Abstract
Despite the well-established concept that an increase in pulsatile GnRH release triggers puberty, the precise signaling mechanism responsible for the pubertal increase in GnRH release remains unclear. A recent study indicates that developmental changes in the network formation between kisspeptin and neurokinin B (NKB) signaling greatly contribute to the pubertal increase in GnRH release in female monkeys. It is, however, unknown whether similar developmental changes in the kisspeptin and NKB network are involved in male puberty. In the current study, we first characterized the pubertal stages in male rhesus monkeys by assessing physiological and hormonal changes during sexual development. Subsequently, we examined the role of the kisspeptin and NKB signaling network in the pubertal increase in GnRH release. Results suggest that while collaborative kisspeptin and NKB signaling to GnRH neurons was active before puberty onset, after initiation of puberty the role of NKB signaling in GnRH neurons diminished and kisspeptin signaling assumed the primary stimulatory role in the regulation of GnRH release in male monkeys. These findings in males differ from those seen in females.
Developmental changes in GnRH release and kisspeptin and neurokinin B signaling in male monkeys are investigated. The network between these two signaling mechanisms undergoes pubertal changes.
The neuroendocrine mechanism triggering the increase in GnRH release at the onset of puberty remains a mystery. Human genetic studies revealed that both kisspeptin and neurokinin B (NKB) play a significant role in puberty (1–3). Previous studies in female monkeys from this laboratory indicate that there are twofold contributions of kisspeptin signaling to the pubertal increase in GnRH release. First, sensitivity of GnRH neurons to human kisspeptin-10 (hKP10), that is, the responsiveness of the kisspeptin receptor (KISS1R), increases at puberty onset (4). Second, kisspeptin release increases at puberty onset (5), parallel to the pubertal increase in GnRH release (6, 7). Furthermore, NKB signaling also contributes to the pubertal increase in GnRH release in female monkeys (8). In this case, a reciprocal, cooperative signaling pathway (kisspeptin signaling is mediated through NKB neurons and NKB signaling is mediated through kisspeptin neurons) is established after, not before, puberty onset in female monkeys.
In general, male puberty occurs at an older age than female puberty. The developmental mechanism governing puberty onset in primates is similar in both sexes, except for subtle differences during the neonatal period and prepubertal hiatal period (9). However, our observations showing the presence of the reciprocal and cooperative signaling mechanism between kisspeptin and NKB neurons for the pubertal increase in GnRH release in female monkeys (8) differs from the report in male monkeys that NKB signaling to GnRH neurons is solely mediated through kisspeptin neurons and there is no kisspeptin signaling mediated through NKB neurons (10). Whereas we measured GnRH and kisspeptin directly from the median eminence (ME) in females, they measured LH levels for assessment of GnRH release in males. Although there is a difference in approaches between our studies and the studies by Ramaswamy et al. (10), further investigation may reveal a potentially important sex difference in kisspeptin and NKB signaling contributing to the pubertal increase in GnRH neurons. Therefore, in the current study, we investigated the role of the GnRH–kisspeptin–NKB signaling network in male puberty, using the same approach employed in the female study.
Materials and Methods
Animals
A total of nine prepubertal and seven pubertal gonadally intact male rhesus monkeys (Macaca mulatta) were used in this study. Because this is the first study looking at male puberty in this laboratory, we obtained detailed physical and hormonal data for the characterization of developmental stages. We obtained weekly body weights, testicular volumes, and serum samples. Serum samples were obtained in the morning (8:00 am) and evening (8:30 pm) for testosterone (T) and LH assessments. All animals were born and raised at the Wisconsin National Primate Research Center. They were housed in pairs (cages 172 × 86 × 86 cm) with a 12-hour light: 12-hour dark cycle and at a controlled temperature (22°C). They were fed Teklad Global Diets no. 2050 (Envigo, Madison, WI) twice daily and water was available ad libitum. Fresh fruit and/or other enrichment was also provided daily. The protocol was approved by the Animal Care and Use Committee, University of Wisconsin–Madison, and all experiments were conducted in accordance with the National Institutes of Health and US Department of Agriculture guidelines.
Experimental design
Four series of in vivo microdialysis experiments were conducted in nine prepubertal (16.4 ± 1.0 months of age) and five pubertal (36.4 ± 1.6 months of age) gonadally intact male rhesus monkeys. The pubertal stage was defined by the physical and hormonal characteristics shown in Fig. 1. The pubertal stage represented in this study is a combination of the early and midpubertal stages. The protocols in these male experiments are similar to the female study reported previously (8).
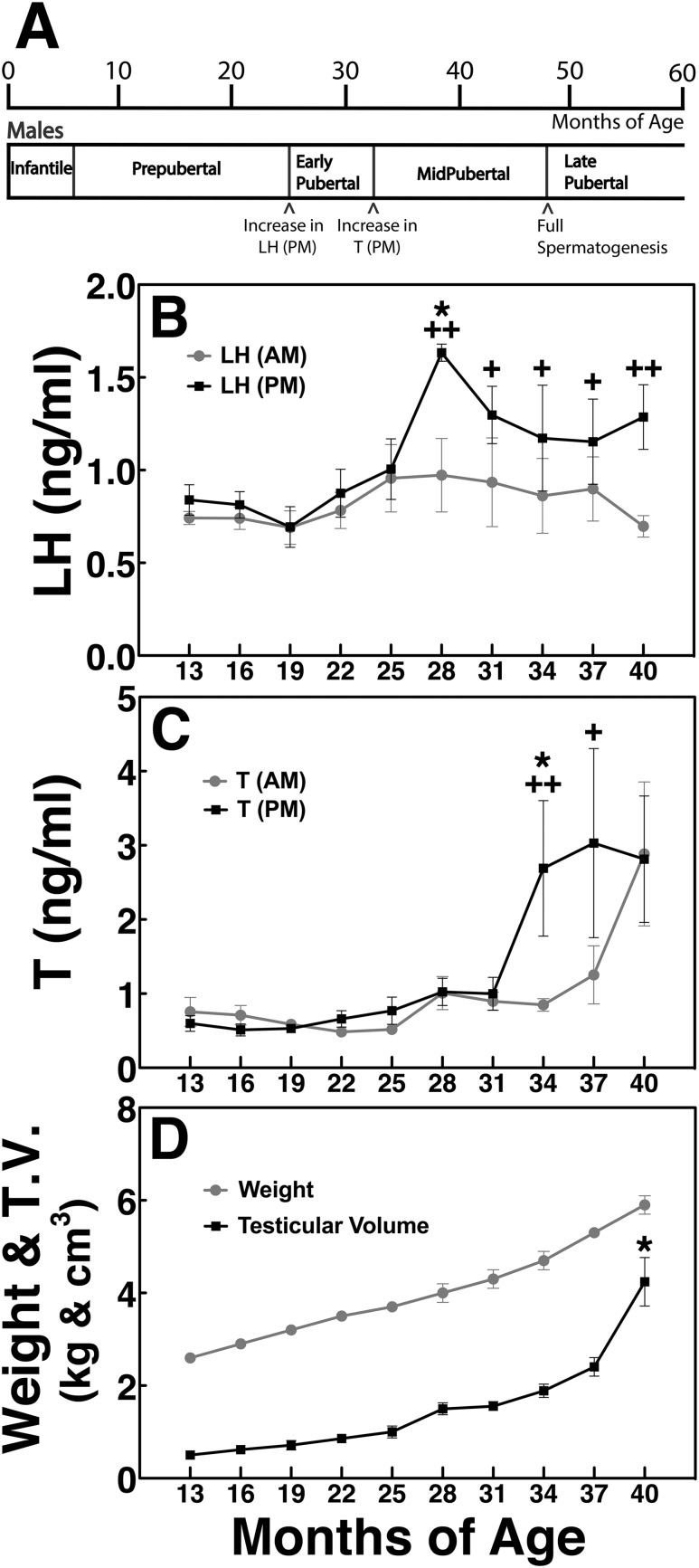
Figure 1.
(A) Developmental stages in male nonhuman primates. Assessment of physiological and hormonal measurements [(B) am and pm LH, (C) am and pm testosterone, (D) body weight and testicular volume] has permitted us to categorize the male pubertal process into the following stages: prepubertal stage (7 to 25 mo of age), early pubertal stage (28 to 34 mo of age), and midpubertal stage (34 to 48 mo of age). The prepubertal stage is defined by low am and pm LH and T levels (≤1 ng/mL for both) occurring at 7 to 25 mo of age and a testicular volume ≤1 cm3. The early pubertal stage is defined by an increase in pm LH levels occurring between 25 and 28 mo of age and a very small increase in testicular volume. The midpubertal stage is defined by consistently elevated pm LH levels, elevated pm T levels, a small increase in testicular volume starting at ∼34 mo of age, and subsequently elevated am T levels followed by a rapid increase in testicular volume occurring between 37 and 48 mo of age. Note that pm LH levels are consistently higher than the am LH levels in all three pubertal stages; however, pm T levels are higher than the am T levels in the midpubertal stage only. *P < 0.05 vs previous stage; +P < 0.05, ++P < 0.01 vs am level. T.V., testicular volume.
Experiment 1
To investigate whether kisspeptin signaling to GnRH neurons undergoes a developmental change in males, we examined the effects of two doses (0.1 and 1 µM) of the kisspeptin agonist hKP10 (112–121 amide; Phoenix Pharmaceuticals, Burlingame, CA) on GnRH release in prepubertal (n = 5) and pubertal (n = 4) monkeys. After a minimum of 60-minute dialysate collections for assessment of baseline GnRH release, hKP10 or vehicle was infused into the ME for 20 minutes, and dialysate sampling was continuously collected for an additional 80 minutes. Control data were obtained from vehicle infusion in the same prepubertal and pubertal animals.
Experiment 2
To determine whether kisspeptin signaling to GnRH neurons is mediated through NKB neurons, and, if so, whether this signaling network undergoes a developmental change, we examined the effects of 0.1 µM hKP10 on GnRH release in the presence or absence of the NKB antagonist SB222200 (1 µM; Sigma-Aldrich, St. Louis, MO) in prepubertal (n = 6) and pubertal (n = 4) monkeys. Previously, we observed that the stimulatory effects of the NKB agonist senktide (0.1 µM) on GnRH release through neurokinin B receptor (NK3R) were blocked by SB222200 (1 µM) in prepubertal and pubertal females (see Supplemental Fig. 1). After the minimum 60-minute control dialysate collections, SB222200 or vehicle was infused for a 60-minute period, during which hKP10 was added for the last 20 minutes, whereas dialysates were continuously collected. As a control, the effects of SB222200 infusion alone were also tested.
