Abstract
Telomeres contain TTAGGG (T; Thymine, A; Adenine and G; Guanine) repetitive sequences and are placed at the end of human chromosomes. Telomere dysfunction is implicated in some age-related and chronic diseases, but its association with total serum lipids and obesity is unknown. Our objective was to determine influenced of total serum lipids on leukocyte telomere lengths (TLs). Participants were selected by cluster sampling from 22 districts of Tehran. The questionnaires were completed by 500 subjects and after the initial assessment in terms of lifestyle, nutrition, home, and job, 300 healthy people, aged 25–40 years were finally selected. TLs and serum level of total lipids were measured by quantitative real-time PCR and the Phillips method, respectively. The average telomere length (T/S) and total lipids were 1.05 ± 0.3 mg/dl and 643.3 ± 70.8 mg/dl, respectively. We found that a one unit difference in the following parameters were associated with kilo base pair differences in TL: Age −0.0002 (95% CI [−0.0022, −0.0018]), BMI −0.0019 (95% CI [−0.0003, −0.0034]), TC 0.0001 (95% CI [−0.0006, −0.0007]), TG −0.0010 (95% CI [−0.0015, −0.0004]), PL 0.0001 (95% CI [−0.0005, −0.0007]), and TSL −0.0003 (95% CI [−0.0008, 0.0001]). Spearman correlation analysis revealed an inverse relationship between TC (R = −0.53; 95% CI [−0.61, −0.44]), TG (R = −0.50; 95% CI [−0.58, −0.41]), PL (R = −0.46; 95% CI [−0.54−0.36]), and TSL (R = −0.63; 95% CI [−0.69, −0.56]) with T/S. Our research suggests that the inverse relationship was found between TL and weight, BMI, age, and TSL which were associated with obesity. High serum lipids concentration may be associated with systemic inflammation and atherosclerosis and may lead to oxidative stress, resulting in telomere shortening.
Keywords: peripheral blood leukocytes, serum lipids concentrations, telomere length
Blood-borne lipids are a risk factor for cardiovascular disease and a leading cause of death in the world. Increased total cholesterol and triglycerides are the most important risk factors for atherosclerosis and cardiovascular disease (The Emerging Risk Factors Collaboration, 2009). The age-related diseases are associated with cells’ aging (Brouilette et al., 2007). The development of atherosclerotic plaques is the main cause of cellular aging; in vitro induction of aging on coronary artery endothelial cells induces the expression of genes involved in atherogenesis. Biological aging and high concentration of blood lipids increase the risk of coronary artery and heart disease (García-Calzón, Moleres, et al., 2014). It is well known that obesity and high level of serum lipids are characterized by systemic inflammation and oxidative stress (Rankin, Andreae, Oliver Chen, & O’Keefe, 2008). The relationships between obesity and shorter telomeres have been indicated by other reports (García-Calzón, Gea, et al., 2014).
Telomeres are complex nuclei-proteins located at the ends of chromosomes, formed by TTAGGG (T; Thymine, A; Adenine and G; Guanine) repetitive sequence in the DNA (García-Calzón, Gea, et al., 2014; Zhan et al., 2017). The role of repetitive sequences is to maintain cellular stability and prevent DNA degradation by exonuclease, and loss of specific genes due to DNA replication repeat. In addition, the sequence prevents chromosome destruction, abnormal gene in combination with each other, and plays an essential role in the maintenance of chromosomes. Areas of telomere at the end of chromosomes are sensitive to the damage by some compounds such as active radical oxygen and contaminants. The average telomere length (TL) in the human body ranges between 10 and 15 kilo base pairs (kb) (Blackburn, Greider, & Szostak, 2006). Shortening of the TL occurs alongside incomplete cell division and DNA duplication (Hahn, 2003). TL is reduced by age, and in each cycle of cell growth, about 50 to 200 base pairs (bp) of TL is reduced (Harte et al., 2012). Therefore, TL is considered as a biomarker of aging (Harte et al., 2012). Short TL in peripheral blood leukocytes (PBLs) indicates the risk of cardiovascular and respiratory diseases (Brouilette et al., 2007; Zhan et al., 2017). Higher cellular lipid level with acute and chronic inflammation causes changes in TL (Lee, Martin, Firpo, & Demerath, 2011; Rehkopf et al., 2016). Cellular inflammation and oxidative stress are reported to be associated with TL (Karimi et al., 2018; Rehkopf et al., 2016). In this condition, cell division capacity is highly increased and TL becomes too short in several stages of cell proliferation. Cells with shorter TL lost the ability of the division and aging, thereby leading to apoptosis (Hohensinner, Goronzy, & Weyand, 2011; Joshu et al., 2015). The inverse relationship between TL and obesity has been reported by several studies (Cui et al., 2013; Lee et al., 2011; Njajou et al., 2012), but others did not report this relationship (Bekaert et al., 2007; Diaz, Mainous, Player, & Everett, 2010). Nevertheless, to the best of our knowledge, no studies have reported the relationship between changes in total serum lipids (TSL) concentration and TL in the male population. Therefore, the aim of this current study was to determine the relationship between TLs with instinct TSL in the male population. In this study, after receiving blood samples from the Tehran active male population, the concentration of TSL and other related factors and TL based on real-time PCR were examined in leukocytes using a cross-sectional study.
Method
Sampling and Questionnaire Study
The study was cross-sectional and studied population included all men aged 25–40 years in Tehran based on a validated questionnaire. More than 500 citizens between 25 and 40 years old were selected by stratified cluster sampling from 22 districts of Tehran and were enrolled in the study. In the present study, only healthy men were selected and those with a history of cardiovascular, or chronic and debilitating diseases or diabetes alongside those treated with plasma lipid-lowering drugs were excluded from the study. Finally, data of 300 subjects were studied. All participants in the study were asked to be on fasting on the visiting day, not have eaten for 12 h the night before visiting, and on the visiting morning, they should have avoided smoking and excessive exercise. In order to collect information, a questionnaire was prepared. Information collected included subjects’ demographic information, questions related to lifestyles such as smoking and physical activity, questions related to specific diseases and history of anti-hyperlipidemic drugs. The examination was done in all subjects as follows: measuring systolic and diastolic blood pressure twice at sitting position after resting for 5 min of resting, measurement of waist and hip circumference, height and weight. BMI was calculated by dividing weight (kg) by height squared (m2) and categorized in three groups: 25 (normal), 25 to 30 (overweight), and ≥30 kg/m2 (obese).
