Abstract
Previous published evidence for the occurrence of an exotic armadillo tick, Amblyomma auricularium (Conil), in Florida is scant, but we found it is fully established and integrated into the state’s tick fauna. We collected 11,192 specimens of this tick from naturalized nine-banded armadillos, Dasypus novemcinctus L., and 14 other species of wild native mammals and birds in Florida, while sampling statewide during 2004 through 2007. In all, we document its specific presence only in 14 contiguous South Florida counties. Moreover, we report the first collections of A. auricularium from the Virginia opossum (Didelphis virginiana Kerr), common raccoon [Procyon lotor (L.)], cotton deermouse [Peromyscus gossypinus (Le Conte)], gray fox [Urocyon cinereoargenteus (Schreber)], eastern spotted skunk [Spilogale putorius (L.)], and white-tailed deer [Odocoileus virginianus (Zimmerman)]. For the first time on birds, we report the collection of this tick from the broad-winged hawk [Buteo platypterus (Vieillot)], northern cardinal [Cardinalis cardinalis (L.)], Carolina wren [Thryothorus ludovicianus (Latham)], gray catbird [Dumetella carolinensis (L.)], and yellow-rumped warbler [Setophaga coronata (L.)]. In addition, we report unattached A. auricularium collected from humans for the first time, and additional new collections from domestic dogs, Canis lupus familiaris L.
Keywords: Dasypus novemcinctus, nine-banded armadillo, surveillance, wildlife tick, Amblyomma auricularium
General published reviews dealing with Amblyomma auricularium (Conil) (Ixodida: Ixodidae) characterize its host specificity as moderately oriented toward armadillos (Xenarthra: Dasypodidae) (Hoogstraal and Aeschlimann 1982) and its geographic range as including Neotropical-Nearctic elements in South America, Central America, and Mexico, with sporadic collections in the United States, i.e., Texas and Florida (Guglielmone et al. 2003a, b). Most documented collections of this tick are from the Dasypodidae, particularly the nine-banded armadillo, Dasypus novemcinctus L., but a wide range of other mammals is reported as less frequent hosts, including wildlife members of the families Myrmecophagidae, Didelphidae, Caviidae, Chinchillidae, Hydrochaeridae, Echimyidae, Cricetidae, Canidae, Mephitidae, and Procyonidae; domestic cattle (Bos primigenius Bojanus), dogs (Canis lupus familiaris L.), horses (Equus ferus caballus L.) (Guglielmone et al. 2003b, Horta et al. 2011), and feral swine (Sus scrofa domesticus Erxleben) (Allan et al. 2001); and experimentally on domestic rabbits (Oryctolagus cuniculus [L.]) (Faccini et al. 2010). In Florida, recognized published reports of A. auricularium are limited to collections from individual armadillos in Glades (Lord and Day,2000) and Hendry (Mertins et al. 2011) Counties, and a single feral swine in Collier County (Allan et al. 2001).
The Southeastern Cooperative Wildlife Disease Study (SCWDS), in collaboration with the U.S. Department of Agriculture, Animal and Plant Health Inspection Service, Veterinary Services (USDA, APHIS, STAS, VS), conducts ectoparasite surveys on free-ranging wildlife in the southeastern United States, with the specific goal of detecting and responding to the otherwise cryptic presence of exotic and invasive ticks at the earliest time possible. For some years, we have concentrated our efforts in the state of Florida, where we have previously documented several new collections of exotic ectoparasites (Hanson et al. 2007, Mertins et al. 2009, Corn et al. 2011, Mertins et al. 2011, Corn et al. 2012). In this report, we document details of the circumscribed occurrence of A. auricularium on a variety of Florida hosts observed during our general and cooperative survey activities in various parts of the state.
Materials and Methods
We systematically collected, identified, and counted ticks and other ectoparasites from wild mammals, birds, reptiles, and amphibians in 63 of Florida’s 67 counties from 2004 through 2007. We sampled no animals in four northern counties (i.e., Calhoun, Escambia, Lafayette, Union), and we sampled only reptiles and amphibians in two additional northern counties (i.e., Gilchrist, Suwanee), but we examined mammals and birds in all other counties.
Mammals were captured via live traps (Havahart, Lititz, PA, and Tomahawk Live Trap Co., Tomahawk, WI) or examined at hunter check stations. Birds were captured via mist nets (Avinet, Inc., Dryden, NY). Reptiles and amphibians were captured by hand. In a few cases, the field biologists conducting these surveys collected additional ticks from themselves. Mesomammals were immobilized chemically using a combination of ketamine hydrochloride and xylazine hydrochloride; birds, reptiles, amphibians, and rodents were restrained manually; and all captured wildlife hosts were released after examination. Live and hunter-killed mammals, birds, reptiles, and amphibians were examined visually, and all ectoparasites seen were collected, stored in 70% ethanol, and identified at the USDA VS National Veterinary Services Laboratories (NVSL), Ames, IA. Field biologists conducting these activities generally examined themselves and their clothing at the end of each workday, collecting and preserving any parasites encountered. Representative voucher specimens for each ectoparasite species collected, including A. auricularium, are deposited in the parasitology reference collection at the NVSL.
In addition, one-third (33.6%) of the ectoparastite collections we examined and identified during the 2004–2007 survey period were from second-party sources, primarily the Florida Fish and Wildlife Conservation Commission (FFWCC) and various wildlife rehabilitation facilities. These samples largely consisted of ectoparasites collected by rehabilitators from ailing wildlife in captivity, or in the field, by cooperating FFWCC personnel, primarily from hunter-killed game animals. The fact that over a third of our total analyzed parasite samples came from cooperator-provided hosts is misleading in that this figure derives mostly from the large numbers of parasite-infested deer and feral swine examined at check stations during hunting seasons. Most importantly, the intensity and rigor of our cooperator-provided parasite acquisitions may have differed from sample to sample and also overall from our own formal survey samples. We analyzed these samples separately from our own.
Finally, we supplemented the information from our systematic survey with records of A. auricularium collections in Florida gathered from the archives of the U.S. National Tick Surveillance Program (NTSP) operated since 1962 by the USDA and maintained at the NVSL. Some of these records were officially documented in abbreviated form in various NTSP annual reports that had limited distribution, and some are previously unpublished. We also pursued and cite an early, unpublished Florida collection of A. auricularium held in the U.S. National Tick Collection (USNTC) curated at Georgia Southern University, Statesboro, GA.
Identifications of A. auricularium specimens were accomplished or confirmed by morphological methods using standard published keys and descriptions (Jones et al. 1972; Amorim and Serra-Freire 2000; Voltzit 2007; Martins et al. 2010, 2014; Guzmán-Cornejo et al. 2011). Initially, we identified A. auricularium nymphs—not described until Martins et al. (2010)—only indirectly, through use of the key in Keirans and Durden (1998), wherein specimens identify to Amblyomma inornatum (Banks), a similar species that occurs in the United States only in South Texas. We tentatively assumed that Florida Amblyomma nymphs—identified in this way as “A. inornatum” and associated with identifiable adult armadillo ticks—were actually A. auricularium, until later confirmation using information in Martins et al. (2010, 2014).
