Abstract
Effectiveness of relapse prevention with antipsychotic drugs has been robustly demonstrated. However, the drawbacks of antipsychotic maintenance treatment have prompted alternative strategies to reduce antipsychotic load. A prominent drawback of antipsychotics is their negative impact on subjective well-being, initiative, and drive related to dopamine D2 blockade. This might compromise functional capacity. First-episode studies from 1980 to 2018, including relevant reviews and meta-analyses, are evaluated, showing a lack of functional outcome data. In addition to relapse rates, which is the primary outcome in the great majority of studies, long-term functional outcome is pivotal, because these two outcome domains may point in opposite directions. The trade-off between relapse rates and functional outcome is discussed by our 2013 dose-reduction study. We conclude that divergent outcomes and various individual risk-profiles preclude the construction of a generic outcome measure. The relationship of relapse and functional outcome is considered, as well as the conceivable role of negative symptoms and some related issues. Future profiling of individual risk/benefit characteristics combined with personal preferences may offer better guidance in antipsychotic pharmacotherapy. More studies are needed to elucidate individual risk profiles, predictive of functional capacity and relapse rates, to draw differential conclusions on long-term risks and benefits of antipsychotics across the spectrum of psychosis.
Keywords: antipsychotic, dose reduction, discontinuation, first episode psychosis, functional outcome, personalized treatment, relapse, recovery, remission
Weighing up the pros and cons of antipsychotic treatment
Antipsychotics are effective in acute psychotic episodes,1 though there is less evidence on long-term treatment.2 Maintenance treatment (MT) is strongly recommended to prevent relapses, though benefits may not countervail against detrimental effects on physical health, brain integrity,3 and daily functioning.4
Drawbacks include side effects, redundant dopamine blockade depriving the individual of the capacity to initiate reward-related behavior5 and perhaps loss of effectivity through development of supersensitivity of the dopamine system.
Notwithstanding the heterogeneity of psychotic disorders, a hyperdopaminergic state appears to be the most prominent common pathway to acute psychosis. However, not all psychotic disorders may be characterized by a persistent proneness to disinhibition of the dopamine system, caused by some primary pathology priming it to derail for example, in case of minor stresses. This implies effectivity of antipsychotics in the majority of acute psychoses, but also questions the necessity of vigorous long-term dopamine blockade in every case. Sparing use of antipsychotics in the long-term might be beneficial to subgroups of patients at least. It was hypothesized that after symptom remission, using lower dosages and discontinuing antipsychotics in selected cases would lead to better functional recovery, probably at the cost of higher relapse rates.6 One randomized trial confirmed this hypothesis,4 while replications are on their way.7 Conceivable implications for antipsychotic treatment strategies are discussed. There is evidence that a minority of 35% of first-episode patients will do well without antipsychotics without relapse.4,8,9 However, at present it is impossible to tell in advance who will be able to do without, and who will need maintenance antipsychotic treatment.10
In the following paragraphs, relevant studies on discontinuation and dose-reduction strategies in first- and multi-episode psychosis from 1 January 1980 to 1 January 2018 are evaluated. The present paper is not a meta-analysis of results (which is only feasible for relapse rates), but we refer to four reviews and meta-analyses published previously.11–14 The focus is on first-episode studies, for reasons discussed in a separate paragraph, but relevant multi-episode studies and reviews were implicated. Apart from a literature search in PubMed, we used references from included articles to find additional relevant dose-reduction, intermittent/targeted treatment and discontinuation trials. Search terms were (schizophrenia [Title/Abstract] OR psychosis [Title/Abstract]) AND (antipsychotic [Title/Abstract] OR neuroleptic [Title/Abstract]) AND (discontinuation [Title/Abstract] OR reduction [Title/Abstract] OR intermittent [Title/Abstract] OR targeted [Title/Abstract]) from 1 January 1980 to 1 January 2018. Search results were filtered to include clinical trials. From 495 studies, 13 randomized controlled trials (RCTs) were included4,6,15–25 and 2 non-randomized/controlled studies.26,27
Historical introduction
The feasibility of antipsychotic discontinuation in patients with nonaffective psychosis has been examined as far back as the 1980s. Three of these studies were in first-episode patients.15–17 Primary outcome was relapse rates, with follow up of 6 months to 2 years. All studies favored MT because of fewer relapses. In trying to achieve dose-reduction without increasing relapse risk, some studies used targeted or intermittent treatment. In these strategies, medication was preserved to periods characterized by recurrent positive symptoms, and as soon as symptoms disappeared dosage was to be discontinued gradually. It should be noted that most of these studies were in multiple-episode patients and patients who took antipsychotics for many years.18–20 Gilbert et al. reviewed the discontinuation literature and found that discontinuation and intermittent treatment invariably led to more relapses. Because advantages that were hoped for (less-severe side effects) failed to materialize, these treatment strategies were discarded.13
Another feature of many studies was that they did not gradually taper the dosage of antipsychotics, but discontinued abruptly. It came up that this could lead to rebound relapses. In 1979, Chouinard and Jones hypothesized these relapses might have been a consequence of dopamine hypersensitization owing to long-term use of antipsychotics.28 In the meantime, Viguera et al. showed that high relapse rates after discontinuation could have been provoked by abrupt discontinuation, instead of gradual tapering.29 The hypothesized dopamine hypersensitivity induced by dopamine D2 blockade was not only thought to lead to rebound psychosis, but also to treatment resistance and tolerance to antipsychotics, such that these agents would lose their effect.30–32 Up to date, the last word has not been spoken about this issue, as we will see later on.
A study by Gitlin et al. in recent onset schizophrenia showed relapse rates did not favor discontinuation strategies compared with MT, though they still thought a discontinuation strategy could work well for a subgroup of patients. They recommended a discontinuation trial in selected cases by gradual tapering antipsychotics and discontinuation if possible.26
The first group to publish RCT data of first-episode patients using targeted treatment were Gaebel et al., by separately looking post hoc at the subset of first-episode patients in a multicenter trial and showed equal 2-year relapse rates in targeted treatment (42%) compared with MT (38%).21
In evaluating the results of the antipsychotic treatment strategies, relapse rates were still the prevailing outcome criterion. Though outcome research changed focus towards consumers’ perspectives and recovery, the evaluation of the effectiveness of antipsychotics still relies almost completely on symptom severity and relapse rates.
In 2001, our group initiated an open RCT comparing dose-reduction/discontinuation (DR) and MT strategies in first-episode psychosis patients after a 6 months symptom remission within the first year of treatment. Primary outcome was functional recovery.
