Abstract
Context
Osteoporosis is a metabolic bone disease. The effect of blood metabolites on the development of osteoporosis remains elusive.
Objective
To explore the relationship between blood metabolites and osteoporosis.
Design and Methods
We used 2286 unrelated white subjects for the discovery samples and 3143 unrelated white subjects from the Framingham Heart Study (FHS) for the replication samples. The bone mineral density (BMD) was measured using dual-energy X-ray absorptiometry. Genome-wide single nucleotide polymorphism (SNP) genotyping was performed using Affymetrix Human SNP Array 6.0 (for discovery samples) and Affymetrix SNP 500K and 50K array (for FHS replication samples). The SNP sets significantly associated with blood metabolites were obtained from a reported whole-genome sequencing study. For each subject, the genetic risk score of the metabolite was calculated from the genotype data of the metabolite-associated SNP sets. Pearson correlation analysis was conducted to evaluate the potential effect of blood metabolites on the variations in bone phenotypes; 10,000 permutations were conducted to calculate the empirical P value and false discovery rate.
Results
We analyzed 481 blood metabolites. We identified multiple blood metabolites associated with hip BMD, such as 1,5-anhydroglucitol (Pdiscovery < 0.0001; Preplication = 0.0361), inosine (Pdiscovery = 0.0018; Preplication = 0.0256), theophylline (Pdiscovery = 0.0048; Preplication = 0.0433, gamma-glutamyl methionine (Pdiscovery = 0.0047; Preplication = 0.0471), 1-linoleoyl-2-arachidonoyl-GPC (18:2/20:4n6; Pdiscovery = 0.0018; Preplication = 0.0390), and X-12127 (Pdiscovery = 0.0002; Preplication = 0.0249).
Conclusions
Our results suggest a modest effect of blood metabolites on the variations of BMD and identified several candidate blood metabolites for osteoporosis.
We calculated a genetic risk score for each metabolite to assess the effects of blood metabolites on osteoporosis and obesity-related phenotypes using the Mendelian randomization analysis.
Osteoporosis is a metabolic bone disease, characterized as low bone mass and increased bone fragility. It has been reported that currently >40 million people are at risk of bone fractures, and the incidence of osteoporotic fractures is increasing rapidly in the United States (1). Bone mineral density (BMD) is commonly used to diagnose osteoporosis and assess individual bone fracture risk (2–4). BMD has a strong heritable component, with an estimated heritability of 50% to 85% (5–8). In recent years, genome-wide association studies identified >100 genetic loci associated with BMD (9, 10). For instance, Estrada et al. (11) identified 56 BMD-associated loci through a large scale genome-wide meta-analysis. By integrating the genome-wide association studies and transcriptomic gene expression data sets, Chen et al. (12) detected several novel candidate genes for osteoporosis. Although a group of susceptibility loci has been identified for BMD, the genetic risks explained by the identified loci are limited, suggesting the existence of undiscovered susceptibility loci for osteoporosis.
Human blood metabolites, defined as the intermediates and products of metabolism, vary widely across different individuals. Noncellular metabolites (from plasma or serum) are commonly used to explore their biological significance and function owing to their convenience of sample collection. Previous studies have attempted to investigate the potential effects of blood metabolites on the development of human complex diseases, such as diabetes and cancer (13, 14). Recently, Long et al. (15) conducted a largescale whole-genome sequencing study to assess the relationships between genetic variations and blood metabolites using comprehensive metabolite profiling of 1960 adults. They identified a group of single nucleotide polymorphisms (SNPs) associated with blood metabolite levels (15).
Interest has increased in the relationships between metabolites and osteoporosis. A recent study observed novel metabolic profiling in postmenopausal women with a low BMD (16). Another prospective study suggested that intact parathyroid hormone and alkaline phosphatase levels were the most important independent factors associated with low BMD of the hip (17). To the best of our knowledge, no largescale study has been conducted to evaluate the relationship between global blood metabolites and osteoporosis.
Mendelian randomization is an epidemiological method in which environmental exposure-related genetic variations as instrumental variables are used to evaluate the association between environmental exposure and disease outcomes (18). Mendelian randomization results in a powerful control for confounding factors (e.g., lifestyle and socioeconomic factors) compared with conventional epidemiologic studies (19). Recently, a Mendelian randomization analysis was widely used to investigate the effect of physiological variables on diseases risks (20, 21).
In the present study, we conducted a Mendelian randomization analysis of 481 blood metabolites to investigate the potential effect of blood metabolites on the variations of BMD and bone areas. Our results might help to reveal the association of blood metabolites with osteoporosis and identify candidate blood metabolites for association with osteoporosis.
