Abstract
BACKGROUND
While oncologists are aware that cancer treatments may impact fertility, referral rates for fertility preservation consultation (FPC) remain poor. The goal of this study was to identify predictors associated with FPC referral.
METHODS
This is a retrospective, cohort study of women aged 18–42 years diagnosed with a new breast, gynecologic, hematologic or gastrointestinal cancer at our institution between January 2008 and May 2010. Exclusion criteria included history of permanent sterilization, documentation of no desire for future children, stage IV disease, short interval (<4 days) between diagnosis and treatment and treatment that posed no threat to fertility. Demographic, socioeconomic and cancer variables were evaluated with respect to FPC. Logistic regression was used to determine the odds of referral for FPC based on specified predictors.
RESULTS
One hundred and ninety-nine patients were eligible for FPC and of those, 41 received FPC (20.6%). Women with breast cancer were 10 times more likely to receive FPC compared with other cancer diagnoses [odds ratio (OR) 10.1; 95% confidence interval (CI) 3.8–26.8]. The odds of FPC referral were approximately two times higher for Caucasian women (OR 2.4; 95% CI 0.9–6.2), three times higher for age <35 years (OR 3.3; 95% CI 1.4–7.7) and four times higher in nulliparous women (OR 4.6; 95% CI 1.9–11.3). There was no association between BMI, income, distance to our institution, being in a relationship and referral for FPC.
CONCLUSIONS
Overall referral rates for FPC are low, and there appear to be significant discrepancies in referral based on ethnicity, age, parity and cancer type. This highlights a need for further provider education and awareness across all oncologic disciplines.
Keywords: fertility preservation, health disparities, cancer
Introduction
In 2008, there were ∼450 000 reproductive-aged cancer survivors in the USA, and 52 000 women under the age of 40 being diagnosed each year (Jemal et al., 2010). In an era of rapidly expanding medical knowledge leading to increased cancer survival rates, there has been amplified emphasis on post-treatment quality of life. Many cancer treatments pose a threat to future fertility, which has been shown to be a significant concern of cancer survivors, as many people feel the ability to have biological children is of great importance to their quality of life (Leiblum et al., 1998; Bryson et al., 2000; Forman et al., 2010). While many patients may be finished with childbearing at the time of diagnosis, there is an increasing body of evidence that suggests information regarding fertility preservation (FP) is desirable to many cancer survivors (Schover, 2005).
These concerns and advances in assisted reproductive technologies (ART) have led to the emerging field of FP, allowing patients to preserve reproductive options before gonadotoxic cancer therapies. Embryo cryopreservation is an established method of using in vitro fertilization (IVF) methods to acquire and fertilize mature oocytes prior to gonadotoxic treatment and freeze them for future implantation. Other experimental options, such as oocyte cryopreservation, ovarian tissue cryopreservation and ovarian suppression with gonadotrophin-releasing hormone agonists have had variable success (Practice Committee of American Society for Reproductive Medicine, 2008). Conservative gynecological surgery, such as trachelectomy in early stage cervical cancer and unilateral oophorectomy in early stage ovarian cancer is another option (Lee et al., 2006).
Recognizing the importance of fertility to cancer survivors, the American Society of Clinical Oncology (ASCO) and the American Society of Reproductive Medicine (ASRM) developed guidelines for oncology professionals (Lee et al., 2006). These guidelines recommend that discussion of treatment-related infertility, basic FP options and referral of appropriate and interested patients to a reproductive specialist for FP consultation (FPC) should occur prior to cancer treatment. The guidelines stress that this should be done at the earliest possible juncture, using clinical judgment (Lee et al., 2006). Even if patients do not pursue FP treatment, studies have suggested that discussion alone may allow patients to make a more educated decision, mourn the loss of fertility and potentially cope better with treatment-related infertility in the future (NIH, 2004; Carter et al., 2005).
