy, and those with lower risk would not undergo biopsy. Even nodules with high-risk features (>70% to 90% estimated risk of malignancy) would not undergo biopsy until the lesion was ≥1 cm in maximal dimension. Solid nodules with hypoechoic echogenicity (10% to 20% estimated risk of malignancy) would receive biopsy if they are ≥1 cm, whereas solid or partially cystic nodules of nonhypoechoic echogenicity (5% to 10% estimated risk of malignancy) would receive FNA biopsy if the nodule is ≥1.5 cm. Spongiform nodules and those with minimal solid content (<3% estimated risk of malignancy) could be monitored without biopsy. Comparison of our data with the ATA adult algorithm yields very different estimates of risk for thyroid cancer in children: 50% to 88% for high-suspicion patterns, 44.7% for solid or partially cystic hypoechoic nodules, 14% to 22% for isoechoic or hyperechoic nodules, and 14% for >25% cystic nodules. No ultrasound features alone or in combination were associated with a <10% risk for thyroid cancer in children.
This study was designed to evaluate the accuracy of thyroid ultrasound in discerning benign from malignant thyroid nodules in children. The relatively high prevalence of thyroid cancer that we identified in our study made this very difficult, even though the study was powered to withstand this. The low prevalence of low-risk features (e.g., an entirely cystic structure) in our sample limit our ability to draw conclusions about their effectiveness in excluding thyroid cancer. Further prospective studies are needed to confirm the true risk associated with these findings.
Limitations
This study was limited by referral bias, which was minimized by our inclusive case-finding approach as described above. There was no significant difference between the prevalence of thyroid cancer between children referred internally or externally (P = 0.66). We also performed sensitivity analyses for each statistical model, and there was no significant difference in findings based on the source of referral. Ascertainment bias also heavily affected our findings, resulting in a higher overall estimate of the prevalence of thyroid cancer, as discussed above. Additionally, the diagnosis of a benign thyroid nodule without surgical pathology is not conclusive, and it is possible that an indolent thyroid cancer may have been misclassified as benign.
Conclusion
Thyroid ultrasound is a powerful and important tool for the evaluation of thyroid nodules, but our analysis was unable to identify any combination of features associated with a “low suspicion” (<10% risk) of thyroid cancer in children. The lowest risk group for thyroid cancer appears to be children with thyroid nodules with >25% cystic composition, but even this group has a higher prevalence of malignancy than is observed in adults. This limitation is underscored by significant intra- and interobserver variability that can be expected even in expert hands. Current pediatric guidelines recommend biopsy of all solid or predominantly solid thyroid nodules ≥1 cm in diameter and nodules of any size with high-risk features; we were unable to identify any group for which reliance on ultrasound features alone was reliable to avoid biopsy.
Acknowledgments
Financial Support: This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant T32DK063688-10 (to A.W.G.).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- ATA
American Thyroid Association
- CI
confidence interval
- OR
odds ratio
References
- 1. Francis GL, Waguespack SG, Bauer AJ, Angelos P, Benvenga S, Cerutti JM, Dinauer CA, Hamilton J, Hay ID, Luster M, Parisi MT, Rachmiel M, Thompson GB, Yamashita S; American Thyroid Association Guidelines Task Force. Management guidelines for children with thyroid nodules and differentiated thyroid cancer. Thyroid. 2015;25:716–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hogan AR, Zhuge Y, Perez EA, Koniaris LG, Lew JI, Sola JE. Pediatric thyroid carcinoma: incidence and outcomes in 1753 patients. J Surg Res. 2009;156(1):167–172. [DOI] [PubMed] [Google Scholar]
