Abstract
Background:
The kynurenine pathway enzymes, breaking down tryptophan, are abundant in placental tissue. These metabolites are involved in immunoregulatory mechanisms, although the role of this pathway in pre-eclampsia (PE) has only begun to be characterized. Here, we determined tryptophan and metabolite levels together with the expression of kynurenine pathway enzymes and inflammatory factors in placental tissue from women with and without PE.
Methods:
Thirty-six placentas (18 PE and 18 controls) were analyzed for expression of kynurenine pathway enzymes indoleamine-2,3-dioxygenase (IDO1 and 2), tryptophan-2,3-dioxygenase (TDO), kynurenine-3-mono-oxygenase (KMO) and quinolinate phosphoribosyltransferase (QPRT) as well as interleukin (IL)-1β, IL-6, and serum amyloid A (SAA). Tryptophan and kynurenine content were measured using high-pressure liquid chromatography and quinolinic acid was measured using gas chromatography-mass spectrometry.
Conclusions:
Tryptophan content was reduced in placentas from women with PE. There was an increased kynurenine/tryptophan ratio in placentas from women with PE but no significant change in downstream metabolites. We confirmed a reduction in IDO1 expression and found a compensatory increase in TDO expression in placentas from women with PE. SAA was reduced in PE placentas compared with controls. Our data show that tryptophan content and the inflammatory mediator SAA are both compromised in placentas from women with PE. Further studies on the role of tryptophan catabolism and mediators of inflammation in sustaining healthy immunobiological pathways in the placenta are warranted.
Keywords: pre-eclampsia; kynurenine pathway; serum amyloid A; indoleamine 2,3-dioxygenase; tryptophan; tryptophan 2,3-dioxygenase
Background
Pre-eclampsia (PE) is one of the most common disorders of pregnancy, affecting around 3% to 7% of all pregnant women. Characterized by pregnancy-onset hypertension, proteinuria, and in severe cases organ dysfunction, PE is a leading cause of maternal death and perinatal morbidity around the world.1 While the exact cause of PE is unknown, evidence suggests that it is connected to aberrant placental implantation.2,3 Inadequate implantation may lead to poor placental perfusion, which can cause hypoxia and inflammatory changes in placental tissue.4–6 Although there is a consensus that PE is linked to inflammatory changes in both plasma and placental tissue, the precise nature and role of these changes in the disease process are not fully understood. It has been proposed that PE is a disorder featuring deficient immunoregulation, and that normal immunoregulatory negative feedback systems (ie, “brakes” of the inflammatory cascade) might not function properly.7
Catabolism of the essential amino acid tryptophan is involved in immunoregulation, and one of its functions within the placenta is the regulation of maternal-fetal tolerance. In the placenta, tryptophan is degraded into nicotinamide adenine dinucleotide (NAD+) by a series of enzymatic reactions, collectively called the kynurenine pathway (Figure 1).8 The activity of the kynurenine pathway increases under inflammatory conditions,9,10 and the different metabolites produced by this pathway can exert potent immunoregulatory functions. The first step of the pathway can be carried out by any of the following three enzymes, indoleamine 2,3-dioxygenase (IDO or IDO1), IDO2, or tryptophan 2,3-dioxygenase (TDO). Of these, IDO is highly expressed in the human placenta and can regulate maternal-fetal tolerance.8,11 Early studies suggest that maternal-fetal tolerance is established by IDO-mediated tryptophan metabolism at the placental interface, leading to a localized state of tryptophan depletion and subsequent anergy of reactive T-cells.11–13 Quinolinic acid, a downstream kynurenine pathway metabolite, can induce apoptosis in T-cells and also stimulate cytokine production and activation of the innate immune system.14
Figure 1.
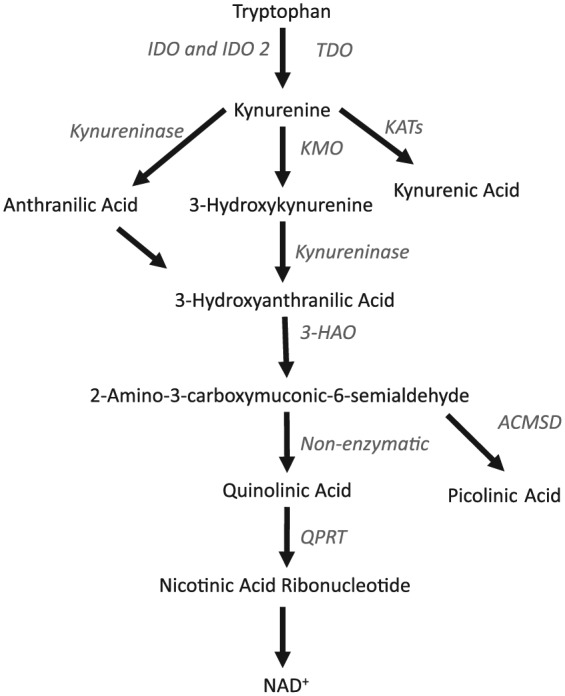
Schematic overview of the Kynurenine pathway, showing the main enzymes and their products. Enzymes are listed in italic font in the figure. ACMSD indicates 2-amino 3-carboxymuconate-6-semialdehyde decarboxylase; 3-HAO, 3-hydroxyanthranilate 3,4-dioxygenase; IDO, indoleamine 2,3-dioxygenase; IDO2, indoleamine 2,3-dioxygenase 2; KATs, kynurenine aminotransferases; KMO, kynurenine 3-monooxygenase; NAD+, nicotinamide adenine dinucleotide; QPRT, quinolinate-phosphoribosyltransferase; TDO, tryptophan 2,3-dioxygenase.
