Abstract
Ovulation is the appropriately timed release of a mature, developmentally competent oocyte from the ovary into the oviduct, where fertilization occurs. Importantly, ovulation is tightly linked with oocyte maturation, demonstrating the interdependency of these two parallel processes, both essential for female fertility. Initiated by pituitary gonadotropins, the ovulatory process is mediated by intrafollicular paracrine factors from the theca, mural, and cumulus granulosa cells, as well as the oocyte itself. The result is the induction of cumulus expansion, proteolysis, angiogenesis, inflammation, and smooth muscle contraction, which are each required for follicular rupture. These complex intercellular communication networks and the essential ovulatory genes have been well defined in mouse models and are highly conserved in primates, including humans. Importantly, recent discoveries in regulation of ovulation highlight new areas of investigation.
A review of cellular events within the ovary that mediate ovulation, with a particular focus on those processes that also influence oocyte maturation and/or acquisition of developmental competence.
Ovulation of an oocyte from the ovary into the oviduct for fertilization is a tightly regulated process, engaging multiple physiological systems to ensure the timely release of only high-quality oocytes at the appropriate time. Within the ovary, each oocyte, which is depended on to grow and acquire the capacity to be fertilized and form a viable embryo—a process known as the acquisition of oocyte developmental competence—is in a follicle surrounded by somatic cells. Once it is grown and competence acquired, the oocyte is released into the oviduct through the culmination of dynamic tissue remodeling, known as ovulation. Ovulation is triggered by the midcycle surge of an LH from the pituitary; yet, remarkably, the effector molecules responsible for the final release of the oocyte from the follicle are still being revealed. Interestingly, many important ovulation genes are also linked to oocyte competence, suggesting that these processes are coordinated, perhaps as a “checkpoint” measure, to ensure oocytes released possess the greatest potential for development.
The preovulatory follicle contains three main, distinct somatic cell lineages. The theca cells are specialized ovarian stromal cells separated from the granulosa layers by the follicular basement membrane, whereas two sublineages of granulosa cells are separated from each other by the follicular antrum (Fig. 1). These granulosa cell sublineages diverge during follicular growth, through physical and biochemical influences, arising from either close contact with the follicular wall to form mural granulosa cells or with the oocyte to form cumulus cells. Importantly, direct communication, as well as paracrine interactions between the oocyte and somatic cells, is necessary for ovulation. In response to the LH surge, the follicle structure is radically remodeled to enable ovulation, and a number of essential parallel processes occur. These include dissolution of the follicular basement membrane, neovascularization, formation of a unique matrix in the complex of cumulus cells surrounding the oocyte (cumulus expansion), and resumption of oocyte meiotic division. The essential gene products that mediate these processes involve a suite of secondary intrafollicular signaling networks, as well as a range of essential effector proteins [such as proteases and the newly synthesized extracellular matrix (ECM)].
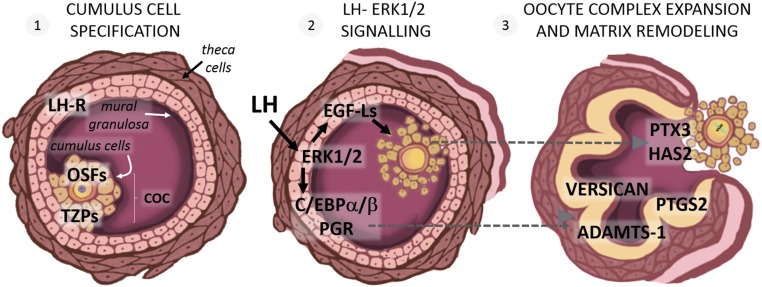
Figure 1.
Essential genes coordinating ovulation and oocyte maturation within the ovarian follicle. (1) Cumulus cell specification occurs as oocyte-secreted factors (OSFs) differentiate their adjacent granulosa cells into the cumulus cell lineage. Cumulus cells directly communicate with the oocyte and vice versa through transzonal projections (TZPs). (2) The LH surge binds LH receptors (LH-R) expressed on mural granulosa cells and activates ERK1/2 kinases, which in turn induce the expression and secretion of epidermal growth factor–like ligands (EGF-Ls), as well as production of a cohort of transcription factors, including CCAAT enhancer–binding protein (C/EBP)α/β and progesterone receptor (PGR). (3) EGF-Ls induce the production of cumulus matrix proteins, such as pentraxin 3 (PTX3) and hyaluronan synthase 2 (HAS2), which cause cumulus oocyte complex (COC) expansion. Granulosa cells produce a suite of inflammatory [prostaglandin synthase 2 (PTGS2)], matrix (versican), and protease [a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS-1)] genes that are essential for ovulation and that are biomarkers of oocyte quality.
This mini-review will summarize our current understanding of the cellular events within the ovary that mediate ovulation, with a particular focus on those processes that also influence oocyte maturation and/or acquisition of developmental competence. The majority of studies revealing specific gene products essential for ovulation have used mouse models, in particular, genetically modified mice with systemic null gene mutations [knockout (KO)] or temporal and cell-specific-targeted gene deletions in oocytes or granulosa cells. Studies using mouse models to identify key ovulatory gene products are emphasized throughout, with studies demonstrating ovulatory mediators in nonhuman primates and/or humans highlighted. What emerges is that there are key phases of folliculogenesis that must be completed to successfully coordinate ovulation and oocyte maturation. (1) The cumulus cell lineage differentiates from the granulosa layer, and bidirectional communication with the oocyte is established. (2) The LH surge initiates multiple signaling cascades originating in granulosa cells that set in motion parallel processes of oocyte maturation and ovulatory gene expression. (3) Granulosa-derived and cumulus cell factors act in concert to breach the follicular apex and release a fully mature oocyte. Each of these sequential phases is required for both oocyte maturation and oocyte release and thus, is essential to female fertility.
The Oocyte Dictates Cumulus Cell Specification
The granulosa cells directly surrounding the oocyte are a specific sublineage, defined as “cumulus cells.” Cumulus and granulosa cells originate from a common progenitor in preantral follicles that differentiate as the cell compartments are physically separated by antrum formation, resulting in the cumulus oocyte complex (COC), surrounded by follicular fluid with granulosa layers lining the follicle wall (Fig. 1). The COC forms a visibly distinguishable complex, comprising three to four layers of cumulus cells (totaling ∼2000 cells), tightly packed together in concentric layers enveloping the oocyte. Cumulus cells have a distinct hormone responsiveness and fate relative to mural granulosa cells and play a unique and critical role, providing a niche environment for development of the pluripotent oocyte and maintaining its meiotic arrest. If the cumulus vestment is removed, even acutely in the final hours of maturation, then the competence of human oocytes to fertilize and develop normally into embryos is severely compromised (1).
