Abstract
Background: Mexico's sugar-sweetened beverage (SSB) intake is among the highest globally. Although evidence shows that increases in SSB intake are linked with increased energy intake, weight gain, and cardiometabolic risks, few randomized clinical trials have been conducted in adults.
Objective: The aim of this study was to determine if replacing SSBs with water affects plasma triglycerides (TGs) (primary outcome), weight, and other cardiometabolic factors.
Methods: We selected overweight/obese (BMI ≥25 and <39 kg/m2) women (18–45 y old) reporting an SSB intake of at least 250 kcal/d living in Cuernavaca, Mexico. Women were randomly allocated to the water and education provision (WEP) group (n = 120) or the education provision (EP)–only group (n = 120). The WEP group received biweekly water deliveries, and both groups received equal monthly nutrition counseling. During nutrition counseling, the WEP group sessions included activities to encourage increased water intake, reduced SSB intake, and substitution of water for SSBs. Repeated 24-h dietary recalls, anthropometric measurements, and fasting blood samples were collected at baseline and at 3, 6, and 9 mo. The Markov–Monte Carlo method was used for multiple imputation; separate mixed-effects models tested each outcome.
Results: An intent-to-treat (ITT) analysis indicated that the WEP group increased water intake and decreased SSB intake significantly over time, but there were no differences in plasma TG concentrations between groups at the end of the intervention (WEP at baseline: 155 ± 2.10 mg/dL; WEP at 9 mo: 149 ± 2.80 mg/dL; EP at baseline: 150 ± 1.90 mg/dL; EP at 9 mo: 161 ± 2.70 mg/dL; P for mean comparisons at 9 mo = 0.10). Secondary analyses showed significant effects on plasma TGs (change from baseline to 9 mo: WEP, −28.9 ± 7.7 mg/dL; EP, 8.5 ± 10.9 mg/dL; P = 0.03) and metabolic syndrome (MetS) prevalence at 9 mo (WEP: 18.1%; EP: 37.7%; P = 0.02) among obese participants.
Conclusions: Providing water and nutritional counseling was effective in increasing water intake and in partially decreasing SSB intake. We found no effect on plasma TGs, weight, and other cardiometabolic risks in the ITT analysis, although the intervention lowered plasma TGs and MetS prevalence among obese participants. Further studies are warranted. This trial was registered at http://www.clinicaltrials.gov as NCT01245010.
Introduction
Overweight and obesity and their related chronic diseases are public health problems in Mexico (1, 2). By 2002, the leading causes of death in the country were coronary heart disease and diabetes (1). The prevalence of metabolic syndrome (MetS)6 among Mexican women aged >20 y was 52.2% in 2006 (3) and that for hypertriglyceridemia was 26.9% (3, 4). In 2012, the combined prevalence of overweight and obesity among women >20 y was 73.0%, with obesity representing 37.5% (2). In the same way that substantial health benefits can be achieved with modest weight losses of 5–7% of initial weight (5, 6), evidence suggests that a decrease in elevated TG concentrations is associated with a decrease in cardiovascular disease risk (7, 8).
Mexico's intake of sugar-sweetened beverages (SSBs) is among the highest worldwide. In 2006, the per capita energy contribution from SSBs in adults was 411 kcal/d, or 22.3% of total energy intake (9–11), and was equally high in 2012, representing 19.0% of total energy intake (12). Evidence from a combination of longitudinal cohorts, small clinical trials, and randomized controlled trials (RCTs) in children shows that increases in SSB intake are linked with increased energy intake, weight gain, and an array of cardiometabolic risks, such as hypertriglyceridemia, low HDL cholesterol, type 2 diabetes, and MetS, among others (13–21). Nevertheless, critics of the results of the limited number of RCTs conducted in adults have argued that more evidence is needed to support conclusions about the negative effects of SSBs on health (22–25). One recent 3-arm RCT in U.S. adults tested the replacement of caloric beverages with noncaloric beverages (water or diet beverages) as a strategy to promote weight loss. The results showed no differences in weight loss from baseline weight in the water group or in the low-caloric beverage group compared with that in the control group. However, in a secondary analysis (combining water and low-caloric beverage vs. control group), participants in the beverage-replacement combined group were 2 times as likely to achieve a 5% weight loss by the end of the intervention (P = 0.04) (26). Evidence, albeit limited, suggests that substituting water for SSBs may facilitate weight loss, especially in subjects participating in weight-loss programs (27). Reduction in total energy intake with the subsequent meal in adults (28), short-term effect of increased satiety, reduced feeling of hunger (29), and increased energy expenditure as a result of water-induced thermogenesis (30, 31) are some of the suggested potential mechanisms.
We conducted a 9-mo clinical trial to determine whether replacement of SSBs with water, through water provision and nutrition counseling, could reduce plasma TG concentrations as the primary outcome and weight and other cardiometabolic risk factors as secondary outcomes in overweight and obese Mexican women. Secondary analyses examined the effect of initial weight status on the primary outcome.
Participants and Methods
Design.
Hernández-Cordero et al. (32) described in detail the methods of this RCT elsewhere. Briefly, this RCT, conducted in Cuernavaca, Mexico, consisted of 2 intervention groups: water and education provision (WEP) and education provision (EP) only. The study was conducted according to the guidelines in the Declaration of Helsinki, and all procedures involving human subjects were approved by the institutional review board of the Mexican National Institute of Public Health. Written informed consent was obtained from all subjects. The study was registered at clinicaltrials.gov (NCT01245010).
Participants.
Women aged 18–45 y with a BMI ≥25 to <39 kg/m2 who reported SSB intakes of at least 250 kcal/d were recruited, randomly allocated to the intervention groups, and followed for 9 mo. An advertising campaign identified potential participants interested in joining the study. Applicants were screened via telephone to determine if they fulfilled the age and BMI criteria. In those who did, three 24-h dietary recalls (nonconsecutive days, including 2 weekdays and 1 weekend day) were administered by trained interviewers to identify their usual intake of SSBs. The procedures for the analysis of the dietary information are explained in detail below. A broader description of exclusion criteria was published elsewhere (32).
Sample size calculation and random assignment.
This study was powered to observe a 31 ± 58 mg/dL decrease, from baseline to the end of the intervention, in plasma TG concentrations and a weight loss of 1.8 ± 3.4 kg. We needed a sample size of 120 cases, which considered 2-sided tests, with 90% power and an α of 0.05, and allowed for >75% attrition. Women fulfilling all selection criteria (n = 240) were randomly assigned to either of the treatment groups through blocked randomization. Assignments to each of the 24 blocks within the groups were made by random numbers generated with Microsoft Office Excel. Each block included 10 participants.
Intervention.
The intervention lasted 9 mo. Because of the characteristics of this intervention, it was not possible to make staff and participants unaware of treatment. The 2 groups were treated identically except that we provided water to the WEP group along with nutrition counseling, including individualized and group meetings targeted to the rationale and strategies to increase water intake, reduce SSB intake, and substitute water for SSBs (see Supplemental Table 1 for detailed characteristics). To ensure water availability, the WEP women received bottled water at home and/or picked it up every 2 wk. We provided 2–3 L of water per participant per day with 1 additional L/d to account for possible consumption by other family members. Women of both groups participated in monthly face-to-face meetings with a dietitian and a psychologist (1 set for each group) either individually or in a group (2–10 participants each). At the end of group meetings, each woman identified her healthy diet goal for the next month. Individual meetings consisted of nutrition counseling with regard to the goal. The WEP and EP groups met separately and received equal attention. For ethical reasons, after final measurements, the EP group participated in an extra meeting with regard to water and SSB intake.
Outcomes and measurements.
The primary outcome was change in plasma TG concentrations over a 9-mo period. The secondary outcomes were change in weight and other MetS indicators: waist circumference, percentage of body fat, fasting glucose, total cholesterol, HDL cholesterol, LDL cholesterol, glycosylated hemoglobin (HbA1c), and blood pressure. In addition, we evaluated serum and urine osmolality and estimated MetS prevalence. MetS was defined according to the International Diabetes Federation (33) as waist circumference >80 cm plus any 2 of the following criteria: TGs >150 mg/dL, HDL cholesterol <50.0 mg/dL, high blood pressure (systolic >130 mm Hg and/or diastolic >85 mm Hg), and fasting glucose >100 mg/dL.
