Abstract
Background
Post-transcriptional gene silencing (PTGS) by short interfering RNA has opened up new directions in the phenotypic mutation of cellular genes. However, its efficacy on non-nuclear genes and its effect on the interferon pathway remain unexplored. Since directed mutation of RNA genomes is not possible through conventional mutagenesis, we have tested sequence-specific 21-nucleotide long double-stranded RNAs (dsRNAs) for their ability to silence cytoplasmic RNA genomes.
Results
Short dsRNAs were generated against specific mRNAs of respiratory syncytial virus, a nonsegmented negative-stranded RNA virus with a cytoplasmic life cycle. At nanomolar concentrations, the dsRNAs specifically abrogated expression of the corresponding viral proteins, and produced the expected mutant phenotype ex vivo. The dsRNAs did not induce an interferon response, and did not inhibit cellular gene expression. The ablation of the viral proteins correlated with the loss of the specific mRNAs. In contrast, viral genomic and antigenomic RNA, which are encapsidated, were not directly affected.
Conclusions
Synthetic inhibitory dsRNAs are effective in specific silencing of RNA genomes that are exclusively cytoplasmic and transcribed by RNA-dependent RNA polymerases. RNA-directed RNA gene silencing does not require cloning, expression, and mutagenesis of viral cDNA, and thus, will allow the generation of phenotypic null mutants of specific RNA viral genes under normal infection conditions and at any point in the infection cycle. This will, for the first time, permit functional genomic studies, attenuated infections, reverse genetic analysis, and studies of host-virus signaling pathways using a wild type RNA virus, unencumbered by any superinfecting virus.
Background
Over the last decade, RNA interference (RNAi), mediated by short interfering double-stranded RNA molecules (siRNA or dsRNA), has been gradually recognized as a major mechanism of post-transcriptional gene silencing (PTGS) in species as diverse as plants, Drosophila, and C. elegans[1]. Recently, 21-nucleotide long dsRNA molecules corresponding to specific mRNA sequences, when introduced into mammalian cells in culture, have been shown to be highly effective in degrading the cognate mRNAs and thus abrogating the expression of the corresponding proteins [2]. Although the exact mechanism of PTGS is currently unknown and is an area of intense research, the successful use of this phenomenon in cultured mammalian cells has raised the exciting prospect that it can be used as a simple strategy for phenotypic ablation of mammalian gene function ex vivo without the time-consuming and expensive construction of transgenic animals.
So far, the genes targeted for siRNA-mediated PTGS have been cellular in origin and thus, the mRNAs were transcribed in the nucleus by cellular DNA-dependent RNA polymerase. In contrast, the vast majority of RNA genomes are transcribed exclusively in the cytoplasm. For example, transcription of RNA viral genomes, with the exception of retroviral RNA, is catalyzed by a virally encoded RNA-dependent RNA polymerase (RdRP) [3]. It remains untested whether the siRNA-mediated PTGS will work on mRNAs that never went through the nucleus. Interestingly, because of the potency of siRNA in some organisms, it has been proposed that they may be replicated by a cellular RdRP activity, although this has been debated [4]. Furthermore, since dsRNA is known to be a potent inducer of the interferon pathway [5,6], it is important to know whether this also occurs in cells in which antisense dsRNA has been introduced.
To answer these questions, we have used a nonsegmented negative-strand RNA (NNR) virus as a test target for RNA-mediated inhibition. We reasoned that, if successful, our studies would additionally contribute a reliable and simple technology for specific gene silencing in cytoplasmic RNA viruses. Traditionally, structure-function analyses of RNA genomes, including those of RNA viruses, have relied on spontaneous mutants found in natural isolates or chemically mutagenized stocks [7]. Mutations in either case are essentially unpredictable and must be mapped by elaborate techniques such as classical complementation analyses or direct sequencing of the genome. Since conventional site-directed mutagenesis requires a DNA template, direct mutational analysis of selected RNA genes is not an option. These obstacles have been largely circumvented by the use of cloned viral cDNA that is then altered by standard DNA-based site-directed mutagenesis procedures [8,9]. Originally designed for influenza virus minigenomes [10], such cDNA-based "reverse genetics" strategy has been adopted in a large number of NNR viruses, including vesicular stomatitis virus (VSV), respiratory syncytial virus (RSV), and measles, to name a few [11-14]. Recently, extension of this approach has resulted in the cloning of full-length viral cDNA capable of producing infectious recombinant virus particles upon transcription.
Despite its revolutionary effect on RNA viral reverse genetics, however, the cDNA-based strategy is not without limitations. First, as implied above, cloning and recombinant expression of the viral genomic RNA and all the viral proteins in the right proportion constitute a long and arduous task, daunting to an average laboratory. The problem is particularly acute for NNR viruses, which have large RNA genomes [8,9]. The 10–15 kb long RNA genomes of these viruses require specific sequences at the 5' and 3' termini for transcription and replication, and must be properly encapsidated by the nucleocapsid protein (N) in order to be recognized by the viral RdRP [15,16]. Thus, any recombinant technology must be able to faithfully reproduce these features of the genome. Moreover, the functional viral RdRP, as detailed later for RSV, is a complex holoenzyme composed of viral as well as cellular proteins [17-23]. Second, many NNR viral genomes and proteins are currently expressed from vaccinia-based cDNA clones, which generally requires superinfection by vaccinia virus [22,23]. Unfortunately, vaccinia virus itself is a major modulator of cellular signaling, including MAP kinase pathways and the actin cytoskeleton [24-26]. It is, therefore, virtually impossible to study the interaction between cellular signaling pathways and NNR viruses in cells that are also superinfected by vaccinia virus [24-26]. Third, mutations in the recombinant DNA are "permanent", and thus, the mutational phenotype cannot be switched on at pre-determined time points in infection. For example, if a viral gene product has essential roles both early and late in infection, its mutational inactivation will fail to reveal the late function, since the mutant virus will never proceed beyond the early stage.
