Abstract
BACKGROUND
Preterm birth (PTB) is hypothesized to be an inflammatory response disease. However, no single factor alone is likely to explain PTB risk. It is more probable that coordinated networks of cytokines affect risk.
METHODS
Therefore, we examined the relationships between amniotic fluid (AF) cytokines/chemokines and related biomarkers in PTB and normal term deliveries in African Americans and Caucasians. Data were obtained from African American (41 preterm labor and 91 term labor) and Caucasian (105 preterm labor and 100 term labor) pregnant mothers. Pro-inflammatory cytokines and related molecules interleukin (IL)-1, IL-6, IL-8, and tumor necrosis factor- (TNF)-α, TNF soluble receptors (sTNFR1 and sTNFR2), and anti-inflammatory cytokine IL-10 that were all previously associated with PTB were studied. Correlations between biomarkers were calculated; differences of correlation coefficients between AF from African American and Caucasian samples in preterm labor and term labor were measured.
RESULTS
Multiple differences were observed between African American and Caucasian preterm and term birth groups. In term birth the strongest differences were between pro- and anti-inflammatory correlations, whereas in PTB differences were equally distributed between pro-inflammatory/anti-inflammatory and pro-inflammatory/pro-inflammatory correlations. Three correlation patterns differed significantly between AF from PTB African Americans with and without microbial invasion of the intra-amniotic cavity (MIAC); no differences were observed in Caucasians with MIAC.
CONCLUSION
Correlation analyses of cytokine measurements suggest coordinated interplay during pregnancy; significant differences exist between African Americans and Caucasians. Such analyses can serve as a means of understanding risk factors in these populations.
Keywords: preterm labor, disparity, cytokines, inflammation, interleukins
Introduction
Cytokine and chemokine networks maintain homoeostasis during pregnancy. Strong inflammatory responses mediated by cytokines and chemokines are involved in pregnancy complications such as preterm birth (PTB). In addition, recent in vitro and in vivo findings suggest that inflammatory responses mediated by cytokines and chemokines may not be the same in different geographic populations, and that this may contribute to the disparity in PTB rates seen between African Americans and Caucasians in the United States (Demissie et al., 2001; Martin et al., 2003; Menon et al., 2006,2007c). It is likely that differences in pregnancy outcomes are affected by differences in the coordination of cytokine regulation (Menon et al., 2007c). Such differences may partially explain the disparity in PTB rates between African Americans and Caucasians. Examining cytokine patterns may therefore be important in understanding PTB risk and ethnic disparity.
Cytokines regulate pregnancy by both autocrine and paracrine mechanisms. Pregnancy can be regarded as maternal immune adaptation to a temporary semi-allogenic graft (the fetus) (Shurin et al., 1999; Thellin et al., 2000). One of the key events during the maintenance of a normal pregnancy is a bias towards the up-regulation of the Th2 immune response that then down-regulates several cellular immune effector functions, including cytotoxic response and inflammatory cytokine release. In contrast, an imbalance of Th1/Th2 production in favor of Th1 can induce labor (Shurin et al., 1999). The predominance of Th2 cytokines during early stages of pregnancy leads to an increased production of progesterone. Progesterone in turn increases Th2 cytokine secretion [such as interleukin (IL)-10 and IL-6] and decreases Th1 and pro-inflammatory cytokines [such as IL-1β, IL-8 and tumor necrosis factor-alpha (TNF-α)], completing the positive feedback loop that maintains pregnancy (Diehl and Rincon, 2002; Ugwumadu, 2002). At term, this balance is shifted in favor of Th1 cytokines that are predominantly pro-inflammatory. A predisposition to a premature shift from anti- to pro-inflammatory balance may explain the roles of risk factors and racial disparity in PTB. This can be assessed by examining the correlation between pro- and anti-inflammatory cytokines in pregnancy.
Several genetic and functional studies have associated both pro- and anti-inflammatory cytokines with PTB, but these studies usually analyzed individual cytokines (Romero et al., 1990; Dudley, 1997; Fortunato et al., 1997; Dizon-Townson et al., 1997; Rizzo et al., 1998; Weiyuan and Li, 1998; Roberts et al., 1999; Simhan et al., 2003; Sadowsky et al., 2006; Speer et al., 2006; Velez et al., 2007; Menon and Fortunato, 2007). These data, and others indicating that PTB is predominantly associated with infection (Averbuch et al., 1995; Hitti et al., 1997; Kniss and Iams, 1998; Clark and Croitoru, 2001; Gardella et al., 2004) and/or an exaggerated inflammatory response (Gomez et al., 1995; Dudley, 1997; Kniss and Iams, 1998; Goldenberg et al., 2000) associated with increased Th1 cytokine concentrations, have led to the hypothesis that PTB is a host inflammatory response disease.
Differences in patterns of cytokine production may contribute to PTB pathophysiology. We have examined the correlations between several cytokines previously shown to be associated with PTB, to assess whether there is evidence for differences in cytokine production patterns between preterm and normal term deliveries and also between ethnic groups (African Americans and Caucasians). Specifically, we have examined the correlations between cytokines/chemokines (IL-1β, IL-6, IL-8, IL-10 and TNF-α) and biomarkers [soluble TNF-R1 (sTNFR1) and soluble TNF-R2 (sTNFR2)]. Correlations were also evaluated with respect to microbial invasion of the amniotic cavity (MIAC).
Materials and Methods
Study population
Subjects were recruited at the Centennial Women’s Hospital, Nashville, TN. Institutional Review Boards at TriStar Nashville, TN and Vanderbilt University, Nashville, TN approved this study. All subjects had singleton live births. Ancestry was identified by self-report and a questionnaire that traces ancestry back two generations from the parents. Individuals who had more than one ancestral group were excluded from the study. Mothers between the ages of 18 and 40 were recruited. Gestational age was determined by last menstrual period and corroborated by ultrasound dating. PTBs (referred to as preterm labor in the rest of the manuscript) were defined as delivery at <360/7 weeks gestation and term births following term labor (referred to as term labor in the rest of the manuscript) were defined as delivery >370/7 with no medical or obstetrical complications during pregnancy. Subjects with multiple gestations, pre-eclampsia, preterm premature rupture of the membranes, placental previa, infant anomalies, gestational diabetes, poly- and oligohydramnios, and other complications including surgeries during pregnancies were excluded. Subjects gave consent and were recruited upon admission to the hospital.
