Abstract
We report a case of a six-year-old boy who presented after a cardiac arrest, likely due to a pulmonary hypertensive crisis in the setting of vitamin C deficiency. After initially presenting with subacute multifocal bone lesions of unknown etiology, he experienced a pulseless electrical activity cardiac arrest while undergoing a diagnostic procedure under sedation. During his post-arrest convalescence, he developed persistent tachycardia and peripheral edema. An echocardiogram revealed findings consistent with significant pulmonary arterial hypertension, which was found to be responsive to inhaled nitric oxide. Laboratory investigation revealed undetectable levels of vitamin C, resulting in disclosure of a history of severe restrictive eating behavior. With ascorbate supplementation, the patient’s pulmonary vasodilators were weaned and discontinued. Given his complete recovery, we suspect that the cardiac arrest and pulmonary hypertension were the consequence of a rare, but reversible, complication of scurvy.
Keywords: Vitamin C, ascorbic acid, nutritional deficiency, disordered eating
Case report
The patient was a six-year-old boy undergoing evaluation for a three-month history of bilateral lower extremity pain and refusal to bear weight. His extensive multidisciplinary work-up was significant only for multifocal areas of bone marrow edema throughout his extremities, pelvis, and vertebrae on magnetic resonance imaging (MRI). Given his continued symptoms, he was scheduled to undergo a bone marrow aspiration and bone biopsy. He was sedated with midazolam and propofol, his airway was secured via laryngeal mask, and he appeared to tolerate the bone marrow aspiration from his posterior iliac crest without any difficulty. However, before proceeding with the bone biopsy, he became acutely tachycardic, hypotensive, and pulseless. He was turned supine, endotracheally intubated, and received ∼30 s of chest compressions, ∼30 mL/kg crystalloid bolus, and phenylephrine and epinephrine boluses. He had return of spontaneous circulation and was started on a dopamine infusion, with stable blood pressures.
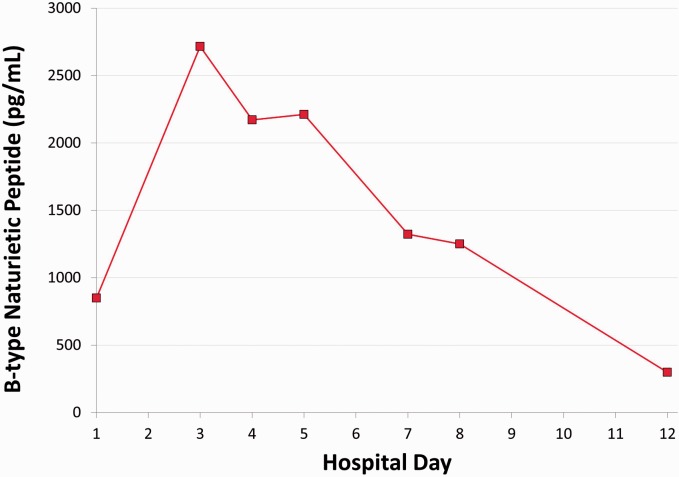
His immediate laboratory evaluation in the PICU was unremarkable with the exception of an elevated B-type natriuretic peptide (BNP; 850 pg/mL; see Fig. 1). The initial post-arrest echocardiogram, performed while the patient was on a dopamine infusion and shortly following epinephrine and phenylephrine boluses, demonstrated tachycardia (138 bpm) with hyperdynamic left ventricular (LV) systolic function (shortening fraction = 61%). Imaging of the right ventricle (RV) was not optimized on this initial study. While mild RV dilation was suspected based on the two-dimensional (2D) imaging, the M-mode quantification of the RV dimension was within normal range (RV/LV diameter ratio is not part of the institution’s standard pediatric echo protocol). There was insufficient tricuspid regurgitation (TR) for estimation of RV pressures, but mild septal flattening was suggested. No assessment of RV function was reported at the time of the initial study. A later, retrospective review of this echocardiogram demonstrated an abnormal RV/LV diameter ratio of 1.7, above the ratio of 1 that has been associated with increased adverse events in pediatric pulmonary hypertension (PH).1 A LV eccentricity index (LVEI) was also abnormal at 1.7; LVEI > 1 in adult PH suggests impaired RV function.2 The pulmonary artery acceleration time (PAAT) in the post-arrest echo showed an abnormal value of 90 ms (normal ≥ 100 ms).
