Abstract
Context
Chronic pancreatitis (CP) is characterized by inflammation, fibrosis, and a loss of pancreatic acinar cells, which can result in exocrine and eventually endocrine deficiency. Pancreatitis has been reported to induce formation of new endocrine cells (neogenesis) in mice. Our recent data have implicated chromogranin A–positive hormone-negative (CPHN) cells as potential evidence of neogenesis in humans.
Objective
We sought to establish if CPHN cells were more abundant in CP in humans.
Design, Setting, and Participants
We investigated the frequency and distribution of CPHN cells and the expression of the chemokine C-X-C motif ligand 10 (CXCL10) and its receptor chemokine C-X-C motif receptor 3 in pancreas of nondiabetic subjects with CP.
Results
CPHN cell frequency in islets was increased sevenfold in CP [2.1% ± 0.67% vs 0.35% ± 0.09% CPHN cells in islets, CP vs nonpancreatitis (NP), P < 0.01], as were the CPHN cells found as scattered cells in the exocrine areas (17.4 ± 2.9 vs 4.2 ± 0.6, CP vs NP, P < 0.001). Polyhormonal endocrine cells were also increased in CP (2.7 ± 1.2 vs 0.1 ± 0.04, CP vs NP, % of polyhormonal cells of total endocrine cells, P < 0.01), as was expression of CXCL10 in α and β cells.
Conclusion
There is increased islet endogenous expression of the inflammation marker CXCL10 in islets in the setting of nondiabetic CP and an increase in polyhormonal (insulin-glucagon expressing) cells. The increase in CPHN cells in CP, often in a lobular distribution, may indicate foci of attempted endocrine cell regeneration.
In nondiabetic CP, endocrine cell changes include an increase in nonhormone expressing cells (possible attempted regeneration), altered identity, and increased expression of CXCL10.
Chronic pancreatitis (CP) is characterized by a progressive loss of acinar tissue from the exocrine compartment of the pancreas, with increasing fibrosis indicative of ongoing inflammation (1, 2). Although the damage is often patchy in the earlier stages, it can extend to involve the entire organ. Classic pathological changes include a variable degree of inflammation, fibrosis, and loss of acinar tissue. Periductal fibrosis, ductal dilatation with foci of calcification, and acinar-to-ductal metaplasia are also commonly seen (3). As a consequence, exocrine deficiency can occur, as can endocrine deficiency, although this usually occurs later in the course of the disease (4–6).
CP is a risk factor for the development of diabetes type 3C. Although the prevalence has been difficult to accurately ascertain, it is projected to account for ∼9% of all patients with diabetes (7). CP is thought to be the underlying cause in nearly 80% of all cases of type 3C diabetes (8).
Chemokines control the essential process of attracting leukocytes to the tissues during inflammation (9, 10). Previously, it has been reported that the existence of chemokine C-X-C motif ligand 10 (CXCL10) and chemokine C-X-C motif receptor 3 (CXCR3) with other chemokine C-X-C motif/CC chemokine subfamily chemokine signatures in humans with CP is associated with the progression of chronic inflammation (11). Moreover, overexpression of another inflammatory initiator, toll-like receptor 4, has been reported to be upregulated in epithelial (pancreatic duct) or endothelial tissues in cerulein-induced pancreatitis in rats (12). Although a lot is known about acinar and ductal cell function and abnormalities in humans with CP (13), not much information is available on changes in identity of islet endocrine cells in CP. Inflammation-induced islet β cell regeneration has previously been reported in rats (14); however, the effect of inflammation in changing islet endocrine cell identity in humans with CP has not previously been investigated.
Several murine studies using a variety of lineage tracing approaches have reported an increase in newly formed endocrine cells from nonendocrine precursors under conditions of induced exocrine pancreas inflammation, for example by duct ligation (15, 16). In several recent studies, we identified chromogranin A–positive hormone-negative (CPHN) cells as potential newly forming endocrine cells. For example, CPHN cells are most abundant in late gestation and early infancy coincident with the most rapid period of endocrine pancreas development. They re-emerge in individuals with type 1 and type 2 diabetes for whom β cell regeneration has been suggested, although this treatment has been insufficient (17–19). To date, the abundance and nature of CPHN cells in humans with CP has not been determined. In the current study, we sought to build on these prior studies to establish if there is an increase in CPHN cells in humans with CP.
We therefore sought to determine whether, in nondiabetic human subjects with CP in whom there is ongoing inflammation in the exocrine compartment but without the confounding effects of diabetes, we would find an increase in the frequency of nonhormone-expressing endocrine cells. Furthermore, we questioned whether, as a consequence of the ongoing exocrine inflammation, there would be evidence of inflammation in the pancreatic islets, even in the absence of diabetes.
Materials and Methods
Human subjects
Sections of pancreas were obtained from the Mayo Clinic autopsy archives with Institutional Review Board permission (IRB no. 15-004992) from both the Mayo Clinic and University of California Los Angeles (UCLA). Two groups of adult human subjects were identified: (1) 38 control subjects without pancreatitis [18 lean and 20 obese; nonpancreatitis (NP)] [the majority (n = 24) of these control subjects were previously reported for quantification of nonhormone expressing endocrine cells] (17, 18), and (2) 19 age- and body mass index (BMI)-matched individuals with CP. To be included, cases were required to have had (1) a full autopsy within 24 hours of death, (2) pancreatic tissue stored that was of adequate size and quality, and (3) no use of glucocorticoids. Cases were excluded if pancreatic tissue had undergone autolysis. Case subjects were identified based on these preferences in the Mayo Clinic autopsy database, and 4-μm sections of pancreas tail from the selected case subjects were obtained and made available to UCLA investigators in a manner coded to conceal the identity of the subjects. The CP and NP control cases used for measurement of β and α cell area percent were matched for age [59.6 ± 4.2 vs 66.3 ± 2.5 years, CP vs NP, P = not significant (NS)], sex (9 men and 10 women in the CP group, 16 men and 22 women in the NP group), and BMI (27.8 ± 1.4 vs 29.4 ± 1.3 kg/m2, CP vs NP, P = NS). The clinical characteristics of these subjects are summarized in Supplemental Table 1. All subjects were nondiabetic. Nondiabetic status was determined by review of each subject’s medical record. Nine of the subjects with CP had a history of alcohol abuse/dependency. No history of alcohol abuse was documented in the remaining 10 subjects with CP.