A member of the Paramyxoviridae family, RSV is a major causative agent of childhood respiratory disease and asthma [27]. Pediatric RSV disease claims about a million lives annually, and no reliable antiviral or vaccine currently exists [27,28]. A need to understand the molecular genetics of the virus and the function of the various gene products has thus been appreciated. Since our laboratory is interested in deciphering the temporal signaling pathways in host-RSV interaction and the role of RSV gene products in the process, we have sought potential alternatives to the cDNA-based approach that might allow us to study the effect of functional loss of a specific RSV gene product during the course of a standard virus infection in cell culture. Using two different RSV gene mRNAs as targets, and another NNR virus (VSV) as well as cellular mRNAs as controls, we show that synthetic dsRNA molecules are highly efficient and specific silencers of cytoplasmic RNA viral gene expression. We also provide the first direct evidence that the 21-nt long dsRNAs do not activate a general interferon response. Our results thus offer a mechanism of specific and direct ablation of RNA-based gene expression and a quicker and simpler alternative to cDNA-based reverse genetics of RNA viruses.
Results
Ablation of viral gene expression by dsRNA against RSV P mRNA
The RNA genome of RSV is about 15 kb long and contains 11 documented protein-coding genes [13]. Three viral proteins are minimally required to reconstitute the functional transcription complex of NNR viruses [3]: the nucleocapsid protein (N) that wraps the negative-strand genome RNA and its full-length complement, the positive-strand antigenome RNA, thus converting them into highly nuclease-resistant, chromatin-like templates; the large protein (L), which is the major subunit of the RdRP; and the phosphoprotein (P), which is the smaller subunit of RdRP and an essential transcription factor of L [19-22]. In RSV, optimal transcription, although not replication, additionally requires the transcription antitermination protein M2-1 [13]. In addition, cellular actin, and to a lesser extent, profilin, are also required for viral transcription [17,18].
The overall steps of a NNR viral macromolecular synthesis in the infected cell are relevant for this paper, and are briefly described here [3]. The L protein is believed to encode the basic RNA polymerization function, and binds to the viral promoter at the 3' end of the genomic RNA to initiate transcription. However, the P protein is essential for the RdRP holoenzyme to exit the promoter and to form a closed complex that is capable of sustained elongation [20]. The preformed RdRP brought in by the infecting viral nucleocapsids catalyzes the first rounds of transcription, known as primary transcription. In its "transcription mode", the viral RdRP starts and stops at the beginning and end, respectively, of each viral gene, and this results in the synthesis of individual gene mRNAs. Unlike the full-length genomic and antigenomic RNA, the mRNAs are 5'-capped, 3'-polyadenylated, and do not bind N protein. Translation of these mRNAs results in de novo synthesis of viral proteins. The availability of large quantities of N protein then allows encapsidation of nascent leader RNA by N. This leads to the switching of the RdRP to the "replication mode", resulting in the synthesis of full-length, encapsidated anti-genomic RNA, which is in turn replicated into more genomic RNA [15]. Thus, the very requirement of N for replication ensures that all full-length genomic and antigenomic RNA are wrapped with N protein, i.e., encapsidated [15]. The new pool of replicated genomic RNA serves as templates for secondary transcription. It should be obvious from the foregoing that the de novo macromolecular synthesis accounts for the major burst of viral protein and RNA in the infected cell. Specifically, if the de novo synthesis of the essential subunits of viral RdRP – such as L or P – is inhibited, it will abolish the bulk of viral transcription and replication, and hence, viral translation [3].
To test the effectiveness of the anti-P dsRNA, we transfected the dsRNA into A549 cells, and infected the cells with RSV. Subsequently, the amount of intracellular P protein was directly monitored by immunoblot analysis using anti-P antibody. Results presented in Fig. 1 show a nearly 90% reduction of P protein using as little as 10 nM dsRNA. Although we have not tested lower amounts of dsRNA for P, the severe loss of P protein at 10 nM dsRNA and only a slightly greater loss with higher dsRNA concentrations (Fig. 1) suggest that it may be possible to cause substantial ablation of P protein at dsRNA concentrations even below 10 nM.
Figure 1.
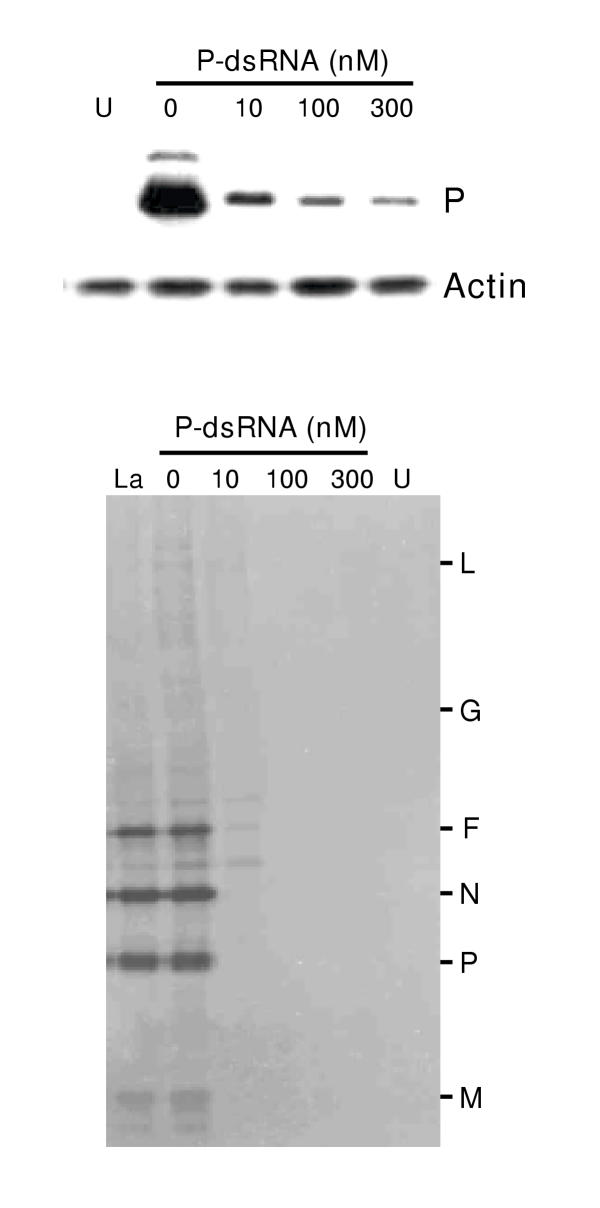
Ablation of RSV P protein by anti-P dsRNA. Transfection of A549 cells with 0 (no dsRNA), 10, 100, or 300 nM dsRNA and infection with RSV were carried out as described under Materials and Methods. Lane 'U' indicates control, uninfected cells. Top: Immunoblot (Western) of total cell extracts was performed with either rabbit anti-P or monoclonal anti-actin antibody (Boehringer-Mannheim), as indicated. Bottom: Viral protein synthesis in dsRNA-treated cells was measured by standard immunoprecipitation procedures as described previously [17]. Infected A549 cells (or uninfected control, lane 'U') were metabolically labeled with 35S-(methionine plus cysteine) at 18 h post-infection, followed by lysis of the cells, precipitation with anti-RSV antibody, and analysis of the labeled proteins by SDS-PAGE and autoradiography. 'La' represents treatment with 100 nM anti-lamin A/C dsRNA. The different viral protein bands are so indicated.
