Abstract
Context
Impaired maternal lipid metabolism in gestational diabetes mellitus (GDM) has detrimental effects on maternal health and fetal growth. We previously reported the excessive expression of adrenomedullin (ADM) and its receptors in GDM adipose tissues compared with normal glucose-tolerant pregnancies. In the present study, we determined the mechanisms underlying enhanced expression of ADM and its receptors.
Design
Omental adipose tissue (OAT) samples were collected from women during cesarian section of term pregnancy with nonoverweight (NOW; n = 9), overweight (OW; n = 8), obese (OBS; n = 10), and GDM (n = 10) status.
Results
The expression of ADM and its receptors was greater in OATs from GDM than from women who were NOW, OW, and OBS. The expression of adipokines, leptin, and resistin were significantly increased, but adiponectin was decreased in OATs from patients with GDM compared with those without GDM. Macrophage infiltration and TNF-α expression were greater in OAT from pregnant women with GDM than in pregnant women without GDM. Furthermore, TNF-α dose dependently increased mRNA for ADM and its receptor components calcitonin receptor-like receptor and receptor activity-modifying proteins 2 and 3 in OAT explants from women who were NOW. Human adipocytes treated with ADM significantly increased glycerol release in culture medium, and the increases of glycerol in culture medium of OAT from women with GDM were attenuated by ADM antagonists, ADM22-52.
Conclusions
Increased macrophage infiltration and TNF-α expression in adipose tissue from GDM, but not from OBS, tissues stimulate ADM and its receptor overexpression, leading to enhanced lipolysis and hyperlipidemia. This might contribute to fetal macrosomia and adiposity in diabetic pregnancies.
We assessed the role of adipose tissue macrophages in enhancing ADM and its receptors and their implications for GDM and obesity.
Gestational diabetes mellitus (GDM) is a common pathologic state affecting 4% to 18% of pregnancies, depending on the criteria used (1). GDM increases the incidence of complications in both the mother and the fetus, including gestational hypertension and preeclampsia for the mother and hyperinsulinemia, macrosomia, cesarian delivery, and hyperglycemia for the infant (2). Although extensive efforts have been made in previous decades, the mechanisms underlying the pathophysiology of GDM remain poorly understood. Evidence from clinical and experimental studies has indicated that maternal adipose tissue dysfunction, characterized by increased lipolysis and abnormal production of adipokines (3), adversely affects the quality and quantity of lipids transferred to the fetus and has profound and detrimental effects on fetal adiposity and growth. However, a detailed understanding of the precise mechanism for impaired lipid homeostasis in GDM remains to be established. We have reported that expression of the multifunctional peptide, adrenomedullin (ADM), and its receptor components, calcitonin receptor-like receptor (CRLR) and receptor activity-modifying proteins 2 (RAMP2) and RAMP3, was greater in omental adipose tissue (OAT) from women with GDM compared with women with normal glucose tolerance during pregnancy (4), and ADM inhibited phosphorylation of insulin receptor-β and insulin receptor substrate-1, indicating a possible role for ADM in lipid dysregulation in women with GDM (4). However, the mechanisms underlying enhanced expression of ADM and its receptors in GDM adipose tissues and its association with lipid dysregulation in diabetic pregnancies remains unclear.
Accumulating evidence has suggested that pregnancy is a state of low-grade systemic inflammation (5), and unbalanced production of adipose tissue-derived cytokines has been implicated in the development of systemic insulin resistance (6–8), indicating roles for fat tissue inflammation and adipokine dysregulation in metabolic disorders. However, the association of fat tissue macrophages and adipokines to the fat mass and the links between adipose tissue inflammation and ADM overexpression are still largely unknown. Therefore, the aims of the present study were to determine whether ADM, its receptor, and adipokines are differentially expressed by adipose tissue in pregnancy with nonoverweight (NOW), overweight (OW), obese (OBS), and GDM status. Also, we sought to determine whether, if so, these alterations are relative to the inflammatory status in adipose tissue and how ADM might affect lipid metabolism in GDM pregnancies. The results of the present study have demonstrated that adipose tissue inflammation, indicated by increased macrophage infiltration and TNF-α production, in women with GDM but not in women who were OBS, might contribute to overexpression of ADM and its receptor in adipose tissue, leading to increased lipolysis and dyslipidemia in GDM and, thus, contributing to fetal macrosomia and adiposity in diabetic pregnancies.
Materials and Methods
Subjects
The Baylor College of Medicine institutional review board approved the present study (approval no. H28527), which was conducted in accordance with the principles of the Declaration of Helsinki. All participants admitted to the Pavilions for Women at Texas Children’s Hospital, who were scheduled for an elective nonlaboring cesarean section for delivery at term, provided written informed consent. OAT was obtained from women with NOW [body mass index (BMI) <25 kg/m2; n = 9], OW (BMI >25 but <30 kg/m2; n = 8), OBS (BMI >30 kg/m2; n = 10), and GDM (n = 10) during the cesarian section from August 2014 to September 2017. All the women were screened for GDM, and patients in the other groups had had negative screening results. The 1-hour glucose screening cutoff for GDM is 140 mg/dL. The 3-hour glucose tolerance test values are fasting, >95 mg/dL; 1 hour, >180 mg/dL; 2 hours, >155 mg/dL; and 3 hours, >140 mg/dL. Pregnant patients were excluded from participating in the study if they had preexisting diabetes, fetal anomalies, multifetal pregnancy, hypertension, preeclampsia, immunosuppressive treatment, or clinical evidence of maternal or fetal infection. The prepregnancy or early pregnancy height and weight for each subject was obtained from the records, and the BMI was calculated using the recorded height and weight, either measured at the first visit during early pregnancy or self-reported as the prepregnancy weight. The relevant clinical details of the subjects are listed in Table 1.
Table 1.
