Abstract
Context
Pituitary carcinoma is a rare and aggressive malignancy with a poor prognosis and few effective treatment options.
Case
A 35-year-old woman presented with an aggressive ACTH-secreting pituitary adenoma that initially responded to concurrent temozolomide and capecitabine prior to metastasizing to the liver. Following treatment with ipilimumab and nivolumab, the tumor volume of the dominant liver metastasis reduced by 92%, and the recurrent intracranial disease regressed by 59%. Simultaneously, her plasma ACTH level decreased from 45,550 pg/mL to 66 pg/mL.
Molecular Evaluation
Both prospective clinical sequencing with Memorial Sloan Kettering–Integrated Mutation Profiling of Actionable Cancer Targets and retrospective whole-exome sequencing were performed to characterize the molecular alterations in the chemotherapy-naive pituitary adenoma and the temozolomide-resistant liver metastasis. The liver metastasis harbored a somatic mutational burden consistent with alkylator-induced hypermutation that was absent from the treatment-naive tumor. Resistance to temozolomide treatment, acquisition of new oncogenic drivers, and subsequent sensitivity to immunotherapy may be attributed to hypermutation.
Conclusion
Combination treatment with ipilimumab and nivolumab may be an effective treatment in pituitary carcinoma. Clinical sequencing of pituitary tumors that have relapsed following treatment with conventional chemotherapy may identify the development of therapy-induced somatic hypermutation, which may be associated with treatment response to immunotherapy.
Pituitary carcinomas may respond to ipilimumab and nivolumab; alkylator-induced hypermutation may contribute to malignant transformation and sensitivity to immunotherapy.
Cushing disease (CD) is a rare endocrine condition associated with an ACTH-secreting pituitary tumor causing excess adrenal cortisol production. Although most pituitary tumors are benign, ∼0.1% to 0.2% are classified as carcinomas, which are defined by the presence of noncontiguous craniospinal or systemic metastasis (1). Pituitary carcinomas with systemic metastases are particularly aggressive malignancies with a median survival of 1 year (2, 3).
Treatment of aggressive and malignant pituitary tumors relies on a combination of surgical resection, radiation therapy (RT), and medical therapies, which are few in number and often ineffective. The only tumor-directed medical therapies available for ACTH-secreting tumors are cabergoline, a dopamine-2 receptor agonist, which reduces circulating cortisol values in 25% to 40% of patients with CD (4–6), and pasireotide, a somatostatin analog that normalizes 24-hour urinary free cortisol values in ∼13% to 25% of patients with CD (7). These therapies are used for benign disease but provide only modest reduction in tumor size and have shown very limited success in the treatment of aggressive and malignant tumors. Temozolomide (TMZ) is an alkylating agent approved for the treatment of glioblastoma that has shown modest activity in pituitary carcinomas, especially when used in combination with capecitabine (8–10). Many patients either do not respond or escape control; hence, there remains a large unmet therapeutic need in this patient population.
The checkpoint inhibitors ipilimumab and nivolumab are effective in the treatment of a number of solid tumor types. A common adverse effect of anti-PD1 and anti-CTLA-4 therapy is the development of hypophysitis, suggesting pituitary endocrine cell susceptibility to these agents. To date, the response of patients with pituitary tumors to immunotherapy has not been reported. Here, we report a patient with a treatment-refractory aggressive ACTH-secreting pituitary carcinoma with sellar, contiguous dural involvement, and hepatic metastases that responded to checkpoint inhibitors, and we investigate the molecular basis for this response. This case suggests a potential role for immunotherapy in the treatment of pituitary carcinoma.
Case Report
A 35-year-old woman presented for management of a right third cranial nerve palsy, hirsutism, and weight gain in the fall of 2011. The patient was found to have a pituitary macroadenoma, elevated 24-hour urine-free cortisol values, and elevated plasma ACTH levels, consistent with CD. She underwent two consecutive subtotal transsphenoidal resections. Over the next year, the residual adenoma enlarged in size, and she received fractionated RT (5040 cGy in 28 fractions). When the tumor grew within months following RT, she underwent a third and a fourth transsphenoidal resection, 27 and 39 months following initial diagnosis.