It is the oocyte that actively directs its surrounding somatic cells into the cumulus lineage. Two oocyte-secreted morphogens, growth and differentiation factor 9 (GDF9) and bone morphogenetic protein 15 (BMP15), regulate cumulus gene expression. One defining characteristic is the repression of Lhcgr gene encoding the LH receptor (LH-R) by these oocyte-derived signals (2), such that mural granulosa and theca cells of preovulatory follicles express LH-R protein at levels typically an order of magnitude higher than cumulus cells. Simultaneously, GDF9 and BMP15 activate the expression of key cumulus-specific metabolic genes, such as glycolysis enzymes (3) and cholesterol biosynthesis enzymes (4), which are essential mediators of oocyte viability and competence. The importance of oocyte-secreted growth factors in specifying the cumulus phenotype is shown when mural granulosa cells treated in vitro with GDF9/BMP15 respond by adopting the cumulus cell gene-expression profile (5). Equally, the removal of the oocyte from COCs or treatment with antagonists of GDF9/BMP15 action results in induction of granulosa-specific and loss of cumulus-specific gene expression (6, 7). In humans, the oocyte-secreted factors are equally important in specifying the cumulus lineage; however, intriguing differences in the bioactivity and relative importance of GDF9 vs BMP15 arise. Whereas rodent BMP15 is relatively inactive, in humans, it is the more active of the two factors (8). It has been proposed that the relative abundance of GDF9 and BMP15 might be a determining factor between mono-ovulation and polyovulation (9), and thus the elucidation of species-specific actions of oocyte growth factors is an important area of investigation.
As cumulus cells provide metabolic and nutritional support for the oocyte, an intimate association with cumulus cells is essential for oocyte survival and healthy development. Cellular projections extended by the cumulus cells traverse the oocyte zona pellucida, forming direct contacts with the oocyte, with terminal gap junctions connecting the cumulus and oocyte plasma membranes. New complexities in this intercellular communication have recently been reported. Formation of the transzonal projections is dependent on signals from the oocyte (10) and may involve membrane fusion between the oocyte and cumulus cells (11). The terminal gap junctions allow transit of small (∼1-kDa) molecules between the oocyte and cumulus cell cytoplasm (12), and the sharing of cytoplasmic contents between cumulus cells and the oocyte is demonstrated when fluorescent dyes injected into one cell type become rapidly visible in both (11, 13). Glucose metabolites are transported, as oocytes cannot independently metabolize glucose and depend on cumulus cells to perform glycolysis and transfer pyruvate intercellularly for energy production in the oocyte mitochondria (14). Other endogenous molecules that are transported from the cumulus cells into oocytes include ATP, cAMP, and cyclic GMP (cGMP), which are needed to maintain oocyte arrest at prophase I of meiosis (also known as the germinal vesicle stage). High cAMP levels, generated in cumulus cells by active adenylate cyclase and transferred to the oocyte via gap junctions, maintain the meiosis-promoting factor complex of cell-cycle proteins in an inactive state (15). Cumulus-derived cGMP, also transferred via gap junctions, inactivates cAMP metabolizing phosphodiesterase 3A in oocytes, further contributing to high-oocyte cAMP levels (16). Thus, cumulus cells play a critical role in directing oocyte progression through meiosis.
A number of studies demonstrate that when oocyte quality or oocyte-cumulus communication is poor, ovulation does not occur. For instance, connexin-37 is a gap junctional protein required for the direct transfer of small molecules between the oocyte and cumulus cells, and in null mutant mice lacking connexin-37, some large preovulatory follicles form but do not ovulate, and oocyte nuclear maturation is deficient (17). This indicates that normal responses to the LH surge cannot occur without sustained communication between oocytes and their surrounding somatic cells. Interestingly, gene products involved in oocyte chromatin modifications also influence ovulation. For instance, two components of the cullin ring-finger ubiquitin E3 ligase 4 complex, which directly binds and regulates the activity of ten eleven translocation enzymes, are required for oocyte viability. Oocyte-specific deletion of the DNA damage–binding protein 1 (DDB1) or DNA damage–binding protein 1 and CUL4-associated factor 1 (DCAF1) subunits in mice results in reduced expression of GDF9 and BMP15, impaired ovulatory gene expression and cumulus expansion, and dramatically reduced ovulation rate (18). Likewise, when histone methylation and transcriptional silencing is prevented by oocyte-specific deletion of Mll2 histone methyltransferase, ovulation is blocked, and oocytes remain trapped in large, unruptured but luteinized follicles (19). Thus, genes that control oocyte quality also influence the capacity for that oocyte to be released. This coordination is regulated, at least in part, by GDF9/BMP15 but likely also involves additional, currently unknown mechanisms that mediate communication between the oocyte and its surrounding somatic cells. This elegant biological crosstalk between the germ cell and somatic cells may act as a checkpoint to ensure that healthy, viable oocytes are the most competent to trigger their own release at ovulation.
The LH Surge Activates a Cascade of Ovulatory Mediators
The LH surge from the pituitary is the trigger that sets in motion and coordinates both the final stages of oocyte maturation, as well as follicular rupture. Circulating LH binds LH-R, a classical G protein–coupled receptor, on mural granulosa cells of mature follicles, triggering activation of multiple intracellular signaling pathways, including protein kinase A, Erk1/2, protein kinase C, and Ras [recently reviewed in Richards and Ascoli (20)]. These distinct signaling cascades are effectors of specific aspects of oocyte maturation and follicular rupture (Fig. 1).
LH-surge activation of the Erk1/2 or MAPK pathway is the key downstream effector that is absolutely required for both oocyte maturation and ovulation. This is demonstrated by the infertility phenotype of mice with inactivation of Erk1 and Erk2 in granulosa cells (21). Ovarian follicles of the double-mutant mice exhibit complete absence of the normal LH-induced responses, wherein >75% of LH target genes were dysregulated, including dramatically impaired expression of cumulus and granulosa cell-specific genes. As such, ovaries were severely affected with no cumulus expansion and a failure of oocytes to undergo germinal vesicle breakdown and resume meiosis. In parallel, ovulation did not occur, granulosa cells failed to undergo luteinization, and there was no detectable increase in progesterone production (21). These defects in both meiosis progression and follicular rupture demonstrate that these related kinases act together in granulosa cells to mediate essential aspects of both oocyte maturation and ovulation.
One of the key LH-surge downstream responses mediated by the ERK1/2 kinases is the transcriptional induction of epidermal growth factor (EGF)–like ligands (EGF-Ls) amphiregulin, epiregulin, and betacellulin in granulosa cells. Secretion of these ligands by granulosa cells is essential to activate the EGF receptor (EGFR) on cumulus cells and initiate the ovulatory response in the COC (22), thereby effectively transmitting the LH-surge signal to the COC, which lacks LH-R and thus, direct responsiveness. There is functional redundancy between amphiregulin, epiregulin, and betacellulin, such that null mutant mice for each of these individual genes are fertile. Inhibition of their common receptor (EGFR), however, blocks ovulation induction by human chorionic gonadotropin (hCG), and oocytes remain arrested in the meiotic prophase and entrapped in follicles surrounded by compact cumulus layers (23, 24). Thus, LH-induced secretion of granulosa cell EGF-Ls transactivates EGFR on cumulus cells to mediate both cumulus expansion and oocyte maturation. It is the physical separation of cumulus cells from the oocyte that impacts the meiosis regulatory machinery by preventing cAMP and cGMP transfer from granulosa cells to the oocyte. Specifically, LH-R activation of adenylate cyclase results in closure of gap junctions, possibly through phosphorylation of the connexin proteins (25) and the loss of direct transzonal communication that occurs with cumulus expansion. Consequently, cAMP and cGMP levels decline within the oocyte. The decreased cGMP releases the inhibition of phosphodiesterase 3A, leading to further cAMP degradation in the oocyte, release of meiosis-promoting factor activity, and resumption of meiosis (20).