Fig. 1.
BNP trend throughout the course of hospitalization. Diagnosis PH occurred at HD5, after which iNO and milrinone were initiated. Vitamin C supplementation was initiated on HD10.
The patient was weaned off inotropic infusions and extubated later on hospital day 1 (HD1). He remained tachycardic, received additional crystalloid boluses, and was noted to have a further increase in BNP on HD3 (2716 pg/mL). He developed poor urine output and peripheral edema and continued to exhibit an elevated BNP (2172 pg/mL) prompting a repeat echocardiogram on HD5.
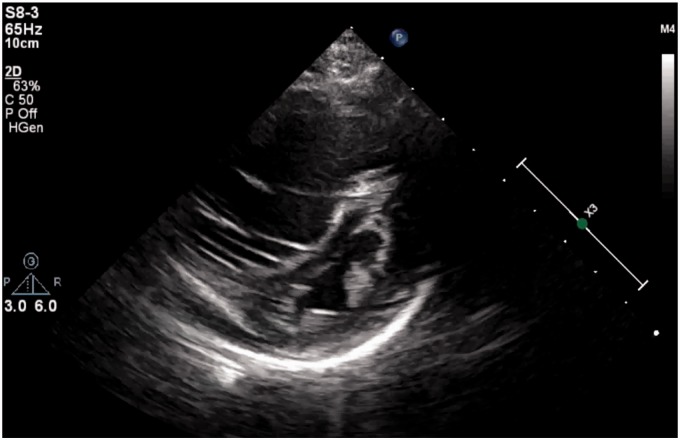
The patient’s HD 5 echocardiogram revealed evidence of significant RV dysfunction with both right atrial and ventricular dilation and prominent septal bowing (see Fig. 2). There was now increased tricuspid regurgitation with a peak TR jet of 68 mmHg (excluding right atrial [RA] pressure) suggesting three-fourths systemic RV pressures (systemic blood pressure of 108/68 mmHg). Concern for PH prompted a retrospective review of the original post-arrest echocardiogram and a more detailed assessment of the RV function. The RV/LV diameter ratio had increased to 2.8, while the LVEI had also increased to 2.85. The PAAT was inadequately assessed on the HD5 study, in part because the Doppler flow pattern in the pulmonary artery was quite low at < 50 cm/s (consistent with low RV output). Tricuspid annular plane systolic excursion (TAPSE) was not evaluated on either study.
Fig. 2.
Echocardiogram on HD5. Frame from parasternal short-axis view on HD5, significant for severely dilated RV and bowing of the intraventricular septum into the LV. Clips can be found in Supplemental Materials.
He was started on a milrinone infusion at 0.5 mcg/kg/min and inhaled nitric oxide (iNO) at 20 parts per million. Over the next 48–72 h, his symptoms and echocardiographic findings improved, most notably with improvement in the RV/LV diameter ratio to 1.5 and LVEI to 1.56 as well as near resolution of tricuspid regurgitation. Computed tomography (CT) arteriogram of the chest, abdomen, and pelvis did not find evidence of pulmonary embolus or vasculitis. Cardiac catheterization on HD9 confirmed mild pulmonary arterial hypertension (Table 1) on milrinone and iNO, consistent with his improving BNP and echocardiographic findings.
Table 1.
Data from cardiac catheterization (on iNO 20, FiO2 21%, milrinone 0.5 mcg/kg/min).
| Right ventricle (mmHg) | 21/4 |
| Left PA (mmHg) | 21/7 (mean 12) |
| Right PA (mmHg) | 24/6 (mean 13) |
| RPA wedge (mean, mmHg) | 4 |
| Rp (Wood units × m2) | 3.5 |
| Qp (L/min/m2) | 3.5 |
Post-catheterization extubation was delayed until HD10 due to significant gingival bleeding at the right maxillary alveolus. Coincidentally, it was at this time that the patient’s vitamin C and vitamin A testing returned at undetectable levels. A subsequent detailed review of his diet history revealed an underappreciated restrictive eating behavior. His daily caloric and protein intake was adequate for age, but his diet lacked fruits, vegetables, vitamins, or fortified foods, resulting in multiple vitamin deficiencies (Table 2). He was started on vitamin C (100 mg, IV, three times daily) and vitamin A supplementation (10,000 U daily). He was also started on sildenafil and subsequently weaned off iNO and milrinone.