Experiment 3
To investigate whether NKB signaling to GnRH and kisspeptin neurons undergoes a developmental change in males, we examined the effects of two doses (0.1 and 10 µM) of the NKB agonist senktide (Sigma-Aldrich) on GnRH release (experiment 3a) and kisspeptin release (experiment 3b) in prepubertal (n = 7) and pubertal (n = 5) monkeys.
Experiment 4
To determine whether NKB signaling to GnRH neurons is mediated through kisspeptin neurons, and, if so, whether this signaling network undergoes a developmental change, we examined the effects of 0.1 µM senktide on GnRH release in the presence or absence of the kisspeptin antagonist peptide 234 (P234; provided by Dr. Robert P. Millar, University of Edinburgh, Edinburgh, United Kingdom) in prepubertal (n = 6) and pubertal (n = 4) males. Again, after the minimum 60-minute control dialysate collections, P234 or vehicle was infused for a 60-minute period, during which senktide was added for the last 20 minutes, whereas dialysates were continuously collected. As a control, the effects of P234 infusion alone were also tested.
In a single microdialysis session, multiple drug (or vehicle) challenges were made at 3- to 4-hour intervals, but the same challenge was never repeated. Experiments 1 and 3 were conducted first, and subsequently experiments 2 and 4 were concurrently conducted. In all experiments, GnRH in dialysates was measured. However, kisspeptin was measured only in experiment 3, as hKP10 and P234 interfere with the kisspeptin assay in experiments 1, 2, and 4.
Microdialysis experiment
Cranial pedestal implantation and guide cannula insertion were conducted as previously described (8, 11). Once in the primate chair, the inner stylet was removed from the guide cannula and a microdialysis probe (stainless steel shaft 96.0 mm in length, 0.6 mm in diameter) with a polyarylethersulfone membrane (20-kDa molecular mass cutoff, 5.0 mm in length, 0.5 mm in diameter) was inserted into the guide cannula, such that the tip of the probe was located in the ME. The placements of the microdialysis probe tip in each age group, assessed by radiographs, were very similar to those described in females (8).
Central nervous system perfusion fluid (artificial cerebrospinal fluid, consisting of 147 mM NaCl, 2.7 mM KCl, 1.2 mM CaCl2, 0.85 mM MgCl2; Harvard Apparatus, Holliston, MA) containing bacitracin (4 U/mL) was infused into the ME through the microdialysis probe at 2 µL/min using a 2.5-mL Hamilton syringe (Hamilton, Reno, NV) and a CMA/102 microdialysis pump (Harvard Apparatus). Dialysate collections were initiated 2 hours after probe insertion and continued for up to 10 hours. Dialysates (40 µL) were collected at 20-minute intervals with a fraction collector (model FC203B; Gilson, Middleton, WI). Samples were immediately aliquoted into two borosilicate tubes (20 µL each), frozen on dry ice, and stored at −80°C for later assay of peptides.
All agonists and antagonists were prepared per the manufacturers’ directions and stored in aliquots at −80°C. On the day of experiment, they were diluted to final concentrations with artificial cerebrospinal fluid and filtered with a 0.22-µm PES filter (Millex GP; Merck, Darmstadt, Germany) for sterility prior to infusion. A previous in vitro study indicated that with the infusion rate at 2 µL/min, 10% to 12% of the administered agonist or antagonist concentration diffused through the probe membrane into the ME (12). As such, local concentrations of agonists/antagonists in the ME would be ∼10% of the infused dose.
During the microdialysis sessions, animals were provided monkey chow, fresh fruit, other enrichment, water ad libitum, and a partner monkey for visualization and vocalization. Animals were returned to their home cage for a minimum of 3 weeks before subsequent microdialysis sessions.
Radioimmunoassay and enzyme immunoassay
Samples were thawed immediately before the assay. Radioimmunoassay (RIA) for GnRH measurement was conducted using the R42 antiserum provided by Dr. Terry Nett (Colorado State University, Fort Collins, CO; RRID: AB_2686919) as previously described (11). Assay sensitivity was 0.02 pg per tube. Intra-assay and interassay coefficients of variation were 8.2% and 10.4%, respectively. RIA for kisspeptin was conducted using the GQ2 antiserum provided by Dr. Stephen Bloom (Imperial College London, London, United Kingdom; RRID: AB_2686920) as previously described (13). Assay sensitivity was 0.05 pg per tube or 1.0 pg/mL. Intra-assay and interassay coefficients of variation were 10.4% and 14.2%, respectively. LH RIA was conducted using the recombinant cynomolgus LH kit from the Hormone and Peptide Program (LH antibody; Torrance, CA; RRID: AB_2728703). The reference standard used was AFP-6936A provided by the National Hormone and Pituitary Program as previously described (14). Assay sensitivity was 0.01 ng per tube. The intra-assay and interassay coefficients of variation were 3.2% and 5.0%, respectively. T enzyme immunoassay was conducted using the T antibody-156 (obtained from C.J. Munro and G.H. Stabenfeldt, University of California Davis School of Veterinary Medicine, Davis, CA; RRID: AB_2728704) as previously described (15). The reference standard used was Sigma T-1500 (Sigma-Aldrich). Assay sensitivity was 0.01 ng per tube. The intra-assay and interassay coefficients of variation were 4.4% and 9.9%, respectively.
Statistical analysis
To establish the developmental stages, monthly mean (±SEM) LH and T levels (both am and pm), body weight, and testicular volumes in each animal were calculated by age. We first noted that there were large individual variations in androgen and LH levels due to seasonal influence described by Wickings and Nieschlag (16). Thus, subsequently we calculated mean (±SEM) at 3-month intervals by age, that is, 25 months = 24 to 26 months of age. Developmental changes in mean GnRH and kisspeptin levels were derived from averaging all values of GnRH and kisspeptin during the second to the third hour control period after the start of microdialysis experiments. Based on the characterization of the developmental stage, mean (±SEM) GnRH and kisspeptin levels were calculated as prepubertal (16.4 ± 1.0 months of age, n = 9), early pubertal (30.5 ± 1.5 months of age, n = 5), and midpubertal (37.3 ± 1.4 months of age, n = 5) stage males.
Because four microdialysis experiments were conducted successively and/or concurrently, most animals were used in two to three experiments. Statistical analysis was conducted with n = 4 to 6 per group in a minimum of three different animals. When data for a treatment were obtained twice in an animal, the mean of the data was used for statistical analysis. Mean (±SEM) GnRH and kisspeptin levels were compared by treatment within a developmental stage and between developmental stages (prepubertal vs pubertal), as well as to baseline and corresponding vehicle infusion levels. The pulsatility of GnRH and kisspeptin release was not assessed in this study, as the samples were obtained in 20-minute intervals. Additionally, area under the curve (AUC) after the initiation of agonist/antagonist challenge was calculated as follows: (1) in each animal, average GnRH or kisspeptin levels during the baseline period (−60 to 0 minutes) was calculated; (2) for each 20-minute sample after initiation of a challenge, the average baseline was subtracted and this value, which represents the amount of GnRH or kisspeptin above baseline per 20 minutes, was summed for the time period up to 100 minutes in each experiment; (3) group mean (±SEM) was calculated for each challenge; and (4) significance between doses within a developmental stage or between developmental stages (prepubertal vs pubertal) at the same dose was determined. For both developmental and microdialysis studies, two-way ANOVA with repeated measures followed by a Bonferroni post hoc test were applied for statistical comparison using GraphPad Prism (GraphPad Software, San Diego, CA). Because of large individual variations, a Kruskal–Wallis nonparametric test was also applied for the comparison between am and pm hormone levels.
Results
Defining the pubertal stage in male rhesus monkeys
In this study we collected data on physiological and hormonal changes in male monkeys between the ages of 11 and 43 months. Based on the results we were able to define the developmental stages as follows (Fig. 1): the prepubertal stage was characterized by low am and pm LH and T levels (≤1 ng/mL for both) seen until 25 months of age, and a testicular volume ≤1 cm3. The early pubertal stage was characterized by a slight increase in pm LH levels starting at 28 months of age (Fig. 1B) and a small increase in testicular volume. The midpubertal stage was characterized by elevated pm LH levels as well as an increase in pm T levels starting at 34 months of age (Fig. 1C) and an increase in testicular volume. In the later part of the midpubertal stage (40 to 48 months) am T levels were also elevated (Fig. 1C), and a large increase in testicular volume was seen (Fig. 1D). By 48 months of age, late pubertal/adult male monkeys attain the capacity of full spermatogenesis (17). Importantly, pm LH levels in all three pubertal monkeys were consistently higher than the am LH levels (Fig. 1B), whereas pm T levels were higher than the am T levels only in the midpubertal stage and am T levels caught up to pm T levels at 40 months of age (Fig. 1C).