The Medication Strategies in First Onset Schizophrenia (Mesifos) trial
This trial consisted of an experimental phase of 2 years starting in 2002, and a 7-year follow-up assessment 5 years after the original trial ended (for details, see the original publications4,6). Relevant characteristics will be summarized here. The original trial intended to examine whether MT according to the guidelines was the best option in remitted first-episode patients, compared with an intention-to-treat DR strategy. We hypothesized that functional capacity would come out better in the DR condition, probably at the cost of higher relapse rates. We recruited all eligible, treatment-naïve first-episode patients (n = 257) in a large catchment area (3.2 million) and were able to include about half of them in the trial (n = 128). The nonincluded patients (n = 129) generally had a worse clinical and social profile and many of these patients prematurely lost contact with mental health services, often soon after first contact. Included patients had never been treated with antipsychotics before, and the duration of untreated psychosis (DUP) ranged from many years to some days, with a relatively short median of 1 month. At baseline 45% had a diagnosis of schizophrenia, which was an underestimation because of the less than 6 months history of symptoms in many cases at entry, and the remainder had different other nonaffective psychotic disorders. After 6 months of positive symptom remission the patients were assigned to either DR or MT strategy, both intention-to-treat. The applied strategy was such that the assigned treatment condition was discussed with patients and family members, including information about the risks and need for monitoring and, in case of DR, implemented in a personalized timeframe that could take weeks or even months. A substantial number of patients were never completely discontinued, because they had recurrent symptoms before complete discontinuation was achieved, or medication was restarted because symptoms recurred after initial discontinuation. During the first 18 months, about 20% of patients in the DR strategy were successfully discontinued without relapse. At 18 months follow up, the 2-year end point of the study was reached.
The results did not show what we hoped for. Though we expected relapse rates in the DR strategy to be higher than in the MT arm, they turned out to be twice as high: 43% against 21%. Relapses were mild and did not lead to more in-patient days in the DR condition. We took 20% of patients in DR successfully off antipsychotic drugs, but there were no functional gains in DR, apart from better vocational functioning bordering on significance. After the trial ended, patients were left to the discretion of their attending clinicians. Five years later, we followed the patients up again. A total of 103 patients were willing to participate. Main outcome of this 7-year follow up was functional recovery during the last 6 months of follow up; we also looked at symptom remission during the same period, relapse rates throughout the whole 7-year follow-up period and antipsychotic dose during the most recent 2 years. In view of the negative results after 2 years, and the absence of any experimental intervention thereafter, we expected the tracks of the original trial conditions to be covered up after the 5-year interval. However, the results after 7 years turned out to be strikingly different.
Patients who originally were in the DR strategy significantly more often recovered functionally than patients who originally received MT: 46.2% against 19.6%. We were not able to find confounders that might have influenced these results. Symptomatic remission was the same in both conditions, 69.2% versus 66.7%. Predictors of functional recovery were less-severe baseline negative symptoms, living together, better baseline social functioning and DR strategy. The only predictor of symptomatic remission was DUP. Another striking finding was that relapse rates in the DR group came on par with the MT group from about 3 years of follow up. The mean antipsychotic dose during the last 2 years of follow up still differed significantly: 2.2 mg daily haloperidol equivalents in former DR patients against 3.6 mg in former MT patients.
This was the first RCT in first-episode patients to show clear-cut outcome improvements as a result of DR strategies. They came to the fore only after longer-term follow up, and only in the domain of functional recovery. In the short term (up to about 3 years), relapse rates were not favorable, but later came on par, and when only looking at symptom severity there were no differences between the outcomes even in the long term.
Critique on alleged methodological flaws
The trial methodology was criticized by Undurraga et al. on three points.33 First, they supposed that during the original 2-year trial the DR and MT strategies did not really differ, because only about 20% of the DR patients were successfully discontinued. This was due to the intention-to-treat open design of our study allowing clinicians to do what would be in the best interest of their patients. Instead of a weakness, this personalized approach might be one of the strong points of our design, and it may be inferred that some of the not-discontinued patients might have been successfully discontinued as well, if not for the reluctance of clinicians, patients and family members. Moreover, they argued MT was carried out by preferential prescription of low-dose second-generation antipsychotics. Nevertheless, the mean haloperidol equivalent daily dosages used during the original trial were 2.1 mg in DR and 2.9 mg in MT: a significant difference (t = −2.395, df 128, p = 0.018). That the groups were certainly not ‘similar in their intervention’ can also be judged by the twice as high relapse rates in the DR condition compared with MT (43% versus 21%) during the original trial, the major drawback of the DR condition at 2-year follow up.
The second point of their critique referred to our inability to fully guarantee the blinding of the raters for the original treatment condition of the patients after 7 years of follow up, which was true but inevitable owing to the open trial design. Patients were aware of their original assignment to either DR or MT and might have disclosed their former assignment during their follow-up assessment 7 years later. Thus, bias is conceivable, but the large difference in outcome is unlikely to be explained by this casual unblinding. The third point was that the vocational functioning outcome of the 2-year trial (insignificantly different) was erroneously supposed to have undone the randomized design for 7-year follow up of the trial. Though all points of critique were refuted by Wunderink and Sytema,34 apart from blinding not being assured, the same arguments were brought up again in a later commentary.2 Actually, no change in randomization took place, because the original conditions and assignments were not changed, and all patients were followed up after being attended for by their clinicians during 5 years. There is no reason to suppose a difference in treatment regimen during this period between patients in both conditions. In addition, Hui and Chen published some comments.35 They suggest our results might have been influenced by nonadherence to antipsychotics, because we did not mention measurement of adherence; we did however estimate adherence by research assistants who were not involved in treatment and had no contact with attending clinicians. We multiplied estimated adherence percentages divided by 100 with prescribed dosages to approximate true intake. Though this might not exclude some deviation from real intake, it is clearly better than no control. They also mention lack of information on additional psychosocial treatments, that might have had an impact on outcome. We did not control for these treatments, but during the first 2 years of the original trial there were no differences in application of these treatments across participating research sites. We also applied randomization per site to minimize the impact of regional differences. Finally, they mention the possible impact of the proportion of diagnostic subgroups, particularly schizophrenia, in the DR and MT groups. There were no significant differences in diagnostic subgroup distributions across the groups however, and a sensitivity analysis excluding the nonschizophrenia patients did not show different results: in patients who were initially diagnosed with schizophrenia the DR strategy was an independent predictor of recovery bordering on significance.
Though the critics do not deem a causal relationship between the early DR strategy and better long-term functional outcome likely, there are no convincing arguments to dispute these results. But obviously robust evidence requires replication in other trials.
Recently started replication trials of antipsychotic DR strategies
To replicate the findings of the Mesifos trial, a number of RCTs are now on their way.
In Melbourne, Australia the Reduce trial (Killackey et al.) has just started, also comparing DR if feasible with MT, in first-episode patients; interesting is the addition to both arms of specific nonpharmacologic relapse-prevention strategies. The effect on brain integrity and cortical thinning is also part of the study.
In Copenhagen, Denmark, the recently started Tailor trial (Nordentoft et al.) has a similar design to the Mesifos trial; the stable remission period before DR or MT is reduced to 3 instead of 6 months, as has also been done in the Reduce trial.7
In the Netherlands, the Hamlett study (Sommer et al.) recently started in first-episode patients who are also included after 3 months of stable remission on antipsychotics.