Results
Between 2004 and 2007, we and our cooperators examined 5,202 wild mammals (42 species), 2,231 wild birds (163 species), 267 wild reptiles (40 species), and 88 wild amphibians (8 species) for ectoparasites during operations conducted in 63 Florida counties. Although most of these animals (3,798 mammals [73.0%] and 345 birds [15.5%]) carried ectoparasites of some sort, we specifically identified 11,148 specimens of A. auricularium from 616 individual infested mammals (10 species) and 44 specimens from 15 birds (5 species; Tables 1 and 2). No reptiles or amphibians were infested by A. auricularium, but 80 of 267 examined reptiles (30%) were infested by other ectoparasites (Corn et al. 2011); none of 88 examined amphibians was infested by ectoparasites.
Table 1.
Amblyomma auricularium ticks collected from surveyed wildlife in Florida, 2004–2007
| Host | Prevalencea | Statistics | Males | Females | Nymphs | Larvae |
|---|---|---|---|---|---|---|
| Didelphidae | ||||||
| Virginia opossum | 361/710 (50.8) | Ticks collected | 6 | 6 | 3453 | 6231 |
| Didelphis virginiana | Animals infested | 3 | 5 | 310 | 267 | |
| Mean intensityb | 2.0 | 1.2 | 11.2 | 23.3 | ||
| Mean abundancec | <0.1 | <0.1 | 4.9 | 8.8 | ||
| Tick burden componentd | 5.1 | 10.3 | 90.1 | 90.3 | ||
| Dasypodidae | ||||||
| Nine-banded armadillo | 9/16 (56.3) | Ticks collected | 95 | 43 | 27 | 92 |
| Dasypus novemcinctus | Animals infested | 10 | 10 | 5 | 3 | |
| Mean intensity | 9.5 | 4.3 | 5.4 | 30.7 | ||
| Mean abundance | 5.9 | 2.7 | 1.7 | 5.8 | ||
| Tick burden component | 80.5 | 74.1 | 0.7 | 1.3 | ||
| Canidae | ||||||
| Gray fox | 4/14 (28.6) | Ticks collected | 0 | 0 | 7 | 17 |
| Urocyon cinereoargenteus | Animals infested | 0 | 0 | 3 | 2 | |
| Mean intensity | 0 | 0 | 2.3 | 8.5 | ||
| Mean abundance | 0 | 0 | 0.5 | 1.2 | ||
| Tick burden component | 0 | 0 | 0.2 | 0.2 | ||
| Mephitidae | ||||||
| Spotted skunk | 16/37 (43.2) | Ticks collected | 1 | 0 | 68 | 55 |
| Spilogale putorius | Animals infested | 1 | 0 | 13 | 10 | |
| Mean intensity | 1.0 | 0 | 5.2 | 5.5 | ||
| Mean abundance | <0.1 | 0 | 1.8 | 1.5 | ||
| Tick burden component | 0.8 | 0 | 1.8 | 0.8 | ||
| Procyonidae | ||||||
| Raccoon | 113/681 (16.6) | Ticks collected | 16 | 9 | 235 | 326 |
| Procyon lotor | Animals infested | 15 | 9 | 72 | 54 | |
| Mean intensity | 1.1 | 1.0 | 3.3 | 6.0 | ||
| Mean abundance | <0.1 | <0.1 | 0.3 | 0.5 | ||
| Tick burden component | 13.6 | 15.5 | 6.1 | 4.7 | ||
| Cricetidae | ||||||
| Cotton deermouse | 17/293 (5.8) | Ticks collected | 0 | 0 | 0 | 31 |
| Peromyscus gossypinus | Animals infested | 0 | 0 | 0 | 17 | |
| Mean intensity | 0 | 0 | 0 | 1.8 | ||
| Mean abundance | 0 | 0 | 0 | 0.1 | ||
| Tick burden component | 0 | 0 | 0 | 0.4 | ||
| Hispid cotton rat | 51/504 (10.1) | Ticks collected | 0 | 0 | 37 | 118 |
| Sigmodon hispidus | Animals infested | 0 | 0 | 22 | 35 | |
| Mean intensity | 0 | 0 | 1.7 | 3.4 | ||
| Mean abundance | 0 | 0 | 0.1 | 0.2 | ||
| Tick burden component | 0 | 0 | 1.0 | 1.7 | ||
| Troglodytidae | ||||||
| Carolina wren | 7/104 (6.7) | Ticks collected | 0 | 0 | 7 | 5 |
| Thryothorus ludovicianus | Animals infested | 0 | 0 | 4 | 4 | |
| Mean intensity | 0 | 0 | 1.8 | 1.3 | ||
| Mean abundance | 0 | 0 | 0.1 | <0.1 | ||
| Tick burden component | 0 | 0 | 0.2 | 0.1 | ||
| Mimidae | ||||||
| Gray catbird | 1/249 (0.4) | Ticks collected | 0 | 0 | 0 | 1 |
| Dumetella carolinensis | Animals infested | 0 | 0 | 0 | 1 | |
| Mean intensity | 0 | 0 | 0 | 1.0 | ||
| Mean abundance | 0 | 0 | 0 | <0.1 | ||
| Tick burden component | 0 | 0 | 0 | <0.1 | ||
| Emberizidae | ||||||
| Yellow-rumped warbler | 1/233 (0.4) | Ticks collected | 0 | 0 | 0 | 1 |
| Dendroica coronata | Animals infested | 0 | 0 | 0 | 1 | |
| Mean intensity | 0 | 0 | 0 | 1.0 | ||
| Mean abundance | 0 | 0 | 0 | <0.1 | ||
| Tick burden component | 0 | 0 | 0 | <0.1 | ||
| Cardinalidae | ||||||
| Northern cardinal | 5/222 (2.3) | Ticks collected | 0 | 0 | 0 | 25 |
| Cardinalis cardinalis | Animals infested | 0 | 0 | 0 | 5 | |
| Mean intensity | 0 | 0 | 0 | 5.0 | ||
| Mean abundance | 0 | 0 | 0 | 0.1 | ||
| Tick burden component | 0 | 0 | 0 | 0.4 | ||
| Total ticks collected | 118 | 58 | 3834 | 6902 |
Data from the 12 counties where A. auricularium was found via SCWDS animal examinations. Both prevalence and tick-burden-component numbers are expressed as percentages.
Prevalence, infested animals per host species/examined animals per host species.
Mean intensity, ticks collected per host species/animals infested per host species.
Mean abundance, ticks collected per host species/animals examined per host species.
Tick burden component, ticks of given life stage collected from given host species/total ticks of given life stage collected from all hosts combined.
Table 2.