Demographic and clinical characteristics
Our study included Caucasians (105 preterm labor and 100 term labor) and African Americans (41 preterm labor and 91 term labor) of non-Hispanic origin. Placental and umbilical cord pathology was performed in all preterm labor to document histological chorioamnionitis and funisitis. The baseline values of demographic and clinical characteristics for African American and Caucasian women with preterm labor and term labor are listed in Table I.
Table I.
Baseline characteristics.
| Variable | Caucasians |
African Americans |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Term labor (n = 100), median [range]a | Preterm labor (n = 105), median [range]a | P-valueb | Term labor (n = 91), median [range]a | Preterm labor (n = 41), median [range]a | P-valueb | |||||
| Maternal age (years) | 28 [18–40] | 28 [18–40] | 0.309 | 24 [18–38] | 25 [18–38] | 0.474 | ||||
| Gestation age (weeks) | 39 [37–42] | 34 [24–36] | <0.001 | 39 [37–41] | 34 [23–36] | <0.001 | ||||
| Birth weight (g) | 3352 [2134–4252] | 2151 [370–3790] | <0.001 | 3255 [2075–4382] | 2267 [746–3685] | <0.001 | ||||
| APGAR 1 | 9 [4–9] | 8 [1–9] | <0.001 | 8 [2–9] | 8 [1–9] | 0.012 | ||||
| APGAR 5 | 9 [8–10] | 9 [1–9] | <0.001 | 9 [7–10] | 9 [6–9] | <0.001 | ||||
| Smoking (Count Y, yes; N, no) | 18Y | 80N | 25Y | 71N | 0.198 | 13Y | 77N | 10Y | 31N | 0.165 |
aRange-upper and lower range of the data; bP-value compares preterm labor to term labor. Non-parametric Mann–Whitney two-sample rank-sum tests were used if data was not normally distributed.
Cytokine and chemokine measurements
Amniotic fluid (AF) samples were collected during labor in both preterm labor and term labor by transvaginal amniocentesis prior to rupture of the membranes and delivery. Samples were collected before preterm or term vaginal deliveries by puncture of intact membrane using a 22-gauge needle prior to artificial rupture of the membranes. A few samples were also collected at the time of cesareans by transabdominal amniocentesis. AF was centrifuged immediately for 10 min at 2000g to remove cellular and particulate matter. Aliquots of AF were stored at –80°C until analysis.
IL-1β, IL-6, IL-8, IL-10, TNF-α and sTNFR1 and sTNFR2 concentrations in the AF were measured by multiplex assay (Biosource International, Camarillo, CA), using Luminex™ (Austin, TX). The details of the assay procedure can be found elsewhere (Menon et al., 2007c; Velez et al., 2007).
MIAC was determined by polymerase chain reaction (PCR) of microbial 16s ribosomal DNA (TaqMan Assay, CA) and correlated with clinical evidence (Hitti et al., 1997; Gardella et al., 2004; Menon et al., 2007c). Microbial species identification, using PCR, was not performed in this study. Preterm labors with clinical evidence of MIAC were defined as those individuals having three or more of the following criteria: abdominal tenderness, temperature >38°C, foul smelling vaginal discharge, an elevated C-reactive protein or histological chorioamnionitis. The distributions of MIAC among preterm labor were: 25 with MIAC and 79 with no MIAC in Caucasians and 10 with MIAC and 31 with no MIAC in African Americans (these distributions are the same in African Americans and Caucasians, P ∼ 1.00). A separate study (Menon et al., 2007a) performed PCR to detect presence of bacteria in a subset of 25 control samples and revealed no evidence of MIAC, ruling out the probability of bacterial contamination during collection of samples.
Analyses were performed separately on African Americans and Caucasians. This was done because our prior analyses strongly support the hypothesis that patterns of cytokine concentrations, as well as the genetic regulation of these concentrations, may be different between these two groups (Menon et al., 2007b, c; Velez et al., 2007).
Statistical analysis
Shapiro–Wilks tests of normality were performed on cytokine measurements. All measurements deviated significantly from normality; as a result non-parametric tests were used for all tests of significance. Mann–Whitney two-sample rank-sum tests (Hollander and Wolfe, 1999) were used to test for statistical differences between cytokines between preterm labor and term labor in each population and in preterm labor stratified by MIAC status. STATA 9.0 statistical software (StataCorp LP, College Station, TX) (StataCorp, 2007) was used for all analyses. Correlations between all pairwise combinations of cytokine measurements were calculated for preterm labor and term labor in African Americans and Caucasians separately. Preterm labor groups were also stratified by MIAC status within each ethnic group. Spearman’s rho, a non-parametric alternative to the correlation coefficient r, was used to calculate correlations using JMP statistical software (JMP Start Statistics, SAS Institute Inc., Canada) (Sall et al., 2005). Differences between correlation coefficients were tested by z-test of the Fisher’s r to z-transformation of the Spearman rho correlation coefficient (Sokal and Rohlf, 1987). This approach has been previously published (Reilly et al., 1994; Asselbergs et al., 2007).
Results
Demographic characteristics
Significant differences between preterm labor and term labor were observed in both African Americans and Caucasians for gestational age (weeks) (P < 0.001 for both), birth weight (g) (P < 0.001 for both), APGAR 1 (Caucasians P < 0.001; African Americans P = 0.012) and APGAR 5 (at 5 min after birth) (P < 0.001 for both) (Table I). APGAR is a method to quickly assess newborn health. The score is a number calculated from a combination of heart rate, respiratory effort, muscle tone, skin color, and response to a catheter in the nostril 1 min after birth (APGAR 1) and 5 min after birth (APGAR 5).
