Abstract
Background: The one-carbon metabolism pathway is highly dependent on a number of B vitamins in order to provide one-carbon units for purine and thymidylate biosynthesis as well as homocysteine remethylation. Previous studies have examined folate and vitamin B-12 deficiency and their effects on formate metabolism; as of yet, to our knowledge, no studies on the effects of riboflavin deficiency on formate metabolism have been published.
Objective: Our objective was to determine the effects of riboflavin deficiency on formate metabolism.
Methods: Weanling male rats were randomly assigned either to control, riboflavin-replete (RR) or to experimental, riboflavin-deficient (RD) versions of the AIN-93G diet for 13 d, at which time a constant infusion of [13C]-formate was carried out to ascertain the effects of deficiency on formate production. Gas chromatography–mass spectrometry was used to measure plasma formate concentration and [13C]-formate enrichment. HPLC, LC–mass spectrometry (MS)/MS, and enzymatic assays were used for the measurement of one-carbon precursors and other metabolites.
Results: RD rats had significantly lower rates of formate production (15%) as well as significantly reduced hepatic methylenetetrahydrofolate reductase activity (69%) and protein concentration (54%) compared with RR rats. There was no difference in plasma formate concentrations between the groups. Plasma serine, a potential one-carbon precursor, was significantly higher in RD rats (467 ± 73 μM) than in RR rats (368 ± 52 μM).
Conclusions: Although deficiencies in folate and vitamin B-12 lead to major changes in plasma formate concentrations, riboflavin deficiency results in no significant difference; this disagrees with the prediction of a published mathematical model. Our observation of a lower rate of formate production is consistent with a role for flavoproteins in this process.
Keywords: one-carbon metabolism, serine, glycine, S-adenosylmethionine, methylenetetrahydrofolate reductase, folate, riboflavin
Introduction
Riboflavin, a water-soluble B vitamin, is a precursor for the synthesis of FMN and FAD. These flavin-derived cofactors play an especially important role in one-carbon metabolism where they participate in the mitochondrial production and the cytosolic incorporation and utilization of one-carbon units. Folate is also a water-soluble B vitamin from which critical cofactors for one-carbon metabolism, the tetrahydrofolates, are derived. The importance of formate to one-carbon metabolism has recently become apparent (1, 2). Previous studies have shown that folate deficiency in rats results in a 5-fold elevation in plasma formate concentration while decreasing formate production by 44% (3, 4).
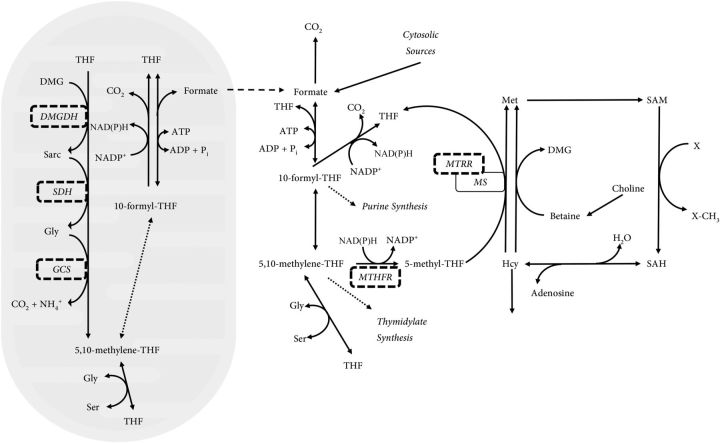
There are a number of flavin-dependent enzymes required in one-carbon metabolism that play important roles in both producing one-carbon units and allowing them to be used for biosynthetic and transmethylation reactions (Figure 1). Mitochondria, which are major sites of intracellular formate production (5), contain 3 flavin-dependent enzymes required for production of one-carbon units from glycine and choline metabolites. Dimethylglycine dehydrogenase, sarcosine dehydrogenase, and the glycine cleavage system (GCS)6 are all flavoenzymes that produce 5,10-methylenetetrahydrofolate (6, 7), which can be converted to formate. The free formate so produced can exit mitochondria and be incorporated into the cytosolic one-carbon tetrahydrofolate pool by cytosolic C1-tetrahydrofolate synthetase (1). To be used for transmethylation, the 5,10-methylenetetrahydrofolate must be irreversibly reduced to 5-methyltetrahydrofolate by the FAD- and NAD(P)H-dependent enzyme methylenetetrahydrofolate reductase (MTHFR). 5-Methyltetrahydrofolate is the substrate for cobalamin-dependent methionine synthase, which methylates homocysteine to methionine. Every 1000–2000 catalytic reactions, the cobalamin cofactor may be oxidized from the cob(I) to the cob(II) state, inactivating methionine synthase (8). Another flavoprotein, methionine synthase reductase is required to reduce the cofactor in an NAD(P)H-dependent manner, returning inactive cob(II) cofactor to its catalytically active cob(I) state (9).
FIGURE 1.