Amblyomma auricularium ticks collected from wildlife provided by cooperators in Florida, 2004–2007
| Host | Prevalencea | Statistics | Males | Females | Nymphs | Larvae |
|---|---|---|---|---|---|---|
| Virginia opossum | 10/48 (20.8) | Ticks collected | 0 | 0 | 40 | 18 |
| Didelphis virginiana | Animals infested | 0 | 0 | 10 | 2 | |
| Nine-banded armadillo | 10/12 (83.3) | Ticks collected | 70 | 55 | 23 | 4 |
| Dasypus novemcinctus | Animals infested | 10 | 9 | 5 | 2 | |
| Gray fox | 2/4 (50.0) | Ticks collected | 4 | 0 | 1 | 0 |
| Urocyon cinereoargenteus | Animals infested | 2 | 0 | 1 | 0 | |
| Raccoon | 4/31 (12.9) | Ticks collected | 1 | 5 | 19 | 2 |
| Procyon lotor | Animals infested | 1 | 2 | 3 | 2 | |
| Florida panther | 2/115 (1.7) | Ticks collected | 1 | 0 | 1 | 1 |
| Puma concolor | Animals infested | 1 | 0 | 1 | 1 | |
| Feral swine | 16/190 (8.4) | Ticks collected | 16 | 12 | 1 | 0 |
| Sus scrofa domesticus | Animals infested | 12 | 6 | 1 | 0 | |
| White-tailed deer | 1/228 (0.4) | Ticks collected | 0 | 0 | 0 | 1 |
| Odocoileus virginianus | Animals infested | 0 | 0 | 0 | 1 | |
| Broad-winged hawk | 1/1 (100.0) | Ticks collected | 0 | 0 | 0 | 5 |
| Buteo platypterus | Animals infested | 0 | 0 | 0 | 1 | |
| Total ticks collected | 92 | 72 | 85 | 31 |
Data from only the 12 counties where A. auricularium was found during statewide activities, and here only for known hosts of the tick examined or provided by cooperators.
Prevalence, infested animals per host species/examined animals per host species, expressed as a percentage.
Overall, although we were particularly looking for the presence of any exotic ticks, we encountered and identified ectoparasites representative of five large taxonomic groups: ticks (Acari: Ixodida), mites (Acari: Mesostigmata, Prostigmata, Astigmatina), lice (Insecta: Phthiraptera), flies (Insecta: Diptera), and fleas (Insecta: Siphonaptera). Speaking only of the identified ticks, we encountered 4 species of Argasidae (i.e., 1 Argas sp., 2 Carios spp., 1 Ornithodoros sp.) and 17 species of Ixodidae (i.e., 8 Amblyomma spp., 2 Dermacentor spp., 1 Haemaphysalis sp., 5 Ixodes spp., 1 Rhipicephalus sp.).
We report the first verified collections of A. auricularium from the following mammalian hosts: Virginia opossum (Didelphis virginiana Kerr), common raccoon (Procyon lotor [L.]), cotton deermouse (Peromyscus gossypinus Le Conte), gray fox (Urocyon cinereoargenteus [Schreber]), eastern spotted skunk (Spilogale putorius [L.]), and white-tailed deer (Odocoileus virginianus [Zimmerman]).
We also report first-time collections of A. auricularium from the following bird hosts: broad-winged hawk (Buteo platypterus [Vieillot]), northern cardinal (Cardinalis cardinalis [L.]), Carolina wren [Thryothorus ludovicianus (Latham)], gray catbird (Dumetella carolinensis [L.]), and yellow-rumped warbler (Setophaga coronata [L.]).
In addition, the field biologists making these collections sometimes found various ticks upon themselves, and they collected A. auricularium specimens that way six times, constituting the first documented collections from humans. In these cases, nymphs were collected three times (n = 9, 5, 1) and larvae three times (n = 1, 1, 1). These collections are not included in the numbers of ticks collected from wildlife in this report. Unlike most of the ticks collected from wildlife hosts, all specimens from humans were unattached and unfed when collected. Based upon fieldwork practices, none of these ticks was on its collector for any more than the day when found, providing limited temporal access for possible attachment. However, even if longer exposures were to happen, we suspect that humans are not very likely hosts for A. auricularium, and these ticks were no more than transient stragglers.
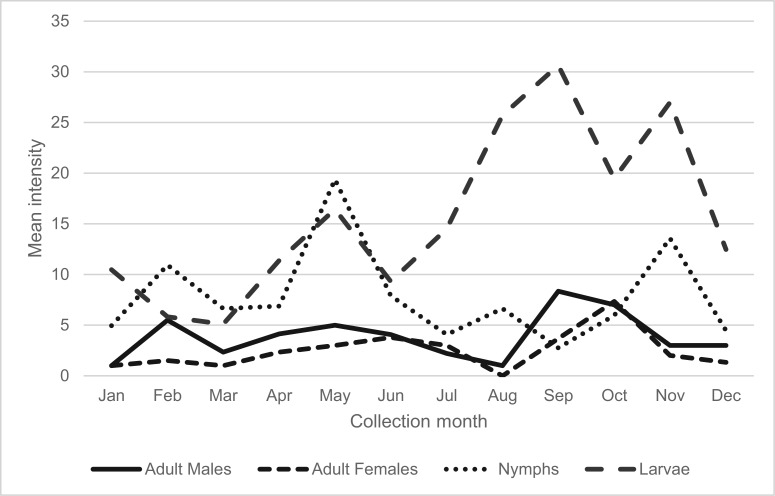
Using the data we accumulated on the A. auricularium suprapopulation in South Florida, i.e., cumulative numbers of all active stages collected from all hosts over 48 mo of host sampling, we assessed possible seasonal variability in occurrences by calculating the mean intensity (Margolis et al. 1982, Bush et al. 1997) of infestation for each life stage on a monthly basis (Fig. 1).
Fig. 1.
Mean intensities of infestations by month for four life stages in the 12-county South Florida suprapopulation of A. auricularium collected from all hosts, 2004–2007.
Compared with our formal 2004–2007 survey results, the NTSP archives provided relatively few Florida collection records for A. auricularium representing other times or sources, i.e., 14 accessions spread over the years 1998, 1999, 2000, and 2010 (Supp.Table 1 [online only]). The primary contributions of these collections were documentation of A. auricularium in two additional new South Florida counties, St. Lucie and Okeechobee, and three additional collection records from domestic dogs. Otherwise, these records merely reinforced our survey findings.
We’ve continued to survey Florida wildlife ectoparasites over the course of several years since 2007, with largely similar results. Not all of these later samples are yet analyzed, but thus far, we have accumulated hundreds of additional collections of A. auricularium from a similar array of wildlife species without documenting any additional new host species or new county records.