Despite the recognized benefits of discussion and referral, in one survey, less than half of cancer patients of reproductive-age recall fertility being discussed during consultation or treatment (Schover et al., 1999). In addition, of those that do recall discussions, many were unsatisfied with the quality and amount of information provided (Schover et al., 1999; Duffy et al., 2005; Peate et al., 2009). To our knowledge, this is the first study of its kind designed to investigate trends of FPC referral patterns by evaluating demographic, socioeconomic and cancer-specific predictive factors associated with FPC.
Materials and Methods
This is a retrospective cohort study designed to assess variables that predict which reproductive-age cancer patients are referred for FPC. The University of North Carolina (UNC) Healthcare Cancer Registry was queried for all women between the ages of 18–42 years diagnosed with cancer between January 2008 and May 2010. The women that were diagnosed with the types of cancer that are most associated with fertility-threatening treatments were further reviewed. These were determined by evaluating which specific cancer types are associated with a high likelihood of systemic gonadotoxic chemotherapy, pelvic radiation or surgery affecting gynecologic organs. The gonadotoxic profile of cancer treatments was determined by published data and fertilehope.org (Fertilehope.org; Sklar, 2005; Oktem and Oktay, 2007). Specifically, we restricted enrollment to women with a new diagnosis of gynecologic (including ovarian, fallopian tube, endometrial, cervical, vaginal, vulvar, choriocarcinoma and primary peritoneal cancers), breast, gastrointestinal or hematologic cancer. Patients with non-gynecological sarcomas would have been included in the study due to the high likelihood of these patients requiring systemic gonadotoxic chemotherapy; however, there were no patients that fit criteria during the study period with this diagnosis. Patients with brain, head and neck, bone, connective tissue, lung, kidney, skin, thyroid and unknown primary cancers were excluded due to low likelihood of requiring gynecologic surgery, pelvic radiation or gonadotoxic systemic therapy. These were excluded in order to conservatively select patients who would benefit from FPC. This study was approved by the UNC institutional review board and it was deemed that consent was not needed.
Exclusion criteria included history of permanent sterilization, documentation of no desire for future children, stage IV disease, short interval (<4 days) between diagnosis and treatment and treatment that posed no threat to fertility. All patients who were included for this study either had documentation that they desired future fertility, or had no documentation of a discussion about fertility plans at all.
Electronic medical records were searched for demographics, socioeconomic and cancer variables, including age, ethnicity, BMI, relationship status, parity, average income based on zip code [acquired by public income tax records (2011)], distance from institution, insurance status (assessed at time of diagnosis), cancer stage, treatment type and timeline.
The women who were referred for and underwent FPC with the Reproductive Endocrinology and Infertility department at UNC were compared with those who did not receive FPC. Those who underwent FPC met with one of two board-certified reproductive endocrinologists who used a standardized FP template. Possible psychological implications of losing fertility and/or fertility after a cancer diagnosis were discussed, as well as FP options offered at our institution, including ovarian suppression during treatment, emergency IVF cycle with either oocyte or embryo cryopreservation, ovarian tissue cryopreservation, conservative surgical treatment for early stage ovarian or cervical gynecologic malignancies, surrogacy and oocyte donation.
Univariate analysis was performed to determine prognostic variables for the primary end-point, FPC, using t-tests and χ2 analysis when appropriate. A value of P < 0.05 was considered statistically significant. We created a multivariable logistic regression model using all variables that were significantly associated with FPC using univariate analysis at P < 0.1. Analysis was performed using STATA statistical software (version 11.0, College Station, TX, USA).
Results
A total of 806 reproductive-age women were identified from the UNC Healthcare Cancer Registry. Of these, 353 were diagnosed with a new primary gynecologic, breast, gastrointestinal or hematologic cancer, and a further 154 of those were excluded based on criteria, leaving 199 for inclusion in the study.