Mean release of GnRH and kisspeptin increases at puberty onset
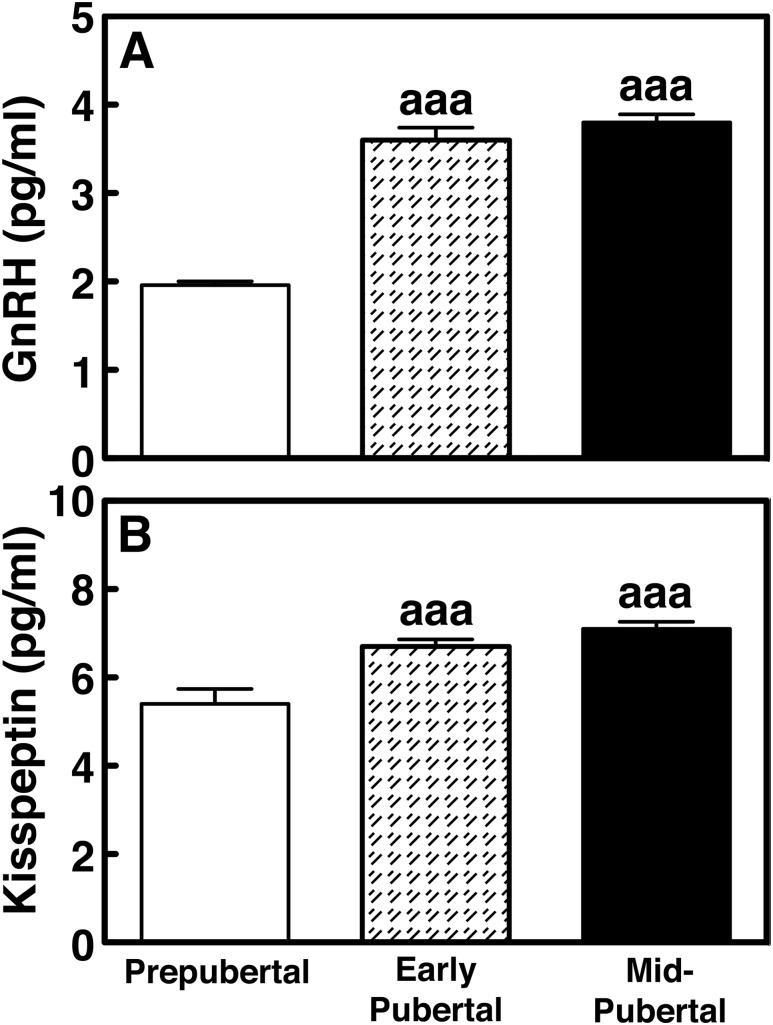
Mean GnRH release levels in early pubertal (3.2 ± 0.1 pg/mL) and midpubertal (3.8 ± 0.1 pg/mL) males were significantly higher (P < 0.001) than that in the prepubertal (2.5 ± 0.1 pg/mL) stage (Fig. 2A). Similarly, kisspeptin release levels in early pubertal (6.5 ± 0.2 pg/mL) and midpubertal (7.1 ± 0.2 pg/mL) males were significantly higher (P < 0.001) than that in prepubertal (5.3 ± 0.3 pg/mL) animals (Fig. 2B).
Figure 2.
Group data (mean ± SEM) showing (A) GnRH and (B) kisspeptin release levels in prepubertal (16.4 ± 1.0 mo of age, n = 9), early pubertal (30.5 ± 1.5 mo of age, n = 5), and midpubertal (36.5 ± 1.3 mo of age, n = 5) stage males. Both GnRH and kisspeptin levels in all pubertal stages were significantly greater (P < 0.001 for all) than those in prepubertal monkeys. aaaP < 0.001 vs prepubertal stage.
Developmental changes in kisspeptin-induced GnRH release (experiment 1)
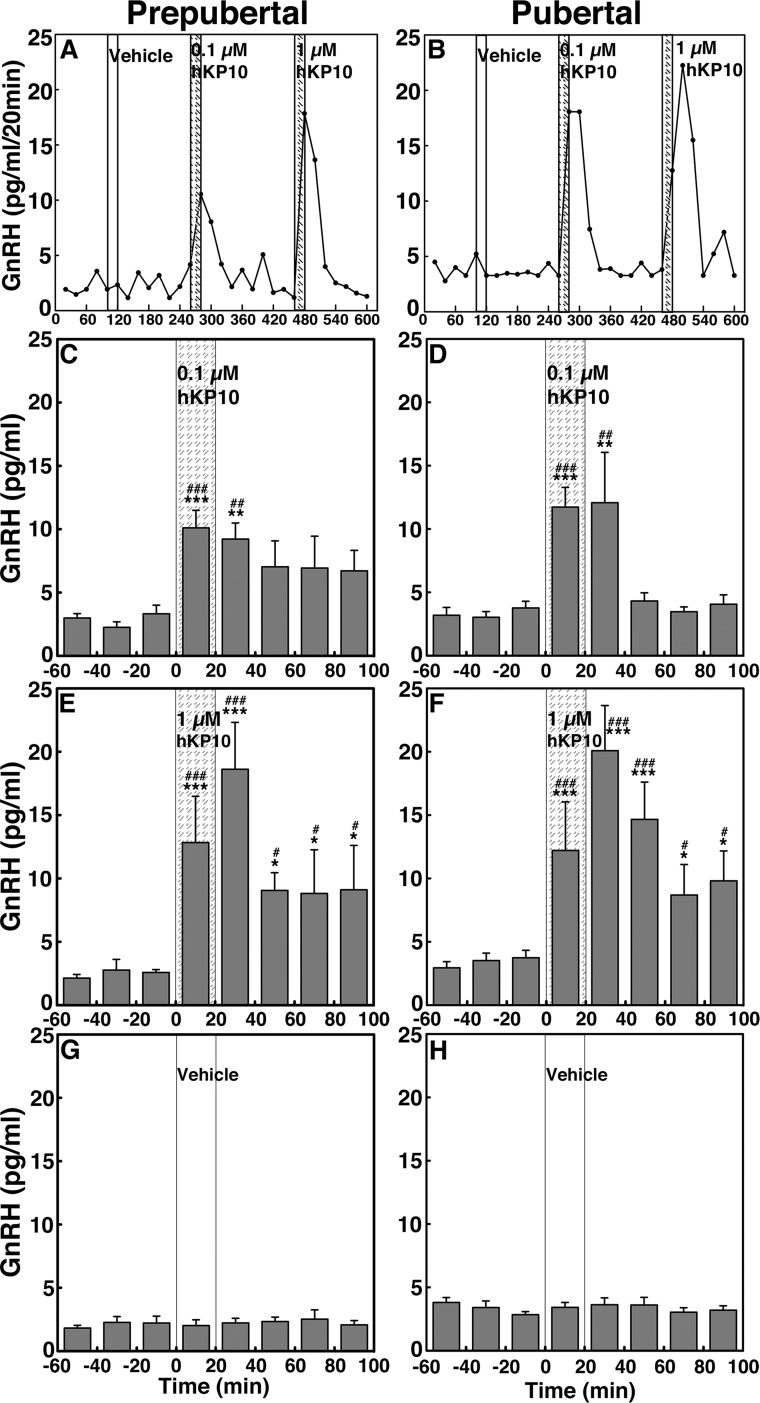
To assess the contribution of kisspeptin signaling to GnRH release across puberty, we examined the effects of two doses of the kisspeptin agonist hKP10 on GnRH release in prepubertal and pubertal male monkeys. In both prepubertal and pubertal monkeys, infusion of 0.1 and 1 µM hKP10 into the ME stimulated GnRH release in a dose-responsive manner (Fig. 3A and 3B, respectively). Group data indicated that the effects of hKP10 at both doses in prepubertal (Fig. 3C and 3E) and pubertal (Fig. 3D and 3F) monkeys were significant (P < 0.001 for all) when compared with vehicle infusion (Fig. 3G and 3H, respectively). Post hoc analysis indicates that the prepubertal GnRH response to 0.1 µM hKP10 persists for 40 minutes (P < 0.001 for 0 to 20 minutes and P < 0.01 for 20 to 40 minutes), and for 100 minutes after the 1 µM hKP10 infusion (P < 0.001 for 0 to 40 minutes and P < 0.05 for 40 to 100 minutes). Durations of the induced GnRH responses to hKP10 were similar in both stages. Additionally, the AUC for GnRH release indicates that hKP10 stimulated GnRH release in a dose-responsive manner in both stages (P < 0.05 and P < 0.001, Fig. 4A and 4B); however, only the 1 µM hKP10-induced GnRH response was significantly greater in pubertal animals than in prepubertal animals (P < 0.001).
Figure 3.
(A and B) Representative cases and group data (mean ± SEM) showing that hKP10 infusion into the ME stimulated GnRH release in a dose-responsive manner in both prepubertal and pubertal (left and right panels, respectively) monkeys. Animals were treated with (C and D) 0.1 µM hKP10, (E and F) 1 µM hKP10, and (G and H) vehicle for control. In prepubertal and pubertal monkeys, both doses of hKP10 infusion significantly (P < 0.001) stimulated GnRH release over baseline and vehicle infusion GnRH levels. Vehicle infusion alone did not alter GnRH release (P > 0.05). *P < 0.05, **P < 0.01, ***P < 0.001 vs vehicle infusion; #P < 0.05, ##P < 0.01, ###P < 0.001 vs before infusion.
Figure 4.
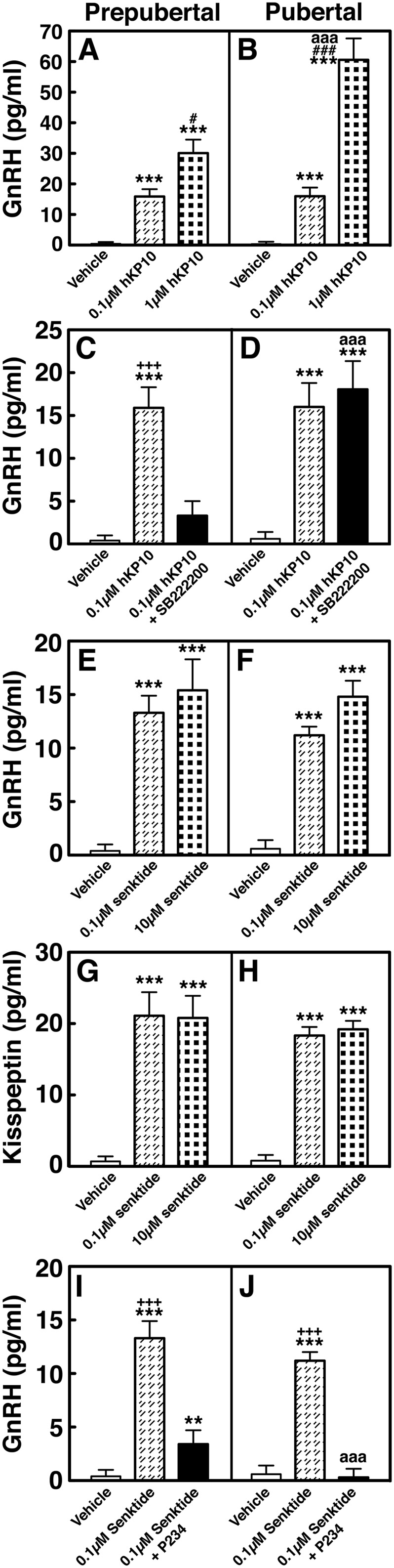
Group data (mean ± SEM) showing changes in AUC of GnRH and kisspeptin release in prepubertal and pubertal (left and right panels, respectively) monkeys. (A and B) In experiment 1 shown in Fig. 3, hKP10 stimulated GnRH release in a dose-responsive manner in prepubertal (P < 0.05) and pubertal (P < 0.001) monkeys and 1 µM hKP10-stimulated GnRH release in pubertal animals was greater (P < 0.001) than that in prepubertal animals. (C and D) In experiment 2 shown in Fig. 5, the presence of NKB antagonist SB222200 (1 µM) blocked the 0.1 µM hKP10-stimulated GnRH release in prepubertal monkeys only (P < 0.001). (E and F) In experiment 3a shown in Fig. 6, 0.1 and 10 µM senktide stimulated GnRH release over vehicle in prepubertal and pubertal monkeys (P < 0.001 for both). (G and H) In experiment 3b shown in Fig. 7, both doses of senktide-stimulated kisspeptin release over vehicle in prepubertal and pubertal (P < 0.001 for both) monkeys. (I and J) In experiment 4 shown in Fig. 8, the presence of kisspeptin antagonist P234 (0.1 µM) attenuated the 0.1 µM senktide-stimulated GnRH release in prepubertal monkeys (P < 0.001), whereas it blocked the senktide-stimulated GnRH release in pubertal monkeys (P < 0.001). Note that in the presence of P234, the senktide-induced GnRH release was significantly different from vehicle control in the prepubertal stage animals (P < 0.01), not pubertal stage animals. **P < 0.01, ***P < 0.001 vs vehicle infusion; #P < 0.05, ###P < 0.001 vs 0.1 µM hKP10 infusion; +++P < 0.001 vs blocking experiment; aaaP < 0.001 vs prepubertal stage.