Patient Characteristics
| Characteristic | Group |
P Value | |||
|---|---|---|---|---|---|
| NOW | OW | OBS | GDM | ||
| Women, n | 9 | 8 | 10 | 10 | NS |
| Ethnicity | NS | ||||
| Non-Hispanic | 9 | 6 | 8 | 6 | |
| Hispanic | 0 | 2 | 2 | 4 | |
| Maternal age, y | 33.4 ± 1.5 | 34.3 ± 1.9 | 31.5 ± 1.3 | 31.4 ± 2.1 | 0.6841 |
| Gestational age, wk | 38.6 ± 0.3 | 38.2 ± 0.6 | 38.8 ± 0.2 | 37.6 ± 0.3 | 0.2298 |
| Fetal sex | NS | ||||
| Male | 4 | 6 | 4 | 5 | |
| Female | 5 | 2 | 6 | 5 | |
| Birth weight, g | 3375 ± 179 | 3274 ± 200 | 3585 ± 317 | 3713 ± 137 | 0.4419 |
| BMI, kg/m2 | 23.1 ± 0.4 | 26.7 ± 0.6 | 32.6 ± 0.7 | 33.8 ± 2.1 | 0.0009 |
| Prepregnancy/first trimester | |||||
| Fasting blood glucose, mg/dL | 110.6 ± 28.4 | 101 ± 16.8 | 114.5 ± 14.4 | 99.3 ± 32.8 | 0.688 |
| Patients taking DM medicine, n | 0 | 0 | 0 | 7 | |
Abbreviation: NS, not significant.
Quantitative RT-PCR
Total RNA was isolated from adipose tissues using TRIzol (Life Technologies, Grand Island, NY), and reverse transcription was performed as previously described (9). Quantitative RT-PCR was performed using TaqMan probes for TNF-α (forward primer: 5′-TCAGGATCATCTTCTCGAACC-3′; reverse primer: 5′-GAGTCCTTCTCACATTGTCTC-3′). The primers used for ADM (catalog no. Hs00181605), CRLR (catalog no. Hs00173787), RAMP2 (catalog no. Hs00359352), and RAMP3 (catalog no. Hs00389130) are commercially available (Life Technologies). Amplification of housekeeping glyceraldehyde 3-phosphate dehydrogenase (forward primer: 5′-GGTCTCCTCTGACTTCAACA-3′; reverse primer: 5′-AGCCAAATTCGTTGTCATAC-3′) served as an endogenous control. The PCR conditions for TaqMan® gene expression was 2 minutes at 50°C and 10 minutes at 95°C for 1 cycle, then 15 seconds at 95°C and 1 minute at 60°C for 40 cycles. All experiments were performed in triplicate. The results were calculated using the 2-ΔΔCT method and expressed in the fold increase or decrease of the gene of interest (10).
Western blotting
Western blotting was performed as described previously (11). In brief, equal amounts of proteins were separated by polyacrylamide gel electrophoresis and then electrotransferred to polyvinylidene difluoride membranes. The membranes were blocked with 5% fat-free milk in Tris-buffered saline plus Tween 20 and were incubated with primary antibodies for CD68, TNF-α, leptin, resistin, adiponectin, and β-actin (Abcam, Cambridge, MA, for all) at 4°C overnight. After washing, the membranes were incubated with horseradish peroxidase-conjugated anti-rabbit or anti-mouse secondary antibody (Southern Biotech, Birmingham, AL). Target proteins were visualized using Pierce-enhanced chemiluminescence detection systems (Thermo Scientific, Waltham, MA). The signals were quantified using the Odyssey Imaging System (LI-COR Biosciences, Lincoln, NE).
Immunofluorescent imaging analysis
Optimal cutting temperature medium-embedded OAT were cut at a 5- to 7-μm thickness and mounted on gelatin-coated slides as previously described (4). The sections were fixed with methanol and acetone mixture (1:1). Next, the first and second primary antibodies, rabbit anti-perilipin monoclonal antibody (Cell Signaling, Beverly, MA) and mouse anti-CD68 monoclonal antibody (Abcam) were applied at 1:1000 dilution, followed by donkey anti-rabbit IgG-TRITC (Life Technology) and goat anti-mouse IgG-FITC (Southern Biotech). The slides were then mounted with mounting-medium containing 4′,6-diamidino-2-phenylindole (Vector Laboratories Inc., Burlingame, CA) and viewed under an Olympus BX51 microscope. The CD68+ macrophages were counted for each specimen, and the number of macrophages per 100 adipocytes (identified as perilipin positive) was calculated and compared between the groups.
Adipose tissue explant culture
The adipose tissues were finely diced and transferred to the wells of 24-well plates containing DMEM (Gibco, Life Technology, Gaithersburg, MD) and cultured in a humidified atmosphere of 21% O2 and 5% CO2 at 37°C for 1 hour (12). After refreshing the medium, the tissues were incubated in the presence or absence of TNF-α (0, 0.1, 1, and 10 ng/mL; Sigma-Aldrich, St. Louis, MO), and tissues were collected after 24 treatments. The adipose tissue from the women with GDM was cultured and treated with ADM22-52 (American Peptide Co., Inc., Sunnyvale, CA) at different doses, and the glycerol release into the cell culture medium was assessed using Free Glycerol Reagent (Sigma Aldrich) in accordance with the manufacturer’s instruction.
Human preadipocyte culture and lipolysis assessment
Primary human preadipocytes (ATCC PCS-210-010; American Type Culture Collection, Manassas, VA) were differentiated into mature adipocytes in wells of 24-well-plates containing adipocyte differentiation medium (Cell Applications, Inc., San Diego, CA) in a 5% CO2 atmosphere at 37°C and treated with ADM (1 nM, 10 nM, and 100 nM), with or without ADM22-52 (1 µM; American Peptide Co., Inc.) and CGRP8-37 (1 µM; American Peptide Co., Inc.). The glycerol release into cell culture medium was assessed using Free Glycerol Reagent (Sigma-Aldrich) in accordance with the manufacturer’s instructions. The absorbance at A540 was read and recorded using Spectrophotometer CLARIO STAR (BMG Labtech, Inc., Cary, NC).
Statistical analysis
All the data are presented as the mean ± SEM. The data were calculated and analyzed using GraphPad Prism (GraphPad, La Jolla, CA). Repeated measures ANOVA (treatment and time as factors) with a Bonferroni post hoc test was used for comparisons between groups. mRNA and protein expression was compared between the control and treatment groups using an unpaired Student t test. Statistical significance was defined as P < 0.05.