Materials and Methods
Discovery samples
A total of 2286 unrelated homogeneous white subjects living in Kansas City and surrounding areas were enrolled in the present study. Subjects with chronic diseases, conditions involving vital organs (e.g., heart, lung, liver, kidney, and brain), and severe endocrine, metabolic, or nutritional diseases were excluded from the present study. In addition, subjects taking antibone resorptive or bone anabolic agents or drugs, such as bisphosphonates, were excluded from our study. BMD (including total body, ulna and radius, hip, and spine) and bone area (including ulna and radius, hip, and spine) were measured using the Hologic 4500W dual energy X-ray absorptiometry system (Hologic Inc., Bedford, MA) that were calibrated daily. All phenotypic values were adjusted for age, sex, height, and weight using a linear regression model. The institutional review board of the University of Missouri Kansas City approved the present study. All the participants signed informed consent documents.
Genome-wide SNP genotyping
Genomic DNA was extracted from peripheral blood leukocytes using the Puregene DNA isolation kit (Gentra Systems, Minneapolis, MN). SNP genotyping was performed using the Genome-Wide Human SNP Array, version 6.0 (Affymetrix, Santa Clara, CA) following the Affymetrix protocol. In brief, 250-ng genomic DNA was digested with restriction enzyme NspI or StyI. Digested DNA was adaptor-ligated and polymerase chain reaction-amplified for each sample. Fragment polymerase chain reaction products were then labeled with biotin, denatured, and hybridized to the arrays. The arrays were scanned using the GeneChip Scanner 3000 7G (Affymetrix). Data management and analysis was conducted using the GeneChip® Command Console® software (Affymetrix). After quality control, we excluded 3930 SNPs with Hardy-Weinberg equilibrium testing P values of < 0.0001 and 145,204 SNPs with minor allele frequencies P values of < 0.01. In addition, 36 study subjects with a SNP call rate of < 0.95 were excluded.
Framingham Heart Study replication samples
The Framingham Heart Study (FHS) cohort was accessed through the database of Genotypes and Phenotypes (available at: https://www.ncbi.nlm.nih.gov/gap; access no. phs000342.v14.p10). In brief, FHS is a longitudinal and prospective cohort comprising >16,000 pedigree participants. The BMD values of the hip and spine were measured using dual-energy X-ray absorptiometry (Lunar Corp., Madison, WI). The participants were genotyped using the high-throughput Affymetrix 500K genotyping array plus a supplemental Affymetrix 50K genotyping array. Two genotype sets were merged together to form a single data set of ∼550,000 SNPs to maximize genotype coverage. Detailed information of the study design and sample recruitment was described in previous studies (22, 23). Specific for the present study, a total of 3143 unrelated white subjects from the FHS were used as replication samples.
Blood metabolite-associated SNP sets
Blood metabolite-associated SNPs and their genetic effects were driven from a largescale whole-genome sequencing study of blood metabolites (15). In brief, Long et al. (15) conducted whole-genome sequencing of 1960 adults to assess the association between genetic variations and blood metabolite levels. The serum samples were collected at three clinical visits for a period of 18 years and were analyzed on a nontarget metabolomics platform. Metabolite profiling was performed using the Metabolon platform (Metabolon, Morrisville, NC). Association tests were conducted using a linear mixed mode. Detailed descriptions of the sample collection, experimental design, quality control, and statistical analysis are available in the previously reported study (15). For the present study, the SNP sets significantly associated with blood metabolites at the genome-wide significance level were selected for individual genetic risk score (GRS) calculation (24, 25). The calculated GRS was then used as the instrumental variable of the corresponding blood metabolite to evaluate the possible association between the blood metabolite and target trait.
Statistical analysis
We analyzed 481 blood metabolites in the present study. Following the standard approach used by recent Mendelian randomization studies (20, 21), the GRS of each metabolite was calculated from SNP genotype data for each study subject. GRSjm denoted the GRS value of the jth metabolite for the mth subject, defined as . βi indicates the effect parameter of the risk allele of the ith significant SNP for the jth metabolite, which was obtained from the previously reported study (15). SNPim was the dosage (0, 1, 2) of the risk allele of the ith SNP for the mth study subject. Using the calculated GRS as the instrumental variable of the blood metabolites, a Pearson correlation analysis of the individual GRS values and BMD values (adjusted for age, sex, height, and weight as covariates) was conducted to evaluate the possible association between each blood metabolite and target trait. Through randomly shuffling of the phenotypic values of study subjects, 10,000 permutations were conducted to obtain the empirical distributions of testing statistics of the Pearson correlation analysis for each pair of blood metabolites and target traits. Empirical P values and the false discovery rate were then calculated from the obtained empirical distributions. The important blood metabolites detected in the discovery samples were further validated in the FHS replication samples. All statistical analyses were performed using R (available at: https://www.r-project.org/).