There are complex interactions between the immune system and the kynurenine pathway metabolites. Recent studies have shown that kynurenine can regulate the immune system through highly specific mechanisms involving binding to the aryl hydrocarbon receptor.13,15–17 In addition, kynurenic acid is an endogenous agonistic ligand of this same receptor and acts in an immunosuppressive manner.18,19 Nilsen et al reported in obese women there is an increase of maternal plasma kynurenic acid levels in early pregnancy which is associated with increased risk of developing PE.20 The authors suggest the elevated kynurenic acid may be a compensatory mechanism of the kynurenine pathway to counteract the pathology of PE.21,22
IDO expression is reduced in placental tissue from women with PE, and in a mouse model, pregnant dams deficient in IDO exhibited phenotypes characteristic of PE, such as proteinuria, endothelial dysfunction, and elevated blood pressure.23,24 Zardoya-Laguardia et al found that reduced levels of IDO in PE were associated with changes in the placental vascular tone.25 While reduced expression of IDO might be coupled to vascular pathophysiology in PE, the enzyme may also be involved in the regulation of oxidative stress in PE.26 To date, this reduction of IDO in PE has not been assessed in the context of the other enzymes of the kynurenine pathway, or in relation to the tissue content of tryptophan, kynurenine and quinolinic acid. To better understand the importance of the kynurenine pathway in PE, we determined tryptophan and metabolite content, as well as the degree of expression and activity of the kynurenine pathway in placental tissue from women with and without PE. We included only placentas that were delivered within the gestational window considered term (37-42 weeks) and further corrected all analyses for exact gestational age within this period. In addition, we investigated the association between the kynurenine pathway and key mediators of inflammation in placental tissue, as inflammatory factors are known to induce the activity of kynurenine pathway enzymes. We measured the inflammatory cytokines interleukin (IL)-1β, IL-6, and serum amyloid A (SAA) in placental tissue. SAA is a mediator of several central aspects of the immune response27 and has a critical role within placental tissue, regulating placental formation and homeostasis by modulating metalloprotease activity and invasion by trophoblasts.28 In this study, our primary hypothesis was that dysregulated tryptophan catabolism would be associated with inflammatory changes in placentas from women diagnosed with PE.
Methods
Clinical study design
This study was approved by the Lund University Institutional Review Board, Lund, Sweden. Placenta samples and clinical information were obtained from a total of 42 women enrolled in our Swedish cohort study between 2003 and 2011.
Women were excluded from the study if they had chronic hypertension. Four of the women gave birth after 42 weeks and were removed from the analysis as they were post-term, and two women were excluded from the analysis due to pre-term birth, defined as birth prior to 37 gestational weeks.
The remaining 36 term placenta samples were analyzed, out of which 18 of the mothers had been diagnosed with late-onset PE and 18 were healthy controls. Late-onset PE was defined as de novo hypertension and proteinuria from 34 weeks of gestation, with blood pressure ⩾140/90 mmHg and proteinuria ⩾300 mg/L, as defined by the International Society of Hypertension in Pregnancy (ISSHP).29 Proteinuria diagnosed by dipstick analysis was accepted as quantification if no other method had been used. All women in the cohort had singleton pregnancies. The prevalence of cesarean section was the same between PE patients and controls. The detailed clinical characteristics of the patient cohort are shown in Table 1. The placentas were collected at the delivery ward of Lund University Hospital, Sweden. Placenta was collected upon delivery, immediately frozen on dry ice, and then stored at −80°C.
Table 1.
Demographic data for the study participants.