Also, subjects with a waist circumference greater than 102 cm were considered as high risk. For the detection of lipid disorders, total triglyceride concentration ≥200 mg/dl and TSL concentrations greater than 600 mg/dl were utilized. The protocol of this study has been approved by the Tehran University of Medical Sciences ethics committee with code IR.TUMS.REC.1395.2586. Before the study, the informed consent form was filled up by all participants. A complete description of the study, objectives, method of study, benefits, and the final result are given to everyone in the study.
Serum Lipids
Serum samples were assessed on the first day of admission of serum lipid levels. Total cholesterol (TC), free cholesterol (FC), and triglycerides (TG) were measured by the kits prepared from PARS Azmon Company. TC was determined via enzymatic colorimetric method utilizing cholesterol esterase and cholesterol oxidase enzymes. FC was determined via enzymatic colorimetric method utilizing cholesterol oxidase and peroxidase enzymes. In the measurement of FC concentration, the cholesterol esterase enzyme was not assessed. According to other articles and the existence of a linear relationship between the PLs and TC, phospholipids (PL) concentration was obtained from PL = (0.766 × TC) + 62.3 mg/dl (Bernert, Turner, Patterson, & Needham, 2007). TSLs are estimated by adding TC, FC, and TG values. Given that the blood cholesterol is found in two forms of esterified and nonesterified fatty acids, the amount of esterified cholesterol was obtained from the difference between TC and FC. Given that the ratio of the average molecular weight of esterified cholesterol divided by the molecular weight of nonesterified cholesterol is equal to 1.677, the TC and FC difference was multiplied by this ratio. Finally, the TSL was estimated by the following equation (Akins, Waldrep, & Bernert, 1989): TSL = 1.677 × (TC – FC) + FC + TG + PL. Moreover, in the case when there is no information regarding the PL, one can use the Phillips method according to the following equations (Bernert et al., 2007): TSL = 1.677 × (0.73TC) + (0.27 × TC) + TG(0.766 × TC) + 62.3. The cumulative dose of smoking is computed by the following equation: pack-years = smoked years × packs per day.
Blood Samples and DNA Extraction
After completing the questionnaires, 10 cc blood samples were taken. Quickly, the serum content of about half of the blood samples was separated by centrifuge (5 min at 4,000 rpm), kept in glass vials, and maintained at −70 °C before testing. Blood samples were collected in EDTA tubes and stored at −20 °C before use. Genomic DNA was extracted from 2 ml of each blood sample by the Salting-Out method. The concentration of extracted DNA and its quantity and purity was assayed using the Nanodrop (Thermo Scientific, Wilmington, DE, USA) and by considering the A260/A280 ratio. For the possibility of destruction of DNA, some extracted samples were poured into electrophorese (1% agarose gel). If the thick DNA band was observed on the agarose gel, extracted DNA was appropriate, but when DNAs create a smear on the gel, re-extraction is performed. All extracted DNA was stored at −70 °C until use.
Relative TL
The relative TL was determined with a real-time PCR method based telomere assay previously described by Cawthon (Cawthon, 2002). In summary, real-time PCR was done using SYBR Premix Ex Taq kit (Takara) and the primer concentrations for the telomere were 270 nM Tel1 [5′-CGG TTT(GTTTGG)5GTT-3′] and 900 nM Tel2 [5′-GGC TTG(CCTTAC)5CCT-3′] and for single-copy gene primers, 300 nM for 36B4u [5′-CCCATTCTATCATCAACGGGTACAA-3′] and 500 nM for 36B4d [5′-CAGCAAGTGGGAAGGTGTAATCC-3′] were used. The reaction was performed three times in duplicate wells for each sample using 25 ng/ml of DNA. In each run, three no-template controls alongside other samples and under the same conditions were included for primer dimer study and correct gene amplification process. Melting curve analysis was used for evaluating the property and verifying the specificity of each run. The standard curve to evaluate the PCR efficiency in each run was utilized by serially diluting one reference DNA sample with deionized water to make six concentrations of DNA ranging from 1.56 to 50 ng/ml. The TL reference value was determined by mixing DNA of 10 randomly selected DNA samples. The TL for each sample was estimated by determining the ratio of the number of telomere repeat copy number (T) to the relative number of 36B4 copies (S) with respect to the same reference DNA sample. The results were expressed in terms of T/S ratios.
Statistical Analyses
Spearman correlation test was employed to examine the relationship between TL and interfering factors. Chi-square test was utilized to compare the frequency of qualitative independent variables in both groups. TL was analyzed as a continuous and categorical variable. Wilcoxon test was employed to study differences in TL as a continuous variable and taking into consideration height, weight, BMI, age, residence in Tehran, place of residence, smoking, cumulative smoking (light smokers (pack-years <30) or heavy smokers (pack-years ≥30), serum levels of TC, TG, FC, PL, and total serum lipid (TSL). The statistical tests were two-sided, and p values less than .05 were considered to be statistically significant. All statistical analyses were carried out using statistical software R 3.2.2 and Stata 12.1.
Results
The questionnaires and blood samples were collected from 300 subjects. The average age was 33.47 ± 4.25 years. The percentage of smokers was 17%–24%. The average concentration of total lipids was 643.3 ± 70.8 mg/dl. The demographic characteristics, TL, Total cholesterol (TCol), Triglycerides (TG), and Phospholipid (PL) in the studied population are present in Table 1.
Table 1.