An overview of one-carbon metabolism showing the role of flavoproteins. The production of mitochondrial 5,10-methylene-THF which may then be converted to 10-formyl-THF and eventually to formate by the actions of MTHFD2L and MTHFD1L, respectively. The mitochondrially derived formate may then be incorporated into the cytosolic THF pool by MTHFD1 and irreversibly reduced to 5-methyl-THF in an NAD(P)H-dependent manner by the FAD-dependent enzyme MTHFR. The 5-methyl-THF is used by MS to remethylate homocysteine, producing methionine. The cobalamin cofactor of MS can be irreversibly oxidized every ∼1000 turns of the methylation cycle and requires the FAD-dependent MTRR to reduce the cofactor to the correct oxidation state. DMG, dimethylglycine; DMGDH, dimethylglycine dehydrogenase; GCS, glycine cleavage system; Hcy, Homocysteine; MS, methionine synthase; MTHFD1, C1-tetrahydrofolate synthetase (cytosolic, trifunctional); MTHFD1L, 10-formyl-tetrahydrofolate synthetase (mitochondrial, monofunctional); MTHFD2L, methylenetetrahydrofolate dehydrogenase 2-Like (mitochondrial, bifunctional); MTHFR, methylenetetrahydrofolate reductase; MTRR, methionine synthase reductase; Pi, inorganic phosphate; SAH, S-adenosylhomocysteine; SAM, S-adenosylmethionine; Sarc, sarcosine; SDH, sarcosine dehydrogenase; THF, tetrahydrofolate; 5-methyl-THF, methyltetrahydrofolate; 5,10-methylene-THF, 5,10-methylenetetrahydrofolate; 10-formyl-THF, 10-formyltetrahydrofolate.
There is growing evidence that formate is not just a metabolic intermediate. In rat models in which formate production has been impaired, neural tube defects become common in offspring (10–12). In each of these models, formate supplementation in the water of the pregnant dam has been shown to moderate (11) or even rescue the neural tube defect phenotype (10, 12).
We are not aware of any studies on the effects of riboflavin on formate metabolism; however, riboflavin deficiency is linked to altered one-carbon metabolism. Narisawa et al. (13) measured the activities of various folate-dependent enzymes in both control and riboflavin-deficient (RD) rats and found large decreases in the activities of MTHFR and methionine synthase; the decrease in methionine synthase may be attributed to impaired reactivation of the cobalamin cofactor of methionine synthase (9).
A review by Powers (14) has identified a number of populations that are susceptible to deficiencies in riboflavin: school children, the elderly, and pregnant or lactating women. In fact, a publication by Sánchez et al. (15) showed that maternal and fetal riboflavin, thiamin, and pyridoxine status were significantly associated with one another in a Spanish population. Although riboflavin-supplementation began in Canada and the United States in the 1940s, not all countries supplement. Individuals with hypothyroidism are at risk of flavin deficiency, regardless of their dietary riboflavin intake. The biosynthesis of flavin kinase, the enzyme responsible for converting dietary riboflavin to its biologically active forms, is regulated by thyroid hormone (16, 17).
Methods
Rats.
Weanling male Sprague-Dawley rats were obtained from Memorial University's breeding colony. They were randomly assigned to either a control riboflavin-replete (RR) diet or the experimental RD diet for 13 d. They were maintained on a 12:12 h light:dark cycle and had free access to food and water for the length of the study. Food intake and body weight were measured every 2 d. All procedures were approved by Memorial University's Institutional Animal Care Committee and conform to Canadian Council on Animal Care guidelines.
Diets.
Rats were maintained on AIN-93G–based diets formulated according to Reeves et al. (18). All diet components were obtained from MP BioMedical with the exceptions of tert-butylhydroquinone, which was obtained from Sigma-Aldrich, and RR vitamin mix (catalog no. 310025) and RD vitamin mix (catalog no. 316753), which were obtained from Dyets Inc. The vitamin mixes were formulated according to the AIN-93 guidelines with the RR vitamin mix providing 6 mg riboflavin/kg diet and the RD vitamin mix providing 0 mg riboflavin/kg diet. Although the RD vitamin mix was riboflavin-free, the casein used in the preparation of the diets contains 7.53 μg riboflavin/g casein (product data sheet, Casein, catalog no. 02901293; MP Biomedicals), which amounts to 1.56 mg riboflavin/kg diet. This equates to ∼50% of the dietary requirement for riboflavin for rats (19).
Cannulation and infusion protocol.
On the morning of day 13 of the experiment, the rats were weighed and anesthesia was induced with 5% isoflurane in oxygen (Isoflo; Abbott Animal Heath) and maintained at 3% isoflurane in oxygen, at a flow rate of 1 L/min. Catheters were placed, one in the iliac vein for infusion, the other in the femoral artery for blood sampling. Catheter patency was maintained by flushing it with 100 U/mL heparin in 0.9% saline. Sodium [13C]-formate (Cambridge Isotopes Ltd.) in isotonic saline was infused at a rate of 9.6 μmol/h for 90 min. Rats were monitored throughout the infusion by using a PhysioSuite with MouseStat pulse oximeter module, and their body temperature was maintained by using the RightTemp module (Kent Scientific).
Sample collection.