| Demographic | Pre-eclampsia (n = 18) | Controls (n = 18) | P-value |
|---|---|---|---|
| Age (mean (SD)) | 29.2 (6.5) | 29.2 (4.0) | 1.0 |
| Body mass index, kg/m2 (mean (SD)) | 28.2 (7.7) | 26.1 (5.1) | .3 |
| Complications/symptoms of PE | |||
| HELLP syndrome (n) | 2 | 0 | |
| Intrauterine growth restriction (n) | 2 | 0 | |
| Proteinuria (n) | 18 | 3a | |
| De novo hypertension (n) | 18 | 1b | |
| Gravidity (mean (SD)) | 2.5 (0.7) | 1.6 (0.5) | .2 |
| Parity (mean (SD)) | 0.3 (0.6) | 0.2 (0.4) | .3 |
| Smoking (n) | |||
| Never | 11 | 15 | |
| Quit before pregnancy | 5 | 2 | |
| Quit during pregnancy | 1 | 0 | |
| Current | 1 | 1 | |
| Co-morbidities (n) | |||
| Psoriasis | 2 | 0 | |
| Cutaneous lupus | 0 | 1 | |
| Asthma | 1 | 1 | |
| Medications (n) | |||
| Anti-hypertensives | 2 | 0 | |
| Antibiotics at labor | 1 | 1 | |
| Corticosteroids | 3 | 0 | |
| Gestational age at delivery (mean (SD)) | 39.1 (1.2) | 39.9 (1.3) | .05 |
| Mode of delivery (n (%)) | |||
| Vaginal | 14 (78%) | 14 (78%) | |
| Cesarean | 4 (12%) | 4 (12%) | |
| Male fetal sex (n (%)) | 7 (39%) | 9 (50%) | |
| Placental weight, g (mean (SD)) | 617.8 (152.1) | 590.9 (100.0) | .5 |
| Newborn birth weight, g (mean (SD)) | 3405.5 (732.6) | 3434.8 (369.3) | .9 |
Abbreviation: HELLP, hemolysis, elevated liver enzymes, low platelet count; PE, pre-eclampsia.
Out of the three controls with proteinuria, one had a urinary tract infection at the time of testing and two had 1+ for proteinuria with no other symptoms.
The control with elevated blood pressure did not have any other symptoms.
qPCR analysis
Placenta samples were homogenized by automatic homogenizing pestle. RNA was extracted by RNeasy kit (Qiagen, Germantown, MD) according to manufacturer’s protocol and stored at −80°C. cDNA was synthesized using 1.5 µg of placental total RNA using the Superscript VILO cDNA Synthesis Kit (Thermofisher, Kalamazoo, MI) according to manufacturer’s recommended protocol. Quantitative real-time PCR (qPCR) was performed to analyze mRNA expression levels of IDO, IDO2, IL-1β, IL-6, TDO, kynurenine-3-mono-oxygenase (KMO), quinolinate phosphoribosyltransferase (QPRT), and SAA—using Taqman Gene Expression assay primer/probe sets (Thermofisher, Kalamazoo, MI) on a StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA). All qPCR samples were run in triplicate. One control placenta sample was excluded due to insufficient RNA yield for analysis by qPCR. Data were analyzed via the comparative threshold cycle (Ct) method as previously described.30
Protein analysis
Placenta samples were homogenized in RIPA lysis buffer (Cell Signaling Technology, Danvers, MA) and diluted to a final concentration of 350 µg/mL, using double the concentration of protease and phosphatase inhibitors as suggested by Meso Scale Discovery (Rockville, MD). IL-1β, IL-6, and SAA were measured using the Meso Scale Discovery platform and run on a Sector 600 in accordance with manufacturer’s instructions. Inter-assay coefficients of variation (% CV) were as follows: IL-1β (1.46%), IL-6 (2.70%), and SAA (2.46%). Intra-assay CVs were as follows: IL-1β (3.02%), IL-6 (3.02%), and SAA (2.89%). Lower detection limits were as follows: IL-1β (0.05 pg/mL), IL-6 (0.04 pg/mL), and SAA (27.90 pg/mL).
Detection of tryptophan, kynurenine, and quinolinic acid
Placenta samples were analyzed using gas chromatography-mass spectrometry to determine quinolinic acid concentrations. Samples were weighed and homogenized using Bertin Minilys® bead tubes containing 10% trichloroacetic acid. Following subsequent centrifugation, 50 μL of the resulting supernatant was added to a glass tube along with deuterated internal standard and processed in accordance with the previously published protocol.31 Subsequently, each sample was injected into a Thermo Trace GC Ultra gas chromatograph interfaced to a Thermo DSQ II mass spectrometer. The inter-/intra-assay (% CV) was 1.41%/1.36% for quinolinic acid. The lower limit of detection was 3 nM.
Tryptophan and kynurenine were analyzed by high-pressure liquid chromatography. Samples were initially processed by the same method stated above. After centrifugation, the supernatant was filtered through a 0.22 µm polytetrafluoroethylene (PTFE) filter and 20 μL was injected into the Thermo Scientific Dionex UltiMate® 3000 (Thermo Scientific, Waltham, MA). The chromatograph separation was achieved on a reversed phase 150 × 3 mm BDS Hypersil C18 column (Thermo Scientific) with 3 µm particle size. Column and pre-column tubing was maintained at 35°C with isocratic elution (0.8 mL/min) of analytes using a mobile phase consisting of 5% methanol in MilliQ water containing 50 mM ammonium acetate (pH 4.65). Tryptophan and kynurenine were detected based on comparison with standards, retention times, and fluorescent detection at 254 nm/404 nm (ex/em) for tryptophan and ultraviolet (UV) emission spectra at 365 nm for kynurenine. Results were analyzed using the Chromeleon™ 7.2 Chromatography Data System (Thermo Scientific). The inter-/intra-assay % CVs were 2.06%/0.50% for tryptophan and 2.58%/1.26% for kynurenine, respectively. Lower limit of detection was 33 nM for both analytes.