Results
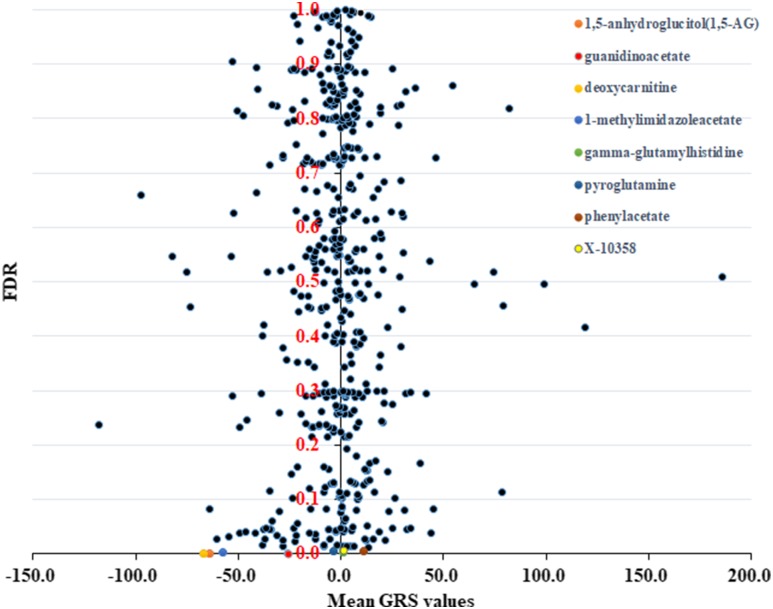
We observed important associations between the total hip BMD and 54 blood metabolites (Supplemental Table 1), including 8 blood metabolites with a false discovery rate of <0.01 (Fig. 1). The eight blood metabolites were 1,5-anhydroglucitol (1,5-AG; Pdiscovery < 0.0001), guanidinoacetate (Pdiscovery = 0.0002), pyroglutamine (Pdiscovery = 0.0002), deoxycarnitine (Pdiscovery < 0.0001), 1-methylimidazoleacetate (Pdiscovery = 0.0002), gamma-glutamylhistidine (Pdiscovery < 0.0001), phenylacetate (Pdiscovery < 0.0001), and X-10358 (Pdiscovery < 0.0001). The 54 blood metabolites identified in the discovery samples were further replicated in the FHS samples. For hip BMD, we also detected association signals for 1,5-AG (Preplication = 0.0361), inosine (Preplication = 0.0256), theophylline (Preplication = 0.0433) gamma-glutamylmethionine (Preplication = 0.0471), 1-linoleoyl-2-arachidonoyl-GPC (18:2/20:4n6) (Preplication = 0.0390), and X-12127 (Preplication = 0.0249). For spinal BMD, association signals were observed for N-acetyltaurine (Preplication = 0.0119), gamma-glutamylmethionine (Preplication = 0.0124), and inosine (Preplication = 0.0178). The absolute values of the Pearson correlation coefficients of all substantial associations were <0.1, indicating the weak effects of blood metabolites on the variations of BMD.
Figure 1.
Plot of Pearson correlation analysis results of total hip BMD and GRS of 481 blood metabolites. Each dot denotes a blood metabolite. The x-axis denotes the average GRS values of 2286 study subjects. The y-axis denotes the false discovery rate (FDR) calculated from the permutations. The top eight blood metabolites are labeled with different colors.
Discussion
To reveal the potential effects of blood metabolites on the development of osteoporosis, we conducted a Mendelian randomization analysis of 481 blood metabolites using 2286 white subjects. We observed modest associations of blood metabolites with the variations of BMD and identified several candidate blood metabolites for an association with osteoporosis. To the best of our knowledge, the present study is the first largescale Mendelian randomization study of blood metabolites for osteoporosis. Our study results could help to reveal the effect of blood metabolites on the development of BMD and provide clues for pathogenetic studies of osteoporosis.
We found that guanidinoacetate, 1,5-AG, and pyroglutamine were substantially associated with total hip BMD. Guanidinoacetate (or glycocyamine) is a metabolite of glycine, as well as the direct precursor of creatine. The biological role of creatine is to facilitate the recycling of adenosine triphosphate, mainly in brain and muscle. Emerging evidence has suggested that creatine supplementation could affect bone biology. For instance, an animal study conducted in young Sprague-Dawley rats suggested that creatine supplementation had beneficial effects on the biological function and structure of bone and increased lumbar BMD (26). A recent study indicated that the combination of 12 months of creatine supplementation and resistance training preserved femoral neck BMD and increased femoral shaft superiosteal width in postmenopausal women (27). The same conclusion was also drawn in the elderly (28), supporting the implication of creatine in maintaining the normal biological function of bone.