For assessment of nonhormone-expressing endocrine cells (CPHN cells) in pancreas, a subgroup of 12 CP and 24 NP cases was selected and matched for age (56.7 ± 5.8 vs 65.0 ± 3.1 years, CP vs NP, P = NS), sex (5 men and 7 women in the CP group, 13 men and 11 women in the NP group), and BMI (25.9 ± 1.5 vs 29.8 ± 1.7 kg/m2, CP vs NP, P = NS) (Supplemental Table 1, indicated by “CPHN subsets”). In the CP subgroup, six subjects had a documented history of alcohol abuse/dependency, and six subjects had no recorded alcohol abuse. For assessment of chemokine (CXCL10) and its receptor CXCR3 expression in the islets of CP or NP, a subgroup of CP and NP (n = 4 in each group) was selected and matched for age and sex (Supplemental Table 1, indicated by “CXCL10/CXCR3”).
The subset of cases for CPHN analysis was chosen to include the spectrum of severity of CP, and the NP cases were matched for age and BMI. Likewise, the four subset cases for chemokine and receptor expression were chosen to cover the spectrum of severity of pancreatitis, from microscopic foci to 100% involvement, and the NP cases were matched appropriately.
Pancreatic tissue processing, histology, and morphometric analyses
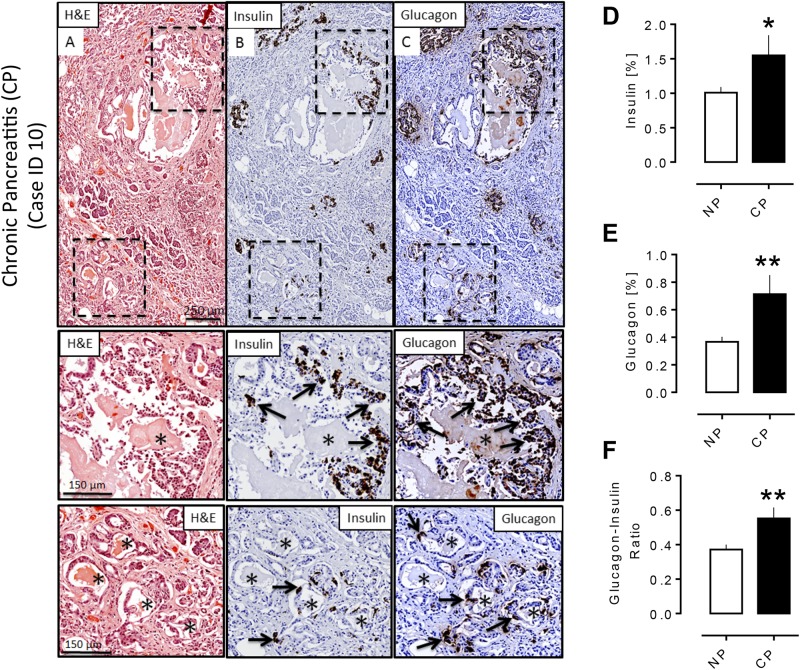
Pancreas was fixed in formaldehyde, embedded in paraffin, and stained for hematoxylin and eosin as well as insulin and glucagon for subsequent analysis as previously described (20). In the CP group, there was variability in the severity of the disease, ranging from mild CP, with foci of disease occupying ∼5% of the tissue area, to severe disease occupying 100% of the tissue section. The classic features of CP, including parenchymal destruction, fibrosis, ductal dilatation, ductuloinsular complexes, and foci of acinar-to-ductal metaplasia, were evident (Fig. 1A–1C). To quantify fractional β and α cell area, the ratios of the insulin-stained area/total pancreas parenchymal area or glucagon-stained area/total pancreas parenchymal area, respectively, were digitally measured as previously described (20). All morphometric analyses were performed in a blinded manner by two independent investigators.
Figure 1.
Pathology of pancreas in a subject with CP. Adjacent sections of pancreas from a subject with CP (40-year-old male, BMI 34.6) stained for (A) hematoxylin and eosin (H&E), (B) insulin (diaminobenzidine) with hematoxylin counterstain, and (C) glucagon (diaminobenzidine) with hematoxylin counterstain, showing typical features of CP (i.e., fibrosis, loss of acinar tissue, dilated pancreatic ducts, and areas of acinar-to ductal metaplasia). Insets show an increase of insulin- and glucagon-positive cells (arrows) in ductal epithelium (upper) and in acinar-to-ductal metaplasia (lower) with a preponderance of glucagon-positive cells in both locations. (D, E) Percentage of (D) insulin and (E) glucagon fractional area in the subjects with CP and without CP (NP). Both insulin and glucagon fractional area were increased in the CP group (insulin area %: 1.55 ± 0.29 vs 1.01 ± 0.08, CP vs NP, *P < 0.05; glucagon area %: 0.71 ± 0.14 vs 0.37 ± 0.04, CP vs NP, **P < 0.005). (F) The glucagon/insulin ratio was increased in subjects with CP (0.55 ± 0.06 vs 0.37 ± 0.03, CP vs NP, **P < 0.005). CP, n = 19; NP, n = 38. *Ductules in an area of acinar-to-ductal metaplasia. Scale bar, 250 μm in lower-power views, 150 μm in insets.