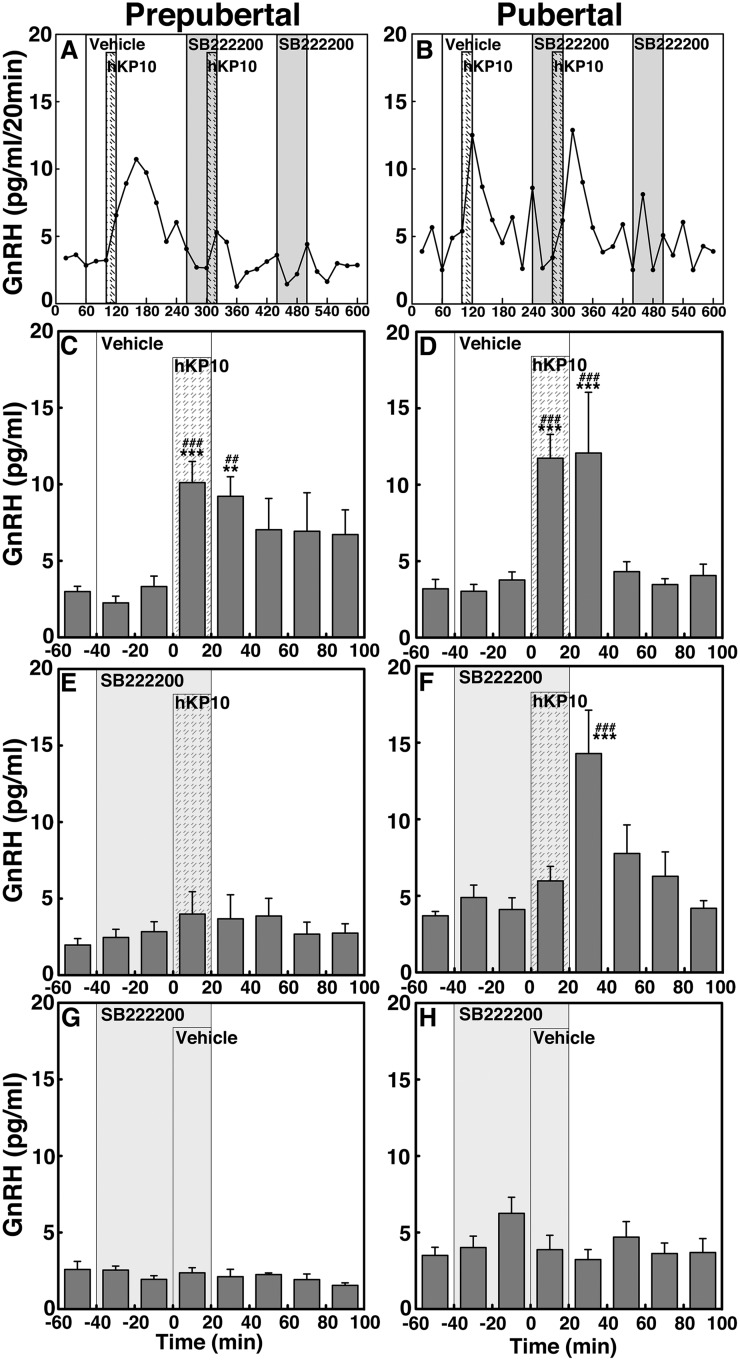
SB222200 suppresses the hKP10-induced GnRH release in prepubertal but not pubertal male monkeys (experiment 2)
To determine whether kisspeptin signaling to GnRH release is mediated through NKB receptor activation and whether the signaling network between kisspeptin and NKB undergoes developmental change, we examined the effects of NKB antagonist SB222200 on the 0.1 µM hKP10-induced GnRH release in prepubertal and pubertal males (Fig. 5). Similar to the results in experiment 1, hKP10 stimulated GnRH release in the presence of vehicle in both prepubertal and pubertal monkeys (P < 0.001 for both stages, Fig. 5A–5D). However, in the presence of SB222200, the expected hKP10-induced GnRH release was abrogated (P > 0.05) in prepubertal monkeys (Fig. 5E). In contrast, in the pubertal stage animals the presence of SB222200 delayed the hKP10-induced GnRH release by 20 minutes (Fig. 5F). That is, the hKP10-induced GnRH release occurred during the first 20 minutes after the hKP10 infusion, rather than during the 20-minute hKP10 infusion. SB222200 infusion alone did not induce any changes (P > 0.05; Fig. 5G and 5H). AUC further indicated that the hKP10-induced GnRH release is blocked when in the presence of SB222200 in prepubertal stage animals only (P < 0.001, Fig. 4C). In contrast, in pubertal monkeys the presence or absence of SB222200 did not significantly (P > 0.05) change the hKP10-induced GnRH release (Fig. 4D).
Figure 5.
(A and B) Representative cases and group data (mean ± SEM) showing modification of the 0.1 µM hKP10-induced GnRH release by the NKB antagonist SB222200 (0.1 µM) in prepubertal and pubertal (left and right panels, respectively) monkeys. Note that hKP10 significantly (P < 0.001) induced GnRH release in the absence of SB222200 in both (C) prepubertal and (D) pubertal monkeys when compared with baseline and vehicle infusion GnRH levels. However, in the presence of SB222200, the hKP10-induced GnRH release was absent (P > 0.05) in (E) prepubertal animals, whereas in (F) pubertal animals, hKP10-stimulated GnRH release (P < 0.001) with a 20-min delay. SB222200 alone did not significantly (P > 0.05) alter GnRH release (G and H). **P < 0.01, ***P < 0.001 vs vehicle infusion; ##P < 0.01, ###P < 0.001 vs before infusion.
Developmental change in NKB-induced GnRH release (experiment 3a)
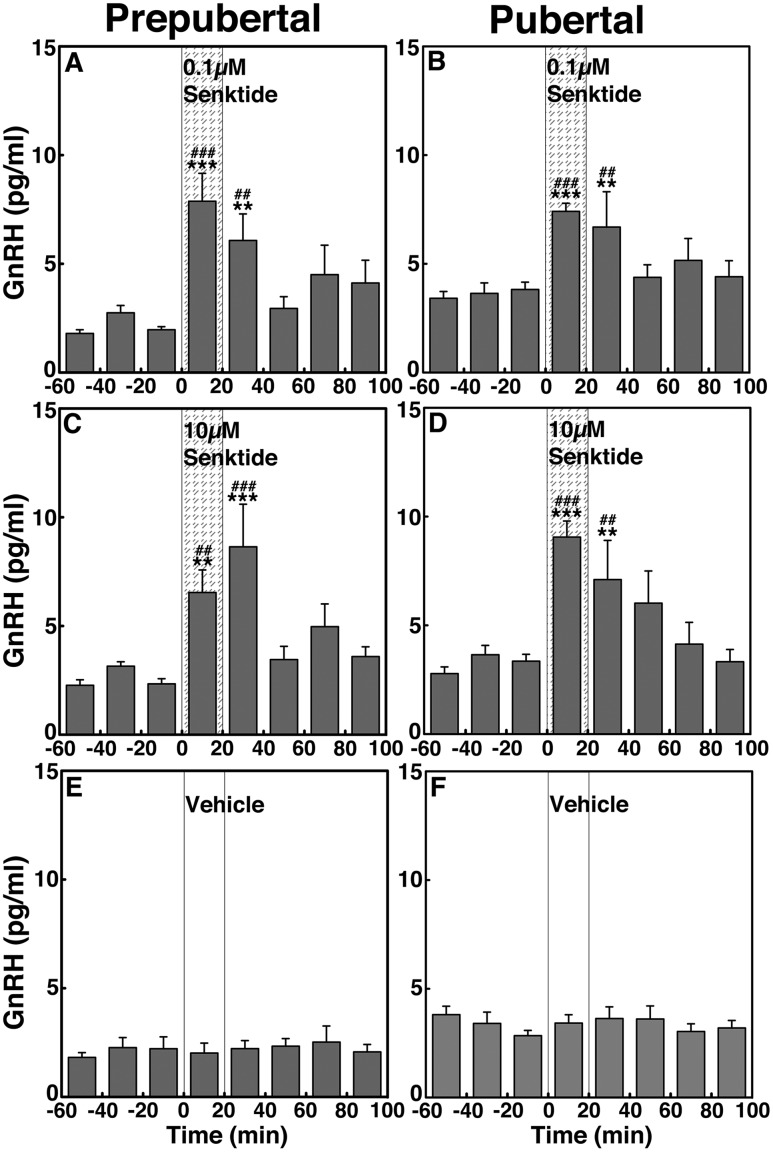
To assess the contribution of NKB signaling to GnRH release across puberty, we examined the effects of two doses of the NKB agonist senktide on GnRH release in prepubertal and pubertal males. In prepubertal animals, infusion of senktide into the ME at both doses (0.1 and 10 µM) stimulated GnRH release (Fig. 6A and 6C). Similarly, in pubertal animals, senktide at both doses stimulated GnRH release (Fig. 6B and 6D). Vehicle infusion did not induce any changes (Fig. 6E and 6F, respectively). In both stages, the effects of 0.1 and 10 µM senktide on GnRH release were significantly larger (P < 0.001 for all) as compared with vehicle infusion. Post hoc analysis indicated that in prepubertal monkeys the senktide-induced increases in GnRH release during the first 40 minutes after initiation were larger (P < 0.001 for 0 to 20 minutes and P < 0.01 for 20 to 40 minutes for the low dose; P < 0.01 for 0 to 20 minutes and P < 0.001 for 20 to 40 minutes for the high dose) than the preinfusion levels as well as the corresponding period of vehicle control. Similarly, in pubertal monkeys, the GnRH responses to senktide during the first 40 minutes after initiation were higher (P < 0.001 for 0 to 20 minutes and P < 0.01 for 20 to 40 minutes for both doses) than the preinfusion levels as well as the corresponding period of vehicle control. AUC indicated that the senktide-induced GnRH responses in prepubertal (Fig. 4E) and pubertal (Fig. 4F) monkeys were not dose-dependent (P > 0.05) and that there was no developmental change between prepubertal and pubertal males (P > 0.05).
