Abstract
Exercise positively impacts mood and symptoms of depression; however, the mechanisms underlying these effects are not fully understood. Recent evidence highlights a potential role for skeletal muscle-derived transcription factors to influence tryptophan metabolism, along the kynurenine pathway, which has important implications in depression. This has important consequences for older adults, whose age-related muscle deterioration may influence this pathway and may increase their risk for depression. Although exercise training has been shown to improve skeletal muscle mass in older adults, whether this also translates into improvements in transcription factors and metabolites related to the kynurenine pathway has yet to be examined. The aim of the present study was to examine the influence of a 12-wk exercise program on skeletal muscle gene expression of transcription factors, kynurenine aminotransferase (KAT) gene expression, and plasma concentrations of tryptophan metabolites (kynurenines) in healthy older men over 65 yr of age. Exercise training significantly increased skeletal muscle gene expression of transcription factors (peroxisome proliferator-activated receptor-γ coactivator 1α, peroxisome proliferator-activated receptor-α, and peroxisome proliferator-activated receptor-δ: 1.77, 1.99, 2.18-fold increases, respectively, P < 0.01] and KAT isoforms 1–4 (6.5, 2.1, 2.2, and 2.6-fold increases, respectively, P ≤ 0.01). Concentrations of plasma kynurenines were not altered. These results demonstrate that 12 wk of exercise training significantly altered skeletal muscle gene expression of transcription factors and gene expression related to the kynurenine pathway, but not circulating kynurenine metabolites in older men. These findings warrant future research to determine whether distinct exercise modalities or varying intensities could induce a shift in the kynurenine pathway in depressed older adults.
Keywords: aging, kynurenine, PGC-1α, physical activity, skeletal muscle
INTRODUCTION
According to the World Health Organization, depression is the single largest contributor to global disability (18). A disproportionately large number of those suffering are older adults, whose disability due to depression is augmented by age-related declines in their physical health and mobility. Traditional pharmaceuticals used in the treatment of depression (i.e., selective serotonin reuptake inhibitors) are often ineffective at reducing primary symptoms (14); these drugs are also associated with adverse side effects (8) and high rates of relapse (10). Exercise may be a beneficial alternative or adjunctive treatment strategy to the pharmaceutical treatment of depression. In addition to its positive effects on physical health and mobility in aging, exercise can reduce symptoms of depression (4, 6, 12). However, the mechanisms underlying these effects are not fully understood, and this information is critical towards determining the effectiveness of exercise as a treatment strategy for depression in older adults.
Preliminary research in younger adults suggests that skeletal muscle may play an important role in the mood-enhancing effects of exercise (1, 15). Specifically, exercise upregulates the expression of skeletal muscle-derived transcription factors that are responsible for promoting the expression of key enzymes that influence tryptophan (Trp) metabolism. Importantly, Trp is the precursor for serotonin (5-HT) synthesis, and alterations in Trp metabolism may contribute to the low central 5-HT concentrations observed in major depressive disorder (MDD) (2). Approximately 95% of Trp metabolism occurs via the kynurenine (KYN) pathway (11). Trp is first degraded into the metabolite KYN. This metabolite is capable of crossing the blood-brain barrier (BBB) and can therefore undergo further metabolism peripherally as well as within the brain. KYN is metabolized along one of two distinct branches of the kynurenine pathway: a neuroprotective branch and a neurotoxic branch. The neuroprotective branch depends on the enzyme kynurenine aminotransferase (KAT), which results in the production of the non-BBB transportable metabolite kynurenic acid (KYNA). In contrast, the neurotoxic branch depends on the enzyme kynurenine monooxygenase (KMO), which shifts the pathway towards the production of potentially neurotoxic metabolites, including 3-hydroxykynurenine (3-HK) and quinolinic acid (QUIN).
