Abstract
Background: Protein intake (PI) may alter adiposity but few studies have explored the age-specific associations of PI with body mass index (BMI).
Objective: We analyzed how PI and breastfeeding relate to BMI in the CLHNS (Cebu Longitudinal Health and Nutrition Survey), an observational Filipino birth cohort (1983–2005).
Methods: Random-effects longitudinal regression models estimated the association of daily breastfeeding frequency and energy-adjusted PI residuals with concurrent BMI z scores (zBMI) measured bimonthly from 2 to 24 mo (n = 2899), and the association of breastfeeding history and PI residuals with concurrent BMI using 5 surveys from 2 to 22 y (n = 2435). Models included statistical interactions between PI, breastfeeding, age, and energy intake and adjusted for potential confounders.
Results: Breastfeeding was associated with higher predicted zBMI at 6 mo (β: 0.491 SD; 95% CI: 0.422, 0.560) and at 18 mo (β: 0.114 SD; 95% CI: 0.032, 0.197). Daily breastfeeding frequency contributed to higher predicted zBMI in younger infants and lower predicted zBMI in later infancy. Those with longer breastfeeding history (19 mo) were significantly smaller at age 11 y (in kg/m2; β: −0.220; 95% CI: −0.342, −0.097) than those with a shorter (4 mo) breastfeeding duration. Total complementary PI was positively associated with predicted zBMI. Complementary animal PI was positively associated with predicted zBMI in nonbreastfed infants. Plant PI was inversely associated with predicted zBMI of nonbreastfed infants at 6 mo. At 22 y contrasts between high (75th percentile) and low (25th percentile) PIs showed that animal PI was associated with higher predicted BMI (β: 0.187; 95% CI: 0.045, 0.329), and total PI was inversely related to predicted BMI (β: −0.008; 95% CI: −0.015, −0.001).
Conclusion: Breastfeeding frequency, breastfeeding history, and PI contribute to BMI from infancy to young adulthood in the CLHNS.
Keywords: BMI, early protein, protein, longitudinal data, life course epidemiology
Introduction
Studies have revealed nuanced age-specific associations of protein intake (PI)6 with body size across the life course. During infancy, metabolic demand for protein is high to support its indispensable structural and functional roles in promoting healthy growth and development (1, 2). In low- and middle-income countries (LMICs), reliance on plant-based proteins and limited intakes of animal protein can also contribute to malnutrition (3). It is well established that protein may reduce risk of undernutrition. However, studies from the developmental origins of health and disease perspective suggest that higher PI in infancy may accelerate weight gain and thus promote obesity in childhood and later life (3, 4), perhaps by promoting adipocyte differentiation and adipogenesis in infancy (5, 6). For example, epidemiologic evidence suggests that infants fed high-protein formula weigh more in the first 2 y of life (7) and have a higher risk of childhood obesity (8). To our knowledge, the effect of PI on infant BMI (in kg/m2) has not yet been explored in LMICs.
Breastfeeding and formula are major sources of protein in infancy. Formula-fed infants are estimated to receive 66–70% more protein (grams per kilogram of body weight) than breastfed infants in the first 6 mo of life (9). Despite or perhaps because of its lower protein content, breastfeeding has been associated with more favorable growth outcomes: a recent meta-analysis that compiled data from 25 studies determined that breastfeeding was associated with lower risk of obesity in childhood (10). It is important to clarify how PI affects concurrent weight gain in settings where breastfeeding and not infant formula is a more common source of protein in early life.
Interestingly, although protein may promote weight gain in early life, an inverse association is apparent in adulthood (11). Plausible mechanisms for this association include the improvement of satiety and the promotion of fat and glucose metabolism (12). Proponents of the protein-leveraging hypothesis suggest that modern diets have lower protein density and are thus less satiating, resulting in increased total energy intake to meet protein needs (12, 13). It is this tradeoff that may be driving the obesity epidemic (13, 14). No study, to our knowledge, has yet examined the postinfancy association of PI with BMI in an LMIC setting.
We present the study of the association of PI with BMI from 2 mo to 22 y. We hypothesized that PI would be positively associated with infant BMI but inversely associated with postinfancy BMI. We anticipated that associations of animal PI with BMI would be larger in magnitude than those for plant PI because amino acid profiles vary by plant and animal source (1). Additionally, we expected that any breastfeeding and breastfeeding frequency would be positively associated with infant BMI, but breastfeeding history would be inversely associated with postinfancy BMI.
Methods
Study population.
The CLHNS (Cebu Longitudinal Health and Nutrition Survey) followed a 1-y birth cohort of infants born in Cebu, Philippines (15). Detailed dietary and anthropometric data were collected at bimonthly intervals between birth and 24 mo (1983–1986) and in 6 subsequent postinfancy surveys: 1991–92 (8.5 y), 1994–95 (11 y), 1998–99 (15 y), 2002 (19 y), and 2005 (22 y) (Supplemental Table 1) (15). There were 3080 singleton infants at baseline and 1888 individuals in 2005.
Primary exposure: PI.