Immunofluorescence staining for CPHN cells, CXCL10, and CXCR3 with insulin and glucagon
Sections (4 μm) were stained for chromogranin A, insulin, glucagon, somatostatin, pancreatic polypeptide, and ghrelin to detect CPHN cells as described previously (17, 18). For CXCL10-CXCR3-insulin or CXCL10-CXCR3-glucagon staining, after antigen retrieval of the paraffin sections of pancreas of CP or NP cases (by following a standard antigen retrieval procedure using citrate buffer), tissues were blocked with blocking buffer (3% bovine serum albumin, 0.2% Triton X-100) for 1 hour at room temperature followed by incubation with a cocktail of rabbit anti-CXCL10 (1:100, Abcam, Cambridge, MA; catalog no. ab9807, RRID: AB_308792), goat anti-CXCR3 (1:300, antibodies-online, Atlanta, GA; catalog no. ABIN334381, RRID: AB_10776441), and guinea pig anti-insulin (1:100, Abcam; catalog no. 7842, RRID: AB_306130) or mouse anti-glucagon (1:1000, Sigma-Aldrich; St. Louis, MO, catalog no. G2654, RRID: AB_259852) prepared in antibody buffer (3% bovine serum albumin in Tris-buffered saline with Tween 20) at 4°C for overnight. The primary antibodies were detected by a cocktail of appropriate secondary antibodies (Jackson ImmunoResearch, Westgrove, PA) conjugated to FITC (1:200 each, to detect CXCL10; catalog no. 711-096-152, RRID: AB_2340597), Cy3 (1:100, to detect CXCR3; catalog no. 705-167-003, RRID: AB_2340414), Alexa 647 [1:100, to detect insulin (catalog no. 706-606-148, RRID: AB_2340477), or glucagon (catalog no. 715-606-151, RRID: AB_2340866)].
For insulin-glucagon-CXCL10 or insulin-glucagon-CXCR3 staining, slides were incubated with a cocktail of the following primary antibodies: guinea pig anti-insulin (1:100, Abcam; catalog no. 7842, RRID: AB_306130), mouse anti-glucagon (1:1000, Sigma-Aldrich; catalog no. G2654, RRID: AB_259852), and rabbit anti-CXCL10 (1:100, Abcam; catalog no. ab9807, RRID: AB_308792) or goat anti-CXCR3 (1:300, antibodies-online; catalog no. ABIN334381, RRID: AB_10776441). The primary antibodies were detected by a cocktail of appropriate secondary antibodies.
Quantification of CPHN cells
To quantify pancreatic endocrine cells in the NP human subjects and those with CP, 50 islets, clusters of endocrine cells, and single endocrine cells per sample were imaged at ×20 magnification using a Leica DM6000 microscope (Leica Microsystems) with a Hamamatsu Orca-ER camera (C4742-80-12AG; Indigo Scientific) and Openlab software (Improvision). Each field of view was calculated to be 0.292 mm2. Endocrine cells and CPHN cells were quantified and analyzed according to our previous reports; the 24 NP cases included here were reported previously as having CPHN cells (17, 18). In brief, islets were selected by starting at the top left corner of the pancreatic tissue section and imaged in a methodical fashion working across the tissue from left to right and back again including all islets encountered, up to a total of 50 islets per subject. Scattered endocrine cells were defined as a grouping of three or fewer chromogranin-positive cells. The mean numbers of endocrine cells within islets for the CP and NP groups were 1956 ± 232 and 2044 ± 192 cells per individual, respectively. The mean number of endocrine cells counted in clusters in the CP and NP groups was 104 ± 12 and 73 ± 7 cells per individual. The mean number of endocrine cells counted in single cells in the CP and NP groups was 52 ± 6 and 41 ± 8 cells per individual. The mean number of CPHN cells per individual identified in islets from the human subjects with CP was 40 ± 11 cells per individual and from the NP subjects was 4.4 ± 1.1 cells per individual. The mean number of CPHN cells in clusters was 17.3 ± 5.0 per individual for the CP group and 3.0 ± 0.5 cells per individual for the NP group. The mean number of CPHN cells in single cells was 14.4 ± 4.1 per individual for the CP group and 2.1 ± 0.4 cells per individual for the NP group.
Quantification of CXCL10 and CXCR3 in α and β cells
To quantify the percentage of islet endocrine cells (α and β cells or both) that express CXCL10 or CXCR3, 20 to 30 randomly selected islets per sample were imaged at ×20. The CXCL10/CXCR3-expressing endocrine cells contained within each islet were manually counted, and the following data were recorded: (1) the number of insulin-positive β cells staining for CXCL10 or CXCR3, (2) the number of glucagon-positive α cells staining for CXCL10 or CXCR3, (3) the number of cells positive for both CXCL10 and CXCR3 that costained for insulin or glucagon, and (4) the number of insulin-glucagon double-positive cells costaining for CXCL10 or CXCR3.
Confocal imaging
Confocal fluorescent images of the immunofluorescent slides were obtained using a Zeiss LSM700 confocal scan head mounted on a Zeiss Axiovert inverted-based microscope with a 20× objective. Sequential excitation at 488, 555, 639, and 405 nm was provided by a pigtail-coupled solid-state laser with polarization-preserving single-mode fiber. Fully automated reflection/fluorescence detection channels, each with highly sensitive photomultiplier tubes, were used for collecting green, red, purple, and blue (4′,6-diamidino-2-phenylindole) in channels one and two, respectively. After sequential excitation, green, red, purple, and blue fluorescent images of the same field or islet were saved and analyzed by Zeiss (ZEN) software. The term “Merged” in the figures refers to the coincidence of green, red, purple, and blue fluorescence as measured by the confocal microscope.
For the confocal z stack, optical sections through the entire selected islets (using a 20× objective) were collected for four channels in axial (z) dimension spaced at intervals of 0.5 μm. Once the islet was selected, points above and below the islet in the z-plane were defined by driving the microscope to a point just out of focus on both the top and bottom of the islet. Images were recorded as a series of .tif files with dimensions of 767 × 767 pixels.
Statistical analysis
Statistical analysis was performed using Student’s t test with GraphPad Prism 6.0 software (GraphPad Software, La Jolla, CA). Data in graphs and tables are presented as means ± standard error of the mean. Findings were assumed statistically significant at P < 0.05.
Results
Insulin and glucagon area measurement
Fractional pancreatic areas immunopositive for insulin and for glucagon were increased in CP (insulin area %: 1.55% ± 0.29% vs 1.01% ± 0.08%, CP vs NP, P < 0.05; glucagon area %: 0.71% ± 0.14% vs 0.37% ± 0.04%, CP vs NP, P < 0.005) (Fig. 1D and 1E). The changes in insulin and glucagon area also resulted in an increase in the glucagon/insulin ratio (0.5 ± 0.06 vs 0.37 ± 0.03, glucagon/insulin ratio, CP vs NP, P < 0.005) (Fig. 1F). This is in accord with a previous publication (21).