Of the 199 eligible women included, 41 (20.6%) underwent FPC (Fig. 1). Demographics including age, ethnicity, BMI, parity, relationship and insurance status of those included are outlined in Table I. Every patient who had a documented desire for future fertility in the oncologic medical record received an FPC. The average age of those receiving FPC was significantly younger than for those who did not (31.5 ± 6.9 versus 34.0 ± 6.3 years; P = 0.025). Over 57% of our eligible study population was Caucasian, 25.6% African American, 9.5% Hispanic and 7.5% other including Asian and Pacific Islander. A higher percentage of FPC eligible Caucasian women received FPC compared with eligible African American, Hispanic or Asian women (26.3, 17.6, 0, 13.3% respectively; P = 0.021). No eligible Hispanic women received FPC (Table I).
Figure 1.
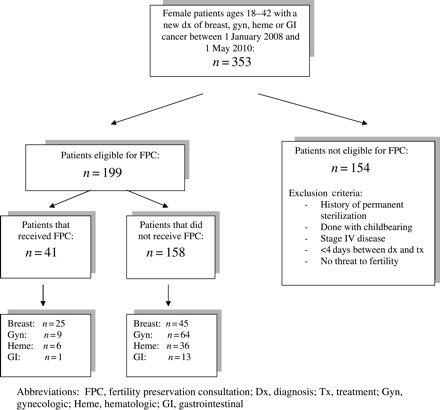
Flow diagram outlining inclusion and exclusion criteria. FPC, fertility preservation consultation; Dx, diagnosis; Tx, treatment; Gyn, gynecologic; Heme, hematologic; GI, gastrointestinal.
Table I.
Patient demographics, grouped by FPC status.
| Demographics | FPC (n = 41), n (%) | Number of FPC (n= 158), n (%) | Total (n = 199) |
|---|---|---|---|
| Age (years) | |||
| <26 | 9 (30.0) | 21 (70.0) | 30 |
| 26–30 | 6 (27.3) | 16 (73.7) | 22 |
| 31–35 | 13 (24.1) | 41 (75.9) | 54 |
| 36–40 | 11 (15.1) | 62 (84.9) | 73 |
| 41–42 | 2 (11.1) | 18 (89.9) | 20 |
| Ethnicity | |||
| Caucasian | 30 (30.7) | 84 (73.7) | 114 |
| African American | 9 (17.6) | 42 (82.4) | 51 |
| Hispanic | 0 (0.0) | 19 (100.0) | 19 |
| Other | 2 (13.3) | 13 (86.7) | 15 |
| Parity | |||
| Nulliparous | 27 (30.7) | 61 (69.3) | 88 |
| 1 child | 12 (28.6) | 30 (71.4) | 42 |
| 2 children | 1 (2.0) | 49 (98.0) | 50 |
| 3+ children | 1 (5.2) | 18 (94.8) | 19 |
| Nulliparous | |||
| Yes | 27 (30.7) | 61 (69.3) | 88 |
| No | 14 (12.6) | 97 (87.4) | 111 |
| Relationship status | |||
| Single | 10 (14.7) | 58 (85.3) | 68 |
| In a relationship | 30 (24.6) | 92 (75.4) | 122 |
| Unknown | 1 (11.1) | 8 (88.9) | 9 |
| Insurance status | |||
| None | 8 (11.6) | 61 (88.4) | 69 |
| Insured | 33 (25.4) | 97 (74.6) | 130 |
| Cancer | |||
| Breast | 25 (35.7) | 45 (64.3) | 70 |
| Gynecologic | 9 (12.3) | 64 (87.7) | 73 |
| Ovarian | 2 | 13 | 15 |
| Endometrial | 4 | 17 | 21 |
| Cervical | 3 | 32 | 35 |
| Vulvar | 0 | 1 | 1 |
| Primary peritoneal | 0 | 1 | 1 |
| Hematologic | 6 (14.3) | 36 (85.7) | 42 |
| Gastrointestinal | 1 (7.1) | 13 (92.9) | 14 |
FPC, fertility preservation consultation.
BMI did not differ significantly between those who received FPC and those who did not (27.3 ± 7.6 versus. 29.9 ± 10.5; P = 0.14). Forty-four percent of FPC eligible women were nulliparous and were four times more likely to receive FPC than women with one or more children [66 versus 39%, odds ratio (OR) 4.78, 95% confidence interval (CI) 1.9–11.9]. There were more consultations among those patients in a relationship than those who were not (24.6 versus 14.7%; P = 0.11); however, this was not statistically significant.