Cytokine measurements
Median cytokine concentrations and ranges are reported for AF concentrations from African Americans and Caucasians in preterm labor and term labor (Table II) and for preterm labor with and without MIAC (Table II). In Caucasians, there were significant differences in IL-8 concentrations between AF of women in preterm and term labor (P = 0.002) and marginal, but statistically non-significant differences in IL-6 (P = 0.070) and TNF-α (P = 0.075) levels. There were no statistically significant differences between AF of Caucasians in preterm labor with and without MIAC (Table II) for any of the biomarkers examined. Multiple significant differences in median analyte concentrations between preterm and term labor were observed in African Americans, including IL-1β (P < 0.001), TNF-α (P < 0.001), sTNFR1 (P = 0.012) and sTNFR2 (P = 0.003) (Table II). Several differences were also observed between African American women in preterm labor with and without MIAC (for IL-1β, P = 0.015; for TNF-α P = 0.007 and for sTNFR2, P = 0.015) (Table II). No differences between preterm labor and term labor were observed in either African Americans or Caucasians for IL-10.
Table II.
Medians and ranges and tests of median differences of cytokine concentrations.
| Cytokine concentrations (pg/ml) | Caucasian | Caucasian | P-value | African American | African American | P-value |
|---|---|---|---|---|---|---|
| Term labor (n = 100), median [range]a | Preterm labor (n = 105), median [range]a | Term labor (n = 91), median [range]a | Preterm labor (n = 41), median [range]a | |||
| IL-1β | 22.64 [0.01–1122.67] | 25.64 [3.81–1008.72] | 0.109 | 23.74 [2.09–432.95] | 237.7 [7.00–1989.9] | <0.001 |
| IL-10 | 22.33 [2.16–525.02] | 20.3 [1.58–817.1] | 0.267 | 12.32 [0.99–3043.49] | 9.57 [1.58–886.21] | 0.439 |
| IL-6b | 2305.71 [108.42–27 269.65] | 3383.51 [118.32–38 734.73] | 0.070 | 2396.60 [160.76–19 611.7] | 22 103.43 [101.46–39 712.64] | 0.780 |
| IL-8 | 654.3 [36.27–17 363.86] | 1224.78 [28.92–10 529] | 0.002 | 729.29 [12.07–15 847.57] | 1617.34 [25.3–31 302.64] | 0.059 |
| TNF-α | 67.62 [1.66–2493.49] | 138.39 [5.36–3508.92] | 0.075 | 67.905 [1.66–4476.42] | 1009.34 [2.09–17 807.91] | <0.001 |
| sTNFR1 | 2156.89 [297.34–16 282.3] | 2388.42 [160.56–15 588.6] | 0.611 | 3249.17 [297.34–12 425.15] | 1891.7 [54.47–18 126.81] | 0.012 |
| sTNFR2 | 2681.47 [152.5–9227.4] | 2581.95 [282.31–10 946.19] | 0.713 | 3294.08 [723.94–10 175.73] | 2025.97 [158.97–9581.8] | 0.003 |
| No MIAC (n = 79) | MIAC (n = 25) | No MIAC (n = 31) | MIAC (n = 10) | |||
| IL-1β | 25.64 [2.26–1008.72] | 28.88 [4.92–1147.63] | 0.538 | 62.26 [7.00–1989.9] | 1179.27 [26.7–1760.33] | 0.015 |
| IL-10 | 21.65 [1.58–817.1] | 16.56 [2.16–282.17] | 0.590 | 7.56 [1.58–428.17] | 37.50 [1.74–886.21] | 0.101 |
| IL-6 | 2648.035 [118.32–38 734.73] | 5211.63 [191.49–33 087] | 0.155 | 3350.75 [101.46–39 712.64] | 1987.6 [407.5–26 347.34] | 0.816 |
| IL-8 | 1186.07[28.92–9876.35] | 1370.23[84.93–10 529] | 0.697 | 1266.87[25.3–31 302.64] | 1709.37 [81.2–7325.4] | 0.851 |
| TNF-α | 139.42 [5.36–2080.66] | 114.83 [ 9.86–3508.92] | 0.547 | 373.68 [2.09–8897.65] | 6234 [5 [209–17 807.91] | 0.007 |
| sTNFR1 | 2042.15 [160.56–14 431.83] | 2942.96 [354.8–15 588.6] | 0.151 | 1160.66 [54.47–18 126.81] | 4976.43 [66.56–10 041.63] | 0.057 |
| sTNFR2 | 2473.68 [282.31–10 946.19] | 2955.9 [392.06–10 094.2] | 0.498 | 1476.82[184.22–8379.87] | 4733.6 [158.97–9581.8] | 0.015 |
aRange- upper and lower range of the data; bOf note, in a larger sampled dataset we observed that IL-6 was statistically significant in our Caucasian population but not in our African American population and the present analysis shows that it trends toward significance (Menon et al., 2007a).
Correlations in AF from African American and Caucasian women in preterm labor and term labor
Multiple significant correlations between cytokine concentrations were observed in both preterm labor and term labor (Table III; Supplementary data, Table SI;Supplementary data, Fig. S1a and b). In AF from Caucasian women in term labor ∼62% of the correlations were statistically significant. Of note, IL-1β AF samples from women in term labor had no significant correlations in Caucasians. In AF from Caucasian women in preterm labor ∼43% of the correlations were statistically significant. Of these, pro-inflammatory cytokine IL-8 had the largest number of significant correlations with other cytokines (IL-8 significantly associated with five cytokines). Anti-inflammatory cytokine IL-10 significantly associated with three as did sTNFR1 and sTNFR2 in Caucasian preterm labor.
Table III.