All measurements were collected at baseline and at 3, 6, and 9 mo except for urine samples and air displacement plethysmography, both of which were measured at baseline and 9 mo, and sociodemographic information, which was collected at baseline only. All assessments were conducted on weekdays between 0700 and 1100 h at the Mexican National Institute of Public Health, except for water delivery and dietary information, which were obtained at the participant's home or another place of her preference.
Fasting blood samples were collected in non-anticoagulated and EDTA tubes (for HbA1c determination). Samples were immediately frozen at −80°C until analysis at the end of the intervention. Urine samples were collected and stored until determination of urine osmolality. All analytic measurements were performed at the Mexican National Institute of Public Health. Plasma TG concentrations were measured after lipase hydrolysis in an automatic analyzer with a tungsten lamp (Prestige 24i; Tokyo Boeki Medical System). The interassay CV was 4.4%. Total cholesterol was determined by using enzymatic hydrolysis and oxidation; the interassay CV was 3.9%. HDL cholesterol was measured by using an enzymatic colorimetric direct method after eliminating chylomicrons, VLDL cholesterol, and LDL cholesterol by enzymatic digestion. Glucose concentrations were measured by using an automatized glucose oxidase method, with an overall interassay CV of 2.1%. The proportion of HbA1c was determined by an immunocolorimetric method in whole blood. Finally, serum and urine osmolality were measured by using freezing point depression with a micro osmometer (Fiske 210 Micro-Sample Osmometer; Advanced Instruments).
Resting blood pressure was measured with a digital sphygmomanometer (Omron model HEM-781 INT) on the right arm after 5 min of rest with the participant seated and her back supported. Three measurements were taken with at least 2 min between each measurement, and the mean was used.
Weight was assessed in tight-fitting swimsuits or spandex shorts without shoes with a Tanita (model BWB-627-A, 100-g precision) digital scale. Height was measured at baseline only by using a calibrated, wall-mounted stadiometer (model 17802, 2-mm precision; Shorr Productions). Waist circumference was measured in a light-weight hospital gown by using a Gulick tape measure. Waist measurements were obtained at 2 points, the midpoint between the sternum and the umbilicus and the iliac crest, following standard procedures (34). Total body fat was evaluated by using air displacement plethysmography (Bod Pod Life Measurement). This technique is reliable and validated for evaluating body composition (35). Subjects were fasting at the time of measurement. The Bod Pod was calibrated before each measurement by using a 49.273-L cylinder. Subjects were tested while wearing a swimsuit and a swimming cap to compress the hair (35, 36). Volume of thoracic capacity was used to correct body volume (corrected body volume = total body volume − thoracic capacity). Body fat mass (in kg) was calculated by using Siri's equation (37).
A 24-h recall assessed dietary intake during a face-to-face interview on 3 nonconsecutive days during the same week (2 weekdays and 1 weekend day). The recall included a complete audit of foods the participant had consumed during the previous 24 h, and specific probes for all beverages and water included measurement cups for a better estimation of liquid intake. We estimated total energy intake from solid foods and beverages as the average of the 3-d intake for each subject according to the Mexican National Institute of Public Health food composition table, with links to and consistency checks with the USDA National Nutrient Database for Standard Reference (38).
Physical activity was measured by an accelerometer (Actigraph GT3X) worn at the waist for at least 8 h for 4 consecutive days. We estimated total metabolic equivalents (METs) per day as the average MET over 24 h. We estimated intervention adherence through the records of participants' attendance at the individual and group meetings, visits, and phone calls.
Sociodemographic information, collected by questionnaire, included age, years of education, and housing condition, such as flooring and roof materials, ownership of home appliances, and number of rooms. We constructed an indicator of socioeconomic status or well-being through a principal components analysis (39). The statistical models included a standardized factor as a continuous variable. This methodology, which has been validated for describing socioeconomic differentiation within a population, allowed us to classify participants' households into socioeconomic groups (39). Supplemental Table 2 summarizes the timing of measurements and contacts.
Adverse events.
We closely monitored the development of any adverse event (any symptom or safety concern requiring medical attention reported by a participant during a contact). Participants reporting potential adverse events were referred to the project's physician.
Statistical analysis.
All analyses were performed by using Stata version 12.1 (StataCorp). We performed an intent-to-treat (ITT) analysis. For continuous variables, the Markov–Monte Carlo method was used to impute missing data, generating 10 imputations. The results from the imputation were combined by using the MI Stata command in all analyses (40, 41). Baseline demographic characteristics, dietary intake, and primary and secondary outcomes were described by treatment group, with means and SDs for continuous variables and percentages for categorical characteristics. The main effects of time, treatment group, and time by treatment group interaction were examined in separate mixed-effects models for each outcome by using the independent structure of the covariance matrix and taking into account the randomization block. Given that we found no differences when considering the randomization block, we present results without it. We tested both the mean outcomes across time between groups and the absolute and relative changes from baseline to the end of the intervention between groups in all outcome variables, both unadjusted and adjusted for baseline characteristics. Because we found no difference with adjusted models, we present unadjusted results only.
Post hoc secondary analyses according to weight status at baseline with the use of the WHO-recommended cutoffs (overweight, BMI ≥25.0–29.9 kg/m2; obese, BMI ≥30.0 kg/m2) were performed to examine the hypothesis that the effect of the intervention would differ across BMI categories, as found in several SSB interventions among young children or adolescents (20, 42). Mean outcomes across time between groups and changes from baseline to 9 mo were tested by the interaction of the group effect and weight status at baseline by using mixed-effects models and linear regression models, respectively. We tested the effect of the intervention on MetS at 9 mo by using logistic regression and its effect modification by weight status at baseline. P values <0.05 were considered significant in all analyses. We present mean ± SEs for continuous variables or as specified and percentages for categorical variables.
Results
ITT analysis
Participants.
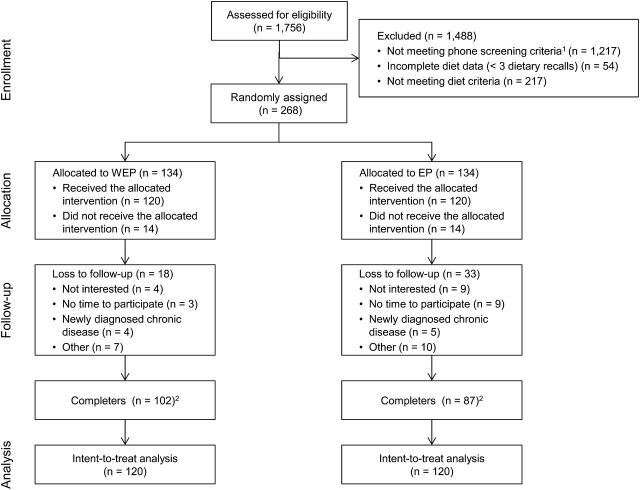
Of the 1756 women screened, 268 fulfilled the selection criteria and were randomly allocated to the WEP or the EP group. From these, 240 agreed to participate in the study, and baseline measurements were taken. The retention rate for participants with baseline measurements was higher in the WEP group (85.0%) than in the EP group (72.5%) (P = 0.03) (Fig. 1). Attendance by the WEP group (mean ± SD: 7.3 ± 2.4 sessions) was greater than that by the EP group (6.4 ± 2.4 sessions) (P = 0.01).
FIGURE 1.
Flow of participants through the trial. 1Phone screening criteria: age and reported BMI (confirmed at study site visit). 2Based on attendance at last appointment. EP, education provision; WEP, water and education provision.
Sociodemographic characteristics and dietary intake among dropouts and women completing the study were similar except for parity, with a greater proportion of nulliparous women finishing the study (28.6% completers, 13.7% dropouts; P = 0.04) (Supplemental Table 3). Baseline characteristics were not different between groups (Tables 1–3). Overall, participants were, on average (±SD), 33.3 ± 6.7 y old and obese (BMI: 31.2 ± 3.7 kg/m2), 26.2% were nulliparous, and 45.0% had completed middle and high school (data not shown).
TABLE 1.