Average income, as determined by home zip code at time of diagnosis, did not differ significantly between groups ($53 526 ± 23 859 versus $51 512 ± 26 896; P = 0.66, Table II). In addition, there was no difference in distance from a patient's home to the UNC Hospital between groups (56.2 ± 48.7 versus 68.3 ± 51.5mi; P = 0.18). However, significantly more patients who received FPC had insurance (80.5 versus 61.4%; P = 0.02).
Table II.
Predictive factors for FPC.
| Predictor | FPC (n= 41) | Number of FPC (n = 158) | P-value |
|---|---|---|---|
| Age (years) | 31.5 ± 6.9 | 34.0 ± 6.3 | 0.025 |
| >30 | 27 (27.8) | 70 (72.2) | 0.014 |
| <30 | 14 (13.7) | 88 (86.3) | |
| Race | |||
| Caucasian | 30 (26.3) | 84 (73.7) | 0.021 |
| Other races | 11 (12.9) | 74 (87.1) | |
| BMI (kg/m2) | 27.3 ± 7.6 | 29.9 ± 10.5 | 0.143 |
| <30 | 29 (23.4) | 94 (76.4) | 0.187 |
| >30 | 12 (15.8) | 64 (84.2) | |
| Parity | |||
| Nulliparous | 27 (30.7) | 61 (69.3) | 0.002 |
| Multiparous | 14 (12.6) | 97 (87.4) | |
| Relationship status | |||
| Single | 10 (14.7) | 58 (85.3) | 0.110 |
| In a relationship | 30 (24.6) | 92 (75.4) | |
| Unknown | 1 (11.1) | 8 (88.9) | |
| Cancer | |||
| Breast cancer | 25 (35.7) | 45 (64.3) | 0.000 |
| Other cancers | 16 (12.4) | 113 (87.6) | |
| Income ($) | 53 526 ± 23 859 | 51 512 ± 26 896 | 0.663 |
| Distance to UNC (mi) | 56.2 ± 48.7 | 68.3 ± 51.5 | 0.177 |
| Time between dx and treatment (days) | 75.8 ± 155.4 | 122.9 ± 277.4 | 0.299 |
Data are mean ± standard deviation or n (%), where appropriate. FPC, fertility preservation consultation; BMI, body mass index; UNC, University of North Carolina; mi, miles; dx, diagnosis.
Breast and gynecologic cancers were the most common primary diagnosis in the FPC eligible group (35.1 and 36.2%, respectively). Of those who received FPC, 60.1% (25/41) had breast cancer, 22.0% (9/41) had a gynecologic primary, 14.6% (6/41) were diagnosed with gastrointestinal cancer and 2.4% (1/41) had a hematologic source. The patients with gynecologic malignancies (n = 73) are broken down by type in Table I. The majority of gynecologic cancers in this study were cervical (47.9%), endometrial (28.8%) and ovarian (20.1%) in origin.
The length of time between diagnosis and cancer treatment did not differ significantly between those who received FPC and those who did not; however, there was a longer duration in those who did not undergo FPC (75.8 ± 155.4 versus 122.9 ± 277.4 days; P = 0.30; Table II).
Multivariable regression analysis showed that age, parity and breast cancer diagnosis was still associated with FPC after controlling for ethnicity and insurance status (Table III). Even after controlling for other factors, women with breast cancer were over 10 times more likely to receive FPC compared with other cancer diagnoses (OR 10.1, 95% CI 3.8–26.8). The odds of FPC referral were ∼2.4 times higher for Caucasian women (OR 2.4, 95% CI 0.9–6.2), 3.3 times higher for age <35 years (OR 3.27, 95% CI 1.3–7.7) and 4.6 times higher in nulliparous women (OR 4.56, 95% CI 1.8–11.3).
Table III.