Demographic Characteristics, the TL, and Serum Lipids in the Studied Population.
| Parameters | n | Mean ± SD | Median | Geometric mean ± SE | Max | 95th percentile |
|---|---|---|---|---|---|---|
| T/S | 300 | 1.13 ± 0.36 | 1.1 | 0.92 ± 0.02 | 2.3 | 1.9 |
| Telomere lengths (bp) | 300 | 5377.7 ± 573.4 | 5325.5 | 5083.3 ± 24.01 | 7227.5 | 6593.5 |
| Height (cm) | 300 | 175.99 ± 9.27 | 178 | 176.86 ± 0.6 | 192 | 188 |
| Weight (kg) | 300 | 78.75 ± 12.69 | 80 | 78.82 ± 0.86 | 115 | 102 |
| BMI (kg/m2) | 300 | 25.57 ± 4.6 | 25.08 | 25.20 ± 0.33 | 40.09 | 33.8 |
| Age (years) | 299 | 33.47 ± 4.25 | 33 | 33.83 ± 0.3 | 42 | 40 |
| Residence, years | 292 | 14.04 ± 9.61 | 12 | 11.59 ± 0.62 | 40 | 34 |
| Live in place, years | 295 | 9.57 ± 7.95 | 7 | 7.61 ± 0.53 | 40 | 29 |
| Mean number of pack-years | 295 | 46.38 ± 46.47 | 36 | 36.2 ± 3.69 | 300 | 102 |
| TCol (mg/dl) | 298 | 185.01 ± 28.8 | 182.5 | 189.4 ± 1.99 | 239 | 234 |
| TG (mg/dl) | 300 | 146.32 ± 36.4 | 150 | 150.44 ± 2.22 | 249.5 | 225 |
| PL (mg/dl) | 300 | 203.4 ± 21.6 | 202.1 | 207.11 ± 1.46 | 245.37 | 241.54 |
Note. PL = phospholipids; TG = triglycerides; TL = telomere length.
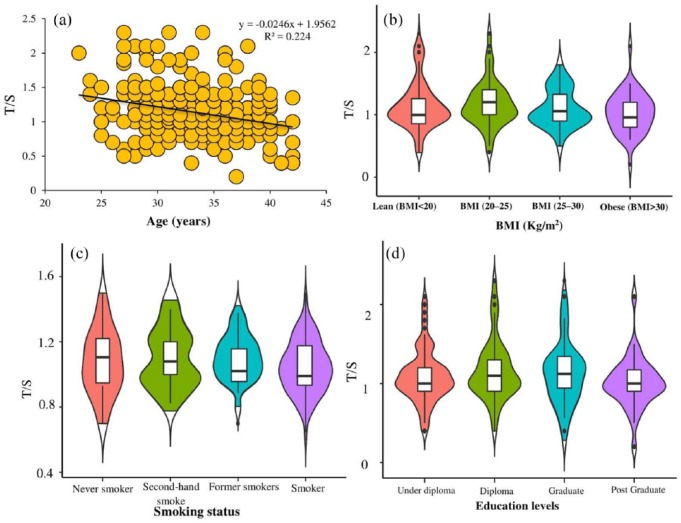
TL in all samples was measured by real-time PCR. Spearman correlation analysis revealed an inverse relationship between age, weight, BMI, place of residence, smoking status, mean pack-years, TC (R = −0.53; 95% CI [−0.61, −0.44]), TG (R = −0.50; 95% CI [−0.58, −0.41]), PL (R = −0.46; 95% CI [−0.54, −0.36]), and TSL (R = −0.63 (95% CI [−0.69, −0.56]) level with TL (Table 2). Shortening of TL with age, BMI, smoking, and quartile of TSL is shown in Figure 1(a)–(d).
Table 2.
Spearman Correlation Matrix With 95% Confidence Interval to Examine the Relationship Between T/S and Interfering Factors.
| T/S (95% CI) | Height (95% CI) | Weight (95% CI) | BMI (95% CI) | Age (95% CI) | Residence (95% CI) | Living (95% CI) | TC (95% CI) | TG (95% CI) | FC (95% CI) | PL (95% CI) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Height | −0.56 [−0.63, −0.48] | 1 | |||||||||
| Weight | −0.46 [−0.55, −0.37] | 0.15 [0.03, 0.25] | 1 | ||||||||
| BMI | −0.05 [−0.16, −0.06] | −0.51 [−0.59, −0.42] | 0.77 [0.72, 0.81] | 1 | |||||||
| Age | −0.42 [−0.51, −0.32] | 0.23 [0.12, 0.34] | 0.27 [0.16, 0.37] | 0.09 [−0.02, 0.20] | 1 | ||||||
| Residence | −0.50 [−0.58, −0.41] | 0.29 [0.18, 0.39] | 0.40 [0.30, 0.49] | 0.15 [0.04, 0.26] | 0.27 [0.16, 0.37] | 1 | |||||
| Living | −0.51 [−0.59, −0.42] | 0.30 [0.20, 0.40] | 0.43 [0.34, 0.52] | 0.18 [0.07, 0.28] | 0.35 [0.24, 0.44] | 0.69 [0.62, 0.74] | 1 | ||||
| TC | −0.53 [−0.61, −0.44] | 0.23 [0.12, 0.33] | 0.44 [0.34, 0.52] | 0.25 [0.14, 0.35] | 0.25 [0.14, 0.35] | 0.41 [0.31, 0.50] | 0.39 [0.29, 0.48] | 1 | |||
| TG | −0.50 [−0.58, −0.41] | 0.22 [0.11, 0.33] | 0.30 [0.19, 0.40] | 0.12 [0.01, 0.23] | 0.38 [0.28, 0.47] | 0.34 [0.23, 0.43] | 0.40 [0.30, 0.49] | 0.40 [0.30, 0.49] | 1 | ||
| FC | −0.49 [−0.57, −0.40] | 0.24 [0.13, 0.34] | 0.34 [0.24, 0.44] | 0.14 [0.02, 0.24] | 0.35 [0.25, 0.45] | 0.34 [0.24, 0.44] | 0.34 [0.23-0.44] | 0.37 [0.26, 0.46] | 0.39 [0.29, 0.48] | 1 | |
| PL | −0.46 [−0.54, −0.36] | 0.14 [0.02, 0.25] | 0.39 [0.29, 0.48] | 0.25 [0.14, 0.36] | 0.22 [0.11, 0.33] | 0.31 [0.21, 0.41] | 0.31 [0.20-0.41] | 0.41 [0.31, 0.50] | 0.30 [0.20, 0.40] | 0.34 [0.24, 0.44] | 1 |
| TSL | −0.63 [−0.69, −0.56] | 0.25 [0.14, 0.35] | 0.48 [0.39, 0.56] | 0.27 [0.16, 0.37] | 0.36 [0.26, 0.46] | 0.46 [0.37, 0.54] | 0.47 [0.38, 0.56] | 0.82 [0.77-0.85] | 0.75 [0.70, 0.80] | 0.36 [0.26, 0.46] | 0.63 [0.56, 0.69] |
Note. PL = phospholipids; TG = triglycerides; TL = telomere length; TSL = total serum lipids; FC = free cholesterol. The significant values (P-value) for T/S with Height, Weight, BMI, Age, Residence, Living in place, TC, TG, PL, TSL were 0.000, 0.000, 0.377, 0.000, 0.000, 0.000, 0.000, 0.000, 0.000 and 0.000, respectively.