Both aerobic (9) and resistance (13) exercise training may bias metabolism of Trp towards the neuroprotective branch by increasing KAT activity. Specifically, skeletal muscle-derived transcriptional coactivators, including peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α), peroxisome proliferator-activated receptor-α (PPARα), and peroxisome proliferator-activated receptor-δ (PPARδ), promote KAT expression (1). Furthermore, skeletal muscle PGC-1α overexpression is associated with a protective shift in Trp metabolism in animal models (1). One prior study in younger adults demonstrated that aerobic exercise-training can induce a protective shift in the kynurenine pathway related to increases in skeletal muscle transcription factors (15). Although this has yet to be examined in older adults, previous research has shown that, in rodents, aging is accompanied by a loss of exercise-induced expression of skeletal muscle PGC-1α (5). If older humans demonstrate a similar loss it may negatively impact the potential for exercise to influence the kynurenine pathway and ultimately impair the mood enhancing properties of physical activity.
The present study examined whether, in a group of healthy, nondepressed older men, a 12-wk combined (resistance + high-intensity interval training) exercise training program would enhance skeletal muscle gene expression of transcriptional coactivators and bias the kynurenine pathway towards the neuroprotective branch. We hypothesized that the exercise training program would increase skeletal muscle gene expression of PGC-1α, PPARα, and PPARδ, which would relate to an increase in KAT gene expression and a decrease in the ratio of QUIN-to-KYNA plasma metabolites, indicating a shift in the kynurenine pathway towards the neuroprotective branch.
MATERIALS AND METHODS
Study design and participants.
This study is a secondary analysis on a subset of participants from a previously published randomized controlled trial (ClinicalTrials.gov NCT02281331). The original study was designed to evaluate the use of a multi-ingredient nutritional supplement combined with an exercise training program on lean body mass and strength in 49 nondepressed (17), healthy older men [≥65 yr, Geriatric Depression Scale: 3.5 (SD 3.3); means (SD)]. All participants had a body mass index within the normal-to-overweight range (between 18.5 and 30.0 kg/m2), normal resting blood pressure or stage 1 hypertension (systolic blood pressure ≤140–159 mmHg, diastolic blood pressure ≤90–99 mmHg), and had not participated in any structured resistance or aerobic exercise training in the previous 6 mo. A full description of the study design has been previously published (3). Briefly, participants received either an experimental protein-based nutritional supplement (n = 25) or a placebo (n = 24) twice per day for 6 wk. All participants then completed a 12-wk progressive exercise training program while continuing to take the supplement and/or placebo twice per day. Blood samples and muscle biopsies were collected at baseline (before beginning supplement or placebo), 7 wk (preexercise training), and 20 wk (postexercise training) following an 8- to 12-h overnight fast (no food or drink except water after midnight the previous night). Subjects were instructed to refrain from strenuous physical activity for 72 h before collection of the blood and muscle samples. For this secondary analysis, blood samples and muscle biopsies were assessed at the original 7-wk time point (before the 12-wk exercise intervention) and at the original 20-wk time point (10 days following cessation of the exercise intervention). Subjects were included for the current secondary analysis based on the availability of muscle tissue samples. As preliminary analysis revealed no differences in gene expression or plasma kynurenines between participants from the original supplement (n = 11) and placebo (n = 14) groups, subjects were collapsed across groups into a single subset of 25 participants. This trial was approved by the Hamilton Integrated Research Ethics Board and complied with the guidelines set out in the Tri-Council policy statement on ethical conduct for research involving humans. All participants were informed of the nature and possible risks of the experimental procedures before their written informed consent was obtained.
Exercise intervention.