In the UK, an even more challenging DR trial started recently, led by Joanna Moncrieff. In this Radar trial, schizophrenia patients with a long-term history of antipsychotic treatment are to be included. These patients will have been treated with higher dosages and polypharmacy of antipsychotics and will more often show treatment resistance. Patients on clozapine will be excluded. This trial is particularly interesting because of the conceivable impact of dopamine hypersensitization in these patients and the possibility of rebound psychosis as a reaction to withdrawal of antipsychotics. Tapering will be done carefully and gradually. Even then, these patients by their mere selection may be more vulnerable because of an easily derailing dopamine system. Nevertheless, also in these patients the action of antipsychotics may have a lack of countervailing benefits in view of treatment resistance and side effects.
All ongoing studies chose functional outcome as primary outcome, and some of them have a follow up of 3 years. There are no results yet from these studies, but they will give better answers to the issue of who needs maintenance antipsychotics, and who can benefit from dose-reduction and discontinuation.
Recent discontinuation studies focusing on relapse rates and symptom severity
In recent years, a few more discontinuation trials have been published, all showing higher relapse rates in discontinued antipsychotic or placebo conditions compared with MT.
Chen et al. randomized 178 remitted first-episode patients to either quetiapine maintenance medication (400 mg/day) or placebo for 12 months; included patients were stably remitted on antipsychotics for 12 months; patients in the maintenance arm relapsed in 41% [95% confidence interval (CI) 29–53] against 79% (95% CI 68–90) in the discontinuation arm, though relapse criteria were very low threshold. There were no significant differences in vocational outcomes after 12 months.22
Boonstra et al. conducted an aborted trial in 20 remitted first-episode patients, showing very high relapse rates (82%) in discontinued patients against 12% in MT,23 and Gaebel et al. randomized 44 patients to MT or intermittent treatment, and 1-year relapse rates were 0% versus 19%, respectively. MT patients were slightly better on Global Assessment of Functioning (GAF) scale scores (78 versus 72) and there were no differences in quality of life or subjective well-being. The authors conclude that about 50% of patients remain well at a significantly lower drug dose and show fewer side effects. Because many patients refuse MT, the authors state that targeted intermittent treatment should be provided in individual cases.24
Leucht et al. performed a meta-analysis comparing antipsychotic treatment and placebo for relapse rates including 65 trials, with data for 6493 patients with schizophrenia. Antipsychotic drugs significantly reduced relapse rates at 1 year [drugs 27% versus placebo 64%; risk ratio (RR) 0.40, 95% CI 0.33–0.49; number needed to benefit (NNTB) 3, 95% CI 2–3]. In a subgroup analysis of first-episode patients they found the same figures (relapse rates in maintenance 26% versus placebo 61%; RR 0.47; 95% CI 0.38–0.58). The effects of antipsychotics compared with placebo decreased with the length of follow up. The authors concluded that the advantages of these drugs must be weighed against their side effects, and that future studies should focus on outcomes of social participation and clarify the long-term morbidity and mortality of these drugs.11
Harrow et al. found in their naturalistic study that in schizophrenia patients not on antipsychotics, neurocognitive functioning was better than in patients on antipsychotics, though the causality of this relationship remains obscure owing to the naturalistic design.36
In 2013, Takeuchi et al. published one of the very few studies examining the effect of antipsychotic dose reduction on neurocognition. They found that in stable schizophrenia patients a 6-month open-label randomized 50% dose reduction of risperidone and olanzapine versus MT improved cognitive functioning.25
Zipursky et al. compared outcome in terms of symptom recurrence rates (lower threshold than relapse) in remitted first-episode patients in MT versus discontinuation in 6 studies. The 1-year recurrence rate in discontinuation was 73% against 3% in MT.12
Mayoral-van Son et al. conducted an open, nonrandomized self-elected discontinuation study in 46 patients who were treated with antipsychotics for at least 18 months, symptomatically remitted for at least 1 year and functionally recovered for at least 6 months, who were stable on the lowest effective dose of antipsychotics for at least 3 months.27 They were compared with 22 patients fulfilling the same criteria, who chose to continue their medication. They were followed up for 3 years, with relapse rates and time to relapse as primary outcome. Relapse rates over 3 years were 67.4% in the discontinuation group versus 31.8% in the maintenance group. However, after 3 years there were no significant differences in the severity of symptoms and functional status between the two groups. When all patients who relapsed were taken together from both groups (n = 38) and compared with nonrelapsing patients from both groups (n = 30), the relapsed patients showed more severe symptomatology (particularly negative symptoms) and poorer functional status at 3 years. Of course, the study was not randomized, and patients who chose to quit their low-dose antipsychotics might have been selected by experiencing more negative effects from their medication, as opposed to patients who chose to stay on them. But interestingly, there were no differences at end point in symptomatic or functional status between the two groups. When separately looking at relapsing patients, there was an association of relapse, negative symptoms, and worse functional outcome.
Takeuchi et al. meta-analyzed 11 placebo-controlled discontinuation trials in long-term patients (n = 2826) and did not use relapse rates (because of different definitions in different trials) but total symptom severity ratings and their 1-year trajectories as an outcome.14 They found maintenance medication did not lose its effect and these patients as a group were almost stable in symptom severity across 1 year of follow up (10.1% worsening of symptoms), as opposed to placebo treatment, causing a linearly increasing level of symptoms (worsening of 49.2%, a significant difference). Antipsychotics did still have an antipsychotic effect in these chronic patients, as should have been less clear in case of dopamine hypersensitivity. The authors found no bump in symptom severity shortly after entering the trial in the placebo condition, too, as might have been an indication of dopamine hypersensitivity. In an editorial comment Stefan Leucht remarks that the results seem to contradict the dopamine hypersensitivity hypothesis, though restricted to multi-episode chronic patients.2 In addition, no data on functioning were presented, and the follow-up period was only 1 year. The authors nonetheless conclude that their findings do not rule out the possibility that a specific group of patients may not require antipsychotic MT, and that antipsychotics may be reduced instead of totally discontinued. This may also correspond to the findings of Mayoral et al., who included patients who at inclusion were symptomatically very stable and functionally recovered on the lowest effective dose of antipsychotics for at least 3 months. The authors refer to naturalistic studies with a follow up of 10 years and more, showing approximately one-third of patients with schizophrenia can remain stable without antipsychotic treatment.8,9
An important recent study by Tiihonen et al. analyzed Swedish nationwide databases to study the risk of rehospitalization and so-called treatment failure in all patients with a schizophrenia diagnosis in Sweden from 1 July 2006 to 31 December 2013 (n = 29,823 total prevalent cohort and n = 4603 incident cohort), eliminating selection bias by within-individual analyses.37 Treatment failure was defined as psychiatric rehospitalization (including due to suicide attempts) or death, but also treatment discontinuation or switch to other antipsychotics. During follow up, 13,042 of 29,823 patients (43.7%) experienced psychiatric hospitalization and 20,225 of 28,189 (71.7%) had treatment failure. Patients using long-term antipsychotic injectables (LAIs) and clozapine were far better off than patients taking oral antipsychotics or no antipsychotics regarding rehospitalization rates with hazard ratios of about 0.5 compared with no use of antipsychotics. This study has many strengths: the huge number of patients evaluated, measuring real-world effectiveness, by also including patients who are not represented in RCTs, and the within-individual analyses to avoid bias. Some critical remarks may be made, however. Treatment discontinuation in a complete population cohort of schizophrenia patients will often be a sign of noncompliance, occurring in patients with persisting symptoms, and being associated with worse physical health and social defeat. This is quite a different population compared with remitted first-episode patients selected to participate in a deliberate dose-reduction program, taking good care of themselves and who are well embedded in treatment programs. Moreover, treatment failure was not only defined as psychiatric rehospitalization (including due to suicide attempts) or death, but also switch to other antipsychotics or treatment discontinuation. Both latter two events may not be an index of treatment failure in all cases, because a switch to another antipsychotic drug as well as discontinuation of antipsychotics may be the next step in a recovery process. More importantly, as the authors acknowledge, there are no data on functioning, nor on symptoms. Prevention of hospitalization by antipsychotics might have a price in terms of lost functional capacity, which is not accounted for. The threshold to be registered in the Swedish nationwide database as a patient diagnosed with schizophrenia is a selection bias: all mild and self-limiting cases of psychotic disorders, not fulfilling the 6 months duration criteria for schizophrenia, for example, first psychotic episodes remitting within a few months without further relapses, were excluded. These nonrelapsing patients amounted to about 35% of first-episode patients in 7 years of follow up in our study, and thus findings may be particularly generalizable to patients with established chronic or multiple-episode course characteristics.