The composition of endocrine and CPHN cells in islets in CP compared with NP controls
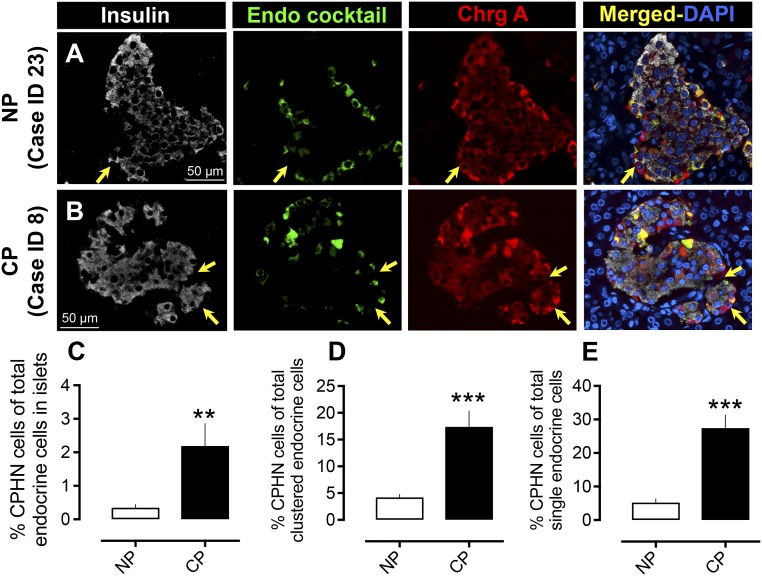
There was no alteration in islet composition in CP in terms of the number of β cells per islet cross-section (19.9 ± 2.6 vs 23.8 ±1.8 β cells per islet cross-section CP vs NP, P = NS) or in the number of endocrine cocktail cells per islet cross-section (13.9 ± 2.3 vs 17.7 ± 2.2 endocrine cells per islet cross-section, CP vs NP, P = NS). We previously reported an increased frequency of CPHN cells in humans with both type 1 and type 2 diabetes as well as in the prediabetic stage in rats (17–19). Because the overall reported prevalence of diabetes is nearly 50% in CP (22, 23), we here investigated the frequency of CPHN cells in subjects with CP but specifically without the confounding effect of diabetes. There was a sevenfold increase in CPHN cells in the islets of subjects with CP (2.1% ± 0.67% vs 0.35% ± 0.09% CPHN cells in islets, CP vs NP, P < 0.01) (Fig. 2A–2C). Within the CP group, the frequency of CPHN cells in islets was not different between the six subjects with documented alcohol abuse and the six subjects with no history of alcohol misuse (2.63% ± 1.06% vs 1.75% ± 0.89%, alcohol abuse vs no known alcohol misuse, P = NS).
Figure 2.
Prevalence of hormone-negative endocrine cells in subjects with CP. Examples of CPHN cells in pancreas from (A) an NP subject and (B) a subject with CP. Individual layers stained for insulin (white), endocrine cocktail (glucagon, somatostatin, pancreatic polypeptide, and ghrelin) (green), chromogranin A (red), and 4′,6-diamidino-2-phenylindole (DAPI) (blue) are shown along with the merged image. Arrows indicate CPHN cells. Scale bars, 50 μm for both (A) and (B). The frequency of nonhormone-expressing endocrine cells was increased in (C) islets in subjects with CP (2.2 ± 0.6 vs 0.34 ± 0.1 CPHN cells per islet cross-section, CP vs NP, **P < 0.0001), in (D) scattered clusters (17.4 ± 2.9 vs 4.2 ± 0.5 cells/mm2, CP vs NP, ***P < 0.0001), and (E) as single cells (27.4 ± 3.8 vs 5.2 ± 1.0 cells/mm2, CP vs NP, **P < 0.001). CP, n = 12; NP, n = 24.
Scattered endocrine cells, with a disproportionate increase in nonhormone expressing cells, are more frequent in pancreas from subjects with CP
In a prior study we noted that isolated endocrine and CPHN cells scattered in the exocrine pancreas were more abundant in obese individuals with type 2 diabetes (18), reproducing the pattern present in the late fetal and early postnatal pancreas in humans (18). In the subjects with CP, we also found an increase in endocrine cells occurring as scattered single cells and clusters (16.9 ± 2.5 vs 11.4 ± 1.3 cells/mm2 pancreas, CP vs NP, P < 0.05). CPHN cells found in clusters were approximately fourfold more frequent in CP (17.4% ± 2.9% vs 4.2% ± 0.5% CPHN cells in clusters, CP vs NP, P < 0.0001); a similar result was apparent when the number of CPHN cells in clusters was converted to unit area (2.64 ± 0.53 vs 0.54 ± 0.10 cells/mm2, CP vs NP, P < 0.0001). CPHN cells found as single cells were approximately fivefold more frequent in CP (27.4% ± 3.8% vs 5.2% ± 1.0% CPHN cells found as single cells, CP vs NP, P < 0.0001); a similar result was apparent when converted to unit area (2.1 ± 0.4 vs 0.3 ± 0.07 cells/mm2, CP vs NP, P < 0.0001) (Fig. 2D and 2E).
Within the CP group, the frequency of CPHN cells in clusters was not different between the six subjects with documented alcohol abuse and the six subjects with no history of alcohol misuse (3.5 ± 0.9 vs 1.8 ± 0.5 cells/mm2, alcohol abuse vs no known alcohol misuse, P = NS). There was no relationship between the percentage of the pancreas affected by CP in the CP group and the number of CPHN cells/mm2 pancreas (r = 0.15, P = NS). A high frequency of CPHN cells tended often to occur in a lobular pattern and may represent foci of attempted repair. This raises the possibility that there may be attempted ongoing endocrine cell regeneration in CP in response to inflammation because this pattern mimics that in late fetal and early neonatal human pancreas.
The relationship between CPHN cells and age, BMI, β cell area %, α cell area %, and the glucagon/insulin ratio
There was no relationship between age and the number of CPHN cells per unit area of pancreas in either the CP or the control groups (Supplemental Fig. 1A). There was a negative correlation of CPHN cells per unit area with BMI in the control subjects (r = 0.50, P < 0.01) (Supplemental Fig. 1B). However, there was a striking positive correlation of CPHN cells with BMI in the CP group (r = 0.70, P < 0.01) (Supplemental Fig. 1B). There was no relationship between CPHN cells and β cell area %, α cell area %, or the glucagon/insulin ratio in subjects with or without pancreatitis.