Statistically significant correlations within preterm labor and within term labor.
| Cytokine 1 | Cytokine 2 | Preterm labor Caucasian |
Term Caucasian |
Preterm labor African American |
Term African American |
||||
|---|---|---|---|---|---|---|---|---|---|
| r | P-value | r | P-value | r | P-value | r | P-value | ||
| IL-1β | IL-8 | 0.153 | 0.136 | 0.197 | 0.055 | 0.281 | 0.088 | 0.394 | <0.001 |
| IL-1β | IL-10 | 0.080 | 0.440 | −0.029 | 0.789 | 0.514 | 0.001 | 0.349 | 0.001 |
| IL-1β | TNF-α | −0.101 | 0.330 | −0.089 | 0.424 | 0.428 | 0.008 | 0.163 | 0.141 |
| IL-1β | sTNFR2 | −0.055 | 0.602 | −0.101 | 0.352 | 0.377 | 0.020 | 0.060 | 0.582 |
| IL-10 | TNF-α | 0.171 | 0.092 | 0.611 | <0.001 | 0.503 | 0.001 | 0.537 | <0.001 |
| IL-10 | sTNFR1 | 0.456 | <0.001 | 0.637 | <0.001 | 0.669 | <0.001 | 0.239 | 0.022 |
| IL-10 | sTNFR2 | 0.480 | <0.001 | 0.660 | <0.001 | 0.736 | <0.001 | 0.337 | 0.001 |
| IL-6 | IL-8 | 0.472 | <0.001 | 0.605 | <0.001 | 0.461 | 0.004 | 0.624 | <0.001 |
| IL-6 | IL-10 | 0.196 | 0.054 | 0.390 | <0.001 | 0.429 | 0.006 | 0.466 | <0.001 |
| IL-6 | TNF-α | 0.311 | 0.002 | 0.366 | 0.001 | −0.199 | 0.245 | 0.362 | 0.001 |
| IL-6 | sTNFR1 | 0.068 | 0.517 | 0.404 | <0.001 | 0.123 | 0.470 | 0.049 | 0.647 |
| IL-6 | sTNFR2 | 0.142 | 0.166 | 0.347 | 0.001 | 0.094 | 0.575 | 0.080 | 0.451 |
| IL-8 | IL-10 | 0.357 | <0.001 | 0.198 | 0.070 | 0.612 | <0.001 | 0.655 | <0.001 |
| IL-8 | TNF-α | 0.420 | <0.001 | 0.114 | 0.308 | 0.091 | 0.593 | 0.374 | 0.001 |
| IL-8 | sTNFR1 | 0.233 | 0.030 | 0.343 | 0.001 | 0.306 | 0.061 | 0.249 | 0.025 |
| IL-8 | sTNFR2 | 0.295 | 0.005 | 0.345 | 0.001 | 0.245 | 0.133 | 0.231 | 0.038 |
| TNF-α | sTNFR1 | 0.119 | 0.258 | 0.260 | 0.017 | 0.360 | 0.031 | 0.292 | 0.006 |
| TNF-α | sTNFR2 | 0.071 | 0.487 | 0.294 | 0.006 | 0.432 | 0.008 | 0.302 | 0.005 |
| sTNFR1 | sTNFR2 | 0.895 | <0.001 | 0.889 | <0.001 | 0.873 | <0.001 | 0.850 | <0.001 |
Bold-faced values are the significant correlations (correlations statistically different from 0).
In African American term labor, 71% of the correlations were statistically significant (Table III; Supplementary data, Table SI). Again IL-8 and IL-10 had the largest number of significant correlations (six for both), followed by TNF-α (five), IL-6 (three), sTNFR1 and sTNFR2 (four each), and finally IL-1β (two). In African American preterm labor, 62% of the correlations were statistically significant. Of these, IL-10 had the largest number of significant correlations (six), followed by TNF-α, sTNFR1, sTNFR2 and IL-1β (each with four positive correlations). The fewest correlations were observed for IL-6 and IL-8 (two each).
Significant correlations in preterm labor with and without MIAC
We next examined correlations in preterm labor with and without MIAC in order to assess whether infection affects the correlation structure (Table IV; Supplementary data, Table SII). In Caucasian preterm labor with MIAC the most significant correlations involved sTNFR1 with sTNFR2 and IL-10 (P-values <0.001). In preterm labor without MIAC five correlations were significant at the P < 0.001 level (Table IV). Three significant correlations were present in preterm labor without MIAC, but not in preterm labor with MIAC: IL-10 and IL-6, TNF-α and IL-6, and TNF-α and IL-8.
Table IV.
Statistically significant correlations for MIAC.
| Cytokine 1 | Cytokine 2 | Caucasian Preterm labor |
African American Preterm labor |
||||||
|---|---|---|---|---|---|---|---|---|---|
| No MIAC |
MIAC |
No MIAC |
MIAC |
||||||
| r | P-value | r | P-value | r | P-value | r | P-value | ||
| IL-1β | IL-10 | 0.022 | 0.855 | 0.267 | 0.229 | 0.350 | 0.058 | 0.905 | 0.002 |
| IL-10 | sTNFR2 | 0.430 | <0.001 | 0.670 | <0.001 | 0.684 | <0.001 | 0.800 | 0.010 |
| IL-10 | sTNFR1 | 0.408 | <0.001 | 0.513 | 0.015 | 0.619 | <0.001 | 0.333 | 0.381 |
| IL-6 | TNF-α | 0.387 | 0.001 | 0.146 | 0.496 | −0.430 | 0.020 | 0.643 | 0.119 |
| IL-6 | IL-8 | 0.449 | <0.001 | 0.425 | 0.049 | 0.405 | 0.029 | 0.667 | 0.050 |
| IL-6 | IL-10 | 0.306 | 0.009 | −0.025 | 0.907 | 0.328 | 0.076 | 0.700 | 0.036 |
| IL-8 | TNF-α | 0.482 | <0.001 | 0.365 | 0.095 | −0.101 | 0.604 | 0.238 | 0.570 |
| IL-8 | sTNFR2 | 0.238 | 0.049 | 0.503 | 0.017 | 0.167 | 0.378 | 0.417 | 0.265 |
| IL-8 | IL-10 | 0.339 | 0.004 | 0.528 | 0.012 | 0.569 | 0.001 | 0.709 | 0.022 |
| sTNFR1 | sTNFR2 | 0.881 | <0.001 | 0.899 | <0.001 | 0.870 | <0.001 | 0.633 | 0.067 |
Bold-faced values are the significant correlations (correlations statistically different from 0).