Before the commencement of infusion, a blood sample was taken from the arterial line to determine the background enrichment of [13C]-formate. On beginning the infusion, 200-μL blood samples were taken every 20 min for the first hour and then every 15 min until the conclusion of the study at 90 min. Fluid loss due to blood sampling was compensated for by the infusion of the isotonic saline containing the sodium [13C]-formate. At the conclusion of the study, a terminal sample was taken from the abdominal aorta into a heparinized syringe, and the liver was rapidly removed and freeze-clamped in liquid nitrogen. Plasma was isolated by centrifugation for 10 min at 3000 × g at 4°C. For the erythrocyte glutathione reductase (EGR) assay, red blood cells were washed 3 times with saline, and 4 volumes of deionized water were added to the packed cells before flash-freezing in liquid nitrogen. All samples were stored at −80°C until analysis.
Confirmation of deficiency.
Riboflavin deficiency was confirmed by measurement of EGR activation coefficient (EGRac) according to the method of Tillotson and Baker (20), which determines the effect of added FAD on EGR activity. Total homocysteine concentration in plasma was determined by the HPLC method of Vester and Rasmussen (21), in which plasma samples were derivatized with 7-fluorobenzo-2-oxa-1,3-diazole-4-sulfonic acid. Total homocysteine was quantified by reference to a standard curve by using mercaptopropionylglycine as an internal standard.
Measurement of MTHFR activity.
MTHFR activity was determined in liver homogenates of RR and RD rats according to the method described by Kang et al. (22). According to this method, the activity of the enzyme was measured in the reverse direction with [Me-14C]-CH3THF as substrate. As the reaction progressed, the [14C]-methyl group was converted to [14C]-formaldehyde. The reaction was terminated with the addition of dimedone and extracted with toluene. The toluene extract was added to 10 mL of scintillation fluid (ScintiVerse L-9588; Fischer Scientific), and radioactive [14C]-formaldehyde was determined in an LKB RakBeta liquid scintillation counter.
MTHFR Western blot.
Liver tissue was homogenized 1:1 in 0.5 M Tris (pH 6.0) containing 0.1 g phenylmethylsulfonyl fluoride/L, 1 μg aprotinin/mL, and 0.01 g leupeptin/L. Gels were run under denaturing conditions by using BioRad Mini-PROTEAN TGX 4–20% gels (catalog no. 456–1096). Proteins were transferred to a polyvinylidene fluoride membrane and probed as follows. A monoclonal mouse anti-rat MTHFR antibody was used for the detection of liver MTHFR (1:1000 dilution, Abcam, ab113637; secondary: goat anti-mouse-HRP conjugate, 1:5000, Santa Cruz Biotechnology, catalog no. sc-2005); actin was used as a loading control (primary: rabbit anti-actin, 1:2000 Sigma-Aldrich, catalogue no. A2066; secondary: goat anti-rabbit-HRP conjugate, 1:5000 Bio-Rad, catalogue no. 170–5046). Membranes were developed by using the BioRad Immun-Star HRP Substrate Kit (catalogue no. 170–5041) and imaged by using the GE ImageQuant system. Band density was quantified by using ImageJ software.
Plasma formate concentration and formate kinetics.
Plasma formate concentration and isotopic enrichment of [13C]-formate were determined by gas chromatography–mass spectrometry according to the method of Lamarre et al. (23) by using 1,2[13C]-acetate as the internal standard. The isotopic enrichment of formate was expressed as a tracer-to-tracee ratio, and the endogenous rate of formate production was calculated by using the equation of Wolfe et al. (24):
 |
where Ra is the endogenous rate of formate production in μmol/h, F is the infusion rate of [13C]-formate in μmol/h, and Ep is the enrichment of [13C]-formate when isotopic enrichment has reached a plateau.
Other measurements.
Plasma amino acids were measured by the HPLC method of Wu and Meininger (25) by using a precolumn derivatization with O-phthaldialdehyde and fluorescence detection. Lactate was measured by using lactate dehydrogenase and alanine aminotransferase as described by Zoll (26) except that deproteinization was carried out by using equal parts plasma and acetonitrile. Hepatic S-adenosylmethionine (SAM) and S-adenosylhomocysteine (SAH) concentrations were measured by reverse-phase HPLC according to the method described by Molloy et al. (27). SAM and SAH were quantified in reference to a standard curve by ultraviolet detection at a wavelength of 254 nm. Plasma choline and betaine were analyzed by LC-MS/MS as previously described by Xiong et al. (28).
Data presentation and statistical analysis.
All data are presented as means ± SDs. Statistical analysis was undertaken by a 2-tailed Student's t test. P < 0.05 was taken to be statistically significant. All analyses were performed by using GraphPad Prism 6 statistical software package.
Results
Characterization of riboflavin deficiency.
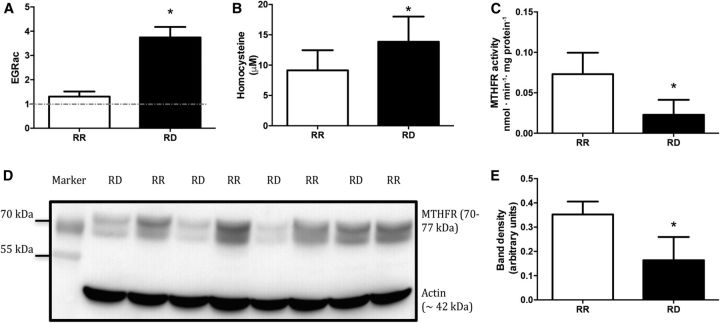
EGRac and total homocysteine were used as markers for assessing riboflavin deficiency. EGRac was higher in the RD rats than in the RR rats (Figure 2A). Plasma homocysteine was also significantly higher in the RD rats than in the RR counterparts (Figure 2B). These results are consistent with other reports of riboflavin deficiency (29, 30).