1,5-AG, a naturally occurring polyol, serves as a valuable complement to frequent self-monitoring or continuous monitoring of plasma glucose to confirm stable glycemic control (29). 1,5-AG can be found in various foods. A previous study of patients with moderately controlled diabetes demonstrated that 1,5-AG was associated with the risk of diabetes in the postprandial state and was more sensitive and specific than fructosamine and hemoglobin A1c (30). In addition, the association between diabetes and bone metabolism has achieved wide attention. Researchers observed that older adults with type 2 diabetes tended to have normal or greater BMD than those without diabetes (31). For instance, Oei et al. (32) found that poor glycemic control in those with type 2 diabetes was associated with a high BMD in narrower bones. It is interesting that the subjects with type 2 diabetes were reported to be associated with an increased fracture risk despite an increased BMD (33).
Theophylline is another common metabolite detected in both discovery samples and replication samples. It is a methylxanthine drug with anti-inflammatory property. Studies of theophylline on bone cells, skeleton, and system calcium homeostasis have resulted in widespread attention. Studies have shown that the parameters related to systemic calcium homeostasis of theophylline-treated animals changed significantly compared with control animals, including increased urinary calcium excretion and decreased total body calcium (34). These results have shown the promotional effects of theophylline on skeletal calcium loss and its acceleration of the development of human osteopenia (34). The same conclusion regarding the detrimental effect of theophylline on bone loss was reported in another study (35).
Pyroglutamine is a notable blood metabolite associated with total hip BMD. Pyroglutamine is a cyclic derivative of glutamine related to pyroglutamic acid. The pituitary glutaminyl cyclotransferase (QPCT) gene encodes an enzyme responsible for the presence of pyroglutamyl residues in neuroendocrine peptides. A study conducted in Japanese subjects found that common polymorphisms in the QPCT gene were significantly associated with BMD in postmenopausal women (36). Huang and Kung (37) also found that rs3770748 in QPCT was associated with spinal BMD in Chinese subjects.
Apart from the top eight metabolites, some other important metabolites were also associated with BMD in our study. For example, gamma-glutamylmethionine, one of the gamma-glutamyl derivatives, was used to examine the transport of gamma-glutamyl amino acids into tissues in the mouse. A previous study conducted in Japanese subjects found that a functional SNP in the vitamin K-dependent gamma-glutamyl carboxylase gene (Arg325Gln) was associated with BMD in elderly Japanese women (23). Gamma-glutamyltransferase is a type of enzyme that plays a key role in the gamma-glutamyl cycle. The serum gamma-glutamyltransferase level within its normal range is inversely correlated with the BMD in the femur neck among postmenopausal women (37).
One limitation of the present study was that the Pearson correlation analysis coefficients of all substantial blood metabolites were <0.1, indicating the limited strength of the association of blood metabolites on the variation of BMD. These results were consistent with previous study results, which demonstrated that osteoporosis is a complex diseases determined by a group of genetic and environmental factors (38, 39). Each factor had limited effects on the risks of osteoporosis (38, 39). In addition, the relatively small sample size might have limited the statistical power of our study. Our study results should be interpreted with caution. Further studies are needed to confirm our findings and clarify the potential molecular mechanisms of the detected associations between blood metabolites and BMD.
In conclusion, using Mendelian randomization analysis, we investigated the effect of global blood metabolites on the variations of BMD. Our study results suggest the modest effects of the associations of blood metabolites on the variation in BMD and identified several candidate blood metabolites for association with osteoporosis. We hope that our study results have provided clues for the pathogenetic studies of osteoporosis.