Between weeks 7 and 18 (inclusive), participants completed a supervised 12-wk progressive exercise program at McMaster University. Each week, participants performed two resistance training sessions (Mondays and Fridays) and one high-intensity interval training (HIIT; Wednesdays) session. Each resistance training session started with a 5-min warm-up on a cycle ergometer followed by three sets of four resistance exercises. Monday sessions consisted of leg press, chest press, horizontal row, and leg extension. Friday sessions consisted of leg press, lateral pull-down, shoulder press, and leg extension. The third set of each exercise was performed to volitional fatigue. Workloads were gradually increased from 65% one-repetition maximum (1RM; 10–12 repetitions) to 80% 1RM (6–8 repetitions) over the first 3 wk of training. Loads were adjusted on the basis of 1RM strength tests every 4 wk or when subjects could complete ≥12 repetitions during the third set of each exercise. HIIT was performed on a cycle ergometer (ISO1000 Upright Bike; SCIFIT, Tulsa, OK) while wearing a heart rate (HR) monitor (H7 Heart Rate Sensor; Polar Electro Canada, Lachine, QC, Canada). Each HIIT session started with a 3-min warm-up at 25 W, followed by 10 × 60 s intervals at a workload corresponding to ~90% maximal HR (HRmax) at a cadence of ≥ 90 rpm. Workload was adjusted as needed to maintain an average HR of ~90% HRmax over the 10 intervals. These high-intensity intervals were interleaved with 60 s of recovery at 25 W at a self-selected pace. Each HIIT session concluded with a 5-min cool-down at 25 W.
Muscle biopsy.
Following an overnight fast (~10 h), a percutaneous muscle biopsy was obtained from the vastus lateralis under local anesthetic (2% Lidocaine) using a 5-mm Bergstrom needle adapted for manual suction. Subjects refrained from exercise for 72 h before the collection of all muscle biopsy samples. Upon excision, a portion of the muscle sample was directly frozen in liquid nitrogen and stored at −80°C until mRNA analysis was performed.
RNA isolation and reverse transcription.
RNA was isolated from 15–25 mg of muscle tissue, using the TRIzol/RNeasy method. All samples were homogenized with 1 ml of TRIzol reagent (Life Technologies, Burlington, ON, Canada), in Lysing Maxtrix D tubes (MP Biomedicals, Solon, OH), with the FastPrep-24 Tissue and Cell Homogenizer (MP Biomedicals, Solon, OH) for a duration of 40 s at a setting of 6 m/s. Following a 5-min room temperature incubation, homogenized samples were stored at −80°C for 1 mo until further processing. After thawing on ice, 200 µl of chloroform (Sigma-Aldrich, Oakville, ON, Canada) was added to each sample, mixed vigorously for 15 s, incubated at room temperature for 5 min, and spun at 12,000 g for 10 min at 4°C. The RNA (aqueous) phase was purified using the E.Z.N.A. Total RNA Kit 1 (Omega Bio-Tek, Norcross, GA) per the manufacturer’s instructions. RNA concentration (ng/ml) and purity (260/280) were determined with the Nano-Drop 1000 Spectrophotometer (Thermo-Fisher Scientific, Rockville, MD). Samples were reverse transcribed using a high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA) in 20-μl reaction volumes, per the manufacturer’s instructions, using an Eppendorf Mastercycler epGradient Thermal Cycler (Eppendorf, Mississauga, ON, Canada) to obtain cDNA for gene expression analysis.
Quantitative real-time PCR.
All quantitative (q)PCR reactions were run in duplicate in 25-µl volumes containing RT SYBR Green qPCR Master Mix (Qiagen Sciences, Valencia, CA), prepared with the epMotion 5075 Eppendorf automated pipetting system (Eppendorf), and carried out using an Eppendorf Realplex2 Master Cycler epgradient. Primers (listed in Table 1) were resuspended in 1× TE buffer (10 mM Tris·HCl and 0.11 mM EDTA) and stored at −20°C before use. Messenger RNA expression was calculated using the 2−∆∆Ct method and expressed as fold change from pre, as described previously (16). Briefly, Ct values were first normalized to the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH). GAPDH expression was not different between the baseline and the posttraining time points. CT values normalized to GAPDH were expressed as ΔΔCt.
Table 1.