Figure 1.
Telomere length shortening with (a) age, (b) BMI, (c) smoking status, and (d) education levels.
The means ± SD of T/S was 1.13 ± 0.36. Higher weight more than 75 kg (p = .05), age over 34 years (p = .03), had lived more than 8 years (p = .056), TG level over 150 mg/dl (p = .04), and FC over 50 mg/dl (p = .036) demonstrated a significant relationship with TL shortening. Results are given in supplementary material (Table S1). Multivariate linear regression coefficients of the association between log T/S and serum lipids (TC, TG, PL, and TSL) adjusted for age, residence, live in place, pack-years, and smoking status are given in supplementary material (Table S2). We found that a one unit difference in the following parameters were associated with kb differences in TL: Age −0.0002 (95% CI [−0.0022, −0.0018]), BMI −0.0019 (95% CI [−0.0003, −0.0034]), TC 0.0001(95% CI [−0.0006, −0.0007]), TG −0.0010 (95% CI [−0.0015, −0.0004]), PL 0.0001 (95% CI [−0.0005, −0.0007]), and TSL −0.0003 (95% CI [−0.0008, −0.0001]). Serum levels of TC, TG, PL, and TSLs with quartiles of TL are present in Figure 2(a)–(d).
Figure 2.
Serum level of total cholesterol (a), triglycerides (b), phospholipids (c), and total serum lipids (d) with quartiles of quartile of telomere length.
The average levels of TC, TG, PL, and TSLs in all samples were 185 ± 28.8, 146.3 ± 36.4, 203.4 ± 21.65, and 624.3 ± 81.4, respectively. The results of the Kruskal–Wallis test was significant (p = .00). Therefore, TL shortening has happened with increasing serum lipids. The mean and median concentration of serum lipids by tertiles of telomere length (base pair [bp]) is present in Table 3.
Table 3.
The Mean and Median Serum Lipid Levels by Tertiles of Telomere Lengths (bp)
| Total serum lipids | Short TL |
Middle TL |
Longest TL |
|||
|---|---|---|---|---|---|---|
| Mean ± SD | Median | Mean ± SD | Median | Mean ± SD | Median | |
| Tcol | 203.2 ± 25.57 | 208.7 | 183.36 ± 25.9 | 181 | 165.17 ± 24.1 | 160 |
| TG | 163.3 ± 33.58 | 155.9 | 146.98 ± 27.3 | 150.5 | 123.31 ± 43.8 | 135 |
| PL | 212.1 ± 21.69 | 213.7 | 205.2 ± 18.9 | 203.24 | 188.78 ± 19.7 | 185.6 |
| TSL | 678.8 ± 71.09 | 677.9 | 622.28 ± 64.8 | 626.4 | 558.86 ± 75.5 | 563.6 |
Note. PL = phospholipids; Tcol = total cholesterol; TG = triglycerides; TL = telomere length; TSL = total serum lipids.
Discussion
Only healthy subjects with no disease who have the least effect on the results of TL were selected. Therefore, the effect of aging and chronic diseases on TL was eliminated. Many studies have shown that shorter telomeres are correlated with age and chronic diseases like dyslipidemia (Aulinas et al., 2015), hypertension (Paik, Kang, Cho, & Shin, 2016), diabetes (Zhao et al., 2014), and smoking status (Courbon et al., 2016). The average age of the study population was 33.47 ± 4.25 years, and the significant reverse relationship was observed between aging and T/S (p = .029). Chance of telomeres shortening was increased with increasing age, BMI (p = .00) and weight (p = .00). TL is dynamic and constantly changing, its length can be changed in both directions during life (Hovatta et al., 2012). Longer TL was seen after a certain period of observation and intervention studies (Nordfjäll et al., 2009; Svenson et al., 2013). According to the study by Svenson et al. (2013), this reduction depends on the original length of TL. In most studies, TL is reduced by aging (Brümmendorf et al., 2001; Hohensinner et al., 2011; Robertson et al., 2000; Wolkowitz et al., 2017), but in the study of Strandberg et al., age was not related to TL (Strandberg et al., 2011). Moreover, obesity, BMI, and smoking had a direct relationship with shorter TL (Strandberg et al., 2011). Although using a wide range of age in telomere studies is common (Aviv, Valdes, & Spector, 2006), these studies may have been biased and reported TL wasn’t corrected. With longer age, cells obesity, inflammatory reactions, oxidative stress, and other affected risk factors on TL shortening, like smoking, are usually increased.
The relationship between BMI and TL is controversial; in some studies, no relationship was observed between the elderly (Bischoff et al., 2006; Fitzpatrick et al., 2007) and middle-aged people (Bekaert et al., 2007; Diaz et al., 2010), but in others, age was seen as an inverse relationship (Cui et al., 2013; Lee et al., 2011; Nordfjäll et al., 2008). The study of Gardner et al. (2005) shows that lower BMI was associated with longer TL in the middle-aged population. In the study of García-Calzón, Gea, et al. (2014), during the intervention period, reduction of BMI was significantly related with longer TL and also the basic amount of TL was increased. The relationship between BMI and TL in young people differs from that of older people, which can be attributed to the effect of age and chronic diseases. Specifically, the study of Lee et al. (2011) showed that average TL in obese patients <30 years was similar to that of the elderly with the age of 60 years. The study of Njajou et al. (2012) after 7 years of follow-up showed that longer TL was associated with a reduction of BMI and body fat percentage.