Due to further growth and incomplete hormonal control despite pasireotide, ketoconazole, and ketoconazole in combination with cabergoline, she was treated with concurrent TMZ and capecitabine; treatment was discontinued after four cycles due to thrombocytopenia and poor tolerance. She had a biochemical and radiographic response to treatment (a 41% decrease in tumor volume and her ACTH level decreased from 266 to 80 pg/mL). Although the pituitary adenoma remained stable in size over the next 2 years, she had worsening hypercortisolemia-induced comorbidities, including diabetes, hypertension, deep vein thrombosis, and pulmonary embolism. Treatment with mifepristone and metyrapone was unsuccessful due to refractory hypokalemia and inability to achieve eucortisolemia; hence, she underwent evaluation for a bilateral adrenalectomy with body CT, which revealed a liver lesion suspicious for malignancy 68 months following initial diagnosis. A biopsy revealed a high-grade neuroendocrine neoplasm that was focally positive for ACTH and exhibited a mitotic index of up to 50%. O-6-methylguanine-DNA methyltransferase (MGMT) immunohistochemistry on this liver specimen demonstrated retained nuclear staining.
Following bilateral adrenalectomy, the patient experienced resolution of the diabetes, hypertension, obesity, and corticosteroid-induced psychosis but developed worsening of the right third nerve palsy. MRI of the sella and brain demonstrated extension of the pituitary tumor along the tentorium, suggestive of Nelson syndrome, and the postoperative MRI of the abdomen showed rapid progression of disease in the liver (Fig. 1a and 1b). She received two additional cycles of capecitabine/TMZ and was reimaged, revealing both intracranial and extracranial progression of disease. The original liver metastasis measured larger, and she was found to harbor additional liver metastases. Following progression on two cycles of carboplatin and etoposide and RT to the intracranial component, she consented to investigational treatment with combination ipilimumab (3 mg/kg every 3 weeks) and nivolumab (1 mg/kg every 3 weeks).
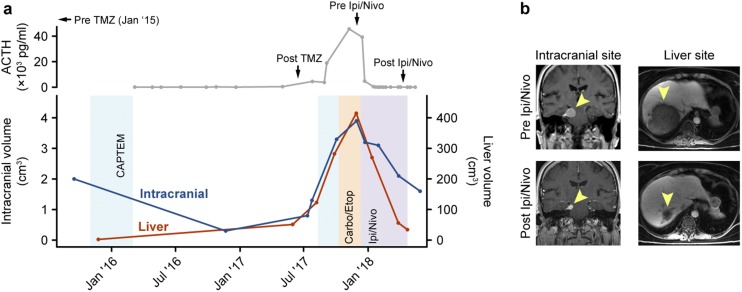
Figure 1.
(a) Volumetric measurements of the intracranial tumor and liver metastasis across the patient’s clinical timeline and corresponding measurements of ACTH show response to treatment with immunotherapy. (b) MRI scans of the intracranial and liver sites before and after combination treatment with ipilimumab and nivolumab, mirroring response in (a). CAPTEM, capecitabine/TMZ; Carbo/Etop, carboplatin/etoposide; Ipi/Nivo, ipilimumab/nivolumab.
Five days after her first infusion of ipilimumab and nivolumab, she developed a fever to 40°C and a mild transaminitis that resolved with high-dose glucocorticoid treatment. Within 1 week of starting ipilimumab and nivolumab, ACTH levels dropped 10-fold, from 45,550 to 4764 pg/mL. Following five treatments with ipilimumab and nivolumab, the dominant hepatic metastasis regressed by 92% (415 to 34 cm3), the recurrent intracranial component decreased by 59% (3.9 to 1.6 cm3), and her plasma ACTH level decreased to 66 pg/mL (Fig. 1a and 1b). Between cycles 4 and 5 of ipilimumab and nivolumab, two of the smaller satellite liver metastases enlarged in size but then stabilized or shrank on subsequent imaging, consistent with pseudoprogression from immunotherapy. Clinically, there was a reversal in the right third nerve palsy. Of note, although the patient was hypopituitary prior to starting immunotherapy, serum TSH values decreased after immunotherapy, with free T4 remaining in the normal range on replacement, suggesting that additional hypophysitis-induced hypopituitarism may have occurred with her treatment. The patient has received five cycles of concurrent ipilimumab and nivolumab, is receiving maintenance nivolumab, and continues to respond without additional immunologic adverse reactions at 6 months of follow-up with an ACTH of 59 pg/mL at the time of this report.