Baseline characteristics of study participants1
| ITT (n = 240) | ||
|---|---|---|
| WEP (n = 120) | EP (n = 120) | |
| Age, y | 33.5 ± 6.7 | 33.3 ± 6.7 |
| Parity, n (%) | ||
| Nulliparous | 34 (28.3) | 27 (22.5) |
| Multiparous | 86 (71.7) | 93 (77.5) |
| Marital status, n (%) | ||
| Married/living with someone | 69 (57.5) | 78 (65.0) |
| Not married/living with someone | 51 (42.5) | 42 (35.0) |
| Education, n (%) | ||
| Incomplete: middle school or less | 4 (3.3) | 6 (5.0) |
| Complete: middle school and high school | 47 (39.1) | 61 (50.8) |
| Technical school | 19 (15.8) | 16 (13.3) |
| Professional or higher | 50 (41.6) | 37 (30.8) |
| BMI classification, n (%) | ||
| Overweight | 56 (46.6) | 54 (45.0) |
| Obese | 64 (53.3) | 66 (55.0) |
| Smoking status, n (%) | ||
| Yes | 39 (32.5) | 36 (30.0) |
| No | 81 (67.5) | 84 (70.0) |
| Socioeconomic level index | 0.11 ± 1.34 | −0.14 ± 1.33 |
Values are means ± SDs unless otherwise indicated. Data were analyzed by using ITT analysis with 10 imputations (n = 240). EP, education provision; ITT, intent-to-treat; WEP, water and education provision.
TABLE 3.
Body composition, dietary intake, and physical activity by intervention group1
| Study time point | P 2 | ||||||
|---|---|---|---|---|---|---|---|
| Baseline3 | 3 mo | 6 mo | 9 mo | Treatment | Time | Treatment × time | |
| Weight, kg | 0.50 | 0.04 | 0.4 | ||||
| WEP | 76.9 ± 0.3 | 76.4 ± 0.3 | 75.6 ± 0.3 | 75.7 ± 0.3 | |||
| EP | 76.0 ± 0.3 | 75.6 ± 0.3 | 75.3 ± 0.3 | 75.3 ± 0.3 | |||
| BMI, kg/m2 | 0.90 | 0.04 | 0.40 | ||||
| WEP | 31.0 ± 0.1 | 30.6 ± 0.1 | 30.5 ± 0.1 | 30.5 ± 0.1 | |||
| EP | 31.0 ± 0.1 | 30.8 ± 0.1 | 30.7 ± 0.1 | 30.7 ± 0.1 | |||
| Body fat,4% | 0.70 | 0.40 | 0.90 | ||||
| WEP | 42.5 ± 0.1 | — | — | 41.6 ± 0.1 | |||
| EP | 42.2 ± 0.1 | — | — | 41.5 ± 0.2 | |||
| Waist circumference, cm | 0.70 | 0.40 | 0.80 | ||||
| WEP | 98.3 ± 0.3 | 97.8 ± 0.3 | 96.9 ± 0.2 | 97.3 ± 0.3 | |||
| EP | 98.5 ± 0.3 | 97.9 ± 0.3 | 97.9 ± 0.3 | 97.3 ± 0.3 | |||
| Total energy intake, kcal/d | 0.30 | <0.001 | 0.90 | ||||
| WEP | 2015 ± 14 | 1461 ± 14 | 1440 ± 13 | 1430 ± 11 | |||
| EP | 2054 ± 15 | 1608 ± 13 | 1577 ± 12 | 1485 ± 11 | |||
| Energy intake from solid foods, kcal/d | 0.09 | 0.10 | 0.07 | ||||
| WEP | 1497 ± 13 | 1212 ± 11 | 1211 ± 11 | 1181 ± 10 | |||
| EP | 1549 ± 13 | 1247 ± 10 | 1203 ± 10 | 1109 ± 8 | |||
| Beverages with sugar | |||||||
| mL/d | 0.40 | <0.001 | <0.001 | ||||
| WEP | 1127 ± 11 | 500.0 ± 11 | 45 ± 12 | 418 ± 11 | |||
| EP | 1094 ± 11 | 807.0 ± 14 | 890 ± 17 | 796 ± 13 | |||
| kcal/d | 0.80 | <0.001 | <0.001 | ||||
| WEP | 407 ± 4 | 164.0 ± 4 | 155.0 ± 4 | 155 ± 4 | |||
| EP | 409 ± 4 | 283.0 ± 6 | 310.0 ± 6 | 292 ± 6 | |||
| % of calories/d | 0.30 | <0.001 | <0.001 | ||||
| WEP | 21.0 ± 0.2 | 10.6 ± 0.2 | 10.1 ± 0.3 | 10.7 ± 0.3 | |||
| EP | 20.0 ± 0.2 | 16.9 ± 0.3 | 19.9 ± 0.4 | 19.3 ± 0.4 | |||
| Water consumption, mL/d | 0.20 | <0.001 | <0.001 | ||||
| WEP | 737 ± 15 | 1713.0 ± 18 | 1842 ± 20 | 1942 ± 22 | |||
| EP | 824 ± 18 | 967.0 ± 18 | 1015 ± 22 | 1065 ± 22 | |||
| Physical activity, METs/d | 0.99 | 0.91 | 0.79 | ||||
| WEP | 1.455 ± 0.004 | 1.445 ± 0.004 | 1.454 ± 0.005 | 1.477 ± 0.011 | |||
| EP | 1.455 ± 0.004 | 1.452 ± 0.004 | 1.489 ± 0.005 | 1.485 ± 0.007 | |||
Values are means ± SEs. EP, education provision; MET, metabolic equivalent; WEP, water and education provision.
Data were analyzed by using intent-to-treat analysis, 10 imputations (n = 240). Repeated-measures mixed-effects model analysis was used to test the mean through time.
There was no difference between groups at baseline in dietary intake or physical activity (all P ≥ 0.1).
Measured at baseline and 9 mo only.
Reported dietary intake and physical activity.
Reported dietary intake is presented in Table 3. Reported water intake increased in both groups, with a greater increase in the WEP group (P-interaction < 0.001). The increase in water intake started early in the intervention (change from baseline to 3 mo: WEP, 976 ± 67 mL/d; EP, 142 ± 67 mL/d; P < 0.001). By the end of the intervention, on average, women in the WEP group increased water intake by 1210 ± 102 mL/d and those in the EP group by 239 ± 91 mL/d (P < 0.001) (Table 4). Even though participants in both groups decreased their SSB intake in all stages, reduction was greater in the WEP group (change from baseline to 9 mo: WEP, −252 ± 19 kcal/d; EP, −115 ± 27 kcal/d; P < 0.001) (Table 4). Women in the EP group tended to have a greater decrease in reported solid food intake (P-interaction = 0.07). Both groups reported a decrease in total energy intake by the end of the intervention, with no difference between groups (change from baseline to 9 mo: WEP, −585 ± 55 kcal/d; EP, −567 ± 66 kcal/d; P = 0.8) (Table 4). Physical activity, measured as METs/d, did not differ between the groups throughout the intervention (Table 3).
TABLE 4.