Statistical analysis
Statistical analyses were performed using R v3.4.3 (https://cran.r-project.org/) and IBM SPSS Statistics v.24 program. Bivariable comparisons in demographic and clinical characteristics were made using Student’s t-tests for continuous variables or Fisher’s exact tests for categorical variables, as appropriate. Multivariable regression analyses were performed to test whether each biomarker was independently associated with PE. To adjust for potential confounding of gestational age, PE and healthy patients were matched based on gestational age using the genetic algorithm in the R package “Matchit” (http://gking.harvard.edu/matchit) and then analyzed via a weighted linear regression (WR), with gestational age as a covariate.32 P-values were then calculated using a likelihood ratio test. Levene’s test was used to test for heteroscedasticity and normality of residuals was verified visually using qq-plots. Analytes were transformed using Box-Cox as needed, based on these regression diagnostics.33
Results
Patient characteristics
Patient demographics, somatic comorbidities, and medications used during pregnancy are listed in Table 1. There were no significant differences in mean age, body mass index, current smoking status, placental weight, newborn birth weight, gestational age at the time of delivery, or in the mode of delivery between women with and without PE. All group-wise comparisons were adjusted for gestational age by weighted regression models.
Tryptophan and metabolite levels in the placenta
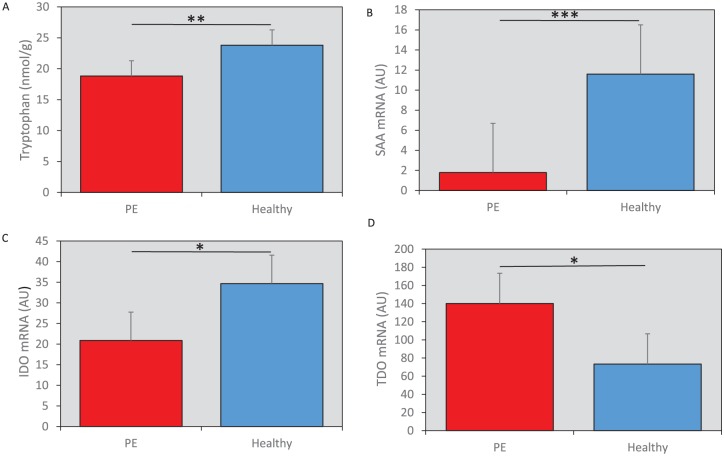
Women with PE exhibited significantly lower levels of placental tryptophan (WR, standardized beta (Sβ) = –0.42, P = .01), compared with the controls (Figure 2A). There was no difference in the level of kynurenine between placenta samples from women with PE and controls (WR, not significant [NS]). However, the activity of the first step of the kynurenine pathway was increased, as evidenced by the kynurenine/tryptophan (KYN/TRP) ratio, in women with PE compared with controls (WR, Sβ = 0.40, P < .01). Levels of quinolinic acid, a downstream metabolite regulated by the enzymes KMO and QPRT, showed no difference between controls and PE placentas (WR, NS). For kynurenine metabolite levels refer to Table 2.
Figure 2.
(A) Tryptophan content was reduced in placentas from women with PE compared with healthy controls (nmol/gram tissue, mean ± 2SEM). (B) mRNA expression of SAA was significantly decreased in placentas from women with PE compared with controls (AU, as defined below, median ± confidence interval). (C) The mRNA expression of IDO1 was reduced in placentas from women with PE compared with controls (AU, mean ± 2SEM). (D) TDO expression was increased in the placentas from women with PE vs the healthy controls (AU, mean ± 2SEM). All mRNA data were analyzed via the comparative threshold cycle method as previously described (see Methods); where the relative mRNA expression in arbitrary units (AU) was obtained by normalization against the expression of the housekeeping gene (GAPDH) in each sample. All data shown in (A) to (D) are raw values and the significance level indicated in the figures is based on the statistical analysis, weighted regression as described in methods. IDO indicates indoleamine-2,3-dioxygenase; PE, pre-eclampsia; SAA, serum amyloid A, TDO, tryptophan 2,3-dioxygenase. *P < .05, **P < .01, ***P < .005.
Table 2.
Kynurenine metabolite levels.
| Metabolite | Pre-eclampsia (n = 18) (mean (SD)) | Controls (n = 18) (mean (SD)) |
|---|---|---|
| Tryptophan (µg/gram tissue) | 3.85 (0.88) | 4.86 (1.30) |
| Kynurenine (µg/gram tissue) | 2.06 (0.84) | 2.21 (0.74) |
| Quinolinic acid (µg/gram tissue) | 0.482 (0.23) | 0.530 (0.31) |
Inflammatory cytokines in placental tissue
To test whether inflammatory markers are upregulated in PE placental tissue, we quantified the expression levels of the pro-inflammatory cytokines IL-1β, IL-6, and SAA by qPCR. SAA mRNA was significantly decreased in the placental tissue from women with PE compared with controls (WR, Sβ = –0.44, P < .005) (Figure 2B). There was no observable difference in the expression of IL-1β or IL-6 mRNA in placental tissue from women with and without PE (WR, NS). We confirmed the SAA mRNA data by measuring protein levels of SAA in the same samples. There was a significant decrease in SAA protein in placentas from women with PE (WR, Sβ = –0.51, P < .005).