Table 2.
Nutrient Levels.
| Laboratory test | Patient value | Normal range |
|---|---|---|
| Vitamin B3 (Niacin, mcg/mL) | 1.47 | 0.5–8.91 |
| Vitamin A (Retinol, mcg/dL) | <20 | 38–106 |
| Vitamin B1 (Thiamine, nmol/L) | 20 | 8–30 |
| Vitamin B12 (pg/mL) | 192 | 180–914 |
| Vitamin B6 (Pyridoxal-5-Phosphate, nmol/L) | 11.9 | 20.0–125.0 |
| Vitamin C (micromol/L) | <5 | 23–114 |
| 25-OH-vitamin D (ng/mL) | 36 | 30–96 |
| Vitamin E (alpha-tocopherol, mg/L) | 7.6 | 2.4–20 |
| Vitamin E (gamma-tocopherol, mg/L) | <2.0 | <4.7 |
| Zinc (mcg/dL) | 60 | 48–129 |
By HD12, the patient’s BNP had normalized and his echo parameters had nearly normalized as well (LV/RV diameter ratio of 0.9, LVEI 1.11–1.18, PAAT 140 ms, TR jet 19 mmHg, mild septal flattening but qualitatively normal RV function). He was later transferred to an inpatient rehabilitation program before being discharged home on sildenafil with follow-up by a pediatric cardiologist and PH specialist. His echocardiogram at six months demonstrated no evidence of PH, and he was weaned off sildenafil. At one year, he remains asymptomatic with a reassuring echocardiogram.
Discussion
Pediatric PH is most commonly associated with cardiac and pulmonary diseases. Among the less common causes, including various hematologic, hepatic, metabolic, oncologic, genetic, and rheumatologic disorders,3 the presentation of PH in the setting of vitamin deficiencies is exceedingly rare, with reports of associations with deficiencies in vitamin D,4,5 thiamine,6 and vitamin C.7–9
To our knowledge, an association between PH and vitamin C deficiency has been described previously in three case reports. In the first two cases, adult patients presented with PH in the setting of vitamin C deficiency and additional deficiencies in thiamine and iron.7,8 In the most recent report, a nine-year-old with severely restricted eating behavior presented similarly to our patient and was found to have deficiencies in vitamin C, thiamine, vitamin B6, and vitamin D.9 Although the link between vitamin C and PH was obscured in these previous reports by coexisting vitamin deficiencies that are also associated with PH,4–6 we posit that the overlap of deficiencies from all four cases now more specifically implicates a relationship between vitamin C metabolism and PH.
Vitamin C (ascorbate) is most commonly found in citrus fruits, vegetables, and breastmilk/formula. It is completely absorbed by the GI tract, with no ability for storage in the body.10 In pediatrics, deficiencies can occur as quickly as over 8–12 weeks,11 primarily in the setting of dietary deficiency (e.g. disordered eating behavior, ketogenic diet, boiled milk for infants) or states of iron overload (e.g. multiple RBC transfusions). There are many potential mechanisms by which ascorbate deficiency could affect pulmonary vasculature. The classic scurvy presentation with bony abnormalities and mucosal breakdown is due to ascorbate’s role in collagen hydroxylation and cross-linking in connective tissues,7 but the contribution of this dysfunctional collagen to pulmonary vascular resistance is not yet defined. Ascorbate is also a co-factor for enzymes that are oxygen sensors for HIF activation,13 has anti-oxidant properties,7 and may play a role in endothelial cell NO production.14,15 We do provide here the first reported trial of inhaled NO for scurvy-associated PH; in only one of the aforementioned cases did the treatment team provide therapy targeted at PH (i.e. sildenafil) which was also noted to have clinical benefit.8
The presentation of PH with other unexplained systemic symptoms should raise flags for a possible nutritional deficiency. While malnutrition-induced PH is likely a rare finding, vitamin repletion may reverse the condition, underscoring the importance of a prompt diagnosis. It is unknown whether unrecognized mild deficiencies in vitamin C may further exacerbate elevated pulmonary vascular resistance in either primary PH or other PH-associated diseases (e.g. chronic thromboembolic diseases, sleep-disordered breathing). We are hopeful that future investigations into the link between vitamin C and PH may provide mechanistic insights and therapeutic strategies to the condition.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Hythem Nawaytou of the UCSF Benioff Children's Hospital Pulmonary Hypertension Program for his expertise and assistance with revising this manuscript. The authors would also like to thank Susan Bessler, PICU clinical dietician at UCSF Benioff Children's Hospital Oakland, for her expertise.