In African American preterm labor with MIAC there were no highly significant correlations (P< 0.001); however, a correlation between IL-10 and IL-1β was significant at P = 0.002 (Table IV). In preterm labor without MIAC IL-10, sTNFR1 and sTNFR2 had the most significant correlations (P< 0.001). Three significant correlations were present only in preterm labor without MIAC: TNF-α and IL-6 (negative correlation), sTNFR1 and IL-10 (positive correlation), and sTNFR2 and sTNFR1 (positive correlation). Correlations present only in African American preterm labor with MIAC are: IL-10 and IL-1β, IL-10 and IL-6, and sTNFR2 and IL-1β (Supplementary data, Table SII).
Analysis of correlation differences
Case–control comparisons within each ethnic population
To assess whether correlations differed between groups, we tested for differences between correlation coefficients (Table V; Supplementary data, Table SIII). In Caucasians three comparisons were significantly different between AF from women in preterm labor and term labor, two between pro-inflammatory cytokines (TNF-α and IL-8, and IL-6 and sTNFR1) and one between pro- and anti-inflammatory analytes (TNF-α and IL-10). In African Americans there were three comparisons significant between AF from women in preterm labor and term labor of which two are between pro- and anti-inflammatory cytokines (sTNFR1 and IL-10, and sTNFR2 and IL-10) and one between pro-inflammatory cytokine (TNF-α and IL-6).
Table V.
Statistically significant correlation differences between both ethnic groups and status groups within an ethnic group.
| Cytokine 1 | Cytokine 2 | Caucasian versus African American, Preterm labor | Caucasian versus African American, Term labor | Caucasian, Preterm versus term labor | African American, Preterm versus term labor |
|---|---|---|---|---|---|
| P-value | P-value | P-value | P-value | ||
| IL-1β | IL-10 | 0.013 | 0.011 | 0.469 | 0.305 |
| IL-1β | sTNFR2 | 0.023 | 0.296 | 0.756 | 0.094 |
| IL-1β | TNF-α | 0.005 | 0.109 | 0.936 | 0.153 |
| IL-1βa | sTNFR1 | 0.015 | 0.878 | 0.416 | 0.055 |
| IL-10 | sTNFR1 | 0.109 | 0.001 | 0.086 | 0.004 |
| IL-10 | sTNFR2 | 0.031 | 0.004 | 0.071 | 0.003 |
| IL-10a | TNF-α | 0.054 | 0.476 | <0.001 | 0.819 |
| IL-6 | sTNFR1 | 0.786 | 0.014 | 0.019 | 0.714 |
| IL-6 | TNF-α | 0.010 | 0.977 | 0.684 | 0.005 |
| IL-8 | IL-10 | 0.083 | <0.001 | 0.258 | 0.722 |
| IL-8 | TNF-α | 0.077 | 0.084 | 0.031 | 0.144 |
| TNF-αa | sTNFR2 | 0.051 | 0.954 | 0.126 | 0.460 |
aBorderline significant in some group comparisons. Bold-faced values are the significant correlation differences.
Significant correlation differences between African Americans and Caucasians within phenotypes
Several differences were observed for correlations between Caucasians and African Americans when comparing AF from women in preterm labor to preterm labor or term labor to term labor (Table V; Supplementary data, Table SIII). Three of eight significant differences between ethnic groups were between pro- and anti-inflammatory cytokines (IL-10 and IL-1β, TNF-α and IL10, sTNFR2 and IL-10) and five were between pro-inflammatory cytokines (TNF-α and IL-1β, TNF-α and IL-6, sTNFR1 and IL-1β, sTNFR2 and IL-1β, sTNFR2 and TNF). This is exemplified by the correlations between TNF-α and IL-6 that had opposite directions in the races (Fig. 1). In term labor there were five correlations with significant differences between Caucasian and African American (Table V).
Figure 1:
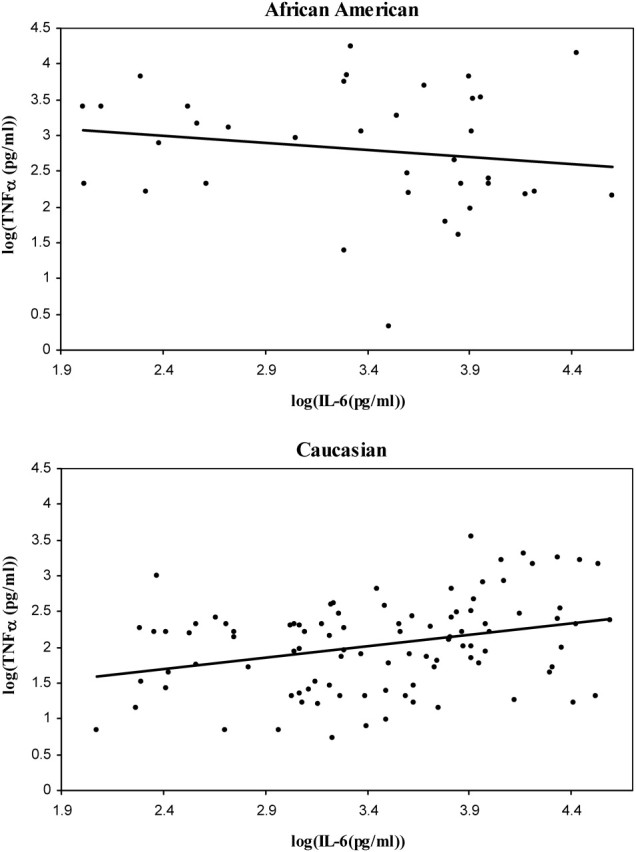
African American and Caucasian scatter plots for TNF-α and IL-6 correlations in preterm labor.