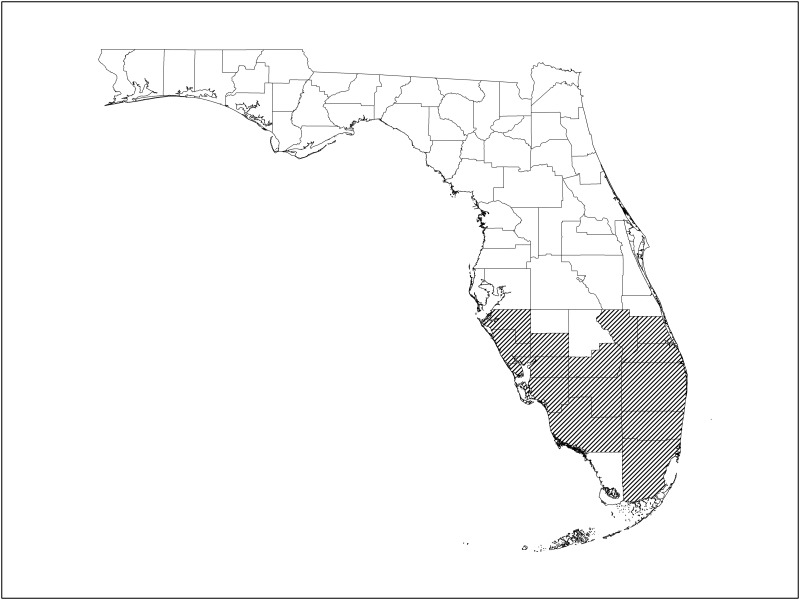
Altogether, we compiled collection records for A. auricularium from 14 contiguous South Florida counties (Fig. 2): Broward, Charlotte, Collier, DeSoto, Glades, Hendry, Lee, Manatee, Martin, Miami-Dade, Okeechobee, Palm Beach, St. Lucie, and Sarasota. Twelve of these county records (i.e., all except Okeechobee and St. Lucie Counties) were based upon tick specimens collected during our formal 2004–2007 survey activities, and survey data from only those 12 counties were used in our assessment of A. auricularium population parameters in Florida (Fig. 1, Tables 1 and 2). We know of no A. auricularium collection records from Florida outside of these 14 counties, and for background context, we provide a truncated summary (Supp. Table 2 [online only]) of our survey efforts in 35 other counties of the state where we sampled known A. auricularium hosts, including 326 opossums, 22 armadillos, 8 gray foxes, 1 spotted skunk, 378 raccoons, 164 cotton deermice, 106 hispid cotton rats, 8 feral swine, 66 Carolina wrens, 40 catbirds, 102 cardinals, and 25 yellow-rumped warblers.
Fig. 2.
South Florida counties (hatched area) from which A. auricularium ticks have been collected.
Discussion
Neither nine-banded armadillos nor their dependent A. auricularium ticks are native residents of the United States, although by the early 1850s, D. novemcinctus were found in the southern tip of Texas, probably having crossed the Rio Grande from Mexico (Lever 1985). Subsequent dispersal movements expanded their U.S. range northwards and eastwards from South Texas to include most of the Southeast south of 38 degrees north latitude (Taulman and Robbins 1996, Hofmann 2005). The first armadillos in peninsular Florida, however, were deliberately introduced during World War I and beyond (Bailey 1924, Lever 1985), although viable feral populations did not establish until about 1924 in Brevard County, following releases from a private zoo in Titusville (Taulman and Robbins 1996).
Comparable evidence about the origins, arrival, and early occurrence of A. auricularium in the United States before the 1980s is lacking, but doubtless, they must have codispersed with the natural range expansion of armadillos into Texas and beyond. Curiously, despite the long-term and present-day common occurrence of armadillos in multiple states situated between and north of Texas and Florida, neither the NTSP nor any other entity has documented the coexistence of A. auricularium there, suggesting lack of tick survivability in these Gulf States and raising the possibility that Florida populations of the tick may have originated not by natural dispersal, but solely from progenitors infesting armadillos released by humans in the state.
The recognized geographic distribution of A. auricularium is largely Neotropical, and most of what we know of the hosts and bionomics of this tick comes from studies in that region (Guglielmone et al. 2003a, b; Guzmán-Cornejo et al. 2011). Until now, data from Nearctic collections of this tick were limited. Forrester (1992) provided the most comprehensive treatment of wildlife parasites in Florida, and he made no mention of A. auricularium on armadillos or on any other resident mammal species. Lord and Day (2000) asserted that their February 1991 collection of adult male and female A. auricularium from D. novemcinctus in Glades County, FL, was the first one documented in the United States, but in fact, two earlier collections were listed in annual reports for the NTSP. Thus, the earliest documented and verifiable A. auricularium collection in the United States consisted of a single female tick taken from a domestic cow in Cameron County, TX, in September 1981, and identified at the NVSL (USDA 1982); this tick is now in the USNTC (accession RML 115881). The other early-published NTSP collection comprised a male and a female tick from a dog in Broward County, FL, in November 1989 (USDA 1994). The 1981 Texas collection also was repeated by Keirans and Durden (2001), and Burridge (2011) reiterated both this Texas collection and the 1989 Florida collection. In addition, L. Beati (personal communication) informs us that the USNTC contains an even earlier, previously unpublished collection of a female A. auricularium taken from D. novemcinctus in Collier County, FL, in November 1980 (accession RML 114937). A still earlier potential collection may be one documented by Irons et al. (1952), who reported finding two Amblyomma inornatum (Banks) ticks (stage not given) on D. novemcinctus examined in Zavala County, TX, during 1950–1951. Although we have found both A. auricularium and A. inornatum on armadillos in South Texas in recent surveys (unpublished data), the former currently are more prevalent, and all stages of both tick species are very similar morphologically, making a species misidentification possible during the earlier Irons et al. (1952) study, when the potential presence of A. auricularium was unsuspected. Current disposition of the Irons et al. specimens is unknown. More recently, Allan et al. (2001) documented collection of a single male A. auricularium from a feral swine, in Collier County, FL, and Mertins et al. (2011) found 23 adult ticks on a D. novemcinctus in Hendry County. Thus, before presentation of our results, A. auricularium was known only sparingly from four counties in Florida (i.e., Broward, Collier, Glades, and Hendry) and from three host species there (i.e., armadillos, dog, and feral swine).
As expected, immigrant D. novemcinctus proved to be an excellent host for all feeding stages of exotic A. auricularium in Florida (Tables 1 and 2). Surprisingly, however, in our formal survey (Table 1), armadillos were the best host species for this tick only by the criterion of numbers of adult ticks they hosted. The largest absolute number and the greatest proportion of all adult ticks collected in our survey came from armadillo hosts, and the mean abundance and mean intensity statistics for these adult infestations were greater for armadillos than for any other sampled host species. However, by nearly every other measure in our survey (Table 1), one or another of the resident Florida mammal host species we examined seemed to better serve the life cycle needs of this exotic tick. Results from the slightly smaller number of armadillo hosts examined by our cooperators (Table 2) found a remarkable overall A. auricularium prevalence on them of 83.3% and again found them the best hosts for adult ticks. In the context of the smaller numbers of all main tick hosts examined by the cooperators, however, armadillos also seemed highly suitable hosts for immature ticks, as well, partially exceeded in this role only by opossums.
Six of the 10 infested mammalian species we or our cooperators encountered represented new host records for A. auricularium, and the taxonomic families Cervidae and Felidae are not among those previously known to include host species for this tick in the neotropics. Among the four mammals in our samples previously recorded as hosts, feral swine and Florida panther, Puma concolor coryi (Bangs), were documented only in Florida (Allan et al. 2001, Shock 2014), but hispid cotton rats and nine-banded armadillos were already documented as Neotropical hosts—several or numerous times, respectively—along with the extant Nearctic collection records for the latter host.