Results
OAT was obtained from the women at the scheduled cesarean section. The demographic data of the participants in the present study are summarized in Table 1. All the women in the NOW group were non-Hispanic. Six non-Hispanic and two Hispanic women were in the OW group, eight non-Hispanic and two Hispanic women were in OBS group, and six non-Hispanic and four Hispanic women were in the GDM group. No substantial differences in maternal age, gestational age, birth weight, and fasting blood glucose between groups. As expected, the prepregnancy/early pregnancy BMI in the OW and NOW groups was lower than that in the OBS group, and no statistically significant differences were found in the BMI between the OBS and GDM groups (P > 0.05). In addition, 7 of 10 women with GDM were prescribed insulin, in addition to dietary management, and their glycemic levels were controlled within the normal levels.
GDM is associated with increased expression of ADM and its receptor in OAT
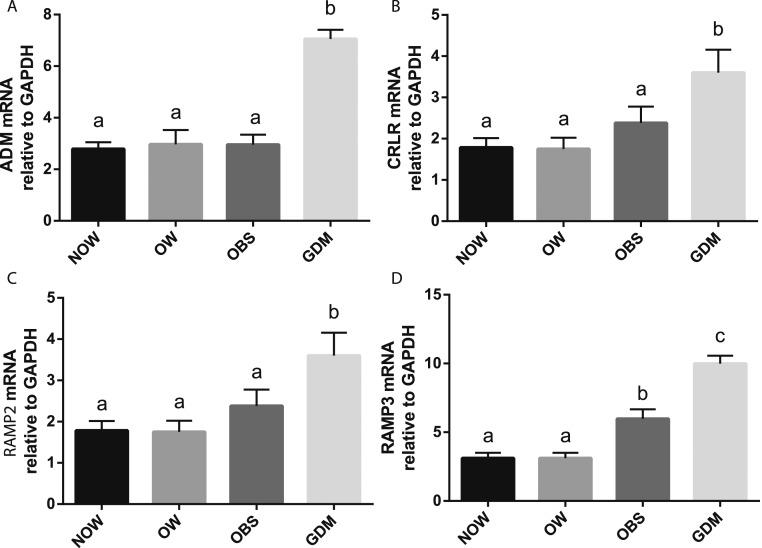
ADM mRNA expression in adipose tissue from the GDM group was substantially greater compared with the NOW, OW, and OBS groups (Fig. 1). The mRNA for the ADM receptor components, CRLR, RAMP2, and RAMP3, were also greater in the adipose tissue from the GDM group compared with the non-GDM groups. However, no statistically significant differences were observed in ADM and its receptor component mRNA of CRLR and RAMP2 between non-GDM groups, irrespective of the BMI.
Figure 1.
ADM and its receptor components in adipose tissue. The mRNA levels of (A) ADM, (B) CRLR, (C) RAMP2, and (D) RAMP3 in OAT from NOW, OW, OBS, and GDM groups were measured using RT-PCR and normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA. Data are displayed as the mean ± SEM. Different letters at the top of each bar indicate statistically significant differences between groups (P < 0.05).
GDM is associated with abnormal adipokine expression in OAT
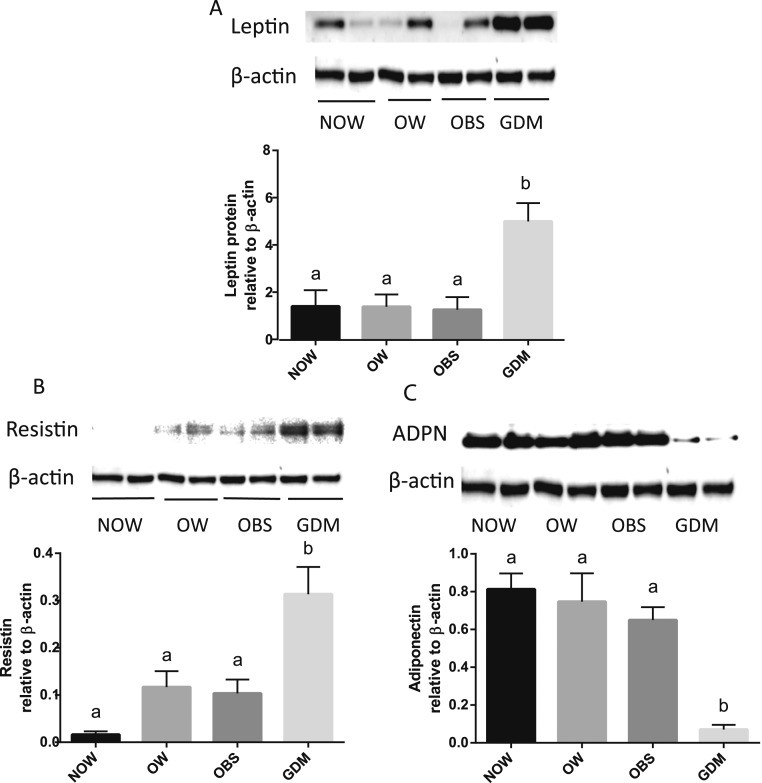
As shown in Fig. 2, leptin, a protein adipokine that plays an important role in the regulation of whole body metabolism, was substantially greater in adipose tissue from GDM compared with the non-GDM groups. In addition, resistin, one of the adipokines implicated in the impairment of glucose tolerance, was also profoundly increased in adipose tissue from the GDM group compared with the non-GDM groups. In contrast, the expression of adiponectin, an insulin-sensitizing and anti-inflammatory adipokine, was significantly lower in the adipose tissue from the GDM group compared with the non-GDM groups, suggesting abnormal adipokine expressions in adipose tissue from those with GDM.
Figure 2.
Leptin, resistin, and adiponectin (ADPN) proteins in adipose tissue. Representative Western immunoblotting of (A) leptin, (B) resistin, and (C) ADPN proteins in OAT from NOW, OW, OBS, and GDM groups are shown. Relative densities of leptin, resistin, and ADPN to β-actin were calculated and compared between groups. Data are displayed as the mean ± SEM. Different letters at the top of each bar indicate statistically significant differences between groups (P < 0.01).