Choosing outcome criteria and timeframe to evaluate treatment: relapse rates or functional recovery?
In the studies mentioned above relapse rates were the primary outcome, and in most studies evaluating the effectiveness of treatment of psychosis they still are. Understandably, because the emphasis of treatment is on the acute and positive psychotic symptoms, and the first psychotic episode is the main reason for entry in specialized mental health services. As a consequence, our first attention is directed to the effectivity of the suppression of these symptoms, and the prevention of them returning on the stage. As we are all aware, positive symptoms are more responsive to antipsychotic treatment, compared with negative symptoms, that tend to be more persistent to any interventions and more stable on the long run.38 But it is the latter component of symptomatology, more than the first, that is associated with worse functional outcome. In our study, less-severe negative symptoms at baseline were the best predictor of functional recovery after 7 years of follow up.4 By overestimating the importance of relapse rates and symptom severity, we might overlook drawbacks and gains in other domains that might be more important from a patient’s perspective.
From a consumers’ point of view, functional outcome is more relevant than symptomatic outcome. Functional recovery is a more demanding concept and does not automatically follow symptomatic remission. Functional recovery may even, in a small percentage (4% according to our data), be achieved without symptomatic remission. In our 7-year follow up of first-episode patients, the majority of functionally recovered patients were also symptomatically remitted, but only a minority of symptomatically remitted patients were also functionally recovered (see Table 1)
Table 1.
Crosstabulation of symptomatic remission and functional recovery at 7 years follow up in a first-episode psychosis cohort (n = 103).
| Functional recovery at 7 years of follow up | Total | ||||
|---|---|---|---|---|---|
| No | Yes | ||||
| Symptomatic remission at 7 years of follow up | No | Count | 29 | 4 | 33 |
| % of total | 28.2 | 3.9 | 32.0 | ||
| Yes | Count | 40 | 30 | 70 | |
| % of total | 38.8 | 29.1 | 68.0 | ||
These figures roughly correspond to the means of symptom remission (58%) and recovery (world overall 38%, only Europe 22%) found in a recent meta-analysis.39
Both from a consumer perspective, and because of its characteristic as an end result of treatment effectiveness and illness severity, functional recovery should be part of the primary outcome of treatment effectiveness evaluation studies in addition to relapse rates and/or symptom severity measures. Functional recovery may be considered a constituent of the recovery concept, including both symptomatic remission and functional recovery.
Another important aspect of outcome evaluation is the timeframe of changes that are expected to be of prime importance. Symptomatic remission may be achieved by 50% of first-episode patients within the first year of treatment, while functional recovery takes longer time to achieve.40,41 We did not find functional improvements at 2 years follow up, and significant functional improvements only came to the fore after a longer follow up, in our case after 7 years.
Assessing functional outcome and criteria for functional recovery
Functional outcome in many studies is only very globally assessed, by means of the GAF scale, the Social Functioning Assessment (SOFAS) scale or the like. Most of these scales are hybrid yielding an overall measure of functioning in all relevant domains, which should at least include three domains: daily living and housekeeping, work and studying, and peer relationships. Poor functioning (below the expected level) in one of these domains will be better reflected by assessing these domains separately, for example, by the Groningen Social Disability Scale (GSDS), assessing functioning in 7 social roles on a four-point scale (0–3), higher scores reflecting more disability. In our study, we chose the GSDS, and criteria for functional recovery were operationalized as no score above 1 (mild or questionable disability) on any item was allowed during a period of 6 months. The observer-rated GSDS takes about 1 hour to complete, which is a drawback for application in routine care.42,43 Self-rated experience sampling methods, using smartphone applications to assess functioning, may offer a better alternative in future, but still have to be developed and tested. For the time being the assessment of functioning using separate GAF-like anchored score charts for the three main domains (self-care and daily living; working and studying; and peer relationships) seems the most feasible compromise. Criteria for functional recovery then would imply only questionable or mild disability on any of these 3 scales, for example, on a scale with a range of 0–100 at least 75 on all 3 of them.
What is the relation of relapse and functional outcome?
Relapse has been considered one of the most important predictors of worse outcome in psychosis. In an often-cited paper by Wiersma et al. it was stated that each relapse entailed a chance of one in six of subsequent treatment resistance.44 If leading to admission in a psychiatric hospital, relapse will also increase the costs of care substantially. The association of relapse and worse outcome or deterioration supported the view that active psychosis was causally related to worse prognosis, already suggested by the association of long DUP with poor prognosis. These findings appeared to support the concept of schizophrenia as a progressive brain disorder. In combination with evidence that both relapse duration and DUP appeared to contribute to cortical thinning45 this supported widespread efforts to start antipsychotic treatment as soon as possible, giving rise to early intervention programs and relapse prevention programs to enable this, from the expectation that reducing DUP and preventing relapse would improve outcome. However, there is no robust evidence that the outcome of psychosis did change very much over the years.46 Recovery rates did not improve over time.39 However, recent efforts to reduce DUP might have had some effect on symptom remission,47 improving in recent years.39
The interpretation that active psychosis negatively impacts on prognosis is quite obvious because of the tempting negative association between the number of relapses after a first episode of psychosis and the chance of recovery. In our study, too, this turned out to be a striking association. We found that the more relapses occurred in an individual patient, the less chance of recovery. No relapse in 7 years follow up yielded a chance of recovery of one in two, one relapse one in four, two relapses one in five, and three or more relapses no recovery at all. At first sight these figures seem to confirm the general assumption of relapse causing worse functional outcome.