Twenty-four–hour dietary recalls provided dietary data. Infant PI was estimated by using 12 recalls that were each administered at bimonthly intervals from birth to 24 mo. Two 24-h recalls were administered at each postinfancy survey when offspring were a mean age of 11, 15, 19, and 22 y. The mean of these 2 recalls was used to represent usual intake. Dietary data collected with multiple 24-h recalls have been shown to strongly correlate with urinary nitrogen, a more accurate proxy for dietary PI (16). Nutrients from breast milk were not quantified, and thus dietary data during infancy only account for estimated nutrient intake from complementary foods. To assess whether the origin or source of protein modified the association between PI and BMI, protein was divided into animal and plant sources. Animal protein came from dairy, eggs, fish, shellfish, meat, and poultry. Plant protein came from vegetables, tubers, legumes, seeds, grains, and fruits. Absolute PI is correlated with energy intake; therefore, PI was expressed as residuals by using the Willett method (17). For each survey, grams of protein consumed were regressed on total energy intake (excluding energy from human breast milk) to obtain energy-independent residuals of PI. Using an absolute specification of PI would have been misleading, because heavier individuals generally eat more and thus have larger PIs. Protein residuals represent that quantity of protein consumed that is not explained by energy intake; therefore, residuals were used as the primary exposure in all regression analyses.
Breastfeeding was analyzed as a separate variable because breast milk is the primary source of energy for these infants and is a key source of protein throughout the first 2 y of life. Breastfeeding frequency was based on the mother's report of the number of times the infant was breastfed in the previous day (this coincided with the period covered by the 24-h recall). Breastfeeding frequency was inversely associated with complementary energy intake and so was expected to be positively correlated with a reasonable approximation of breast-milk intake. A dummy variable for no breastfeeding (1 = nonbreastfed, 0 = breastfed) was also included, because we hypothesized that the estimated effect of any breastfeeding compared with no breastfeeding was much larger in magnitude than the effect of an additional episode of breastfeeding among breastfed infants. The breastfeeding frequency variable was also truncated at the 95th percentile to diminish the impact of implausibly large values and potential outliers. In the postinfancy analyses, breastfeeding history was represented by the duration of breastfeeding in years.
Primary outcome: BMI.
BMI served as the outcome because it is a reasonable proxy for adiposity (18). In infancy, the WHO growth standards were used to create BMI z scores (zBMIs). zBMIs served as the primary outcome to avoid the challenges of modeling the complex BMI-age association in infancy (19). BMI was used for postinfancy analyses because it is a more interpretable characterization of body mass in children and adults, and participants in this birth cohort were approximately the same age at any given survey (20). Women were excluded from a survey if they were pregnant when it was conducted.
Confounders.
Confounders were selected based on previous research that suggested that they potentially confound diet-BMI associations. These included maternal education, height, and age at baseline; offspring sex and birth weight; time-varying offspring age and education; and time-varying household assets and urbanicity. The composite score of assets ranged from 0 to 11 and was included as a measure of socioeconomic status; assets used to construct the score were electricity, house, material of house, air conditioner, television, tape recorder, refrigerator, electric fan, jeepney, car, and clothing iron. Quantifying assets in this manner has been shown to be a simple but robust proxy of socioeconomic status in LMICs (21). The urbanicity score was derived from community-level variables based on data from the CLHNS; this validated continuous variable is significantly associated with breastfeeding, diet, and other health-related variables and captures differences related to urban- or rural-dwelling (22).
Statistical methodology.
t Tests were used to estimate whether breastfed and nonbreastfed infants had statistically significantly different intakes of complementary macronutrients from 2 to 24 mo. To estimate the association of breastfeeding and PI with BMI, we used random-effects longitudinal regression models with a robust variance estimator. The primary exposures were residuals from protein, plant protein, or animal protein. In appreciation of the hypothesized dynamic associations of breastfeeding with infant BMI, separate models were run for the early-life period from 2 to 24 mo and the postinfancy period of 2–22 y. All models were first specified to match hypothesized synergies among dietary factors and elucidate associations between diet and BMI that were modified by age. Thus, we modeled BMI using 2-way product terms or statistical interactions for protein residuals, energy intake, current breastfeeding frequency or historic breastfeeding, and age. Models were adjusted for the sociodemographic and anthropometric variables related to the offspring, mother, and household; these variables were previously described in the confounders. Postinfancy models were additionally adjusted for BMI at the age of 2 y to obtain an estimate of the association of protein with BMI independent of body size in early life. Postinfancy models were additionally adjusted for residuals of carbohydrate intake because they were found to confound the protein-BMI association. All models were adjusted for the above-described confounders. In the first 2 y, 2899 infants provided data from a mean of 10.4 surveys of a possible 12 surveys. From age 2 to 22 y, 2435 offspring contributed data from a mean of 4.1 surveys of a possible 5 surveys. Analyses were executed by using Stata 14 (StataCorp, LP).
To elucidate the implications of age interactions, β coefficients from each model were used to estimate the difference in predicted BMI for those with high compared with low PI. Survey-specific values for the 75th or 25th percentile were designated as high or low PI, respectively. Wald tests were executed to assess whether the predicted differences in BMI for these high compared with low PI contrasts were statistically significantly different from 0 (23). The β coefficients from regression analyses were used to predict these differences. Rather than testing whether the difference in BMI between high and low PI was equal to zero at each of the 17 surveys, we only tested these differences at 2 time points each in the infancy (6 and 18 mo) and postinfancy (11 and 22 y) analyses. For the infancy period, we chose 6 and 18 mo because complementary feeding is advised by the WHO to begin at age 6 mo (24), and complementary foods predominate in the diet of children at age 18 mo, thus making these 2 time points reasonable to assess joint associations of both breastfeeding and PI with BMI. For the postinfancy period, ages 11 and 22 y were arbitrarily selected as examples of the PI -BMI association.
Results
Mothers in the CLHNS had received (mean ± SD) 7.56 ± 3.72 y of formal education at the time of the offspring's birth (Table 1). Urbanicity score was low at birth (30.58 ± 12.61 mo of a possible range of 0–70 mo), but the sample became more urban by age 22 y (25). Infants were breastfed for a mean ± SD of 12.39 ± 8.32 mo.