FIGURE 2.
Confirmation of riboflavin deficiency after feeding rats RD diet for 13 d. (A) EGRac measurements of RR (n = 9) and RD (n = 8) rats. (B) Total homocysteine concentrations of RR (n = 9) and RD (n = 8) rats. (C) Hepatic MTHFR activity in RR and RD rats (n = 6). (D) Western blot of MTHFR and actin from rat liver homogenate with molecular weight–marker overlay (n = 4). (E) Densitometric analysis of MTHFR protein concentrations, normalized to the density of the corresponding actin band (n = 4). Values are means ± SDs. *Significant difference from RR, P < 0.05. EGRac, erythrocyte glutathione reductase activation coefficient; MTHFR, methylenetetrahydrofolate reductase; RD, riboflavin-deficient; RR, riboflavin-replete.
MTHFR activity and Western blot.
We found that the MTHFR activity in the livers of rats fed an RD diet was significantly lower than in the livers of the RR controls (Figure 2C). We also observed that the abundance of MTHFR protein (normalized to actin) was significantly lower in RD rats than in the RR rats (Figure 2D, E).
13C-formate infusion studies: plasma formate concentration, isotopic enrichment, and production of formate.
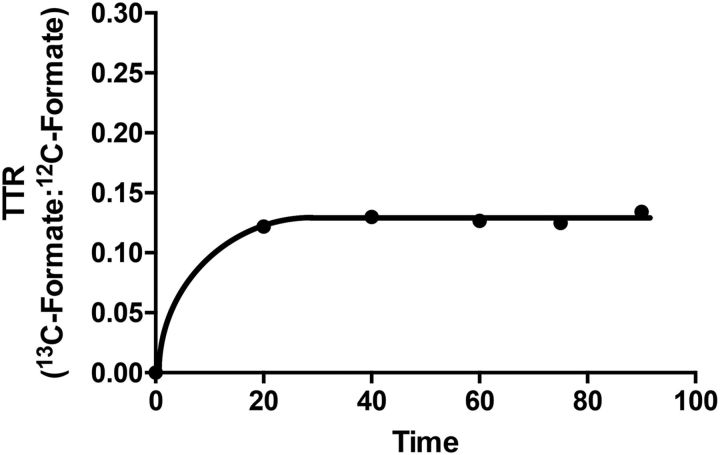
A plateau of isotopic enrichment was reached between 20 and 40 min in both groups (Figure 3). Table 1 provides a summary of the infusion experiments. No significant difference in plasma formate concentration was noted between the RR and RD groups of rats. The RD rats had a significantly higher plateau enrichment of 13C-formate than their RR counterparts. RD rats showed significantly lower endogenous rates of formate production than RR rats.
FIGURE 3.
Plasma [13C]-formate enrichment in a typical 90-min infusion of [13C]-formate in an anesthetized riboflavin-replete rat. The TTR is the ratio between the AUC of [13C]-formate and unlabeled formate after correction for the background natural abundance. TTR, tracer-to-tracee ratio.
TABLE 1.
In vivo formate kinetics in plasma of RR and RD rats1
| RR (n = 9) | RD (n = 9) | |
|---|---|---|
| Plateau isotopic formate, 13C:12C | 0.129 ± 0.027 | 0.192 ± 0.038* |
| Endogenous rate of formate production, μmol ⋅ h−1; ⋅ 100 g−1 | 36.3 ± 4.4 | 30.8 ± 4.4* |
| Plateau formate concentration, μM | 50.2 ± 6.6 | 52.8 ± 13.1 |
Values are means ± SDs. *Different from RR, P < 0.05. RD, riboflavin-deficient; RR, riboflavin-replete.
One-carbon precursors and other metabolites.
Table 2 shows plasma concentrations of one-carbon precursors and other metabolites. Of the plasma amino acids, only serine and alanine concentrations were significantly different, both being elevated in the RR rats. Glycine concentrations tended to be higher in the RD rats although this did not reach statistical significance (P = 0.051); there was no difference in the concentrations of methionine, choline, or betaine.
TABLE 2.
Plasma potential one-carbon precursors and other metabolites in RR and RD rats1
| RR (n = 8) | RD (n = 9) | |
|---|---|---|
| Serine, μM | 368 ± 52 | 467 ± 73* |
| Glycine, μM | 261 ± 99 | 361 ± 95 |
| Methionine, μM | 83.4 ± 11.6 | 76.8 ± 17.5 |
| Choline, μM | 29.4 ± 2.0 | 28.8 ± 1.7 |
| Betaine, μM | 152 ± 40 | 131 ± 34 |
| Alanine, μM | 447 ± 284 | 776 ± 296* |
| Lactate, mM | 0.82 ± 0.33 | 1.10 ± 0.34 |
Values are means ± SDs. *Different from RR, P < 0.05. RD, riboflavin-deficient; RR, riboflavin-replete.