In this study, a significant inverse relationship was found between TL and TSL concentration and its components. The risk of TL shortening was significantly increased by TC, TG, FC, and TSL concentration. According to previous reports, the higher concentration of lipids was associated with TL shortening and atherosclerosis (O’Donnell et al., 2008; Okuda et al., 2002; Samani, Boultby, Butler, Thompson, & Goodall, 2001). Higher serum level of cholesterol may cause narrowing of arteries and reducing the blood supply to the heart, thereby resulting in heart attack, heart diseases, chest pain, and coronary artery disease (Law, Wald, & Rudnicka, 2003). In addition, cardiovascular diseases are associated with shorter TL (Brouilette et al., 2007; Fitzpatrick et al., 2007). In the study of Bentose et al., an inverse relationship was found between TL and fat parameters (TC and TG; Benetos et al., 2001). In other studies, the high serum levels of TGs and PLs are associated with obesity, atherosclerosis, and oxidative stress (Pan et al., 2004; The Emerging Risk Factors Collaboration, 2009). High concentration of blood and abdominal fat leads to increase systemic oxidative stress in cells, which is associated with lower antioxidant, the higher level of lipid-markers (Palmieri, Grattagliano, Portincasa, & Palasciano, 2006). Inflammatory markers are also increased, leading to release of cytokines like TNF-a and IL-6 (Fontana, Eagon, Trujillo, Scherer, & Klein, 2007; Pou et al., 2007). The atherogenic properties of TC and TG caused a pro-inflammatory environment, thereby leading to TL shorting (Epel, 2009). Higher blood cholesterol is associated with cellular damage, chronic subclinical inflammation, and cell replication leading to telomere shortening (Melamed et al., 1999). Usually, men are exposed to more stress than women. Chronic stress in adult men with a modern lifestyle is associated with increased obesity, bulimia, and metabolic syndrome (Chrousos, 2000). Moreover, the simultaneous increase in cortisol and insulin hormones causes visceral fat accumulation over time, and by several mechanisms leads to cell aging (Chrousos, 2000).
Higher serum lipids cause increase weight and obesity (Heilbronn et al., 2013). In a study of the effect of weight loss on the TL, it was suggested that weight loss prevented the shortening of telomere and DNA damage (Laimer et al., 2016) which is in line with the results of our study. According to the report of Kim et al. (2009), obesity may accelerate aging and cause telomere shortening. In several studies, the relationship between TL and obesity-related parameters has been investigated, and the results revealed that obesity increases cell damage and telomere shortening (Fitzpatrick et al., 2007; O’Donnell et al., 2008). In a cross-sectional study on 18- 76-year-old women, obesity and smoking were associated with shorter telomeres (Valdes et al., 2005). Obesity was also considered as a major factor in aging of adipose tissue and metabolic diseases, such as higher pro-inflammatory cytokines, insulin resistance, diabetes and cardiovascular diseases (Després & Lemieux, 2006; Minamino et al., 2009; Trayhurn & Wood, 2004). But this relationship was not seen in several other studies (Brouilette, Singh, Thompson, Goodall, & Samani, 2003; McGrath, Wong, Michaud, Hunter, & De Vivo, 2007).
In a report by Njajio et al. (2012), it was suggested that shorter TL may be a risk factor for obesity in the elderly. But in a cohort study by Bekaert et al., 2007, this relationship was not observed between men and women aged 35–55 years (Bekaert et al., 2007), and in another study, this relationship was only seen among women (Nordfjäll et al., 2008). In some studies, there was no relationship between telomeres and obesity; in the study of (Epel et al., 2006), an inverse relationship was found between obesity and telomerase activity (Epel et al., 2006). Activated p53 gene in fat tissue plays an important role in the aging of adipose tissue and increased inflammation and also plays an important role regarding the prevention and treatment of obesity and obesity-related aging (Minamino et al., 2009). In this regard, the result of the in vitro study showed that subcutaneous fatty cells TL in patients who were obese were lower than lean patients (Moreno-Navarrete, Ortega, Sabater, Ricart, & Fernández-Real, 2010). The harmful effects of obesity on TL can be created by different mechanisms (Epel, 2009). Exposure to oxidative stress and inflammation also accelerated the process of telomere damage (Houben, Moonen, van Schooten, & Hageman, 2008) and premature aging caused by stress. Obesity and increased concentration of serum lipids were associated with oxidative stress (Houben et al., 2008; Minamino et al., 2009). Nevertheless, behavioral factors like smoking (Valdes et al., 2005), physical activity (Du et al., 2012), socioeconomic status (Cherkas et al., 2006), and diet (Crous-Bou et al., 2014) are confounding factors in the TL. Smoking is known to be associated with systemic inflammation and oxidative stress (Houben et al., 2008; Valdes et al., 2005). It is probable that the high destruction of TL in smokers is part of an oxidant-induced senescence phenomenon (Karimi, Yunesian, Nabizadeh, Mehdipour, & Aghaie, 2017).
Considering the reduction of the TL in each cell division cycle, the study of a vast range of age can lead to improper TL estimation. Therefore, in this study, with the objective of fixing the problems of previous studies, the peoples’ age was selected in the range of 25–40 years. The method of measuring TL (qPCR rather than Southern-blot) and properties of the samples are another origin of conflicts in TL-related studies. Furthermore, the method of DNA extraction and quality of DNA extracted could also affect the results. The phenol-chloroform method usually a more damaging effect on the DNA than the salting-out method (Demeke & Jenkins, 2010). The benefits of this study include modifying problems of previous studies, limiting the age range, and removing the influence of other confounding factors.
Conclusions
In conclusion, the results of this study demonstrated that an inverse relationship was found between TL and weight, BMI, age, and TSL which were associated with obesity. High serum lipids concentration may be associated with systemic inflammation and atherosclerosis and may lead to oxidative stress, resulting in telomere shortening. It seems that the effect of serum lipids on TL is influenced by many factors (including age, smoking, disease status, living conditions, nutritional status). Further well-designed cohort studies with larger sample size could greatly contribute to the study results.
Supplemental Material
Supplemental material, supplementary_material_Table_ for Serum Level of Total Lipids and Telomere Length in the Male Population: A Cross-Sectional Study by Behrooz Karimi, Masud Yunesian, Ramin Nabizadeh and Parvin Mehdipour in American Journal of Men’s Health
Acknowledgments
The authors gratefully acknowledge the scientific and technical assistance provided by Dr. Azarnejad and Dr. Alizadeh.
