Abstract
Context
Treatment of postmenopausal osteoporosis with teriparatide parathyroid hormone amino terminal 1-34 increases bone formation and improves bone microarchitecture. A possible modulator of action is periostin. In vitro experiments have shown that periostin might regulate osteoblast differentiation and bone formation through Wnt signaling. The effect of teriparatide on periostin is not currently known.
Objectives
To determine the effect of teriparatide treatment on circulating levels of periostin and other regulators of bone formation and investigate how changes in periostin relate to changes in bone turnover markers, regulators of bone formation, and bone mineral density (BMD).
Participants and Design
Twenty women with osteoporosis; a 2-year open-label single-arm study.
Intervention
Teriparatide 20 µg was administered by subcutaneous injection daily for 104 weeks. Periostin, sclerostin, and Dickkopf-related protein 1, procollagen type I N-terminal propeptide (PINP), and C-telopeptide of type I collagen were measured in fasting serum collected at baseline (two visits) and then at weeks 1, 2, 4, 12, 26, 52, 78, and 104. BMD was measured at the lumbar spine, total hip, and femoral neck using dual energy x-ray absorptiometry.
Results
Periostin levels increased by 6.6% [95% confidence interval (CI), −0.4 to 13.5] after 26 weeks of teriparatide treatment and significantly by 12.5% (95% CI, 3.3 to 21.0; P < 0.01) after 52 weeks. The change in periostin correlated positively with the change in the lumbar spine BMD at week 52 (r = 0.567; 95% CI, 0.137 to 0.817; P < 0.05) and femoral neck BMD at week 104 (r = 0.682; 95% CI, 0.261 to 0.885; P < 0.01).
Conclusions
Teriparatide therapy increases periostin secretion; it is unclear whether this increase mediates the effect of the drug on bone.
Teriparatide therapy increases periostin secretion in postmenopausal women with osteoporosis. It is unclear whether this increase mediates the effect of the drug on bone.
Teriparatide, parathyroid hormone (PTH) amino terminal (PTH 1-34), is an anabolic agent that stimulates bone remodeling. It increases bone formation and, subsequently, bone resorption (1–3). It improves bone microarchitecture, increasing cortical thickness at the radius and tibia and trabecular connectivity (4–6). It can also induce bone formation on the periosteal surface and, therefore, have an effect on bone size, geometry, and strength (7–11).
The molecular mechanisms underlying the anabolic action of teriparatide remain unclear. A possible modulator of the increased bone formation observed with teriparatide treatment is periostin (12). In bone, it is primarily expressed in the periosteum of long bones and by osteocytes. Its expression is regulated by factors involved in bone homeostasis such as mechanical strain, PTH, growth factors, and cytokines (12, 13).
Periostin enhances the activation of lysyl oxidase and regulates collagen cross-link formation (14). Through interaction with receptor tyrosine kinases, several signaling pathways are initiated to activate transcription factors Notch1 and β-catenin (15, 16). One pathway regulated in this manner is the Wnt signaling pathway. The results from in vitro experiments suggest that periostin might increase bone formation through Wnt signaling by direct action on β-catenin (16, 17), inhibition of osteocyte sclerostin expression (a Wnt inhibitor) (18), or direct interaction with sclerostin (19). Periostin null mice have altered bone microarchitecture, lower bone mineral density (BMD), and reduced bone strength and bone turnover (12, 14, 20).
The expression of periostin is influenced by PTH treatment (21). In vitro and in vivo experiments have demonstrated that periostin is upregulated in response to PTH in human osteoblast cultures, in mice, and in patients with hyperparathyroidism (18, 22–24). The increases in some cortical bone parameters might be partly mediated by the inhibition of sclerostin (25) or by directly activating β-catenin Wnt signaling, increasing osteoblast differentiation and, hence, bone formation (18).
Clinical studies have demonstrated that postmenopausal women with osteoporosis treated with teriparatide have increased levels of Dickkopf-related protein 1 (DKK-1) (a Wnt signaling inhibitor); however, the data for sclerostin are more varied (26–30). The observed increase in DKK-1 suggests that Wnt signaling shows some response to teriparatide and could possibly explain the reduction in the anabolic effect seen after 12 months of treatment with teriparatide (29). To the best of our knowledge, no current data are available describing the effects of teriparatide on circulating levels of periostin in postmenopausal women with osteoporosis. We hypothesized that teriparatide might stimulate periostin expression and that the increase in periostin contributes to the anabolic action of teriparatide through interaction with DKK-1, sclerostin, and Wnt signaling.
Circulating levels of periostin have previously been measured in serum and plasma in several clinical studies using two commercially available enzyme-linked immunosorbent assays (ELISA) (31–34). More recently, in 2016, a third assay was developed (35). To the best of our knowledge, the present study is the first to report the effects of osteoporosis treatment on periostin using this assay.
Our aims were to determine the effect of teriparatide treatment on circulating levels of periostin and the regulators of bone formation in postmenopausal women with osteoporosis, and to explore the changes in periostin in relation to the changes in bone turnover markers, regulators of bone formation, BMD, and cortical thickness.
Study Design and Methods
We conducted a 2-year open-label single-arm study to investigate the mechanisms of action of teriparatide in postmenopausal women with osteoporosis (MOAT study). The study was registered with ClinicalTrials.gov (ClinicalTrials.gov no. NCT01293292) and with the European Union Drug Regulating Authorities Clinical Trials (EudraCT no. 2010-021009-19).