Odds Ratios for Predictors Associated with FPC
| Eligible for FPC (n = 199) |
Odds ratio | 95% CI | ||
|---|---|---|---|---|
| FPC (n = 41) | Number of FPC (n = 158) | |||
| Age <35 years | 27 (66%) | 70 (44%) | 3.27 | 1.38–7.72 |
| Caucasian | 30 (73%) | 84 (53%) | 2.42 | 0.94–6.22 |
| Insured | 33 (80%) | 97 (61%) | 1.40 | 0.52–3.78 |
| Nulliparous | 27 (66%) | 61 (39%) | 4.56 | 1.85–11.25 |
| Breast cancer | 25 (61%) | 45 (28%) | 10.09 | 3.79–26.83 |
FPC, fertility preservation consultation.
Of the 41 women who received FPC, 11 (26.8%) underwent FP treatment with 7 undergoing embryo cryopreservation and 4 undergoing ovarian tissue cryopreservation. Of the patients who underwent FP treatment with embryo cryopreservation during the study period, two attempted pregnancy by the time of publication. One was successful from a frozen IVF cycle and one had a failed attempt with a surrogate. No women who underwent ovarian tissue cryopreservation had attempted pregnancy by the time of publication of this study.
Discussion
In the devastating event of a new cancer diagnosis, knowledge of FP options is important to many women (Dunn and Steginga, 2000), and access to FPC and FP treatment appear to be limited. However, the exact barriers to referral for FPC appear to be complex. This is the first study to demonstrate that specific socio-demographic patient characteristics and cancer variables are associated with referral for FPC.
Socioeconomic disparities in access to healthcare have been established in many disciplines of medicine (Brown et al., 2000), including infertility assessment and ART (Seifer et al., 2008; Seifer et al., 2010). In one study designed to assess the trends of racial disparities in ART from 2010, they found significant disparities in access to ART and IVF outcomes between Caucasian and African American women (Seifer et al., 2010). And although the racial gap of those presenting for care had narrowed when comparing IVF data from 1999–2000 to 2004–2006, there seemed to be a widening disparity in outcomes, highlighting a need for increased awareness and directed research (Seifer et al., 2010).
Several studies have queried oncologists about referrals to FPC. A nationwide survey of 249 responding oncologists from 2010 found that even though 95% of oncologists report that they routinely discuss fertility consequences of cancer treatments, over half (61.1%) rarely or never refer patients for FPC (Forman et al., 2010). In addition, 30% of oncologists responded that they rarely consider a woman's desire for future fertility when planning treatment (Forman et al., 2010). Other studies have queried oncologists to evaluate potential etiologies for the frequently inadequate discussions surrounding FP and referrals for FPC, and have found that prioritizing discussion of immediate or life-threatening complications, feeling that a delay in cancer treatment for FP is not warranted, lack of knowledge, training or time for discussion and physician attitudes may play a role (Lee et al., 2006; Quinn et al., 2007). Prognosis, severity of disease, type of cancer and treatment may also be factors, and the data regarding specific treatment-related infertility rates is poor, which may contribute to discomfort discussing the issue. Sociological factors and physician perception of patients may also play a role, including assuming patients may not be interested in FP, that they cannot afford it, or that there are other barriers to care (Quinn et al., 2009; Lee et al., 2011).
According to the United States Census data for the year 2000, 69.1% of people considered themselves white or Caucasian, 12.3% black or African American, 12.5% Hispanic and 10.1% other (McKinnon, 2010). The North Carolina data have a similar breakdown of Caucasian (69.3%) versus minorities (30.7%); however, this population has an increased number of African American residents (22.1 versus 12.3%) and a decreased number of Hispanic residents (4.7 versus 12.5%; McKinnon, 2010). Our population of FPC eligible reproductive-aged women (n = 199) included ∼45% minorities (25.6% African American, 9.5% Hispanic and 7.5% other), representing an increased number of minorities when compared with North Carolina and national statistics. Despite almost half of our patients representing minorities, our data shows <22% of FPCs occurred in African American women, 0% in Hispanic women and 5% in other ethnicities. Moreover, when controlling for other significant factors, Caucasian women were twice as likely to receive FPC as women of other ethnicities. There are many potential explanations for this, including ethnic disparities in access to care, clinician perceived economic status, language barriers, health literacy and cultural beliefs regarding oncologic and/or reproductive issues (Brown et al., 2000; Richardson and Norris, 2010; Fedewa et al., 2011).