The phenotypic effect of loss of P was further examined by measuring progeny viral titer, overall viral protein synthesis, and syncytium formation, as described under Materials and Methods. Yield of progeny virus in 20 nM dsRNA-treated cells was found to be reduced by 10 fold, and was reduced by at least 104 fold at 100 and 300 nM dsRNA (data not shown). De novo viral protein synthesis was measured by metabolic labeling with S35-Met/Cys followed by immunoprecipitation. As shown in Fig. 1, all viral proteins detectable in the precipitate were drastically diminished in the dsRNA-treated cells, as would be expected in the event of a loss of the P protein. The inhibition of viral growth was further reflected in the essentially complete loss of cell fusion (syncytia) in the treated cells (Fig. 2). In fact, the RSV-infected anti-P dsRNA-treated cells were morphologically indistinguishable from control uninfected ones even at 5 days post-infection, which was the longest time period for which they were observed. The presence of equal amounts of actin in all the samples confirmed that the observed inhibition of viral proteins is not due to a general degradation of proteins.
Figure 2.
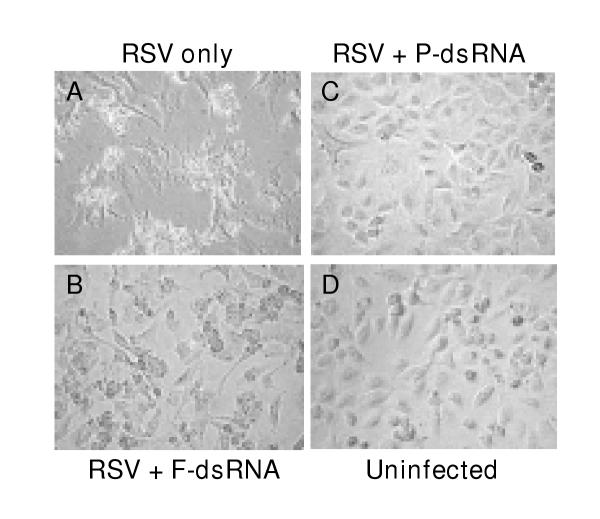
Effect of dsRNA on the cell fusion activity of RSV. A549 monolayers were transfected with 20 nM of anti-P or anti-F dsRNA and infected with RSV as described under Materials and Methods. At 40 h p.i., the monolayers were examined under a Nikon TS100F phase-contrast microscope at 40× magnification and digitally photographed with a Nikon Coolpix 995 camera. Note the syncytia in 'A', cytopathic effect without syncytia in 'B', and monolayers that appear unaffected and identical in 'C' and 'D'.
The specificity of dsRNA activity was further tested by using a dsRNA against cellular lamin A/C that was earlier shown to specifically abrogate lamin A/C synthesis in a variety of cultured cell lines [2]. As shown in Fig. 1 (lane 'La'), the anti-lamin dsRNA, while abrogating lamin protein (data not shown) had no effect on RSV protein synthesis. Furthermore, a mismatched anti-P dsRNA in which the lowercase A-U base pair (see the dsRNA sequences in Materials and Methods) was altered to a G-C pair also failed to inhibit viral translation (data not shown), confirming that a perfect match is needed for the dsRNA effect, hence its extreme specificity of action.
Lack of syncytium in RSV-infected cells treated with anti-F dsRNA
Fusion of the infected cells is a hallmark of all Paramyxoviruses including RSV (as also in some other viruses, such as HIV), and the resultant mass of fused cells is referred as a syncytium, from which respiratory syncytial virus derives its middle name. The fusion protein F is by far the most important viral glycoprotein that is central to the cell fusion activity [29]. Since the P and F proteins have such diverse roles in viral life cycle, we decided to investigate the effect of dsRNA on F as a second test gene, and also to compare and contrast the two respective phenotypes.
First, to test the effectiveness of the anti-F dsRNA intracellularly, we probed the infected cell monolayer with anti-F antibody by indirect immunofluorescence (Fig. 3). Results clearly demonstrated the abundant synthesis of F protein as cytoplasmic fluorescence in cells that were not treated with dsRNA; the nuclei of the same cells could be visualized by staining with DAPI. In contrast, cells treated with just 3 nM anti-F dsRNA showed a substantial loss of F stain. At 20 nM dsRNA, F protein was undetectable.
Figure 3.
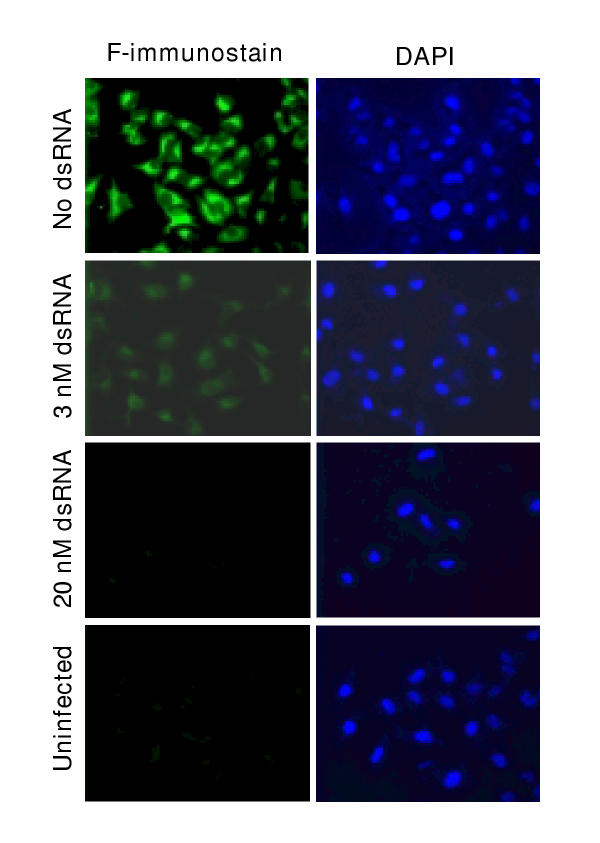
Ablation of RSV F by anti-F dsRNA. Anti-F dsRNA, RSV infection, and immunostaining of A549 monolayer were performed as described under Materials and Methods. Right panel shows the nuclear staining of the same cells using DAPI (Blue). Note the substantial reduction of F (Green) with as low as 3 nM anti-F dsRNA, and reduction to background levels by 20 nM dsRNA.