Footnotes
Author Contributions: B.K. and M.Y. designed the study, participated in the data collection, and wrote the main manuscript. M.Y. and R.N. performed the statistical analysis and assisted in the data collection and approved the final version. P.M. participated in its design and helped to draft the manuscript and contributed to the raw data collection. All authors reviewed and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Ph.D. Programs Foundation of Public Health, Tehran University of Medical Sciences, Iran (Grant No. 9211150001). The protocol of this study has been approved by the Tehran University of Medical Sciences ethics committee with code IR.TUMS.REC.1395.2586. Before the study, the informed consent form was filled up by all participants. A complete description of the study, objectives, method of study, benefits and the final result are given to everyone in the study.
Supplemental Material: Supplemental material for this article is available online.
References
- Akins J. R., Waldrep K., Bernert J. T. (1989). The estimation of total serum lipids by a completely enzymatic ‘summation’ method. Clinica Chimica Acta, 184(3), 219–226. [DOI] [PubMed] [Google Scholar]
- Aulinas A., Ramírez M.-J., Barahona M.-J., Valassi E., Resmini E., Mato E., … Webb S. M. (2015). Dyslipidemia and chronic inflammation markers are correlated with telomere length shortening in Cushing’s syndrome. PLoS One, 10(3), e0120185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv A., Valdes A. M., Spector T. D. (2006). Human telomere biology: Pitfalls of moving from the laboratory to epidemiology. International Journal of Epidemiology, 35(6), 1424–1429. [DOI] [PubMed] [Google Scholar]
- Bekaert S., De Meyer T., Rietzschel E. R., De Buyzere M. L., De Bacquer D., Langlois M., … Van Oostveldt P. (2007). Telomere length and cardiovascular risk factors in a middle-aged population free of overt cardiovascular disease. Aging Cell, 6(5), 639–647. [DOI] [PubMed] [Google Scholar]
- Benetos A., Okuda K., Lajemi M., Kimura M., Thomas F., Skurnick J., … Aviv A. (2001). Telomere length as an indicator of biological aging: The gender effect and relation with pulse pressure and pulse wave velocity. Hypertension, 37(2), 381–385. [DOI] [PubMed] [Google Scholar]
- Bernert J. T., Turner W. E., Patterson D. G., Needham L. L. (2007). Calculation of serum “total lipid” concentrations for the adjustment of persistent organohalogen toxicant measurements in human samples. Chemosphere, 68(5), 824–831. [DOI] [PubMed] [Google Scholar]
- Bischoff C., Petersen H. C., Graakjaer J., Andersen-Ranberg K., Vaupel J. W., Bohr V. A., … Christensen K. (2006). No association between telomere length and survival among the elderly and oldest old. Epidemiology, 17(2), 190–194. [DOI] [PubMed] [Google Scholar]
- Blackburn E. H., Greider C. W., Szostak J. W. (2006). Telomeres and telomerase: The path from maize, Tetrahymena and yeast to human cancer and aging. Nature Medicine, 12(10), 1133–1138. [DOI] [PubMed] [Google Scholar]
- Brouilette S. W., Singh R. K., Thompson J. R., Goodall A. H., Samani N. J. (2003). White cell telomere length and risk of premature myocardial infarction. Arteriosclerosis, Thrombosis, and Vascular Biology, 23(5), 842–846. [DOI] [PubMed] [Google Scholar]
- Brouilette S. W., Moore J. S., McMahon A. D., Thompson J. R., Ford I., Shepherd J., … Samani N. J. (2007). Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland primary prevention study: A nested case-control study. The Lancet, 369(9556), 107–114. [DOI] [PubMed] [Google Scholar]
- Brümmendorf T. H., Rufer N., Holyoake T. L., Maciejewski J., Barnett M. J., Eaves C. J., … Lansdorp P. M. (2001). Telomere length dynamics in normal individuals and in patients with hematopoietic stem cell-associated disorders. Annals of the New York Academy of Sciences, 938(1), 293–304. [DOI] [PubMed] [Google Scholar]
- Cawthon R. M. (2002). Telomere measurement by quantitative PCR. Nucleic Acids Research, 30(10), e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherkas L. F., Aviv A., Valdes A. M., Hunkin J. L., Gardner J. P., Surdulescu G. L., … Spector T. D. (2006). The effects of social status on biological aging as measured by white-blood-cell telomere length. Aging Cell, 5(5), 361–365. [DOI] [PubMed] [Google Scholar]
- Chrousos G. P. (2000). The role of stress and the hypothalamic–pituitary–adrenal axis in the pathogenesis of the metabolic syndrome: Neuro-endocrine and target tissue-related causes. International Journal of Obesity, 24(S2), S50–S55. [DOI] [PubMed] [Google Scholar]
- Courbon D., Bizard E., Marcos E., Adnot S., Demoly P., Pin I., … Andujar P. (2016). Associations between smoking, telomere length and lung function decline: Findings from a population-based longitudinal study. Pneumologie, 4, 5. [DOI] [PubMed] [Google Scholar]
- Crous-Bou M., Fung T. T., Prescott J., Julin B., Du M., Sun Q., … De Vivo I. (2014). Mediterranean diet and telomere length in nurses’ health study: Population based cohort study. BMJ, 349, g6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y., Gao Y.-T., Cai Q., Qu S., Cai H., Li H.-L., … Zheng W. (2013). Associations of leukocyte telomere length with body anthropometric indices and weight change in Chinese women. Obesity, 21(12), 2582–2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeke T., Jenkins G. R. (2010). Influence of DNA extraction methods, PCR inhibitors and quantification methods on real-time PCR assay of biotechnology-derived traits. Analytical and Bioanalytical Chemistry, 396(6), 1977–1990. [DOI] [PubMed] [Google Scholar]
- Després J.-P., Lemieux I. (2006). Abdominal obesity and metabolic syndrome. Nature, 444(7121), 881–887. [DOI] [PubMed] [Google Scholar]
- Diaz V. A., Mainous A. G., Player M. S., Everett C. J. (2010). Telomere length and adiposity in a racially diverse sample. International Journal of Obesity, 34(2), 261–265. [DOI] [PubMed] [Google Scholar]