Among our new host records, only cotton deermice and eastern spotted skunks occupy exclusively Nearctic geographic distributions, isolating them from exposure to A. auricularium infestation until the relatively recent invasion of their ranges by the ticks and their primary armadillo hosts. The natural distributions of Virginia opossum, common raccoon, and white-tailed deer, however, extend southwards to various extents into the Neotropical Region of Mexico, Central, and even South America, meaning that these mammals have historically shared parts of their geographic ranges with A. auricularium. (Although ours are the first known collections of A. auricularium from raccoons, while we were preparing this report, Burmúdez et al. [2015] reported that they collected a single male [stage uncertain, possibly a nymph that molted] from a P. lotor in Panama during the period 2010–2014.) Why opossums, raccoons, and deer have not been recorded previously as hosts for the tick is unknown, although our survey results show that the prevalence of this tick on Florida deer, at least, seems extremely low, suggesting that cervids may not be very acceptable hosts. Our cooperators examined 228 deer in the 12 infested Florida counties, and they found 1,159 ticks of five different species on them, but we identified only one larval A. auricularium within this effort (Table 2).
On the other hand, our observations show that Virginia opossums are highly acceptable hosts for A. auricularium, even rivaling sympatric Florida armadillos, according to several measures (Table 1). Our trapping methods had limited success in catching armadillos, yielding over 20 times as many opossums as armadillos for examination statewide, and 44 times as many in the infested counties, but within the latter unequal samples, the overall prevalences of A. auricularium on the two host species were close, 50.8 vs. 56.3%, respectively (Table 1). The mean intensities and mean abundances of the immature stages on the two host species display somewhat mutually similar trends (though largely favoring opossums), as well, but our numerical data clearly show that adult A. auricularium are much more likely to infest Florida armadillos than sympatric opossums, whereas immature stages seem to notably infest opossums over armadillos. In fact, ∼90% of all immature A. auricularium we collected during our survey were taken from abundant opossum hosts, whereas armadillos supplied <2%. Although we have no independent data on the actual relative population densities of armadillos and opossums in the areas we surveyed, our tick sampling data seem to suggest that opossums, in their abundance, may now serve as hosts every bit as important for the long-term survival of A. auricularium in South Florida as do armadillos.
Other sampled Florida mesomammals seem to serve well as A. auricularium hosts, too, although their overall importance may not rank with opossums and armadillos (Table 1). Most notably, the overall prevalence of A. auricularium on spotted skunks, 43.2%, was the highest we measured for any encountered host species beyond opossums and armadillos. However, the number of skunks examined was relatively small, composing <4% of the number of sampled opossums statewide, and 5.2% in the 12 infested counties, but equal to 80% of the examined armadillo numbers statewide, and 230% in the infested counties. Our skunks yielded only one adult tick, but immature stages were prevalent on them, especially the nymphs, which occurred at a mean intensity 96% of that seen on infested armadillos, about 46% of that on opossums, and higher than on any of the other host species sampled. Mean abundance of nymphal ticks on spotted skunks was higher than that on any other host save for opossums, as well, suggesting that both opossums and skunks are highly suitable hosts for nymphs. The statistics for larval numbers on spotted skunks tell a similar story of relative host suitability, but the relatively small evident population size of available skunk hosts, and the small proportion of our total tick collections (especially adult ticks) taken from skunks suggest that these animals probably are only marginally important in sustaining A. auricularium in Florida.
Beyond skunks, we examined 1,098 raccoons statewide during our survey and 681 in the 12 infested counties (Table 1), slightly less than the number of 710 local opossums sampled, but the overall prevalence of A. auricularium on them was only 16.6%, equal to 32.7% of the rate on opossums and 38.4% of the rate on skunks. As seen in all host species in the survey but armadillos, the mean abundance of adult ticks on raccoons was low, but still, the proportion of all adult ticks we collected in our survey that came from raccoons (14.2%) was second only to that taken from armadillos (78.4%). Even opossums, which seem to be highly suitable hosts for immature A. auricularium, yielded only 6.8% of the adult ticks in our survey. Among all other hosts, only feral swine examined by our cooperators yielded adult ticks in such notable numbers, i.e., 28 ticks from 16 infested animals in the 12 infested counties (Table 2). These data together suggest that raccoons, because of their abundance and evident relative suitability as hosts for adult ticks, may be an important resource in the life cycle of A. auricularium in Florida. The population statistics for immature A. auricularium stages on raccoons (Table 1) seem less convincing about the relative importance of this host for these ticks in the Florida environment, suggesting that raccoons host moderate numbers of both nymphs and larvae, but generally at lower mean intensities and mean abundances than other sympatric mesomammals.
We and our cooperators examined 28 gray foxes during statewide operations, but only 14 of them were in the formal survey in infested counties (Table 1), and overall A. auricularium prevalence in the infested counties was 28.6% on foxes. Although our sampling efforts suggest that gray fox populations in South Florida may be relatively small, our observations indicate a substantial proportion of them are infested by small numbers of the tick. The geographic ranges of Virginia opossums, common raccoons, and gray foxes all extend into parts of the Neotropical Region of Mexico and Central America, but of these three mesomammals now shown to be suitable hosts for A. auricularium in Florida, only U. cinereoargenteus is previously recorded as infested by this tick (one nymph) in the tropical environment (Varma 1973). The reliability of this early, immature tick identification and host record is subject to considerable skepticism, however, because the nymph of A. auricularium was not formally described until recently (Martins et al. 2010). In any case, the gray fox probably is not a notably important host for A. auricularium anywhere that they are sympatric.
The largest mammals we and our cooperators examined generally seemed to be infrequent hosts for A. auricularium (Table 2); however, although many of these animals were examined during the fall hunting season, we don’t think that seasonal depression in tick host-seeking activities (Fig. 1) affected these results because all stages of the tick were present on other wildlife during this time period. Just one of 971 white-tailed deer examined statewide (228 in the 12 infested counties) yielded a single larval specimen. This species is previously unreported as a host for this tick, but deer seem to be of negligible importance to its ecology in Florida. One hundred fourteen American black bears, Ursus americanus Pallas, also were among the larger mammals opportunistically examined by cooperators statewide (five in the 12 infested counties), but no A. auricularium was found on them.
Of the larger host animals examined, only feral swine bore notable numbers of A. auricularium (Table 2), almost exclusively adults present on >8% of cooperator-examined animals in the 12 infested counties. In fact, the absolute number of adult ticks found on cooperator-sampled feral swine (28, the equivalent of 15.9% of total adults collected in our own formal survey in the same area) was exceeded only by the numbers on sympatric survey-sampled armadillos (138, or 78.4% of total), and it actually surpassed the number on surveyed raccoons (25, or 14.2% of total), despite the fact that both opossums and raccoons—two often infested native wildlife hosts—were examined in numbers ∼3.5 times larger than the 190 sampled sympatric feral swine. Armadillos are undoubtedly the primary host used by adult A. auricularium in Florida, but despite their presumed smaller population density in surveyed areas, feral swine may be marginally important hosts, as well. Indeed, except for armadillos, the only previously well-documented Florida host for this tick was a feral swine (Allan et al. 2001). We also found a single obscure, older literature record for a collection of A. auricularium from a “hog” (not identified as feral/domestic) in Central America (Tonn et al. 1963). Perchance some shared but unrecognized similarities in behaviors or habitat use increase the likelihood that both feral swine and armadillos will encounter and acquire questing adult A. auricularium ticks in the Florida environment.