Second, immunoblot analysis (Fig. 4, top panel) revealed that anti-F dsRNA, at concentrations as low as 20 nM, produced a severe reduction in F protein levels. Again, no effect was seen on cellular profilin, ruling out a general protein loss. The anti-F dsRNA also had no effect on P protein levels, suggesting that such dsRNAs do not activate a general antiviral response that might abrogate all viral mRNA translation. This was further corroborated by the direct measurement of de novo viral protein synthesis by metabolic labeling (Fig. 4, bottom panel). Results showed that the synthesis of F only was affected while all other viral proteins were translated in normal amounts, which is in agreement with the notion that F has little or no role in intracellular viral macromolecular synthesis.
Figure 4.
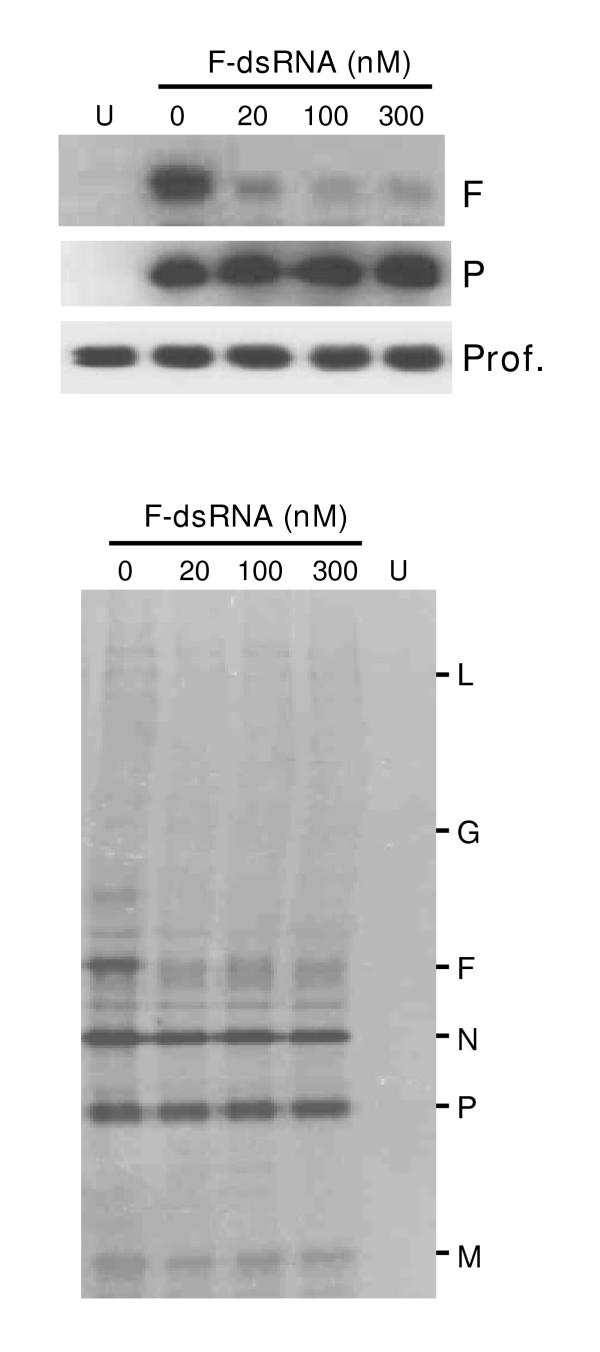
Specificity of anti-F dsRNA. Experiments were done essentially as described for Fig. 2. A549 cells were transfected with the indicated amounts of anti-F dsRNA followed by infection by RSV as described under Materials and Methods. Top: Immunoblot of total cell extracts to detect RSV F, RSV P, and profilin; Bottom: Autoradiograph showing immunoprecipitated metabolically 35S-labeled RSV proteins. 'U' represents uninfected cells. Note the specific loss of F protein, but no effect on other proteins.
Finally, the phenotype of F protein loss was tested by examining syncytia formation, and as presented in Fig. 2, no syncytia could be discerned in anti-F dsRNA-treated cells (Panel B). However, a cytopathic effect was still visible, which is most likely the result of intracellular replication of the virus. This demonstrates an interesting contrast with the anti-P dsRNA (Panel C), which inhibited all viral gene expression, and therefore, the resultant monolayer exhibited essentially the same appearance as the uninfected one (Panel D).
Direct measurement of intracellular F mRNA by semi-quantitative RT-PCR showed a nearly 15-fold loss caused by anti-F dsRNA (Fig. 5, top panel). Similar RT-PCR of viral genomic RNA, viral P mRNA, or cellular actin mRNA revealed essentially no reduction, suggesting that the dsRNA did not activate a general antiviral response, and did not directly target genomic RNA. Together, these results directly demonstrate that the dsRNAs promote ablation of the specific mRNA target, which most likely underlies the loss of the respective proteins.
Figure 5.