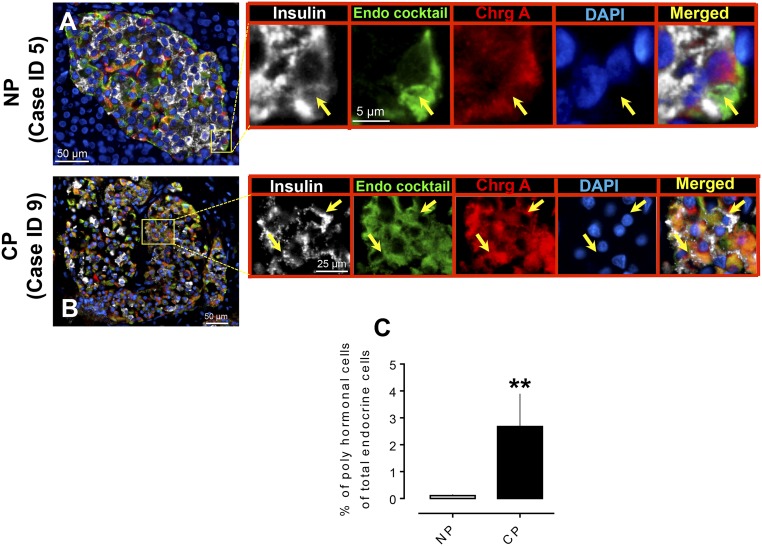
Frequency of polyhormonal cells in CP
We used immunofluorescence staining of insulin and a cocktail of antibodies to noninsulin islet hormones (glucagon, somatostatin, pancreatic polypeptide, and ghrelin) to identify insulin-expressing cells that express other islet hormones in CP (n = 14) and NP (which included lean and obese subjects with no diabetes, n = 22) (Fig. 3). Consistent with our prior results (17), these polyhormonal endocrine cells in islets were more frequent in CP compared with NP (% of polyhormonal cells of total endocrine cells in islets, 2.7 ± 1.2 vs 0.1 ± 0.04, CP vs NP, P < 0.01) (Fig. 3C).
Figure 3.
Expression of polyhormonal cells in islets in human subjects with CP. Representative merged images showing coexpression of insulin, endocrine cocktail, and chromogranin A along with 4′,6-diamidino-2-phenylindole (DAPI) in the pancreas from (A) an NP subject and (B) a subject with CP. Insets: Higher magnification of the indicated region in the low-power images; arrows indicate the polyhormonal cells that express insulin (white), endocrine cocktail (glucagon, somatostatin, pancreatic polypeptide, and ghrelin) (green), chromogranin A (red), and DAPI (blue). Individual layers are shown along with the merged image. Scale bars, 50 μm for both (A) and (B) in lower-power views, 5 μm (A) and 25 μm (B) in insets. (C) The percentage of polyhormonal cells of total endocrine cells in CP was higher compared with NP. **P < 0.01. CP, n = 13; NP, n = 22.
Coexpression of CXCL10 and its receptor CXCR3 with insulin and glucagon in islets of subjects with CP and NP
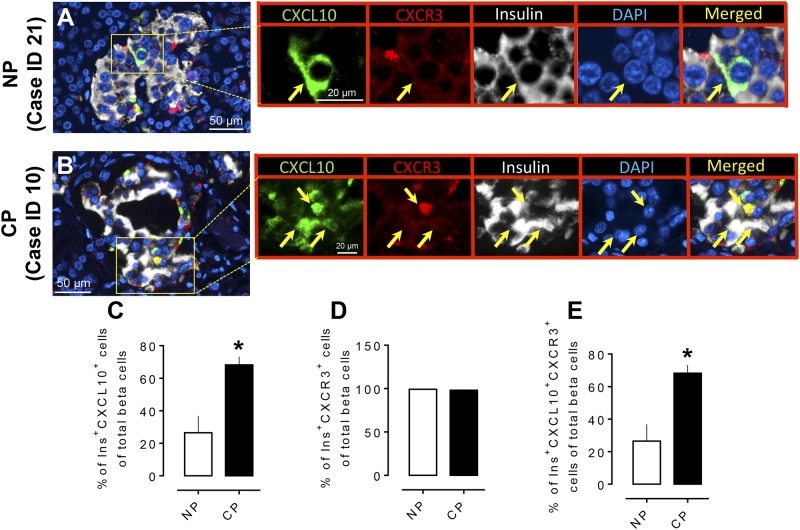
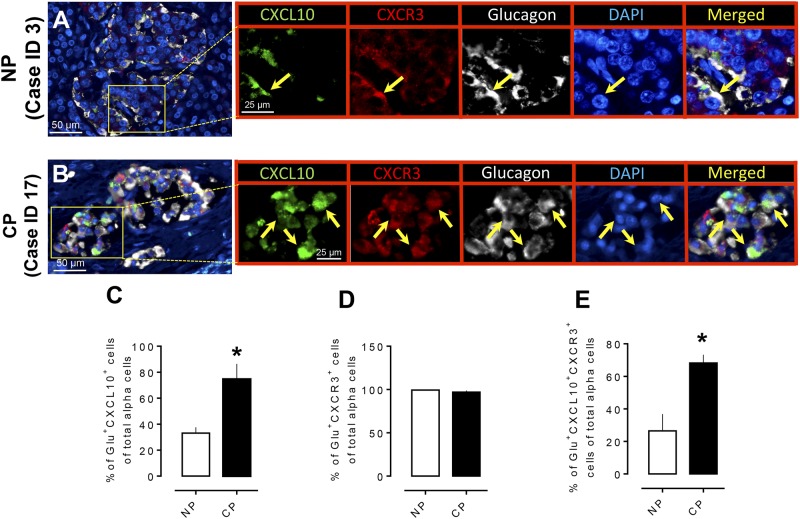
The chemokine CXCL10 has been reported to be involved in the progression of pancreatitis-like injury in mice after murine retroviral infection (24). β cells that are exposed to cytokines express chemokines, which are also produced in islets in the setting of insulitis in mice (25). Moreover, CXCL10 is produced by murine β cells (25) and is increasingly detectable in serum of newly diagnosed or prediabetic human subjects (26). Because we reported an increased number of hormone-negative and polyhormonal endocrine cells in the prediabetic state in the human islet amyloid polypeptide (HIP) rat model of type 2 diabetes (17), we questioned whether the inflammatory chemokine CXCL10 has a role in modulating pancreatic endocrine cell trans- or de-differentiation in humans with CP. We found that both β (Fig. 4A and 4B) and α (Fig. 5A and 5B) cells expressed CXCL10 in islets of subjects with and without CP and that the percentage of both β and α cells expressing CXCL10 increased in subjects with CP compared with NP subjects (% of Ins+CXCL10+ cells of total β cells, 68.2% ± 5.0% vs 26.4% ± 10.3%, CP vs NP, P < 0.05, and % of Glu+CXCL10+ cells of total α cells, 74.6% ± 11.8% vs 33.0% ± 4.4%, CP vs NP, P < 0.05) (Figs. 4C and 5C). We next evaluated the expression of CXCR3 in insulin- and glucagon-producing cells in CP and NP cases. Consistent with previous reports (27), our data indicate that >95% of α and β cells (both in CP and NP) express CXCR3 (Figs. 4D and 5D); however, the percentage of β and α cells that express both CXCL10 and CXCR3 was higher in CP compared with NP (68.2% ± 5.0% vs 26.4% ± 10.3% of Ins+CXCL10+CXCR3+ cells of total β cells, CP vs NP, P < 0.05, and 74.3% ± 11.8% vs 33.02% ± 4.5% of Glu+CXCL10+CXCR3+ cells of total α cells, CP vs NP, P < 0.05) (Figs. 4E and 5E). The above data suggest that the CXCL10-CXCR3 interaction is higher in β and α cells in CP and might be associated with endocrine cell trans-differentiation in CP, resulting in an increased α to β pancreatic fractional area.