Primer sequences for quantitative real-time PCR analysis
| Gene Name | Sense Primer Sequence (5′–3′) | Antisense Primer Sequence (5′–3′) |
|---|---|---|
| PGC1α | CAGCCTCTTTGCCCAGATCTT | TCACTGCACCACTTGAGTCCAC |
| PPARα | CATCACGGACACGCTTTCAC | CCACAGGATAAGTCACCGAGG |
| PPARδ | ACTGAGTTCGCCAAGAGCATC | ACGCCATACTTGAGAAGGGTAA |
| KAT1 | CCAGTGGATGGTCTACGACG | CTCCCGTTCAAAGCTCTCG |
| KAT2 | AATTACGCACGGTTCATCACG | TCCTCTGCTCAATATGTCAGTCA |
| KAT3 | ATCCTTGTGACAGTAGGAGCA | GGGCTCATAGCAGTCATAGAAAG |
| KAT4 | AAGAGGGACACCAATAGCAAAAA | GCAGAACGTAAGGCTTTCCAT |
| GAPDH | CCTCCTGCACCACCAACTGCTT | GAGGGGCCATCCACAGTCTTCT |
GAPDH, glyceraldehyde-3-phosphate dehydrogenase; PGC-1α, peroxisome proliferator-activated receptor-γ coactivator-1α; PPARα, peroxisome proliferator-activated receptor-α; PPARδ, peroxisome proliferator-activated receptor-δ; KAT1, kynurenine aminotransferase 1; KAT2, kynurenine aminotransferase 2; KAT3, kynurenine aminotransferase 3; KAT4, kynurenine aminotransferase 4.
Venous blood sampling.
Blood samples (~10 ml) were obtained from an antecubital vein immediately before the muscle biopsy procedure. Samples were collected into lithium heparin-coated tubes, mixed by inversion, and centrifuged at 1,500 rpm for 10 min at 4°C. Aliquots of plasma were stored at −80°C until analysis.
Plasma analyses.
Plasma kynurenine was measured using the Kynurenine ELISA (ImmuSmol, Pessac, France). Plasma kynurenic acid and quinolinic acid were measured using the KYNA ELISA and QUIN ELISA, respectively (Cloud-Clone, TX). All samples were measured undiluted in triplicate, and all standards were run in duplicate. Absorbance of each plate was measured at 450 nm, with a reference wavelength of 540 nm using a Multiskan GO UV/Vis microplate spectrophotometer (Thermo-Fisher Scientific).
Statistical analyses.
The change in skeletal muscle gene and protein expression, and plasma kynurenines, were assessed using multivariate repeated measures ANOVA with a within-subjects factor of time (pre, post). Statistical significance was set at P ≤ 0.05. Univariate post-hoc analyses were then performed to determine which variables changed over time. Change scores are presented as means (SD). The association between the changes in skeletal muscle gene and protein expression, and plasma kynurenines, were analyzed using Pearson Correlations. Statistical analyses were performed using SPSS (IBM SPSS Statistics for Windows (version 23.0; IBM, Armonk, NY).
RESULTS
Multivariate repeated-measures ANOVA showed a significant multivariate effect for the 11 latent variables F(11,6) = 5.03, P = 0.03, Cohens d = 6.07. Univariate level post hoc tests were then conducted to identify the specific dependent variables that changed over time.
Participant characteristics: physiological and performance parameters.
A complete description of participant physiological and performance parameters has been previously published (3). In brief, participants in the supplement and control groups had baseline body mass indices (BMI) of 28.9 and 28.1, respectively. BMI was not significantly altered in either group following the intervention. Aerobic fitness was assessed by a peak oxygen consumption (V̇o2 peak) test on a cycle ergometer. Significant improvements from baseline to postintervention were observed for both supplement and control groups by respective changes in peak of 23.8 ml·kg−1·min−1 (SD 0.8)–26.2 ml·kg−1·min−1 (SD 1.2) and 24.4 ml·kg−1·min−1 (SD 0.9) – 26.4 ml·kg−1·min−1 (SD 1.4) and peak power 154 W (SD 5)−164 W (SD 7) and 158 W (SD 7)–178 W (SD 10). Isotonic muscle strength was assessed by 1 repetition maximums (1RMs) for the following exercises: leg press, chest press, lateral pull-down, horizontal row, shoulder press, and leg extension. Significant improvements, from baseline to postintervention, were demonstrated in both the supplement and control groups by changes in the sum of all 1RMs, which included 23% and 21% increases, respectively.
Skeletal muscle gene and transcription factor expression.