(a) Correlations between TNF-α and IL-6 in African Americans in preterm samples; (b) Correlations between TNF-α and IL-6 in Caucasian preterm samples. Log transformed values were used for illustrative purposes, as nonparametric analyses were performed for all correlation analyses.
Significant correlation differences for preterm labor with and without MIAC
In Caucasians, correlations between AF from women in preterm labor and term labor that showed significant differences demonstrated no such effect between preterm labor with and without MIAC (Supplementary data, Table SIV). However, in African Americans three correlations showed differences between AF from women in preterm labor with and without MIAC (Supplementary data, Table SIV). Of these correlations that between TNF-α and IL-6 was in opposite directions in the MIAC (positive correlation) and no MIAC groups (negative correlation).
Discussion
Cytokine production patterns were analyzed in PTB and in two ethnic groups by assessing correlation structures in control versus case AF, in both Caucasians and African Americans. The goal of this analysis was to better elucidate the role of cytokine networks in PTB. Our data revealed significant differences between correlations in AF from African Americans and Caucasians in both preterm labor and term labor, suggesting disparity in immune response. In addition, within an ethnic group we found several differences between preterm labor and term labor. Certain patterns suggested some indications of pro-inflammatory versus anti-inflammatory effects that merit follow-up in future studies. In the present study, IL-6 was defined as a pro-inflammatory cytokine in line with the substantial amount of literature supporting this role. However, some reports suggest that it can be considered either a pro- or anti-inflammatory cytokine (Xing et al., 1998; Smirnova et al., 2002).
Within Caucasians, the strongest evidence for differences in correlation between AF of women in preterm labor and term labor was provided by TNF-α and IL-10. The differential coordination of IL-10 and TNF-α is of potential significance because IL-10 is a regulator of TNF-α, and its production is known to be significantly reduced in the placenta of patients at term compared with first and second-trimester tissues (Hanna et al., 2000; Romero et al., 2007). Previous studies of decidual leukocytes have shown TNF-α and IL-10 concentrations to be highly correlated during normal labor, with correlations of ∼0.6 (Gustafsson et al., 2006), indicating a balanced immune status between pro- and anti-inflammatory cytokines. Our results are consistent with these previous reports in term labor of both Caucasians and African Americans. However, this correlation is not observed in preterm labor in either ethnic group, suggesting a trend toward immune imbalance in preterm labor.
In African Americans notable differences between correlation coefficients were observed between IL-6 and TNF-α (−0.20 in preterm labor and 0.36 in term labor). In AF from women with term birth, the two cytokine concentrations were positively correlated, suggesting a generalized inflammatory status during labor. However, the correlation coefficient is negative in preterm labor with the increase in TNF-α not being coordinated with the IL-6 concentrations, indicating an overwhelming TNF-α response.
Another intriguing result is for IL-1β. In comparisons between preterm labor groups, correlation coefficients indicated that African Americans and Caucasians differed significantly between IL-1β and TNF-α, sTNFR1, and sTNFR2. These results suggest that the IL-1β is coordinately co-regulated with several other cytokines in AF of African American women with preterm labor; however, this co-regulation is absent in Caucasians, again indicating a difference in the underlying processes leading to PTB in different ethnic groups. Recent animal model studies documented that IL-1β and TNF-α are inducers of preterm labor, whereas IL-6 and IL-8 failed to induce labor even after prolonged administration, although both IL-6 and IL-8 produced inflammatory changes in the fetal membranes and lungs. Our data in African Americans support these findings and suggest that preterm labor may be mediated predominantly by TNF-α and IL-1β (Sadowsky et al., 2003).
Correlation differences were observed between immune function regulators in African Americans. IL-10 was significantly more correlated with sTNFR1 and sTNFR2 in preterm labor than term labor, supporting the operation of immunoinhibitory mechanisms during PTB. However, a pro-inflammatory surge, represented by increases in TNF-α and IL-1β, may overwhelm the inhibitory mechanisms, further supporting the claim that PTB is a host inflammatory disease.
The potential role of infection in African American compared with Caucasian pregnancies is also illustrated by evidence for differences between correlations in preterm labor with and without MIAC in the two groups. We emphasize that definition of MIAC was not based solely on the detection of bacterial DNA in the AF, but also on clinical evidence for the presence of MIAC that correlated with the PCR data. However, it is possible that we could not detect presence of microbes between the membranes or in biofilms in the amniotic fluid. We did not find strong evidence for correlation differences in Caucasian preterm labor with and without MIAC. This suggests the coordinated cytokine activity in Caucasian preterm labor is not significantly affected by the presence of infection. Although certain Caucasian genetic predispositions may cause changes in individual cytokine concentrations in the presence of MIAC (Velez et al., 2007), infection alone does not appear to explain the observed heterogeneity in Caucasian preterm-term labor cytokine correlations. In contrast, there was evidence for heterogeneity between the presence and absence of MIAC in African American preterm labor, although differences were observed in only 14% of our comparisons (3/21). We caution, however, that these secondary analyses may not be adequately powered to detect effects of biological importance and that our results with respect to MIAC are preliminary.
Our data provide preliminary evidence for differences between Caucasian and African American populations in concentration patterns of cytokines and cytokine-related biomarkers. This is especially true in preterm labor, suggesting that differences in PTB rate may be due to differences in the underlying etiology. From the present study we cannot conclude whether the mother or fetus is contributing to the AF pool of cytokines, since an immune response can be initiated by both mother and fetus and cytokines within the AF are therefore the net effect of the two. Finally, in assessing patterns of coordinated cytokine production it appears that the two populations have fewer correlation differences in term labor than in preterm labor. Based on our findings we hypothesize that the pathways leading to PTB may be different in the two ethnic groups.
Supplementary material
Supplementary material is available at HUMREP Journal online.
Funding
This study was supported by a grant from Thrasher Research Funds to S.J.F.