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Supplemental Materials
Supplemental video 1. Echocardiogram on HD5 (parasternal short-axis view). Supplemental video 2. Echocardiogram on HD5 (parasternal long-axis view). Supplemental video 3. Echocardiogram on HD5 (apical four-chamber view).
References
- 1.Jone P-N, Hinzman J, Wagner BD, et al. Right ventricular to left ventricular diameter ratio at end-systole in evaluating outcomes in children with pulmonary hypertension. J Am Soc Echocardiogr 2014; 27(2): 172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koestenberger M, Friedberg MK, Nestaas E, et al. Transthoracic echocardiography in the evaluation of pediatric pulmonary hypertension and ventricular dysfunction. Pulm Circ 2016; 6(1): 15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abman SH, Hansmann G, Archer SL, et al. Pediatric pulmonary hypertension: guidelines from the American Heart Association and the American Thoracic Society. Circulation 2015; 132: 2037–2099. [DOI] [PubMed] [Google Scholar]
- 4.Mirdamadi A, Moshkdar P. Benefits from the correction of vitamin D deficiency in patients with pulmonary hypertension. Caspian J Intern Med 2016; 7(4): 253–259. [PMC free article] [PubMed] [Google Scholar]
- 5.Demir M, Uyan U, Keceoclu S, et al. The relationship between vitamin D deficiency and pulmonary hypertension. Prague Med Rep 2013; 114(3): 154–161. [DOI] [PubMed] [Google Scholar]
- 6.Park JH, Lee JH, Jeong JO, et al. Thiamine deficiency as a rare cause of reversible severe pulmonary hypertension. Int J Cardiol 2007; 121(1): e1–3. [DOI] [PubMed] [Google Scholar]
- 7.Mertens MT, Gertner E. Rheumatic manifestations of scurvy: a report of three recent cases in a major urban center and a review. Semin Arthritis Rheum 2011; 41(2): 286–290. [DOI] [PubMed] [Google Scholar]
- 8.Kupari M, Rapola J. Reversible pulmonary hypertension associated with vitamin C deficiency. Chest 2012; 142(1): 225–227. [DOI] [PubMed] [Google Scholar]
- 9.Duvall MG, Pikman Y, Kantor DB, et al. Pulmonary hypertension associated with scurvy and vitamin deficiencies in an autistic child. Pediatrics 2013; 132: e1699–e1703. [DOI] [PubMed] [Google Scholar]
- 10.Levine M, Rumsey SC, Daruwala R. Criteria and recommendations for vitamin C intake. JAMA 1999; 281(15): 1415–1423. [DOI] [PubMed] [Google Scholar]
- 11.Agarwal A, Shaharyar A, Kumar A, et al. Scurvy in pediatric age group - a disease often forgotten? J Clin Orthop Trauma 2015; 6(2): 101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinstein M, Vavyn P, Zlotkin S. An orange a day keeps the doctor away: scurvy in the year 2000. Pediatrics 2001; 108(3): E55. [DOI] [PubMed] [Google Scholar]
- 13.Kuiper C, Dachs GU, Currie MJ, et al. Intracellular ascorbate enhances hypoxia inducible factor (HIF)-hydroxylase activity and preferentially suppresses the HIF-1 transcriptional response. Free Radic Biol Med 2014; 69: 308–317. [DOI] [PubMed] [Google Scholar]
- 14.Heller B, Unbehaun A, Schellenberg B, et al. L-ascorbic acid potentiates endothelial nitric oxide synthesis via a chemical stabilization of tetrahydrobiopterin. J Biol Chem 2001; 276(1): 40–47. [DOI] [PubMed] [Google Scholar]
- 15.Holowatz LA. Ascorbic acid: what do we really NO? J App Physiol 2011; 11(6): 1542–1543. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.