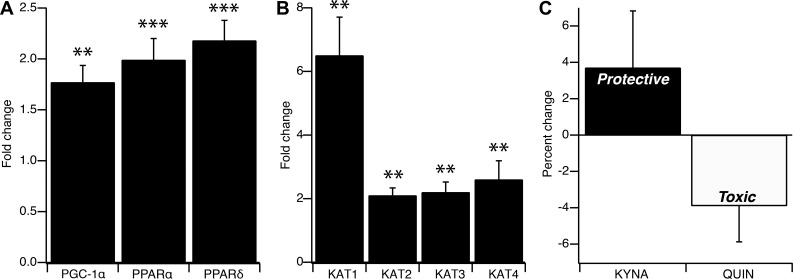
Figure 1A illustrates significant exercise training-induced increases in transcriptional coactivators PGC-1α (1.77-fold increase, P < 0.01, Cohens d = 1.71), PPARα (1.99-fold increase; P < 0.001, Cohens d = 3.22), and PPARδ (2.18-fold increase; P < 0.001; Cohens d = 3.59). Significant increases were also shown for all 4 KAT isoforms including KAT1 (6.5-fold increase, P = 0.01, Cohens d = 1.39), KAT2 (2.1-fold increase, P < 0.01, Cohens d = 1.88), KAT3 (2.2-fold increase, P < 0.01, Cohens d = 1.81), and KAT4 (2.6-fold increase, P < 0.01; Cohens d = 1.75; Fig. 1B). Importantly, increases in each transcription factor (PGC-1α, PPARα, PPARδ) were significantly correlated with increases in each KAT isoform (KAT 1–4) (see Table 2).
Fig. 1.
A and B: fold changes post- versus preexercise training in expression of skeletal muscle transcription coactivators PGC-1α, PPARα, and PPARδ (A) and skeletal muscle gene expression of KAT isoforms 1–4 (B). C: percent change in plasma metabolites KYNA and QUIN post- versus preexercise training. PGC-1α, peroxisome proliferator-activated receptor-γ coactivator 1α; PPAR, peroxisome proliferator-activated receptor; KAT, kynurenine amino transferase; KYNA, kynurenic acid; QUIN, quinolinic acid. Repeated-measures ANOVA; n = 25. Error bars represent SE. **P ≤ 0.01, ***P ≤ 0.001.
Table 2.
Skeletal muscle gene and transcription factor expression correlation matrix
| ΔPGC-1α | ΔPPARα | ΔPPARδ | ΔKAT1 | ΔKAT2 | ΔKAT3 | |
|---|---|---|---|---|---|---|
| ΔPPARα | 0.53* | |||||
| ΔPPARδ | 0.53* | 0.80† | ||||
| ΔKAT1 | 0.41 | 0.71† | 0.53* | |||
| ΔKAT2 | 0.55* | 0.60† | 0.54† | 0.88† | ||
| ΔKAT3 | 0.46* | 0.52* | 0.53* | 0.62† | 0.70† | |
| ΔKAT4 | 0.49* | 0.73† | 0.53* | 0.75† | 0.71† | 0.57† |
Values are Pearson correlation r values; n = 25. PGC-1α, peroxisome proliferator-activated receptor-γ coactivator-1α; PPARα, peroxisome proliferator-activated receptor-α; PPARδ, peroxisome proliferator-activated receptor-δ; KAT1, kynurenine aminotransferase 1; KAT2, kynurenine aminotransferase 2; KAT3, kynurenine aminotransferase 3; KAT4, kynurenine aminotransferase 4.
P ≤ 0.05;
P ≤ 0.01.
Plasma kynurenines.
The change in plasma kynurenines following intervention showed moderate effect sizes; however, no changes reached statistical significance (Fig. 1C). Kynurenine was reduced from 651.3 to 603.3 ng/ml (7.4% decrease, P = 0.09, Cohens d = 0.90), KYNA was increased from 154.0 to 159.7 ng/ml (3.7% increase, P = 0.59, Cohens d = 0.27), and QUIN was reduced from 163.7 to 157.3 ng/ml (3.9% decrease, P = 0.31, Cohens d = 0.53). The QUIN/KYNA ratio was reduced by 5.7% (P = 0.47, Cohens d = 0.38). Changes in skeletal muscle gene and transcription factor expression were not correlated with the changes in any of the plasma kynurenines.