However, this association does not necessarily mean a causal relationship. If relapse rates would be co-determined by a factor that also has a negative impact on functional outcome, the negative association of relapse and worse outcome could, at least in part, be attributed to this common confounder. Relapse then might be both a consequence of this confounder, as well as cause of functional deterioration by itself.
Is there a case for negative symptoms?
When determining the predictors of functional recovery after 7 years of follow up in our study, the multivariate regression analysis with functional recovery as dependent variable showed independent contributions of the original treatment condition, favoring DR above MT with antipsychotics, better social functioning, living together, and less-severe baseline negative symptoms.4
Predictors of relapse have not yet been established unequivocally.48 Treatment noncompliance and self-elected discontinuation of antipsychotics is one of the causes of relapse most frequently mentioned in literature.49–54 Now DR was what we deliberately aimed at in one of the treatment arms in our study. We did indeed find higher relapse rates in the DR arm initially (43% against 21% in the first 18 months of the trial), but the relapse rates came on par with those in (also relatively low-dose) MT after about 3 years of follow up, and remained on an equal level until the end of follow up (Figure 1). The difference in mean daily dose between the arms the last 2 years of the 7 years follow up was 2.2 versus 3.6 mg equivalents of haloperidol per day.
Figure 1.
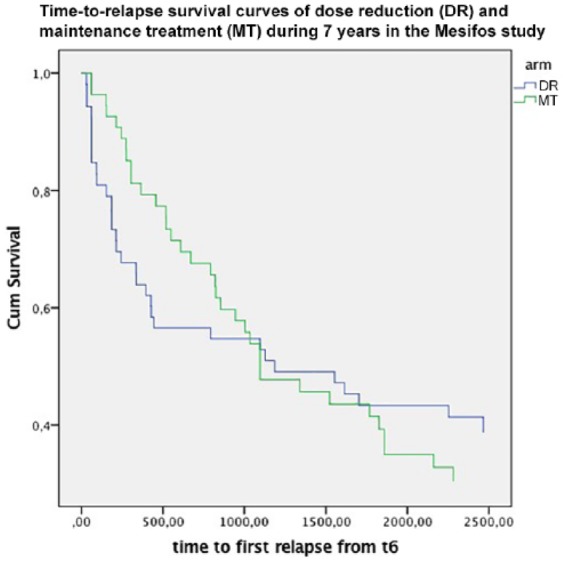
Survival curves of first relapse in remitted patients with a first-episode psychosis (n = 103) who were assigned to dose-reduction/discontinuation strategy (blue line) or maintenance treatment (green line) during the first 18 months and followed up for 7 years.
We interpreted this finding as relapses being postponed by maintenance antipsychotics, but not set off. Moreover, because DR yielded better recovery rates at 7 years of follow up compared with MT, initially higher relapse rates in DR did not negatively impact on symptomatic and functional outcome. A recent large nationwide study though showed that antipsychotic maintenance with LAIs and clozapine had a risk of rehospitalization compared with no use of medication of about 50%.37 Thus, our conclusion has to be that antipsychotic medication has a relapse preventing effect, and that probably the equalizing relapse rates in our study have to do with the equal relapse preventing properties of low-dose and very low-dose treatment. Moreover, the patients that were discontinued or stably treated with low-dose antipsychotics in the DR strategy either probably did not need any relapse prevention, because of their stable dopamine system, or were empirically selected to stay well on low dosages. But the interesting question is what intrinsic mechanisms, apart from insufficient treatment, cause relapse. This probably has to do with instability and proneness to derailment of the mesolimbic dopamine system. One interesting factor in this respect might be negative symptoms.
There are only a few studies showing negative symptoms predict relapse,55,56 but related concepts, worse premorbid adaptation and social withdrawal, were predictors of relapse in quite a number of studies.50,52,57,58 What could be the rationale behind the role of negative symptoms? Negative symptoms might be considered a proxy of an ill-wired brain. Negative symptoms, most often emerging several years before the first psychotic episode, might be related to a primary disturbance caused by glutamatergic and GABA-ergic dysfunction, and excitation–inhibition imbalance in cortical and hippocampal areas,59 leading in turn to inadequate upregulation of ventral tegmental dopaminergic burst activity,60 put through the mesolimbic tracts and resulting in episodic positive symptoms. Negative symptoms thus might represent a sign of relapse-proneness, a vulnerability to develop positive symptoms, but also a sign of imbalanced cortical excitation/inhibition that would negatively impact on functioning.
Following this somewhat speculative reasoning, prevention of relapses is still of the utmost importance as one of few amenable factors, probably having an independent impact on outcome of its own, but functional outcome would not only depend on the prevention of relapses but also to a certain extent on the neuropathology associated with the preexisting negative symptoms.
The deleterious consequences of relapse itself on outcome would be less prominent than often assumed.
In addition, the association of long DUP with worse outcome might have the same confounder, because long DUP is associated with negative symptoms.61 Negative symptoms might also be a proxy of a primary pathology that might cause the relation between brain changes on the one, and relapse duration and DUP on the other hand.
Last but not least, the worse prognostic features of schizophrenia compared with other nonaffective psychotic disorders might also be determined by negative symptoms. After all the latter will often lead to a schizophrenia diagnosis.
Instead of preselecting patients in terms of diagnostic classifications within the schizophrenia spectrum, to study the course and outcome of psychosis one should include the whole group of schizophrenia spectrum psychoses and examine the contributions of the symptomatic characteristics and other markers that might be important in view of prognosis and outcome, irrespective the diagnostic classification.
Selection bias in discontinuation trials: first-episode or multiple-episode patients in a discontinuation trial?
To study the prognostic meaning of certain characteristics of patients and their impact on outcome one should not preselect the sample of patients in such a way that all selected patients do already have less-favorable characteristics or are already on the route to chronicity. The dynamics of the trajectories to more- or less-severe psychopathology can only be differentiated and critical factors can only be descried if different profiles are present in the selected sample, and if different outcomes will be the result.
This implies that for this purpose we should preferably include first-episode patients because of their unknown course characteristics, the lack of iatrogenic influences on course and symptomatology, and their pristine dopamine system, not in any way changed by antipsychotic treatment (see next paragraph).
But what exactly are first episode patients? Our stepped care mental health systems rapidly select the patients with more severe, more resistant symptomatology, and tend to overlook patients with transient or less-persistent symptoms, or psychotic episodes limited to only one episode that remits favorably. There is a preselection of the more resistant, relapse prone patients in the sampling methods, particularly when multiple-episode patients are sampled. These patients will more often show disinhibited dopamine regulation related to hypothesized defects in excitation–inhibition balance, and probably have a worse prognostic profile.