Increased infiltration of macrophages in OAT from GDM group
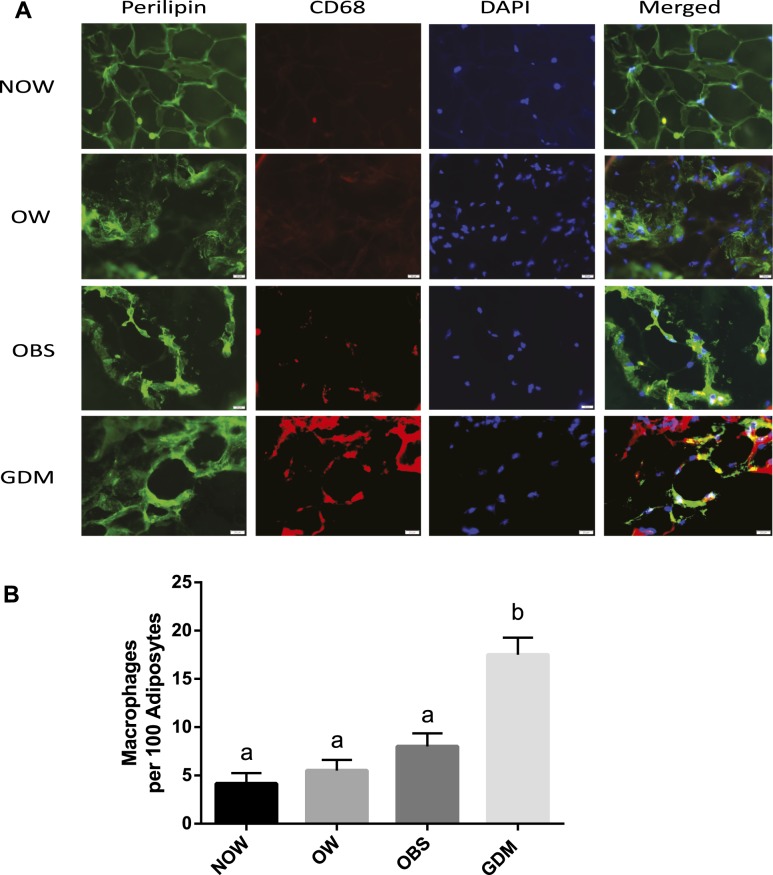
Immunofluorescent imaging was performed to assess the inflammatory status in the adipose tissue. As shown in Fig. 3, macrophages, identified by CD68 antibody in red, were present in the adipose tissue from subjects with NOW, OW, OBS, and GDM. Compared with the NOW, OW, and OBS groups, the GDM group displayed a substantial increase in macrophage infiltration into the adipose tissue. Furthermore, the number of infiltrated macrophages per 100 adipocytes and CD68 protein expression were significantly increased in the GDM group compared with the non-GDM groups, confirming the increased adipose tissue inflammation in those with GDM.
Figure 3.
Macrophage infiltration in adipose tissue. (A) OAT biopsies from NOW, OW, OBS, and GDM groups were subjected to immunofluorescent staining using anti-perilipin (a marker of adipocytes; green) and anti-CD68 antibodies (a marker of macrophages; red). The adipocyte nucleus was identified by 4′,6-diamidino-2-phenylindole (DAPI; blue; original magnification ×400). (B) Increased macrophages in OAT from GDM. The CD68+ macrophages were counted for each specimen, and the number of macrophages per 100 adipocytes was calculated and compared. Data are displayed as the mean ± SEM. Different letters at the top of each bar indicate statistically significant differences between groups (P < 0.01).
GDM is associated with increased CD68 and TNF-α expression in OAT
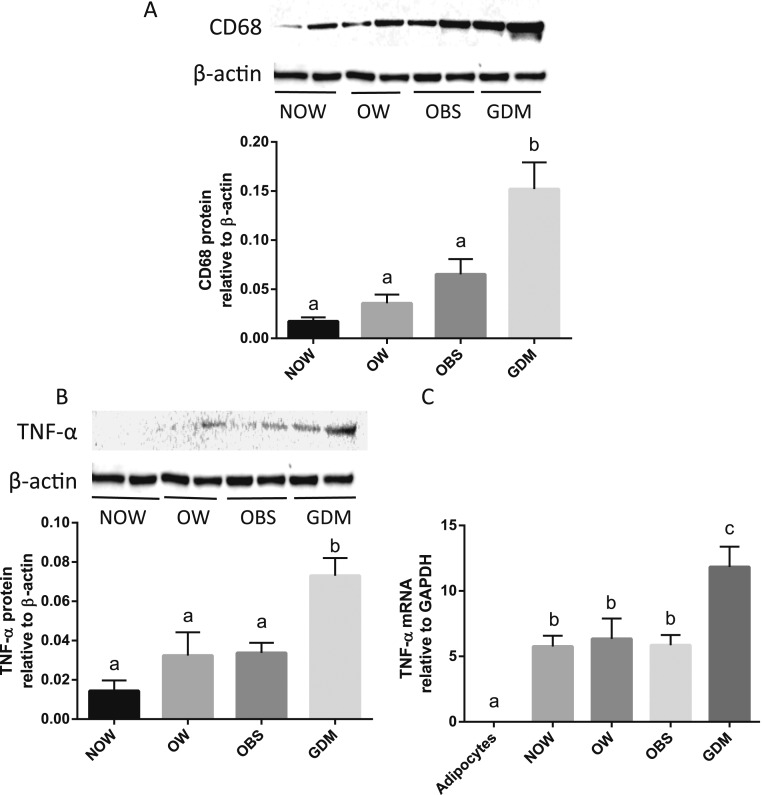
Western blots and quantitative PCR were performed to evaluate the alterations in CD68 and TNF-α levels in OAT among those with and without GDM. As shown in Fig. 4, the proteins for CD68 and TNF-α and the mRNA for TNF-α were substantially greater in the OAT from the GDM group compared with the non-GDM group. Because TNF-α mRNA was is in human adipocytes, enhanced expression of TNF-α in adipose tissue indicates increased macrophage infiltration in the adipose tissue from the women with GDM.
Figure 4.
Expression of CD68 and TNF-α in adipose tissue. Representative Western immunoblotting of (A) CD68 and (B) TNF-α proteins in OAT from NOW, OW, OBS, and GDM groups are shown. Relative densities of CD68 and TNF-α to β-actin were calculated and compared between groups. (C) The mRNA levels of TNF-α in adipose tissue were measured using RT-PCR and were normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA expression. Data are displayed as the mean ± SEM. Different letters at the top of each bar indicate statistically significant differences between groups (P < 0.01).