Change in metabolic syndrome indicators, hydration status, and dietary intake by intervention group1
| Change from baseline | P | |||||
|---|---|---|---|---|---|---|
| 3 mo | 6 mo | 9 mo | 3 mo | 6 mo | 9 mo | |
| Plasma TGs, mg/dL | 0.70 | 0.30 | 0.30 | |||
| WEP | 5.60 ± 8.00 | −2.40 ± 9.10 | −5.70 ± 10.0 | |||
| EP | 1.80 ± 6.60 | 13.6 ± 11.3 | 10.7 ± 9.90 | |||
| Total cholesterol, mg/dL | 0.60 | 0.60 | 0.70 | |||
| WEP | 1.50 ± 4.70 | 5.80 ± 5.60 | 1.90 ± 8.70 | |||
| EP | 5.70 ± 5.20 | 1.20 ± 7.60 | −3.00 ± 8.90 | |||
| LDL cholesterol, mg/dL | 0.50 | 0.30 | 0.40 | |||
| WEP | 1.10 ± 3.50 | 4.90 ± 4.00 | 0.200 ± 4.00 | |||
| EP | 4.60 ± 3.70 | −1.60 ± 4.60 | −5.00 ± 4.70 | |||
| HDL cholesterol, mg/dL | 0.40 | 0.90 | 0.60 | |||
| WEP | −0.300 ± 1.30 | 1.90 ± 8.50 | 0.300 ± 1.40 | |||
| EP | 1.20 ± 1.40 | 1.10 ± 4.60 | −1.70 ± 1.80 | |||
| Fasting plasma glucose, mg/dL | 0.90 | 0.90 | 0.90 | |||
| WEP | 0.0400 ± 1.50 | 0.60 ± 1.90 | 1.20 ± 1.70 | |||
| EP | 0.200 ± 1.40 | 0.90 ± 2.20 | 1.70 ± 2.80 | |||
| HbA1c,2% | — | — | 0.30 | |||
| WEP | — | — | −0.0300 ± 0.0300 | |||
| EP | — | — | 0.0200 ± 0.0300 | |||
| Systolic blood pressure, mm Hg | 0.30 | 0.50 | 0.60 | |||
| WEP | 0.0500 ± 0.900 | −2.40 ± 1.00 | −0.90 ± 1.20 | |||
| EP | −1.30 ± 1.00 | −3.80 ± 1.40 | −2.80 ± 1.70 | |||
| Diastolic blood pressure, mm Hg | 0.50 | 0.50 | 0.80 | |||
| WEP | −1.00 ± 0.900 | −3.70 ± 1.00 | −3.40 ± 1.10 | |||
| EP | −1.90 ± 0.800 | −4.70 ± 1.10 | −3.90 ± 1.50 | |||
| Serum osmolality,2mOsm/kg | — | — | 0.80 | |||
| WEP | — | — | −2.60 ± 3.30 | |||
| EP | — | — | −1.30 ± 3.00 | |||
| Urine osmolality,2mOsm/kg | — | — | 0.008 | |||
| WEP | — | — | −131 ± 38.0 | |||
| EP | — | — | 3.10 ± 42.5 | |||
| Weight, kg | 0.06 | 0.10 | 0.40 | |||
| WEP | −1.0 ± 0.2 | −1.4 ± 0.3 | −1.2 ± 0.4 | |||
| EP | −0.5 ± 0.2 | −0.7 ± 0.3 | −0.8 ± 0.4 | |||
| BMI, kg/m2 | 0.07 | 0.10 | 0.40 | |||
| WEP | −0.40 ± 0.08 | −0.54 ± 0.11 | −0.50 ± 0.14 | |||
| EP | −0.20 ± 0.08 | −0.30 ± 0.14 | −0.33 ± 0.15 | |||
| Body fat,2% | — | — | 0.90 | |||
| WEP | — | — | −0.8 ± 0.4 | |||
| EP | — | — | −0.8 ± 0.6 | |||
| Waist circumference, cm | 0.10 | 0.20 | 0.70 | |||
| WEP | −1.0 ± 0.3 | −1.4 ± 0.3 | −1.0 ± 0.4 | |||
| EP | −0.5 ± 0.3 | −0.6 ± 0.5 | −1.3 ± 0.7 | |||
| Total energy intake, kcal/d | 0.20 | 0.30 | 0.80 | |||
| WEP | −553 ± 56 | −575 ± 54 | −585 ± 55 | |||
| EP | −447 ± 52 | −478 ± 60 | −567 ± 66 | |||
| Energy intake from solid foods, kcal/d | 0.80 | 0.40 | 0.10 | |||
| WEP | −285 ± 46 | −285 ± 46 | −315 ± 47 | |||
| EP | −302 ± 44 | −347 ± 53 | −440 ± 58 | |||
| Beverages with sugar | ||||||
| mL/d | <0.001 | <0.001 | <0.001 | |||
| WEP | −628 ± 50 | −675 ± 50 | −709 ± 59 | |||
| EP | −286 ± 53 | −198 ± 71 | −297 ± 59 | |||
| kcal/d | <0.001 | <0.001 | 0.001 | |||
| WEP | −243 ± 17 | −252 ± 16 | −252 ± 19 | |||
| EP | −125 ± 20 | −96 ± 24 | −115 ± 27 | |||
| % of calories/d | <0.001 | <0.001 | <0.001 | |||
| WEP | −10.5 ± 0.9 | −11.0 ± 1.2 | −10.4 ± 1.3 | |||
| EP | −3.5 ± 1.0 | −0.4 ± 1.6 | −1.0 ± 1.5 | |||
| Water consumption, mL/d | <0.001 | <0.001 | <0.001 | |||
| WEP | 976 ± 67 | 1,109 ± 88 | 1,210 ± 102 | |||
| EP | 142 ± 67 | 190 ± 92 | 239 ± 91 | |||
Values are means ± SEs. Data were analyzed by using intent-to-treat analysis, 10 imputations. Changes were calculated from baseline to 3, 6, and 9 mo without adjustments for covariates; n = 120 for WEP and n = 120 for EP. A repeated-measures mixed-effects model analysis was used to test the mean through time, all P > 0.05 (data not shown). EP, education provision; HbA1c, glycosylated hemoglobin; mOsm, milliosmole; WEP, water and education provision.
Measured at baseline and 9 mo only.
Outcomes.
The effects of the intervention on the study outcomes are shown in Table 2. The primary outcome, change in plasma TG concentrations, did not differ between the groups (P-interaction = 0.10). There was no significant change at any stage between the groups (Table 4).
TABLE 2.
Metabolic syndrome risk indicators and hydration status by intervention group1
| Study time point | P 2 | ||||||
|---|---|---|---|---|---|---|---|
| Baseline3 | 3 mo | 6 mo | 9 mo | Treatment | Time | Treatment × time | |
| Plasma TGs, mg/dL | 0.20 | 0.20 | 0.10 | ||||
| WEP | 155 ± 2.10 | 161 ± 2.60 | 157 ± 2.30 | 149 ± 2.80 | |||
| EP | 150 ± 1.90 | 152 ± 1.70 | 164 ± 2.60 | 161 ± 2.70 | |||
| Plasma total cholesterol, mg/dL | 0.30 | 0.50 | 0.30 | ||||
| WEP | 188 ± 1.30 | 190 ± 1.20 | 191 ± 1.30 | 187 ± 1.20 | |||
| EP | 188 ± 1.30 | 195 ± 1.40 | 192 ± 1.40 | 186 ± 1.20 | |||
| Plasma LDL cholesterol, mg/dL | 0.10 | 0.20 | 0.09 | ||||
| WEP | 103 ± 0.900 | 105 ± 0.900 | 106 ± 0.900 | 105 ± 0.900 | |||
| EP | 106 ± 0.800 | 111 ± 1.00 | 106 ± 1.10 | 102 ± 0.900 | |||
| Plasma HDL cholesterol, mg/dL | 0.30 | 0.10 | 0.10 | ||||
| WEP | 52.5 ± 0.400 | 52.6 ± 0.400 | 55.0 ± 0.400 | 52.7 ± 0.400 | |||
| EP | 53.2 ± 0.300 | 53.5 ± 0.400 | 52.7 ± 0.400 | 51.5 ± 0.300 | |||
| Fasting plasma glucose, mg/dL | 0.90 | 0.80 | 0.90 | ||||
| WEP | 90.2 ± 0.400 | 90.0 ± 0.300 | 90.5 ± 0.300 | 90.7 ± 0.400 | |||
| EP | 90.2 ± 0.300 | 90.3 ± 0.300 | 90.5 ± 0.400 | 91.1 ± 0.300 | |||
| HbA1c,4% | 0.30 | 0.20 | 0.30 | ||||
| WEP | 5.80 ± 0.0100 | — | — | 5.82 ± 0.0100 | |||
| EP | 5.80 ± 0.0100 | — | — | 5.80 ± 0.0100 | |||
| Systolic blood pressure, mm Hg | 0.10 | 0.80 | 0.30 | ||||
| WEP | 100 ± 0.300 | 100 ± 0.300 | 97.6 ± 0.300 | 98.0 ± 0.300 | |||
| EP | 102 ± 0.300 | 100 ± 0.300 | 98.3 ± 0.300 | 98.6 ± 0.400 | |||
| Diastolic blood pressure, mm Hg | 0.20 | 0.10 | 0.40 | ||||
| WEP | 68.3 ± 0.800 | 67.3 ± 0.200 | 64.6 ± 0.200 | 63.9 ± 0.200 | |||
| EP | 70.0 ± 0.700 | 68.1 ± 0.200 | 65.3 ± 0.300 | 65.5 ± 0.300 | |||
| Serum osmolality,4mOsm/kg | 0.40 | 0.60 | 0.90 | ||||
| WEP | 296 ± 0.800 | — | — | 293 ± 0.500 | |||
| EP | 293 ± 0.600 | 291 ± 0.400 | |||||
| Urine osmolality,4mOsm/kg | 0.30 | 0.01 | 0.05 | ||||
| WEP | 707 ± 7.70 | — | — | 581 ± 7.70 | |||
| EP | 701 ± 6.60 | — | — | 701 ± 9.20 | |||
Values are means ± SEs. EP, education provision; HbA1c, glycosylated hemoglobin; mOsm, milliosmole; WEP, water and education provision.