TABLE 1.
Select demographic characteristics at birth in 3080 offspring of the Cebu Longitudinal Health and Nutrition Survey (1983–2005)1
| Covariate | Values |
|---|---|
| Maternal education, y | 7.6 ± 3.7 |
| Maternal height, cm | 150.6 ± 5.0 |
| Household assets2 | 2.5 ± 1.9 |
| Household urbanicity3 | 30.6 ± 12.6 |
| Offspring weight, kg | 3.0 ± 0.4 |
| Offspring length or height, cm | 49.1 ± 2.1 |
| Offspring BMI, kg/m2 | 15.6 ± 1.2 |
| Breastfeeding duration, mo | 12.4 ± 8.3 |
Values are means ± SDs, n = 3080.
Score ranges from 1 to 11.
Score ranges from 0 to 70.
Macronutrient intakes and food groups contributing to PI.
Between 2 and 4 mo, infant foods and dairy products were the primary contributors of complementary PI. As the infants grew, grains and vegetables were added to the complementary diet, and by 24 mo, meat, poultry, and seafood also became top contributors to complementary PI. In postinfancy surveys, meat, poultry, seafood, and grains were consistently the top contributors of total PI for all 4 surveys from 11 to 22 y. Mean macronutrient intakes from complementary foods increased from 2 to 24 mo (Supplemental Table 2). As expected, the results of the t tests indicate that there were statistically significant differences in the complementary macronutrient intakes for breastfed and nonbreastfed infants. For example, at any given survey between 2 and 24 mo, mean intakes of fat, carbohydrates, and protein were significantly higher in nonbreastfed infants. The same was true for fat density and protein density from 2 to 20 mo, but the complementary diets of breastfed infants were characterized by higher carbohydrate density from 2 to 24 mo. Mean absolute intakes of protein, fat, carbohydrates, and energy tended to increase as the cohort grew older (Supplemental Table 3).
Results of the longitudinal regression of BMI on PI.
The longitudinal regression analyses revealed many associations of PI and breastfeeding with BMI from age 2 mo to 22 y (Table 2). The magnitude of these associations varied for total, animal, and plant PI. Because of the many significant statistical interactions, postestimation tests were used to estimate differences in BMI for those with high compared with low breast-milk intake and PI. The following are the results of these postestimation tests.
TABLE 2.
Associations of total, animal, and plant protein intake residuals with standardized BMI from 2 to 24 mo and BMI from 2 to 22 y in offspring of Cebu Longitudinal Health and Nutrition Survey (1983–2005)1
| Total protein2 | Animal protein | Plant protein | |
|---|---|---|---|
| Variables associated with standardized BMI from 2 to 24 mo3 | |||
| Nonbreastfed, relative to any breastfeeding | −0.81* (−0.92, −0.70) | −0.81* (−0.92, −0.70) | −0.82* (−0.94, −0.71) |
| Protein residuals/d | 0.14* (0.03, 0.24) | 0.03 (−0.07, 0.14) | −0.15* (−0.29, −0.00) |
| Nonbreastfed × protein residuals/d | 0.08 (−0.01, 0.16) | 0.09* (0.00, 0.18) | −0.05 (−0.17, 0.07) |
| Energy intake, 1000 kcal/d | −0.08 (−0.25, 0.09) | −0.10 (−0.27, 0.06) | −0.08 (−0.25, 0.09) |
| Nonbreastfed × energy intake/1000 kcal | 0.28* (0.13, 0.43) | 0.30* (0.15, 0.45) | 0.27* (0.11, 0.43) |
| Protein residuals/d × energy intake/1000 kcal | −0.02 (−0.09, 0.04) | −0.03 (−0.10, 0.04) | −0.07 (-0.17, 0.03) |
| Age, mo | 0.01* (0.00, 0.01) | 0.01* (0.00, 0.01) | 0.01* (0.00, 0.01) |
| Nonbreastfed × age, mo | 0.03* (0.03, 0.04) | 0.03* (0.03, 0.04) | 0.03* (0.03, 0.04) |
| Energy intake, 1000 kcal/d × age, mo | 0.01* (0.00, 0.01) | 0.01* (0.00, 0.02) | 0.01* (0.00, 0.01) |
| Breastfeeding frequency/d4 | 0.09* (0.05, 0.13) | 0.09* (0.05, 0.13) | 0.09* (0.05, 0.13) |
| Breastfeeding frequency/d × protein residuals/d | 0.00 (−0.05, 0.05) | 0.01 (−0.04, 0.07) | 0.06 (−0.01, 0.14) |
| Breastfeeding frequency/d × energy intake/1000 kcal | −0.05 (−0.14, 0.04) | −0.05 (−0.15, 0.04) | −0.09 (−0.19, 0.01) |
| Breastfeeding frequency/d × age, mo | −0.01* (−0.01, 0.00) | −0.01* (−0.01, -0.00) | −0.01* (-0.01, −0.00) |
| Protein residuals × age, mo | −0.01* (−0.01, −0.01) | 0.00 (−0.01, 0.00) | 0.02* (0.01, 0.02) |
| Plant protein in animal models and animal protein in plant models residuals/d | — | 0.06* (0.03, 0.09) | 0.06* (0.03, 0.09) |
| Variables associated with BMI from 2 to 22 y | |||
| Protein residuals/d | −0.16* (−0.27, −0.06) | −0.12* (−0.23, −0.00) | 0.01 (−0.27, 0.29) |
| Energy intake, 1000 kcal/d | −2.13* (−2.27, −2.00) | −0.50* (−0.72, −0.27) | −0.51* (−0.73, −0.28) |
| Age, y | 0.05* (0.03, 0.07) | 0.36* (0.32, 0.40) | 0.36* (0.32, 0.41) |
| Breastfeeding duration, y | −0.48* (−0.55, −0.41) | −0.48* (−0.72, −0.23) | −0.46* (−0.71, −0.22) |
| Protein residuals/d × energy intake/1000 kcal | 0.06* (0.02, 0.11) | 0.04* (0.01, 0.07) | −0.07 (−0.15, 0.01) |
| Protein residuals/d × age, y | 0.00 (−0.00, 0.01) | 0.01 (−0.00, 0.01) | 0.01 (−0.01, 0.02) |
| Energy intake, 1000 kcal/d × age, y | 0.13* (0.12, 0.14) | 0.03* (0.02, 0.05) | 0.04* (0.02, 0.05) |
| Breastfeeding duration, y × age, y | 0.03* (0.02, 0.04) | 0.03* (0.01, 0.04) | 0.02* (0.01, 0.04) |
| Plant protein in animal models and animal protein in plant models residuals/d | — | −0.05 (−0.13, 0.03) | 0.06* (0.02, 0.09) |
Values are regression β coefficients (95% CIs). Analyses were conducted by using data from 2899 participants (infancy analyses from 2 to 24 mo) and 2435 offspring (postinfancy analyses from 2 to 22 y). *P < 0.05.