Kynurenine enzyme levels in the placental tissue
We analyzed the mRNA expression of the kynurenine pathway enzymes IDO, IDO2, TDO, KMO, and QPRT in placental tissue. Placental IDO mRNA levels were significantly lower in women with PE compared with controls (WR, Sβ = –0.33, P < .05) (Figure 2C). Interestingly, there was increased expression of TDO mRNA in placental tissue from the women with PE (WR, Sβ = 0.40, P < .05) (Figure 2D). There were no significant differences in the expression level of IDO2, KMO, or QPRT in placental tissue of women with and without PE (WR, NS).
Associations between IDO and inflammatory factors in placenta
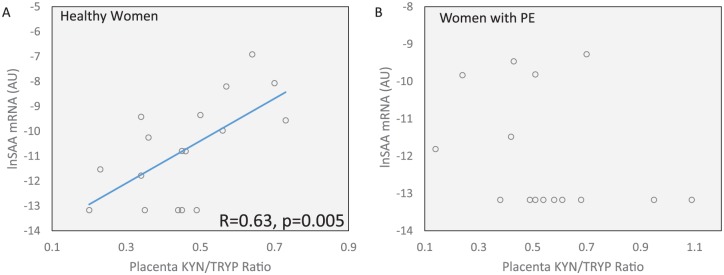
SAA mRNA correlated positively with activity of the first step of the kynurenine pathway, as measured by the KYN/TRP ratio in healthy placental tissue (Pearson’s R = 0.63, P = .005, Figure 3A). There was no such correlation in the PE placental tissue (Pearson’s R = –0.30, NS, Figure 3B).
Figure 3.
(A) The mRNA expression of SAA in healthy placental tissue correlates significantly with the activity of the first step of the kynurenine pathway, as indicated by the KYN/TRP ratio (Pearson’s R = 0.63, P < .005). (B) There was no association between the mRNA expression of SAA and the kynurenine pathway activity in placentas from women with PE. KYN/TRP indicates kynurenine/tryptophan; PE, pre-eclampsia; SAA, serum amyloid A.
Discussion
In this study, we detected altered tryptophan catabolism in placentas from women with PE. To our knowledge, tryptophan content in the placenta has not previously been determined in PE. We found that tryptophan content was significantly reduced in PE placental tissue, resulting in a significant increase in the KYN/TRP ratio. This ratio often indicates increased activity of the kynurenine pathway. The enzymatic conversion of tryptophan into kynurenine can be performed independently by IDO, IDO2, or TDO, of which IDO and TDO have been previously been detected in term placenta.34 Using qPCR, we confirmed lower mRNA expression of IDO in PE placentas, in agreement with previously published data.23,24 While IDO2 expression was unaltered, we found a significant increase in the expression of TDO in women with PE, which may account for the reduced levels of tryptophan observed in this study. Interestingly, similar compensatory mechanisms within the regulation of the kynurenine pathway enzymes have been observed in other settings.35,36
Our results give rise to the question of what the biological differences between IDO and TDO are, and whether these could have any relevance in the pathobiological mechanisms of PE. IDO and TDO both catalyze the breakdown of tryptophan into kynurenine, but the enzymes are different in terms of genomic location, tissue expression, structure, and mode of activation.37 Several cytokines can induce IDO, whereas TDO can be induced by tryptophan, the cofactor heme, as well as by glucocorticoids.38 Increased TDO expression in human placenta has also been observed under infectious conditions.39 Both enzymes involve oxidation of the substrate tryptophan, although the exact catalytic process is not yet fully established. The reaction may differ in the formation of catalytic ferrous-oxy complexes.40 IDO has anti-oxidant properties, as the superoxide anion is used as the oxygen source in IDO’s enzymatic reaction to cleave the pyrrole ring of tryptophan.41–43 A previous study found decreased levels of IDO in placentas from women with PE and suggested that the protective function of IDO in PE is not related to immunoregulation, but to its anti-oxidant properties.26 It is established that oxidative stress is involved in the pathogenesis of PE and one can speculate that an upregulation of TDO cannot completely compensate for a reduction of IDO in this respect, given their different biological properties.39,42,44,45 Future studies should address the differential regulation of IDO and TDO in placental tissue and determine their mechanistic roles in pathological conditions, such as PE.
Although 95% of tryptophan is degraded through the kynurenine pathway, it is possible that the reduced levels of tryptophan we observed are a result of increased serotonin production. The placenta is a major source of fetal serotonin, and disruption of placental serotonin synthesis can impact fetal forebrain development.46 Interestingly, previous studies have demonstrated that placental serotonin levels are increased in women with PE during pregnancy but the effect of this increase remains unclear.47,48 While we were unable to measure serotonin levels in our study, we recommend that future studies of this nature include measurements of placental serotonin levels, to gain a better understanding of this metabolites role in PE.