Supplementary Material
Acknowledgments
Financial Support: The present study was partially supported by the National Natural Scientific Foundation of China (grants 81472925 and 81673112), Technology Research and Development Program of Shaanxi Province of China (grant 2013KJXX-51), and Fundamental Research Funds for the Central Universities. Q.T., H.S., and H.W.D. were partially supported by grants from the National Institutes of Health (grants R01AR057049, R01AR059781, D43TW009107, P20 GM109036, R01MH107354, R01MH104680, and R01GM109068), Edward G. Schlieder Endowment fund, and Tsai and Kung endowment fund to Tulane University. The Framingham Heart Study is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with Boston University (contract no. N01-HC-25195). The present report was not prepared in collaboration with investigators of the Framingham Heart Study and does not necessarily reflect the opinions or views of the Framingham Heart Study, Boston University, or NHLBI. Funding for SHARe Affymetrix genotyping was provided by the NHLBI (contract N02-HL-64278). SHARe Illumina genotyping was provided under an agreement between Illumina and Boston University. Funding support for the Framingham Whole Body and Regional Dual X-ray Absorptiometry data set was provided by National Institutes of Health (grant R01 AR/AG 41398). The data sets used for the analyses described in our report were obtained from database of Genotypes and Phenotypes (available at: http://www.ncbi.nlm.nih.gov/sites/entrez?db=gap through dbGaP accession phs000342.v14.p10).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- 1,5-AG
1,5-anhydroglucitol
- BMD
bone mineral density
- FHS
Framingham Heart Study
- GRS
genetic risk score
- QPCT
pituitary glutaminyl cyclase cyclotransferase
- SNP
single nucleotide polymorphism
References
- 1. Bartl MR, Bartl PMC. Epidemiology of Osteoporotic Fractures. Cham, Switzerland: Springer International Publishing; 2017:585–597. [Google Scholar]
- 2. Sinha R, Bukhari M. OP0292 the diagnosis of osteoporosis using BMD and T score measurements at specific skeletal sites. Ann Rheum Dis. 2014;73(Suppl 2):172. [Google Scholar]
- 3. Johnell O, Kanis JA, Oden A, Johansson H, De Laet C, Delmas P, Eisman JA, Fujiwara S, Kroger H, Mellstrom D, Meunier PJ, Melton LJ III, O’Neill T, Pols H, Reeve J, Silman A, Tenenhouse A. Predictive value of BMD for hip and other fractures. J Bone Miner Res. 2005;20(7):1185–1194. [DOI] [PubMed] [Google Scholar]
- 4. Steier M, Shefer G, Hoshen M, Feldman B, Balicer R. Bone mass density, yes or no: the effectiveness of BMD test in decreasing the risk for hip fractures in different demographic groups in Israel. Bone Abstracts. 2016;5:298. [Google Scholar]
- 5. Pocock NA, Eisman JA, Hopper JL, Yeates MG, Sambrook PN, Eberl S. Genetic determinants of bone mass in adults. A twin study. J Clin Invest. 1987;80(3):706–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Knapp KM, Andrew T, MacGregor AJ, Blake GM, Fogelman I, Spector TD. An investigation of unique and shared gene effects on speed of sound and bone density using axial transmission quantitative ultrasound and DXA in twins. J Bone Miner Res. 2003;18(8):1525–1530. [DOI] [PubMed] [Google Scholar]
- 7. Brandi ML, Bianchi ML, Eisman JA, Glorieux F, Adami S, Fiore CE, Nuti R, Ortolani S. Genetics of osteoporosis. Calcif Tissue Int. 1994;55(3):161–163. [DOI] [PubMed] [Google Scholar]
- 8. Healthy ageing twin study reveals role of genetic factors on BMD Bonekey Rep. 2012;1:163–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sabik OL, Farber CR. Using GWAS to identify novel therapeutic targets for osteoporosis. Transl Res. 2017;181:15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hegarty KG, Drummond FJ, Daly M, Shanahan F, Molloy MG. GREB1 genetic variants are associated with bone mineral density in Caucasians. J Bone Miner Metab. 2018;36(2):189–199. [DOI] [PubMed] [Google Scholar]