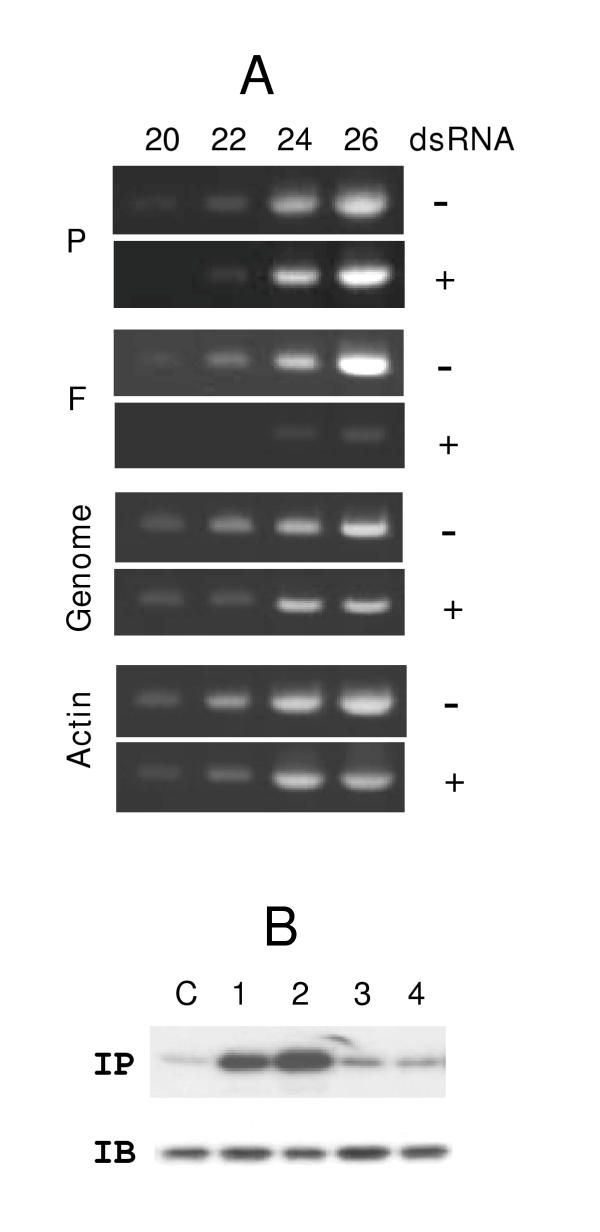
Induction of target mRNA degradation but not interferon response by dsRNA. Panel A: Semi-quantitative RT-PCR to measure the indicated RSV gene mRNA and genomic RNA in A549 cells were performed as described under Materials and Methods. Where indicated (labeled '+'), anti-F dsRNA was used at a concentration of 20 nM. PCR samples were taken at the end of the number of cycles indicated on top (20, 22, 24, and 26). Actin mRNA was also quantitated as a control. Note that in dsRNA-untreated cells (labeled '-') the F band is visible even at 20 cycles, whereas in the treated cells, appearance of a comparable intensity required 4 additional PCR cycles, i.e., 16-fold more amplification. Panel B: Assay of eIF-2α phosphorylation. Metabolic 32P-labeling and immunoprecipitation (IP) analysis of eIF-2 have been described in Materials and Methods. An autoradiograph of the gel is shown. The cells were treated with no RNA (lane C), 100 nM thapsigargin (lane 1), 100 nM A23178 (lane 2), 50 nM anti-P dsRNA (lane 3), or 50 nM anti-F dsRNA (lane 4). Note the increased phosphorylation of eIF-2 in lanes 1 and 2 only. The immunoblot (IB) shows that the total amount of eIF-2α protein was not affected by the treatments.
Anti-RSV dsRNAs do not activate an interferon response
As mentioned before, cytoplasmic dsRNA can trigger a series of signaling reactions that lead to interferon (IFN) synthesis [5,6]. In the "interferon response", dsRNA molecules activate protein kinase PKR and 2',5'-oligonucleotide synthetase. One of the effects of PKR is to phosphorylate the α subunit of the general translation initiation factor eIF-2, which constitutes a major mechanism for global translation arrest. The 2', 5'-oligonucleotide synthetase activates RNase L that in turn catalyses non-specific degradation of mRNA. Since NNR viruses co-opt the cellular translation machinery, the interferon response thus causes severe inhibition of viral translation. Interestingly, the IFN response requires long dsRNA [5,6], and it has been conjectured that the improved specificity of the 21-nucleotide long dsRNA in cultured mammalian cells is probably due to their inability to activate the IFN response [2]. We provide several lines of direct and indirect evidence that the dsRNAs described here did not activate a general IFN response. First, the inhibitory effect of each dsRNA was gene-specific (Figs. 1, 2, 4). Second, growth of VSV is known to be highly sensitive to IFN, however, its replication in A549 cells was not affected by any of the dsRNAs described here (data not shown). Lastly, elevated phosphorylation of eIF-2α has been used as a diagnostic marker of IFN response [30]. We, therefore, examined the phosphorylation status of eIF-2α in A549 cells following transfection with these dsRNAs. Results (Fig. 5, bottom panel) showed no increase in phosphorylation. In positive controls, A549 cell treated with calcium mobilizers (thapsigargin or A23178) did increase eIF-2 phosphorylation, as has been shown earlier [30]. These results provide the first direct evidence that the 21-nt long double-stranded siRNAs fail to trigger interferon response in mammalian cells, and hence, can be used as specific antiviral agents.
Discussion
In this communication, we establish 21-nucleotide long double-stranded interfering RNA as a viable tool to ablate specific cytoplasmic RNAs, as exemplified by the mRNAs of a RNA virus. The major findings are: (i) The ablation is highly efficient: nanomolar concentrations of dsRNA can lead to a 10–20 fold reduction of the corresponding protein. The two RSV mRNAs that we have targeted here, namely P and F, are relatively abundant viral mRNAs. In fact, they respectively represent the 4th and 5th most abundant viral mRNAs in the infected cell. Thus, we predict that an even lower concentration of dsRNA, perhaps in the sub-nanomolar range, might be able to destroy NNR viral mRNAs that are rarer, such as those of RSV M2 and L proteins. This is currently being tested. It is to be noted that a chemical is generally considered a promising pharmaceutical if it is effective at sub-micromolar concentrations, (ii) The effect is highly specific: dsRNA against one viral mRNA did not affect other viral mRNAs or the cellular genes tested here. In this regard, dsRNA generally surpasses the standard antisense technology based on oligodeoxynucleotides (ODNs), whose specificity has often been debated. Moreover, since the most potent antisense ODNs rely on the degradation of target RNA by RNase H [31], which is a predominantly nuclear enzyme, this mechanism is not available to RNA viruses that are strictly cytoplasmic. (iii) Lack of interferon response: We provide direct experimental evidence that the 21-nt dsRNA does not trigger an IFN response, which is an advantage because an IFN response would have caused a general and non-specific inhibition of all cap-dependent translation [5]. (iv) Regulation any time: Although we have not tested this specifically, one can, in principle, transfect the virus-infected cell with dsRNA at any time point during infection. This will allow ablation of a specific protein at different times in infection and allow one to determine if the same "mutation" may have early and late phenotypes. In addition, this will allow mutational analysis of essential genes, genetic deletion of which may result in a nonviable virus. In this regard, the dsRNA technique is akin to a temperature-shift experiment using a temperature-sensitive mutant virus, however, as implied earlier, conditional lethal mutations are not available for the vast majority of viral genes. (v) Ease of use: The dsRNA approach is relatively simple to design, and its application in cell culture only requires the standard transfection technology that already exists. (vi) Multiple targets: It is also possible to deplete multiple cellular or viral gene mRNAs in any combination, either simultaneously or in a temporal order, and thus ask questions about the interaction between the phenotypes. (vii) Normal infection environment: As we have contended, a major benefit of this approach in viral reverse genetics is that one can start with standard wild type virus (or even a relatively uncharacterized field isolate) with no requirements of recombinant expression, and thus, the cellular milieu is only minimally perturbed, if at all.