We also detected significantly reduced levels of SAA mRNA expression in placental tissue from women with PE and observed a concordant reduction in SAA protein levels. SAA is an acute-phase protein that increases in response to tissue damage and activates toll-like receptor 2 and toll-like receptor 4, both of which are present in the placenta.49 Binding of these receptors by SAA stimulates the production of several pro- and anti-inflammatory cytokines, such as IL-6 and IL-10, respectively.27 Several previous studies have found a reduction of IL-10 in placentas from women with PE.50–52 To our knowledge, this is the first time that SAA has been measured in placental samples from PE patients. In addition to being an inflammatory regulator, SAA also plays an important role in the placental microenvironment through its ability to induce trophoblast invasion and metalloprotease activity, two essential processes in placental formation, and homeostasis.28 Since PE is linked to poor placental implantation and development, our findings of decreased SAA in placentas from women with PE are concordant with the findings by Sandri et al and imply that SAA could be a pathognomonic factor in the development of PE.28 Interestingly, the levels of SAA correlated significantly with the activity of the first step of the kynurenine pathway (KYN/TRP ratio) in healthy placentas, but not those diagnosed with PE. Therefore, it is possible that SAA is a novel regulator of IDO activity, capable of inducing IDO in placental tissue, although this remains to be causally determined in experimental model systems.
This study has several strengths and limitations. As a strength, we measured a range of kynurenine pathway enzymes, including three separate enzymes capable of degrading tryptophan, as well as both the substrate (tryptophan) and metabolite (kynurenine) to understand the complex nature of changes involved in the first step of the kynurenine pathway in placentas from women with PE. Importantly, the expression of IDO and the other kynurenine pathway enzymes change over pregnancy, and it is therefore difficult to establish group differences unless there is a careful matching for gestational age.53 This can be challenging, especially since the diagnosis of PE is often associated with pre-term delivery. In this study, we only included women with term pregnancies, and we further corrected all statistical analyses for exact gestational age. These adjustments are important to establish associations between biological factors and diseases of the placenta, as the placental environment undergoes profound changes throughout pregnancy, particularly in the weeks leading up to birth. A limitation to our study is the comparatively low number of subjects (n = 36), as well as being a clinical correlative study, that is unable to establish causal relationships. In future studies of tryptophan catabolism in placental tissue, it will be important to measure substrate, metabolites, and enzymes of the kynurenine pathway in the same sample to help interpretation of the observed changes in PE.
Conclusions
In summary, we detected a reduction in tryptophan content and SAA expression in placental tissue from women with PE compared with controls. While IDO expression was reduced, TDO expression was increased, and the KYN/TRP ratio, indicative of kynurenine pathway activity, was increased in PE placental tissue. We observed no changes in the expression of the downstream pathway enzymes KMO and QPRT, suggesting that altered tryptophan catabolism in PE does not affect the production of downstream metabolites of the kynurenine pathway, and may involve additional tryptophan catabolic pathways. The biological role of placental tryptophan, the kynurenine pathway enzymes, and their interaction with inflammatory factors in placental tissue in PE warrant further investigation.
Footnotes
Funding:This work was supported by the National Institutes of Health grant R01-MH104622 (LB); Van Andel Research Institute (LB); the Swedish Research Council, ALF; the Wallenberg- Ragnar Soderbergs- and Maggie Stephens Foundations (SRH). None of these institutions had any further role in the design of the study or in the decision to submit the work for publication.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: SAK performed experiments, data analysis, and wrote the first draft of the manuscript. SK and JG performed experiments. PH, EYB, ESM, KR, and AF are subject matter experts who contributed to revisions and commented on the manuscript. ZM performed statistical analysis. MJ, GK, SRH collected samples and clinical data. LB oversaw experimental, statistical, and manuscript operations. All authors read and approved the final version of the manuscript.