Other genes downstream of ERK1/2 activation are essential, specifically for follicular rupture. Two transcription factors, CCAAT enhancer–binding protein (C/EBP)α/β, have been shown in KO mouse studies to be dependent on induction by Erk1/2 and highly important for ovulation (21). The combination of the CEBPaKO and granulosa-specific CEBPbKO leads to a complete failure to ovulate similar to the Erk1/2 KO (26), demonstrating that these two transcription factors together are key mediators of follicular rupture. Unlike the Erk1/2 double mutants, the CEBPa/gcbKO have some degree of cumulus expansion and normal oocyte maturation with oocytes progressing to metaphase II following hCG treatment (26). Thus, the main roles of C/EBPα/β appear to be in mediating ovulatory responses in the granulosa compartment rather than regulating ovulatory actions in the COC.
Progesterone receptor (PGR) is also acutely induced after the LH surge via protein kinase A/cAMP response element–binding protein-mediated transactivation and Erk1/2-dependent signaling (21). Once induced in mural granulosa cells by LH, PGR—a steroid receptor transcription factor—is immediately activated by the high local concentration of progesterone (P4), translocates to the nucleus, and initiates transcription of downstream targets critical for follicular rupture. Mice with a targeted deletion of the Pgr gene [PGR KO (PRKO)] exhibit normal follicle growth and luteinization but a complete and specific block in ovulation (27). The role of PGR is restricted to mediating follicular rupture, as activation of cumulus expansion and oocyte meiosis occurs normally, and oocytes extracted from PRKO follicles are able to be fertilized. The key role of PGR in follicular rupture is highly conserved across vertebrates, including rhesus monkeys (28, 29) and humans (30). Knockdown of PGR mRNA in the dominant preovulatory follicles of rhesus macaques also results in a complete anovulatory phenotype (28). Clinically, PGR antagonists, such as RU486 (mifepristone), ulipristal acetate, and valaprisan, can block ovulation and are effective contraceptives (31, 32). Although PGR is essential for follicular rupture, P4 influences oocyte maturation, independent of PGR, in some species. For instance, progesterone agonist treatment can promote oocyte maturation to metaphase II in rhesus macaque follicles, even in the absence of the LH surge (33).
Thus, the LH surge is the systemic endocrine signal that acts on ovarian granulosa cells to set in motion both the resumption of meiosis and follicular rupture. Although LH-R initiates multiple signaling pathways in mural granulosa cells to trigger massive follicular changes, LH-R activation of ERK1/2 is the key signal connecting ovulation and oocyte quality. ERK1/2, in turn, generates bifurcating signaling cascades (Fig. 1) that (1) initiate resumption of meiosis and oocyte maturation and (2) induce transcription factors that culminate in unique complexes, mediating expression of a suite of ovulatory genes. ERK1/2 regulation of oocyte maturation is, at least in part, via induction of EGF-Ls that are essential for cumulus expansion, changes in cGMP and cAMP, and oocyte meiosis resumption. In parallel, ERK1/2 activation controls ovulation via the induction of transcription factors C/EBPα/β and PGR, essential for the induction of effector genes involved in rupture and remodeling of the ovarian follicle. Whereas LH-induced transcription factors C/EBPs and PGR are critical for follicular remodeling and rupture, specifically by acting in granulosa cells, they do not appear to have roles in regulating oocyte quality or the resumption of meiosis.
Matrix Production and Proteolytic Remodeling Combine to Release a Meiotically Mature Oocyte
As the follicle responds to the LH surge, one of the most visually striking and important events is COC expansion. The COC mass expands many-fold in volume through the rapid induction of genes encoding structural proteins, proteoglycans, and glycosaminoglycan-synthesizing enzymes, resulting in formation of a highly specialized ECM enveloping the cumulus layers [reviewed in Russell and Robker (34)]. As cumulus cells are not directly responsive to the LH surge, COC expansion is activated via secondary paracrine signals, namely, the EGF-Ls from granulosa cells (22) and prostaglandins from granulosa and cumulus cells (30). Importantly, oocyte-derived factors are a required, permissive signal for cumulus gene expression, as detailed above, such that expansion cannot occur without the requisite oocyte-mediated specification of cumulus cells during follicular growth,
The expansion of the COC in periovulatory follicles involves the very rapid production of a suite of ECM proteins to form a unique and specialized matrix. Interestingly, this cumulus matrix is generated by combinatorial synthesis and intercalation of cumulus-, serum-, and granulosa-derived proteins [reviewed in Brown et al. (35)]. The backbone of the expanded cumulus matrix is hyaluronan (HA), a large disaccharide chain common to many ECMs. HA synthase 2 (HAS2), an enzyme induced in cumulus cells by EGF-L, FSH, and prostaglandins, assembles the long disaccharide HA chains (up to megadaltons) and extrudes it into the extracellular space. Simultaneously, inter-α trypsin inhibitor (IαI), a circulating protein complex secreted by the liver, enters the follicle, as a result of heightened vascular permeability, and forms covalent bonds with HA. TNF-α–induced protein 6 (TNFAIP6), secreted by cumulus cells, binds HA through a link domain and helps catalyze IαI-HA bond formation. Also secreted by cumulus cells, pentraxin 3 forms a decameric complex, interacting with TNFAIP6 molecules to crosslink and stabilize the HA matrix. Mural granulosa cells responding to the LH surge secrete versican (36), a large, aggregating proteoglycan with chondroitin-sulfate side chains that becomes incorporated into the cumulus matrix through HA-binding link domains (36). Concurrent with their rapid production of surrounding ECM, the cumulus cells themselves adopt a striking increase in adhesive capacity, as well as migratory and invasive activity (37).
Animal models of cumulus matrix disruption frequently show impaired ovulation, demonstrating that COC expansion is a critical ovulatory event. For instance, treatment of mice with inhibitors of the HA synthetic pathway (38) or short HA oligosaccharides that adsorb TNFAIP6 HA-binding activity (39) blocks COC expansion and ovulation. Loss of either TNFAIP6 or IαI synthesis results in almost identical phenotypes, wherein the HA produced by cumulus cells is not stabilized, and the COC fails to undergo expansion in vitro or in vivo (40). Disruption of IαI binding to HA results in ∼50% reduced ovulation, as well as a deficiency in fertilization capacity (41). Tnfaip6 null mutant mice (40) and one of two lines of Ptx3 null mice (42) also exhibit significantly impaired ovulation. Thus, alterations to any of a number of matrix components disrupts the structural integrity of the cumulus matrix and blocks ovulation, demonstrating that cumulus expansion is essential for oocyte release.