The treatment was teriparatide (Forsteo; Eli Lilly and Company, Basingstoke, United Kingdom), 20 µg by subcutaneous injection daily. To assess compliance, patients were asked to return the used teriparatide syringes. A 100,000 IU cholecalciferol (vitamin D3) load was given orally at baseline before starting teriparatide and every 6 months throughout the study period. The patients also received a daily supplement of 600 mg of calcium and 400 IU of vitamin D (marketed as AdCal D3; ProStrakan, now Kyowa Kirin, Inc., Tokyo, Japan) or an equivalent preparation. The summary of product characteristics instructions for treatment, including information about dosing and interactions, was followed. The response to teriparatide therapy was assessed by measurements of bone density [using quantitative computed tomography, high-resolution peripheral quantitative computed tomography (HR-pQCT), and dual-energy x-ray absorptiometry (DXA)], and bone turnover markers. The study was performed at the Centre for Biomedical Research, Northern General Hospital (Sheffield, UK). Patients with osteoporosis were identified from Sheffield Metabolic Bone Clinic referrals and general practice registers.
Twenty postmenopausal women with osteoporosis were recruited. The inclusion criteria were a BMD T-score (at the lumbar spine or total hip) of −2.5 or less, ≥5 years since the last menstrual period, ambulatory, able and willing to participate in the study and provide written informed consent, and a serum 25OH vitamin D3 >50 nmol/L (after the 100,000 U cholecalciferol bolus). All participants were bisphosphonate naive. Other exclusion criteria were diseases, treatments, and/or lifestyle factors know to affect bone metabolism. The North West 2 Research Ethics Committee–Liverpool Central and the Medicines and Healthcare Products Regulatory Agency, UK, approved the present study, and all participants gave fully informed written consent before participation. All investigations were performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments, and in accordance with the International Conference on Harmonization Good Clinical Practice guidelines.
Laboratory methods
Venous blood samples were collected from each patient, between 8:00 and 10:00 am after an overnight fast at baseline (two visits) and at weeks 1, 2, 4, 12, 26, 52, 78, and 104. The serum and plasma were separated and stored at −70°C until the biochemical measurements were performed.
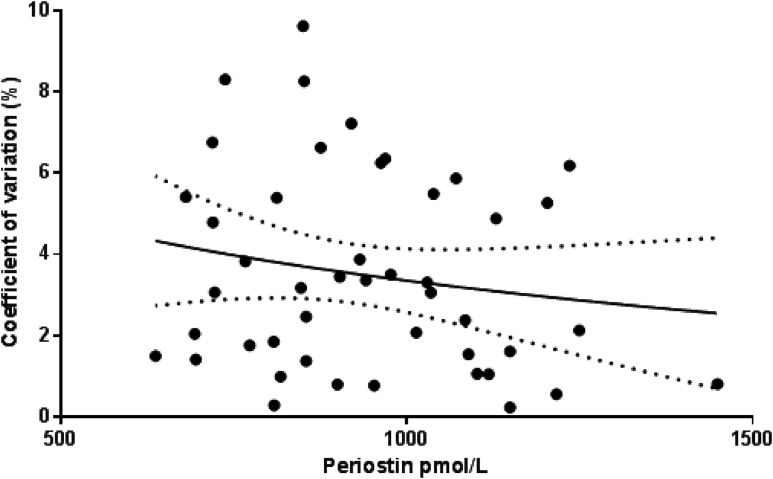
Periostin was measured in plasma using a recently released (June 2016) ELISA from Biomedica Gruppe (Vienna, Austria). The ELISA recognizes all seven known splice variants of human periostin, and the calibrators and controls are produced in a human serum matrix. It uses a mouse monoclonal antibody directed against the midregion and a goat polyclonal antibody directed against the epitopes that spread across the whole periostin molecule and that are mostly conserved between the isoforms. The range for the assay is 125 to 4000 pmol/L, the intra-assay precision was 5%, and the assay was linear with spike samples (range, 99% to 115%). A precision profile to assess the assay was performed. Fifty samples were each measured in duplicate. The standard deviation and coefficient of variation were calculated for each duplicate (Fig. 1). The samples used had not been through a previous freeze-thaw cycle. Sclerostin and DKK-1 were measured in serum using a manual sandwich ELISA from Biomedica Gruppe, with an interassay precision of 5% to 6%.
Figure 1.
The precision profile of the assay showing the percentage of the coefficient of variation at different concentrations of periostin.
Procollagen type I N-terminal propeptide (PINP), C-telopeptide of type I collagen (CTX), bone alkaline phosphatase, tartrate-resistant acid phosphatase 5b, 1,25 (OH)2D, and 25OHD were measured in serum using the IDS-iSYS automated immunoassays (Immunodiagnostic Systems, Boldon, United Kingdom). The interassay coefficients of variation were 3% to 7%.
BMD and HR-pQCT methods
The areal BMD (in g/cm2) of the lumbar spine, femoral neck and total body was measured at baseline and 26, 52, and 104 weeks using DXA using a Discovery A densitometer (Hologic Inc., Bedford, MA).
Images of the distal radius and tibia (nondominant, nonfractured limb) were obtained using HR-pQCT. The cortical thickness (in mm) was measured at the radius and tibia using the standard in-built software (version 6.0; Scanco Medical AG, Zurich, Switzerland). Detailed methods and quality control assessments have been previously reported (36).
Statistical analysis
On the basis of a 1-standard deviation change in lumbar spine BMD from baseline and a 10% withdrawal annually, we calculated that 20 participants would provide >90% power to detect a change at the 5% significance level. A similar power was calculated for the bone formation markers. Early bone formation marker changes (14 and 28 days) in excess of 1 standard deviation have previously been reported in 15 patients treated with teriparatide (37).