SAM and SAH.
No significant difference in hepatic SAM or SAH content was found between the diets, nor was there a significant difference in the SAM:SAH ratio (Table 3).
TABLE 3.
Hepatic SAM and SAH concentrations from RR and RD rats1
| RR (n = 8) | RD (n = 9) | |
|---|---|---|
| SAM, nmol/g liver | 126 ± 37 | 105 ± 31 |
| SAH, nmol/g liver | 33.5 ± 3.7 | 33.1 ± 6.0 |
| SAM:SAH | 4.1 ± 1.7 | 3.1 ± 0.8 |
Values are means ± SDs. RD, riboflavin-deficient; RR, riboflavin-replete; SAH, S-adenosylhomocysteine; SAM, S-adenosylmethionine.
Discussion
The principal findings in this study were the decreased rates of endogenous formate production in RD rats and the unchanged plasma formate concentrations. An in silico model of one-carbon and glutathione metabolism developed by Reed et al. (31) and Nijhout et al. (32) allows for the modeling of a variety of defects in one-carbon metabolism, by either decreasing enzymatic activity in parts of the metabolic pathway or by modifying the availability of tetrahydrofolate and cobalamin cofactors. This computer model of one-carbon metabolism predicts a 26% decrease in intracellular formate concentration when the activities of the flavoenzymes highlighted in Figure 1 are set to 50% of normal. The measured MTHFR activity in rats fed an RD diet is decreased to <50% of control, and as such we would expect a ≥20% decrease in formate, which we would expect to be reflected by a lower plasma formate concentration. This was not found to be the case. The model has the formate concentration parameter set to 500 μM. Based on our own studies normal plasma formate concentration is closer to 50 μM (3, 4, 23). As a result of this, the model variables may lead to an overestimation of the activity of enzymes that directly produce and use formate. In addition, this marker and others in the model were derived from studies on either breast cancer or leukemia cell lines by Morrison and Allegra (33), a possible limitation in the application of this model to normal rats.
The MTHFR 677C→T single nucleotide polymorphism (TT genotype) is perhaps the most widely studied of the polymorphisms in genes of one-carbon metabolism. Individuals homozygous for the single nucleotide polymorphism have an MTHFR protein, which in a low-folate environment is unstable. This instability is due to subunit dissociation and loss of the flavin-cofactor and catalytic activity (34). Wilcken et al. (35) estimate the worldwide prevalence of homozygosity for the TT genotype is ∼13% but ranges from 2.7% to 32% depending on ethnic background. Studies performed in Northern Ireland have focused on the connection between riboflavin status and the 677C→T polymorphism. McNulty et al. (36) showed that in subjects with the TT genotype, low riboflavin intake led to much higher homocysteine concentrations than in subjects with either the CT or CC genotype. Subjects with the TT genotype who had medium or high intakes of riboflavin had plasma homocysteine concentrations that were comparable to both the CC and CT genotypes. Further studies showed that individuals suffering from hypertension and who were homozygous for the TT genotype exhibited normalized blood pressure when given riboflavin supplements (37–39).
Our experiments revealed a lower abundance of MTHFR protein in the livers of rats fed the RD diet for 13 d. This may in part be attributable to increased catabolism of the apoenzyme. Indeed, a study by Martínez-Limón et al. (40) demonstrates that RD treatment of a murine melanoma cell line results in the destabilization of the flavoproteome. Their data showed that cells cultured in a riboflavin-free medium had dramatic reductions in a number of flavoenzymes; MTHFR and methionine synthase reductase were both among the top 10 most-affected proteins. In the presence of a proteasomal inhibitor, this degradation was not seen. This indicates that the destabilization of these flavoproteins, because of the lack of flavin cofactor, most likely leads to ubiquitination of the enzyme and degradation in a proteasomal-dependent fashion.
Our observation of a decrease in the endogenous rate of formate production is most easily explained by a decrease in mitochondrial formate production. Because dimethylglycine dehydrogenase, sarcosine dehydrogenase, and GCS are all mitochondrial, flavin-dependent enzymes, it is reasonable to expect that the activities of these enzymes would decrease when riboflavin is deficient. The decreased enzymatic activity would result in a lower production of 5,10-methylenetetrahydrofolate and ultimately formate from their respective substrates. Indeed, we saw a strong trend toward increased plasma glycine concentrations in RD rats, suggesting a decrease in GCS activity. In a study by White et al. (41) it was found that tissue concentrations of serine and glycine mirrored one another, and indeed this may have been the case in our study. Because the reaction catalyzed by serine hydroxymethyltransferase is fully reversible in vivo, an increase in glycine provides one possible explanation for the elevated plasma serine concentrations we observed in RD rats.