TNF-α stimulates mRNA for ADM and its receptor components in OAT explants
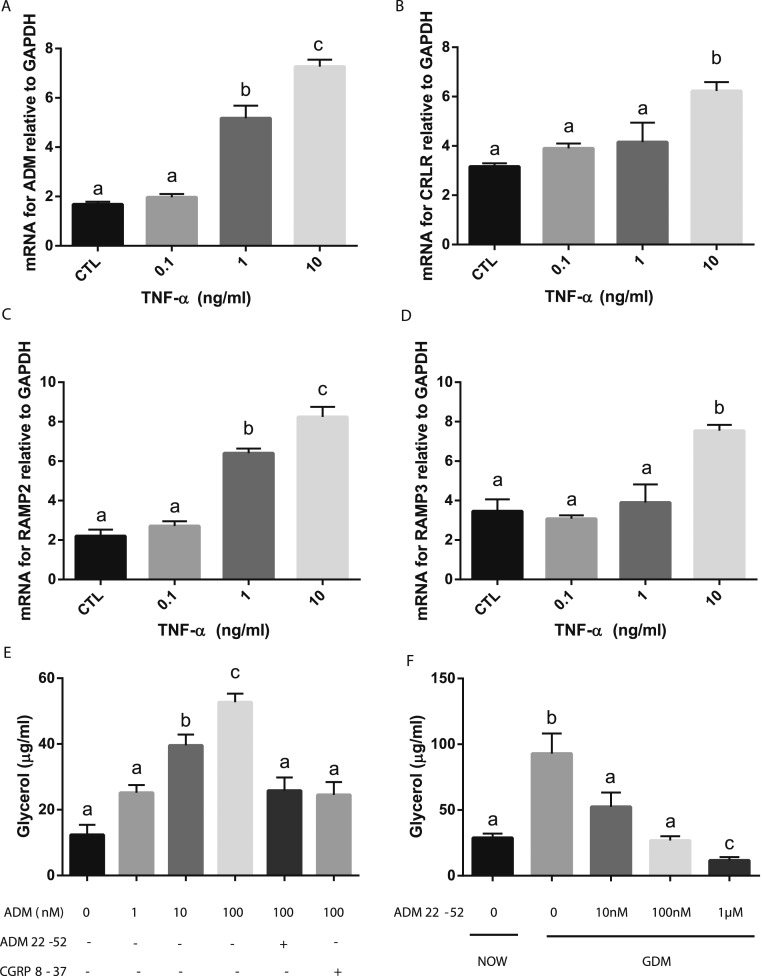
To determine the influence of increased inflammation on ADM and its receptors in adipose tissue, we treated the adipose tissue from the NOW group with TNF-α. ADM mRNA expression was increased by TNF-α in a dose-dependent manner (Fig. 5A). Similarly, TNF-α elevated the expression of mRNA for CRLR, RAMP2, and RAMP3 in a dose-dependent manner (Fig. 5B–5D), indicating that the elevated levels of TNF-α in those with GDM might contribute, at least in part, to the enhanced expression of ADM and its receptors in adipose tissue.
Figure 5.
The effects of TNF-α on ADM and its receptors and ADM on lipolysis in adipocytes. OAT from women who were NOW (n = 6) were incubated with increasing doses of TNF-α for 24 h, and the mRNA levels for (A) ADM, (B) CRLR, (C) RAMP2, and (D) RAMP3 were determined using RT-PCR and normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA. (E) In addition, human adipocytes were treated with ADM in the presence or absence of its antagonist, ADM22-52 (1 µM) and CGRP8-37 (1 µM) for 24 h, and the glycerol levels in culture medium were determined using Free Glycerol Reagents. (F) Furthermore, OAT from women with GDM (n = 6) were incubated with different doses of ADM22-52 for 24 h, and the glycerol level was determined using Free Glycerol Reagent. Data are displayed as the mean ± SEM. Different letters at the top of each bar indicate statistically significant differences between groups (P < 0.01). CGRP, calcitonin gene-related peptide; CTL, control.
ADM stimulates lipolysis and ADM22-52 attenuate lipolytic status in OAT from patients with GDM
To assess the effect of ADM on lipid metabolism, we treated the human adipocytes with ADM for 24 hours. We found that ADM stimulated glycerol release in the culture medium in a dose-dependent manner, and these increases were reduced by preincubation with the ADM antagonist ADM22-52 and CGRP8-37 (Fig. 5E), indicating that ADM stimulates lipolysis in adipocytes, and these effects are reversible by ADM antagonists. Furthermore, basal glycerol release by adipose tissue from the GDM pregnancies was much greater than that from the non-GDM pregnancies (Fig. 5F), and the ADM antagonist, ADM22-52, dose dependently inhibited the glycerol release by adipose tissue from the patients with GDM, suggesting that blockade of ADM actions with its antagonist can improve the lipid metabolic status in women with GDM.
Discussion
The present study has demonstrated that ADM and its receptors are greater in OATs from the GDM group than in the NOW, OW, and OBS groups. The expression of leptin and resistin was substantially increased, but adiponectin was decreased in OAT from the GDM group compared with the non-GDM groups. Macrophage infiltration and TNF-α expression was greater in the OAT from pregnant women with GDM than in the women without GDM. Furthermore, TNF-α dose dependently increased mRNA for ADM and its receptor components CRLR, RAMP2, and RAMP3 in OAT explants from the NOW group. ADM significantly increased adipocyte glycerol release in culture medium, and the increases in glycerol in OAT from women with GDM were attenuated by the ADM antagonists, ADM22-52. Therefore, we propose that the increased macrophage infiltration and TNF-α expression in adipose tissue from the GDM group, but not the OBS group, might contribute to enhanced expression of ADM and its receptors, leading to increased lipolysis and hyperlipidemia, which might contribute to fetal macrosomia and adiposity in women with GDM.