The baseline characteristics are described as the mean and standard deviation for each variable (Table 1). Periostin, sclerostin, DKK-1, PINP, and CTX are expressed as the mean percentage of change from baseline [and 95% confidence intervals (CIs)] at each visit. One-way analysis of variance was used to test for between-group differences. Bonferroni post hoc analysis was used to test for differences between the different time points. Spearman rank coefficients were used to test for correlations between changes in periostin and changes in other variables. A P value of < 0.05 was considered to indicate statistical significance in all tests. Statistical analysis was performed with SPSS for Windows, version 21 (IBM Corp., Armonk, NY).
Table 1.
Baseline Characteristics of MOAT Study Participants (n = 20 Postmenopausal Women With Osteoporosis)
| Variable | Mean ± SD |
|---|---|
| Age, y | 65.8 ± 5.1 |
| Height, cm | 161.2 ± 4.6 |
| Weight, kg | 64.3 ± 8.5 |
| BMI, kg/m2 | 24.8 ± 4.0 |
| Lumbar spine BMD T-score | −2.8 ± 0.3 |
| Total hip BMD T-score | −1.5 ± 0.6 |
| β-CTX, ng/mL | 0.55 ± 0.20 |
| Intact PINP, ng/mL | 60.4 ± 17.2 |
| Bone TRAP 5b, IU/L | 5.1 ± 0.8 |
| Bone ALP, ng/mL | 20.8 ± 4.5 |
| Osteocalcin, ng/mL | 24.1 ± 8.4 |
| PTH, pg/mL | 34.3 ± 10.2 |
| 25(OH)D, ng/mL | 34.0 ± 8.7 |
| 1,25(OH)2D, pg/mL | 62.0 ± 17.6 |
| Periostin, pmol/L | 955.1 ± 224.3 |
| Sclerostin, pmol/L | 20.3 ± 11.4 |
| DKK-1, pmol/L | 34.0 ± 11.9 |
| Radius Ct.Th., mm | 0.48 ± 0.12 |
| Tibia Ct.Th., mm | 0.79 ± 0.22 |
Abbreviations: ALP, alkaline phosphatase; Ct.Th., cortical thickness; MOAT, mechanisms of action of teriparatide in postmenopausal women with osteoporosis; SD, standard deviation; TRAP 5b, tartrate-resistant acid phosphatase.
Effect of teriparatide on periostin
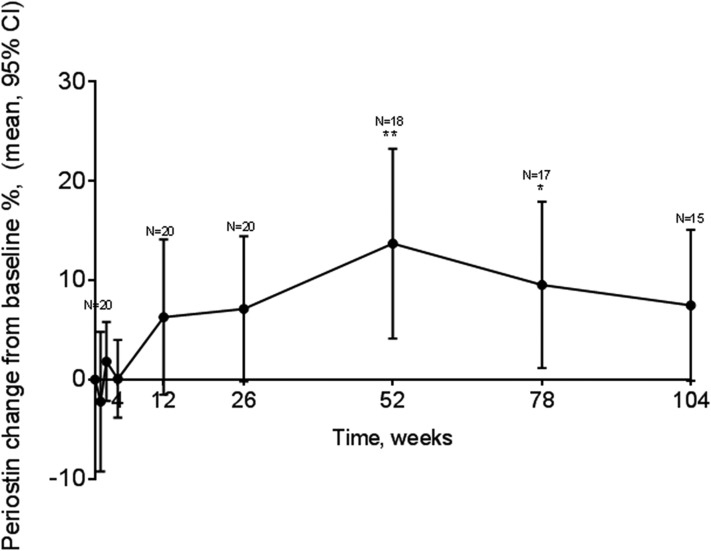
The periostin levels had increased at 26 weeks after teriparatide treatment by 6.6% (Fig. 2). It remained increased for the remainder of the study period, with a statistically significant increase at 52 weeks of 12.5% (95% CI, 3.3% to 21%; P < 0.01). Of the 20 patients, 15 had periostin data available at the end of the study. To assess the effect of the five patients with missing data at week 104, we used the week 26 time point because all 20 patients had periostin data available at that point. The mean percentage of change in the levels of periostin from baseline to week 26 for these five patients was 1.96%. The mean percentage of change for the remaining 15 was 7.4%.
Figure 2.
Changes in serum circulating levels of periostin over a 104-week treatment period with teriparatide. Data presented as the mean percentage of change and 95% CI from baseline (0) (n = 20) at several time points. *P < 0.05; **P < 0.01.
Effect of teriparatide on regulators of bone formation
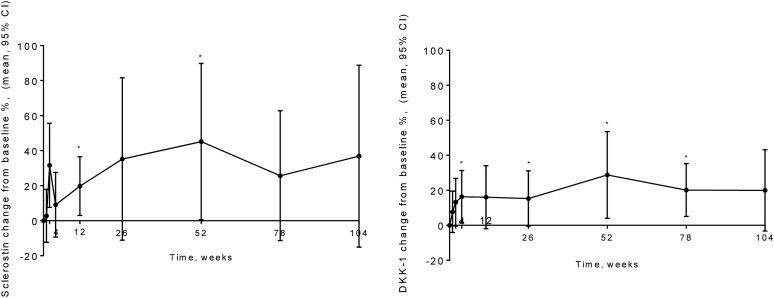
Sclerostin was increased at 12 weeks after baseline by 20.8% (95% CI, 3.2% to 38.4%; P < 0.05). It was increased through the remainder of the study period, with a peak increase at 52 weeks of 28.9% (95% CI, −1.5 to 59.2). DKK-1 was increased at 26 weeks after baseline by 15.3% (95% CI, −0.5 to 31.1). It was increased for the remainder of the study period, with a statistically significant increase at 52 weeks of 28.8% (95% CI, 3.9% to 53.6%; P < 0.05; Fig. 3).