Prostaglandins are another major class of intrafollicular signaling molecules critical for ovulation, at least in part, through their influence on cumulus expansion (43). The enzyme that catalyzes production of prostaglandin precursors from membrane lipids, prostaglandin synthase 2 (Ptgs2; also known as cyclooxygenase 2), is strongly and rapidly induced in granulosa cells after the LH surge in most mammalian species, from rodents to rhesus macaques (44) and humans (30). Deletion of the Ptgs2 gene in mice results in defective cumulus expansion, reduced ovulation, and female infertility (45). Likewise, nonsteroidal anti-inflammatory drugs, such as indomethacin, which inhibit cyclooxygenase 2 activity, also inhibit ovulation, leading to the development of products using high-dose nonsteroidal anti-inflammatory drugs as emergency contraceptives (46, 47). The predominant prostaglandin produced by granulosa cells is prostaglandin E2 (PGE2), which interacts with the type 2 PGE2 receptor (EP2) to induce the expression of cumulus genes critical for ovulation (48), such as TNFAIP6, in mice, primates, and humans (45–47). EP2 null mice also have reduced ovulation and disrupted cumulus gene expression (49) but less severely disrupted fertility than Ptgs2 nulls, which produce no prostaglandins, indicating that PGE-EP2 signaling is important, but other prostaglandins also mediate substantial effects. In primates and humans, PGE2 and the type 1 PGE2 receptor and EP2 receptors are implicated as the most important and have been shown to mediate aspects of follicular rupture and angiogenesis (50). Drug classes that block the interaction of PGE with its receptors have also been investigated in mouse and primate models with similar signs of potential ovulation blocking efficacy (51, 52).
Interestingly, these same cumulus expansion processes that are essential for ovulation also influence oocyte maturation. For instance, the proteoglycan versican possesses two EGF-like domains that stimulate COC activation through EGFR signaling (53). The importance of cumulus gene expression for health of the oocyte is further demonstrated by a range of studies showing that the expression level of key cumulus genes is directly associated with the developmental outcomes for that oocyte. Genes for which expression is positively correlated with high-quality oocyte outcomes, i.e., pregnancy and live birth in humans, include Has2, Tnfaip6, Ptx3, Vcan, and several others (35, 54–58). Reduced cumulus matrix gene expression associated with impaired developmental competence could be a reflection of poor oocyte signals specifying cumulus responsiveness or suboptimal granulosa responses to the LH surge. Regardless, reduced cumulus gene expression is considered a key marker for oocyte quality.
In addition to the dramatic changes in the COC at ovulation, the entire follicle undergoes profound remodeling, including proteolytic degradation, inflammation, and angiogenesis. Proteolytic enzymes are thought to be responsible for the degradation of the follicle wall at ovulation, as broad-spectrum matrix metalloproteinase inhibitors reduce ovulation when administered into the ovarian bursa (59) or to in vitro-perfused rat ovaries (60) or injected into primate ovaries (61). Although it is likely that redundant actions of several proteases with overlapping substrate specificity are coordinately involved in matrix degradation [reviewed Russell and Robker (34)], a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS-1) is a metalloprotease that has emerged to play an important individual role in mediating ovulation (62). Adamts-1 gene expression is induced by PGR in periovulatory follicles of rodents (27), monkeys (61), and humans (63), and this is likely one of the mechanisms by which PGR controls ovulation. The proenzyme of ADAMTS-1 is synthesized by the mural granulosa cells, yet the secreted, active form is selectively localized to the matrix and cumulus cells of the expanded COC (62). Versican is a target substrate of the protease ADAMTS-1, and mice lacking ADAMTS-1 have reduced versican cleavage in their COCs (62). Furthermore, Adamts-1 null mutant mice have highly reduced ovulation rates, and oocytes remain trapped within luteinized follicles (64, 65), suggesting that versican cleavage may be necessary to release the COC from the follicle. ADAMTS-1 may also influence oocyte quality, as in humans, cumulus and granulosa cell expression of Adamts-1 is associated with oocyte fertilization capacity (66). Supporting a role in oocyte quality, Adamts-1 null mice have increased oocyte dysmorphogenesis, dramatically reduced rates of fertilization, and increased incidence of aberrant zygotes (65). How Adamts-1 might influence oocyte developmental competence is not clear, but this could be via its proteolytic remodeling of the cumulus matrix or direct actions of its EGF-like protein domains. Interestingly, the EGF-L factors are synthesized in granulosa cells as integral membrane proteins, and proteolytic cleavage of these precursor forms is required to permit interaction with their cognate receptors on cumulus cells (23). Physiologically, members of the matrix metalloproteinase, ADAM, and ADAMTS protease families are likely to be the key processing enzymes that regulate this event.
As ADAMTS-1 null mice do not have complete anovulatory infertility, whereas PRKOs do, PGR must have additional roles in the ovulatory cascade, in addition to the induction of Adamts-1. For instance, PGR induces other downstream transcription factors that are critical effectors of ovulation. Peroxisome proliferator–activated receptor (PPAR)γ and hypoxia-inducible factor (HIF)1a, HIF1b, and HIF2a were identified as PGR-regulated genes via microarray (67). PPARγ is induced in mouse granulosa cells in response to hCG but not in PRKO mice (67). When PPARγ expression was prevented by deleting the gene in periovulatory granulosa cells, ovulation was reduced by almost 80%, as were downstream target genes of PPARγ, including Et-2, cGKII, and IL-6 (all previously identified as downstream of PGR regulation) but not Adamts-1 (67). Likewise, the HIF transcription factors are induced in granulosa cells in response to the LH surge but not in PRKO mice (68). The treatment of mice with an HIF inhibitor echinomycin, concurrent with hCG, dramatically inhibited ovulation (69), indicating HIF transcription factors are essential mediators of ovulation. HIF target genes, again known to be downstream of PGR, include endothelin 2 (Edn2), Vegfa, and Cxcr4. These genes, in particular, may mediate vascular endothelial and neoangiogenic changes that contribute to ovulation.
Angiogenesis in the vasculature surrounding the follicle is essential for ovulation and is primarily regulated through induction of vascular endothelial growth factor (VEGF) expression in response to the LH surge in granulosa cells of most species. The blocking of the action of VEGF and hence, angiogenesis causes failure of follicle-wall remodeling and ovulation and entrapment of oocytes in unruptured and poorly luteinized follicles (50). Thus, VEGF-mediated angiogenic remodeling of the follicle wall is necessary for efficient follicle rupture, including in primates. Furthermore, recent studies show that vasoconstriction is required for ovulation, as the ovulatory follicles of mice lacking vascular smooth muscle cells fail to rupture in response to an ovulatory stimulus (70). Smooth muscle activating endothelins may be an important initiator of the follicular smooth muscle contractions (and ovulation), as contractions in rat ovarian tissue strips can be triggered by treatment with endothelin 2 (Edn2), and endothelin antagonists block ovulation (71). A null mutation of Edn2 in mice causes a substantial reduction in the numbers of oocytes released into the oviducts, indicating that the smooth muscle contraction or other actions of endothelins are important mediators of ovulation (71). Expression of endothelins and the presence of smooth muscle within the theca layer have been documented in human ovarian follicles (72). Edn2 is strongly induced in granulosa cells in the final stages of the periovulatory period and dependent on the intrafollicular induction of PGR (73). Echinomycin inhibition of HIF reduces Edn2 induction and prevents vasoconstriction in the follicle wall and thinning of the follicular apex structure, both of which are reversible with exogenous Edn2 (70), indicating that regulation of smooth muscle vascular events may be the mechanism by which HIFs regulate ovulation.