- 3. Rivkees SA, Mazzaferri EL, Verburg FA, Reiners C, Luster M, Breuer CK, Dinauer CA, Udelsman R. The treatment of differentiated thyroid cancer in children: emphasis on surgical approach and radioactive iodine therapy. Endocr Rev. 2011;32(6):798–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vergamini LB, Frazier AL, Abrantes FL, Ribeiro KB, Rodriguez-Galindo C. Increase in the incidence of differentiated thyroid carcinoma in children, adolescents, and young adults: a population-based study. J Pediatr. 2014;164(6):1481–1485. [DOI] [PubMed] [Google Scholar]
- 5. Gupta A, Ly S, Castroneves LA, Frates MC, Benson CB, Feldman HA, Wassner AJ, Smith JR, Marqusee E, Alexander EK, Barletta J, Doubilet PM, Peters HE, Webb S, Modi BP, Paltiel HJ, Kozakewich H, Cibas ES, Moore FD Jr, Shamberger RC, Larsen PR, Huang SA. A standardized assessment of thyroid nodules in children confirms higher cancer prevalence than in adults. J Clin Endocrinol Metab. 2013;98(8):3238–3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Al Nofal A, Gionfriddo MR, Javed A, Haydour Q, Brito JP, Prokop LJ, Pittock ST, Murad MH. Accuracy of thyroid nodule sonography for the detection of thyroid cancer in children: systematic review and meta-analysis. Clin Endocrinol (Oxf). 2016;84(3):423–430. [DOI] [PubMed] [Google Scholar]
- 7. Cappelli C, Castellano M, Pirola I, Cumetti D, Agosti B, Gandossi E, Agabiti Rosei E. The predictive value of ultrasound findings in the management of thyroid nodules. QJM. 2007;100(1):29–35. [DOI] [PubMed] [Google Scholar]
- 8. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L 2015. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mussa A, De Andrea M, Motta M, Mormile A, Palestini N, Corrias A.. Predictors of malignancy in children with thyroid nodules. J Pediatr. 2015;167:886–892. [DOI] [PubMed] [Google Scholar]
- 10. Lyshchik A, Drozd V, Demidchik Y, Reiners C. Diagnosis of thyroid cancer in children: value of gray-scale and power doppler US. Radiology. 2005;235(2):604–613. [DOI] [PubMed] [Google Scholar]
- 11. Corrias A, Mussa A, Baronio F, Arrigo T, Salerno M, Segni M, Vigone MC, Gastaldi R, Zirilli G, Tuli G, Beccaria L, Iughetti L, Einaudi S, Weber G, De Luca F, Cassio A; Study Group for Thyroid Diseases of Italian Society for Pediatric Endocrinology and Diabetology (SIEDP/ISPED) . Diagnostic features of thyroid nodules in pediatrics. Arch Pediatr Adolesc Med. 2010;164(8):714–719. [DOI] [PubMed] [Google Scholar]
- 12. Lim-Dunham JE, Erdem Toslak I, Alsabban K, Aziz A, Martin B, Okur G, Longo KC. Ultrasound risk stratification for malignancy using the 2015 American Thyroid Association Management Guidelines for Children with Thyroid Nodules and Differentiated Thyroid Cancer. Pediatr Radiol. 2017;47(4):429–436. [DOI] [PubMed] [Google Scholar]
- 13. Koltin D, O’Gorman CS, Murphy A, Ngan B, Daneman A, Navarro OM, García C, Atenafu EG, Wasserman JD, Hamilton J, Rachmiel M. Pediatric thyroid nodules: ultrasonographic characteristics and inter-observer variability in prediction of malignancy. J Pediatr Endocrinol Metab. 2016;29(7):789–794. [DOI] [PubMed] [Google Scholar]
- 14. Wienke JR, Chong WK, Fielding JR, Zou KH, Mittelstaedt CA. Sonographic features of benign thyroid nodules: interobserver reliability and overlap with malignancy. J Ultrasound Med. 2003;22(10):1027–1031. [DOI] [PubMed] [Google Scholar]
- 15. Niedziela M. Pathogenesis, diagnosis and management of thyroid nodules in children. Endocr Relat Cancer. 2006;13(2):427–453. [DOI] [PubMed] [Google Scholar]
- 16. Moon WJ, Jung SL, Lee JH, Na DG, Baek JH, Lee YH, Kim J, Kim HS, Byun JS, Lee DH; Thyroid Study Group, Korean Society of Neuro- and Head and Neck Radiology . Benign and malignant thyroid nodules: US differentiation--multicenter retrospective study. Radiology. 2008;247(3):762–770. [DOI] [PubMed] [Google Scholar]
- 17. Frates MC, Benson CB, Doubilet PM, Kunreuther E, Contreras M, Cibas ES, Orcutt J, Moore FD Jr, Larsen PR, Marqusee E, Alexander EK. Prevalence and distribution of carcinoma in patients with solitary and multiple thyroid nodules on sonography. J Clin Endocrinol Metab. 2006;91(9):3411–3417. [DOI] [PubMed] [Google Scholar]