Lastly, the lack of effect of the anti-F dsRNA on viral genome replication deserves special attention. As described earlier (in the beginning of the Results section), intracellular NNR viral replication generates both negative- and positive-strand full-length genomic RNA, each of which should find complementarity to the appropriate strand of the anti-F dsRNA. In general, genome-length RNAs of both positive and negative sense could, therefore, be potential targets for RNA interference, resulting in severe inhibition of replication. We speculate that the genomic and antigenomic RNA of NNR viruses escape the onslaught of dsRNA because they are tightly wrapped with the nucleocapsid protein N, which makes them inaccessible to the dsRNA and/or the RNAi silencing complex (RISC). As mentioned before, the N-encapsidated genome-length RNAs are indeed extremely resistant to nucleases, to the extent that formation of such nuclease-resistant RNA products is in fact considered a defining criterion for viral replication in vitro and in vivo[32]. While the resistance of the viral genomic RNA to dsRNA has important ramifications for antiviral therapy, it is also clear that the dsRNA approach cannot be used against cis-acting NNR viral genomic sequences such as the intergenic regions, for which the cDNA-based approach will continue to be the method of choice [16]. It is obvious that a creative combination of the two techniques will lead to exciting possibilities in the reverse genetics of RNA viruses and in the antiviral regimen.
Conclusions
Properly designed synthetic 21-nucleotide long double-stranded RNA (dsRNA) molecules can effectively and specifically abrogate translation of target RNAs without activating a general interferon response. When applied against mRNAs of cytoplasmic RNA viruses, the dsRNAs caused degradation of the specific viral mRNA and resultant ablation of the specific viral protein. The technique is quick, simple, and can be used against wild type RNA viruses in standard tissue culture at any point in the infection cycle. Post-transcriptional gene silencing by such dsRNA molecules should facilitate the reverse genetics and functional genomics of RNA genomes.
Materials and methods
Double-stranded RNA
The following dsRNA sequences with 3'-dT extensions were synthesized against RSV P, RSV F, and cellular lamin A/C mRNA sequences (Accession numbers Ml1486, M22643, and X03444, respectively), following the design recommendations of Tuschl and coworkers [2]:
P: 5' CGAUAAUAUaACUGCAAGATT 3'
3' TTGCUAUUAUAuUGACGUUCU 5'
F: 5' UGCUGUAACAGAAUUGCAGTT 3'
3' TTACGACAUUGUCUUAACGUC 5'
Lamin: 5' CUGGACUUCCAGAAGAACATT 3'
3' TTGACCUGAAGGUCUUCUUGU 5'
The two T's at the 3'-end of all RNAs were 2'-deoxythymidines. The dT residues most likely provided stability against RNases [2], since they produced a more reproducible and sustained effect at a substantially lower concentration, compared to RNAs that contained all ribonucleotides but an otherwise identical sequence (data not shown). All oligonucleotide were synthesized by Dharmacon Research (Lafayette, CO) using their recommended 2'-ACE protection chemistry, and then gel-purified. Deprotection of the RNA and hybridization of the two strands were carried out according to the manufacturer's protocol. In the mutant dsRNA (for P), the lowercase a-u base pair was changed to g-c (see Results).
Transfection with dsRNA and RSV infection
The dsRNAs were introduced in cells essentially as described [2]. Briefly, A549 cells were propagated in standard MEM (Life Technologies) supplemented with 10% fetal bovine serum (FBS) and penicillin-streptomycin. Twenty-four h before transfection, cells were trypsinized and transferred to 12-well plates. Transfection with dsRNAs was carried out with OligofectAMINE Reagent (Life Technologies) in OPTIMEM I (Life Technologies) as described by the manufacturer for adherent cell lines. Indicated amounts of dsRNA, formulated into liposomes, were applied per well, and the final volume was 500 μl per well. Cells were incubated for 6 h after transfection, and then infected with RSV Long strain at an m.o.i. of 4 [17,33], and FBS was added back to 1% to supplement the growth of the virus. A second transfection was performed after 5 h of infection and FBS was again restored to 1% in 12 h after second transfection. At 40 to 48 h after the first transfection, cells were photographed, or processed for immunostaining (see below), RNA isolation, or immunoblot, as and where indicated.
Indirect immunofluorescence
Rabbit anti-F antibody was raised against a synthetic peptide, and was a kind gift from Dr. James E. Crowe, Jr. (Vanderbilt University, TN). Rabbit polyclonal antibody against RSV-P protein has been described [21]. Indirect immunofluorescence and nuclear staining with DAPI was performed essentially as described previously [33]. A549 cells in monolayer, grown on cover slips, were washed in PBS and fixed in ice-cold 10% trichloracetic acid for 15 min, followed by successive washes in cold 70%, 90% and absolute ethanol for 3 min each. After one more PBS washing, the fixed cells were incubated for 45 min at room temperature with anti-P or anti-F protein antibody in PBS. Thereafter, cells were washed three times for 5 min in PBS and incubated for 45 min at room temperature with TRITC- or FITC-conjugated anti-rabbit IgG secondary antibody (Sigma) diluted 1:75. Nonspecific binding was eliminated by three washes in PBS. Where mentioned, nuclei were stained with DAPI (Sigma) after the final PBS wash. Cells were visualized and the images digitally captured in an Olympus BMAX Epifluorescence microscope using a 100× oil-immersion objective and appropriate filters [12,33].