Dopamine hypersensitization
However, there is one other conceivable reason to select treatment-naïve first-episode patients in a discontinuation/dose reduction trial: dopamine hypersensitization. At present, there is still debate about the significance of dopamine hypersensitization. In animal studies, it has been shown that using dopamine D2 blockers may induce post-synaptic upregulation of D2 receptors, making the dopamine system even more sensitive to dopamine activity, and less responsive to antipsychotic drugs.30,31 This might create tolerance to the effects of antipsychotics, causing a need for higher dosing of these drugs to maintain their effectiveness and, importantly, a rebound hypersensitivity when lowering the dosage of antipsychotic drugs. It is not exactly known how fast hypersensitivity may develop, though this may be in terms of weeks or months. Dopamine hypersensitivity implies the need for gradual tapering of antipsychotics, to give the dopamine system time to adjust to lower dosages. From this viewpoint, one should apply antipsychotics for the shortest possible time to prevent hypersensitivity developing. Some authors suggest the use of partial dopamine agonists, such as aripiprazole, to prevent upregulation of D2 receptors.1 Others have suggested to use extended dosing of antipsychotics, once every few days, to prevent the dopamine system from developing hypersensitivity.62 Most discontinuation trials that are being conducted at present start the experimental discontinuation phase after 3 months of antipsychotic treatment, as opposed to the 6 months that we took in our trial. It is not clear whether treatment for a certain period is recommendable, or whether the tapering could as well be started as soon as the positive symptoms waned sufficiently. It has been shown that tapering before remission has been achieved led to more relapses and longer time to remission.63
The study by Takeuchi et al. mentioned previously, pooling symptomatic trajectories in patients on and off antipsychotic drugs showed linearly increasing symptoms in withdrawn multiple-episode patients, did not show an initial bump in symptom severity, as would have probably been the case if hypersensitization was an important phenomenon.14 Leucht et al. concluded these results do cast doubt on both rebound psychosis after withdrawal and loss of effectiveness of antipsychotics, as would be expected in case of hypersensitization.2
Implications for antipsychotic treatment strategies to date
To date there is overwhelming evidence that antipsychotic drugs, particularly on the short term in acutely ill and relapsing patients, but also in patients with long-term schizophrenia, are effective in preventing relapse and symptoms. There are reasonable doubts about the clinical relevance of dopamine hypersensitization, given the stabilizing effects in chronic patients as opposed to placebo. However, we know less about the costs of relapse prevention in terms of physical health and mortality, side effects, brain changes, and functional capacity. We are faced with a dilemma.
There is also good evidence that about 35% of all first-episode patients will do well without long-term antipsychotics; in these patients, after the adequate treatment of the episode to remission, the antipsychotics can be gradually tapered without recurrent symptoms or relapse.
At present, we are not able to determine who will need antipsychotic MT and who will be able to do without.10 That is why both guidelines and clinical practice tend to put everyone in the same box.
The problem is that we still have insufficient knowledge to individually profile our patients presenting with a first-episode psychosis in terms of pathological pathways with different prognostic features and treatment options.
Some speculations were presented on the cortical excitation–inhibition imbalance (a putative glutamatergic/GABA-ergic deficit with interneuron dysfunction) that might lead to a structurally vulnerable, easy-derailing dopamine system and psychosis. Different trajectories to psychosis might play a role as well, solely or in combination, for example, cannabis-induced or trauma-related dopaminergic responses, also leading to psychosis through the final hyperdopaminergic pathway. It could be that a structural vulnerability of the dopamine system creates a need for maintenance protection by antipsychotics, whereas in other trajectories the dopamine system may have the potential to stabilize. Apart from that, other primarily nondopaminergic pathologies, such as NMDA-R encephalitis, might be at the core of a small number of disorganized or psychotic states.
Anyway, until we understand more about the routes to psychosis probably the best guide to antipsychotic treatment strategies apart from general evidence on relapse rates and the scant data on functional outcome, is preliminary personal risk-profiling (course characteristics, former relapses, danger during relapse) together with personal preferences. If former relapses were not clearly elicited by self-elected or deliberate antipsychotic discontinuation, if they did not lead to dangerous situations, and if positive symptoms are below the remission threshold for at least 3 months and increased relapse risks are acceptable, gradual tapering the antipsychotic dosage to half the daily defined dosage and less under careful monitoring can give a decisive answer whether continuous prophylaxis is needed or may be considered redundant. Substantial dose reduction is often feasible, and approximately 35% of patients presenting with a first episode of psychosis will even be able to discontinue completely, without experiencing a relapse.
It is important to realize that substantial dose reduction might offer an equal improvement of functional capacity and alleviation of other side effects as complete discontinuation would have done.
Perhaps the question should not be whether to maintain or discontinue antipsychotics, but to find the lowest effective dosage to optimally prevent both relapses and side effects, and to allow optimal functional recovery. Finally, we have to conclude that a future differential approach, based on individualized profiling of the psychosis spectrum, is crucial to improve outcome of psychotic disorders. Even small steps forward will easily outperform the current one-size-fits-all approach.
Footnotes
Funding: The original trials by our group were funded by ZON-mw, nr. DO945-01-001, Eli Lilly NL, Janssen Cilag NL and Friesland Mental Health Services.
Conflict of interest statement: The author declares that there is no conflict of interest.