Figure 6.
Group data (mean ± SEM) showing that senktide infusion into the ME stimulated GnRH release in both prepubertal and pubertal (left and right panels, respectively) monkeys. Animals were treated with (A and B) 0.1 µM senktide, (C and D) 10 µM senktide, and (E and F) vehicle. In prepubertal and pubertal animals, both doses of senktide significantly (P < 0.001) induced GnRH release over baseline and vehicle infusion GnRH levels. Note that the senktide-induced GnRH release was not dose-responsive. Vehicle infusion alone did not alter GnRH release (P > 0.05). **P < 0.01, ***P < 0.001 vs vehicle infusion; ##P < 0.01, ###P < 0.001 vs before infusion.
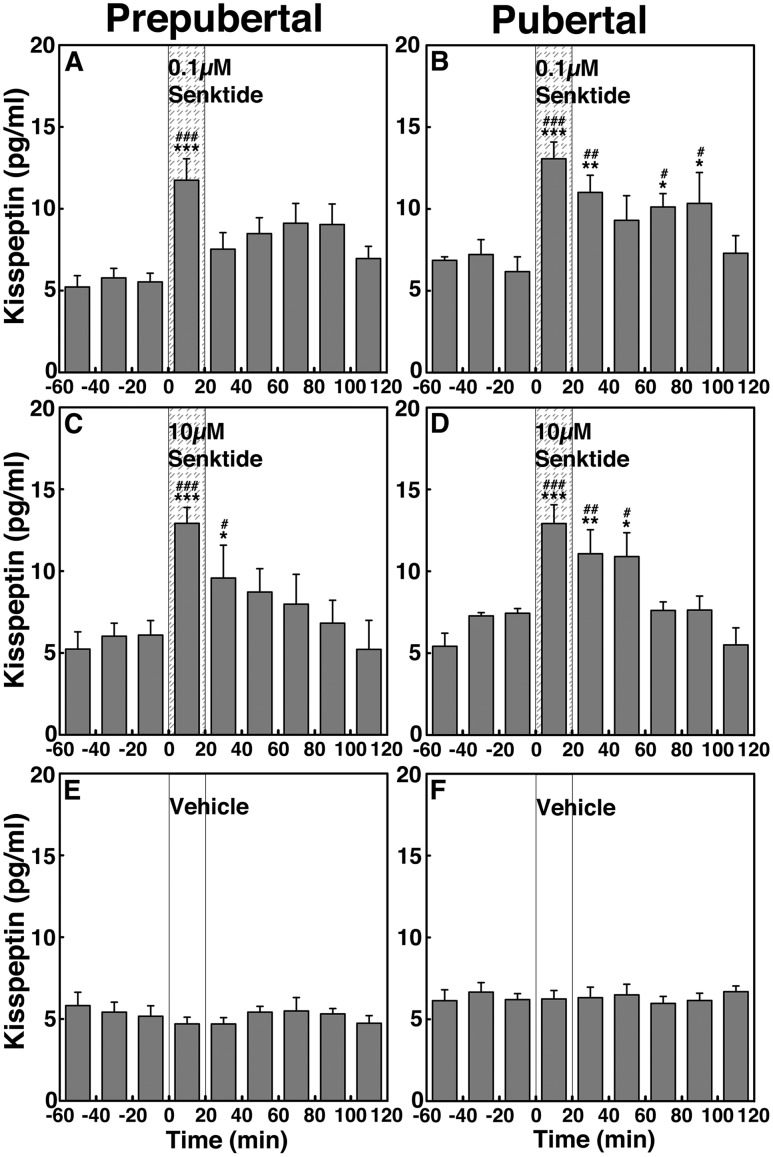
Developmental change in NKB-induced kisspeptin release (experiment 3b)
To assess the contribution of NKB signaling to kisspeptin release across puberty, we examined the effects of senktide on kisspeptin release in prepubertal and pubertal males. Parallel to the senktide-induced GnRH release, senktide significantly (P < 0.001 for all) stimulated kisspeptin release in prepubertal (Fig. 7A and 7C) and pubertal (Fig. 7B and 7D) monkeys when compared with vehicle control (Fig. 7E and 7F). Interestingly, in pubertal monkeys, senktide at both 0.1 and 10 µM induced a prolonged elevation of kisspeptin increases up to 100 minutes or 60 minutes, respectively. Post hoc analysis further indicated that in prepubertal males, kisspeptin responses to senktide at 0.1 µM during the 20 minutes after initiation of infusion and 10 µM during the 20 minutes and 20 to 40 minutes after initiation of infusion were larger (P < 0.001 to P < 0.01, respectively) than preinfusion levels as well as with the corresponding period of vehicle control. In pubertal males kisspeptin responses to senktide at 0.1 µM induced significant kisspeptin increases up to 100 minutes after initiation of infusion, which were significantly larger (P < 0.001 to P < 0.05) than in preinfusion levels as well as with the corresponding period of vehicle control. Similarly, senktide at 10 µM induced kisspeptin increases up to 60 minutes after initiation of infusion (P < 0.001 to P < 0.05). AUC for kisspeptin indicated that similar to the senktide effects on GnRH release, the senktide-induced kisspeptin release did not significantly change (P > 0.05) by dose in both stages (Fig. 4G and 4H). Furthermore, comparison between the two developmental stages indicated that the stimulated kisspeptin release in pubertal animals was not significantly different (P > 0.05) than that in the prepubertal animals at a respective dose.
Figure 7.
Group data (mean ± SEM) showing that senktide infusion into the ME stimulated kisspeptin release in both prepubertal and pubertal (left and right panels, respectively) monkeys. Animals were treated with (A and B) 0.1 µM senktide, (C and D) 10 µM senktide, and (E and F) vehicle. In prepubertal and pubertal animals, both doses of senktide significantly (P < 0.001) induced kisspeptin release over baseline and vehicle infusion kisspeptin levels. Note that the senktide-induced kisspeptin release was not dose-responsive. Vehicle infusion alone did not alter kisspeptin release (P > 0.05). *P < 0.05, **P < 0.01, ***P < 0.001 vs vehicle infusion; #P < 0.05; ##P < 0.01, ###P < 0.001 vs before infusion.
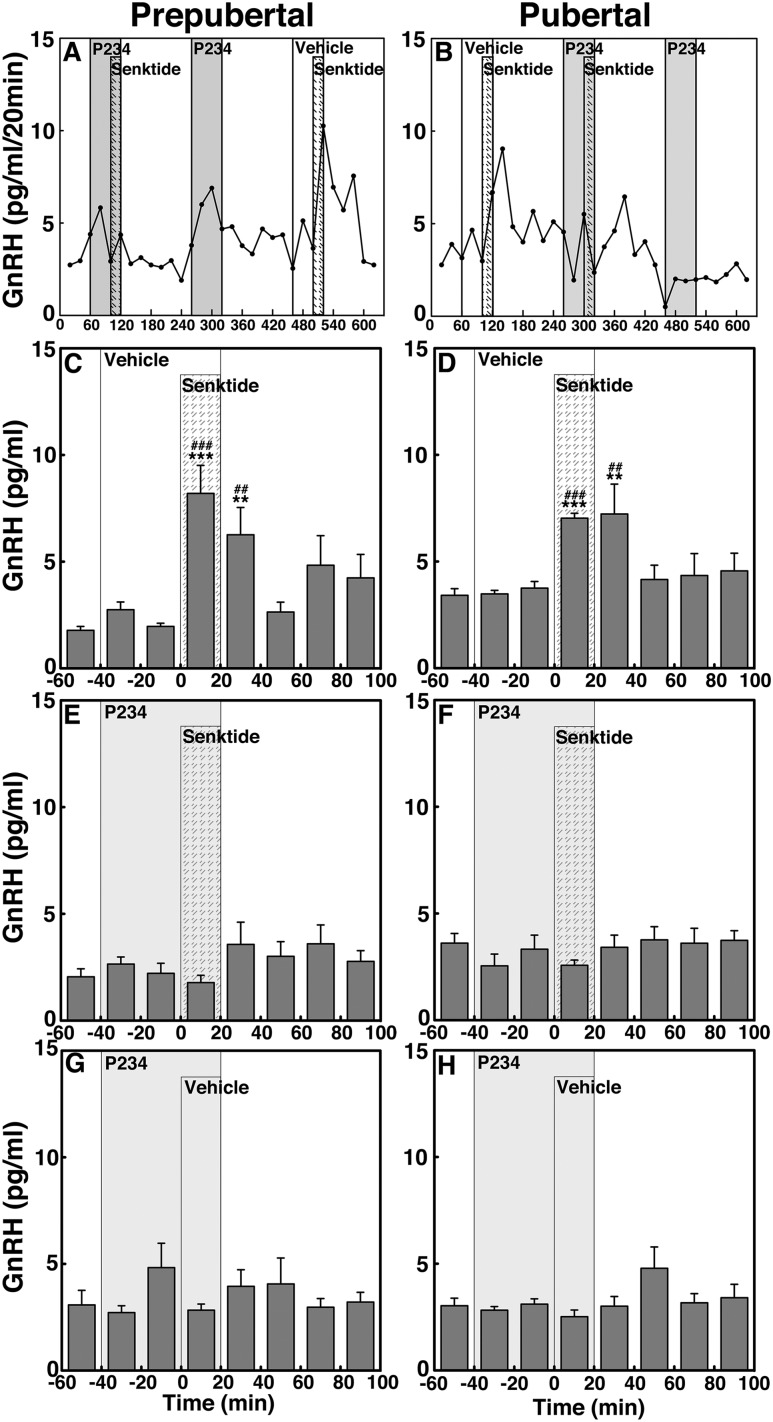
P234 blocks the senktide-induced GnRH release in both prepubertal and pubertal male monkeys (experiment 4)
To determine whether NKB signaling to GnRH release is mediated through kisspeptin receptor activation and whether the signaling network between NKB and kisspeptin undergoes developmental change, we examined the effects of 0.1 µM senktide on GnRH release in the presence or absence of the kisspeptin antagonist P234 in prepubertal and pubertal male monkeys (Fig. 8). Consistent with the results in experiment 3a, senktide in the absence of P234 (vehicle control) stimulated GnRH release in both prepubertal and pubertal monkeys (P < 0.001 for both, Fig. 8A–8D). In contrast, in the presence of P234 the senktide-induced GnRH release was blocked (P > 0.05) in prepubertal and pubertal animals (Fig. 8E and 8F, respectively). P234 infusion alone did not induce any changes (P > 0.05; Fig. 8G and 8H). Examination of AUC further indicated that the senktide-induced GnRH release in the presence of P234 was significantly smaller (P < 0.001) than in the absence of P234 in both prepubertal and pubertal animals (Fig. 4I and 4J). Importantly, however, note that unlike in pubertal animals, AUC indicated that P234 did not completely block the senktide-induced GnRH release in prepubertal animals (P < 0.01 over vehicle).