Human pregnancy, especially in the third trimester, is characterized by maternal hyperinsulinemia and insulin resistance, which is an adaptation required to meet the needs of the growing fetus (13). In women with GDM, however, peripheral insulin resistance is even more pronounced, which results in greater substrate availability for fetal growth and development (14). In the present study, the clinical characteristics of the patients (Table 1) were similar to those in previous reports showing a greater fetal birth weight in women with GDM compared with women without GDM (15, 16), implying a trend toward fetal overgrowth in GDM pregnancies. In addition, no substantial differences in BMI were found between the OBS and GDM subjects, indicating that the adipose tissue mass in women with GDM and OBS is similar. Thus, in the present study, OBS subjects could serve as a BMI-matched control to women with GDM in lipid metabolic assessments.
Plasma ADM concentrations are elevated in patients with type 2 diabetes mellitus (17) and in the amniotic fluid from diabetic pregnancies compared with uncomplicated pregnancies (18), suggesting the possible involvement of ADM in the pathogenesis of insulin resistance seen in those with diabetes. The present study showed that the mRNA expression for ADM and its receptor components CRLR, RAMP2, and RAMP3 is significantly greater in the OAT from patients with GDM compared with the NOW, OW, and BMI-matched OBS subjects (Fig. 1). These results suggest that the increases in ADM and its receptor components in OAT from women with GDM are related to the GDM and not to the adipose tissue mass or BMI.
Adipose tissue has been recognized as more than simply a storage depot for lipids. It is an active endocrine organ secreting adipokines (19). Altered adipokine secretions have been considered prime candidates for direct involvement in the pathophysiology of GDM (19). Leptin is a protein hormone produced by adipocytes that plays an important role in the regulation of whole body metabolism (20), including the influence on insulin secretion, glucose usage, glycogen synthesis, and fatty acid metabolism (20). Resistin, a hormone expressed abundantly in adipocytes (21), is thought to impair glucose tolerance and has shown a positive correlation between obesity and insulin resistance in pregnancy (22). Another adipokine, adiponectin, secreted exclusively from adipose tissue (20), is an insulin-sensitizing and anti-inflammatory adipokine that stimulates glucose uptake in skeletal muscle and reduces hepatic glucose production (23, 24). The present study revealed that the expression of leptin and resistin in OAT was substantially increased in women with GDM compared with women without GDM, irrespective of their BMI (Fig. 2). In contrast, the expression of adiponectin in adipose tissue was significantly reduced in the GDM compared with the non-GDM pregnancies, suggesting abnormal adipokine levels in women with GDM. Our previous results revealed that ADM stimulates leptin and resistin mRNA expressions in OAT from women with a normal pregnancy (4). Thus, increased mRNA expression of leptin and resistin in OAT from women with GDM could be reduced by ADM22-52 treatment in vitro. These results suggest that ADM might play a role in regulating adipocyte leptin and resistin productions and that the adverse effects of leptin and resistin in GDM could be attenuated by ADM antagonists.
Chronic low-degree adipose tissue inflammation is a crucial contributor to the pathogenesis of insulin resistance and metabolic diseases (25). The infiltrated immune cells play critical roles in modulating adipose tissue inflammation (6). During this procedure, macrophages undergo a phenotypic switch from anti-inflammatory status (M2) to a proinflammatory (M1) status, which results in the development of tissue inflammation and systemic insulin resistance (26). Despite the increased production of proinflammatory molecules in human pregnancy, the contribution of macrophages in fat tissue to the pathophysiology of GDM and its related morbidities are still largely unknown. The present study has demonstrated that GDM pregnancies are associated with a greater rate of macrophages infiltrating in the OAT (Fig. 3), with no substantial differences among the NOW, OW, and OBS subjects, suggesting that an inflammatory stress is imposed on the adipose tissue and that such low-grade chronic inflammation might participate in the complex metabolic changes in GDM. However, the lack of an association between macrophage infiltration and BMI in women who were OBS might indicate that adipose tissue macrophage infiltration is not dependent on the amount of the fat mass, but rather on factors beyond the fat mass, such as (1) different expression levels of monocyte chemoattractant protein-1, one of the chemokines produced in large amounts by adipocytes and attracting macrophage migration (27); (2) altered adipokine production, such as adiponectin, which has anti-inflammatory properties (23); and (3) local proliferation and differentiation of macrophages (26). The mechanisms underlying the altered OAT inflammation in GDM warrant further investigation.
Adipose tissue inflammation is attributable in large part to the proinflammatory actions of macrophages through synthesis and release of TNF-α (28). Increased levels of TNF-α occur owing to oxidative stress and the inflammatory changes induced by hyperglycemia in GDM (21). Studies have found substantially elevated TNF-α in the serum of patients with GDM compared with BMI-matched controls (29). The present study revealed that the protein and mRNA for TNF-α were significantly greater in the GDM group compared with the non-GDM groups (Fig. 4). Also, TNF-α dose dependently increased mRNA expression for both ADM and its receptor components CRLR, RAMP2, and RAMP3 in the adipose tissue explants (Fig. 5A–5D). Thus, we propose that the increased adipose tissue TNF-α levels in women with GDM might not only directly contribute to insulin resistance but also adversely affect the metabolic status via increased ADM and its receptor levels in OAT. However, the poor glycemic control would be a substantial factor for increased inflammation; thus, women with good glycemic control might have less inflammation. Our results showed that 7 of 10 of the women with GDM were taking insulin medication, and their blood glucose was well controlled and within the normal range. However, the macrophage infiltration and TNF-α levels were still greater in the GDM group than in the other groups, indicating that factors other than glucose could also contribute to the adipose tissue inflammation. These factors might include, but are not limited to, increased oxidative stress and adipose tissue nitric oxide production. The underlying mechanisms warrant further investigation.
GDM is characterized by increased lipolysis and elevated circulating free fatty acids (FFAs) (30). Excessive FFAs from adipose tissue increase insulin resistance during pregnancy (31) and have been associated with fetal overgrowth, especially of adipose tissue (32), suggesting that the neonatal birth weight correlates positively with the FFA level. By measuring the glycerol production, the breakdown product of triglycerides, we have demonstrated that ADM dose dependently stimulates lipolysis in human adipocytes (Fig. 5E). Also, this effect was abolished in the presence of ADM’s antagonists, ADM22-52 and CGRP8-37, indicating the specificity of ADM effects. Furthermore, basal glycerol release by adipose tissue from women with GDM was much greater than that from non-GDM adipose tissue (Fig. 5F). Also, the ADM antagonist, ADM22-52, dose dependently inhibited glycerol release by the adipose tissue from the patients with GDM. Thus, we propose that increased ADM might contribute to a state of hyperlipidemia consistent with the insulin resistance seen in GDM and that the alterations in the lipid profile on the maternal side would affect the quantity and/or quality of lipids being transferred to the fetus, leading to fetal macrosomia. Thus, the blockade of ADM actions with its antagonist could improve the lipid metabolic status in women with GDM.