Figure 4.
Expression of the chemokine CXCL10 and its receptor (CXCR3) in pancreatic β cells in CP. Representative merged images showing coexpression of CXCL10 and CXCR3 with insulin in the pancreas from (A) an NP subject and (B) a subject with CP. Insets: Higher magnification of the indicated region in the low-power images, with arrows indicating the cells that express CXCL10 (green), CXCR3 (red), insulin (white), 4′,6-diamidino-2-phenylindole (DAPI) (blue), and merged (yellow). Scale bar, 50 μm in lower-power views, 20 μm in insets. (C) The percentage of β cells that express CXCL10 was higher in CP compared with NP. (D) There was no change in the frequency of β cells that express the receptor (CXCR3) in CP or NP. (E) The percentage of Ins+CXCL10+CXCR3+ (triple-positive cells) was higher in CP compared with NP. *P < 0.05. CP, n = 4; NP, n = 4.
Figure 5.
Expression of the chemokine CXCL10 and its receptor (CXCR3) in pancreatic α cells in CP. Representative merged images showing coexpression of CXCL10 and CXCR3 with glucagon in the pancreas from (A) an NP subject and (B) a subject with CP. Insets: Higher magnification of the indicated region in the low-power images, with arrows indicating the cells that express CXCL10 (green), CXCR3 (red), glucagon (white), 4′,6-diamidino-2-phenylindole (DAPI) (blue), and merged (yellow). Scale bar, 50 μm in lower-power views, 25 μm in insets. (C) The percentage of α cells that express CXCL10 was higher in CP compared with NP. (D) There was no change of percentage of α cells that express receptor (CXCR3) in CP or in NP. (E) The percentage of Glu+CXCL10+CXCR3+ (triple-positive cells) was higher in CP compared with NP. *P < 0.05. CP, n = 4; NP, n = 4.
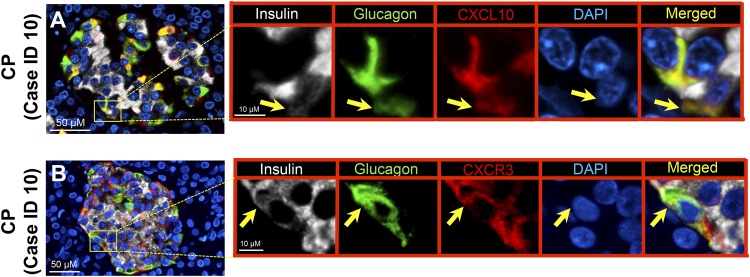
Expression of CXCL10 or CXCR3 in insulin-glucagon double-positive cells in CP: islet-derived chemokine mediated trans-differentiation?
CXCL10 and CXCR3 have been reported to be upregulated in the pancreas of subjects with CP (11). However, their association with the morphological changes of islet endocrine cells has not been previously explored. Because we found increased expression of CXCL10 and CXCL10-CXCR3 coexpressed α and β cells in CP, we questioned whether α-β double-positive cells express CXCL10 or CXCR3. To investigate this, the same cases of NP (n = 4) and CP (n = 4) that were used to analyze CXCL10/CXCR3 were stained with insulin, glucagon, and CXCL10 or CXCR3. In the case of CP, 1096 ± 463 (mean ± standard error of the mean) endocrine cells (α cells + β cells) were counted, and among them 48 ± 13 cells were double positive (Ins+Glu+). For the NP cases, 1523 ± 298 endocrine cells were examined, and 1.7 ± 0.5 cells were double positive. As anticipated, whenever an α-β double-positive cell was found, it was positive for CXCL10 and CXCR3 (Fig. 6; Supplemental Figs. 2 and 3). Colocalization of insulin and glucagon double-positive cells with CXCL10 was also confirmed by confocal z-stack images (Supplemental Fig. 4A and 4B).
Figure 6.
Expression of chemokine (CXCL10) and its receptor (CXCR3) in α-β double-positive cells. Representative merged images showing (A) coexpression of insulin-glucagon-CXCL10 and (B) insulin-glucagon-CXCR3 in subjects with CP. Insets: Higher magnification of the indicated region in the low-power images, with arrows indicating (A) the cells that express insulin (white), glucagon (green), CXCL10 (red), 4′,6-diamidino-2-phenylindole (DAPI) (blue), and merged (yellow) and (B) insulin (white), glucagon (green), CXCR3 (red), DAPI (blue), and merged (yellow). Scale bar, 50 μm in lower-power views, 10 μm in insets.