Figure 8.
(A and B) Representative cases and group data (mean ± SEM) showing modification of the 0.1 µM senktide-induced GnRH release by the kisspeptin antagonist P234 (0.1 µM) in prepubertal and pubertal (left and right panels, respectively) monkeys. Note that senktide significantly (P < 0.001) induced GnRH release in the absence of P234 in both (C) prepubertal and (D) pubertal monkeys when compared with baseline and vehicle infusion GnRH levels. However, in the presence of P234, the senktide-induced GnRH release was greatly attenuated in (E) prepubertal animals and absent in (F) pubertal animals (P > 0.05). (G and H) P234 alone did not significantly (P > 0.05) alter GnRH release. **P < 0.01, ***P < 0.001 vs vehicle infusion; ##P < 0.01, ###P < 0.001 vs before infusion.
Discussion
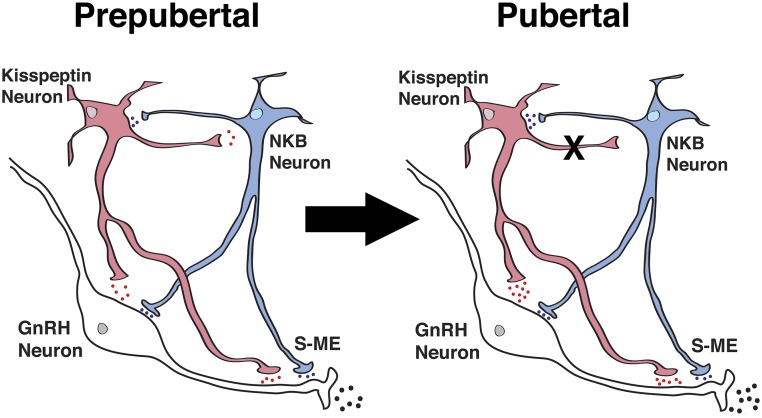
In the current study, we first defined the developmental stage in male rhesus monkeys by assessment of physiological and hormonal changes during sexual development. Subsequently, we examined the roles of kisspeptin and NKB signaling in the regulation of the pubertal increase in GnRH release by measuring release of GnRH and kisspeptin. Our findings are summarized as follows: first, similar to females, the mean release of GnRH and kisspeptin in pubertal males was higher than that in prepubertal males. Second, infusion of the kisspeptin agonist hKP10 into the ME stimulated GnRH release in a dose-responsive manner in both prepubertal and pubertal males, and the hKP10-induced GnRH release in pubertal males was greater than that in prepubertal males. Third, infusion of the NKB agonist senktide also stimulated release of GnRH and kisspeptin in prepubertal and pubertal males, but the stimulated GnRH and kisspeptin responses were not dose responsive. The duration of the senktide-induced kisspeptin release in pubertal males was, however, longer than that in prepubertal males. Fourth, the hKP10-induced GnRH release was blocked by the NKB antagonist SB222200 in prepubertal, but not pubertal, males. Fifth, the senktide-induced GnRH release was blocked by the kisspeptin antagonist P234 in both prepubertal and pubertal male monkeys. Taken together, these results suggest that although the regulatory mechanism for GnRH release by collaborative kisspeptin and NKB signaling exists before puberty, after puberty onset kisspeptin signaling mediated through NKB neurons is lost and kisspeptin signaling assumes the primary stimulatory role in the regulation of GnRH release in male rhesus monkeys (Fig. 9).
Figure 9.
Schematic diagram showing the developmental changes in kisspeptin (red) and NKB (blue) signaling to GnRH neurons in the S-ME in prepubertal (left) and pubertal (right) male monkeys. During the prepubertal period, kisspeptin and GnRH neurons are under tonic inhibition. Note that the “X” between kisspeptin and NKB neurons indicates the absence of a signaling pathway and the blue, red, and black dots indicate the relative amount of neuropeptide release. Also note that kisspeptin singling to NKB neurons is hypothetical, as we did not measure the kisspeptin-induced NKB release in the current study. S-ME, stalk–median eminence.
To determine the developmental changes in GnRH and kisspeptin release across male puberty, we first classified the animals into prepubertal, early pubertal, and midpubertal stages by assessing changes in circulating LH and T levels and testicular volume (Fig. 1A). Previously, based on testicular development and spermatogenesis, Plant and colleagues (18, 19) established the early pubertal stage in male rhesus monkeys starting at 42 months of age. However, male puberty in rhesus monkeys appears to start between 25 and 28 months of age, as in the current study we found that pm levels of LH at 28 months of age were significantly higher than those at 25 months of age. Our classification of male puberty is consistent with that reported by Lomniczi et al. (20) in orchidectomized males by measurement of LH levels.
In testicular intact neonatal males transiently high gonadotropin and T levels and transient proliferation of Sertoli cells are seen (21–23). Plant (22) showed that the frequency and amplitude of LH release in neonatal males are as high as in adult males. The activated pituitary–testicular axis during the neonatal period, commonly known as mini-puberty, is the consequence of elevated kisspeptin signaling to GnRH neurons (24). Subsequently, the gonadal steroid-independent “central inhibition” results in the suppression of gonadotropin release, which is the characteristic of the prepubertal period in male monkeys (25). Similar steroid-independent central inhibition on release of GnRH and LH has also been shown in prepubertal female rhesus monkeys (26), although activity of the GnRH neurosecretory system during the neonatal period is far less than that in males (27).
Direct measurement of neuropeptides in the ME indicate that both mean release of GnRH and kisspeptin in early, middle, and late pubertal males were significantly higher than those in prepubertal counterparts. In fact, similar parallel developmental increases in GnRH and kisspeptin levels were reported in female rhesus monkeys (4, 5), except the timetable shifts to slightly older ages in males. Because hKP10 stimulated GnRH release in a dose-responsive manner and kisspeptin signaling in pubertal males was larger than in prepubertal males (Fig. 3), a larger GnRH release in pubertal males is attributable to a greater release of kisspeptin and higher sensitivity of KISS1R on GnRH neurons. In other words, similar to females (4, 5), the pubertal increase in GnRH release in male monkeys is, at least in part, due to a larger kisspeptin release and higher sensitivity of KISS1R.
Comparison of the results from the current study in males and a previous study in females (8) reveals a subtle sex difference in the role of kisspeptin signaling in puberty. GnRH neurons in females are 10-fold more sensitive to kisspeptin signaling than in males. For instance, in gonadally intact prepubertal and pubertal males, GnRH responses to hKP10 occur at the doses of 0.1 and 1 µM, whereas similar GnRH responses to hKP10 in gonadally intact prepubertal and pubertal females take place at 0.01 and 0.1 µM doses (4, 8). Likewise, whereas the GnRH response to hKP10 in pubertal females is maximized at 0.1 µM (4, 8), the same phenomenon in males is seen at 1 µM. Furthermore, developmental amplification of the hKP10-induced GnRH release, that is, a larger magnitude of GnRH response to hKP10 in pubertal animals over prepubertal animals, is limited to only the high dose of hKP10 in males, and the response was much smaller than that in females.
In the current study we found that senktide stimulates release of both GnRH and kisspeptin in prepubertal and pubertal males. However, the senktide-stimulated release of GnRH and kisspeptin was not dose-dependent. Importantly, there was no developmental amplification of the senktide-induced release of GnRH and kisspeptin. These findings sharply contrast those in females. Senktide-induced release of GnRH and kisspeptin was dose-dependent and there was a twofold developmental amplification of the senktide-induced kisspeptin release in females (8). Additionally, the high-dose (10 µM) senktide-induced kisspeptin release in pubertal females (8) was fourfold larger than that in males. Therefore, it appears that the response of the female kisspeptin system to NKB signaling is sensitive to circulating estradiol, whereas there is little influence of gonadal steroids in this context in males. This speculation is based on our previous observations showing that ovariectomy dramatically attenuated the GnRH response to kisspeptin in pubertal, but not prepubertal, females (4) and our preliminary data that castration did not change GnRH response to kisspeptin in pubertal males (28). Additional studies are necessary to clarify the role of gonadal steroids in sex differences in NKB and kisspeptin signaling.