Figure 3.
Changes in serum circulating levels of (left) sclerostin and (right) DKK-1 over a 104-week treatment period with teriparatide. Data presented as mean percentage of change and 95% CI from baseline (0) (n = 20) at several time points. *P < 0.05 compared with baseline.
Effect of teriparatide on bone turnover markers
PINP and CTX increased significantly during teriparatide treatment, with peaks at week 52 by 204% (95% CI, 119% to 289%) and 227% (95% CI, 116% to 338%), respectively (P < 0.001).
Periostin correlations
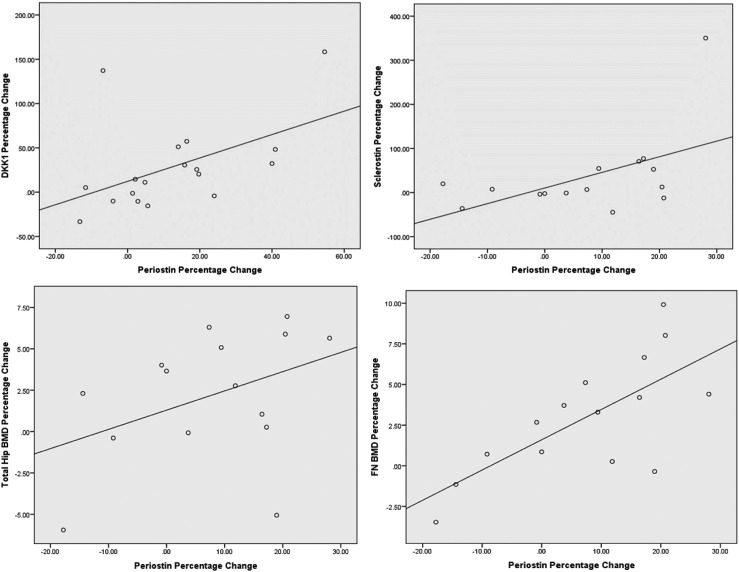
The change in periostin levels correlated positively with the changes in sclerostin at week 104 (r = 0.518; 95% CI, 0.008 to 0.814; P < 0.05) and DKK-1 at week 52 (r = 0.494; 95% CI, 0.036 to 0.780; P < 0.05; Table 2; Fig. 4). The correlation coefficients were recalculated with the outliers removed, and the changes in the periostin levels did not correlate with sclerostin (r = 0.386; 95% CI, −0.81 to 0.760) or DKK-1 (r = 0.205; 95% CI, −0.305 to 0.624; P > 0.05). The changes in periostin did not correlate with the change in bone turnover markers or cortical thickness at the radius or tibia. The changes in periostin levels at week 52 correlated positively with the changes in total hip BMD and femoral neck BMD at week 104 (r = 0.547; 95% CI, 0.070 to 0.820; P < 0.05; and r = 0.682; 95% CI, 0.261 to 0.885; P < 0.01), respectively (Table 2; Fig. 4).
Table 2.
Spearman Rank Correlations (95% CIs) Between Changes in Periostin and PINP, CTX, Regulators of Bone Formation, and BMD Assessed From Percentage of Changes From Baseline at Weeks 52 and 104
| Variable | Week 52 Periostin |
Week 104 Periostin |
||
|---|---|---|---|---|
| Spearman Correlation Coefficient (95% CI) | P Value | Spearman Correlation Coefficient (95% CI) | P Value | |
| PINP | 0.217 (−0.332 to 0.656) | 0.438 | −0.167 (−0.676 to 0.450) | 0.603 |
| CTX | 0.157 (−0.334 to 0.581) | 0.533 | −0.201 (−0.677 to 0.393) | 0.509 |
| Sclerostin | 0.026 (−0.446 to 0.486) | 0.918 | 0.518 (0.008– 0.814)a | 0.048 |
| DKK-1 | 0.494 (0.036– 0.780)a | 0.037 | 0.323 (−0.226 to 0.716) | 0.240 |
| Week 104 BMD | ||||
| Femoral neck | 0.424 (−0.091 to 0.760) | 0.102 | 0.682 (0.261– 0.885)b | 0.005 |
| Total hip | 0.547 (0.070– 0.820) | 0.028a | 0.461 (−0.067 to 0.787) | 0.084 |
| Lumbar spine | 0.432 (−0.081 to 0.764) | 0.094 | 0.486 (−0.034 to 0.799) | 0.066 |
P < 0.05, statistically significant correlation.
P < 0.01, statistically significant correlation.
Figure 4.
Graphs showing changes between periostin and DKK-1, sclerostin, total hip BMD, and femoral neck BMD.
Discussion
Teriparatide and periostin
In the present study, we found that teriparatide treatment of postmenopausal women with osteoporosis significantly increased the circulating levels of periostin. This offers some support to our hypothesis that the anabolic actions of teriparatide might be partially related to periostin. The results of a number of in vitro and animal experiments support our findings. Periostin messenger RNA was upregulated in osteoblasts by PTH (21). Periostin expression was also increased when PTH was added to the IDG-SW3 cell line (12), osteocytes in vivo (18), and human osteoblasts (22). However, our study, to the best of our knowledge, is the first to show that the levels of periostin are also increased by teriparatide treatment in postmenopausal women.