- Du M., Prescott J., Kraft P., Han J., Giovannucci E., Hankinson S. E., De Vivo I. (2012). Physical activity, sedentary behavior, and leukocyte telomere length in women. American Journal of Epidemiology, 175(5), 414–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel E. S. (2009). Psychological and metabolic stress: A recipe for accelerated cellular aging. Hormones (Athens), 8(1), 7–22. [DOI] [PubMed] [Google Scholar]
- Epel E. S., Lin J., Wilhelm F. H., Wolkowitz O. M., Cawthon R., Adler N. E., … Blackburn E. H. (2006). Cell aging in relation to stress arousal and cardiovascular disease risk factors. Psychoneuroendocrinology, 31(3), 277–287. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick A. L., Kronmal R. A., Gardner J. P., Psaty B. M., Jenny N. S., Tracy R. P., … Aviv A. (2007). Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. American Journal of Epidemiology, 165(1), 14–21. [DOI] [PubMed] [Google Scholar]
- Fontana L., Eagon J. C., Trujillo M. E., Scherer P. E., Klein S. (2007). Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes, 56(4), 1010–1013. [DOI] [PubMed] [Google Scholar]
- García-Calzón S., Gea A., Razquin C., Corella D., Lamuela-Raventós R. M., Martínez J. A., … Marti A. (2014). Longitudinal association of telomere length and obesity indices in an intervention study with a Mediterranean diet: The PREDIMED-NAVARRA trial. International Journal of Obesity, 38(2), 177–182. [DOI] [PubMed] [Google Scholar]
- García-Calzón S., Moleres A., Marcos A., Campoy C., Moreno L. A., Azcona-Sanjulián M. C., … Marti A. (2014). Telomere length as a biomarker for adiposity changes after a multidisciplinary intervention in overweight/obese adolescents: The EVASYON study. PLoS One, 9(2), e89828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner J. P., Li S., Srinivasan S. R., Chen W., Kimura M., Lu X., … Aviv A. (2005). Rise in insulin resistance is associated with escalated telomere attrition. Circulation, 111(17), 2171–2177. [DOI] [PubMed] [Google Scholar]
- Hahn W. C. (2003). Role of telomeres and telomerase in the pathogenesis of human cancer. Journal of Clinical Oncology, 21(10), 2034–2043. [DOI] [PubMed] [Google Scholar]
- Harte A. L., da Silva N. F., Miller M. A., Cappuccio F. P., Kelly A., O’Hare J. P., … McTernan P. G. (2012). Telomere length attrition, a marker of biological senescence, is inversely correlated with triglycerides and cholesterol in South Asian males with type 2 diabetes mellitus. Experimental Diabetes Research, 2012, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronn L. K., Coster A. C. F., Campbell L. V., Greenfield J. R., Lange K., Christopher M. J., … Samocha-Bonet D. (2013). The effect of short-term overfeeding on serum lipids in healthy humans. Obesity, 21(12), E649–E659. [DOI] [PubMed] [Google Scholar]
- Hohensinner P. J., Goronzy J. J., Weyand C. M. (2011). Telomere dysfunction, autoimmunity and aging. Aging and Disease, 2(6), 524–537. [PMC free article] [PubMed] [Google Scholar]
- Houben J. M. J., Moonen H. J. J., van Schooten F. J., Hageman G. J. (2008). Telomere length assessment: Biomarker of chronic oxidative stress? Free Radical Biology and Medicine, 44(3), 235–246. [DOI] [PubMed] [Google Scholar]
- Hovatta I., de Mello V. D. F., Kananen L., Lindström J., Eriksson J. G., Ilanne-Parikka P., … Uusitupa M. (2012). Leukocyte telomere length in the Finnish diabetes prevention study. PLoS One, 7(4), e34948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshu C. E., Peskoe S. B., Heaphy C. M., Kenfield S. A., Van Blarigan E. L., Mucci L. A., … Platz E. A. (2015). Prediagnostic obesity and physical inactivity are associated with shorter telomere length in prostate stromal cells. Cancer Prevention Research, 8(8), 737–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi B., Nabizadeh R., Yunesian M., Mehdipour P., Rastkari N., Aghaie A. (2018). Foods, dietary patterns and occupational class and leukocyte telomere length in the male population. American Journal of Men’s Health, 12(2), 479–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi B., Yunesian M., Nabizadeh R., Mehdipour P., Aghaie A. (2017). Is leukocyte telomere length related with lung cancer risk?: A meta-analysis. Iranian Biomedical Journal, 21(3), 142–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Parks C. G., DeRoo L. A., Chen H., Taylor J. A., Cawthon R. M., Sandler D. P. (2009). Obesity and weight gain in adulthood and telomere length. Cancer Epidemiology, Biomarkers & Prevention, 18(3), 816–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laimer M., Melmer A., Lamina C., Raschenberger J., Adamovski P., Engl J., … Ebenbichler C. (2016). Telomere length increase after weight loss induced by bariatric surgery: Results from a 10 year prospective study. International Journal of Obesity, 40(5), 773–778. [DOI] [PubMed] [Google Scholar]
- Law M. R., Wald N. J., Rudnicka A. R. (2003). Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: Systematic review and meta-analysis. BMJ, 326(7404), 1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M., Martin H., Firpo M. A., Demerath E. W. (2011). Inverse association between adiposity and telomere length: The Fels Longitudinal Study. American Journal of Human Biology, 23(1), 100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath M., Wong J. Y. Y., Michaud D., Hunter D. J., De Vivo I. (2007). Telomere length, cigarette smoking, and bladder cancer risk in men and women. Cancer Epidemiology, Biomarkers & Prevention, 16(4), 815–819. [DOI] [PubMed] [Google Scholar]
- Melamed S., Ugarten U., Shirom A., Kahana L., Lerman Y., Froom P. (1999). Chronic burnout, somatic arousal and elevated salivary cortisol levels. Journal of Psychosomatic Research, 46(6), 591–598. [DOI] [PubMed] [Google Scholar]
- Minamino T., Orimo M., Shimizu I., Kunieda T., Yokoyama M., Ito T., … Komuro I. (2009). A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nature Medicine, 15(9), 1082–1087. [DOI] [PubMed] [Google Scholar]