Data were analyzed by using intent-to-treat analysis, 10 imputations (n = 240). Repeated-measures mixed-effects model analysis was used to test the mean through time.
There was no difference between groups at baseline in any of the outcomes or indicators of hydration status (all P ≥ 0.1).
Measured at baseline and at 9 mo only.
Women in both groups lost weight, with no difference between groups (P-interaction = 0.40) (Table 3). By the end of the intervention, mean weight loss was −1.2 ± 0.4 kg in the WEP group and −0.8 ± 0.4 kg in the EP group (P = 0.40) (Table 4). Changes in other outcomes (waist circumference, percentage of body fat, total cholesterol, LDL cholesterol, HDL cholesterol, fasting plasma glucose, HbA1c, systolic and diastolic blood pressure) were not significant (Tables 2–4).
Indicators of hydration status.
Urine osmolality in the WEP group improved significantly at 9 mo (−131 ± 38.0 mOsm/kg; P = 0.008) compared with than in the EP group (3.10 ± 42.5 mOsm/kg; P = 0.8). Neither group had changes in serum osmolality (P = 0.9) (Tables 2 and 4).
Results by weight status at baseline (secondary analysis)
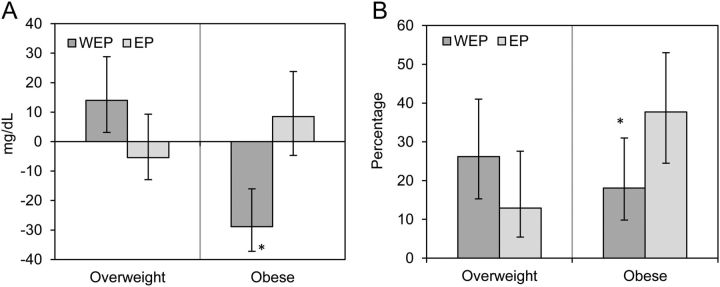
We tested the effect of the intervention considering the weight status at baseline in the primary and secondary outcomes and found significant results for the primary outcome (plasma TGs) and MetS prevalence. There was no difference in TG concentration at baseline by weight status (144 ± 7.60 vs. 160 ± 6.00 mg/dL for overweight and obese participants, respectively; P = 0.09). A significant treatment × time effect appeared when we considered baseline BMI (overweight vs. obese) for TG concentrations throughout the intervention (P-interaction = 0.02) (Supplemental Table 4). The effect of the intervention differed between women who started the intervention while overweight (BMI ≥25.0–29.9 kg/m2) and those who started while obese (BMI >30 kg/m2). Among the latter participants, TG concentrations decreased from baseline to 9 mo in the WEP group (−28.9 ± 7.70 mg/dL; P value for change <0.001), with no change in the EP group (8.50 ± 10.9 mg/dL; P value for change = 0.4) (Fig. 2A). There was no difference in MetS prevalence at baseline by weight status (24.5% and 33.8% for overweight and obese, respectively; P = 0.1). The effect of the intervention on MetS prevalence differed by baseline weight status after adjusting for change in physical activity from baseline to 9 mo and for age (P-interaction = 0.02). The estimated MetS prevalence at 9 mo was lower in obese women in the WEP group (18.1%) than in those in the EP group (37.7%) (P value for comparison between groups in obese women = 0.05) (Fig. 2B).
FIGURE 2.
Effects of the intervention at 9 mo on the basis of baseline weight status on plasma TG changes from baseline to 9 mo (A) and prevalence of metabolic syndrome at 9 mo (B). Values are means ± SEs (A) and percentages (95% CIs) (B). The model for panel A is a simple linear regression model including as an outcome variable the change in plasma TGs from baseline to 9 mo and as predictor variables the treatment group (WEP = 1, EP = 0) and BMI at baseline (obese = 1, overweight = 0). Interaction term: treatment × BMI at baseline; n = 184. The model for panel B is a logistic regression model, adjusted by prevalence of metabolic syndrome at baseline, treatment (WEP = 1, EP = 0), BMI at baseline (obese = 1, overweight = 0), change in physical activity from baseline to 9 mo, age at baseline, and interaction of treatment × BMI at baseline; n = 179. *Different from EP, P < 0.05. EP, education provision; WEP, water and education provision.
Adverse events
Twenty-two participants from the WEP group reported an adverse event during the intervention. The most common adverse events reported were tiredness, nausea, stress, or frequent urge to urinate. The project's physician assessed their severity and relatedness to the intervention. All participants with reported adverse events were treated and monitored until they improved. No subjects were removed from the study because of an adverse event.
Discussion
This clinical trial showed a significant increase in reported water intake in both the WEP and the EP groups, with the greatest increase in the WEP group supported by an improvement in urine osmolality after 9 mo. Both groups reported significant declines in total energy and SSB intake, and both groups demonstrated a reduction in weight and BMI over time. However, no significant improvements in plasma TG concentrations, weight, or other cardiometabolic risk indicators were observed by intervention group in the ITT analysis.
A possible explanation for the lack of effect in the overall sample is the incomplete replacement of SSB consumption. Even though the participants in the WEP group increased water intake, they did not completely replace SSB consumption, which was still considerable at the end of the intervention (155 ± 4 kcal/d or 418 ± 11 mL/d). There is evidence that a reduction in SSB intake of 355 mL/d is associated with weight losses of 0.5 kg at 6 mo (95% CI: 0.1, 0.8 kg; P = 0.006) and 0.7 kg at 18 mo (95% CI: 0.2, 1.1 kg; P = 0.003) (43). Our results, which showed increased water intake but incomplete substitution for SSBs, are consistent with results from 2 other studies: 1 in Cuernavaca, Mexico (44), the city of our study, and the other in The Netherlands (45), in which changes in water consumption did not result in changes in SSB intake. The first, a cross-sectional qualitative study, explored knowledge of the benefits of water intake among adults with low and high SSB intakes. Participants had similar water intake amounts whether their SSB consumption was low or high, suggesting that drinking water does not necessarily replace SSB consumption (44). The second, a secondary analysis of an RCT in adolescents, showed that a reduction in SSB intake was not explained by an increase in the consumption of water or diet drinks (45).
Another explanation relates to the fact that SSB intake decreased in both groups and that a considerable percentage of women (36.8%) in the EP group had a water intake >1.2 L/d at the end of the intervention (S. Rodríguez-Ramírez, T. González-Cossio, M. Mendez, K. Tucker, I. Méndez-Ramírez, S. Hernández-Cordero, B. Popkin, unpublished data, 2013). The reduction in SSB intake and increase in water intake in the EP group was unexpected, because those participants did not receive information on healthy beverage consumption. Despite our requests that WEP participants not discuss the intervention with the EP participants, contamination from the WEP to the EP group is possible, which would make both groups very similar and affect the intervention results. The nutrition counseling that both groups received did not address weight loss or changes in beverage consumption patterns but instead covered general topics, such as sodium intake, fat content in the diet (unsaturated vs. saturated), and including vegetables in the diet. Nevertheless, it is possible that women in the EP group were motivated by joining this weight-loss study and decided to modify some behaviors that are related to a healthier lifestyle (e.g., increasing water intake or reducing SSBs, topics that received extensive media coverage in Mexico during this period). We adhered to strict attention control limits for both groups.
Another potential explanation relates to the total energy intake of participants. In addition to the decrease in SSB intake in the EP group, the participants in this group tended to have a greater decrease in energy intake from solid foods than did those in the WEP group. Thus, even with the WEP group's greater reduction in SSB calories, average total energy intake did not differ between groups at the end of the intervention.
Finally, the fact that a large percentage of women (50%) entered the study with TG concentrations in the normal range (<150 mg/dL) might explain the intervention's lack of effect. There is evidence suggesting that the beneficial changes in lipid profile depend on initial concentrations, with a greater response among those with higher concentrations at the beginning of any intervention (46, 47). Thus, only half of our study population had the potential to reduce TG concentrations.