DISCUSSION
The present study is the first to evaluate the influence of a 12-wk exercise training program on skeletal muscle transcription factors and aspects of the kynurenine pathway in older men. The exercise intervention resulted in significant increases in the expression of the skeletal muscle transcription factors PGC-1α, PPARα, and PPARδ, which is in accordance with previous work in young adults. Critically, these changes correlated positively with increased gene expression of all KAT isoforms; however, the plasma concentrations of kynurenines were not significantly altered in response to the exercise training program. Despite the lack of change in plasma kynurenines, the significant increase in skeletal muscle gene expression is encouraging, given that previous work in animal models showed a loss in exercise-induced increases with aging (5). These changes would be expected to help facilitate a shift towards the neuroprotective branch of the kynurenine pathway. Although this was not achieved in the present study, we will herein discuss potential limitations in the study design related to the timing of sample collection that may have obscured a true physiological effect.
As hypothesized, we observed an exercise training-induced increase in skeletal muscle transcriptional coactivators. This was related to an increase in the expression of KAT isoforms, upon which the neuroprotective branch depends. However, for these changes to be regarded as reflective of a true neuroprotective shift, we would also have needed to observe a decrease in plasma concentrations of QUIN and an increase in plasma concentrations of KYNA. Although the QUIN/KYNA ratio was reduced by a medium-effect size following exercise training, the changes in circulating KYNA and QUIN were not statistically significant. This lack of significant alterations in metabolites may have been due to several factors, including the timing of sample collection, exercise training parameters, and the use of nondepressed participants. Blood samples were collected following muscle biopsies 10 days following the cessation of the exercise intervention. As plasma kynurenic acid has been shown to be rapidly excreted by the kidneys following exercise, the most dramatic elevations in plasma concentrations would likely have been apparent near the end or immediately following an exercise bout (7). It may be possible that the changes in plasma kynurenines were purely transient in nature and therefore not detected in the current analysis. Future research should evaluate the temporal dynamics of the kynurenine pathway during and following an acute bout in this population. Furthermore, although significant increases in KAT gene expression were demonstrated, limited tissue sample availability precluded the examination of KAT protein expression, and it is therefore not possible to confirm whether corresponding increases in KAT protein were achieved.
Although both aerobic (9) and resistance (13) exercise has been shown to bias Trp metabolism towards the neuroprotective branch, research is limited regarding the most effective modality, volume, and intensity (particularly in the older adult population). It may be possible that the multimodal exercise intervention employed in the present study, which consisted of both resistance training and high-intensity interval training, was not sufficient to induce substantial enough changes to produce lasting effects. Alternatively, the training volume of twice per week and/or the intensity within those training sessions may not have been sufficient.
Although these results provide evidence for the ability of exercise to enhance skeletal muscle gene expression related to the kynurenine pathway in older adults, our sample was relatively small and consisted of only men who were not depressed. Older adults with depression may have an even greater skeletal muscle transcriptional deficit and thus be more apt to experience a dramatic shift in the kynurenine pathway with exercise. Furthermore, as plasma kynurenines were assessed 10 days following the final exercise bout, it is not possible to comment on potential transient changes in the acute phase following exercise. Larger scale exercise trials, which assess both transient and resting state biochemical changes are needed to examine how changes in these fundamental biochemical processes impact depressive symptoms.
In conclusion, older men who engaged in a new 12-wk combined exercise training program had significant increases in skeletal muscle transcriptional coactivators and gene expression related to the kynurenine pathway. Plasma concentrations of kynurenines were, however, not significantly altered. Despite this, the significant exercise training-induced increase in the expression of skeletal muscle transcription factors and KAT in older adults is encouraging, given the potential implications related to kynurenine pathway regulation. Future studies are warranted to explore the impact of various exercise modalities and intensities on transient changes of such factors in depressed older adults.