Ex vivo phosphorylation assay
Metabolic labeling of cells and immunoprecipitation was performed essentially as described previously [21,30]. In brief, confluent monolayers of A549 cells were transfected with appropriate dsRNA (at 20 nM final) as described above, and at 8 hr post-transfection, were washed with a phosphate-free buffer (120 mM NaCl, 5 mM KCl, 1.5 mM MgCl2, 0.25 mM CaCl2, 25 mM NaHCO3, 20 mM HEPES [pH 7.4]) and labeled with 32P-orthophosphate (500 μCi/ml) (Amersham) in Dulbecco-modified Eagle MEM lacking both phosphate and pyruvate. Where mentioned, thapsigargin or A23178 was used at final concentrations of 100 nM, and was added to the cells 15 min before addition of the 32P-containing medium containing the same concentration of the drugs. After a 1 h labeling period, all cells were washed with phosphate-buffered saline without Ca2+ and Mg2+ and disrupted as described previously to obtain the total lysate. The eIF-2 was precipitated with a polyclonal antibody against human eIF-2α (C-20) (Santa Cruz Biotech) in the presence of protein G-Sepharose (Amersham) for 1 h at 10°C, and then processed as described previously [21]. The immunoprecipitates were subjected to SDS-PAGE followed by autoradiography to examine eIF-2 phosphorylation.
To monitor total eIF-2α in the cell, a parallel A549 monolayer was treated identically except that the labeled orthophosphate was omitted. Portions of the total cell lysate were analyzed by SDS-PAGE followed by immunoblot with anti-eIF-2α antibody. All immunoblots (Western blots) were performed essentially as described [17]. Thapsigargin and A23178 were from Sigma-Aldrich.
Reverse transcription-PCR (RT-PCR)
Quantitation of specific mRNAs by RT-PCR was done essentially as described [34]. Briefly, total RNA was isolated from trypsinized cell monolayers using the Quickprep Micro™ mRNA purification kit from Pharmacia Biotech (Piscataway, NJ). Equal amounts of total RNA were subjected to reverse transcription using the C. therm. RT system (Roche Molecular Biochemicals, Indianapolis, IN) at 65°C for 1 hr in the presence of 5 units of RNasin (Promega), followed by PCR through increasing number of cycles. The primers were based on RSV (Acc# M74568) and actin (X00351) sequences, and are as follows (sense and antisense, respectively):
P: 5'CCCTTTTCTAAACTATACAAAGAAACC3' and 5'AGCAGATGTAGGTCCTGCACTTG3';
F: 5'AGTGTAATGGAACAGATGCCAAGG3' and 5'GCAGGACCTTAGATACAGCAGTG3';
RSV genomic RNA: 5'AATGACCAATTATATGAATCAATTATCTG3' and 5'GTTGACCAGGAATGTAAATGTGGC3';
β-actin: 5'CCTCACCCTGAAGTACCCCATC3' and 5'GCCGTGGTGGTGAAGCTGTAGC3'. The RT-PCR products were 198, 274, 307, and 420 bp long, respectively, for these mRNAs. The amount of RNA was optimized for the individual genes, and PCR samples were withdrawn at 20, 22, 24, and 26 cycles of amplification and analyzed by standard agarose gel electrophoresis followed by ethidium bromide staining.
Acknowledgments
Acknowledgements
We thank Dr. James E. Crowe for the anti-F antibody. This research was supported in part by a National Research Service Award (to V.B.) from the National Institute of Allergy and Infectious Diseases, NIH. The technical assistance of Anja Oldenburg is appreciated.
Contributor Information
Vira Bitko, Email: vbitko@jaguar1.usouthal.edu.
Sailen Barik, Email: sbarik@jaguar1.usouthal.edu.
References
- Hammond SM, Caudy AA, Hannon GJ. Post-transcriptional gene silencing by double-stranded RNA. Nat Rev Genet. 2001;2:110–119. doi: 10.1038/35052556. [DOI] [PubMed] [Google Scholar]
- Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- Banerjee AK, Barik S, De BP. Gene expression of nonsegmented negative-strand RNA viruses. Pharmacol Ther. 1991;51:47–70. doi: 10.1016/0163-7258(91)90041-j. [DOI] [PubMed] [Google Scholar]
- Cogoni C, Macino G. Post-transcriptional gene silencing across kingdoms. Curr Opin Genet Dev. 2000;10:638–643. doi: 10.1016/s0959-437x(00)00134-9. [DOI] [PubMed] [Google Scholar]
- Geiss G, Jin G, Guo J, Bumgarner R, Katze MG, Sen GC. A comprehensive view of regulation of gene expression by double-stranded RNA-mediated cell signaling. J Biol Chem. 2001;276:30178–30182. doi: 10.1074/jbc.c100137200. [DOI] [PubMed] [Google Scholar]
- Kumar M, Carmichael GG. Antisense RNA: function and fate of duplex RNA in cells of higher eukaryotes. Microbiol Mol Biol Rev. 1998;62:1415–1434. doi: 10.1128/mmbr.62.4.1415-1434.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe JE, Jr, Bui PT, London WT, Davis AR, Hung PP, Chanock RM, Murphy BR. Satisfactorily attenuated and protective mutants derived from a partially attenuated cold-passaged respiratory syncytial virus mutant by introduction of additional attenuating mutations during chemical mutagenesis. Vaccine. 1994;12:691–699. doi: 10.1016/0264-410x(94)90218-6. [DOI] [PubMed] [Google Scholar]
- Pekosz A, He B, Lamb RA. Reverse genetics of negative-strand RNA viruses: Closing the circle. Proc Natl Acad Sci USA. 1999;96:8804–8806. doi: 10.1073/pnas.96.16.8804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marriott AC, Easton AJ. Reverse genetics of the Paramyxoviridae. Adv Virus Res. 1999;53:321–340. [PubMed] [Google Scholar]
- Luytjes W, Krystal M, Enami M, Pavin JD, Palese P. Amplification, expression, and packaging of foreign gene by influenza virus. Cell. 1989;59:1107–1113. doi: 10.1016/0092-8674(89)90766-6. [DOI] [PubMed] [Google Scholar]