ORCID iD: Lex Wunderink  https://orcid.org/0000-0002-4150-4681
https://orcid.org/0000-0002-4150-4681
References
- 1. Murray RM, Quattrone D, Natesan S, et al. Should psychiatrists be more cautious about the long-term prophylactic use of antipsychotics? Br J Psychiatry 2016; 209: 361–365. [DOI] [PubMed] [Google Scholar]
- 2. Leucht S, Davis JM. Do antipsychotic drugs lose their efficacy for relapse prevention over time? Br J Psychiatry 2017; 211: 127–129. [DOI] [PubMed] [Google Scholar]
- 3. Huhtaniska S, Jääskeläinen E, Hirvonen N, et al. Long-term antipsychotic use and brain changes in schizophrenia - a systematic review and meta-analysis. Hum Psychopharmacol 2017; 32: e2574. [DOI] [PubMed] [Google Scholar]
- 4. Wunderink L, Nieboer RM, Wiersma D, et al. Recovery in remitted first-episode psychosis at 7 years of follow-up of an early dose reduction/discontinuation or maintenance treatment strategy: long-term follow-up of a 2-year randomized clinical trial. JAMA Psychiatry 2013; 70: 913–920. [DOI] [PubMed] [Google Scholar]
- 5. McGorry P, Alvarez-Jimenez M, Killackey E. Antipsychotic medication during the critical period following remission from first-episode psychosis: less is more. JAMA Psychiatry 2013; 70: 898–900. [DOI] [PubMed] [Google Scholar]
- 6. Wunderink L, Nienhuis FJ, Sytema S, et al. Guided discontinuation versus maintenance treatment in remitted first-episode psychosis: relapse rates and functional outcome. J Clin Psychiatry 2007; 68: 654–661. [DOI] [PubMed] [Google Scholar]
- 7. Stürup AE, Jensen HD, Dolmer S, et al. TAILOR - tapered discontinuation versus maintenance therapy of antipsychotic medication in patients with newly diagnosed schizophrenia or persistent delusional disorder in remission of psychotic symptoms: study protocol for a randomized clinical trial. Trials 2017; 18: 445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moilanen J, Haapea M, Miettunen J, et al. Characteristics of subjects with schizophrenia spectrum disorder with and without antipsychotic medication - a 10-year follow-up of the northern Finland 1966 birth cohort study. Eur Psychiatry 2013; 28: 53–58. [DOI] [PubMed] [Google Scholar]
- 9. Wils RS, Gotfredsen DR, Hjorthøj C, et al. Antipsychotic medication and remission of psychotic symptoms 10years after a first-episode psychosis. Schizophr Res 2017; 182: 42–48. [DOI] [PubMed] [Google Scholar]
- 10. Wunderink L. Who needs antipsychotic maintenance treatment and who does not? Our need to profile and personalize the treatment of first episode psychosis. Schizophr Res 2018; 197: 65–66. [DOI] [PubMed] [Google Scholar]
- 11. Leucht S, Tardy M, Komossa K, et al. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. Lancet 2012; 379: 2063–2071. [DOI] [PubMed] [Google Scholar]
- 12. Zipursky RB, Menezes NM, Streiner DL. Risk of symptom recurrence with medication discontinuation in first-episode psychosis: a systematic review. Schizophr Res 2014; 152: 408–414. [DOI] [PubMed] [Google Scholar]
- 13. Gilbert PL, Harris MJ, McAdams LA, et al. Neuroleptic withdrawal in schizophrenic patients. A review of the literature. Arch Gen Psychiatry 1995; 52: 173–188. [DOI] [PubMed] [Google Scholar]
- 14. Takeuchi H, Kantor N, Sanches M, et al. One-year symptom trajectories in patients with stable schizophrenia maintained on antipsychotics versus placebo: meta-analysis. Br J Psychiatry 2017; 211: 137–143. [DOI] [PubMed] [Google Scholar]
- 15. Kane JM, Rifkin A, Quitkin F, et al. Fluphenazine vs placebo in patients with remitted, acute first-episode schizophrenia. Arch Gen Psychiatry 1982; 39: 70–73. [DOI] [PubMed] [Google Scholar]
- 16. Crow TJ, Macmillan JF, Johnson AL, et al. A randomised controlled trial of prophylactic neuroleptic treatment. Br J Psychiatry 1986; 148: 120–127. [DOI] [PubMed] [Google Scholar]
- 17. McCreadie RG, Wiles D, Grant S, et al. The Scottish first episode schizophrenia study. VII. Two-year follow-up. Scottish schizophrenia research group. Acta Psychiatr Scand 1989; 80: 597–602. [DOI] [PubMed] [Google Scholar]
- 18. Carpenter WT, Hanlon TE, Heinrichs DW, et al. Continuous versus targeted medication in schizophrenic outpatients: outcome results. Am J Psychiatry 1990; 147: 1138–1148. [DOI] [PubMed] [Google Scholar]
- 19. Herz MI, Glazer WM, Mostert MA, et al. Intermittent vs. maintenance medication in schizophrenia. Two-year results. Arch Gen Psychiatry 1991; 48: 333–339. [DOI] [PubMed] [Google Scholar]
- 20. Jolley AG, Hirsch SR, Morrison E, et al. Trial of brief intermittent neuroleptic prophylaxis for selected schizophrenic outpatients: clinical and social outcome at two years. BMJ 1990; 301: 837–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gaebel W, Janner M, Frommann N, et al. First vs multiple episode schizophrenia: two-year outcome of intermittent and maintenance medication strategies. Schizophr Res 2002; 53: 145–159. [DOI] [PubMed] [Google Scholar]
- 22. Chen EYH, Hui CLM, Lam MML, et al. Maintenance treatment with quetiapine versus discontinuation after one year of treatment in patients with remitted first episode psychosis: randomised controlled trial. BMJ 2010; 341: c4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boonstra G, Burger H, Grobbee DE, et al. Antipsychotic prophylaxis is needed after remission from a first psychotic episode in schizophrenia patients: results from an aborted randomised trial. Int J Psychiatry Clin Pract 2011; 15: 128–134. [DOI] [PubMed] [Google Scholar]
- 24. Gaebel W, Riesbeck M, Wolwer W, et al. Relapse prevention in first-episode schizophrenia–maintenance vs intermittent drug treatment with prodrome-based early intervention: results of a randomized controlled trial within the German research network on schizophrenia. J Clin Psychiatry 2011; 72: 205–218. [DOI] [PubMed] [Google Scholar]
- 25. Takeuchi H, Suzuki T, Remington G, et al. Effects of risperidone and olanzapine dose reduction on cognitive function in stable patients with schizophrenia: an open-label, randomized, controlled, pilot study. Schizophr Bull 2013; 39: 993–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gitlin M, Nuechterlein K, Subotnik KL, et al. Clinical outcome following neuroleptic discontinuation in patients with remitted recent-onset schizophrenia. Am J Psychiatry 2001; 158: 1835–1842. [DOI] [PubMed] [Google Scholar]
- 27. Mayoral-van Son J, de la Foz VO, Martinez-Garcia O, et al. Clinical outcome after antipsychotic treatment discontinuation in functionally recovered first-episode nonaffective psychosis individuals: a 3-year naturalistic follow-up study. J Clin Psychiatry 2016; 77: 492–500. [DOI] [PubMed] [Google Scholar]
- 28. Chouinard G, Jones BD. Evidence of brain dopamine deficiency in schizophrenia. Can J Psychiatry 1979; 24: 661–667. [DOI] [PubMed] [Google Scholar]
- 29. Viguera AC, Baldessarini RJ, Hegarty JD, et al. Clinical risk following abrupt and gradual withdrawal of maintenance neuroleptic treatment. Arch Gen Psychiatry 1997; 54: 49–55. [DOI] [PubMed] [Google Scholar]
- 30. Moncrieff J. Does antipsychotic withdrawal provoke psychosis? Review of the literature on rapid onset psychosis (supersensitivity psychosis) and withdrawal-related relapse. Acta Psychiatr Scand 2006; 114: 3–13. [DOI] [PubMed] [Google Scholar]