As expected, we found that younger and nulliparous patients were more likely to receive FPC. This may reflect the fact that older women may be more likely to have completed childbearing. However, for the subset of older patients who desire future fertility, education and information on reproductive options is essential given that they are at higher risk than younger women of deleterious fertility effects from cancer treatments. In addition, it is important to consider that patients with one or more children may still have a desire to increase their family size.
In this study, patients with breast cancer were over 10 times more likely to receive FPC than women with other cancers. There were no significant differences in age, parity or ethnicity of those diagnosed with breast cancer from other cancers. The difference observed may be due to close collaboration between breast oncologists and FP providers at our institution, an increased body of literature regarding breast cancer and FP, and no surgical involvement of reproductive organs in treatment.
Despite an equal number of patients being diagnosed with a primary gynecologic cancer as breast cancer in the same timeframe, gynecologic cancer patients made up less than one quarter of patients who received FPC. One hypothesis is that gynecologic oncologists may feel more comfortable discussing reproductive options with their patients and offering conservative surgical treatments on their own, and do not feel the need to refer unless FP treatment is desired. This is supported by the fact that four patients with cervical cancer in this study underwent trachelectomy, a conservative surgical option for early stage cervical cancer, with only one of them receiving an FPC. While the outcome may have been the same, these patients may have benefit from counseling with a reproductive specialist in a formal FPC to discuss other FP options and the difficulty and implications of getting pregnant after trachelectomy. Other gynecologic cancers also have special considerations—low-stage ovarian cancers may be able to undergo conservative surgical management with unilateral oophorectomy and endometrial cancer patients may be able to undergo egg or embryo banking before hysterectomy/oophorectomy to allow for surrogacy. It is important to note that in the case of many gynecological cancers, final diagnosis and staging is often not concluded until after fertility-damaging surgery has occurred, highlighting a need for earlier patient education and evaluation for referral.
In order to account for poor oncologic prognosis and need for immediate treatment, patients with stage IV disease and those who received treatment within 4 days of diagnosis were excluded in this study. Being cognizant of the fact that each cancer is staged differently and that stage may not accurately depict the acuity of the individual situation, we excluded advance stage disease to err on the side of more conservative enrollment criteria, and to make the findings more generalizable. There has been concern that providing FPC and subsequent FP treatment could delay potentially life-saving cancer treatment. However, our data shows that there is no significant difference in overall time from cancer diagnosis to initiation of cancer treatments in those that received FPC and those that did not. Alternatively, a trend towards the opposite was observed. This may be due to the large proportion of patients with breast cancer who received FPC, where diagnosis, surgery, staging, chemotherapy and radiation are on a relatively established timeline. On the other hand, each of the different gynecologic, hematologic and gastrointestinal cancers has individual staging and treatment criteria and cancer stage may not exactly portray the acuity of the situation.
This study has several limitations. These patients were seen at a single institution, which may limit the generalizability of these findings. However, this subset of patients was seen at a tertiary care institution that serves as a referral center from many areas. FPC and FP treatment are not an option at many smaller institutions and outlying areas, and if data were able to be collected from these areas, the discrepancies may be even more impressive. Another limitation was relying on medical records to provide documentation of conversations about fertility between oncologists and patients. In patient records with no documentation of conversations about fertility risks, we assumed that it was not discussed, when it may have been, and not recorded. This may have especially affected the gynecologic oncology data, where providers are more likely to take a full obstetrical and gynecologic history than in other specialties. Another limitation is that this study only includes those patients who presented for consultation—perhaps other patients may have been referred for FPC but did not schedule or present for the consultation itself. A patient may also have been offered FPC and declined without documentation in the medical record. Therefore, it is difficult to differentiate between the lack of information and referral to FPC from the clinician, inadequate medical records and documentation, or reluctance on the part of the patient. However, from a medico-legal standpoint, we believe all reproductive-aged women with cancer should have documentation about discussion of fertility preferences in the medical record. An additional limitation is that economic status is difficult to assess using zip code of residence at time of diagnosis, since average income is based on income tax reports filed, which may not represent each patient accurately. Also, patient educational status was not evaluated for in this study and may play a role in referral patterns.