Materials and Methods
Memorial Sloan Kettering–Integrated Mutation Profiling of Actionable Cancer Targets and exome sequencing
All clinical sequencing included in this report was performed via a research protocol that was approved by the Memorial Sloan Kettering Cancer Center Institutional Review Board. The patient provided written informed consent for tumor sequencing and review of medical records (NCT01775072). Prospective clinical sequencing on Memorial Sloan Kettering–Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT) (a targeted sequencing platform covering 468 cancer-associated genes and select intronic regions for detection of recurrent gene fusion events) was performed in Clinical Laboratory Improvement Amendments (CLIA)-certified environments, as previously described (11, 12), for the following patient specimens: (1) the TMZ-naive pituitary tumor from the final transsphenoidal resection and (2) the TMZ-exposed liver lesion. All variants detected by MSK-IMPACT were manually reviewed. In addition, the analysis of germline variants was performed in a subset of 76 genes, with manual variant review by members of the clinical genetics service.
For research purposes, the cDNA libraries from the aforementioned assay were subjected to exome sequencing as previously described (13). Briefly, after target capture using SureSelect Human All Exon V6 (Agilent Technologies, Santa Clara, CA) and sequencing on HiSeq 2500 (Illumina, San Diego, CA) to generate paired-end 125-bp reads, reads were aligned to genome assembly b37 using BWA-MEM (v0.7.5a) (14) and subsequently processed using GATK (GATK suite v3.3-0, Picard tools v2.9) (15) best practices. The mean target coverage in the tumor specimens was 129× and 179×, respectively, and 82× in the normal sample. Somatic single-nucleotide variants (SNVs) were called with MuTect (v1.14) (16), and Pindel (v0.2.5a7) (17) and VarDict (v.1.5.1) were used for detection of insertions/deletions. Variants were annotated with VEP (v88) using vcf2maf (v1.6.14). Filtering of false-positive mutation calls was performed using a set of filters.
Genomic variants were classified as oncogenic or likely oncogenic based on annotation with OncoKB. Mutational burden was estimated as the total number of mutations per megabases of targeted regions.
Immunohistochemistry
Immunohistochemistry for MSH6 (clone 44, ready-to-use; Ventana Medical Systems, Oro Valley, AZ) was performed on the Ventana platform. Loss of staining was defined as no nuclear labeling in any of the tumor cells examined.
Immunohistochemistry for PD-L1 (clone E1L3N, dilution 1:250; Cell Signaling Technology, Danvers, MA) was performed on the Bond III platform (Leica Biosystems, Wetzlar, Germany). Membranous staining in either the tumor cells or the tumor-associated immune cells was counted as positivity.
For both stains, appropriate internal and external controls were applied.
Radiology response review
Radiographic response was determined by a board-certified radiologist who manually performed volumetric analysis using iNtuition 4.4.13 (TeraRecon, Foster City, CA) while blinded to the clinical history.
Results
Prospective sequencing of the sellar tumor using MSK-IMPACT revealed no somatic mutations, a focal amplification of CCND3, and a homozygous deletion of PTPRD. This tumor was typical of previously sequenced pituitary adenomas, which tend to harbor few mutations (18). MSK-IMPACT testing of the liver metastasis demonstrated the same amplification of CCND3 and homozygous deletion of PTPRD but also 105 somatic mutations that were not present in the pretreatment sellar tumor.
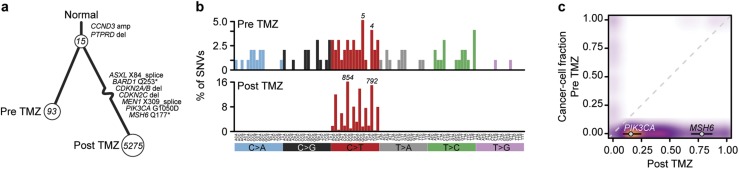
MSK-IMPACT interrogates 468 cancer-associated genes and select intronic regions. To explore the clonal relatedness of these tumors in greater detail, we performed whole-exome sequencing on both tumor specimens along with a matched blood normal (the matched normal being a sample of nontumoral tissue that is used to distinguish somatic from germline mutations). Exome sequencing revealed that the sellar tumor and liver metastasis shared 15 somatic mutations, revealing their common origin and clonal relationships (Fig. 2a). Notably, neither tumor harbored a mutation in USP8, a recurrently mutated gene in ACTH-secreting pituitary adenomas (19, 20). The TMZ-naive sellar tumor harbored 93 mutations that were private to that specimen, indicating ongoing subclonal evolution. The TMZ-exposed liver metastasis was hypermutated, harboring 5275 mutations (93 mutations/Mb) that were not detected in the pretreatment specimen. TMZ is an alkylating agent that causes C>T/G>A transitions primarily at CpC and CpT dinucleotides. Indeed, mutational signature decomposition analysis revealed that 76% of the mutations in the liver metastasis had the signature of TMZ-induced hypermutation (Fig. 2b). Moreover, no pathogenic or likely pathogenic allele was identified from germline analysis in this patient that could explain the somatic hypermutation identified.