Discussion
CPHN cells are highly abundant during new endocrine cell formation in fetal and neonatal life. Lineage tracing studies in rodent models have shown β cell neogenesis in the context of exocrine pancreas inflammation. In the current study, we sought to investigate the relationship between the inflammation present in the exocrine pancreas in nondiabetic subjects with CP with the frequency of CPHN cells.
We confirmed the previous findings that pancreatic α and β cell fractional area was increased in CP, likely as a consequence of loss of acinar tissue (21), along with increased frequency of endocrine cells expressing both insulin and glucagon. We then determined that there is a marked increase in nonhormone-expressing endocrine cells in CP in islets but particularly as single cells or small clusters scattered throughout the exocrine pancreas. Although these CPHN cells may be newly forming cells, it is possible that these cells are dedifferentiating endocrine cells in response to stress secondary to the inflammatory milieu present in CP. Because lineage tracing is not possible in humans, this possibility cannot be discounted. However, the distribution of CPHN cells, being most frequent as scattered cells in the exocrine pancreas rather than in established islets, weighs in favor of them being newly forming cells because this pattern recapitulates that seen in the neonatal human pancreas when β cells are increasing in number (18). Given the increase in endocrine cells costaining for insulin and glucagon in the setting of CP, a third possibility is the bihormonal cells emerging from CPHN cells along with the possibility of α cells transdifferentiating into β cells.
This led us to investigate the endocrine compartment of the pancreas for key chemokines known to be upregulated in the exocrine compartment in CP. We found that >95% of both α and β cells express CXCR3, with no difference between CP and NP groups. However, the percentage of α and β cells expressing CXCL10 was markedly increased in this cohort of nondiabetic subjects with CP.
Given the loss of pancreatic volume in CP, it would be optimal to relate these findings, particularly the α and β cell areas, to total pancreatic weight. However, at autopsy, typically only a single block from pancreas tail is collected, and, because the entire pancreas is not routinely removed, pancreatic weights were not available.
Our findings suggest that the well-documented inflammation in the exocrine pancreas affects all compartments of the pancreas. One consequence may be the activation of regenerative programs, perhaps resulting in an increase in newly forming endocrine cells. These findings are in keeping with our recently published work in the human islet amyloid polypeptide rat model of type 2 diabetes (HIP rat) where we demonstrated a twofold increase in replication in the pancreatic duct gland (PDG) compartment in the prediabetic state (28). The fact that this increased PDG replication was present in the HIP rat before the deranged metabolism accompanying diabetes implies that islet inflammation is a sufficient stimulus to induce ductal replication, and we reported that this is also the case in pancreas from human brain-dead organ donors in whom there was increased PDG and ductal replication in both type 1 (29) and type 2 diabetes (28). In the setting of the exocrine pancreatic inflammation of CP, we now demonstrate that inflammation is present in pancreatic islets even in the nondiabetic state.
Supplementary Material
Acknowledgments
We thank Bonnie Lui from the Larry L Hillblom Islet Research Center at UCLA for editorial assistance.
Financial Support: This work was supported by National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases Grant DK077967 and by Larry Hillblom Foundation Grant 2014-D-001-NET to P.C.B.
Author Contributions: A.S.M.M., M.C., J.C., A.O., S.D., and A.E.B. performed the studies, microscopy, and morphological analysis. A.S.M.M., S.D., S.M.D., P.C.B., R.A.R., and A.E.B. researched the data, wrote the manuscript, reviewed the manuscript, edited the manuscript, and contributed to the discussion.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- BMI
body mass index
- CP
chronic pancreatitis
- CPHN
chromogranin A–positive hormone-negative
- CXCL10
chemokine C-X-C motif ligand 10
- CXCR3
chemokine C-X-C motif receptor 3
- HIP
human islet amyloid polypeptide
- NP
nonpancreatitis
- NS
not significant
- PDG
pancreatic duct gland
References
- 1. Klöppel G, Maillet B. The morphological basis for the evolution of acute pancreatitis into chronic pancreatitis. Virchows Arch A Pathol Anat Histopathol. 1992;420(1):1–4. [DOI] [PubMed] [Google Scholar]
- 2. Steer ML, Waxman I, Freedman S. Chronic pancreatitis. N Engl J Med. 1995;332(22):1482–1490. [DOI] [PubMed] [Google Scholar]
- 3. Sarner M, Cotton PB. Classification of pancreatitis. Gut. 1984;25(7):756–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lankisch PG, Löhr-Happe A, Otto J, Creutzfeldt W. Natural course in chronic pancreatitis: pain, exocrine and endocrine pancreatic insufficiency and prognosis of the disease. Digestion. 1993;54(3):148–155. [DOI] [PubMed] [Google Scholar]
- 5. Ammann RW, Akovbiantz A, Largiader F, Schueler G. Course and outcome of chronic pancreatitis. Longitudinal study of a mixed medical-surgical series of 245 patients. Gastroenterology. 1984;86(5 Pt 1):820–828. [PubMed] [Google Scholar]
- 6. Miyake H, Harada H, Kunichika K, Ochi K, Kimura I. Clinical course and prognosis of chronic pancreatitis. Pancreas. 1987;2(4):378–385. [DOI] [PubMed] [Google Scholar]