Thus, C/EBPα/β and PGR are required to induce several critical ovulatory genes, such as HIFs, PPARγ, Adamts-1, Edn2, Vegfa, and Ptgs2, which in turn contribute to follicular rupture, likely via induction of ECM remodeling, vasoconstriction, angiogenesis, and smooth muscle contraction. Likewise, PGR regulates aspects of inflammation at ovulation (74)—namely, cytokine production and immune-cell infiltration, which characterize follicular rupture. Interestingly, the depletion of dendritic leukocytes, either systemically or locally in the ovary, dramatically reduced hCG-induced ovulation rates (75). Detailed assessment of ovarian function showed that cumulus expansion was poor and ovulatory gene expression reduced; however vascular permeability was also impaired and Vegfc expression reduced (75). This suggests that dendritic cells influence endothelial cell and vascular basement membrane permeability/function and the subsequent availability of serum components to stabilize the expanded cumulus matrix. Importantly, the follicular processes of vasoconstriction, angiogenesis, inflammation, and smooth muscle contraction at ovulation appear to be specific for follicular rupture and have not been associated with oocyte meiotic maturation or acquisition of developmental competence.
Future Directions
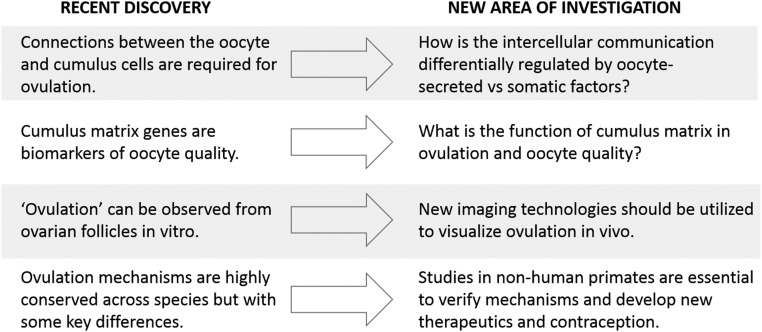
The coordinated induction of multiple cell physiological processes in the ovary tightly links ovulation with the acquisition of oocyte quality. Both processes are triggered by the master endocrine factor, LH, acting on mural granulosa cells to induce an array of effector proteins derived from granulosa cells, cumulus cells, and the circulation, enabling the release of a fertilizable oocyte. Ovulation is a rapid, tightly regulated, highly conserved, and essential biological process, yet, there are a number of outstanding questions that remain to be answered about how it is controlled (Fig. 2). For one, ovulation is exceedingly difficult to observe in vivo and, although some exciting in vitro ovulation methods have recently been developed (11, 76), they have their limitations. Creative use of newer, advanced imaging systems and biological labels should be able to capture more details about the physiological events occurring throughout the follicle in the exact moment of oocyte release.
Figure 2.
Recent advances provide new insights into the biological mechanisms linking ovulation and oocyte quality but also lead to new areas of investigation. Novel modes of communication between oocytes and somatic cells are emerging, yet their regulation by mechanisms intrinsic to the oocyte vs external maternal factors needs to be dissected. Cumulus expansion is essential for ovulation, and cumulus matrix genes are consistently identified as biomarkers of oocyte developmental competence; yet the function of this unique matrix is not understood. Ovulation can be observed in vitro using a few specialized culture systems, yet visualization in vivo would provide a more holistic view of follicular events, such as vascularization and inflammation. The discovery of leukemia inhibitory factor as a mediator of ovulation in primates highlights that there are some differences with mouse models, which likely also include the bioactivity of oocyte-secreted factors.
Intrafollicular induction of EGF-Ls, C/EBPα/β, and PGR transcription factors and inflammatory prostaglandins are pivotal mediators of the required cell-tissue remodeling changes. Cumulus expansion is essential for ovulation, yet exactly how this extensive ECM drives ovulation is not clear. Proteolytic activity is essential for follicular rupture, to cleave and thus activate the EGF-Ls as well as to degrade structural ECM proteins; however, the identity of all of the essential proteases is still emerging. Interestingly, muscle contraction is emerging as a possible additional essential mechanism for the expanded oocyte complex to be released from the apex of the follicle. The verification of the importance of this mechanism, as well as other molecular mediators, in primates is essential to develop new strategies to manipulate ovulation clinically. Recent findings indicating primate-specific regulators of ovulation (77) highlight the need for further work in this area.
Importantly, a dialogue between the oocyte and its surrounding somatic cells synchronizes the acquisition of oocyte developmental competence with its release from the follicle. New details are emerging, but there is still much to learn about the structural intricacies of this bidirectional communication and the diversity of messengers. This dialogue results in oocyte specification of the cumulus cell phenotype, which permits later cumulus cell–specific production of matrix proteins. These integrate with matrix proteins from granulosa and serum to achieve cumulus expansion, thereby interrupting direct oocyte–cumulus cell communication and allowing resumption of meiosis. With the specification of cumulus cell responses at ovulation, the oocyte is able, at least partly, to control its own release, potentially ensuring that ovulated oocytes are those with high developmental competence. Thus, ovulation is a complex process with multiple physiological systems contributing to ensure the release of an oocyte with the highest developmental potential at the right time for fertilization and the establishment of pregnancy.