Figure 2.
(a) Sample tree showing clonal relationship of the pre-TMZ primary specimen and the posttreatment liver metastasis, with numbers in circles indicating mutations acquired from the previous branch point. The two tumors share 15 mutations. The post-TMZ sample acquired a large number of private mutations, typical of therapy-induced hypermutation. Mutations considered likely oncogenic are highlighted. (b) The substitution types (colored labels) and trinucleotide context (vertical labels) of SNVs in both samples, as a fraction of total mutations. An enrichment for C>T/G>A transitions, characteristic of alkylator-induced hypermutation, was present in the posttreatment but not pretreatment sample. Numbers above bars indicate absolute number of mutations in that bin. (c) Estimates of fraction of cancer cells harboring mutations in both samples, represented as a three-dimensional density plot, showing the large number of mutations acquired in the post-TMZ sample. Whereas the mutation in MSH6 was nearly clonal in the liver specimen, the acquired mutation in PIK3CA was found in a minority of the cell population.
Consistent with prior work exploring the mechanisms of TMZ resistance and TMZ-induced hypermutation in gliomas (21), we identified a truncating homozygous MSH6 mutation present in most cancer cells in the TMZ-hypermutated liver metastasis absent in the treatment-naive sellar tumor. Immunohistochemistry on the two specimens confirmed MSH6 loss in the hypermutated liver metastasis, whereas the TMZ-naive sellar tumor demonstrated retained expression of MSH6. In addition, the liver metastasis was evaluated for PDL1 expression by immunohistochemistry, demonstrating <1% staining.
The sequenced liver metastasis reveals activation of pathways that were quiescent in the sellar tumor. In addition to developing additional cell cycle alterations, including a deletion at the CDKN2A/B locus, the liver metastasis demonstrated pathway activation of the PI3K pathway via a subclonal PIK3CA G1050D hotspot mutation, which is directly attributed to mutagenesis induced by alkylator chemotherapy (Fig. 2a and 2c).
Discussion
Clinical experience with immunotherapy suggests that the pituitary cells may be susceptible to checkpoint inhibitors. In patients treated with ipilimumab for a nonpituitary neoplasm, the rate of hypophysitis ranges from 4% to 15% (22–25). It has been postulated that this immunogenicity is in part mediated by ectopic expression of CTLA-4 on pituitary endocrine cells, leading to complement activation and the development of antipituitary antibodies (26). The addition of nivolumab to ipilimumab appears to potentiate the development of hypophysitis (25). We hypothesize that the susceptibility of pituitary cells to checkpoint inhibitors in part explains our patient’s robust radiographic and biochemical response and that checkpoint inhibition should be a treatment consideration, especially in tumors that have developed resistance to TMZ.
TMZ is an alkylating chemotherapy that creates O6-methylguanine adducts and induces apoptosis in the presence of a functional DNA repair system (27). Treatment with TMZ can induce a mutation in an MMR gene, resulting in the accumulation of genetic lesions, also known as hypermutation (28). Aggressive pituitary adenomas that initially respond to TMZ may become resistant, as seen in this case. In gliomas, it has been suggested that hypermutation can lead to the acquisition of new oncogenic drivers, resulting in a more aggressive tumor clone (21). This may be reflected by the subclonal PIK3CA G1050D mutation in this patient’s liver metastasis as it is a known hotspot mutation. It has been previously shown that mutational burden correlates with treatment response to checkpoint inhibitors in metastatic melanoma, possibly due to the creation of neoantigens (29). TMZ-induced hypermutation may make pituitary adenomas, which are already uniquely immunogenic, further sensitive to treatment with immunotherapy.