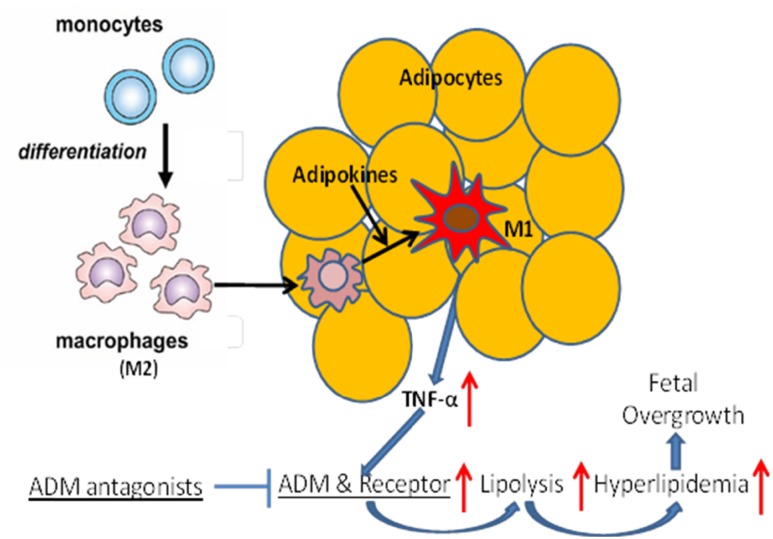
Based on our results, we speculate that in GDM (Fig. 6), monocyte-differentiated macrophages (M2) migrate into adipose tissue, and local factors, such as adipokines, activate anti-inflammatory M2 to proinflammatory M1 and produce increasing amounts of proinflammatory cytokines, such as TNF-α. This increase in TNF-α would then stimulate expression of ADM and its receptors in adipose tissue, leading to a maternal lipolytic status and hyperlipidemia and contribute to fetal overgrowth. This is further supported by our previous studies in which we demonstrated that lipolysis is increased in OAT explants from normal glucose-tolerant women when incubated with TNF-α and was reversed by ADM22-52. Therefore, the increased proinflammatory status in GDM induces elevations in lipolysis in adipose tissue through the ADM system and blockade of ADM actions could improve the lipid dysregulation seen in GDM. In the present study, we focused primarily on the OAT from women with GDM and with different BMI status to assess whether lipid metabolism is altered by the adipose tissue mass or GDM status and whether the ADM system in involved in inflation-related increases in lipolysis in GDM. It is unclear whether similar changes would also be present in other fat depots such as subcutaneous fat and their contribution to the overall increases in FFA levels seen in women with GDM. Future studies are in progress to address these limitations.
Figure 6.
Schematic illustration of ADM action in human adipose tissue. Monocyte-differentiated macrophages (M2) migrate into adipose tissue. Local factors, such as adipokines, activate anti-inflammatory M2 to proinflammatory M1 and produce increasing amounts of proinflammatory cytokines, such as TNF-α, which stimulate expression of ADM and its receptors in adipose tissue, leading to maternal lipolytic status, hyperlipidemia, and fetal overgrowth. Blockade of ADM actions could improve the ADM-related lipid dysregulation seen in GDM.
The results from the present study support the notion that macrophage infiltration into OAT is an inflammatory feature of fat depot and could play a role in stimulating the lipolytic actions of the ADM system, linking adipose tissue dysfunction in GDM. In contrast, the lack of an association between the BMI and macrophage infiltration and TNF-α level in OAT might indicate that the GDM-related adipose tissue inflammation is not dependent on the amount of the fat mass but that the inflammation–ADM–pathway plays an important role in the pathophysiology of GDM. We therefore propose that manipulation of ADM, its receptors, and/or its actions in adipocytes might represent another approach in reducing the risk of GDM and fetal overgrowth.
Acknowledgments
The findings from the present study have been orally presented at the 38th Society for Maternal-Fetal Medicine Annual Meeting, 3 February 2018, Dallas, Texas. The authors thank Dr. Manu Banadakoppa and Ms. Meena Balakrishnan for their technical assistance in RT-PCR and Western blots and Ms. Uma Yallampalli and Ms. Sandra Dale for their assistance in reagent ordering and manuscript preparation.