Previous research in our laboratory into formate metabolism in folate-deficient rats demonstrated that folate deficiency results in reduced rates of formate production concurrent with an elevated plasma formate concentration (4). Although formate was still being produced, albeit at a lower rate, a major defect of formate incorporation and removal was caused by the reduced availability of folate cofactors. This resulted in the observed elevation of plasma formate concentrations. In the present study, if formate was being produced at a lower rate, it must also have been used at a lower rate to account for the unchanged plasma formate concentrations. Because these RD rats had adequate folate intake, there was no defect of formate incorporation, rather a defect in the utilization of one-carbon units by MTHFR. Another major distinction between the 2 conditions may be the folate-dependent catabolism of one-carbon units. The cytosolic 10f-tetrahydrofolate dehydrogenase enzyme will be unable to catabolize excess formate as efficiently in folate-deficient rats because of the reduced ability to incorporate formate into the cytosolic tetrahydrofolate pool; this should be unaffected in RD rats.
Riboflavin deficiency has wide-ranging effects on energy metabolism; it is not surprising that it results in some level of dysfunction in the electron transport chain (42). The pyruvate dehydrogenase complex, which is responsible for the conversion of pyruvate to acetyl-CoA will also be affected by riboflavin deficiency by virtue of the FAD-dependent dihydrolipoamide dehydrogenase subunit of the complex (43, 44). With decreased flux through the pyruvate dehydrogenase complex we would expect to see increased pyruvate concentrations. Pyruvate is a substrate for both alanine aminotransferase and lactate dehydrogenase, which produce alanine and lactate respectively (45, 46). Although lactate concentrations were not significantly different in RD rats, alanine was significantly higher; this provides evidence for an elevation of pyruvate concentrations secondary to reduced pyruvate dehydrogenase complex activity.
This is, to our knowledge, the first published study examining the role of riboflavin in formate metabolism. Compared with our prior studies on folate and vitamin B-12 deficiencies, we see clear differences in how a deficiency in any of these B vitamins may have a negative impact on the production or utilization of one-carbon units. In light of the critical role that formate plays in fetal development, it may be of some value to reevaluate the importance of adequate riboflavin status in women of childbearing age.
Acknowledgments
We thank Theerawat Pongnopparat for his assistance with Western blotting. We also thank Cornelia Ulrich, Mike Reed, and Fred Nijhout, codevelopers of the mathematical model of one-carbon metabolism; they developed the software with the support of NIH grant RO1 CA105437 and National Science Foundation grant DMS010987. LM, SGL, MEB, and JTB designed the research and analyzed the data; LM, SGL, RPdS, and RLJ conducted the research; LM, MEB, and JTB wrote the manuscript and had primary responsibility for the final content. All authors read and approved the final manuscript.
Abbreviations
- EGR
erythrocyte glutathione reductase
- EGRac
erythrocyte glutathione reductase activity coefficient
- GCS
glycine cleavage system
- MTHFR
methylenetetrahydrofolate reductase
- RD
riboflavin-deficient
- RR
riboflavin-replete
- SAH
S-adenosylhomocysteine
- SAM
S-adenosylmethionine
- THF
tetrahydrofolate
Footnotes
Supported by the Canadian Institutes of Health Research (RNL119954, 133501, MRN133254) and the Research and Development Corporation of Newfoundland and Labrador (5404-1433-101, 00783-002).
References
- 1. Brosnan ME, MacMillan L, Stevens JR, Brosnan JT. Division of labour: how does folate metabolism partition between one-carbon metabolism and amino acid oxidation? Biochem J 2015;472:135–46. [DOI] [PubMed] [Google Scholar]
- 2. Brosnan ME, Brosnan JT. Formate: the neglected member of one-carbon metabolism. Annu Rev Nutr 2016;36:369–88. [DOI] [PubMed] [Google Scholar]
- 3. Lamarre SG, Molloy AM, Reinke SN, Sykes BD, Brosnan ME, Brosnan JT. Formate can differentiate between hyperhomocysteinemia due to impaired remethylation and impaired transsulfuration. Am J Physiol Endocrinol Metab 2012;302:E61–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Morrow GP, MacMillan L, Lamarre SG, Young SK, MacFarlane AJ, Brosnan ME, Brosnan JT. In vivo kinetics of formate metabolism in folate-deficient and folate-replete Rats. J Biol Chem 2015;290:2244–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pike ST, Rajendra R, Artzt K, Appling DR. Mitochondrial C1-tetrahydrofolate synthase (MTHFD1L) supports the flow of mitochondrial one-carbon units into the methyl cycle in embryos. J Biol Chem 2010;285:4612–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Porter DH, Cook RJ, Wagner C. Enzymatic properties of dimethylglycine dehydrogenase and sarcosine dehydrogenase from rat liver. Arch Biochem Biophys 1985;243:396–407. [DOI] [PubMed] [Google Scholar]