Supplementary Material
Reference
- Asselbergs FW, Williams SM, Hebert PR, Coffey CS, Hillege HL, Navis G, Vaughan DE, van Gilst WH, Moore JH. Gender-specific correlations of plasminogen activator inhibitor-1 and tissue plasminogen activator levels with cardiovascular disease-related traits. J Thromb Haemost. 2007;5:313–320. doi: 10.1111/j.1538-7836.2007.02311.x. [DOI] [PubMed] [Google Scholar]
- Averbuch B, Mazor M, Shoham-Vardi I, Chaim W, Vardi H, Horowitz S, Shuster M. Intra-uterine infection in women with preterm premature rupture of membranes: maternal and neonatal characteristics. Eur J Obstet Gynecol Reprod Biol. 1995;62:25–29. doi: 10.1016/0301-2115(95)02176-8. [DOI] [PubMed] [Google Scholar]
- Clark DA, Croitoru K. TH1/TH2,3 imbalance due to cytokine-producing NK, gammadelta T and NK-gammadelta T cells in murine pregnancy decidua in success or failure of pregnancy. Am J Reprod Immunol. 2001;45:257–265. doi: 10.1111/j.8755-8920.2001.450501.x. [DOI] [PubMed] [Google Scholar]
- Demissie K, Rhoads GG, Ananth CV, Alexander GR, Kramer MS, Kogan MD, Joseph KS. Trends in preterm birth and neonatal mortality among blacks and whites in the United States from 1989 to 1997. Am J Epidemiol. 2001;154:307–315. doi: 10.1093/aje/154.4.307. [DOI] [PubMed] [Google Scholar]
- Diehl S, Rincon M. The two faces of IL-6 on Th1/Th2 differentiation. Mol Immunol. 2002;39:531–536. doi: 10.1016/s0161-5890(02)00210-9. [DOI] [PubMed] [Google Scholar]
- Dizon-Townson DS, Major H, Varner M, Ward K. A promoter mutation that increases transcription of the tumor necrosis factor-alpha gene is not associated with preterm delivery. Am J Obstet Gynecol. 1997;177:810–813. doi: 10.1016/s0002-9378(97)70273-4. [DOI] [PubMed] [Google Scholar]
- Dudley DJ. Pre-term labor: an intra-uterine inflammatory response syndrome? J Reprod Immunol. 1997;36:93–109. doi: 10.1016/s0165-0378(97)00065-x. [DOI] [PubMed] [Google Scholar]
- Fortunato SJ, Menon R, Lombardi SJ. Collagenolytic enzymes (gelatinases) and their inhibitors in human amniochorionic membrane. Am J Obstet Gynecol. 1997;177:731–741. doi: 10.1016/s0002-9378(97)70260-6. [DOI] [PubMed] [Google Scholar]
- Gardella C, Riley DE, Hitti J, Agnew K, Krieger JN, Eschenbach D. Identification and sequencing of bacterial rDNAs in culture-negative amniotic fluid from women in premature labor. Am J Perinatol. 2004;21:319–323. doi: 10.1055/s-2004-831884. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Andrews WW, Mercer BM, Moawad AH, Meis PJ, Iams JD, Das A, Caritis SN, Roberts JM, Miodovnik M, et al. The preterm prediction study: granulocyte colony-stimulating factor and spontaneous preterm birth. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol. 2000;182:625–630. doi: 10.1067/mob.2000.104210. [DOI] [PubMed] [Google Scholar]
- Gomez R, Ghezzi F, Romero R, Munoz H, Tolosa JE, Rojas I. Premature labor and intra-amniotic infection. Clinical aspects and role of the cytokines in diagnosis and pathophysiology. Clin Perinatol. 1995;22:281–342. [PubMed] [Google Scholar]
- Gustafsson C, Hummerdal P, Matthiesen L, Berg G, Ekerfelt C, Ernerudh J. Cytokine secretion in decidual mononuclear cells from term human pregnancy with or without labour: ELISPOT detection of IFN-gamma, IL-4, IL-10, TGF-beta and TNF-alpha. J Reprod Immunol. 2006;71:41–56. doi: 10.1016/j.jri.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Hanna N, Hanna I, Hleb M, Wagner E, Dougherty J, Balkundi D, Padbury J, Sharma S. Gestational age-dependent expression of IL-10 and its receptor in human placental tissues and isolated cytotrophoblasts. J Immunol. 2000;164:5721–5728. doi: 10.4049/jimmunol.164.11.5721. [DOI] [PubMed] [Google Scholar]
- Hitti J, Riley DE, Krohn MA, Hillier SL, Agnew KJ, Krieger JN, Eschenbach DA. Broad-spectrum bacterial rDNA polymerase chain reaction assay for detecting amniotic fluid infection among women in premature labor. Clin Infect Dis. 1997;24:1228–1232. doi: 10.1086/513669. [DOI] [PubMed] [Google Scholar]
- Hollander M, Wolfe DA. 2nd edn. 1999. Nonparametric Statistical Analysis. [Google Scholar]
- Kniss DA, Iams JD. Regulation of parturition update. Endocrine and paracrine effectors of term and preterm labor. Clin Perinatol. 1998;25:819–836. [PubMed] [Google Scholar]
- Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Munson ML. Births: final data for 2002. Natl Vital Stat Rep. 2003;52:1–113. [PubMed] [Google Scholar]
- Menon R, Fortunato SJ. Infection and the role of inflammation in preterm premature rupture of the membranes. Best Pract Res Clin Obstet Gynaecol. 2007;21:467–478. doi: 10.1016/j.bpobgyn.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Menon R, Merialdi M, Lombardi SJ, Fortunato SJ. Differences in the placental membrane cytokine response: a possible explanation for the racial disparity in preterm birth. Am J Reprod Immunol. 2006;56:112–118. doi: 10.1111/j.1600-0897.2006.00394.x. [DOI] [PubMed] [Google Scholar]
- Menon R, Camargo C, Thorsen P, Fortunator SJ. Amniotic fluid interleukin-6 increase is an indicator of preterm birth in Caucasians but not in blacks. Am J Obstet Gynecol. 2007;a doi: 10.1016/j.ajog.2007.06.071. In Press. [DOI] [PubMed] [Google Scholar]