References
- 1. World Health Organization. WHO Recommendations for Prevention and Treatment of Pre-eclampsia and Eclampsia. Geneva, Switzerland: WHO; 2011. [PubMed] [Google Scholar]
- 2. James-Allan LB, Whitley GS, Leslie K, Wallace AE, Cartwright JE. Decidual cell regulation of trophoblast is altered in pregnancies at risk of pre-eclampsia. J Mol Endocrinol. 2018;60:239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Espino Y, Sosa S, Flores-Pliego A, et al. New insights into the role of matrix metalloproteinases in preeclampsia. Int J Mol Sci. 2017;18:E1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Al-Jameil N, Aziz Khan F, Fareed Khan M, Tabassum H. A brief overview of preeclampsia. J Clin Med Res. 2014;6:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Redman CW. Current topic: pre-eclampsia and the placenta. Placenta. 1991;12:301–308. [DOI] [PubMed] [Google Scholar]
- 6. Ahn H, Park J, Gilman-Sachs A, Kwak-Kim J. Immunologic characteristics of preeclampsia, a comprehensive review. Am J Reprod Immunol. 2011;65:377–394. [DOI] [PubMed] [Google Scholar]
- 7. Ahmed A, Ramma W. Unravelling the theories of pre-eclampsia: are the protective pathways the new paradigm. Br J Pharmacol. 2015;172:1574–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Manuelpillai U, Nicholls T, Wallace EM, Phillips DJ, Guillemin G, Walker D. Increased mRNA expression of kynurenine pathway enzymes in human placentae exposed to bacterial endotoxin. Adv Exp Med Biol. 2003;527:85–89. [DOI] [PubMed] [Google Scholar]
- 9. Schwieler L, Larsson MK, Skogh E, et al. Increased levels of IL—6 in the cerebrospinal fluid of patients with chronic schizophrenia—significance for activation of the kynurenine pathway. J Psychiatry Neurosci. 2015;40:126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Urata Y, Koga K, Hirota Y, et al. IL-1beta increases expression of tryptophan 2,3-dioxygenase and stimulates tryptophan catabolism in endometrioma stromal cells. Am J Reprod Immunol. 2014;72:496–503. [DOI] [PubMed] [Google Scholar]
- 11. Sedlmayr P, Blaschitz A, Wintersteiger R, et al. Localization of indoleamine 2,3-dioxygenase in human female reproductive organs and the placenta. Mol Hum Reprod. 2002;8:385–391. [DOI] [PubMed] [Google Scholar]
- 12. Munn DH, Zhou M, Attwood JT, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. [DOI] [PubMed] [Google Scholar]
- 13. Badawy AA, Namboodiri AM, Moffett JR. The end of the road for the tryptophan depletion concept in pregnancy and infection. Clin Sci (Lond). 2016;130:1327–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lugo-Huitron R, Ugalde Muniz P, Pineda B, Pedraza-Chaverri J, Rios C, Perez-de la, Cruz V. Quinolinic acid: an endogenous neurotoxin with multiple targets. Oxid Med Cell Longev. 2013;2013:104024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fallarino F, Grohmann U, Vacca C, et al. T cell apoptosis by tryptophan catabolism. Cell Death Differ. 2002;9:1069–1077. [DOI] [PubMed] [Google Scholar]
- 16. Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol. 2010;185:3190–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yeung AW, Terentis AC, King NJ, Thomas SR. Role of indoleamine 2,3-dioxygenase in health and disease. Clin Sci (Lond). 2015;129:601–672. [DOI] [PubMed] [Google Scholar]
- 18. DiNatale BC, Murray IA, Schroeder JC, et al. Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicol Sci. 2010;115:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wirthgen E, Hoeflich A, Rebl A, Gunther J. Kynurenic acid: the Janus-faced role of an immunomodulatory tryptophan metabolite and its link to pathological conditions. Front Immunol. 2017;8:1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nilsen RM, Bjorke-Monsen AL, Midttun O, et al. Maternal tryptophan and kynurenine pathway metabolites and risk of preeclampsia. Obstet Gynecol. 2012;119:1243–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stone TW, Darlington LG. Endogenous kynurenines as targets for drug discovery and development. Nat Rev Drug Discov. 2002;1:609–620. [DOI] [PubMed] [Google Scholar]
- 22. Zavalza-Gomez AB. Obesity and oxidative stress: a direct link to preeclampsia. Arch Gynecol Obstet. 2011;283:415–422. [DOI] [PubMed] [Google Scholar]
- 23. Iwahashi N, Yamamoto M, Nanjo S, Toujima S, Minami S, Ino K. Downregulation of indoleamine 2, 3-dioxygenase expression in the villous stromal endothelial cells of placentas with preeclampsia. J Reprod Immunol. 2017;119:54–60. [DOI] [PubMed] [Google Scholar]
- 24. Santillan MK, Pelham CJ, Ketsawatsomkron P, et al. Pregnant mice lacking indoleamine 2,3-dioxygenase exhibit preeclampsia phenotypes. Physiol Rep. 2015;3:e12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zardoya-Laguardia P, Blaschitz A, Hirschmugl B, et al. Endothelial indoleamine 2,3-dioxygenase-1 regulates the placental vascular tone and is deficient in intrauterine growth restriction and pre-eclampsia. Sci Rep. 2018;8:5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nishizawa H, Suzuki M, Pryor-Koishi K, et al. Impact of indoleamine 2,3-dioxygenase on the antioxidant system in the placentas of severely pre-eclamptic patients. Syst Biol Reprod Med. 2011;57:174–178. [DOI] [PubMed] [Google Scholar]