- 7. Motokawa Y, Kikuchi G. Glycine metabolism in rat liver mitochondria. V. Intramitochondrial localization of the reversible glycine cleavage system and serine hydroxymethyltransferase. Arch Biochem Biophys 1971;146:461–4. [DOI] [PubMed] [Google Scholar]
- 8. Wolthers KR, Scrutton NS. Protein interactions in the human methionine synthase-methionine synthase reductase complex and implications for the mechanism of enzyme reactivation. Biochemistry 2007;46:6696–709. [DOI] [PubMed] [Google Scholar]
- 9. Olteanu H, Banerjee R. Human methionine synthase reductase, a soluble P-450 reductase-like dual flavoprotein, is sufficient for NADPH-dependent methionine synthase activation. J Biol Chem 2001;276:35558–63. [DOI] [PubMed] [Google Scholar]
- 10. Narisawa A, Komatsuzaki S, Kikuchi A, Niihori T, Aoki Y, Fujiwara K, Tanemura M, Hata A, Suzuki Y, Relton CL, et al. . Mutations in genes encoding the glycine cleavage system predispose to neural tube defects in mice and humans. Hum Mol Genet 2012;21:1496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Momb J, Lewandowski JP, Bryant JD, Fitch R, Surman DR, Vokes SA, Appling DR. Deletion of Mthfd1l causes embryonic lethality and neural tube and craniofacial defects in mice. Proc Natl Acad Sci USA 2013;110:549–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pai YJ, Leung KY, Savery D, Hutchin T, Prunty H, Heales S, Brosnan ME, Brosnan JT, Copp AJ, Greene ND. Glycine decarboxylase deficiency causes neural tube defects and features of non-ketotic hyperglycinemia in mice. Nat Commun 2015;6:6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Narisawa K, Tamura T, Tanno K, Ohara K, Arakawa T. Tetrahydrofolate-dependent enzyme activities of the rat liver in riboflavin deficiency. Tohoku J Exp Med 1968;94:417–30. [DOI] [PubMed] [Google Scholar]
- 14. Powers HJ. Riboflavin (vitamin B-2) and health. Am J Clin Nutr 2003;77:1352–60. [DOI] [PubMed] [Google Scholar]
- 15. Sánchez DJ, Murphy MM, Bosch-Sabater J, Fernandez-Ballart J. Enzymic evaluation of thiamin, riboflavin and pyridoxine status of parturient mothers and their newborn infants in a Mediterranean area of Spain. Eur J Clin Nutr 1999;53:27–38. [DOI] [PubMed] [Google Scholar]
- 16. Rivlin RS, Menendez C, Langdon RG. Biochemical similarities between hypothyroidism and riboflavin deficiency. Endocrinology 1968;83:461–9. [DOI] [PubMed] [Google Scholar]
- 17. Cimino JA, Jhangiani S, Schwartz E, Cooperman JM. Riboflavin metabolism in the hypothyroid human adult. Proc Soc Exp Biol Med 1987;184:151–3. [DOI] [PubMed] [Google Scholar]
- 18. Reeves PG, Nielsen FH, Fahey GC Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 1993;123:1939–51. [DOI] [PubMed] [Google Scholar]
- 19. National Research Council Nutrient requirements of laboratory animals: fourth revised edition. Washington (DC): National Academies Press; 1995. [PubMed] [Google Scholar]
- 20. Tillotson JA, Baker EM. An enzymatic measurement of the riboflavin status in man. Am J Clin Nutr 1972;25:425–31. [DOI] [PubMed] [Google Scholar]
- 21. Vester B, Rasmussen K. High performance liquid chromatography method for rapid and accurate determination of homocysteine in plasma and serum. Eur J Clin Chem Clin Biochem 1991;29:549–54. [DOI] [PubMed] [Google Scholar]
- 22. Kang SS, Wong PW, Zhou JM, Sora J, Lessick M, Ruggie N, Grcevich G. Thermolabile methylenetetrahydrofolate reductase in patients with coronary artery disease. Metabolism 1988;37:611–3. [DOI] [PubMed] [Google Scholar]
- 23. Lamarre SG, MacMillan L, Morrow GP, Randell E, Pongnopparat T, Brosnan ME, Brosnan JT. An isotope-dilution, GC-MS assay for formate and its application to human and animal metabolism. Amino Acids 2014;46:1885–91. [DOI] [PubMed] [Google Scholar]
- 24. Wolfe RR, Goodenough RD, Wolfe MH, Royle GT, Nadel ER. Isotopic analysis of leucine and urea metabolism in exercising humans. J Appl Physiol 1982;52:458–66. [DOI] [PubMed] [Google Scholar]
- 25. Wu G, Meininger CJ. Analysis of citrulline, arginine, and methylarginines using high-performance liquid chromatography. Methods Enzymol 2008;440:177–89. [DOI] [PubMed] [Google Scholar]
- 26. Zoll F. L-lactate: determination with LDH, GPT and NAD. In: Bergmeyer HU. editor. Methods of enzymatic analysis. Vol. 3, 2nd ed Amsterdam: Elsevier Inc.; 1974. p. 1475–77. [Google Scholar]
- 27. Molloy AM, Weir DG, Kennedy G, Kennedy S, Scott JM. A new high performance liquid chromatographic method for the simultaneous measurement of S-adenosylmethionine and S-adenosylhomocysteine. Concentrations in pig tissues after inactivation of methionine synthase by nitrous oxide. Biomed Chromatogr 1990;4:257–60. [DOI] [PubMed] [Google Scholar]