Acknowledgments
Financial Support: R.L.R. and D.L.R. are funded by National Health and Medical Research Council Senior Research Fellowships. Funding for J.D.H. was provided by National Institutes of Health Grants HD020869 and OD011092.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- ADAMTS-1
a disintegrin and metalloproteinase with thrombospondin motifs
- BMP15
bone morphogenetic protein 15
- C/EBP
CCAAT enhancer–binding protein
- cGMP
cyclic GMP
- COC
cumulus oocyte complex
- DCAF1
DNA damage–binding protein 1 and CUL4-associated factor 1
- DDB1
DNA damage–binding protein 1
- ECM
extracellular matrix
- Edn2
endothelin 2
- EGF
epidermal growth factor
- EGF-L
epidermal growth factor–like ligands
- EGFR
epidermal growth factor receptor
- EP2
type 2 prostaglandin E2 receptor
- GDF9
growth and differentiation factor 9
- HA
hyaluronan
- HAS2
hyaluronan synthase 2
- hCG
human chorionic gonadotropin
- HIF
hypoxia-inducible factor
- IαI
inter-α trypsin inhibitor
- KO
knockout
- LH-R
LH receptor
- PGE2
prostaglandin E2
- PGR
progesterone receptor
- PPAR
peroxisome proliferator–activated receptor
- PRKO
progesterone receptor knockout
- TNFAIP6
TNF-α–induced protein 6
- VEGF
vascular endothelial growth factor
References
- 1. Zhang A, Xu B, Sun Y, Lu X, Niu Z, Chen Q, Feng Y, Xu C. The effect of human cumulus cells on the maturation and developmental potential of immature oocytes in ICSI cycles. J Assist Reprod Genet. 2012;29(4):313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Eppig JJ, Wigglesworth K, Pendola F, Hirao Y. Murine oocytes suppress expression of luteinizing hormone receptor messenger ribonucleic acid by granulosa cells. Biol Reprod. 1997;56(4):976–984. [DOI] [PubMed] [Google Scholar]
- 3. Sugiura K, Su YQ, Diaz FJ, Pangas SA, Sharma S, Wigglesworth K, O’Brien MJ, Matzuk MM, Shimasaki S, Eppig JJ. Oocyte-derived BMP15 and FGFs cooperate to promote glycolysis in cumulus cells. Development. 2007;134(14):2593–2603. [DOI] [PubMed] [Google Scholar]
- 4. Su YQ, Sugiura K, Wigglesworth K, O’Brien MJ, Affourtit JP, Pangas SA, Matzuk MM, Eppig JJ. Oocyte regulation of metabolic cooperativity between mouse cumulus cells and oocytes: BMP15 and GDF9 control cholesterol biosynthesis in cumulus cells. Development. 2008;135(1):111–121. [DOI] [PubMed] [Google Scholar]
- 5. Peng J, Li Q, Wigglesworth K, Rangarajan A, Kattamuri C, Peterson RT, Eppig JJ, Thompson TB, Matzuk MM. Growth differentiation factor 9:bone morphogenetic protein 15 heterodimers are potent regulators of ovarian functions. Proc Natl Acad Sci USA. 2013;110(8):E776–E785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Watson LN, Mottershead DG, Dunning KR, Robker RL, Gilchrist RB, Russell DL. Heparan sulfate proteoglycans regulate responses to oocyte paracrine signals in ovarian follicle morphogenesis. Endocrinology. 2012;153(9):4544–4555. [DOI] [PubMed] [Google Scholar]
- 7. Emori C, Wigglesworth K, Fujii W, Naito K, Eppig JJ, Sugiura K. Cooperative effects of 17β-estradiol and oocyte-derived paracrine factors on the transcriptome of mouse cumulus cells. Endocrinology. 2013;154(12):4859–4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peng J, Wigglesworth K, Rangarajan A, Eppig JJ, Thompson TB, Matzuk MM. Amino acid 72 of mouse and human GDF9 mature domain is responsible for altered homodimer bioactivities but has subtle effects on GDF9:BMP15 heterodimer activities. Biol Reprod. 2014;91(6):142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Crawford JL, McNatty KP. The ratio of growth differentiation factor 9: bone morphogenetic protein 15 mRNA expression is tightly co-regulated and differs between species over a wide range of ovulation rates. Mol Cell Endocrinol. 2012;348(1):339–343. [DOI] [PubMed] [Google Scholar]
- 10. El-Hayek S, Yang Q, Abbassi L, FitzHarris G, Clarke HJ. Mammalian oocytes locally remodel follicular architecture to provide the foundation for germline-soma communication. Curr Biol. 2018;28(7):1124–1131.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Komatsu K, Masubuchi S. Mouse oocytes connect with granulosa cells by fusing with cell membranes and form a large complex during follicle development [published ahead of print 2018 March 24]. Biol Reprod. doi: 10.1093/biolre/ioy072. [DOI] [PMC free article] [PubMed]
- 12. Sela-Abramovich S, Edry I, Galiani D, Nevo N, Dekel N. Disruption of gap junctional communication within the ovarian follicle induces oocyte maturation. Endocrinology. 2006;147(5):2280–2286. [DOI] [PubMed] [Google Scholar]
- 13. Li TY, Colley D, Barr KJ, Yee SP, Kidder GM. Rescue of oogenesis in Cx37-null mutant mice by oocyte-specific replacement with Cx43. J Cell Sci. 2007;120(Pt 23):4117–4125. [DOI] [PubMed] [Google Scholar]
- 14. Johnson MT, Freeman EA, Gardner DK, Hunt PA. Oxidative metabolism of pyruvate is required for meiotic maturation of murine oocytes in vivo. Biol Reprod. 2007;77(1):2–8. [DOI] [PubMed] [Google Scholar]
- 15. Grieco D, Porcellini A, Avvedimento EV, Gottesman ME. Requirement for cAMP-PKA pathway activation by M phase-promoting factor in the transition from mitosis to interphase. Science. 1996;271(5256):1718–1723. [DOI] [PubMed] [Google Scholar]
- 16. Shuhaibar LC, Egbert JR, Norris RP, Lampe PD, Nikolaev VO, Thunemann M, Wen L, Feil R, Jaffe LA. Intercellular signaling via cyclic GMP diffusion through gap junctions restarts meiosis in mouse ovarian follicles. Proc Natl Acad Sci USA. 2015;112(17):5527–5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Simon AM, Goodenough DA, Li E, Paul DL. Female infertility in mice lacking connexin 37. Nature. 1997;385(6616):525–529. [DOI] [PubMed] [Google Scholar]
- 18. Yu C, Xu YW, Sha QQ, Fan HY. CRL4DCAF1 is required in activated oocytes for follicle maintenance and ovulation. Mol Hum Reprod. 2015;21(2):195–205. [DOI] [PubMed] [Google Scholar]
- 19. Andreu-Vieyra CV, Chen R, Agno JE, Glaser S, Anastassiadis K, Stewart AF, Matzuk MM. MLL2 is required in oocytes for bulk histone 3 lysine 4 trimethylation and transcriptional silencing. PLoS Biol. 2010;8(8):e1000453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Richards JS, Ascoli M. Endocrine, paracrine, and autocrine signaling pathways that regulate ovulation. Trends Endocrinol Metab. 2018;29(5):313–325. [DOI] [PubMed] [Google Scholar]
- 21. Fan HY, Liu Z, Shimada M, Sterneck E, Johnson PF, Hedrick SM, Richards JS. MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science. 2009;324(5929):938–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004;303(5658):682–684. [DOI] [PubMed] [Google Scholar]
- 23. Ashkenazi H, Cao X, Motola S, Popliker M, Conti M, Tsafriri A. Epidermal growth factor family members: endogenous mediators of the ovulatory response. Endocrinology. 2005;146(1):77–84. [DOI] [PubMed] [Google Scholar]