The finding that hKP10-induced GnRH release was blocked by the presence of SB222200 in prepubertal, but not pubertal, males suggests that in prepubertal males kisspeptin-induced GnRH release is, in part, mediated through NKB signaling, whereas in pubertal males this pathway is no longer available (Fig. 9). In contrast, senktide-induced GnRH release was completely blocked by P234 in pubertal males and greatly attenuated in prepubertal males. These results indicate that in males NKB signaling to GnRH neurons mediated through kisspeptin neurons is present throughout sexual maturation to adulthood, but kisspeptin signaling mediated through NKB neurons is limited to the prepubertal period (Fig. 9). We speculate that the reciprocal pathways are important during the neonatal mini-puberty period in males, when activity of GnRH neurons (and LH release) is as high as that in adults (22). Perhaps subsequent central inhibition during the prepubertal stage masks the activity of the reciprocal pathways until the time of puberty onset. At the onset of puberty, the reciprocal pathways in the prepubertal stage are remodeled to a simpler kisspeptin-dominant pathway. The mechanism of the pathway remodeling remains to be investigated. Nevertheless, these developmental changes in the interactions between kisspeptin and NKB signaling in males are significantly different from those in females (8). In prepubertal females there are no reciprocal pathways until the time of puberty onset, perhaps reflecting a less robust mini-puberty. The reciprocal pathways accelerate the pubertal increase in GnRH release and provide mechanisms for regulation of the complex adult female reproductive functions, including cyclic ovulation and pregnancy.
The regulation of GnRH release by estrogens and androgens is thought to be mediated through kisspeptin and NKB neurons. This concept is established by the reports showing that (1) whereas GnRH neurons express neither estrogen receptor α nor androgen receptors, kisspeptin and NKB neurons do (29–32), and (2) cell bodies and/or fibers of GnRH neurons in rodents, ruminants, and primates express Kiss1r or KISS1R (33–37) and Nk3r or NK3R (38, 39). Additionally, kisspeptin neurons express Nk3r or NK3R (40, 41), but not Kiss1r (42). NKB neurons express Nk3r (43, 44), but whether they express Kiss1r or KISS1R is presently unclear. In adult females, a larger number of kisspeptin neurons in the arcuate nucleus express NKB in several species, including humans, whereas a smaller number of kisspeptin neurons in the arcuate nucleus contained NKB in males (40, 45–47). Thus, it is reasonable to assume that sex differences in the neurocircuitry between kisspeptin and NKB signaling at puberty in the current study are the consequence of changes in an increased number and activity of kisspeptin and NKB neurons and/or availability of a larger number of KISS1R and NK3R regulated by the pubertal increase in estradiol in females and androgen increase in males.
In general, the number of kisspeptin and NKB neurons in neonatal rodent and human brains in the gonadally intact condition are either not present or much smaller than those in pubertal and adult brains, respectively (48–50). For example, kisspeptin neurons in the anteroventral periventricular nucleus are not found in P10, start to increase by P25, and reach a maximum in number at or shortly after puberty in both male and female mice (48, 49). Analysis of the postmortem human brain also indicates that there are relatively smaller numbers of NKB neurons in the infundibular nucleus and ME in gonadally intact male and female infants (50), and the number increases at the age of puberty (46, 50). Collectively, it appears that the numbers of both kisspeptin and NKB neurons increase at puberty onset in gonadally intact primates and nonprimate species.
There are significant sex differences in kisspeptin and NKB signaling. The number of kisspeptin and NKB neurons in human females is larger than in males, and this female-dominant difference in the kisspeptin number is greater than that in NKB neurons (51). Female-dominant expression of NKB neurons in the arcuate nucleus in sheep and rats are also reported (31, 52, 53). Moreover, appositions of synaptic kisspeptin and NKB inputs to GnRH neurons in human adult females are greater than those in males (51). Consistent with our monkey studies, in female rats, LH responsiveness to senktide in females is larger than that in males throughout the developmental stages, although male rats lose LH responsiveness to senktide after puberty onset (53). The sex difference in the number of kisspeptin and NKB neurons in mice is dependent on circulating gonadal steroids (49, 54). In primates as well, a larger release of GnRH in response to kisspeptin signaling and kisspeptin response to NKB signaling in females is due to the pubertal increase in circulating estradiol. In contrast, the pubertal increase in androgens in males appears to influence kisspeptin and NKB signaling to a much smaller degree. These speculations, however, need to be experimentally examined.
With measurement of LH release, upstream NKB signaling over kisspeptin signaling has been reported in both rats and male monkeys (10, 55). However, a fast scan cyclic voltammetry study in mice indicates that activation of Nk3r results in GnRH release independent of kisspeptin signaling at the ME where GnRH neuroterminals are present, but not in the preoptic area where GnRH cell bodies are present (39). Therefore, in primates NKB signaling may directly modify GnRH release in the ME, where the NKB fibers appose GnRH neuroterminal fibers (46) and where our microdialysis cannula tip is located.
In summary, results from this study demonstrate that in male monkeys the reciprocal kisspeptin and NKB signaling mechanism is present before puberty onset, but at puberty onset kisspeptin signaling takes over the stimulatory role in the pubertal increase in GnRH release. This is in contrast to that in females, where collaborative kisspeptin and NKB signaling to GnRH neurons is established after puberty onset. The mechanism underlying this sexual dimorphism remains to be investigated.
Supplementary Material
Acknowledgments
We thank Dustin Richter, Lucille Kohlenberg, William Lundeen, and Ryan Anderson for technical assistance as well as the veterinarians and veterinary technicians of the Wisconsin National Primate Research Center. The authors also recognize the technical assistance of Hemanta Shrestha of Assay Services for iodination of reagents used in the RIAs.
Financial Support: This work was supported by National Institutes of Health Grant R01HD011355 (to E.T.), National Institutes of Health Grants R01HD043341 and P50HD028138 (to S.B.S.) from the Eunice Kennedy Shriver Institute of Child Health and Human Development, and by National Institutes of Health Grants R25GM083252 and T32HD041921 (to J.P.G.) for predoctoral training. The work was made possible by support from the National Institutes of Health Office of the Director for the Wisconsin National Primate Research Center (Grant OD011106). S.B.S. is a Robert and Laura Reynolds Research Scholar.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- AUC
area under the curve
- hKP10
human kisspeptin-10
- KISS1R
kisspeptin receptor
- ME
median eminence
- NKB
neurokinin B
- NK3R
neurokinin B receptor
- P234
peptide 234
- RIA
radioimmunoassay
- T
testosterone
References
- 1. Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MB, Crowley WF Jr, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349(17):1614–1627. [DOI] [PubMed] [Google Scholar]
- 2. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100(19):10972–10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Imamoglu S, Akalin NS, Yuksel B, O’Rahilly S, Semple RK. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for neurokinin B in the central control of reproduction. Nat Genet. 2009;41(3):354–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guerriero KA, Keen KL, Millar RP, Terasawa E. Developmental changes in GnRH release in response to kisspeptin agonist and antagonist in female rhesus monkeys (Macaca mulatta): implication for the mechanism of puberty. Endocrinology. 2012;153(2):825–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guerriero KA, Keen KL, Terasawa E. Developmental increase in kisspeptin-54 release in vivo is independent of the pubertal increase in estradiol in female rhesus monkeys (Macaca mulatta). Endocrinology. 2012;153(4):1887–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Watanabe G, Terasawa E. In vivo release of luteinizing hormone releasing hormone increases with puberty in the female rhesus monkey. Endocrinology. 1989;125(1):92–99. [DOI] [PubMed] [Google Scholar]
- 7. Chongthammakun S, Claypool LE, Terasawa E. Ovariectomy increases in vivo luteinizing hormone-releasing hormone release in pubertal, but not prepubertal, female rhesus monkeys. J Neuroendocrinol. 1993;5(1):41–50. [DOI] [PubMed] [Google Scholar]
- 8. Garcia JP, Guerriero KA, Keen KL, Kenealy BP, Seminara SB, Terasawa E. Kisspeptin and neurokinin B signaling network underlies the pubertal increase in GnRH release in female rhesus monkeys. Endocrinology. 2017;158(10):3269–3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Plant T, Terasawa E, Witchel S. Puberty in non-human primates and man. In: Plant TM, Zeleznik AJ, eds. Knobil and Neill’s Physiology of Reproduction. Vol. 2. 4th ed. New York, NY: Academic Press; 2015:1487–1536.