β coefficients show the average change in BMI or standardized BMI associated with a 1-unit increase in the indicated variable. For example, all variables held equal, nonbreastfed infants had a BMI z score that was smaller, or −0.81 (95% CI: −0.92, −0.70) heavier than breastfed infants.
Standardized BMI or BMI z scores were based on the WHO growth standards (19).
Breastfeeding frequency shown in 5-session units.
Any breastfeeding and breastfeeding frequency during infancy.
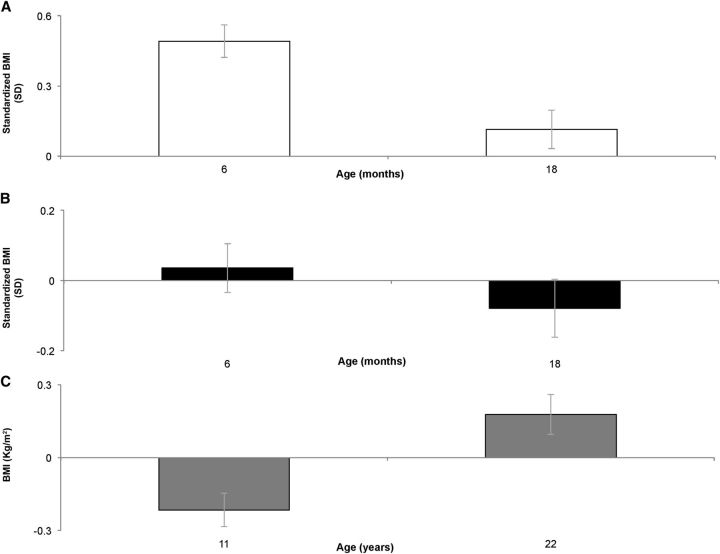
Breastfeeding showed significant, positive, age-dependent associations with infant zBMI (Figure 1). Compared with nonbreastfed infants, breastfed infants had a predicted zBMI that was ∼0.5 SD higher than their breastfed counterparts at 6 mo (β: 0.491 SD; 95% CI: 0.422, 0.560), but this positive association was mitigated with age. By 18 mo, predicted BMI of breastfed infants was only 0.114 SD (95% CI: 0.032, 0.197) higher than nonbreastfed infants. Additionally, among breastfed infants, an additional 5 feedings/d was associated with higher predicted zBMI at 6 mo (β: 0.036 SD; 95% CI: 0.012, 0.060). However, by 18 mo an additional 5 feedings was associated with lower predicted zBMI (β: −0.079 SD; 95% CI:−0.109, −0.049).
FIGURE 1.
Breastfeeding, breastfeeding frequency, and breastfeeding duration are associated with predicted BMI from 2 to 22 y in the Cebu Longitudinal Health and Nutritional Survey (1983–2005). Postestimation Wald tests estimated the difference in (A) predicted standardized infant BMI for being breastfed compared with nonbreastfed (□), (B) predicted standardized infant BMI for receiving an additional 5 breastfeeding episodes/d (■), and (C) predicted postinfancy BMI for those with long (75th percentile) compared with short (25th percentile) breastfeeding duration, the equivalent to an additional 15 mo of breastfeeding (■). Standardized BMIs or BMI z scores were based on the WHO growth standards (19).
Complementary PI in infancy.