- Moreno-Navarrete J. M., Ortega F., Sabater M., Ricart W., Fernández-Real J. M. (2010). Telomere length of subcutaneous adipose tissue cells is shorter in obese and formerly obese subjects. International Journal of Obesity, 34(8), 1345–1348. [DOI] [PubMed] [Google Scholar]
- Njajou O. T., Cawthon R. M., Blackburn E. H., Harris T. B., Li R., Sanders J. L., … Hsueh W.-C. (2012). Shorter telomeres are associated with obesity and weight gain in the elderly. International Journal of Obesity, 36(9), 1176–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordfjäll K., Eliasson M., Stegmayr B., Melander O., Nilsson P., Roos G. (2008). Telomere length is associated with obesity parameters but with a gender difference. Obesity, 16(12), 2682–2689. [DOI] [PubMed] [Google Scholar]
- Nordfjäll K., Svenson U., Norrback K.-F., Adolfsson R., Lenner P., Roos G. (2009). The individual blood cell telomere attrition rate is telomere length dependent. PLoS Genetics, 5(2), e1000375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell C. J., Demissie S., Kimura M., Levy D., Gardner J. P., White C., … Aviv A. (2008). Leukocyte telomere length and carotid artery intimal medial thickness: The Framingham Heart Study. Arteriosclerosis, Thrombosis, and Vascular Biology, 28(6), 1165–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda K., Bardeguez A., Gardner J. P., Rodriguez P., Ganesh V., Kimura M., … Aviv A. (2002). Telomere length in the newborn. Pediatric Research, 52(3), 377–381. [DOI] [PubMed] [Google Scholar]
- Paik J. K., Kang R., Cho Y., Shin M.-J. (2016). Association between genetic variations affecting mean telomere length and the prevalence of hypertension and coronary heart disease in Koreans. Clinical Nutrition Research, 5(4), 249–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri V. O., Grattagliano I., Portincasa P., Palasciano G. (2006). Systemic oxidative alterations are associated with visceral adiposity and liver steatosis in patients with metabolic syndrome. The Journal of Nutrition, 136(12), 3022–3026. [DOI] [PubMed] [Google Scholar]
- Pan M., Cederbaum A. I., Zhang Y.-L., Ginsberg H. N., Williams K. J., Fisher E. A. (2004). Lipid peroxidation and oxidant stress regulate hepatic apolipoprotein B degradation and VLDL production. The Journal of Clinical Investigation, 113(9), 1277–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pou K. M., Massaro J. M., Hoffmann U., Vasan R. S., Maurovich-Horvat P., Larson M. G., … Fox C. S. (2007). Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress. Circulation, 116(11), 1234–1241. [DOI] [PubMed] [Google Scholar]
- Rankin J. W., Andreae M. C., Oliver Chen C.-Y., O’Keefe S. F. (2008). Effect of raisin consumption on oxidative stress and inflammation in obesity. Diabetes, Obesity and Metabolism, 10(11), 1086–1096. [DOI] [PubMed] [Google Scholar]
- Rehkopf D. H., Needham B. L., Lin J., Blackburn E. H., Zota A. R., Wojcicki J. M., Epel E. S. (2016). Leukocyte telomere length in relation to 17 biomarkers of cardiovascular disease risk: A cross-sectional study of US adults. PLoS Medicine, 13(11), e1002188. doi: 10.1371/journal.pmed.1002188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J. D., Gale R. E., Wynn R. F., Dougal M., Linch D. C., Testa N. G., Chopra R. (2000). Dynamics of telomere shortening in neutrophils and T lymphocytes during ageing and the relationship to skewed X chromosome inactivation patterns. British Journal of Haematology, 109(2), 272–279. [DOI] [PubMed] [Google Scholar]
- Samani N. J., Boultby R., Butler R., Thompson J. R., Goodall A. H. (2001). Telomere shortening in atherosclerosis. The Lancet, 358(9280), 472–473. [DOI] [PubMed] [Google Scholar]
- Strandberg T. E., Saijonmaa O., Tilvis R. S., Pitkälä K. H., Strandberg A. Y., Miettinen T. A., Fyhrquist F. (2011). Association of telomere length in older men with mortality and midlife body mass index and smoking. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences, 66A(7), 815–820. [DOI] [PubMed] [Google Scholar]
- Svenson U., Grönlund E., Söderström I., Sitaram R. T., Ljungberg B., Roos G. (2013). Telomere length in relation to immunological parameters in patients with renal cell carcinoma. PLoS One, 8(2), e55543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Emerging Risk Factors Collaboration. (2009). Major lipids, apolipoproteins, and risk of vascular disease. JAMA, 302(18), 1993–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trayhurn P., Wood I. S. (2004). Adipokines: Inflammation and the pleiotropic role of white adipose tissue. British Journal of Nutrition, 92(3), 347–355. [DOI] [PubMed] [Google Scholar]
- Valdes A. M., Andrew T., Gardner J. P., Kimura M., Oelsner E., Cherkas L. F., … Spector T. D. (2005). Obesity, cigarette smoking, and telomere length in women. The Lancet, 366(9486), 662–664. [DOI] [PubMed] [Google Scholar]
- Wolkowitz O. M., Jeste D. V., Martin A. S., Lin J., Daly R. E., Reuter C., Kraemer H. (2017). Leukocyte telomere length: Effects of schizophrenia, age, and gender. Journal of Psychiatric Research, 85, 42–48. [DOI] [PubMed] [Google Scholar]
- Zhan Y., Karlsson I. K., Karlsson R., Tillander A., Reynolds C. A., Pedersen N. L., Hägg S. (2017). Exploring the causal pathway from telomere length to coronary heart disease: A network Mendelian randomization study. Circulation Research, 121(3), 214–219. doi: 10.1161/circresaha.116.310517 [DOI] [PubMed] [Google Scholar]
- Zhao J., Zhu Y., Lin J., Matsuguchi T., Blackburn E., Zhang Y., … Howard B. V. (2014). Short leukocyte telomere length predicts risk of diabetes in American Indians: The strong heart family study. Diabetes, 63(1), 354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, supplementary_material_Table_ for Serum Level of Total Lipids and Telomere Length in the Male Population: A Cross-Sectional Study by Behrooz Karimi, Masud Yunesian, Ramin Nabizadeh and Parvin Mehdipour in American Journal of Men’s Health