- 7. Ewald N, Hardt PD. Diagnosis and treatment of diabetes mellitus in chronic pancreatitis. World J Gastroenterol. 2013;19(42):7276–7281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ewald N, Kaufmann C, Raspe A, Kloer HU, Bretzel RG, Hardt PD. Prevalence of diabetes mellitus secondary to pancreatic diseases (type 3c). Diabetes Metab Res Rev. 2012;28(4):338–342. [DOI] [PubMed] [Google Scholar]
- 9. Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392(6676):565–568. [DOI] [PubMed] [Google Scholar]
- 10. Luster AD. Chemokines: chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338(7):436–445. [DOI] [PubMed] [Google Scholar]
- 11. Singh L, Bakshi DK, Majumdar S, Vasishta RK, Arora SK, Wig JD. Expression of interferon-gamma-inducible protein-10 and its receptor CXCR3 in chronic pancreatitis. Pancreatology. 2007;7(5-6):479–490. [DOI] [PubMed] [Google Scholar]
- 12. Li Y, Zhou ZG, Xia QJ, Zhang J, Li HG, Cao GQ, Wang R, Lu YL, Hu TZ. Toll-like receptor 4 detected in exocrine pancreas and the change of expression in cerulein-induced pancreatitis. Pancreas. 2005;30(4):375–381. [DOI] [PubMed] [Google Scholar]
- 13. Stevens T, Dumot JA, Zuccaro G Jr, Vargo JJ, Parsi MA, Lopez R, Kirchner HL, Purich E, Conwell DL. Evaluation of duct-cell and acinar-cell function and endosonographic abnormalities in patients with suspected chronic pancreatitis. Clin Gastroenterol Hepatol. 2009;7(1):114–119. [DOI] [PubMed] [Google Scholar]
- 14. Lampeter EF, Gurniak M, Brocker U, Klemens C, Tubes M, Friemann J, Kolb H. Regeneration of beta-cells in response to islet inflammation. Exp Clin Endocrinol Diabetes. 1995;103(S 02, Suppl 2):74–78. [DOI] [PubMed] [Google Scholar]
- 15. Xu X, D’Hoker J, Stangé G, Bonné S, De Leu N, Xiao X, Van de Casteele M, Mellitzer G, Ling Z, Pipeleers D, Bouwens L, Scharfmann R, Gradwohl G, Heimberg H. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132(2):197–207. [DOI] [PubMed] [Google Scholar]
- 16. Huch M, Bonfanti P, Boj SF, Sato T, Loomans CJ, van de Wetering M, Sojoodi M, Li VS, Schuijers J, Gracanin A, Ringnalda F, Begthel H, Hamer K, Mulder J, van Es JH, de Koning E, Vries RG, Heimberg H, Clevers H. Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis. EMBO J. 2013;32(20):2708–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Md Moin AS, Dhawan S, Cory M, Butler PC, Rizza RA, Butler AE. Increased frequency of hormone negative and polyhormonal endocrine cells in lean individuals with type 2 diabetes. J Clin Endocrinol Metab. 2016;101(10):3628–3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Butler AE, Dhawan S, Hoang J, Cory M, Zeng K, Fritsch H, Meier JJ, Rizza RA, Butler PC. β-cell deficit in obese type 2 diabetes, a minor role of β-cell dedifferentiation and degranulation. J Clin Endocrinol Metab. 2016;101(2):523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Md Moin AS, Dhawan S, Shieh C, Butler PC, Cory M, Butler AE. Increased hormone-negative endocrine cells in the pancreas in type 1 diabetes. J Clin Endocrinol Metab. 2016;101(9):3487–3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52(1):102–110. [DOI] [PubMed] [Google Scholar]
- 21. Klöppel G, Bommer G, Commandeur G, Heitz P. The endocrine pancreas in chronic pancreatitis: immunocytochemical and ultrastructural studies. Virchows Arch A Pathol Anat Histol. 1978;377(2):157–174. [DOI] [PubMed] [Google Scholar]
- 22. Wang W, Guo Y, Liao Z, Zou DW, Jin ZD, Zou DJ, Jin G, Hu XG, Li ZS. Occurrence of and risk factors for diabetes mellitus in Chinese patients with chronic pancreatitis. Pancreas. 2011;40(2):206–212. [DOI] [PubMed] [Google Scholar]
- 23. Lowenfels AB, Maisonneuve P, Cavallini G, Ammann RW, Lankisch PG, Andersen JR, Dimagno EP, Andrén-Sandberg A, Domellöf L; International Pancreatitis Study Group . Pancreatitis and the risk of pancreatic cancer. N Engl J Med. 1993;328(20):1433–1437. [DOI] [PubMed] [Google Scholar]
- 24. Kawauchi Y, Suzuki K, Watanabe S, Yamagiwa S, Yoneyama H, Han GD, Palaniyandi SS, Veeraveedu PT, Watanabe K, Kawachi H, Okada Y, Shimizu F, Asakura H, Aoyagi Y, Narumi S. Role of IP-10/CXCL10 in the progression of pancreatitis-like injury in mice after murine retroviral infection. Am J Physiol Gastrointest Liver Physiol. 2006;291(2):G345–G354. [DOI] [PubMed] [Google Scholar]
- 25. Frigerio S, Junt T, Lu B, Gerard C, Zumsteg U, Holländer GA, Piali L. Beta cells are responsible for CXCR3-mediated T-cell infiltration in insulitis. Nat Med. 2002;8(12):1414–1420. [DOI] [PubMed] [Google Scholar]
- 26. Nicoletti F, Conget I, Di Mauro M, Di Marco R, Mazzarino MC, Bendtzen K, Messina A, Gomis R. Serum concentrations of the interferon-gamma-inducible chemokine IP-10/CXCL10 are augmented in both newly diagnosed Type I diabetes mellitus patients and subjects at risk of developing the disease. Diabetologia. 2002;45(8):1107–1110. [DOI] [PubMed] [Google Scholar]
- 27. Morimoto J, Yoneyama H, Shimada A, Shigihara T, Yamada S, Oikawa Y, Matsushima K, Saruta T, Narumi S. CXC chemokine ligand 10 neutralization suppresses the occurrence of diabetes in nonobese diabetic mice through enhanced beta cell proliferation without affecting insulitis. J Immunol. 2004;173(11):7017–7024. [DOI] [PubMed] [Google Scholar]
- 28. Schludi B, Moin ASM, Montemurro C, Gurlo T, Matveyenko AV, Kirakossian D, Dawson DW, Dry SM, Butler PC, Butler AE. Islet inflammation and ductal proliferation may be linked to increased pancreatitis risk in type 2 diabetes. JCI Insight. 2017;2(13):e92282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moin AS, Butler PC, Butler AE. Increased proliferation of the pancreatic duct gland compartment in type 1 diabetes. J Clin Endocrinol Metab. 2017;102(1):200–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.