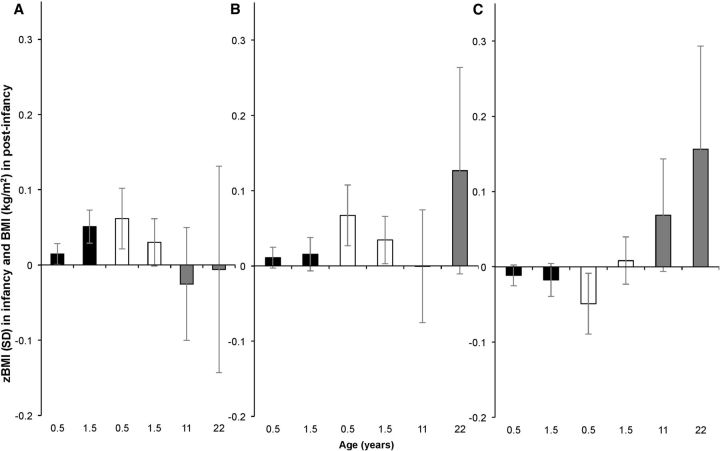
Total complementary PI was also significantly associated with zBMI (Figure 2). At 6 mo, those receiving high quantities of complementary protein (at the 75th percentile of protein residuals) had greater predicted zBMIs than those who received low quantities (at the 25th percentile) (β: 0.015 SD; 95% CI: 0.007, 0.022 and β: 0.062 SD; 95% CI: 0.038, 0.085 for breastfed and nonbreastfed infants, respectively). Findings were similar at 18 mo: PI was positively associated with zBMI in breastfed infants (β: 0.051 SD; 95% CI: 0.024, 0.078) and tended to be positively associated in nonbreastfed infants (β: 0.030 SD; 95% CI: −0.001, 0.061; P < 0.10).
FIGURE 2.
Protein intake residuals are significantly associated with predicted body size from 2 to 22 y in the Cebu Longitudinal Health and Nutritional Survey (1983–2005). Postestimation Wald tests were conducted to estimate the difference between those with high and low protein intake at 6 and 18 mo and 11 and 22 y. Graphs show the difference in predicted body size between those with high (at the 75th) or low (at the 25th) percentile of protein intake from (A) all nonhuman milk sources, (B) all animal protein sources and, (C) all plant sources. Contrasts are shown for breastfed infants (■), nonbreastfed infants (□), and postinfancy analyses (■). Standardized BMI was the outcome in analyses from 2 to 24 mo. BMI was the outcome for postinfancy analyses (2–22 y). Standardized BMIs or BMI z scores were based on the WHO growth standards (19). zBMI, BMI z score.
Source-specific associations were only significant in contrasts among nonbreastfed infants. Although animal PI was positively associated with BMI at 6 and 18 mo (β: 0.067 SD; 95% CI: 0.027, 0.107 and β: 0.034 SD; 95% CI: 0.003, 0.066, respectively), plant PI was inversely associated with BMI at 6 mo (β: −0.049 SD; 95% CI: −0.080, −0.018) and showed modest, positive associations with BMI at 18 mo (β: 0.008 SD; 95% CI: 0.001, 0.015).
Breastfeeding duration in postinfancy analyses.
As shown in Figure 1, at age 11 y offspring with longer breastfeeding duration (at the 75th percentile or 19 mo) had significantly lower BMI (in kg/m2; β: −0.220; 95% CI: −0.342, −0.097) than those with shorter breastfeeding duration (at the 25th percentile or 4 mo). Breastfeeding duration was not associated with BMI at age 22 y (β: 0.178; 95% CI: 20.077, 0.433; P = 0.17).
PI and BMI in postinfancy analyses.
There was a modest, inverse association between total PI and BMI at age 22 y (β: −0.008; 95% CI: −0.015, −0.001) (Figure 2). Plant PI was not significantly associated with BMI in postinfancy analyses, whereas animal PI was positively associated with BMI at age 22 y (β: 0.187; 95% CI: 0.045, 0.329).
Discussion
We estimated the association of PI with BMI from infancy to early adulthood in a large Filipino birth cohort. The analyses revealed modest associations of protein from birth to 22 y and important modifications of the PI-adiposity association by protein source and age of PI. Our analyses also revealed time-varying contributions of breastfeeding frequency and breastfeeding history to growth and BMI.
Age-dependent associations of breastfeeding frequency and duration with BMI during infancy.
We found that the strongest contributions of breastfeeding to zBMI occurred in the earlier months. Other studies showed similar time-varying associations of breastfeeding with infant weight outcomes (26–28), including another CLHNS study (29). Socioeconomically disadvantaged mothers in the CLHNS were more likely to breastfeed but may have fed their infants lower-quality complementary foods (30). The macronutrient composition of the infant diets varied significantly by breastfeeding status. In the second year of life when the infants' needs exceed that which breast milk can provide, differences in the quality of complementary foods may explain why the benefit of breastfeeding was substantially attenuated in older infants. We included relevant socioeconomic variables to control for confounding but cannot rule out remaining residual confounding.
Duration of breastfeeding was inversely associated with postinfancy BMI.
Consistent with other studies (10, 31–33), we found a significant, inverse association of breastfeeding duration with BMI at age 11 y. Although these findings may hint at some protection against obesity, <1% of participants were obese (i.e., had a standardized BMI >2) at age 11 y. With this negligible obesity prevalence, this seems to be a tendency for those who were breastfed longest to be thinner. Because breastfeeding duration in this cohort was inversely associated with socioeconomic status, these socioeconomic differences may have driven divergent dietary patterns in childhood and thus led to thinness or lower BMI. Breastfeeding may also improve the infants' ability to regulate food intake, potentially programming improved food intake patterns in later life (34, 35). Another possibility is that those who were breastfed longest were thinner adolescents because they received less complementary protein during infancy and so did not experience the obesogenic programming that underpins the early protein-adiposity developmental origins of health and disease hypothesis (5, 6).
Dietary PI was associated with BMI in an age- and source-dependent manner.