The generalizability of this patient’s treatment response to other patients with pituitary tumors requires further study. This patient’s response to ipilimumab and nivolumab should prompt a clinical trial to better define the patient population with pituitary tumors that would most benefit from checkpoint inhibition.
Acknowledgments
We thank James Fagin for helpful discussions and comments on this manuscript.
Financial Support: This research was funded in part through the National Institutes of Health/National Cancer Institute Cancer Center Support Grant P30 CA008748.
Disclosure Summary: M.P. reports that he is on the scientific advisory board and has received honoraria from Bristol-Myers Squibb. The remaining authors have nothing to disclose.
Glossary
Abbreviations:
- CD
Cushing disease
- MSK-IMPACT
Memorial Sloan Kettering–Integrated Mutation Profiling of Actionable Cancer Targets
- RT
radiation therapy
- TMZ
temozolomide
References
- 1. Zada G, Woodmansee WW, Ramkissoon S, Amadio J, Nose V, Laws ER Jr. Atypical pituitary adenomas: incidence, clinical characteristics, and implications. J Neurosurg. 2011;114(2):336–344. [DOI] [PubMed] [Google Scholar]
- 2. Pernicone PJ, Scheithauer BW, Sebo TJ, Kovacs KT, Horvath E, Young WF Jr, Lloyd RV, Davis DH, Guthrie BL, Schoene WC. Pituitary carcinoma: a clinicopathologic study of 15 cases. Cancer. 1997;79(4):804–812. [DOI] [PubMed] [Google Scholar]
- 3. Ragel BT, Couldwell WT. Pituitary carcinoma: a review of the literature. Neurosurg Focus. 2004;16(4):E7. [DOI] [PubMed] [Google Scholar]
- 4. Lila AR, Gopal RA, Acharya SV, George J, Sarathi V, Bandgar T, Menon PS, Shah NS. Efficacy of cabergoline in uncured (persistent or recurrent) Cushing disease after pituitary surgical treatment with or without radiotherapy. Endocr Pract. 2010;16(6):968–976. [DOI] [PubMed] [Google Scholar]
- 5. Pivonello R, De Martino MC, Cappabianca P, De Leo M, Faggiano A, Lombardi G, Hofland LJ, Lamberts SW, Colao A. The medical treatment of Cushing’s disease: effectiveness of chronic treatment with the dopamine agonist cabergoline in patients unsuccessfully treated by surgery. J Clin Endocrinol Metab. 2009;94(1):223–230. [DOI] [PubMed] [Google Scholar]
- 6. Burman P, Edén-Engström B, Ekman B, Karlsson FA, Schwarcz E, Wahlberg J. Limited value of cabergoline in Cushing’s disease: a prospective study of a 6-week treatment in 20 patients. Eur J Endocrinol. 2015;174(1):17–24. [DOI] [PubMed] [Google Scholar]
- 7. Colao A. Improvement of cardiac parameters in patients with acromegaly treated with medical therapies. Pituitary. 2011;15(1):50–58. [DOI] [PubMed] [Google Scholar]
- 8. Raverot G, Sturm N, de Fraipont F, Muller M, Salenave S, Caron P, Chabre O, Chanson P, Cortet-Rudelli C, Assaker R, Dufour H, Gaillard S, François P, Jouanneau E, Passagia JG, Bernier M, Cornélius A, Figarella-Branger D, Trouillas J, Borson-Chazot F, Brue T. Temozolomide treatment in aggressive pituitary tumors and pituitary carcinomas: a French multicenter experience. J Clin Endocrinol Metab. 2010;95(10):4592–4599. [DOI] [PubMed] [Google Scholar]
- 9. Bengtsson D, Schrøder HD, Andersen M, Maiter D, Berinder K, Feldt Rasmussen U, Rasmussen ÅK, Johannsson G, Hoybye C, van der Lely AJ, Petersson M, Ragnarsson O, Burman P. Long-term outcome and MGMT as a predictive marker in 24 patients with atypical pituitary adenomas and pituitary carcinomas given treatment with temozolomide. J Clin Endocrinol Metab. 2015;100(4):1689–1698. [DOI] [PubMed] [Google Scholar]