Teriparatide and regulators of bone formation
Substantial increases in circulating levels of the Wnt inhibitors sclerostin and DKK-1 were also observed. Therefore, the anabolic action of teriparatide is unlikely to be mediated by these regulators of bone formation. In rat bone and osteoblast experiments, sclerostin and DKK-1 decrease in response to intermittent PTH (38–40). However, our data are consistent with some previous clinical studies of teriparatide (26, 29). Idolazzi et al. (26) reported statistically significant increases in DKK-1 and sclerostin of ~15% and ~5%, respectively, at 12 months from baseline (P < 0.01) using ELISAs (Biomedica Medizinprodukte, Vienna, Austria). Similarly, Anastasilakis et al. (28), reported a statistically significant increase in DKK-1 by ~30% at 12 months from baseline (P < 0.05). Others have reported an acute decrease in sclerostin from baseline 4 hours after injection of 12.7% ± 1.9% (P < 0.0001) in 27 nonosteoporotic, healthy postmenopausal women treated with teriparatide, measured using Luminex kits (Millipore/Linco, St. Charles, MO) (30) or that the levels remained unchanged (27, 29). The differences in the data could be attributed to the different assays, study populations, and timing of the injections.
We observed that the DKK-1 levels remained increased after 52 weeks of treatment. This finding supports the concept that the anabolic effects of teriparatide might be downregulated through continuous increased secretion of DKK-1 to achieve homeostasis through the mechanostat (29). This observation is supported by, and might explain the partial reduction in, bone turnover markers that we, and others (29, 41–43), observed in the second year of the treatment period.
The increase in DKK-1 might be partly due to the increase in 1,25 dihydroxy-vitamin D [1,25(OH)2D3] observed with teriparatide treatment; it has been reported that 1,25 (OH)2D3 induces DKK-1 gene expression and protein production in cell culture (44). We observed a substantial increase in 1,25(OH)2D3 but these changes did not correlate with changes in DKK-1 (data not shown).
It is important to recognize the limitation that sclerostin and DKK-1 in circulation might not accurately reflect their concentration at the tissue level, and this could contribute to the conflicting observations.
Periostin correlations
Changes in periostin during teriparatide treatment correlated positively with changes in sclerostin and DKK-1. This finding does not support our second hypothesis that periostin mediates the effects of teriparatide by inhibiting these regulators of bone formation. This is in contrast to the results from mouse experiments (18) in which expression of sclerostin was inhibited by PTH in the bones of periostin knockout mice. However, it might be that periostin does mediate bone formation and Wnt signaling through direct stimulation of β-catenin (45) or by downregulating the action of sclerostin (19). Both of these explanations would not necessarily be reflected by the circulating levels of sclerostin.
Changes in periostin did not correlate with the changes in bone turnover markers. Similar findings were demonstrated by others using a different periostin ELISA. Bonnet et al. (33) reported that periostin did not correlate with PINP, CTX, PTH, sclerostin, or serum 25-hydroxyvitamin D using 432 healthy subjects from the Geneva Retired Workers Cohort. This lack of correlation does not necessarily imply that periostin is not a mediator of the bone anabolic effect of teriparatide on the periosteum. Periosteal bone formation only forms a small part of the bone formation in response to teriparatide treatment and the increase in PINP is also related to the trabecular bone changes. The increase in bone formation markers observed might have been due to the increase in the rate of bone remodeling. An increase in DXA BMD at the spine of 7% to 10% after 18 months of treatment has been reported (4, 46). The lack of correlation between periostin and bone formation markers might have resulted from the higher ratio of trabecular to cortical bone at this site (47).
Furthermore, the changes in periostin were positively correlated with changes in BMD at the proximal femur. This is an important finding owing to the lack of correlation with PINP. Changes in BMD in the spine might be related to changes in bone turnover markers (43, 48), with changes in periostin related to changes in BMD at the hip. This finding has not previously been explored, and it could be that the action of teriparatide on BMD is mediated through periostin. This suggests a stronger relationship to the hip structure owing to the greater presence of cortical bone. No changes were found in the cortical thickness and cortical density at the radius and tibia with teriparatide treatment, and these did not correlate with changes in periostin. In contrast, one study reported substantial increases in cortical thickness with teriparatide treatment using HR-pQCT (4) and histomorphometry methods (11), implying that bone formation is markedly increased in cortical bone. Our study was powered for DXA BMD and bone turnover markers. The sample size and measurement variability will have affected the ability to detect these changes.
The present study was performed to explore the role of periostin during teriparatide treatment. The present study had some limitations such as the small number of patients receiving only one dose of teriparatide and the lack of a control group receiving no treatment. By the end of the study period, data were missing for five patients because the patients withdrew from the study. If the five patients had remained in the study until week 104, we would have expected the mean change to be lower. However, despite these limitations, some of the results obtained were consistent with those from previous studies.
Conclusions
To the best of our knowledge, ours is the first study to describe the circulating periostin response to teriparatide treatment in osteoporosis and explore the correlations of periostin with bone turnover markers, Wnt inhibitors, BMD, and cortical thickness. We conclude that periostin increased in response to teriparatide. This increase might partly mediate the anabolic mechanism of action on BMD, but it does not affect bone formation through Wnt signaling because it does not reduce the sclerostin and DKK-1 levels.
Acknowledgments
Financial Support: The present study was funded by a National Institute for Health Research Bone Biomedical Research Unit award (National Institute for Health Research; available at: http://dx.doi.org/10.13039/501100000272) to R.E. The study drug costs were provided by Eli Lilly and Company. The periostin assay kits were provided by Biomedica Gruppe. The bone turnover markers were provided by Immunodiagnostics Systems.
Clinical Trial Information: ClinicalTrials.gov no. NCT01293292 (registered 4 February 2011) and EudraCT no. 2010-021009-19 (registered 21 September 2010).