As we hypothesized, PI was positively associated with infant zBMI. Randomized clinical trials conducted in the European Childhood Obesity Trial study show similar findings: the high-protein group received 2.9 or 4.4 g/100 kcal, and the low-protein group received 1.77 and 2.2 g/100 kcal for infant and follow-on formula, respectively (7, 36). Compared with the low-protein control group, infants in the high-protein treatment arm gained significantly more weight during the first 6 mo of life (36) and had significantly greater weight-for-length z scores (7). Although figures are not directly translatable to the protein residuals used in our observational cohort, similar contrasts were made in our cohort because, for example, 6-mo-old Cebu infants in the highest quintile of protein residuals received a median of 3.6 g complementary protein/100 kcal compared with a median of 1.6 g/100 kcal in the lowest quintile. Furthermore, a recent study in the CLHNS found that protein composition (grams per kilocalorie) was positively associated with infant weight but not length (37). Although this prior study did not consider the important role of breastfeeding in CLHNS, its findings suggest that protein may exert its effects on infant BMI by driving weight gain rather than changes in linear growth in this population (37).
As hypothesized, total PI was inversely associated with postinfancy BMI but only in contrasts conducted for the survey at age 22 y. The US cross-sectional study using NHANES data also found that being in a higher decile of PI was associated with lower BMI (−0.47) and waist circumference (−0.53 cm) (38). This NHANES magnitude was larger than ours; however, it may be because protein was associated with both length and weight in these Filipino participants from age 8 to 22 y (37). If protein simultaneously affects these two anthropometric indicators of growth, this may explain why its effects on BMI were so modest. The modest estimated effects may also be due to our use of protein residuals instead of absolute PI. Further research is needed to clarify how protein influences postinfancy BMI.
We also found evidence that the protein-BMI association varied by protein source. In nonbreastfed infants at 6 mo, animal PI was positively associated with zBMI whereas plant PI was inversely associated with zBMI. The rice and corn gruels that characterized these infants' diet did not support their growth needs (29, 39). This reinforces the importance of recommending animal-source foods during the complementary feeding period to stave off undernutrition. In fact, interventions that specifically recommend including animal-source foods in the complementary diet work better to promote diet quality and infant nutritional status than do vague recommendations to increase protein (40, 41). This finding thus underscores the WHO recommendations of animal-source complementary foods (25). In our postinfancy analyses, similar positive associations were evident for animal protein and BMI whereas plant PI was nonsignificant. These results concord with other studies. A Belgian study found a positive association of animal protein with adult BMI and waist circumference, whereas plant protein was inversely associated with these outcomes (42). Additionally, whereas no significant associations were observed in our 11-y contrasts, a study in European adolescents found daily plant PI (grams) was strongly and inversely associated with cross-sectional body fat percentage, although modest inverse associations also existed for animal PI (43). Our null findings for plant protein may be because of differences in dietary pattern between CLHNS participants and the European adolescents in that study. For example, at age ∼15–17.5 y those adolescents consumed more total protein (∼98 compared with 49 g/d), more plant protein (39 compared with 26 g/d), and more animal protein (58 compared with 33 g/d) (43) compared with the Filipino offspring (age 15 y).
The merits of this study must be taken within context of important substantive and technical limitations. The major limitation is that our dietary data did not include nutrients from breast milk and thus underestimated total intake. Breast-milk protein content (grams and percentage of energy) tends to decrease over time (44). With this in mind, we underestimated total PI in breastfed infants, and this underestimation was greater in earlier months of life; this might have attenuated our associations. Furthermore, breastfeeding frequency has been shown to be inversely associated with total 24-h breast-milk PI (45). This implies that even if an additional breastfeeding session will increase total breast-milk PI, this association is nonlinear and not perfectly positive. By explicitly showing how related variables, such as any breastfeeding and breastfeeding frequency, relate to zBMI, we at least provide additional albeit imperfect proxies for how breast-milk protein relates to zBMI in infancy. Another limitation is that we are missing the 2- to 11-y period and thus excluded the adiposity rebound, a potentially vulnerable window during which PI may program risk of later obesity (46). Finally, it is worth reiterating that the protein-BMI effects estimated here do not necessarily reflect enhanced obesity risk but may indicate reduced risk of stunting or wasting or even enhanced growth in lean tissue.
Despite the limitations of these analyses, there are key, noteworthy strengths of this study. To our knowledge, this is the first exploration of the age- and source-specific associations of protein with BMI that was conducted in an LMIC. We included product terms in order to elucidate any age-specific associations of PI and modifications by breastfeeding status. We further analyzed animal- and plant-specific PI to detect any modifications by source. The longitudinal richness of this study and the careful attention paid to dietary details make this a significant contributor to the literature.
Altogether, our findings highlight the role of PI and different protein sources in modifying growth and BMI from infancy to young adulthood in a single Filipino birth cohort.
Supplementary Material
Acknowledgments
We thank Allison Aiello and Barry Popkin for their insights throughout this project. MW designed the research, analyzed the data, and wrote the final paper; LA provided necessary databases; MAM, DS-A, and LA provided guidance in the analysis of data and interpretation of the research findings and critically revised the manuscript; and LA was responsible for the final content. All authors read and approved the final manuscript.
Abbreviations
- CLHNS
Cebu Longitudinal Health and Nutrition Survey
- LMIC
low- and middle-income country
- PI
protein intake
- zBMI
BMI z score
Footnotes
Supported by Howard Hughes Medical Institute International Student Research Fellowship and the Carolina Population Center (R24 HD050924).