At our institution, we have not yet had enough FP patients undergo ovarian tissue cryopreservation or oocyte cryopreservation to publish success rates in the literature to date. However, our IVF pregnancy rates with fresh and frozen cycles are on par with the national average in the USA. An increase in FPC and FP treatment volume would allow for success rates specific to FP treatment to be published, which may positively affect referral rates.
There is growing substantiation that fertility after cancer treatment is important to patients and the field of FP is progressing. This study suggests that ethnicity, sociological influences and cancer site-specific factors may contribute to disparities in referral patterns for FPC, showing there is a need for further provider education and awareness across all oncologic specialties. Future studies evaluating patient and provider views on referral to FPC based on socio-demographic factors may provide further insight into these discrepancies, and provide a starting point to provide access to all interested reproductive-aged patients.
Authors' roles
L.R.G. provided contribution to conception and design of the study, acquisition of data, analysis and interpretation of data, drafting and revision of the article. U.B. and J.K. provided contribution to conception and design of the study, acquisition of data, analysis and interpretation of data, and revision of the article. J.E.M. provided contribution to conception and design of the study, analysis and interpretation of data, and revision of the article.
Funding
There was no financial support for this study.
Conflict of interest
None of the authors have any financial disclosures.
References
- Melissa Data. 2011 http://www.melissadata.com/lookups/TaxZip.asp?zip=44860. (August 2011, date last accessed) [Google Scholar]
- Brown R, Ojeda V, Wyn R, Levan R. Racial and Ethnic Disparities in Access to Health Insurance and Health Care. 2000. http://wwwkfforg/uninsured/upload/Racial-and-Ethnic-Disparities-in-Access-to-Health-Insurance-and-Health-Care-Reportpdf .
- Bryson CA, Sykes DH, Traub AI. In vitro fertilization: a long-term follow-up after treatment failure. Hum Fertil (Camb) 2000;3:214–220. doi: 10.1080/1464727002000199011. [DOI] [PubMed] [Google Scholar]
- Carter J, Rowland K, Chi D, Brown C, Abu-Rustum N, Castiel M, Barakat R. Gynecologic cancer treatment and the impact of cancer-related infertility. Gynecol Oncol. 2005;97:90–95. doi: 10.1016/j.ygyno.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Duffy CM, Allen SM, Clark MA. Discussions regarding reproductive health for young women with breast cancer undergoing chemotherapy. J Clin Oncol. 2005;23:766–773. doi: 10.1200/JCO.2005.01.134. [DOI] [PubMed] [Google Scholar]
- Dunn J, Steginga SK. Young women's experience of breast cancer: defining young and identifying concerns. Psycho Oncology. 2000;9:137–146. doi: 10.1002/(sici)1099-1611(200003/04)9:2<137::aid-pon442>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Fedewa SA, Lerro C, Chase D, Ward EM. Insurance status and racial differences in uterine cancer survival: a study of patients in the National Cancer Database. Gynecol Oncol. 2011;122:63–68. doi: 10.1016/j.ygyno.2011.03.010. [DOI] [PubMed] [Google Scholar]
- Fertile Hope. Healthcare Professionals, Fertility Risks. Fertilehope.org (February 2012, date last accessed)