- 28. Xiong Y, Zhao YY, Goruk S, Oilund K, Field CJ, Jacobs RL, Curtis JM. Validation of an LC-MS/MS method for the quantification of choline-related compounds and phospholipids in foods and tissues. J Chromatogr B Analyt Technol Biomed Life Sci 2012;911:170–9. [DOI] [PubMed] [Google Scholar]
- 29. Prentice AM, Bates CJ. A biochemical evaluation of the erythrocyte glutathione reductase (EC 1.6.4.2) test for riboflavin status. 2. Dose-response relationships in chronic marginal deficiency. Br J Nutr 1981;45:53–65. [DOI] [PubMed] [Google Scholar]
- 30. Jacques PF, Bostom AG, Wilson PW, Rich S, Rosenberg IH, Selhub J. Determinants of plasma total homocysteine concentration in the Framingham Offspring cohort. Am J Clin Nutr 2001;73:613–21. [DOI] [PubMed] [Google Scholar]
- 31. Reed MC, Nijhout HF, Neuhouser ML, Gregory JF III, Shane B, James SJ, Boynton A, Ulrich CM. A mathematical model gives insights into nutritional and genetic aspects of folate-mediated one-carbon metabolism. J Nutr 2006;136:2653–61. [DOI] [PubMed] [Google Scholar]
- 32. Nijhout HF, Reed MC, Ulrich CM. Mathematical models of folate-mediated one-carbon metabolism. Vitam Horm 2008;79:45–82. [DOI] [PubMed] [Google Scholar]
- 33. Morrison PF, Allegra CJ. Folate cycle kinetics in human breast cancer cells. J Biol Chem 1989;264:10552–66. [PubMed] [Google Scholar]
- 34. Yamada K, Chen Z, Rozen R, Matthews RG. Effects of common polymorphisms on the properties of recombinant human methylenetetrahydrofolate reductase. Proc Natl Acad Sci USA 2001;98:14853–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wilcken B, Bamforth F, Li Z, Zhu H, Ritvanen A, Renlund M, Stoll C, Alembik Y, Dott B, Czeizel AE, et al. . Geographical and ethnic variation of the 677C>T allele of 5,10 methylenetetrahydrofolate reductase (MTHFR): findings from over 7000 newborns from 16 areas world wide. J Med Genet 2003;40:619–25. Erratum in: J Med Genet 2004;41:400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McNulty H, McKinley MC, Wilson B, McPartlin J, Strain JJ, Weir DG, Scott JM. Impaired functioning of thermolabile methylenetetrahydrofolate reductase is dependent on riboflavin status: implications for riboflavin requirements. Am J Clin Nutr 2002;76:436–41. [DOI] [PubMed] [Google Scholar]
- 37. Horigan G, McNulty H, Ward M, Strain JJ, Purvis J, Scott JM. Riboflavin lowers blood pressure in cardiovascular disease patients homozygous for the 677C→T polymorphism in MTHFR. J Hypertens 2010;28:478–86. [DOI] [PubMed] [Google Scholar]
- 38. Wilson CP, Ward M, McNulty H, Strain JJ, Trouton TG, Horigan G, Purvis J, Scott JM. Riboflavin offers a targeted strategy for managing hypertension in patients with the MTHFR 677TT genotype: a 4-y follow-up. Am J Clin Nutr 2012;95:766–72. [DOI] [PubMed] [Google Scholar]
- 39. Wilson CP, McNulty H, Ward M, Strain JJ, Trouton TG, Hoeft BA, Weber P, Roos FF, Horigan G, McAnena L, et al. . Blood pressure in treated hypertensive individuals with the MTHFR 677TT genotype is responsive to intervention with riboflavin: findings of a targeted randomized trial. Hypertension 2013;61:1302–8. [DOI] [PubMed] [Google Scholar]
- 40. Martínez-Limón A, Alriquet M, Lang WH, Calloni G, Wittig I, Vabulas RM. Recognition of enzymes lacking bound cofactor by protein quality control. Proc Natl Acad Sci USA 2016;113:12156–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. White PJ, Lapworth AL, An J, Wang L, McGarrah RW, Stevens RD, Ilkayeva O, George T, Muehlbauer MJ, Bain JR, et al. . Branched-chain amino acid restriction in Zucker-fatty rats improves muscle insulin sensitivity by enhancing efficiency of fatty acid oxidation and acyl-glycine export. Mol Metab 2016;5:538–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Beyer RE, Lamberg SL, Neyman MA. The effect of riboflavin deficiency and galactoflavin feeding on oxidative phosphorylation and related reactions in rat liver mitochondria. Can J Biochem Physiol 1961;39:73–88. [Google Scholar]
- 43. Ishikawa E, Oliver RM, Reed LJ. Alpha-Keto acid dehydrogenase complexes, V. Macromolecular organization of pyruvate and alpha-ketoglutarate dehydrogenase complexes isolated from beef kidney mitochondria. Proc Natl Acad Sci USA 1966;56:534–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Patel MS, Roche TE. Molecular biology and biochemistry of pyruvate dehydrogenase complexes. FASEB J 1990;4:3224–33. [DOI] [PubMed] [Google Scholar]
- 45. Karmen A, Wroblewski F, Ladue JS. Transaminase activity in human blood. J Clin Invest 1955;34:126–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Markert CL. Lactate dehydrogenase. Biochemistry and function of lactate dehydrogenase. Cell Biochem Funct 1984;2:131–4. [DOI] [PubMed] [Google Scholar]