- Menon R, Thorsen P, Vogel I, Jacobsson B, Williams SM, Fortunato SJ. Increased bioavailability of TNF-alpha in African Americans during in vitro infection: predisposing evidence for immune imbalance. Placenta. 2007;b 28:946–950. doi: 10.1016/j.placenta.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Menon R, Williams SM, Fortunato SJ. Amniotic fluid interleukin-1beta and interleukin-8 concentrations: racial disparity in preterm birth. Reprod Sci. 2007;c 14:253–259. doi: 10.1177/1933719107301336. [DOI] [PubMed] [Google Scholar]
- Reilly SL, Ferrell RE, Sing CF. The gender-specific apolipoprotein E genotype influence on the distribution of plasma lipids and apolipoproteins in the population of Rochester, MN. III. Correlations and covariances. Am J Hum Genet. 1994;55:1001–1018. [PMC free article] [PubMed] [Google Scholar]
- Rizzo G, Capponi A, Vlachopoulou A, Angelini E, Grassi C, Romanini C. Interleukin-6 concentrations in cervical secretions in the prediction of intrauterine infection in preterm premature rupture of the membranes. Gynecol Obstet Invest. 1998;46:91–95. doi: 10.1159/000010009. [DOI] [PubMed] [Google Scholar]
- Roberts AK, Monzon-Bordonaba F, Van Deerlin PG, Holder J, Macones GA, Morgan MA, Strauss JF, III, Parry S. Association of polymorphism within the promoter of the tumor necrosis factor alpha gene with increased risk of preterm premature rupture of the fetal membranes. Am J Obstet Gynecol. 1999;180:1297–1302. doi: 10.1016/s0002-9378(99)70632-0. [DOI] [PubMed] [Google Scholar]
- Romero R, Avila C, Santhanam U, Sehgal PB. Amniotic fluid interleukin 6 in preterm labor. Association with infection. J Clin Invest. 1990;85:1392–1400. doi: 10.1172/JCI114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25:21–39. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowsky DW, Novy MJ, Witkin SS, Gravett MG. Dexamethasone or interleukin-10 blocks interleukin-1beta-induced uterine contractions in pregnant rhesus monkeys. Am J Obstet Gynecol. 2003;188:252–263. doi: 10.1067/mob.2003.70. [DOI] [PubMed] [Google Scholar]
- Sadowsky DW, Adams KM, Gravett MG, Witkin SS, Novy MJ. Preterm labor is induced by intraamniotic infusions of interleukin-1beta and tumor necrosis factor-alpha but not by interleukin-6 or interleukin-8 in a nonhuman primate model. Am J Obstet Gynecol. 2006;195:1578–1589. doi: 10.1016/j.ajog.2006.06.072. [DOI] [PubMed] [Google Scholar]
- Sall J, Creighton L, Lehman A. JMP Start Statistics. 3rd edn. Canada: SAS Institute Inc; 2005. [Google Scholar]
- Shurin MR, Lu L, Kalinski P, Stewart-Akers AM, Lotze MT. Th1/Th2 balance in cancer, transplantation and pregnancy. Springer Semin Immunopathol. 1999;21:339–359. doi: 10.1007/BF00812261. [DOI] [PubMed] [Google Scholar]
- Simhan HN, Krohn MA, Roberts JM, Zeevi A, Caritis SN. Interleukin-6 promoter -174 polymorphism and spontaneous preterm birth. Am J Obstet Gynecol. 2003;189:915–918. doi: 10.1067/s0002-9378(03)00843-3. [DOI] [PubMed] [Google Scholar]
- Smirnova MG, Kiselev SL, Gnuchev NV, Birchall JP, Pearson JP. Role of the pro-inflammatory cytokines tumor necrosis factor-alpha, interleukin-1 beta, interleukin-6 and interleukin-8 in the pathogenesis of the otitis media with effusion. Eur Cytokine Netw. 2002;13:161–172. [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. 2nd edn. New York: W.H. Freeman and Company; 1987. Introduction to Biostatistics. [Google Scholar]
- Speer EM, Gentile DA, Zeevi A, Pillage G, Huo D, Skoner DP. Role of single nucleotide polymorphisms of cytokine genes in spontaneous preterm delivery. Hum Immunol. 2006;67:915–923. doi: 10.1016/j.humimm.2006.08.291. [DOI] [PubMed] [Google Scholar]
- StataCorp. Stata Statistical Software: Release 9. Texas: College Station; 2007. [Google Scholar]
- Thellin O, Coumans B, Zorzi W, Igout A, Heinen E. Tolerance to the foeto-placental ‘graft’: ten ways to support a child for nine months. Curr Opin Immunol. 2000;12:731–737. doi: 10.1016/s0952-7915(00)00170-9. [DOI] [PubMed] [Google Scholar]
- Ugwumadu AH. Bacterial vaginosis in pregnancy. Curr Opin Obstet Gynecol. 2002;14:115–118. doi: 10.1097/00001703-200204000-00003. [DOI] [PubMed] [Google Scholar]
- Velez DR, Menon R, Thorsen P, Jiang L, Simhan H, Morgan N, Fortunato SJ, Williams SM. Ethnic differences in interleukin 6 (IL-6) and IL6 receptor genes in spontaneous preterm birth and effects on amniotic fluid protein levels. Ann Hum Genet. 2007;71:586–600. doi: 10.1111/j.1469-1809.2007.00352.x. [DOI] [PubMed] [Google Scholar]
- Weiyuan Z, Li W. Study of interleukin-6 and tumor necrosis factor-alpha levels in maternal serum and amniotic fluid of patients with premature rupture of membranes. J Perinat Med. 1998;26:491–494. doi: 10.1515/jpme.1998.26.6.491. [DOI] [PubMed] [Google Scholar]
- Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, Lei XF, Achong MK. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Invest. 1998;101:311–320. doi: 10.1172/JCI1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