Limited observations in the Neotropical Region previously suggested that small mammals and various armadillo species probably were the primary hosts for immature stages of A. auricularium (Guglielmone et al. 2003b). During the course of our statewide ectoparasite survey in Florida, we trapped and processed 1,373 rodents representing 20 species, although in the 12 infested counties, we sampled only 928 rodents from 13 species. Among the sampled animals, only two species, cotton deermice and hispid cotton rats, yielded A. auricularium specimens, all immature (Table 1). Tick prevalence and mean intensity on cotton deermice were both moderately low, and all collected ticks were larvae. The tick numbers for hispid cotton rats were somewhat larger, with an infestation rate of 10.1% and a mean intensity of infestation nearly double that of ticks on sampled cotton deermice. In general, our results suggest that small mammals do not seem to play an important role in maintenance of A. auricularium populations in Florida, with only hispid cotton rats sustaining notable levels of infestation.
Until recently, avian hosts were unknown for A. auricularium. After we finished our field work and while we were preparing this report, however, two reports of this tick infesting Neotropical birds appeared. Lugarini et al. (2015) studied ticks on endemic, nonmigratory birds at four sites in the coastal Atlantic Forest and Caatinga ecoregions of northeastern Brazil between 2010 and 2013. They examined a total of 1,984 birds and found 959 immature ticks of five species on 106 infested hosts. Amblyomma auricularium were present in three of the four study sites, and nymphs (17 total) or larvae (14 total) were collected at low intensities from 17 avian species. Cohen et al. (2015) reported three collections of immature A. auricularium on spring immigrant songbirds arriving in Gulf Coastal Texas, USA, from Central America, including one tick each on gray catbird, ovenbird [Seirus aurocapilla (L.)], and painted bunting [Passerina ciris (L.)] hosts. These workers examined 3,844 individual captures of 85 bird species in their study during 2013 and 2014. Our earlier, statewide ectoparasite survey activities in Florida, combined with samples from cooperators, included examinations of 2,231 individual birds representing 163 avian species, and a small number (15) of the sampled birds yielded a few immature specimens of the tick (Tables 1 and 2), constituting four new host records and an additional, but actually the earliest, collection of A. auricularium on a catbird. All of our A. auricularium-infested birds were encountered only within the 12 South Florida counties where infested mammals also were encountered, suggesting that the avian infestations probably were acquired locally. Within the 12-county area, we and our cooperators sampled 1,603 individual birds representing 127 species. All five infested avian species sustain geographic distributions that overlap Neotropical areas where A. auricularium also occurs, but only the catbird is otherwise recorded as a host for this tick (Cohen et al. 2015). Three of the five avian host species are noted for foraging extensively on the ground in leaf litter, where they were most likely to have acquired their infestations. However, neither yellow-rumped warblers nor broad-winged hawks spend much time on the ground, greatly limiting their exposure to questing ticks. Only Carolina wrens accumulated an infestation rate over 5% in the infested 12-county area (Table 1), and only they were infested by both nymphs and larvae. In general, however, our results suggest that birds probably are rare and unimportant hosts in the A. auricularium life cycle in Florida.
In our survey, 80 of 267 captured and examined reptiles were infested by ectoparasites of some sort, but none of them bore A. auricularium. We found one published report (Guzmán-Cornejo et al. 2011) of a female A. auricularium in the Mexican Colección Nacional de Ácaros, allegedly found in 1980 on an “iguana” (no species named) in Michoacán, Mexico. Any other association with reptiles is unknown to us, and we think that poikilothermic animals probably are rarely used and unsuitable hosts for this tick.
Contrary to some published skepticism (Guglielmone et al. 2003b, Saraiva et al. 2013), we found that A. auricularium is not only widely established in South Florida, but it has become a fully integrated member of the wildlife tick fauna of the state. Although introduced nine-banded armadillos may still serve as the primary hosts for adult A. auricularium, several native mesomammals also host the adults, and some of them evidently serve as even better hosts for the immature stages. We also found small to moderate numbers of the tick feeding on rodents, birds, and two larger mammals. In our statewide survey, we found flourishing populations of A. auricularium in only the southern half of peninsular Florida. This observation, combined with the seeming lack of documented A. auricularium collections on armadillos in states north of Texas and Florida, suggests to us that perhaps this tick is unable to survive prevailing winter conditions north of South Texas and South Florida, and thus, prevalent populations of the tick in South Florida may derive not from natural dispersion through the Gulf States from Texas, but instead, from progenitors on armadillos historically and independently introduced by humans into the area. Migrant birds might be an alternative source of immature A. auricularium arriving in Florida from Neotropical origins, but based upon our sampling results and those of the only other study finding this tick on migratory avian hosts (Cohen et al. 2015), we think the observed small numbers of such immigrant ticks are less likely to found new extralimital breeding populations than they are to enhance the genetic diversity of an existing such population. Cohen et al. (2015) project from their data that >19 million immature Neotropical ticks probably arrive annually in North America on migrating birds, but to date, no evidence exists that any exotic tick species has invaded and established a Nearctic population as a result.
Using monthly mean intensity of infestation as an index of possible seasonal variation in activities by the various life stages of A. auricularium (Fig. 1), we observed generally depressed tick numbers during the winter months, particularly in December and January, although the lowest larval intensities came during February and March. Intensities of both adult males and females were relatively low and stable throughout the year. Infestations by immature stages, however, showed more distinct seasonal patterns, with nymphal activity generally highest in the first half of the year, and larval activity greatest in the last half of the year. Nymphal infestation intensities peaked in May, with a secondary peak in November, whereas larval infestations were most intense in August through November and secondarily so again in May.
Other exotic ticks with tropical origins previously have been introduced and established in Florida, e.g., Amblyomma dissimile Koch; A. rotundatum Koch; the tropical horse tick, Anocentor nitens (Neumann); and the southern cattle tick, Rhipicephalus (Boophilus) microplus (Canestrini). From what we know of their past and/or present geographic distributions in the state (Marshall et al. 1963, Strickland and Gerrish 1964, Oliver et al. 1993, Foster et al. 2000), climatic factors may limit the northern survivability of these ticks in the state, as well. Two of the species, A. nitens and R. microplus, were economically important and previously extirpated from Florida, but both historically and presently, all four species had or have their strongest presence in the southern half of the peninsula.
The anomalous absence of A. auricularium in only one South Florida county (i.e., the most southerly, Monroe) is curious and not readily explicable. We examined 757 potential host animals in Monroe County, including 237 individuals of the known mammalian host species (Supp. Table 2 [online only]), and third most of any county in our survey, but none was infested. Environmental conditions in the southern tip of Florida are decidedly different from those prevailing further north, and perhaps some undetermined feature(s) of this unique environment is/are inimical to the local survival of A. aruicularium. Moreover, the absence of any armadillos in our captured and examined potential hosts in Monroe County may be an important aspect of this outcome.