Disclosure Summary: R.E. received consulting fees from Amgen, AstraZeneca, Chronos, GSK, Immunodiagnostic Systems, Fonterra Brands, Ono Pharma, Lilly, Bayer, Janssen Research, Alere, CL Biosystems, Teijin, D-Star, Roche Diagnostics, and Inverness Medical, and grant support from Amgen, Alexion, Immunodiagnostic Systems, Roche, and AstraZeneca. E.V.M. received consultancy fees, research funding, and/or speaker fees from ActiveSignal, Amgen, AstraZeneca, Consilient Healthcare, GlaxoSmithKline, Hologic, Internis, Lilly, Medtronic, Merck, Novartis, Pfizer, Roche, Sanofi-Aventis, Servier, Synexus,Tethys, UCB, and Warner Chilcott, and research support from ARUK, I3 Innovus, MRC, IOF, and Unilever. J.S.W. received lecture fees from Lilly, drug and placebo for a clinical trial from Prostrakan, consulting fees from Mereo Biopharma and Shire, and grant funding from Alexion and Immunodiagnostic Systems. N.F.A.P. received lecture fees and fees for participation on the advisory board for Lilly, and lecture fees from Amgen and advisory board payment from Internis and ProStrakan.
Glossary
Abbreviations:
- 1,25(OH)2D3
1,25 dihydroxy-vitamin D
- BMD
bone mineral density
- CI
confidence interval
- CTX
C-telopeptide of type I collagen
- DKK-1
Dickkopf-related protein 1
- DXA
dual-energy x-ray absorptiometry
- ELISA
enzyme-linked immunosorbent assays
- HR-pQCT
high-resolution peripheral quantitative computed tomography
- PINP
procollagen type I N-terminal propeptide
- PTH
parathyroid hormone
References
- 1. McClung MR, San Martin J, Miller PD, Civitelli R, Bandeira F, Omizo M, Donley DW, Dalsky GP, Eriksen EF. Opposite bone remodeling effects of teriparatide and alendronate in increasing bone mass. Arch Intern Med. 2005;165(15):1762–1768. [DOI] [PubMed] [Google Scholar]
- 2. Arlot M, Meunier PJ, Boivin G, Haddock L, Tamayo J, Correa-Rotter R, Jasqui S, Donley DW, Dalsky GP, Martin JS, Eriksen EF. Differential effects of teriparatide and alendronate on bone remodeling in postmenopausal women assessed by histomorphometric parameters. J Bone Miner Res. 2005;20(7):1244–1253. [DOI] [PubMed] [Google Scholar]
- 3. Lindsay R, Cosman F, Zhou H, Bostrom MP, Shen VW, Cruz JD, Nieves JW, Dempster DW. A novel tetracycline labeling schedule for longitudinal evaluation of the short-term effects of anabolic therapy with a single iliac crest bone biopsy: early actions of teriparatide. J Bone Miner Res. 2006;21(3):366–373. [DOI] [PubMed] [Google Scholar]
- 4. Hansen S, Hauge EM, Beck Jensen JE, Brixen K. Differing effects of PTH 1-34, PTH 1-84, and zoledronic acid on bone microarchitecture and estimated strength in postmenopausal women with osteoporosis: an 18-month open-labeled observational study using HR-pQCT. J Bone Miner Res. 2013;28(4):736–745. [DOI] [PubMed] [Google Scholar]
- 5. Jiang Y, Zhao JJ, Mitlak BH, Wang O, Genant HK, Eriksen EF. Recombinant human parathyroid hormone (1-34) [teriparatide] improves both cortical and cancellous bone structure. J Bone Miner Res. 2003;18(11):1932–1941. [DOI] [PubMed] [Google Scholar]
- 6. Dempster DW, Cosman F, Kurland ES, Zhou H, Nieves J, Woelfert L, Shane E, Plavetić K, Müller R, Bilezikian J, Lindsay R. Effects of daily treatment with parathyroid hormone on bone microarchitecture and turnover in patients with osteoporosis: a paired biopsy study. J Bone Miner Res. 2001;16(10):1846–1853. [DOI] [PubMed] [Google Scholar]
- 7. Lindsay R, Zhou H, Cosman F, Nieves J, Dempster DW, Hodsman AB. Effects of a one-month treatment with PTH(1-34) on bone formation on cancellous, endocortical, and periosteal surfaces of the human ilium. J Bone Miner Res. 2007;22(4):495–502. [DOI] [PubMed] [Google Scholar]
- 8. Parfitt AM. Parathyroid hormone and periosteal bone expansion. J Bone Miner Res. 2002;17(10):1741–1743. [DOI] [PubMed] [Google Scholar]
- 9. Zanchetta JR, Bogado CE, Ferretti JL, Wang O, Wilson MG, Sato M, Gaich GA, Dalsky GP, Myers SL. Effects of teriparatide [recombinant human parathyroid hormone (1-34)] on cortical bone in postmenopausal women with osteoporosis. J Bone Miner Res. 2003;18(3):539–543. [DOI] [PubMed] [Google Scholar]
- 10. Dempster DW, Zhou H, Recker RR, Brown JP, Recknor CP, Lewiecki EM, Miller PD, Rao SD, Kendler DL, Lindsay R, Krege JH, Alam J, Taylor KA, Janos B, Ruff VA. Differential effects of teriparatide and denosumab on intact PTH and bone formation indices: AVA Osteoporosis study. J Clin Endocrinol Metab. 2016;101(4):1353–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ma YL, Zeng QQ, Chiang AY, Burr D, Li J, Dobnig H, Fahrleitner-Pammer A, Michalská D, Marin F, Pavo I, Stepan JJ. Effects of teriparatide on cortical histomorphometric variables in postmenopausal women with or without prior alendronate treatment. Bone. 2014;59:139–147. [DOI] [PubMed] [Google Scholar]