- 31. Yin J, Barr AM, Ramos-Miguel A, et al. Antipsychotic induced dopamine supersensitivity psychosis: a comprehensive review. Curr Neuropharmacol 2017; 15: 174–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chouinard G, Samaha AN, Chouinard VA, et al. Antipsychotic-induced dopamine supersensitivity psychosis: pharmacology, criteria, and therapy. Psychother Psychosom 2017; 86: 189–219. [DOI] [PubMed] [Google Scholar]
- 33. Undurraga J, Murru A, Vieta E. Early Medication discontinuation on long-term recovery outcome in first-episode psychosis. JAMA Psychiatry 2014; 71: 206–207. [DOI] [PubMed] [Google Scholar]
- 34. Wunderink L, Sytema S. Early Medication discontinuation on long-term recovery outcome in first-episode psychosis, reply. JAMA Psychiatry 2014; 71: 208–209. [DOI] [PubMed] [Google Scholar]
- 35. Hui CL, Chen EY. Early medication discontinuation on long-term recovery outcome in first-episode psychosis. JAMA Psychiatry 2014; 71: 207–208. [DOI] [PubMed] [Google Scholar]
- 36. Harrow M, Jobe TH, Faull RN. Do all schizophrenia patients need antipsychotic treatment continuously throughout their lifetime? A 20-year longitudinal study. Psychol Med 2012; 42: 2145–2155. [DOI] [PubMed] [Google Scholar]
- 37. Tiihonen J, Mittendorfer-Rutz E, Majak M, et al. Real-world effectiveness of antipsychotic treatments in a nationwide cohort of 29 823 patients with schizophrenia. JAMA Psychiatry 2017; 74: 686–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Boonstra N, Klaassen R, Sytema S, et al. Duration of untreated psychosis and negative symptoms - A systematic review and meta-analysis of individual patient data. Schizophr Res 2012; 142: 12–19. [DOI] [PubMed] [Google Scholar]
- 39. Lally J, Ajnakina O, Stubbs B, et al. Remission and recovery from first-episode psychosis in adults: systematic review and meta-analysis of long-term outcome studies. Br J Psychiatry 2017; 211: 350–358. [DOI] [PubMed] [Google Scholar]
- 40. Wunderink L, Nienhuis FJ, Sytema S, et al. Predictive validity of proposed remission criteria in first-episode schizophrenic patients responding to antipsychotics. Schizophr Bull 2007; 33: 792–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wunderink L, Sytema S, Nienhuis FJ, et al. Clinical recovery in first-episode psychosis. Schizophr Bull 2009; 35: 362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wiersma D, de Jong A, Kraaijkamp HJM, et al. GSDS II, the Groningen social disabilities schedule, second version. Groningen: Department of Psychiatry, Rijksuniversiteit Groningen, 1990. [Google Scholar]
- 43. Schutzwohl M, Jarosz-Nowak J, Briscoe J, et al. Inter-rater reliability of the brief psychiatric rating scale and the Groningen social disabilities schedule in a European multi-site randomized controlled trial on the effectiveness of acute psychiatric day hospitals. Int J Methods Psychiatr Res 2003; 12: 197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wiersma D, Nienhuis FJ, Slooff CJ, et al. Natural course of schizophrenic disorders: a 15-year followup of a Dutch incidence cohort. Schizophr Bull 1998; 24: 75–85. [DOI] [PubMed] [Google Scholar]
- 45. Andreasen NC, Liu D, Ziebell S, et al. Relapse duration, treatment intensity, and brain tissue loss in schizophrenia: a prospective longitudinal MRI study. Am J Psychiatry 2013; 170: 609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fusar-Poli P, McGorry PD, Kane JM. Improving outcomes of first-episode psychosis: an overview. World Psychiatry 2017; 16: 251–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hegelstad WTV, Larsen TK, Auestad B, et al. Long-term follow-up of the TIPS early detection in psychosis study: effects on 10-year outcome. Am J Psychiatry 2012; 169: 374–380. [DOI] [PubMed] [Google Scholar]
- 48. Bowtell M, Eaton S, Thien K, et al. Rates and predictors of relapse following discontinuation of antipsychotic medication after a first episode of psychosis. Schizophr Res 2018; 195: 231–236. [DOI] [PubMed] [Google Scholar]
- 49. Robinson DG, Woerner MG, Alvir JM, et al. Predictors of treatment response from a first episode of schizophrenia or schizoaffective disorder. Am J Psychiatry 1999; 156: 544–549. [DOI] [PubMed] [Google Scholar]
- 50. Üçok A, Polat A, Genç A, et al. One year outcome in first episode schizophrenia predictors of relapse. Eur Arch Psychiatry Clin Neurosci. Epub ahead of print 18 July 2005. [DOI] [PubMed] [Google Scholar]
- 51. Gearing R, Mian I, Sholonsky A, et al. Developing a risk-model of time to first-relapse for children and adolescents with a psychotic disorder. J Nerv Ment Dis 2009; 197: 6–14. [DOI] [PubMed] [Google Scholar]
- 52. Álvarez-Jiménez M, Gleeson JF, Henry LP, et al. Road to full recovery: longitudinal relationship between symptomatic remission and psychosocial recovery in first-episode psychosis over 7.5 years. Psychol Med 2012; 42: 595–606. [DOI] [PubMed] [Google Scholar]
- 53. Hui CL, Tang JY, Leung CM, et al. A 3-year retrospective cohort study of predictors of relapse in first-episode psychosis in Hong Kong. Aust N Z J Psychiatry 2013; 47: 746–753. [DOI] [PubMed] [Google Scholar]
- 54. Pelayo-Terán JM, Gajardo Galán VG, de la Ortiz-García de la Foz V, et al. Rates and predictors of relapse in first-episode non-affective psychosis: a 3-year longitudinal study in a specialized intervention program (PAFIP). Eur Arch Psychiatry Clin Neurosci 2017; 267: 315–323. [DOI] [PubMed] [Google Scholar]
- 55. Dyck DG, Short RA, Hendryx MS, et al. Management of negative symptoms among patients with schizophrenia attending multiple-family groups. Psychiatr Serv 2000; 51: 513–519. [DOI] [PubMed] [Google Scholar]
- 56. Altamura AC, Bassetti R, Sassella F, et al. Duration of untreated psychosis as a predictor of outcome in first-episode schizophrenia: a retrospective study. Schizophr Res 2001; 52: 29–36. [DOI] [PubMed] [Google Scholar]
- 57. Robinson D, Woerner MG, Alvir JM, et al. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry 1999; 56: 241–247. [DOI] [PubMed] [Google Scholar]
- 58. Alvarez-Jimenez M, O’Donoghue B, Thompson A, et al. Beyond clinical remission in first episode psychosis: Thoughts on antipsychotic maintenance vs. Guided discontinuation in the functional recovery era. CNS Drugs 2016; 30: 357–368. [DOI] [PubMed] [Google Scholar]
- 59. Howes O, McCutcheon R, Stone J. Glutamate and dopamine in schizophrenia: an update for the 21st century. J Psychopharmacol 2015; 29: 97–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lodge DJ, Grace AA. Hippocampal dysregulation of dopamine system function and the pathophysiology of schizophrenia. Trends Pharmacol Sci 2011; 32: 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Larsen TK, Moe LC, Vibe-Hansen L, et al. Premorbid functioning versus duration of untreated psychosis in 1 year outcome in first-episode psychosis. Schizophr Res 2000; 45: 1–9. [DOI] [PubMed] [Google Scholar]
- 62. Remington G, Kapur S. Antipsychotic dosing: how much but also how often? Schizophr Bull 2010; 36: 900–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Winton-Brown TT, Elanjithara T, Power P, et al. Five-fold increased risk of relapse following breaks in antipsychotic treatment of first episode psychosis. Schizophr Res 2017; 179: 50–56. [DOI] [PubMed] [Google Scholar]