Few RCTs have addressed a research question similar to ours in adult populations. Tate et al. (26) studied the replacement of caloric beverages with water or diet beverages as a method to lose weight and improve some cardiometabolic indicators over 6 mo in U.S. overweight and obese adults and included attention controls. There was an improvement in hydration status in the water group, as in our study, measured by urine osmolality, but no significant differences in other metabolic indicators in the ITT analysis except for a significant improvement in fasting glucose in the water group compared with the control. In addition, Tate et al. found a significantly greater likelihood of a 5% weight loss in the 2 intervention arms in a secondary analysis. Another RCT in women and men aged 55–75 y tested the hypothesis that premeal water consumption (500 mL/meal · d−1) would lead to greater weight loss in U.S. overweight and obese individuals consuming a hypocaloric diet than in those who consumed the same hypocaloric diet only (i.e., without premeal water consumption) during 12 wk (48). Adults in the premeal water group had a greater weight loss than did those adhering to the hypocaloric diet only. The authors concluded that, when combined with a hypocaloric diet, water intake of 500 mL before each meal leads to a greater weight loss than a hypocaloric diet alone in adults. The water intake may cause a reduction in energy intake from the meal. The difference between these trial results and our study results might be explained by the difference in the ages of the study populations [participants in the Dennis et al. (48) trial were 55–75 y old vs. 18–45 y old in our trial], the length of the follow-up (3 vs. 9 mo, respectively), and the specific instructions provided to participants (intake of 500 mL of water per meal vs. increase in water intake and decrease in SSB intake, respectively). Finally, in a recently published 12-wk weight-loss phase of a 1-y RCT in overweight and obese U.S. women and men, researchers tested the hypothesis that the amount of weight lost (over 12 wk) and maintained (for 9 mo) in a behavioral management program would be equivalent in participants consuming nonnutritive sweetened beverages compared with those consuming water (49). The authors reported that both groups lost weight during the 12-wk weight-loss phase, with a greater weight loss among the participants in the nonnutritive sweetened beverages group (mean ± SD: 5.95 ± 3.94 kg) than among those in the water group (4.09 ± 3.74 kg; P < 0.0001). The results of this trial are difficult to interpret and compare with ours, because no dietary data (food or beverage intake) are included and the follow-up period is shorter (no data on the maintenance period were presented).
Our study has some limitations. As discussed by Hernández-Cordero et al. (32), ideally a clinical trial should have a blinded design. However, in food intervention studies this is not possible. The unblinded design might result in an overestimation of the effect of the intervention if there is overreporting of an outcome measure or a change in the promoted behavior. We discuss the latter below. As for the outcome variables, those that are subjective are often found to be biased (50), in contrast with physiologic outcomes, which correspond to our primary outcome and the other cardiometabolic risk factors measured in our study. For dietary information, which we used to evaluate change in beverage intake (including water and SSBs) and dietary intake, we treated all participants identically in interviews to reduce the potential bias of collecting this information differentially. Another potential bias due to the unblinded design is performance bias, which results from a systematic difference in the group follow-ups (51). To reduce this bias, we treated all participants according to a strict protocol. In addition, there is a greater chance of attrition bias. In our study, the EP group had a lower retention rate. The potential effect of a low retention rate is selection bias, which we minimized by using an ITT analysis in our main analysis (51). Another potential limitation that might explain the lack of effect in the ITT analysis is the fact that our control group (the EP group) received nutrition counseling. We decided to include nutrition counseling for the EP group to ensure attention control comparability between the groups, so that the only differences between them were the water provision and the additional information on increasing water intake while decreasing SSB intake. However, even though the topics included in the counseling did not address weight loss, both groups might have been willing to modify dietary behaviors not addressed by the intervention (i.e., the EP group increased water intake). A potential way to overcome this could have been to include a third comparison group, which would have made the study more expensive and logistically more complex.
Another potential limitation of our study is misreporting. In-depth analyses of our dietary data suggest a high proportion of underreporting, defined by the disparity between reported energy intake and predicted energy requirements from doubly labeled water equations adjusted for energy deficits on the basis of weight changes and total energy expenditure (S. Rodríguez-Ramírez, M. Mendez, S. Hernández-Cordero, T. González de Cossio, B. Popkin, unpublished data, 2013). These analyses indicate that underreporting increased from 11% of the sample at baseline to 42% by the end of the intervention. The misreporting made it difficult to interpret the potential impact of dietary changes throughout the study on the lack of effect of the intervention in the ITT analysis. In addition to the misreporting expected in a weight-loss trial, described in other studies (54–59), in our study the underreporting of SSB consumption might be higher in the WEP group given that the intervention discouraged SSB consumption, a phenomenon that others have reported (58, 59).
Although percentage of body fat was not 1 of our main outcomes, the Siri equation that was used to estimate it has not been validated in Hispanics but has been used by other scholars (60, 61). Finally, another potential limitation is the restriction of our study population to women. This puts a constraint on the conclusions we can draw from our results, which are applicable only to overweight and obese women.
Secondary analyses suggest that weight status at baseline was an effect modifier of TG change during follow-up and of the MetS at the end of the study. Plasma TG concentrations and the MetS decreased among obese women in the WEP group. This change among obese women is possibly explained by a greater physiologic response to a modification intervention in subjects with greater risk (i.e., heavier initial weight), as others have suggested (62). A pilot RCT found that baseline BMI was an effect modifier in an intervention to examine the effect of decreasing SSB consumption on body weight in adolescents. Among subjects in the upper BMI tertile, BMI change differed significantly between the intervention and control groups (20). Similar results were reported in a child cohort in the United States (42). Another possibility is a stronger desire for behavior change among obese subjects in our trial. Although initial higher weight predicted low compliance in weight-management treatments (63, 64), in our study the change in SSB intake was greater among women with a BMI >30 kg/m2, even after considering the potential effect of underreporting, as defined above (change from baseline to 9 mo in plausible reporters: overweight WEP, −236 ± 31 kcal/d; overweight EP, −148 ± 41 kcal/d; P = 0.090; obese WEP, −228 ± 41 kcal/d; obese EP, −42 ± 40 kcal/d; P = 0.003). Water intake was similar. Obese women in the WEP group had the highest water intake at 9 mo (plausible reporters: overweight WEP, 1532 ± 123 mL/d; overweight EP, 1043 ± 124 mL/d; P < 0.008; obese WEP, 2071 ± 133 mL/d; obese EP, 905 ± 148 mL/d; P < 0.001). The results of secondary analyses are worth mentioning considering the high impact in countries such as Mexico, where the obesity prevalence is considerably high—37.5% of Mexican women in 2012 (2). Furthermore, the existing evidence of the association of hypertriglyceridemia with risk of coronary heart disease (65, 66) and the potential benefits of decreasing TG concentrations (8) highlights the importance of the findings of this study. However, these results should be considered cautiously, because further investigation is needed.
In conclusion, overall, this study found that providing water and nutritional counseling was effective in increasing water intake but insufficient to achieve a complete substitution of water for SSBs among these overweight and obese Mexican women, which may have contributed to the lack of change in plasma TGs, weight, and other cardiometabolic risks in the ITT analysis. Other potential explanations of the lack of effect are that both groups decreased SSB intake, resulting in a great proportion of the EP group having an SSB intake similar to the WEP group; total energy intake did not differ between groups because of the trend of decreased energy intake from solid foods among women in the EP group; and the baseline mean plasma TG values were near normal in our study population. Secondary analyses suggest that the intervention lowered plasma TGs and MetS among obese women only. The results of both the ITT and secondary analyses indicate the need for more research in efficacy trials focused on the effect of SSB intake reduction on MetS risks and the possible differential effect according to initial weight status.
Supplementary Material
Acknowledgments
The authors thank Donna Miles, PhD, for statistical and programming assistance in handling the multiple-imputation work and for file preparation; Phil Bardsley, PhD, for his assistance; and Frances Dancy, BSc, for her administrative assistance in the preparation of this manuscript. None of these persons were compensated for their contributions. S.H.-C. and B.P. had full access to all of the data in the study, take responsibility for the integrity of the data and the accuracy of the data analysis, and wrote the manuscript and had primary responsibility for the final content; B.P., S.H.-C., S.B., S.R.-R., M.A.V.-B., T.G.d.C., and J.R.D. designed the research; S.H.-C., S.R.-R., and M.A.V.-B. conducted the research; S.H.-C., B.P., S.B., J.R.D., and T.G.d.C. proposed and approved modification of the protocol during the development of the project; S.H.-C., S.R.-R., and B.P. analyzed the data; and S.B. and J.R.D. gave key insights into data interpretation and into the final manuscript. All authors read and approved the final manuscript.