- Whelan SP, Ball LA, Barr JN, Wertz GT. Efficient recovery of infectious vesicular stomatitis virus entirely from cDNA clones. Proc Natl Acad Sci USA. 1995;92:8388–8392. doi: 10.1073/pnas.92.18.8388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson N, Stillman E, Whitt M, Rose J. Recombinant vesicular stomatitis viruses from DNA. Proc Natl Acad Sci USA. 1995;92:4477–4481. doi: 10.1073/pnas.92.10.4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins PL, Hill MG, Camargo E, Grosfeld H, Chanock RM, Murphy BR. Production of infectious human respiratory syncytial virus from cloned cDNA confirms an essential role for the transcription elongation factor from the 5' proximal open reading frame of the M2 mRNA in gene expression and provides a capability for vaccine development. Proc Natl Acad Sci USA. 1995;92:11563–11567. doi: 10.1073/pnas.92.25.11563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radecke F, Spielhofer P, Schneider H, Kaelin K, Huber M, Dotsch C, Christiansen G, Billeter MA. Rescue of measles viruses from cloned DNA. EMBO J. 1995;14:5773–5784. doi: 10.1002/j.1460-2075.1995.tb00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz GM, Howard MB, Davis N, Patton J. The switch from transcription to replication of a negative-strand RNA virus. Cold Spring Harb Symp Quant Biol. 1987;52:367–371. doi: 10.1101/sqb.1987.052.01.042. [DOI] [PubMed] [Google Scholar]
- Pattnaik AK, Ball LA, LeGrone A, Wertz GW. The termini of VSV DI particle RNAs are sufficient to signal RNA encapsidation, replication, and budding to generate infectious particles. Virology. 1995;206:760–764. doi: 10.1016/S0042-6822(95)80005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke E, Dupuy L, Wall C, Barik S. Role of cellular actin in the gene expression and morphogenesis of human respiratory syncytial virus. Virology. 1998;252:137–148. doi: 10.1006/viro.1998.9471. [DOI] [PubMed] [Google Scholar]
- Burke E, Mahoney NM, Almo SC, Barik S. Profilin is required for optimal actin-dependent transcription of respiratory syncytial virus genome RNA. J Virol. 2000;74:669–675. doi: 10.1128/jvi.74.2.669-675.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barik S, McLean T, Dupuy LC. Phosphorylation of Ser232 directly regulates the transcriptional activity of the P protein of human respiratory syncytial virus: phosphorylation of Ser237 may play an accessory role. Virology. 1995;213:405–412. doi: 10.1006/viro.1995.0013. [DOI] [PubMed] [Google Scholar]
- Dupuy LC, Dobson S, Bitko V, Barik S. Casein kinase 2-mediated phosphorylation of respiratory syncytial virus phosphoprotein P is essential for the transcription elongation activity of the viral polymerase; phosphorylation by casein kinase 1 occurs mainly at Ser215 and is without effect. J Virol. 1999;73:8384–8392. doi: 10.1128/jvi.73.10.8384-8392.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumder B, Barik S. Requirement of casein kinase ll-mediated phosphorylation for the transcriptional activity of human respiratory syncytial viral phosphoprotein P: transdominant negative phenotype of phosphorylation-defective P mutants. Virology. 1994;205:104–111. doi: 10.1006/viro.1994.1624. [DOI] [PubMed] [Google Scholar]
- Yu Q, Hardy RW, Wertz GW. Functional cDNA clones of the human respiratory syncytial (RS) virus N, P, and L proteins support replication of RS virus genomic RNA analogs and define minimal trans-acting requirements for RNA replication. J Virol. 1995;69:2412–2419. doi: 10.1128/jvi.69.4.2412-2419.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosfeld H, Hill MG, Collins PL. RNA replication by respiratory syncytial virus (RSV) is directed by the N, P, and L proteins; transcription also occurs under these conditions but requires RSV superinfection for efficient synthesis of full-length mRNA. J Virol. 1995;69:5677–5686. doi: 10.1128/jvi.69.9.5677-5686.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Magalhaes JC, Andrade AA, Silva PN, Sousa LP, Ropert C, Ferreira PC, Kroon EG, Gazzinelli RT, Bonjardim CA. A mitogenic signal triggered at an early stage of vaccinia virus infection: implication of MEK/ERK and protein kinase A in virus multiplication. J Biol Chem. 2001;276:38353–38360. doi: 10.1074/jbc.M100183200. [DOI] [PubMed] [Google Scholar]
- Dramsi S, Cossart P. Intracellular pathogens and the actin cytoskeleton. Annu Rev Cell Dev Biol. 1998;14:137–166. doi: 10.1146/annurev.cellbio.14.1.137. [DOI] [PubMed] [Google Scholar]
- Frischknecht F, Moreau V, Rottger S, Gonfloni S, Reckmann I, Superti-Furga G, Way M. Actin-based motility of vaccinia virus mimics receptor tyrosine kinase signalling. Nature. 1999;401:926–929. doi: 10.1038/44860. [DOI] [PubMed] [Google Scholar]
- Falsey AR, Walsh EE. Respiratory syncytial virus infection in adults. Clin Microbiol Rev. 2000;13:371–384. doi: 10.1128/cmr.13.3.371-384.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn JS. Respiratory syncytial virus vaccine development. Curr Opin Pediatr. 2000;12:257–262. doi: 10.1097/00008480-200006000-00015. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Reyes L, Ruiz-Arguello MB, Garcia-Barreno B, Calder L, Lopez JA, Albar JP, Skehel JJ, Wiley DC, Melero JA. Cleavage of the human respiratory syncytial virus fusion protein at two distinct sites is required for activation of membrane fusion. Proc Natl Acad Sci USA. 2001;98:9859–9864. doi: 10.1073/pnas.151098198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava SP, Davies MV, Kaufman RJ. Calcium depletion from the endoplasmic reticulum activates the double-stranded RNA-dependent protein kinase (PKR) to inhibit protein synthesis. J Biol Chem. 1995;270:16619–16624. doi: 10.1074/jbc.270.28.16619. [DOI] [PubMed] [Google Scholar]
- Bennett CF, Cowsert LM. Application of antisense oligonucleotides for gene functionalization and target validation. Curr Opin Mol Ther. 1999;1:359–371. [PubMed] [Google Scholar]
- Moyer SA, Smallwood-Kentro S, Haddad A, Prevec L. Assembly and transcription of synthetic vesicular stomatitis virus nucleocapsids. J Virol. 1991;65:2170–2178. doi: 10.1128/jvi.65.5.2170-2178.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitko V, Barik S. An endoplasmic reticulum-specific stress-activated caspase (caspase-12) is implicated in the apoptosis of A549 epithelial cells by respiratory syncytial virus. J Cell Biochem. 2001;80:441–454. doi: 10.1002/1097-4644(20010301)80:3<441::aid-jcb170>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Fuchs B, Zhang K, Schabel A, Bolander ME, Sarkar G. Identification of twenty-two candidate markers for human osteogenic sarcoma. Gene. 2001;278:245–252. doi: 10.1016/s0378-1119(01)00731-4. [DOI] [PubMed] [Google Scholar]