- 10. Zacharia BE, Gulati AP, Bruce JN, Carminucci AS, Wardlaw SL, Siegelin M, Remotti H, Lignelli A, Fine RL. High response rates and prolonged survival in patients with corticotroph pituitary tumors and refractory Cushing disease from capecitabine and temozolomide (CAPTEM): a case series. Neurosurgery.2014;74(4):E447–E455; discussion E455. [DOI] [PubMed]
- 11. Zehir A, Benayed R, Shah RH, Syed A, Middha S, Kim HR, Srinivasan P, Gao J, Chakravarty D, Devlin SM, Hellmann MD, Barron DA, Schram AM, Hameed M, Dogan S, Ross DS, Hechtman JF, DeLair DF, Yao J, Mandelker DL, Cheng DT, Chandramohan R, Mohanty AS, Ptashkin RN, Jayakumaran G, Prasad M, Syed MH, Rema AB, Liu ZY, Nafa K, Borsu L, Sadowska J, Casanova J, Bacares R, Kiecka IJ, Razumova A, Son JB, Stewart L, Baldi T, Mullaney KA, Al-Ahmadie H, Vakiani E, Abeshouse AA, Penson AV, Jonsson P, Camacho N, Chang MT, Won HH, Gross BE, Kundra R, Heins ZJ, Chen HW, Phillips S, Zhang H, Wang J, Ochoa A, Wills J, Eubank M, Thomas SB, Gardos SM, Reales DN, Galle J, Durany R, Cambria R, Abida W, Cercek A, Feldman DR, Gounder MM, Hakimi AA, Harding JJ, Iyer G, Janjigian YY, Jordan EJ, Kelly CM, Lowery MA, Morris LGT, Omuro AM, Raj N, Razavi P, Shoushtari AN, Shukla N, Soumerai TE, Varghese AM, Yaeger R, Coleman J, Bochner B, Riely GJ, Saltz LB, Scher HI, Sabbatini PJ, Robson ME, Klimstra DS, Taylor BS, Baselga J, Schultz N, Hyman DM, Arcila ME, Solit DB, Ladanyi M, Berger MF. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med. 2017;23(6):703–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cheng DT, Mitchell TN, Zehir A, Shah RH, Benayed R, Syed A, Chandramohan R, Liu ZY, Won HH, Scott SN, Brannon AR, O’Reilly C, Sadowska J, Casanova J, Yannes A, Hechtman JF, Yao J, Song W, Ross DS, Oultache A, Dogan S, Borsu L, Hameed M, Nafa K, Arcila ME, Ladanyi M, Berger MF. Memorial Sloan Kettering–Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn. 2015;17(3):251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Al-Ahmadie HA, Iyer G, Lee BH, Scott SN, Mehra R, Bagrodia A, Jordan EJ, Gao SP, Ramirez R, Cha EK, Desai NB, Zabor EC, Ostrovnaya I, Gopalan A, Chen YB, Fine SW, Tickoo SK, Gandhi A, Hreiki J, Viale A, Arcila ME, Dalbagni G, Rosenberg JE, Bochner BH, Bajorin DF, Berger MF, Reuter VE, Taylor BS, Solit DB. Frequent somatic CDH1 loss-of-function mutations in plasmacytoid variant bladder cancer. Nat Genet. 2016;48(4):356–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25(14):1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. DePristo MA, Banks E, Poplin R, Garimella KV, Maguire JR, Hartl C, Philippakis AA, del Angel G, Rivas MA, Hanna M, McKenna A, Fennell TJ, Kernytsky AM, Sivachenko AY, Cibulskis K, Gabriel SB, Altshuler D, Daly MJ. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011;43(5):491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cibulskis K, Lawrence MS, Carter SL, Sivachenko A, Jaffe D, Sougnez C, Gabriel S, Meyerson M, Lander ES, Getz G. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat Biotechnol. 2013;31(3):213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ye K, Schulz MH, Long Q, Apweiler R, Ning Z. Pindel: a pattern growth approach to detect break points of large deletions and medium sized insertions from paired-end short reads. Bioinformatics. 