- 27. Eklund KK, Niemi K, Kovanen PT. Immune functions of serum amyloid A. Crit Rev Immunol. 2012;32:335–348. [DOI] [PubMed] [Google Scholar]
- 28. Sandri S, Urban Borbely A, Fernandes I, et al. Serum amyloid A in the placenta and its role in trophoblast invasion. PLoS ONE. 2014;9:e90881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brown MA, Lindheimer MD, de Swiet M, Van Assche A, Moutquin JM. The classification and diagnosis of the hypertensive disorders of pregnancy: statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Hypertens Pregnancy. 2001;20:IX–XIV. [DOI] [PubMed] [Google Scholar]
- 30. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. [DOI] [PubMed] [Google Scholar]
- 31. Smythe GA, Braga O, Brew BJ, et al. Concurrent quantification of quinolinic, picolinic, and nicotinic acids using electron-capture negative-ion gas chromatography-mass spectrometry. Anal Biochem. 2002;301:21–26. [DOI] [PubMed] [Google Scholar]
- 32. Ho D, Imai K, King G, Stuart E. Matching as nonparametric preprocessing for reducing model dependence in parametric causal inference. Polit Anal. 2007;15:199–236. [Google Scholar]
- 33. Box GEP, Cox DR. An analysis of transformation. J Roy Stat Soc B. 1964;26:211–252. [Google Scholar]
- 34. Sedlmayr P, Blaschitz A, Stocker R. The role of placental tryptophan catabolism. Front Immunol. 2014;5:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Braidy N, Guillemin GJ, Mansour H, Chan-Ling T, Grant R. Changes in kynurenine pathway metabolism in the brain, liver and kidney of aged female Wistar rats. FEBS J. 2011;278:4425–4434. [DOI] [PubMed] [Google Scholar]
- 36. Lim CK, Bilgin A, Lovejoy DB, et al. Kynurenine pathway metabolomics predicts and provides mechanistic insight into multiple sclerosis progression. Sci Rep. 2017;7:41473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rafice SA, Chauhan N, Efimov I, Basran J, Raven EL. Oxidation of L-tryptophan in biology: a comparison between tryptophan 2,3-dioxygenase and indoleamine 2,3-dioxygenase. Biochem Soc Trans. 2009;37:408–412. [DOI] [PubMed] [Google Scholar]
- 38. Badawy AA. Kynurenine pathway of tryptophan metabolism: regulatory and functional aspects [published online ahead of print March 15, 2017]. Int J Tryptophan Res. doi: 10.1177/1178646917691938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Myatt L. Review: reactive oxygen and nitrogen species and functional adaptation of the placenta. Placenta. 2010;31:S66–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nienhaus K, Nienhaus GU. Different mechanisms of catalytic complex formation in two L-tryptophan processing dioxygenases. Front Mol Biosci. 2017;4:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Thomas SR, Stocker R. Antioxidant activities and redox regulation of interferon-gamma-induced tryptophan metabolism in human monocytes and macrophages. Adv Exp Med Biol. 1999;467:541–552. [DOI] [PubMed] [Google Scholar]
- 42. Thomas SR, Stocker R. Redox reactions related to indoleamine 2,3-dioxygenase and tryptophan metabolism along the kynurenine pathway. Redox Rep. 1999;4:199–220. [DOI] [PubMed] [Google Scholar]
- 43. Dang Y, Dale WE, Brown OR. Comparative effects of oxygen on indoleamine 2,3-dioxygenase and tryptophan 2,3-dioxygenase of the kynurenine pathway. Free Radic Biol Med. 2000;28:615–624. [DOI] [PubMed] [Google Scholar]
- 44. Jauniaux E, Poston L, Burton GJ. Placental-related diseases of pregnancy: involvement of oxidative stress and implications in human evolution. Hum Reprod Update. 2006;12:747–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Perkins AV. Endogenous anti-oxidants in pregnancy and preeclampsia. Aust N Z J Obstet Gynaecol. 2006;46:77–83. [DOI] [PubMed] [Google Scholar]
- 46. Bonnin A, Goeden N, Chen K, et al. A transient placental source of serotonin for the fetal forebrain. Nature. 2011;472:347–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Senior JB, Fahim I, Sullivan FM, Robson JM. Possible role of 5-hydroxytryptamine in toxaemia of pregnancy. Lancet. 1963;2:553–554. [DOI] [PubMed] [Google Scholar]
- 48. Wang Y, Walsh SW. Hormonal and related mechanisms for preeclampsia of pregnancy. Endocrinologist. 1997;7:238–244. [Google Scholar]
- 49. Hayati AR, Mohamed AE, Tan GC. An immunohistochemical study of Toll-like receptors 2 and 4 in placenta with and without infection. Malays J Pathol. 2010;32:13–19. [PubMed] [Google Scholar]
- 50. Hennessy A, Pilmore HL, Simmons LA, Painter DM. A deficiency of placental IL-10 in preeclampsia. J Immunol. 1999;163:3491–3495. [PubMed] [Google Scholar]
- 51. Rein DT, Breidenbach M, Honscheid B, et al. Preeclamptic women are deficient of interleukin-10 as assessed by cytokine release of trophoblast cells in vitro. Cytokine. 2003;23:119–125. [DOI] [PubMed] [Google Scholar]
- 52. Makris A, Xu B, Yu B, Thornton C, Hennessy A. Placental deficiency of interleukin-10 (IL-10) in preeclampsia and its relationship to an IL10 promoter polymorphism. Placenta. 2006;27:445–451. [DOI] [PubMed] [Google Scholar]
- 53. Badawy AA. Tryptophan metabolism, disposition and utilization in pregnancy. Biosci Rep. 2015;35:e00261. [DOI] [PMC free article] [PubMed] [Google Scholar]