References
- 1. Institute of Medicine of the National Academies Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington (DC): The National Academies Press; 2005. [Google Scholar]
- 2. Brabin B, Coulter J. Nutrition-associated disease. In: Manson's tropical diseases. London: Saunders; 2003. p. 561–80. [Google Scholar]
- 3. Michaelsen KF, Larnkjær A, Mølgaard C. Amount and quality of dietary proteins during the first two years of life in relation to NCD risk in adulthood: nutrition, metabolism, and cardiovascular diseases. Nutr Metab Cardiovasc Dis 2012;22:781–6. [DOI] [PubMed] [Google Scholar]
- 4. Monteiro POA, Victora CG. Rapid growth in infancy and childhood and obesity in later life: a systematic review. Obes Rev 2005;6:143–54. [DOI] [PubMed] [Google Scholar]
- 5. Hauner H, Wabitsch M, Zwiauer K, Widhalm K, Pfeiffer EF. Adipogenic activity in sera from obese children before and after weight reduction. Am J Clin Nutr 1989;50:63–7. [DOI] [PubMed] [Google Scholar]
- 6. Koletzko B, Broekaert I, Demmelmair H, Franke J, Hannibal I, Oberle D, Schiess S, Baumann BT, Verwied-Jorky S. Protein intake in the first year of life: a risk factor for later obesity?: The EU childhood obesity project. In: Early nutrition and its later consequences: new opportunities. Dordrecht (Netherlands): Springer Netherlands; 2005. p. 69–79. [DOI] [PubMed] [Google Scholar]
- 7. Koletzko B, Dobrzanska A, Sengier A, Langhendries J, Rolland Cachera M, Grote V, von Kries R, Closa R, Escribano J, Scaglioni S, et al. . Lower protein in infant formula is associated with lower weight up to age 2 y: a randomized clinical trial. Am J Clin Nutr 2009;89:1836–45. [DOI] [PubMed] [Google Scholar]
- 8. Weber M, Grote V, Closa-Monasterolo R, Escribano J, Langhendries J, Dain E, Giovannini M, Verduci E, Gruszfeld D, Socha P, et al. . Lower protein content in infant formula reduces BMI and obesity risk at school age: follow-up of a randomized trial. Am J Clin Nutr 2014;99:1041–51. [DOI] [PubMed] [Google Scholar]
- 9. Heinig MJ, Nommsen LA, Peerson JM, Lonnerdal B, Dewey KG. Energy and protein intakes of breast-fed and formula-fed infants during the first year of life and their association with growth velocity: The DARLING Study. Am J Clin Nutr 1993;58:152–61. [DOI] [PubMed] [Google Scholar]
- 10. Yan J, Liu L, Zhu Y, Huang G, Wang PP. The association between breastfeeding and childhood obesity: a meta-analysis. BMC Public Health 2014;14:1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hu FB. Protein, body weight, and cardiovascular health. Am J Clin Nutr 2005;82:242S–7S. [DOI] [PubMed] [Google Scholar]
- 12. Pesta DH, Samuel VT. A high-protein diet for reducing body fat: mechanisms and possible caveats. Nutr Metab (Lond) 2014;11:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Simpson SJ, Raubenheimer D. Macronutrient balance and lifespan. Aging 2009;1:875–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Simpson SJ, Batley R. Geometric analysis of macronutrient intake in humans: the power of protein? Appetite 2003;41:123–40. [DOI] [PubMed] [Google Scholar]
- 15. Adair LS, Hindin MJ, Popkin BM, Akin JS, Guilkey DK, Gultiano SA, Borja J, Perez L, Kuzawa CW, McDade TW. Cohort profile: the Cebu longitudinal health and nutrition survey. Int J Epidemiol 2011;40:619–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Freedman LS, Commins JM, Moler JE, Arab L, Baer DJ, Kipnis V, Midthune D, Moshfegh AJ, Neuhouser ML, Prentice RL, et al. . Pooled results from 5 validation studies of dietary self-report instruments using recovery biomarkers for energy and protein intake. Am J Epidemiol 2014;180:172–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hu FB, Manson JE, Willett WC. Types of dietary fat and risk of coronary heart disease: a critical review. J Am Coll Nutr 2001;20:5–19. [DOI] [PubMed] [Google Scholar]
- 18. Bouchard C. BMI, fat mass, abdominal adiposity and visceral fat: where is the ‘beef’? Int J Obes (Lond) 2007;31:1552–3. [DOI] [PubMed] [Google Scholar]
- 19. WHO Multicentre Growth Reference Study Group WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr Suppl 2006;95:76–85. [DOI] [PubMed] [Google Scholar]
- 20. Berkey CS, Laird NM. Nonlinear growth curve analysis: estimating the population parameters. Ann Hum Biol 1986;13:111–28. [DOI] [PubMed] [Google Scholar]
- 21. Bollen KA, Glanville JL, Stecklov G. Economic status proxies in studies of fertility in developing countries: does the measure matter? Popul Stud (Camb) 2002;56:81–96. [DOI] [PubMed] [Google Scholar]
- 22. Dahly DL, Adair LS. Quantifying the urban environment: a scale measure of urbanicity outperforms the urban–rural dichotomy. Soc Sci Med 2007;64:1407–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. StataCorp Stata 13 base reference manual. College Station (TX): Stata Press; 2013. [Google Scholar]
- 24. WHO Indicators for assessing infant and young child feeding practices: part III country profiles. Geneva (Switzerland): WHO; 2010. [Google Scholar]
- 25. WHO Infant and young child feeding: model chapter for textbooks for medical students and allied health professionals. Geneva (Switzerland): WHO; 2009. [PubMed] [Google Scholar]