- 12. Bonnet N, Garnero P, Ferrari S. Periostin action in bone. Mol Cell Endocrinol. 2016;432:75–82. [DOI] [PubMed] [Google Scholar]
- 13. Bonnet N, Standley KN, Bianchi EN, Stadelmann V, Foti M, Conway SJ, Ferrari SL. The matricellular protein periostin is required for Sost inhibition and the anabolic response to mechanical loading and physical activity. J Biol Chem. 2009;284(51):35939–35950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maruhashi T, Kii I, Saito M, Kudo A. Interaction between periostin and BMP-1 promotes proteolytic activation of lysyl oxidase. J Biol Chem. 2010;285(17):13294–13303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ghatak S, Misra S, Norris RA, Moreno-Rodriguez RA, Hoffman S, Levine RA, Hascall VC, Markwald RR. Periostin induces intracellular cross-talk between kinases and hyaluronan in atrioventricular valvulogenesis. J Biol Chem. 2014;289(12):8545–8561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morra L, Moch H. Periostin expression and epithelial-mesenchymal transition in cancer: a review and an update. Virchows Arch. 2011;459(5):465–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tkatchenko TV, Moreno-Rodriguez RA, Conway SJ, Molkentin JD, Markwald RR, Tkatchenko AV. Lack of periostin leads to suppression of Notch1 signaling and calcific aortic valve disease. Physiol Genomics. 2009;39(3):160–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bonnet N, Conway SJ, Ferrari SL. Regulation of beta catenin signaling and parathyroid hormone anabolic effects in bone by the matricellular protein periostin. Proc Natl Acad Sci USA. 2012;109(37):15048–15053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chan CSL, Cheah KSE, Tanner JA, Leung CM, Chan D. Periostin interacts with sclerostin and inhibits its antagonistic effect on Wnt signalling. International Bone & Mineral Society (IBMS) Davos Workshops. Davos, Switzerland: International Bone & Mineral Society; 2008. [Google Scholar]
- 20. Kudo A. Periostin in fibrillogenesis for tissue regeneration: periostin actions inside and outside the cell. Cell Mol Life Sci. 2011;68(19):3201–3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fortunati D, Reppe S, Fjeldheim AK, Nielsen M, Gautvik VT, Gautvik KM. Periostin is a collagen associated bone matrix protein regulated by parathyroid hormone. J Int Soc Matrix Biol. 2010;29(7):594–601. [DOI] [PubMed] [Google Scholar]
- 22. Bianchi EN, Ferrari SL. Beta-arrestin2 regulates parathyroid hormone effects on a p38 MAPK and NFkappaB gene expression network in osteoblasts. Bone. 2009;45(4):716–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Onyia JE, Helvering LM, Gelbert L, Wei T, Huang S, Chen P, Dow ER, Maran A, Zhang M, Lotinun S, Lin X, Halladay DL, Miles RR, Kulkarni NH, Ambrose EM, Ma YL, Frolik CA, Sato M, Bryant HU, Turner RT. Molecular profile of catabolic versus anabolic treatment regimens of parathyroid hormone (PTH) in rat bone: an analysis by DNA microarray. J Cell Biochem. 2005;95(2):403–418. [DOI] [PubMed] [Google Scholar]
- 24. Reppe S, Stilgren L, Olstad OK, Brixen K, Nissen-Meyer LS, Gautvik KM, Abrahamsen B. Gene expression profiles give insight into the molecular pathology of bone in primary hyperparathyroidism. Bone. 2006;39(1):189–198. [DOI] [PubMed] [Google Scholar]
- 25. Robling AG, Kedlaya R, Ellis SN, Childress PJ, Bidwell JP, Bellido T, Turner CH. Anabolic and catabolic regimens of human parathyroid hormone 1-34 elicit bone- and envelope-specific attenuation of skeletal effects in Sost-deficient mice. Endocrinology. 2011;152(8):2963–2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Idolazzi L, Rossini M, Viapiana O, Braga V, Fassio A, Benini C, Kunnathully V, Adami S, Gatti D. Teriparatide and denosumab combination therapy and skeletal metabolism. Osteoporosis Int. 2016;27(11):3301–3307. [DOI] [PubMed] [Google Scholar]
- 27. Polyzos SA, Anastasilakis AD, Bratengeier C, Woloszczuk W, Papatheodorou A, Terpos E. Serum sclerostin levels positively correlate with lumbar spinal bone mineral density in postmenopausal women—the six-month effect of risedronate and teriparatide. Osteoporosis Int. 2012;23(3):1171–1176. [DOI] [PubMed] [Google Scholar]
- 28. Anastasilakis AD, Polyzos SA, Avramidis A, Toulis KA, Papatheodorou A, Terpos E. The effect of teriparatide on serum Dickkopf-1 levels in postmenopausal women with established osteoporosis. Clin Endocrinol (Oxf). 2010;72(6):752–757. [DOI] [PubMed] [Google Scholar]
- 29. Gatti D, Viapiana O, Idolazzi L, Fracassi E, Rossini M, Adami S. The waning of teriparatide effect on bone formation markers in postmenopausal osteoporosis is associated with increasing serum levels of DKK1. J Clin Endocrinol Metab. 2011;96(5):1555–1559. [DOI] [PubMed] [Google Scholar]