Abbreviations
- EP
education provision
- HbA1c
glycosylated hemoglobin
- ITT
intent-to-treat
- MET
metabolic equivalent
- MetS
metabolic syndrome
- RCT
randomized controlled trial
- SSB
sugar-sweetened beverage
- WEP
water and education provision
Footnotes
Supported in part by a grant from the Danone Research Center to the National Institute of Public Health, Cuernavaca, Mexico, which provided water for the intervention and partially supported S.H.-C.'s research sabbatical at the University of North Carolina at Chapel Hill. The Danone Research Center had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation or approval of themanuscript.
References
- 1. Villalpando S, Rull-Rodrigo J. The status of non-transmissible chronic disease in Mexico based on the National Health and Nutrition Survey 2006. Salud Publica Mex 2010;52:S2–3. [DOI] [PubMed] [Google Scholar]
- 2. Barquera S, Campos-Nonato I, Hernández-Barrera L, Rivera Dommarco J. Encuesta Nacional de Salud y Nutrición 2012. Evidencia para la política pública en salud. Obesidad en adultos: los retos de la cuesta abajo. Cuernavaca (México): Instituto Nacional de Salud Pública; 2012. [Google Scholar]
- 3. Rojas R, Aguilar-Salinas CA, Aída Jiménez-Corona A, Shamah-Levy T, Rauda J, Ávila-Burgos L, Villalpando S, Lazcano Ponce E. Metabolic syndrome in Mexican adults: results from the National Health and Nutrition Survey 2006. Salud Publica Mex 2010;52:S11–8. [DOI] [PubMed] [Google Scholar]
- 4. Aguilar-Salinas CA, Gómez-Pérez FJ, Rull J, Villalpando S, Barquera S, Rojas R. Prevalence of dyslipidemias in the Mexican National Health and Nutrition Survey 2006. Salud Publica Mex 2010;52:S44–53. [DOI] [PubMed] [Google Scholar]
- 5. Appel LJ, Moore TJ, Obarzanek E, Vollmer W, Svetkey L, Sacks F, Bray G, Vogt T, Cutler J, Windhauser M, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med 1997;336:1117–24. [DOI] [PubMed] [Google Scholar]
- 6. Obarzanek E, Sacks F, Vollmer W, Bray G, Miller E, Lin P, Karanja N, Most-Windhauser M, Moore T, Swain J, et al. Effects on blood lipids of a blood pressure-lowering diet: the Dietary Approaches to Stop Hypertension (DASH) Trial. Am J Clin Nutr 2001;74:80–9. [DOI] [PubMed] [Google Scholar]
- 7. Tirosh A, Rudich A, Schochat T, Tekes-Manova D, Israelí E, Henkin Y. Changes in triglyceride levels and risk of coronary disease in young men. Ann Intern Med 2007;147:377–85. [DOI] [PubMed] [Google Scholar]
- 8. Criqui MH. Triglycerides and coronary heart disease revisited (again). Ann Intern Med 2007;147:425–7. [DOI] [PubMed] [Google Scholar]
- 9. Barquera S, Hernández L, Tolentino ML, Espinosa J, Leroy J, Rivera J, Popkin BM. Energy from beverages is on the rise among Mexican adolescents and adults. J Nutr 2008;138:2454–61. [DOI] [PubMed] [Google Scholar]
- 10. Barquera S, Campirano F, Bonvecchio A, Hernández L, Rivera J, Popkin B. Caloric beverage consumption patterns in Mexican children. Nutr J 2010;9:47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Popkin BM, Adair LS, Ng SW. Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev 2012;70:3–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stern D, Piernas C, Barquera S, Rivera J, Popkin B. Caloric beverages were major source of energy among children and adults in Mexico, 1999–2012. J Nutr 2014;144:949–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ebbeling CB, Feldman HA, Chomitz VR, Antonelli TA, Gortmaker SL, Psgamoam S, Ludwig DS. A randomized trial of sugar-sweetened beverages and adolescents body weight. N Engl J Med 2012;367:1407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Malik VS, Popkin BM, Bray GA, Despres JP, Willett WC, Hu FB. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care 2010;33:2477–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rajpathak SN, Gupta LS, Waddell EN, Upadhyay UD, Wildman RP, Kaplan R, Wassertheil-Smoller S, Wylie-Rosett J. Elevated risk of type 2 diabetes and metabolic syndrome among Asians and South Asians: results from the 2004 New York City HANES. Ethn Dis 2010;20:225–30. [PubMed] [Google Scholar]
- 16. Johnson RK, Appel L, Brands M, Howard B, Lefevre M, Lustig R, Sacks F, Steffen L, Wylie-Rosett J. Dietary sugar intake and cardiovascular health: a scientific statement from the American Heart Association. Circulation 2009;120:1011–20. [DOI] [PubMed] [Google Scholar]
- 17. Stanhope KL, Schwarz J, Keim N, Griffen S, Bremer A, Graham J, Hatcher B, Cox C, Dyachenko A, Zhang W, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest 2009;119:1322–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vartanian LR, Schwartz MB, Brownell KD. Effects of soft drink consumption on nutrition and health: a systematic review and meta-analysis. Am J Public Health 2007;97:667–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dhingra R, Sullivan L, Jacques P, Wang T, Fox C, Meigs J, D'Agostino R, Gaziano J, Vasan R. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-aged adults in the community. Circulation 2007;116:480–8. [DOI] [PubMed] [Google Scholar]
- 20. Ebbeling CB, Feldman HA, Osganian SK, Chomitz VR, Ellenbogen SJ, Ludwig DS. Effects of decreasing sugar-sweetened beverage consumption on body weight in adolescents: a randomized, controlled pilot study. Pediatrics 2006;117:673–80. [DOI] [PubMed] [Google Scholar]
- 21. Malik VS, Pan A, Willett W, Hu F. Sugar-sweetened beverages and weight gain in children and adults: a systematic review and meta-analysis. Am J Clin Nutr 2013;98:1084–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mattes RD, Shikany JM, Kaiser KA, Allison DB. Nutritively sweetened beverage consumption and body weight: a systematic review and meta-analysis of randomized experiments. Obes Rev 2011;12:346–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Allison DB, Mattes RD. Nutritively sweetened beverage consumption and obesity: the need for solid evidence on a fluid issue. JAMA 2009;301:318–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hu FB. Resolved: there is sufficient scientific evidence that decreasing sugar-sweetened beverage consumption will reduce the prevalence of obesity and obesity-related diseases. Obes Rev 2013;14:606–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kaiser KA, Shikany JM, Keating KD, Allison DB. Will reducing sugar-sweetened beverage consumption reduce obesity? Evidence supporting conjecture is strong, but evidence when testing effect is weak. Obes Rev 2013;14:620–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tate DF, Turner-McGrievy G, Lyons E, Stevens J, Erickson K, Polzien K, Diamond M, Wang X, Popkin B. Replacing caloric beverages with water or diet beverages for weight loss in adults: main results of the Choose Healthy Options Consciously Everyday (CHOICE) randomized clinical trial. Am J Clin Nutr 2012;95:555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Muckelbauer R, Sarganas G, Gruneis A, Müller-Nordhom J. Association between water consumption and body weight outcomes: a systematic review. Am J Clin Nutr 2013;97:667–76. [DOI] [PubMed] [Google Scholar]
- 28. Daniels MC, Popkin B. The impact of water intake on energy intake and weight status: a systematic review. Nutr Rev 2010;68:505–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dennis EA, Flack K, Davy B. Beverage consumption and adult weight management: a review. Eat Behav 2009;10:237–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vij VA, Joshi A. Effect of “water induced thermogenesis” on body weight, body mass index and body composition of overweight subjects. J Clin Diagn Res. 2009;7:1894–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Boschmann M, Steiniger J, Hille U, Tank J, Adams F, Sharma A, Klaus S, Luft F, Jordan J. Water induced thermogenesis. J Clin Endocrinol Metab 2013;88:6015–9. [DOI] [PubMed] [Google Scholar]