- 11. Estrada K, Styrkarsdottir U, Evangelou E, Hsu YH, Duncan EL, Ntzani EE, Oei L, Albagha OM, Amin N, Kemp JP, Koller DL, Li G, Liu CT, Minster RL, Moayyeri A, Vandenput L, Willner D, Xiao SM, Yerges-Armstrong LM, Zheng HF, Alonso N, Eriksson J, Kammerer CM, Kaptoge SK, Leo PJ, Thorleifsson G, Wilson SG, Wilson JF, Aalto V, Alen M, Aragaki AK, Aspelund T, Center JR, Dailiana Z, Duggan DJ, Garcia M, Garcia-Giralt N, Giroux S, Hallmans G, Hocking LJ, Husted LB, Jameson KA, Khusainova R, Kim GS, Kooperberg C, Koromila T, Kruk M, Laaksonen M, Lacroix AZ, Lee SH, Leung PC, Lewis JR, Masi L, Mencej-Bedrac S, Nguyen TV, Nogues X, Patel MS, Prezelj J, Rose LM, Scollen S, Siggeirsdottir K, Smith AV, Svensson O, Trompet S, Trummer O, van Schoor NM, Woo J, Zhu K, Balcells S, Brandi ML, Buckley BM, Cheng S, Christiansen C, Cooper C, Dedoussis G, Ford I, Frost M, Goltzman D, González-Macías J, Kähönen M, Karlsson M, Khusnutdinova E, Koh JM, Kollia P, Langdahl BL, Leslie WD, Lips P, Ljunggren Ö, Lorenc RS, Marc J, Mellström D, Obermayer-Pietsch B, Olmos JM, Pettersson-Kymmer U, Reid DM, Riancho JA, Ridker PM, Rousseau F, Slagboom PE, Tang NL, Urreizti R, Van Hul W, Viikari J, Zarrabeitia MT, Aulchenko YS, Castano-Betancourt M, Grundberg E, Herrera L, Ingvarsson T, Johannsdottir H, Kwan T, Li R, Luben R, Medina-Gómez C, Palsson ST, Reppe S, Rotter JI, Sigurdsson G, van Meurs JB, Verlaan D, Williams FM, Wood AR, Zhou Y, Gautvik KM, Pastinen T, Raychaudhuri S, Cauley JA, Chasman DI, Clark GR, Cummings SR, Danoy P, Dennison EM, Eastell R, Eisman JA, Gudnason V, Hofman A, Jackson RD, Jones G, Jukema JW, Khaw KT, Lehtimäki T, Liu Y, Lorentzon M, McCloskey E, Mitchell BD, Nandakumar K, Nicholson GC, Oostra BA, Peacock M, Pols HA, Prince RL, Raitakari O, Reid IR, Robbins J, Sambrook PN, Sham PC, Shuldiner AR, Tylavsky FA, van Duijn CM, Wareham NJ, Cupples LA, Econs MJ, Evans DM, Harris TB, Kung AW, Psaty BM, Reeve J, Spector TD, Streeten EA, Zillikens MC, Thorsteinsdottir U, Ohlsson C, Karasik D, Richards JB, Brown MA, Stefansson K, Uitterlinden AG, Ralston SH, Ioannidis JP, Kiel DP, Rivadeneira F. Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat Genet. 2012;44(5):491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen YC, Guo YF, He H, Lin X, Wang XF, Zhou R, Li WT, Pan DY, Shen J, Deng HW. Integrative analysis of genomics and transcriptome data to identify potential functional genes of BMDs in females. J Bone Miner Res. 2016;31(5):1041–1049. [DOI] [PubMed] [Google Scholar]
- 13. Larsson SC, Burgess S, Michaëlsson K. Association of genetic variants related to serum calcium levels with coronary artery disease and myocardial infarction. JAMA. 2017;318(4):371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cole TJ, Freeman JV, Preece MA. Body mass index reference curves for the UK, 1990. Arch Dis Child. 1995;73(1):25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Long T, Hicks M, Yu HC, Biggs WH, Kirkness EF, Menni C, Zierer J, Small KS, Mangino M, Messier H, Brewerton S, Turpaz Y, Perkins BA, Evans AM, Miller LA, Guo L, Caskey CT, Schork NJ, Garner C, Spector TD, Venter JC, Telenti A. Whole-genome sequencing identifies common-to-rare variants associated with human blood metabolites. Nat Genet. 2017;49(4):568–578. [DOI] [PubMed] [Google Scholar]
- 16. Miyamoto T, Hirayama A, Sato Y, Koboyashi T, Katsuyama E, Kanagawa H, Miyamoto H, Mori T, Yoshida S, Fujie A, Morita M, Watanabe R, Tando T, Miyamoto K, Tsuji T, Funayama A, Nakamura M, Matsumoto M, Soga T, Tomita M, Toyama Y. A serum metabolomics-based profile in low bone mineral density postmenopausal women. Bone. 2017;95:1–4. [DOI] [PubMed] [Google Scholar]