- Forman EJ, Anders CK, Behera MA. A nationwide survey of oncologists regarding treatment-related infertility and fertility preservation in female cancer patients. Fertil Steril. 2010;94:1652–1656. doi: 10.1016/j.fertnstert.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, Beck LN, Brennan LV, Oktay K. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917–2931. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- Lee S, Heytens E, Moy F, Ozkavukcu S, Oktay K. Determinants of access to fertility preservation in women with breast cancer. Fertil Steril. 2011;95:1932–1936. doi: 10.1016/j.fertnstert.2011.01.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiblum SR, Aviv A, Hamer R. Life after infertility treatment: a long-term investigation of marital and sexual function. Hum Reprod. 1998;13:3569–3574. doi: 10.1093/humrep/13.12.3569. [DOI] [PubMed] [Google Scholar]
- McKinnon J. The Black Population: 2000. Census brief. 2010. http://wwwcensusgov/population/www/cen2000/briefshtml. (October 2010, date last accessed)
- NIH. US Department of Health and Human Services; 2004. Living Beyond Cancer: Finding a New Balance; pp. 1–87. President's Cancer Panel 2003–2004 Annual Report https://cissecure.nci.nih.gov/ncipubs/detail.aspx?prodid=P986 . [Google Scholar]
- Oktem O, Oktay K. Quantitative assessment of the impact of chemotherapy on ovarian follicle reserve and stromal function. Cancer. 2007;110:2222–2229. doi: 10.1002/cncr.23071. [DOI] [PubMed] [Google Scholar]
- Peate M, Meiser B, Hickey M, Friedlander M. The fertility-related concerns, needs and preferences of younger women with breast cancer: a systematic review. Breast Cancer Res Treat. 2009;116:215–223. doi: 10.1007/s10549-009-0401-6. [DOI] [PubMed] [Google Scholar]
- Practice Committee of American Society for Reproductive Medicine, P. C. o. S. f. A. R. T. Ovarian tissue and oocyte cryopreservation. Fertil Steril. 2008;90:S241–S246. doi: 10.1016/j.fertnstert.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Quinn GP, Vadaparampil ST, Gwede CK, Miree C, King LM, Clayton HB, Wilson C, Munster P. Discussion of fertility preservation with newly diagnosed patients: oncologists' views. J Cancer Surviv. 2007;1:146–155. doi: 10.1007/s11764-007-0019-9. [DOI] [PubMed] [Google Scholar]
- Quinn GP, Vadaparampil ST, Lee JH, Jacobsen PB, Bepler G, Lancaster J, Keefe DL, Albrecht TL. Physician referral for fertility preservation in oncology patients: a national study of practice behaviors. J Clin Oncol. 2009;27:5952–5957. doi: 10.1200/JCO.2009.23.0250. [DOI] [PubMed] [Google Scholar]
- Richardson LD, Norris M. Access to health and health care: how race and ethnicity matter. Mt Sinai J Med. 2010;77:166–177. doi: 10.1002/msj.20174. [DOI] [PubMed] [Google Scholar]
- Schover LR. Motivation for parenthood after cancer: a review. J Natl Cancer InstMonogr. 2005;34:2–5. doi: 10.1093/jncimonographs/lgi010. [DOI] [PubMed] [Google Scholar]
- Schover LR, Rybicki LA, Martin BA, Bringelsen KA. Having children after cancer. A pilot survey of survivors' attitudes and experiences. Cancer. 1999;86:697–709. doi: 10.1002/(sici)1097-0142(19990815)86:4<697::aid-cncr20>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Seifer DB, Frazier LM, Grainger DA. Disparity in assisted reproductive technologies outcomes in black women compared with white women. Fertil Steril. 2008;90:1701–1710. doi: 10.1016/j.fertnstert.2007.08.024. [DOI] [PubMed] [Google Scholar]
- Seifer DB, Zackula R, Grainger DA. Trends of racial disparities in assisted reproductive technology outcomes in black women compared with white women: Society for Assisted Reproductive Technology 1999 and 2000 vs. 2004–2006. Fertil Steril. 2010;93:626–635. doi: 10.1016/j.fertnstert.2009.02.084. [DOI] [PubMed] [Google Scholar]
- Sklar C. Maintenance of ovarian function and risk of premature menopause related to cancer treatment. J Natl Cancer Inst. 2005;34:25–27. doi: 10.1093/jncimonographs/lgi018. [DOI] [PubMed] [Google Scholar]