- 32. Hernández-Cordero S, González-Castell D, Rodríguez-Ramírez S, Villanueva-Borbolla MA, Unar M, Barquera S, González de Cossío T, Rivera-Dommarco J. Design and challenges of a randomized controlled trial for reducing risk factors of metabolic syndrome in Mexican women through water intake. Salud Publica Mex 2013;55:595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. International Diabetes Federation The IDF consensus worldwide definition of metabolic syndrome. Brussels: International Diabetes Federation, 2006. [Google Scholar]
- 34. Lohman TG, Roche AF, Martorell R. Anthropometric standardization reference manual. Champaign (IL): Human Kinetics Publishers; 1988. [Google Scholar]
- 35. Fields DA, Higgins P, Hunter G. Assessment of body composition by air-displacement plethysmography: influence of body temperature and moisture. Dyn Med 2004;3:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Higgins PB, Fields D, Hunter G, Gower B. Effect of scalp and facial hair on air displacement plethysmography estimates of percentage of body fat. Obes Res 2001;9:326–30. [DOI] [PubMed] [Google Scholar]
- 37. Siri WE. Body composition from fluid spaces and density: analysis of methods. Nutrition 1961;9:480–91. [PubMed] [Google Scholar]
- 38. USDA Food and nutrient database for dietary studies, 4.1. Beltsville (MD), Agricultural Research Service, Food Surveys Research Group; 2010. [Google Scholar]
- 39. Vyas S, Kumaranayake L. Constructing socio-economic status indices: how to use principal components analysis. Health Policy Plan 2006;21:459–68. [DOI] [PubMed] [Google Scholar]
- 40. Schafer JL, Graham J. Missing data: our view of the state of the art. Psychol Methods 2002;7:147–77. [PubMed] [Google Scholar]
- 41. Mackinnon A. The use and reporting of multiple imputation in medical research—a review. J Intern Med 2010;268:586–93. [DOI] [PubMed] [Google Scholar]
- 42. Welsh JA, Cogswell M, Rogers S, Rockett H, Mei Z, Grummer-Strawn L. Overweight among low-income preschool children associated with the consumption of sweet drinks: Missouri, 1999–2002. Pediatrics 2005;115:e223–9. [DOI] [PubMed] [Google Scholar]
- 43. Chen L, Appel L, Loria C, Lin P, Champagne C, Elmer P, Ard J, Mitchell D, Batch B, Svetkey L, et al. Reduction in consumption of sugar-sweetened beverages is associated with weight loss: the PREMIER trial. Am J Clin Nutr 2009;89:1299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Espinosa J, Aguilar-Tamayo M, Monterrubio E, Barquera S. Conocimiento cotidiano de adultos Mexicanos acerca de la ingesta de agua simple. Contribucion a politicas en educacion para la salud. Congreso de Salud Publica de Mexico. Cuernavaca (Mexico): Instituto Nacional de Salud Pública; 2012. [Google Scholar]
- 45. Veitch J, Singh A, Van Staralen M, Van Mechelen W, Brug J, Chin A, Paw M. Reduction in sugar-sweetened beverages is not associated with more water or diet drinks. Public Health Nutr 2011;14:1388–93. [DOI] [PubMed] [Google Scholar]
- 46. Osterman J, Lin T, Nankin H, Brown K, Hornung C. Serum cholesterol profiles during treatment of obese outpatients with a very low calorie diet: effect of initial cholesterol levels. Int J Obes Relat Metab Disord 1992;16:49–58. [PubMed] [Google Scholar]
- 47. Nasiff-Hadad A, Barceló M, González F, Fernández-Britto J, Paula B. Modificaciones de los lípidos y lipoproteínas del plasma en obesos dislipidémicos sometidos a reducción ponderal a corto plazo con la dieta Cambridge. Revista Cubana Investigaciones Biomédicas 2002;21:221–7. [Google Scholar]
- 48. Dennis EA, Dengo A, Comber D, Flack K, Savla J, Davy K, Davy B. Water consumption increases weight loss during a hypocaloric diet intervention in middle-aged and older adults. Obesity (Silver Spring) 2010;18:300–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Peter JC, Wyatt H, Foster G, Pan Z, Wojtanowski A, Vander V, Herring S, Brill C, Hill J. The effects of water and non-nutritive sweetened beverages on weight loss during a 12-week weight loss treatment program. Obesity (Silver Spring) 2014;22:1415–21. [DOI] [PubMed] [Google Scholar]
- 50. Higgins JPT, Green S. editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated 2011 Mar]. The Cochrane Collaboration; 2011. Available from: www.cochrane-handbook.org. [Google Scholar]
- 51. Jüni P, Altman D, Egger M. Assessing the quality of controlled clinical trials. BMJ 2001;323:42–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ferrari P, Slimani N, Ciampi A, Trichopoulou A, Naska A, Lauria C, Veglia F, Bueno-de-Mesquita H, Ocké M, Brustad M, et al. Evaluation of under- and overreporting of energy intake in the 24-hour diet recalls in the European Prospective Investigation into Cancer and Nutrition (EPIC). Public Health Nutr 2002;5:1329–45. [DOI] [PubMed] [Google Scholar]
- 53. Brehm BJ, Spang S, Lattin B, Seeley R, Daniels S, D'Alessio D. The role of energy expenditure in the differential weight loss in obese women on low-fat and low-carbohydrate diets. J Clin Endocrinol Metab 2005;90:1475–82. [DOI] [PubMed] [Google Scholar]
- 54. Lissner L. Measuring food intake in studies of obesity. Public Health Nutr 2002;5:889–92. [DOI] [PubMed] [Google Scholar]
- 55. Macdiarmid J, Blundell J. Assessing dietary intake: who, what and why of under-reporting. Nutr Res Rev 1998;11:231–53. [DOI] [PubMed] [Google Scholar]
- 56. Mendez MA, Wynter S, Wilks R, Forrester T. Under-and overreporting of energy is related to obesity, lifestyle factors and food intakes in Jamaican adults. Public Health Nutr 2004;7:9–19. [DOI] [PubMed] [Google Scholar]
- 57. Mendez MA, Popkin B, Buckland G, Schroder H, Amiano P, Barricarte A, Huerta J, Quiros J, Sanchez M, Gonzalez C. Alternative methods of accounting for underreporting and overreporting when measuring dietary intake-obesity relations. Am J Epidemiol 2011;173:448–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hebert JR, Ebbeling CB, Matthews C, Hurley T, Ma Y, Druker S, Clemow L. Systematic error in middle-aged women's estimates of energy intake: comparing three self-report measures to total energy expenditure from doubly labeled water. Ann Epidemiol 2002;12:577–86. [DOI] [PubMed] [Google Scholar]
- 59. Kristal AR, Andrilla H, Koepsell T, Diehr P, Cheadle A. Dietary assessment instruments are susceptible to intervention-associated response set bias. J Am Diet Assoc 1998;98:40–3. [DOI] [PubMed] [Google Scholar]
- 60. Vella CA, Ontiveros D, Zubia R, Bader J. Acculturation and metabolic syndrome risk factors in young Mexican and Mexican-American women. J Immigr Minor Health 2011;13:119–26. [DOI] [PubMed] [Google Scholar]
- 61. Vella CA, Zubia R, Ontiveros D, Cruz M. Physical activity, cardiorespiratory fitness, and metabolic syndrome in young Mexican and Mexican-American women. Appl Physiol Nutr Metab 2009;34:10–7. [DOI] [PubMed] [Google Scholar]
- 62. Hall KD, Sacks G, Chandramohan D, Chow C, Wang C, Gotmaker S, Swinburn B. Quantification of the effect of energy imbalance on bodyweight. Lancet 2011;378:826–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bautista-Castaño I, Molina-Cabrillana J, Montoya-Alonso J, Serra-Majem L. Variables predictive of adherence to diet and physical activity recommendations in the treatment of obesity and overweight, in a group of Spanish subjects. Int J Obes Relat Metab Disord 2004;28:697–705. [DOI] [PubMed] [Google Scholar]
- 64. Teixeira PJ, Going S, Houtkooper L, Cussler E, Metcalfe L, Blew R, Sardinha L, Lohman T. Pretreatment predictors of attrition and successful weight management in women. Int J Obes Relat Metab Disord 2004;28:1124–33. [DOI] [PubMed] [Google Scholar]
- 65. Hokanson JE, Austin M. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a meta-analysis of population-based prospective studies. J Cardiovasc Risk 1996;3:213–9. [PubMed] [Google Scholar]
- 66. Dayspring TD. Understanding hypertriglyceridemia in women: clinical impact and management with prescription omega-3-acid ethyl esters. Int J Womens Health 2011;3:87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.