- 17. Elmaataoui A, Benghabrite A, El Maghraoui A, Chabraoui L, Ouzzif Z. Relationship between sex hormone levels, bone mineral density and bone turnover markers in healthy Moroccan men: a cross-sectional study. Pan Afr Med J. 2015;22:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Davey Smith G, Ebrahim S. What can mendelian randomisation tell us about modifiable behavioural and environmental exposures? BMJ. 2005;330(7499):1076–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Burgess S, Daniel RM, Butterworth AS, Thompson SG; EPIC-InterAct Consortium . Network Mendelian randomization: using genetic variants as instrumental variables to investigate mediation in causal pathways. Int J Epidemiol. 2015;44(2):484–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Reed ZE, Micali N, Bulik CM, Davey Smith G, Wade KH. Assessing the causal role of adiposity on disordered eating in childhood, adolescence, and adulthood: a Mendelian randomization analysis. Am J Clin Nutr. 2017;106(3):764–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, Jensen MK, Hindy G, Hólm H, Ding EL, Johnson T, Schunkert H, Samani NJ, Clarke R, Hopewell JC, Thompson JF, Li M, Thorleifsson G, Newton-Cheh C, Musunuru K, Pirruccello JP, Saleheen D, Chen L, Stewart A, Schillert A, Thorsteinsdottir U, Thorgeirsson G, Anand S, Engert JC, Morgan T, Spertus J, Stoll M, Berger K, Martinelli N, Girelli D, McKeown PP, Patterson CC, Epstein SE, Devaney J, Burnett MS, Mooser V, Ripatti S, Surakka I, Nieminen MS, Sinisalo J, Lokki ML, Perola M, Havulinna A, de Faire U, Gigante B, Ingelsson E, Zeller T, Wild P, de Bakker PI, Klungel OH, Maitland-van der Zee AH, Peters BJ, de Boer A, Grobbee DE, Kamphuisen PW, Deneer VH, Elbers CC, Onland-Moret NC, Hofker MH, Wijmenga C, Verschuren WM, Boer JM, van der Schouw YT, Rasheed A, Frossard P, Demissie S, Willer C, Do R, Ordovas JM, Abecasis GR, Boehnke M, Mohlke KL, Daly MJ, Guiducci C, Burtt NP, Surti A, Gonzalez E, Purcell S, Gabriel S, Marrugat J, Peden J, Erdmann J, Diemert P, Willenborg C, König IR, Fischer M, Hengstenberg C, Ziegler A, Buysschaert I, Lambrechts D, Van de Werf F, Fox KA, El Mokhtari NE, Rubin D, Schrezenmeir J, Schreiber S, Schäfer A, Danesh J, Blankenberg S, Roberts R, McPherson R, Watkins H, Hall AS, Overvad K, Rimm E, Boerwinkle E, Tybjaerg-Hansen A, Cupples LA, Reilly MP, Melander O, Mannucci PM, Ardissino D, Siscovick D, Elosua R, Stefansson K, O’Donnell CJ, Salomaa V, Rader DJ, Peltonen L, Schwartz SM, Altshuler D, Kathiresan S. Plasma HDL cholesterol and risk of myocardial infarction: a Mendelian randomisation study. Lancet. 2012;380(9841):572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cupples LA, Arruda HT, Benjamin EJ, D’Agostino RB Sr, Demissie S, DeStefano AL, Dupuis J, Falls KM, Fox CS, Gottlieb DJ, Govindaraju DR, Guo CY, Heard-Costa NL, Hwang SJ, Kathiresan S, Kiel DP, Laramie JM, Larson MG, Levy D, Liu CY, Lunetta KL, Mailman MD, Manning AK, Meigs JB, Murabito JM, Newton-Cheh C, O’Connor GT, O’Donnell CJ, Pandey M, Seshadri S, Vasan RS, Wang ZY, Wilk JB, Wolf PA, Yang Q, Atwood LD. The Framingham Heart Study 100K SNP genome-wide association study resource: overview of 17 phenotype working group reports. BMC Med Genet. 2007;8(Suppl 1):S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pei YF, Xie ZG, Wang XY, Hu WZ, Li LB, Ran S, Lin Y, Hai R, Shen H, Tian Q, Zhang YH, Lei SF, Papasian CJ, Deng HW, Zhang L. Association of 3q13.32 variants with hip trochanter and intertrochanter bone mineral density identified by a genome-wide association study. Osteoporos Int. 2016;27(11):3343–3354. [DOI] [PubMed] [Google Scholar]
- 24. Thanassoulis G, Peloso GM, Pencina MJ, Hoffmann U, Fox CS, Cupples LA, Levy D, D’Agostino RB, Hwang SJ, O’Donnell CJ. A genetic risk score is associated with incident cardiovascular disease and coronary artery calcium: the Framingham Heart Study. Circ Cardiovasc Genet. 2012;5(1):113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hivert M-F, Jablonski KA, Perreault L, Saxena R, McAteer JB, Franks PW, Hamman RF, Kahn SE, Haffner S, DIAGRAM Consortium; Meigs JB, Altshuler D, Knowler WC, Florez JC; Diabetes Prevention Program Research Group . Updated genetic score based on 34 confirmed type 2 diabetes loci is associated with diabetes incidence and regression to normoglycemia in the diabetes prevention program. Diabetes. 2011;60(4):1340–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Antolic A, Roy BD, Tarnoplosky MA, Zernicke RF, Wohl GR, Shaughnessy SG, Bourgeois JM. Creatine monohydrate increases bone mineral density in young Sprague-Dawley rats. Med Sci Sports Exerc. 2007;39(5):816–820. [DOI] [PubMed] [Google Scholar]