- 30. Drake MT, Srinivasan B, Mödder UI, Peterson JM, McCready LK, Riggs BL, Dwyer D, Stolina M, Kostenuik P, Khosla S. Effects of parathyroid hormone treatment on circulating sclerostin levels in postmenopausal women. J Clin Endocrinol Metab. 2010;95(11):5056–5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Anastasilakis AD, Polyzos SA, Makras P, Savvides M, Sakellariou GT, Gkiomisi A, Papatheodorou A, Terpos E. Circulating periostin levels do not differ between postmenopausal women with normal and low bone mass and are not affected by zoledronic acid treatment. Horm Metab Res. 2014;46(2):145–149. [DOI] [PubMed] [Google Scholar]
- 32. Rousseau JC, Sornay-Rendu E, Bertholon C, Chapurlat R, Garnero P. Serum periostin is associated with fracture risk in postmenopausal women: a 7-year prospective analysis of the OFELY study. J Clin Endocrinol Metab. 2014;99(7):2533–2539. [DOI] [PubMed] [Google Scholar]
- 33. Bonnet N, Biver E, Durosier C, Chevalley T, Rizzoli R, Ferrari S. Additive genetic effects on circulating periostin contribute to the heritability of bone microstructure. J Clin Endocrinol Metab. 2015;100(7):E1014–E1021. [DOI] [PubMed] [Google Scholar]
- 34. Kim BJ, Rhee Y, Kim CH, Baek KH, Min YK, Kim DY, Ahn SH, Kim H, Lee SH, Lee SY, Kang MI, Koh JM. Plasma periostin associates significantly with non-vertebral but not vertebral fractures in postmenopausal women: clinical evidence for the different effects of periostin depending on the skeletal site. Bone. 2015;81:435–441. [DOI] [PubMed] [Google Scholar]
- 35. Gadermaier E, Tesarz M, Suciu AA, Wallwitz J, Berg G, Himmler G. Characterization of a sandwich ELISA for the quantification of all human periostin isoforms. Journal of clinical laboratory analysis. 2017. [DOI] [PMC free article] [PubMed]
- 36. Paggiosi MA, Eastell R, Walsh JS. Precision of high-resolution peripheral quantitative computed tomography measurement variables: influence of gender, examination site, and age. Calcif Tissue Int. 2014;94(2):191–201. [DOI] [PubMed] [Google Scholar]
- 37. Glover SJ, Eastell R, McCloskey EV, Rogers A, Garnero P, Lowery J, Belleli R, Wright TM, John MR. Rapid and robust response of biochemical markers of bone formation to teriparatide therapy. Bone. 2009;45(6):1053–1058. [DOI] [PubMed] [Google Scholar]
- 38. Kulkarni NH, Halladay DL, Miles RR, Gilbert LM, Frolik CA, Galvin RJ, Martin TJ, Gillespie MT, Onyia JE. Effects of parathyroid hormone on Wnt signaling pathway in bone. J Cell Biochem. 2005;95(6):1178–1190. [DOI] [PubMed] [Google Scholar]
- 39. Silvestrini G, Ballanti P, Leopizzi M, Sebastiani M, Berni S, Di Vito M, Bonucci E. Effects of intermittent parathyroid hormone (PTH) administration on SOST mRNA and protein in rat bone. J Mol Histol. 2007;38(4):261–269. [DOI] [PubMed] [Google Scholar]
- 40. Kakar S, Einhorn TA, Vora S, Miara LJ, Hon G, Wigner NA, Lindsey R. Enhanced chondrogenesis and Wnt signaling in PTH-treated fractures. J Bone Mineral Res. 2007;22(12):1903–1912. [DOI] [PubMed] [Google Scholar]
- 41. Rubin MR, Bilezikian JP. The anabolic effects of parathyroid hormone therapy. Clin Geriatr Med. 2003;19(2):415–432. [DOI] [PubMed] [Google Scholar]
- 42. Cosman F, Nieves J, Woelfert L, Formica C, Gordon S, Shen V, Lindsay R. Parathyroid hormone added to established hormone therapy: effects on vertebral fracture and maintenance of bone mass after parathyroid hormone withdrawal. J Bone Miner Res. 2001;16(5):925–931. [DOI] [PubMed] [Google Scholar]
- 43. Finkelstein JS, Wyland JJ, Lee H, Neer RM. Effects of teriparatide, alendronate, or both in women with postmenopausal osteoporosis. J Clin Endocrinol Metab. 2010;95(4):1838–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Aguilera O, Peña C, García JM, Larriba MJ, Ordóñez-Morán P, Navarro D, Barbáchano A, López de Silanes I, Ballestar E, Fraga MF, Esteller M, Gamallo C, Bonilla F, González-Sancho JM, Muñoz A. The Wnt antagonist DICKKOPF-1 gene is induced by 1alpha,25-dihydroxyvitamin D3 associated to the differentiation of human colon cancer cells. Carcinogenesis. 2007;28(9):1877–1884. [DOI] [PubMed] [Google Scholar]
- 45. Haertel-Wiesmann M, Liang Y, Fantl WJ, Williams LT. Regulation of cyclooxygenase-2 and periostin by Wnt-3 in mouse mammary epithelial cells. J Biol Chem. 2000;275(41):32046–32051. [DOI] [PubMed] [Google Scholar]
- 46. Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344(19):1434–1441. [DOI] [PubMed] [Google Scholar]
- 47. Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3(Suppl 3):S131–S139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Leder BZ, Tsai JN, Uihlein AV, Burnett-Bowie SA, Zhu Y, Foley K, Lee H, Neer RM. Two years of denosumab and teriparatide administration in postmenopausal women with osteoporosis (the DATA Extension study): a randomized controlled trial. J Clin Endocrinol Metab. 2014;99(5):1694–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]