- 26. Hediger ML, Overpeck MD, Ruan WJ, Troendle JF. Early infant feeding and growth status of US-born infants and children aged 4–71 mo: analyses from the third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr 2000;72:159–67. [DOI] [PubMed] [Google Scholar]
- 27. Kramer MS, Guo T, Platt RW, Shapiro S, Collet J, Chalmers B, Hodnett E, Sevkovskaya Z, Dzikovich I, Vanilovich I, et al. . Breastfeeding and infant growth: biology or bias? Pediatrics 2002;110:343–7. [DOI] [PubMed] [Google Scholar]
- 28. Dewey KG, Anderson MA, Dewey KG, Frongillo E, Garza C, Haschke F, Kramer M, Whitehead RG, Winichagoon P, Peerson JM, et al. . Growth of breast-fed infants deviates from current reference data: a pooled analysis of US, Canadian, and European data sets. Pediatrics 1995;96:495–503. [PubMed] [Google Scholar]
- 29. Wright MJ, Bentley M, Mendez M, Adair L. The interactive association of dietary diversity scores and breast-feeding status with weight and length in Filipino infants aged 6–24 months. Public Health Nutr 2015;18:1762–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Adair LS, Popkin BM, Guilkey DK. The duration of breast-feeding: how is it affected by biological, sociodemographic, health sector, and food industry factors? Demography 1993;30:63–80. [PubMed] [Google Scholar]
- 31. Stolzer JM. Breastfeeding and obesity: a meta-analysis. Open J Prev Med 2011;1:88–93. [Google Scholar]
- 32. Parikh N, Hwang SJ, Ingelsson E, Benjamin EJ, Fox CS, Vasan RS, Murabito JM. Breastfeeding in infancy and adult cardiovascular disease risk factors. Am J Med 2009;122:656–63. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Harder T, Bergmann R, Kallischnigg G, Plagemann A. Duration of breastfeeding and risk of overweight: a meta-analysis. Am J Epidemiol 2005;162:397–403. [DOI] [PubMed] [Google Scholar]
- 34. Li R, Fein SB, Grummer-Strawn LM. Do infants fed from bottles lack self-regulation of milk intake compared with directly breastfed infants? Pediatrics 2010;125:e1386–93. [DOI] [PubMed] [Google Scholar]
- 35. DiSantis KI, Collins BN, Fisher JO, Davey A. Do infants fed directly from the breast have improved appetite regulation and slower growth during early childhood compared with infants fed from a bottle? Int J Behav Nutr Phys Act 2011;8:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Escribano J, Closa-Monasterolo R, Luque V, Ferre N, Mendez-Riera G, Koletzko B, Grote V, Demmelmair H, Bluck L, Wright A, et al. . Effect of protein intake and weight gain velocity on body fat mass at 6 months of age: the EU Childhood Obesity Programme. Int J Obes (Lond) 2012;36:548–53. [DOI] [PubMed] [Google Scholar]
- 37. Bhargava A. Protein and micronutrient intakes are associated with child growth and morbidity from infancy to adulthood in the Philippines. J Nutr 2016;146:133–41. [DOI] [PubMed] [Google Scholar]
- 38. Pasiakos SM, Lieberman HR, Fulgoni VL 3rd. Higher-protein diets are associated with higher HDL cholesterol and lower BMI and waist circumference in US adults. J Nutr 2015;145:605–14. [DOI] [PubMed] [Google Scholar]
- 39. Perlas LA, Gibson RS, Adair LS. Macronutrient and selected vitamin intakes from complementary foods of infants and toddlers from Cebu, Philippines. Int J Food Sci Nutr 2004;55:1–15. [DOI] [PubMed] [Google Scholar]
- 40. Daelmans B, Mangasaryan N, Martines J, Saadeh R, Casanovas C, Arabi M. Strengthening actions to improve feeding of infants and young children 6 to 23 months of age: summary of a recent World Health Organization/UNICEF technical meeting, Geneva, 6–9 October 2008. Food Nutr Bull 2009;30:S236–8. [DOI] [PubMed] [Google Scholar]
- 41. Dewey KG, Adu-Afarwuah S. Systematic review of the efficacy and effectiveness of complementary feeding interventions in developing countries. Matern Child Nutr 2008;4Suppl 1:24–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lin Y, Bolca S, Vandevijvere S, De Vriese S, Mouratidou T, De Neve M, Polet A, Van Oyen H, Van Camp J, De Backer G. Plant and animal protein intake and its association with overweight and obesity among the Belgian population. Br J Nutr 2011;105:1106–16. [DOI] [PubMed] [Google Scholar]
- 43. Lin Y, Mouratidou T, Vereecken C, Kersting M, Bolca S, de Moraes AC, Cuenca-García M, Moreno LA, González-Gross M, Valtueña J, et al. . Dietary animal and plant protein intakes and their associations with obesity and cardio-metabolic indicators in European adolescents: the HELENA cross-sectional study. Nutr J 2015;14:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Grote V, Verduci E, Scaglioni S, Vecchi F, Contarini G, Giovannini M, Koletzko B, Agostoni C. European Childhood Obesity Project Breast milk composition and infant nutrient intakes during the first 12 months of life. Eur J Clin Nutr 2016;70:250–6. [DOI] [PubMed] [Google Scholar]
- 45. Khan S, Hepworth AR, Prime DK, Lai CT, Trengove NJ, Hartmann PE. Variation in fat, lactose, and protein composition in breast milk over 24 hours: associations with infant feeding patterns. J Hum Lact 2013;29:81–9. [DOI] [PubMed] [Google Scholar]
- 46. Günther ALB, Remer T, Kroke A, Buyken AE. Early protein intake and later obesity risk: which protein sources at which time points throughout infancy and childhood are important for body mass index and body fat percentage at 7 y of age? Am J Clin Nutr 2007;86:1765–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.