Financial Support: The present study was supported by the National Institutes of Health (grants HD091503 and HL58144 to C.Y.).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- ADM
adrenomedullin
- BMI
body mass index
- CRLR
calcitonin receptor-like receptor
- FFA
free fatty acid
- GDM
gestational diabetes mellitus
- NOW
nonoverweight
- OAT
omental adipose tissue
- OBS
obese
- OW
overweight
- RAMP
receptor activity-modifying protein
References
- 1. Cundy T, Ackermann E, Ryan EA. Gestational diabetes: new criteria may triple the prevalence but effect on outcomes is unclear. BMJ. 2014;348(6):g1567. [DOI] [PubMed] [Google Scholar]
- 2. Dabelea D, Hanson RL, Lindsay RS, Pettitt DJ, Imperatore G, Gabir MM, Roumain J, Bennett PH, Knowler WC. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes. 2000;49(12):2208–2211. [DOI] [PubMed] [Google Scholar]
- 3. Herrera E, Ortega-Senovilla H. Disturbances in lipid metabolism in diabetic pregnancy—are these the cause of the problem? Best Pract Res Clin Endocrinol Metab. 2010;24(4):515–525. [DOI] [PubMed] [Google Scholar]
- 4. Dong Y, Betancourt A, Belfort M, Yallampalli C. Targeting adrenomedullin to improve lipid homeostasis in diabetic pregnancies. J Clin Endocrinol Metab. 2017;102(9):3425–3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lekva T, Bollerslev J, Norwitz ER, Aukrust P, Henriksen T, Ueland T. Aortic stiffness and cardiovascular risk in women with previous gestational diabetes mellitus. PLoS One. 2015;10(8):e0136892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Finucane OM, Reynolds CM, McGillicuddy FC, Roche HM. Insights into the role of macrophage migration inhibitory factor in obesity and insulin resistance. Proc Nutr Soc. 2012;71(4):622–633. [DOI] [PubMed] [Google Scholar]
- 8. Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, Capeau J, Feve B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17(1):4–12. [PubMed] [Google Scholar]
- 9. Dong Y, Betancourt A, Chauhan M, Balakrishnan M, Lugo F, Anderson ML, Espinoza J, Fox K, Belfort M, Yallampalli C. Pregnancy increases relaxation in human omental arteries to the CGRP family of peptides. Biol Reprod. 2015;93(6):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arocho A, Chen B, Ladanyi M, Pan Q. Validation of the 2-DeltaDeltaCt calculation as an alternate method of data analysis for quantitative PCR of BCR-ABL P210 transcripts. Diagn Mol Pathol. 2006;15(1):56–61. [DOI] [PubMed] [Google Scholar]
- 11. Chauhan M, Balakrishnan M, Chan R, Yallampalli C. Adrenomedullin 2 (ADM2) regulates mucin 1 at the maternal-fetal interface in human pregnancy. Biol Reprod. 2015;93(6):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barker G, Lim R, Georgiou HM, Lappas M. Omentin-1 is decreased in maternal plasma, placenta and adipose tissue of women with pre-existing obesity. PLoS One. 2012;7(8):e42943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Buchanan TA, Xiang AH. Gestational diabetes mellitus. J Clin Invest. 2005;115(3):485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lain KY, Catalano PM. Metabolic changes in pregnancy. Clin Obstet Gynecol. 2007;50(4):938–948. [DOI] [PubMed] [Google Scholar]
- 15. Boghossian NS, Yeung E, Albert PS, Mendola P, Laughon SK, Hinkle SN, Zhang C. Changes in diabetes status between pregnancies and impact on subsequent newborn outcomes. Am J Obstet Gynecol. 2014;210(5):431.e1–431.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kitajima M, Oka S, Yasuhi I, Fukuda M, Rii Y, Ishimaru T. Maternal serum triglyceride at 24–32 weeks’ gestation and newborn weight in nondiabetic women with positive diabetic screens. Obstet Gynecol. 2001;97(5 Pt 1):776–780. [DOI] [PubMed] [Google Scholar]
- 17. Hayashi M, Shimosawa T, Isaka M, Yamada S, Fujita R, Fujita T. Plasma adrenomedullin in diabetes. Lancet. 1997;350(9089):1449–1450. [DOI] [PubMed] [Google Scholar]
- 18. Di Iorio R, Marinoni E, Urban G, Costantini A, Cosmi EV, Letizia C. Fetomaternal adrenomedullin levels in diabetic pregnancy. Horm Metab Res. 2001;33(8):486–490. [DOI] [PubMed] [Google Scholar]
- 19. Fasshauer M, Blüher M, Stumvoll M. Adipokines in gestational diabetes. Lancet Diabetes Endocrinol. 2014;2(6):488–499. [DOI] [PubMed] [Google Scholar]
- 20. Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol. 2010;316(2):129–139. [DOI] [PubMed] [Google Scholar]
- 21. Briana DD, Malamitsi-Puchner A. Reviews: adipocytokines in normal and complicated pregnancies. Reprod Sci. 2009;16(10):921–937. [DOI] [PubMed] [Google Scholar]
- 22. Wójcik M, Chmielewska-Kassassir M, Grzywnowicz K, Woźniak L, Cypryk K. The relationship between adipose tissue-derived hormones and gestational diabetes mellitus (GDM). Endokrynol Pol. 2014;65(2):134–142. [DOI] [PubMed] [Google Scholar]
- 23. Miehle K, Stepan H, Fasshauer M. Leptin, adiponectin and other adipokines in gestational diabetes mellitus and pre-eclampsia. Clin Endocrinol (Oxf). 2012;76(1):2–11. [DOI] [PubMed] [Google Scholar]
- 24. Georgiou HM, Lappas M, Georgiou GM, Marita A, Bryant VJ, Hiscock R, Permezel M, Khalil Z, Rice GE. Screening for biomarkers predictive of gestational diabetes mellitus. Acta Diabetol. 2008;45(3):157–165. [DOI] [PubMed] [Google Scholar]
- 25. Johnson AM, Olefsky JM. The origins and drivers of insulin resistance. Cell. 2013;152(4):673–684. [DOI] [PubMed] [Google Scholar]
- 26. Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117(1):175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Engin AB. Adipocyte-macrophage cross-talk in obesity. Adv Exp Med Biol. 2017;960:327–343. [DOI] [PubMed] [Google Scholar]
- 28. Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83(3):847–850. [DOI] [PubMed] [Google Scholar]
- 29. Xu J, Zhao YH, Chen YP, Yuan XL, Wang J, Zhu H, Lu CM. Maternal circulating concentrations of tumor necrosis factor-α, leptin, and adiponectin in gestational diabetes mellitus: a systematic review and meta-analysis. Sci World J. 2014;2014:926932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Catalano PM, Nizielski SE, Shao J, Preston L, Qiao L, Friedman JE. Downregulated IRS-1 and PPARgamma in obese women with gestational diabetes: relationship to FFA during pregnancy. Am J Physiol Endocrinol Metab. 2002;282(3):E522–E533. [DOI] [PubMed] [Google Scholar]
- 31. Bergmann K, Sypniewska G. Diabetes as a complication of adipose tissue dysfunction: is there a role for potential new biomarkers? Clin Chem Lab Med. 2013;51(1):177–185. [DOI] [PubMed] [Google Scholar]
- 32. Knopp RH, Bergelin RO, Wahl PW, Walden CE. Relationships of infant birth size to maternal lipoproteins, apoproteins, fuels, hormones, clinical chemistries, and body weight at 36 weeks gestation. Diabetes. 1985;34(Suppl 2):71–77. [DOI] [PubMed] [Google Scholar]