- 10. Ramaswamy S, Seminara SB, Plant TM. Evidence from the agonadal juvenile male rhesus monkey (Macaca mulatta) for the view that the action of neurokinin B to trigger gonadotropin-releasing hormone release is upstream from the kisspeptin receptor. Neuroendocrinology. 2011;94(3):237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gearing M, Terasawa E. Luteinizing hormone releasing hormone (LHRH) neuroterminals mapped using the push-pull perfusion method in the rhesus monkey. Brain Res Bull. 1988;21(1):117–121. [DOI] [PubMed] [Google Scholar]
- 12. Frost SI, Keen KL, Levine JE, Terasawa E. Microdialysis methods for in vivo neuropeptide measurement in the stalk-median eminence in the rhesus monkey. J Neurosci Methods. 2008;168(1):26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Keen KL, Wegner FH, Bloom SR, Ghatei MA, Terasawa E. An increase in kisspeptin-54 release occurs with the pubertal increase in luteinizing hormone-releasing hormone-1 release in the stalk-median eminence of female rhesus monkeys in vivo. Endocrinology. 2008;149(8):4151–4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mizuno M, Terasawa E. Search for neural substrates mediating inhibitory effects of oestrogen on pulsatile luteinising hormone-releasing hormone release in vivo in ovariectomized female rhesus monkeys (Macaca mulatta). J Neuroendocrinol. 2005;17(4):238–245. [DOI] [PubMed] [Google Scholar]
- 15. Ziegler TE, Wegner FH, Carlson AA, Lazaro-Perea C, Snowdon CT. Prolactin levels during the periparturitional period in the biparental cotton-top tamarin (Saguinus oedipus): interactions with gender, androgen levels, and parenting. Horm Behav. 2000;38(2):111–122. [DOI] [PubMed] [Google Scholar]
- 16. Wickings EJ, Nieschlag E. Seasonality in endocrine and exocrine testicular function of the adult rhesus monkey (Macaca mulatta) maintained in a controlled laboratory environment. Int J Androl. 1980;3(1):87–104. [DOI] [PubMed] [Google Scholar]
- 17. Plant TM. Undifferentiated primate spermatogonia and their endocrine control. Trends Endocrinol Metab. 2010;21(8):488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Plant TM, Ramaswamy S, Simorangkir D, Marshall GR. Postnatal and pubertal development of the rhesus monkey (Macaca mulatta) testis. Ann N Y Acad Sci. 2005;1061(1):149–162. [DOI] [PubMed] [Google Scholar]
- 19. Simorangkir DR, Ramaswamy S, Marshall GR, Roslund R, Plant TM. Sertoli cell differentiation in rhesus monkey (Macaca mulatta) is an early event in puberty and precedes attainment of the adult complement of undifferentiated spermatogonia. Reproduction. 2012;143(4):513–522. [DOI] [PubMed] [Google Scholar]
- 20. Lomniczi A, Wright H, Castellano JM, Matagne V, Toro CA, Ramaswamy S, Plant TM, Ojeda SR. Epigenetic regulation of puberty via zinc finger protein-mediated transcriptional repression. Nat Commun. 2015;6(1):10195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mann DR, Davis-DaSilva M, Wallen K, Coan P, Evans DE, Collins DC. Blockade of neonatal activation of the pituitary-testicular axis with continuous administration of a gonadotropin-releasing hormone agonist in male rhesus monkeys. J Clin Endocrinol Metab. 1984;59(2):207–211. [DOI] [PubMed] [Google Scholar]
- 22. Plant TM. A study of the role of the postnatal testes in determining the ontogeny of gonadotropin secretion in the male rhesus monkey (Macaca mulatta). Endocrinology. 1985;116(4):1341–1350. [DOI] [PubMed] [Google Scholar]
- 23. Simorangkir DR, Marshall GR, Plant TM. Sertoli cell proliferation during prepubertal development in the rhesus monkey (Macaca mulatta) is maximal during infancy when gonadotropin secretion is robust. J Clin Endocrinol Metab. 2003;88(10):4984–4989. [DOI] [PubMed] [Google Scholar]
- 24. Ramaswamy S, Dwarki K, Ali B, Gibbs RB, Plant TM. The decline in pulsatile GnRH release, as reflected by circulating LH concentrations, during the infant-juvenile transition in the agonadal male rhesus monkey (Macaca mulatta) is associated with a reduction in kisspeptin content of KNDy neurons of the arcuate nucleus in the hypothalamus. Endocrinology. 2013;154(5):1845–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Plant TM, Barker-Gibb ML. Neurobiological mechanisms of puberty in higher primates. Hum Reprod Update. 2004;10(1):67–77. [DOI] [PubMed] [Google Scholar]
- 26. Terasawa E, Fernandez DL. Neurobiological mechanisms of the onset of puberty in primates. Endocr Rev. 2001;22(1):111–151. [DOI] [PubMed] [Google Scholar]
- 27. Plant TM. A striking sex difference in the gonadotropin response to gonadectomy during infantile development in the rhesus monkey (Macaca mulatta). Endocrinology. 1986;119(2):539–545. [DOI] [PubMed] [Google Scholar]
- 28. Garcia JP, Keen KL, Seminara SB, Terasawa E. Gonadal steroid independent and dependent changes in pubertal increases in hypothalamic neurokinin B (NKB) and gonadotropin releasing hormone (GnRH) in male rhesus monkeys. Annual Meeting of the Endocrine Society; 17–20 March 2018; Chicago, IL. Abstract 623.
- 29. Ciofi P, Krause JE, Prins GS, Mazzuca M. Presence of nuclear androgen receptor-like immunoreactivity in neurokinin B-containing neurons of the hypothalamic arcuate nucleus of the adult male rat. Neurosci Lett. 1994;182(2):193–196. [DOI] [PubMed] [Google Scholar]
- 30. Dellovade TL, Merchenthaler I. Estrogen regulation of neurokinin B gene expression in the mouse arcuate nucleus is mediated by estrogen receptor alpha. Endocrinology. 2004;145(2):736–742. [DOI] [PubMed] [Google Scholar]
- 31. Goubillon ML, Forsdike RA, Robinson JE, Ciofi P, Caraty A, Herbison AE. Identification of neurokinin B-expressing neurons as an highly estrogen-receptive, sexually dimorphic cell group in the ovine arcuate nucleus. Endocrinology. 2000;141(11):4218–4225. [DOI] [PubMed] [Google Scholar]
- 32. Sandoval-Guzmán T, Stalcup ST, Krajewski SJ, Voytko ML, Rance NE. Effects of ovariectomy on the neuroendocrine axes regulating reproduction and energy balance in young cynomolgus macaques. J Neuroendocrinol. 2004;16(2):146–153. [DOI] [PubMed] [Google Scholar]
- 33. Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80(4):264–272. [DOI] [PubMed] [Google Scholar]
- 34. Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25(49):11349–11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A, Aparicio SA. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA. 2005;102(5):1761–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bellingham M, Fowler PA, Amezaga MR, Rhind SM, Cotinot C, Mandon-Pepin B, Sharpe RM, Evans NP. Exposure to a complex cocktail of environmental endocrine-disrupting compounds disturbs the kisspeptin/GPR54 system in ovine hypothalamus and pituitary gland. Environ Health Perspect. 2009;117(10):1556–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci USA. 2005;102(6):2129–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Krajewski SJ, Anderson MJ, Iles-Shih L, Chen KJ, Urbanski HF, Rance NE. Morphologic evidence that neurokinin B modulates gonadotropin-releasing hormone secretion via neurokinin 3 receptors in the rat median eminence. J Comp Neurol. 2005;489(3):372–386. [DOI] [PubMed] [Google Scholar]
- 39. Gaskins GT, Glanowska KM, Moenter SM. Activation of neurokinin 3 receptors stimulates GnRH release in a location-dependent but kisspeptin-independent manner in adult mice. Endocrinology. 2013;154(11):3984–3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29(38):11859–11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. de Croft S, Boehm U, Herbison AE. Neurokinin B activates arcuate kisspeptin neurons through multiple tachykinin receptors in the male mouse. Endocrinology. 2013;154(8):2750–2760. [DOI] [PubMed] [Google Scholar]
- 42. Higo S, Iijima N, Ozawa H. Characterization of Kiss1r (Gpr54)-expressing neurones in the arcuate nucleus of the female rat hypothalamus. J Neuroendocrinol. 2017;29(2). [DOI] [PubMed] [Google Scholar]
- 43. Burke MC, Letts PA, Krajewski SJ, Rance NE. Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: morphologic evidence of interrelated function within the arcuate nucleus. J Comp Neurol. 2006;498(5):712–726. [DOI] [PubMed] [Google Scholar]
- 44. Amstalden M, Coolen LM, Hemmerle AM, Billings HJ, Connors JM, Goodman RL, Lehman MN. Neurokinin 3 receptor immunoreactivity in the septal region, preoptic area and hypothalamus of the female sheep: colocalisation in neurokinin B cells of the arcuate nucleus but not in gonadotrophin-releasing hormone neurones. J Neuroendocrinol. 2010;22(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CV, Jafarzadehshirazi MR, Pereira A, Iqbal J, Caraty A, Ciofi P, Clarke IJ. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology. 2007;148(12):5752–5760. [DOI] [PubMed] [Google Scholar]
- 46. Hrabovszky E, Ciofi P, Vida B, Horvath MC, Keller E, Caraty A, Bloom SR, Ghatei MA, Dhillo WS, Liposits Z, Kallo I. The kisspeptin system of the human hypothalamus: sexual dimorphism and relationship with gonadotropin-releasing hormone and neurokinin B neurons. Eur J Neurosci. 2010;31(11):1984–1998. [DOI] [PubMed] [Google Scholar]
- 47. Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin NA, Plant TM. Neurokinin B stimulates GnRH release in the male monkey (Macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus. Endocrinology. 2010;151(9):4494–4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147(12):5817–5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Semaan SJ, Tolson KP, Kauffman AS. The development of kisspeptin circuits in the mammalian brain. Adv Exp Med Biol. 2013;784:221–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Taziaux M, Swaab DF, Bakker J. Sex differences in the neurokinin B system in the human infundibular nucleus. J Clin Endocrinol Metab. 2012;97(12):E2210–E2220. [DOI] [PubMed] [Google Scholar]
- 51. Hrabovszky E, Molnár CS, Sipos MT, Vida B, Ciofi P, Borsay BA, Sarkadi L, Herczeg L, Bloom SR, Ghatei MA, Dhillo WS, Kalló I, Liposits Z. Sexual dimorphism of kisspeptin and neurokinin B immunoreactive neurons in the infundibular nucleus of aged men and women. Front Endocrinol (Lausanne). 2011;2:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cheng G, Coolen LM, Padmanabhan V, Goodman RL, Lehman MN. The kisspeptin/neurokinin B/dynorphin (KNDy) cell population of the arcuate nucleus: sex differences and effects of prenatal testosterone in sheep. Endocrinology. 2010;151(1):301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ruiz-Pino F, Navarro VM, Bentsen AH, Garcia-Galiano D, Sanchez-Garrido MA, Ciofi P, Steiner RA, Mikkelsen JD, Pinilla L, Tena-Sempere M. Neurokinin B and the control of the gonadotropic axis in the rat: developmental changes, sexual dimorphism, and regulation by gonadal steroids. Endocrinology. 2012;153(10):4818–4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Clarkson J. Effects of estradiol on kisspeptin neurons during puberty. Front Neuroendocrinol. 2013;34(2):120–131. [DOI] [PubMed] [Google Scholar]
- 55. García-Galiano D, van Ingen Schenau D, Leon S, Krajnc-Franken MA, Manfredi-Lozano M, Romero-Ruiz A, Navarro VM, Gaytan F, van Noort PI, Pinilla L, Blomenröhr M, Tena-Sempere M. Kisspeptin signaling is indispensable for neurokinin B, but not glutamate, stimulation of gonadotropin secretion in mice. Endocrinology. 2012;153(1):316–328. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.