- 24. Hsieh M, Thao K, Conti M. Genetic dissection of epidermal growth factor receptor signaling during luteinizing hormone-induced oocyte maturation. PLoS One. 2011;6(6):e21574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Norris RP, Freudzon M, Nikolaev VO, Jaffe LA. Epidermal growth factor receptor kinase activity is required for gap junction closure and for part of the decrease in ovarian follicle cGMP in response to LH. Reproduction. 2010;140(5):655–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fan HY, Liu Z, Johnson PF, Richards JS. CCAAT/enhancer-binding proteins (C/EBP)-α and -β are essential for ovulation, luteinization, and the expression of key target genes. Mol Endocrinol. 2011;25(2):253–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Robker RL, Russell DL, Espey LL, Lydon JP, O’Malley BW, Richards JS. Progesterone-regulated genes in the ovulation process: ADAMTS-1 and cathepsin L proteases. Proc Natl Acad Sci USA. 2000;97(9):4689–4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bishop CV, Hennebold JD, Kahl CA, Stouffer RL. Knockdown of progesterone receptor (PGR) in macaque granulosa cells disrupts ovulation and progesterone production. Biol Reprod. 2016;94(5):109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stouffer RL. Pre-ovulatory events in the rhesus monkey follicle during ovulation induction. Reprod Biomed Online. 2002;4(Suppl 3):1–4. [DOI] [PubMed] [Google Scholar]
- 30. Choi Y, Park JY, Wilson K, Rosewell KL, Brännström M, Akin JW, Curry TE Jr, Jo M. The expression of CXCR4 is induced by the luteinizing hormone surge and mediated by progesterone receptors in human preovulatory granulosa cells. Biol Reprod. 2017;96(6):1256–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jesam C, Cochon L, Salvatierra AM, Williams A, Kapp N, Levy-Gompel D, Brache V. A prospective, open-label, multicenter study to assess the pharmacodynamics and safety of repeated use of 30 mg ulipristal acetate. Contraception. 2016;93(4):310–316. [DOI] [PubMed] [Google Scholar]
- 32. Schütt B, Schultze-Mosgau MH, Draeger C, Chang X, Löwen S, Kaiser A, Rohde B. Effect of the novel selective progesterone receptor modulator vilaprisan on ovarian activity in healthy women. J Clin Pharmacol. 2018;58(2):228–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Borman SM, Chaffin CL, Schwinof KM, Stouffer RL, Zelinski-Wooten MB. Progesterone promotes oocyte maturation, but not ovulation, in nonhuman primate follicles without a gonadotropin surge. Biol Reprod. 2004;71(1):366–373. [DOI] [PubMed] [Google Scholar]
- 34. Russell DL, Robker RL. Molecular mechanisms of ovulation: co-ordination through the cumulus complex. Hum Reprod Update. 2007;13(3):289–312. [DOI] [PubMed] [Google Scholar]
- 35. Brown HM, Dunning KR, Sutton-McDowall M, Gilchrist RB, Thompson JG, Russell DL. Failure to launch: aberrant cumulus gene expression during oocyte in vitro maturation. Reproduction. 2017;153(3):R109–R120. [DOI] [PubMed] [Google Scholar]
- 36. Russell DL, Ochsner SA, Hsieh M, Mulders S, Richards JS. Hormone-regulated expression and localization of versican in the rodent ovary. Endocrinology. 2003;144(3):1020–1031. [DOI] [PubMed] [Google Scholar]
- 37. Akison LK, Alvino ER, Dunning KR, Robker RL, Russell DL. Transient invasive migration in mouse cumulus oocyte complexes induced at ovulation by luteinizing hormone. Biol Reprod. 2012;86(4):125. [DOI] [PubMed] [Google Scholar]
- 38. Chen L, Russell PT, Larsen WJ. Functional significance of cumulus expansion in the mouse: roles for the preovulatory synthesis of hyaluronic acid within the cumulus mass. Mol Reprod Dev. 1993;34(1):87–93. [DOI] [PubMed] [Google Scholar]
- 39. Hess KA, Chen L, Larsen WJ. Inter-alpha-inhibitor binding to hyaluronan in the cumulus extracellular matrix is required for optimal ovulation and development of mouse oocytes. Biol Reprod. 1999;61(2):436–443. [DOI] [PubMed] [Google Scholar]
- 40. Fülöp C, Szántó S, Mukhopadhyay D, Bárdos T, Kamath RV, Rugg MS, Day AJ, Salustri A, Hascall VC, Glant TT, Mikecz K. Impaired cumulus mucification and female sterility in tumor necrosis factor-induced protein-6 deficient mice. Development. 2003;130(10):2253–2261. [DOI] [PubMed] [Google Scholar]
- 41.Zhuo L, Salustri A, Atsumi F, Kawano M, Wu J, Shen L, Ogura A, Yasue H, Hascall VC, Kimata K. Role of serum-derived hyaluronan-associated protein in the construction of cumulus matrix and oocyte maturation. In: Balazs EA, Hascall VC, eds. Hyaluronan: Structure, Metabolism, Biological Activities, Therapeutic Applications. Edgewater, NJ: MBI Press; 2005:731–735.
- 42. Varani S, Elvin JA, Yan C, DeMayo J, DeMayo FJ, Horton HF, Byrne MC, Matzuk MM. Knockout of pentraxin 3, a downstream target of growth differentiation factor-9, causes female subfertility. Mol Endocrinol. 2002;16(6):1154–1167. [DOI] [PubMed] [Google Scholar]
- 43. Sirois J, Sayasith K, Brown KA, Stock AE, Bouchard N, Doré M. Cyclooxygenase-2 and its role in ovulation: a 2004 account. Hum Reprod Update. 2004;10(5):373–385. [DOI] [PubMed] [Google Scholar]
- 44. Stouffer RL, Xu F, Duffy DM. Molecular control of ovulation and luteinization in the primate follicle. Front Biosci. 2007;12:297–307. [DOI] [PubMed] [Google Scholar]
- 45. Ochsner SA, Russell DL, Day AJ, Breyer RM, Richards JS. Decreased expression of tumor necrosis factor-alpha-stimulated gene 6 in cumulus cells of the cyclooxygenase-2 and EP2 null mice. Endocrinology. 2003;144(3):1008–1019. [DOI] [PubMed] [Google Scholar]
- 46. Jesam C, Salvatierra AM, Schwartz JL, Fuentes A, Croxatto HB. Effect of oral administration of a continuous 18 day regimen of meloxicam on ovulation: experience of a randomized controlled trial. Contraception. 2014;90(2):168–173. [DOI] [PubMed] [Google Scholar]
- 47. McCann NC, Lynch TJ, Kim SO, Duffy DM. The COX-2 inhibitor meloxicam prevents pregnancy when administered as an emergency contraceptive to nonhuman primates. Contraception. 2013;88(6):744–748. [DOI] [PubMed] [Google Scholar]
- 48. Segi E, Haraguchi K, Sugimoto Y, Tsuji M, Tsunekawa H, Tamba S, Tsuboi K, Tanaka S, Ichikawa A. Expression of messenger RNA for prostaglandin E receptor subtypes EP4/EP2 and cyclooxygenase isozymes in mouse periovulatory follicles and oviducts during superovulation. Biol Reprod. 2003;68(3):804–811. [DOI] [PubMed] [Google Scholar]
- 49. Tamba S, Yodoi R, Morimoto K, Inazumi T, Sukeno M, Segi-Nishida E, Okuno Y, Tsujimoto G, Narumiya S, Sugimoto Y. Expression profiling of cumulus cells reveals functional changes during ovulation and central roles of prostaglandin EP2 receptor in cAMP signaling. Biochimie. 2010;92(6):665–675. [DOI] [PubMed] [Google Scholar]