- 27. Chilibeck PD, Candow DG, Landeryou T, Kaviani M, Paus-Jenssen L. Effects of creatine and resistance training on bone health in postmenopausal women. Med Sci Sports Exerc 2015;47(8):1587–1596. [DOI] [PubMed] [Google Scholar]
- 28. Candow DG, Chilibeck PD. Potential of creatine supplementation for improving aging bone health. J Nutr Health Aging. 2010;14(2):149–153. [DOI] [PubMed] [Google Scholar]
- 29. Yamanouchi T, Ogata N, Tagaya T, Kawasaki T, Sekino N, Funato H, Akaoka L, Miyashita H. Clinical usefulness of serum 1,5-anhydroglucitol in monitoring glycaemic control. Lancet. 1996;347(9014):1514–1518. [DOI] [PubMed] [Google Scholar]
- 30. Dungan KM, Buse JB, Largay J, Kelly MM, Button EA, Kato S, Wittlin S. 1,5-Anhydroglucitol and postprandial hyperglycemia as measured by continuous glucose monitoring system in moderately controlled patients with diabetes. Diabetes Care. 2006;29(6):1214–1219. [DOI] [PubMed] [Google Scholar]
- 31. Samelson EJ, Demissie S, Cupples LA, Zhang X, Xu H, Liu CT, Boyd SK, McLean RR, Broe KE, Kiel DP, Bouxsein ML. Diabetes and deficits in cortical bone density, microarchitecture, and bone size: Framingham HR‐pQCT study. J Bone Miner Res 2018;33(1):54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Oei L, Zillikens MC, Dehghan A, Buitendijk GH, Castaño-Betancourt MC, Estrada K, Stolk L, Oei EH, van Meurs JB, Janssen JA, Hofman A, van Leeuwen JP, Witteman JC, Pols HA, Uitterlinden AG, Klaver CC, Franco OH, Rivadeneira F. High bone mineral density and fracture risk in type 2 diabetes as skeletal complications of inadequate glucose control: the Rotterdam study. Diabetes Care. 2013;36(6):1619–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. de Liefde II, van der Klift M, de Laet CE, van Daele PL, Hofman A, Pols HA. Bone mineral density and fracture risk in type-2 diabetes mellitus: the Rotterdam Study. Osteoporos Int. 2005;16(12):1713–1720. [DOI] [PubMed] [Google Scholar]
- 34. Fortenbery EJ, McDermott MT, Duncan WE. Effect of theophylline on calcium metabolism and circulating vitamin D metabolites. J Bone Miner Res. 1990;5(4):321–324. [DOI] [PubMed] [Google Scholar]
- 35. Pal S, Khan K, China SP, Mittal M, Porwal K, Shrivastava R, Taneja I, Hossain Z, Mandalapu D, Gayen JR, Wahajuddin M, Sharma VL, Trivedi AK, Sanyal S, Bhadauria S, Godbole MM, Gupta SK, Chattopadhyay N. Theophylline, a methylxanthine drug induces osteopenia and alters calciotropic hormones, and prophylactic vitamin D treatment protects against these changes in rats. Toxicol Appl Pharmacol. 2016;295:12–25. [DOI] [PubMed] [Google Scholar]
- 36. Ezura Y, Kajita M, Ishida R, Yoshida S, Yoshida H, Suzuki T, Hosoi T, Inoue S, Shiraki M, Orimo H, Emi M. Association of multiple nucleotide variations in the pituitary glutaminyl cyclase gene (QPCT) with low radial BMD in adult women. J Bone Miner Res. 2004;19(8):1296–1301. [DOI] [PubMed] [Google Scholar]
- 37. Huang QY, Kung AW. The association of common polymorphisms in the QPCT gene with bone mineral density in the Chinese population. J Hum Genet. 2007;52(9):757–762. [DOI] [PubMed] [Google Scholar]
- 38. Xu XH, Dong SS, Guo Y, Yang TL, Lei SF, Papasian CJ, Zhao M, Deng HW. Molecular genetic studies of gene identification for osteoporosis: the 2009 update. Endocr Rev. 2010;31(4):447–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rivadeneira F, Styrkársdottir U, Estrada K, Halldórsson BV, Hsu YH, Richards JB, Zillikens MC, Kavvoura FK, Amin N, Aulchenko YS, Cupples LA, Deloukas P, Demissie S, Grundberg E, Hofman A, Kong A, Karasik D, van Meurs JB, Oostra B, Pastinen T, Pols HA, Sigurdsson G, Soranzo N, Thorleifsson G, Thorsteinsdottir U, Williams FM, Wilson SG, Zhou Y, Ralston SH, van Duijn CM, Spector T, Kiel DP, Stefansson K, Ioannidis JP, Uitterlinden AG; Genetic Factors for Osteoporosis (GEFOS) Consortium . Twenty bone-mineral-density loci identified by large-scale meta-analysis of genome-wide association studies. Nat Genet. 2009;41(11):1199–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.