Finally, in our wildlife survey, we incidentally noted a remarkable infrequency of the lone star tick, Anblyomma americanum (L.), in South Florida samples. Smith (1977) and Allan et al. (2001) also made similar observations. During the four years of our survey, from the 14 South Florida counties where we found A. auricularium, plus Monroe County (where we didn’t), we encountered only 12 A. americanum collections from five wildlife host species. Throughout other parts of the Southeast, including northern Florida, A. americanum is frequent to nearly ubiquitous in ectoparasite samples from wildlife, making its near absence in South Florida a curious and unexplained phenomenon. However, the notable paucity of A. americanum ticks in South Florida habitats and on wildlife hosts there seems to leave largely untapped a major local environmental resource that these ticks usually exploit efficiently elsewhere. Possible scramble competition for habitat and hosts between sympatric tick species is not a well-documented phenomenon, and furthermore, published records of lone star ticks infesting armadillos are practically nonexistent. Indeed, Brennan (1945) observed that armadillos in Texas are of no importance and not suitable hosts for lone star ticks, and we found absolutely no A. americanum specimens on our sampled armadillos anywhere in Florida, so we doubt that direct A. americanum–A. auricularium interactions are at play on this key host in South Florida. Instead, given our observations showing that invasive South Florida A. auricularium ticks now feed on a notable but selective segment of the broad range of hosts normally used by lone star ticks, we are tempted to suggest that perhaps the surprising rise of A. auricularium populations here (except in Monroe County) was facilitated by unimpeded exploitation of a variety of abundant, available host resources in a salubrious new environment.
Supplementary Material
Acknowledgments
We thank SCWDS staff members R. Aldridge, B. Barton, A. Bladh, A. Byrd, J. Demarco, S. Edwards, A. Finfera, J. Hampshire, B. Hanson, M. Lang, S. Letcher, A. Mahoney, C. Okraska, and R. Vargas for assistance in wildlife capture and tick collection in the field. L. Beati graciously assisted with confirmation of early A. auricularium records in the USNTC. We also thank the private landowners, and local, county, state, and federal agencies who have cooperated with us and allowed us to conduct surveys on their lands, including the Florida Fish and Wildlife Conservation Commission and U.S. Fish and Wildlife Service. The capture and examination of wild birds, mammals, reptiles, and amphibians was done under Protocols A2004-10006 and A2007-10054 approved by the Institutional Animal Care and Use Committee of the University of Georgia; Florida Fish and Wildlife Conservation Commission Permits WX03378 and WX07021; and Federal Fish and Wildlife Permit No. MB779238. Funding for this project was provided through Cooperative Agreements 0391130808CA, 0491130808CA, 0591130808CA, 0691130808CA, and 0791130808CA, Veterinary Services, Animal and Plant Health Inspection Service, U.S. Department of Agriculture. Additional funds were provided through sponsorship from the fish and wildlife agencies of Alabama, Arkansas, Florida, Georgia, Kansas, Kentucky, Louisiana, Maryland, Mississippi, Missouri, North Carolina, Puerto Rico, South Carolina, Tennessee, Virginia, and West Virginia; through the Federal Aid to Wildlife Restoration Act (50 Stat. 917) and Grant Agreement 06ERAG0005, Biological Resources Division, U.S. Geological Survey, U.S. Department of the Interior; and through Cooperative Agreements 0396130032CA, 0496130032CA, 0596130032CA,0696130032CA, 0796130032CA, Veterinary Services, Animal and Plant Health Inspection Service, U.S. Department of Agriculture.
References Cited
- Allan S. A., Simmons L.-A., Burridge M. J. 2001. Ixodid ticks on white-tailed deer and feral swine in Florida. J. Vector Ecol. 26: 93–102. [PubMed] [Google Scholar]
- Amorim M., Serra-Freire N. M. 2000. Morphological description of tick larval stage (Acari: Ixodidae). 7. Amblyomma auricularium (Conil, 1878). Entomología y Vectores 7: 297–309. [Google Scholar]
- Bailey H. H. 1924. The armadillo in Florida and how it reached there. J. Mammal. 5: 2665. [Google Scholar]
- Burmúdez C. S. E., Esser H. J., R. Miranda C., Moreno R. S. 2015. Wild carnivores (Mammalia) as hosts for ticks (Ixodida) in Panama. Syst. Appl. Acarol. 20: 13–19. [Google Scholar]
- Brennan J. M. 1945. Field investigations pertinent to Bullis fever. The lone star tick, Amblyomma americanum (Linnaeus, 1758). Notes and observations from Camp Bullis, Texas. Texas Rep. Biol. Med. 3: 204–226. [Google Scholar]
- Burridge M. L. 2011. Non-native and invasive ticks: threats to human and animal health in the United States. Univ. Press of Florida, Gainesville, FL. [Google Scholar]
- Bush A. O., Lafferty K. D., Lotz J. M., Shostak A. W. 1997. Parasitology meets ecology on its own terms: Margolis et al. revisited. J. Parasitol. 83: 575–583. [PubMed] [Google Scholar]
- Cohen E. B., Auckland L. D., Marra P. P., Hamer S. A. 2015. Avian migrants facilitate invasions of Neotropical ticks and tick-borne pathogens into the United States. Appl. Environ. Microbiol. 81: 8366–8376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corn J. L., Mertins J. W., Hanson B., Snow S. 2011. First reports of ectoparasites collected from wild-caught exotic reptiles in Florida. J. Med. Entomol. 48: 94–100. [DOI] [PubMed] [Google Scholar]
- Corn J. L., Hanson B. A., Okraska C. R., Muizneiks B., Morgan V., Mertins J. W. 2012. First at-large record of Amblyomma parvum (Acari: Ixodidae) in the United States. Syst. Appl. Acarol. 17: 3–6. [Google Scholar]
- Faccini J. L. H., Cardoso A. C. B., Onofrio V. C., Labruna M. B., Barros-Battesti D. M. 2010. The life cycle of Amblyomma auricularium (Acari: Ixodidae) using rabbits (Oryctolagus cuniculus) as experimental host. Exp. Appl. Acarol. 50: 71–77. [DOI] [PubMed] [Google Scholar]
- Forrester D. J. 1992. Parasites and diseases of wild mammals in Florida. Univ. Press of Florida, Gainesville, FL. [Google Scholar]
- Foster G. W., Moler P. E., Kinsella J. M., Terrell S. P., Forrester D. J. 2000. Parasites of indigo snakes (Drymarchon corais couperi) from Florida, U.S.A. Comp. Parasitol. 67: 124–128. [Google Scholar]