- 50. Trau HA, Brännström M, Curry TE Jr, Duffy DM. Prostaglandin E2 and vascular endothelial growth factor A mediate angiogenesis of human ovarian follicular endothelial cells. Hum Reprod. 2016;31(2):436–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Duffy DM. Novel contraceptive targets to inhibit ovulation: the prostaglandin E2 pathway. Hum Reprod Update. 2015;21(5):652–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Peluffo MC, Stanley J, Braeuer N, Rotgeri A, Fritzemeier KH, Fuhrmann U, Buchmann B, Adevai T, Murphy MJ, Zelinski MB, Lindenthal B, Hennebold JD, Stouffer RL. A prostaglandin E2 receptor antagonist prevents pregnancies during a preclinical contraceptive trial with female macaques. Hum Reprod. 2014;29(7):1400–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dunning KR, Watson LN, Zhang VJ, Brown HM, Kaczmarek AK, Robker RL, Russell DL. Activation of mouse cumulus-oocyte complex maturation in vitro through EGF-like activity of versican. Biol Reprod. 2015;92(5):116. [DOI] [PubMed] [Google Scholar]
- 54. McKenzie LJ, Pangas SA, Carson SA, Kovanci E, Cisneros P, Buster JE, Amato P, Matzuk MM. Human cumulus granulosa cell gene expression: a predictor of fertilization and embryo selection in women undergoing IVF. Hum Reprod. 2004;19(12):2869–2874. [DOI] [PubMed] [Google Scholar]
- 55. Gebhardt KM, Feil DK, Dunning KR, Lane M, Russell DL. Human cumulus cell gene expression as a biomarker of pregnancy outcome after single embryo transfer. Fertil Steril. 2011;96(1):47–52.e2. [DOI] [PubMed] [Google Scholar]
- 56. Wathlet S, Adriaenssens T, Segers I, Verheyen G, Janssens R, Coucke W, Devroey P, Smitz J. New candidate genes to predict pregnancy outcome in single embryo transfer cycles when using cumulus cell gene expression. Fertil Steril. 2012;98(2):432–439.e1-4. [DOI] [PubMed] [Google Scholar]
- 57. Anderson RA, Sciorio R, Kinnell H, Bayne RA, Thong KJ, de Sousa PA, Pickering S. Cumulus gene expression as a predictor of human oocyte fertilisation, embryo development and competence to establish a pregnancy. Reproduction. 2009;138(4):629–637. [DOI] [PubMed] [Google Scholar]
- 58. Assou S, Haouzi D, Mahmoud K, Aouacheria A, Guillemin Y, Pantesco V, Rème T, Dechaud H, De Vos J, Hamamah S. A non-invasive test for assessing embryo potential by gene expression profiles of human cumulus cells: a proof of concept study. Mol Hum Reprod. 2008;14(12):711–719. [DOI] [PubMed] [Google Scholar]
- 59. Reich R, Tsafriri A, Mechanic GL. The involvement of collagenolysis in ovulation in the rat. Endocrinology. 1985;116(2):522–527. [DOI] [PubMed] [Google Scholar]
- 60. Butler TA, Zhu C, Mueller RA, Fuller GC, Lemaire WJ, Woessner JF Jr. Inhibition of ovulation in the perfused rat ovary by the synthetic collagenase inhibitor SC 44463. Biol Reprod. 1991;44(6):1183–1188. [DOI] [PubMed] [Google Scholar]
- 61. Peluffo MC, Murphy MJ, Baughman ST, Stouffer RL, Hennebold JD. Systematic analysis of protease gene expression in the rhesus macaque ovulatory follicle: metalloproteinase involvement in follicle rupture. Endocrinology. 2011;152(10):3963–3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Russell DL, Doyle KM, Ochsner SA, Sandy JD, Richards JS. Processing and localization of ADAMTS-1 and proteolytic cleavage of versican during cumulus matrix expansion and ovulation. J Biol Chem. 2003;278(43):42330–42339. [DOI] [PubMed] [Google Scholar]
- 63. Rosewell KL, Al-Alem L, Zakerkish F, McCord L, Akin JW, Chaffin CL, Brännström M, Curry TE Jr. Induction of proteinases in the human preovulatory follicle of the menstrual cycle by human chorionic gonadotropin. Fertil Steril. 2015;103(3):826–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mittaz L, Russell DL, Wilson T, Brasted M, Tkalcevic J, Salamonsen LA, Hertzog PJ, Pritchard MA. Adamts-1 is essential for the development and function of the urogenital system. Biol Reprod. 2004;70(4):1096–1105. [DOI] [PubMed] [Google Scholar]
- 65. Brown HM, Dunning KR, Robker RL, Boerboom D, Pritchard M, Lane M, Russell DL. ADAMTS1 cleavage of versican mediates essential structural remodeling of the ovarian follicle and cumulus-oocyte matrix during ovulation in mice. Biol Reprod. 2010;83(4):549–557. [DOI] [PubMed] [Google Scholar]
- 66. Xiao S, Li Y, Li T, Chen M, Xu Y, Wen Y, Zhou C. Evidence for decreased expression of ADAMTS-1 associated with impaired oocyte quality in PCOS patients. J Clin Endocrinol Metab. 2014;99(6):E1015–E1021. [DOI] [PubMed] [Google Scholar]
- 67. Kim J, Sato M, Li Q, Lydon JP, Demayo FJ, Bagchi IC, Bagchi MK. Peroxisome proliferator-activated receptor gamma is a target of progesterone regulation in the preovulatory follicles and controls ovulation in mice. Mol Cell Biol. 2008;28(5):1770–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kim J, Bagchi IC, Bagchi MK. Signaling by hypoxia-inducible factors is critical for ovulation in mice. Endocrinology. 2009;150(7):3392–3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kim J, Bagchi IC, Bagchi MK. Control of ovulation in mice by progesterone receptor-regulated gene networks. Mol Hum Reprod. 2009;15(12):821–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Migone FF, Cowan RG, Williams RM, Gorse KJ, Zipfel WR, Quirk SM. In vivo imaging reveals an essential role of vasoconstriction in rupture of the ovarian follicle at ovulation. Proc Natl Acad Sci USA. 2016;113(8):2294–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cacioppo JA, Lin PP, Hannon PR, McDougle DR, Gal A, Ko C. Granulosa cell endothelin-2 expression is fundamental for ovulatory follicle rupture. Sci Rep. 2017;7(1):817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Choi DH, Kim EK, Kim KH, Lee KA, Kang DW, Kim HY, Bridges P, Ko C. Expression pattern of endothelin system components and localization of smooth muscle cells in the human pre-ovulatory follicle. Hum Reprod. 2011;26(5):1171–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Palanisamy GS, Cheon YP, Kim J, Kannan A, Li Q, Sato M, Mantena SR, Sitruk-Ware RL, Bagchi MK, Bagchi IC. A novel pathway involving progesterone receptor, endothelin-2, and endothelin receptor B controls ovulation in mice. Mol Endocrinol. 2006;20(11):2784–2795. [DOI] [PubMed] [Google Scholar]
- 74. Akison LK, Robertson SA, Gonzalez MB, Richards JS, Smith CW, Russell DL, Robker RL. Regulation of the ovarian inflammatory response at ovulation by nuclear progesterone receptor. Am J Reprod Immunol. 2018;79(6):e12835. [DOI] [PubMed] [Google Scholar]
- 75. Cohen-Fredarow A, Tadmor A, Raz T, Meterani N, Addadi Y, Nevo N, Solomonov I, Sagi I, Mor G, Neeman M, Dekel N. Ovarian dendritic cells act as a double-edged pro-ovulatory and anti-inflammatory sword. Mol Endocrinol. 2014;28(7):1039–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Skory RM, Xu Y, Shea LD, Woodruff TK. Engineering the ovarian cycle using in vitro follicle culture. Hum Reprod. 2015;30(6):1386–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Murphy MJ, Halow NG, Royer PA, Hennebold JD. Leukemia inhibitory factor is necessary for ovulation in female rhesus macaques. Endocrinology. 2016;157(11):4378–4387. [DOI] [PMC free article] [PubMed] [Google Scholar]