2009;25(21):2865–2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Song ZJ, Reitman ZJ, Ma ZY, Chen JH, Zhang QL, Shou XF, Huang CX, Wang YF, Li SQ, Mao Y, Zhou LF, Lian BF, Yan H, Shi YY, Zhao Y. The genome-wide mutational landscape of pituitary adenomas. Cell Res. 2016;26(11):1255–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ma ZY, Song ZJ, Chen JH, Wang YF, Li SQ, Zhou LF, Mao Y, Li YM, Hu RG, Zhang ZY, Ye HY, Shen M, Shou XF, Li ZQ, Peng H, Wang QZ, Zhou DZ, Qin XL, Ji J, Zheng J, Chen H, Wang Y, Geng DY, Tang WJ, Fu CW, Shi ZF, Zhang YC, Ye Z, He WQ, Zhang QL, Tang QS, Xie R, Shen JW, Wen ZJ, Zhou J, Wang T, Huang S, Qiu HJ, Qiao ND, Zhang Y, Pan L, Bao WM, Liu YC, Huang CX, Shi YY, Zhao Y. Recurrent gain-of-function USP8 mutations in Cushing’s disease. Cell Res. 2015;25(3):306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Reincke M, Sbiera S, Hayakawa A, Theodoropoulou M, Osswald A, Beuschlein F, Meitinger T, Mizuno-Yamasaki E, Kawaguchi K, Saeki Y, Tanaka K, Wieland T, Graf E, Saeger W, Ronchi CL, Allolio B, Buchfelder M, Strom TM, Fassnacht M, Komada M. Mutations in the deubiquitinase gene USP8 cause Cushing’s disease. Nat Genet. 2014;47(1):31–38. [DOI] [PubMed] [Google Scholar]
- 21. Johnson BE, Mazor T, Hong C, Barnes M, Aihara K, McLean CY, Fouse SD, Yamamoto S, Ueda H, Tatsuno K, Asthana S, Jalbert LE, Nelson SJ, Bollen AW, Gustafson WC, Charron E, Weiss WA, Smirnov IV, Song JS, Olshen AB, Cha S, Zhao Y, Moore RA, Mungall AJ, Jones SJM, Hirst M, Marra MA, Saito N, Aburatani H, Mukasa A, Berger MS, Chang SM, Taylor BS, Costello JF. Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science. 2013;343(6167):189–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Albarel F, Gaudy C, Castinetti F, Carré T, Morange I, Conte-Devolx B, Grob JJ, Brue T. Long-term follow-up of ipilimumab-induced hypophysitis, a common adverse event of the anti-CTLA-4 antibody in melanoma. Eur J Endocrinol. 2014;172(2):195–204. [DOI] [PubMed] [Google Scholar]
- 23. Faje AT, Sullivan R, Lawrence D, Tritos NA, Fadden R, Klibanski A, Nachtigall L. Ipilimumab-induced hypophysitis: a detailed longitudinal analysis in a large cohort of patients with metastatic melanoma. J Clin Endocrinol Metab. 2014;99(11):4078–4085. [DOI] [PubMed] [Google Scholar]
- 24. Min L, Hodi FS, Giobbie-Hurder A, Ott PA, Luke JJ, Donahue H, Davis M, Carroll RS, Kaiser UB. Systemic high-dose corticosteroid treatment does not improve the outcome of ipilimumab-related hypophysitis: a retrospective cohort study. Clin Cancer Res. 2014;21(4):749–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, Lao CD, Wagstaff J, Schadendorf D, Ferrucci PF, Smylie M, Dummer R, Hill A, Hogg D, Haanen J, Carlino MS, Bechter O, Maio M, Marquez-Rodas I, Guidoboni M, McArthur G, Lebbé C, Ascierto PA, Long GV, Cebon J, Sosman J, Postow MA, Callahan MK, Walker D, Rollin L, Bhore R, Hodi FS, Larkin J. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377(14):1345–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Iwama S, De Remigis A, Callahan MK, Slovin SF, Wolchok JD, Caturegli P. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci Transl Med. 2014;6(230):230ra45. [DOI] [PubMed] [Google Scholar]
- 27. Karran P, Bignami M. Self-destruction and tolerance in resistance of mammalian cells to alkylation damage. Nucleic Acids Res. 1992;20(12):2933–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cahill DP, Levine KK, Betensky RA, Codd PJ, Romany CA, Reavie LB, Batchelor TT, Futreal PA, Stratton MR, Curry WT, Iafrate AJ, Louis DN. Loss of the mismatch repair protein MSH6 in human glioblastomas is associated with tumor progression during temozolomide treatment. Clin Cancer Res. 2007;13(7):2038–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, Hollmann TJ, Bruggeman C, Kannan K, Li Y, Elipenahli C, Liu C, Harbison CT, Wang L, Ribas A, Wolchok JD, Chan TA. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371(23):2189–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]