- Guglielmone A. A., Estrada-Peña A., Keirans J. E., Robbins R. G. 2003a. Ticks (Acari: Ixodida) of the Neotropical Zoogeographic Region. International Consortium on Ticks and Tick-borne Diseases/Atalanta, Houten, The Netherlands. [Google Scholar]
- Guglielmone A. A., Estrada-Peña A., Luciani C. A., Mangold A. J., Keirans J. E. 2003b. Hosts and distribution of Amblyomma auricularium (Conil 1878) and Amblyomma pseudoconcolor Aragão, 1908 (Acari: Ixodidae). Exp. Appl. Acarol. 29: 131–139. [DOI] [PubMed] [Google Scholar]
- Guzmán-Cornejo C., Robbins R. G., Guglielmone A. A., Montiel-Parra G., Pérez T. M. 2011. The Amblyomma (Acari: Ixodida: Ixodidae) of Mexico: Identification keys, distribution and hosts. Zootaxa 2998: 16–38. [Google Scholar]
- Hanson B. A., Frank P. A., Mertins J. W., Corn J. L. 2007. Tick paralysis of a snake caused by Amblyomma rotundatum (Acari: Ixodidae). J. Med. Entomol. 44: 155–157. [DOI] [PubMed] [Google Scholar]
- Hofmann J. E. 2005. A survey for the nine-banded armadillo (Dasypus novemcinctus) in Illinois. Ill. Dep. Nat. Res. Ctrol Biodiv. Tech. Rep. 16: 1–29. [Google Scholar]
- Hoogstraal H., Aeschlimann A. 1982. Tick-host specificity. Bulletin de la Société Entomologique Suisse 55: 5–32. [Google Scholar]
- Horta M. C., Nascimento G. F., Martins T. F., Labruna M. B., Machado L.C.P., Nicola P. A. 2011. Ticks (Acari: Ixodida) parasitizing free-living wild animals in the Caatinga biome in the State of Pernambuco, northeastern Brazil. Syst. Appl. Acarol. 16: 207–211. [Google Scholar]
- Irons J. V., Eads R. B., Johnson C. W., Walker O. L., Norris M. A.1952. Southwest Texas Q fever studies. J. Parasitol. 38: 1–5. [PubMed] [Google Scholar]
- Jones E. K., Clifford C. M., Keirans J. E., Kohls G. M. 1972. The ticks of Venezuela (Acarina: Ixodoidea) with a key to the species of Amblyomma of the Western Hemisphere. Brigham Young Univ. Sci. Bull. Biol. Ser. 17: 1–40. [Google Scholar]
- Keirans J. E., Durden L. A. 1998. Illustrated key to nymphs of the tick genus Amblyomma (Acari: Ixodidae) found in the United States. J. Med. Entomol. 35: 489–495. [DOI] [PubMed] [Google Scholar]
- Keirans J. E., Durden L. A. 2001. Invasion: exotic ticks (Acari: Argasidae, Ixodidae) imported into the United States. A review and new records. J. Med. Entomol. 38: 850–861. [DOI] [PubMed] [Google Scholar]
- Lever C. 1985. Naturalized mammals of the world. Longman Group Ltd, London, England. [Google Scholar]
- Lord C. C., Day J. F. 2000. First record of Amblyomma auricularium (Acari: Ixodidae) in the United States. J. Med. Entomol. 37: 977–978. [DOI] [PubMed] [Google Scholar]
- Lugarini C., Martins T. F., Ogrzewalska M., Vasconcelos N. C. T., Ellis V. A., Oliveira J. B., Pinter A., Labruna M. B., Silva J. C. R. 2015. Rickettsial agents in avian ixodid ticks in northeast Brazil. Ticks Tick-Borne Dis. 6: 364–375. [DOI] [PubMed] [Google Scholar]
- Margolis L., Esch G. W., Holmes J. C., Kuris A. M., Schad G. A. 1982. The use of ecological terms in parasitology (report of an ad hoc committee of the American Society of Parasitologists). J. Parsitol. 68: 131–133. [Google Scholar]
- Marshall C. M., Seaman G. A., Hayes F. A. 1963. A critique on the tropical cattle fever tick controversy and its relationship to white-tailed deer. Trans. N. Am. Wildl. Nat. Resour. Conf. 28: 225–232. [Google Scholar]
- Martins T. F., Onofrio V. C., Barros-Battesti D. M., Labruna M. B. 2010. Nymphs of the genus Amblyomma (Acari: Ixodidae) of Brazil: descriptions, redescriptions, and identification key. Ticks Tick-Borne Dis. 1: 75–99. [DOI] [PubMed] [Google Scholar]
- Martins T. F., Labruna M. B., Mangold A. J., Cafrune M. M., Guglielmone A. A., Nava S. 2014. Taxonomic key to nymphs of the genus Amblyomma (Acari: Ixodidae) in Argentina, with description and redescription of the nymphal stage of four Amblyomma species. Ticks Tick-Borne Dis. 5: 753–770. [DOI] [PubMed] [Google Scholar]
- Mertins J. W., Hanson B. A., Corn J. L. 2009. Whartonacarus floridensis sp. n. Acarina: Trombiculidae), with a generic review and the first record of Whartonacarus chiggers from the continental United States. J. Med. Entomol. 46: 1260–1268. [DOI] [PubMed] [Google Scholar]
- Mertins J. W., Hanson B. A., Corn J. L. 2011. Echimyopus dasypus Fain et al. (Acari: Astigmatina: Echimyopodidae) from a nine-banded armadillo, Dasypus novemcinctus L. (Mammalia: Dasypodidae), in Florida, USA. Syst. Appl. Acarol. 16: 252–254. [Google Scholar]
- Oliver J. H., Jr., Hayes M. P., Keirans J. E., Lavender D. R. 1993. Establishment of the foreign parthenogenetic tick Amblyomma rotundatum (Acari: Ixodidae) in Florida. J. Parasitol. 79: 786–790. [PubMed] [Google Scholar]
- Saraiva D. G., Nieri-Bastos F. A., Horta M. C., Soares H. S., Nicola P. A., Pereira L.C.M., Labruna M. B. 2013. Rickettsia amblyommii infecting Amblyomma auricularium ticks in Pernambuco, northeastern Brazil: isolation, transovarial transmission, and transstadial perpetuation. Vector Borne Zoonotic Dis. 13: 615–618. [DOI] [PubMed] [Google Scholar]
- Shock B. C. 2014. Studying the natural history of the endangered Florida puma: ectoparasites, piroplasms, and genetic diversity. Ph.D. dissertation, University of Georgia, Athens.
- Smith J. S. 1977. A survey of ticks infesting white-tailed deer in 12 southeastern states. M.S. thesis, University of Georgia, Athens.
- Strickland R. K., Gerrish R. R. 1964. Distribution of the tropical horse tick in the United States, with notes on associated cases of equine piroplasmosis. J. Am. Vet. Med. Assoc. 144: 875–878. [PubMed] [Google Scholar]
- Taulman J. F., Robbins L. W. 1996. Recent range expansion and distributional limits of the nine-banded armadillo (Dasypus novemcinctus) in the United States. J. Biogeog. 23: 635–648. [Google Scholar]
- Tonn R. J., Kohls G. M., Arnold K. 1963. Ectoparasites of birds and mammals of Costa Rica 2. Ticks. Rev. Biol. Trop. 11: 217–220. [Google Scholar]
- (USDA) U. S. Department of Agriculture. 1982. National tick surveillance program: calendar years 1980 and 1981. Vet. Ser. APHIS 91-39: 26.
- (USDA) U. S. Department of Agriculture. 1994. National Tick Surveillance Program: calendar years 1988 and 1989. Anim. Plant Health Inspect. Ser. APHIS 91-45-005.
- Varma M. G. R. 1973. Ticks (Ixodidae) of British Honduras. Trans. R. Soc. Trop. Med. Hyg. 67: 92–102. [DOI] [PubMed] [Google Scholar]
- Voltzit O. V. 2007. A review of Neotropical Amblyomma species (Acari: Ixodidae). Acarina 15: 3–134. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.