GRANTS
This study was supported, in part, by Canadian Institutes of Health Research Grant MOP-123296 (S. M. Phillips).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.J.A., J.P.N., and J.J.H. conceived and designed research; D.J.A., J.P.N., T.S., K.E.B., D.K., S.M.P., and G.P. performed experiments; D.J.A., J.P.N., and J.J.H. analyzed data; D.J.A., J.P.N., and J.J.H. interpreted results of experiments; D.J.A. and J.J.H. prepared figures; D.J.A. and J.P.N. drafted manuscript; D.J.A., J.P.N., T.S., K.E.B., D.K., S.M.P., G.P., and J.J.H. edited and revised manuscript; D.J.A., J.P.N., T.S., K.E.B., D.K., S.M.P., G.P., and J.J.H. approved final version of manuscript.
REFERENCES
- 1.Agudelo LZ, Femenía T, Orhan F, Porsmyr-Palmertz M, Goiny M, Martinez-Redondo V, Correia JC, Izadi M, Bhat M, Schuppe-Koistinen I, Pettersson AT, Ferreira DMS, Krook A, Barres R, Zierath JR, Erhardt S, Lindskog M, Ruas JL. Skeletal muscle PGC-1α1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell 159: 33–45, 2014. [Erratum in Cell 160: 351, 2015.] doi: 10.1016/j.cell.2014.07.051. [DOI] [PubMed] [Google Scholar]
- 2.Allison DJ, Ditor DS. The common inflammatory etiology of depression and cognitive impairment: a therapeutic target. J Neuroinflammation 11: 151, 2014. doi: 10.1186/s12974-014-0151-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bell KE, Snijders T, Zulyniak M, Kumbhare D, Parise G, Chabowski A, Phillips SM. A whey protein-based multi-ingredient nutritional supplement stimulates gains in lean body mass and strength in healthy older men: a randomized controlled trial. PLoS One 12: e0181387, 2017. doi: 10.1371/journal.pone.0181387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carek PJ, Laibstain SE, Carek SM. Exercise for the treatment of depression and anxiety. Int J Psychiatry Med 41: 15–28, 2011. doi: 10.2190/PM.41.1.c. [DOI] [PubMed] [Google Scholar]
- 5.Derbré F, Gomez-Cabrera MC, Nascimento AL, Sanchis-Gomar F, Martinez-Bello VE, Tresguerres JAF, Fuentes T, Gratas-Delamarche A, Monsalve M, Viña J. Age associated low mitochondrial biogenesis may be explained by lack of response of PGC-1α to exercise training. Age (Dordr) 34: 669–679, 2012. doi: 10.1007/s11357-011-9264-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunn AL, Trivedi MH, Kampert JB, Clark CG, Chambliss HO. Exercise treatment for depression: efficacy and dose response. Am J Prev Med 28: 1–8, 2005. doi: 10.1016/j.amepre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Erhardt S, Olsson SK, Engberg G. Pharmacological manipulation of kynurenic acid: potential in the treatment of psychiatric disorders. CNS Drugs 23: 91–101, 2009. doi: 10.2165/00023210-200923020-00001. [DOI] [PubMed] [Google Scholar]
- 8.Fava GA, Offidani E. The mechanisms of tolerance in antidepressant action. Prog Neuropsychopharmacol Biol Psychiatry 35: 1593–1602, 2011. doi: 10.1016/j.pnpbp.2010.07.026. [DOI] [PubMed] [Google Scholar]
- 9.Jung S, Kim K. Exercise-induced PGC-1α transcriptional factors in skeletal muscle. Integr Med Res 3: 155–160, 2014. doi: 10.1016/j.imr.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nierenberg AA, Alpert JE. Depressive breakthrough. Psychiatr Clin North Am 23: 731–742, 2000. doi: 10.1016/S0193-953X(05)70194-5. [DOI] [PubMed] [Google Scholar]
- 11.Oxenkrug GF. Tryptophan kynurenine metabolism as a common mediator of genetic and environmental impacts in major depressive disorder: the serotonin hypothesis revisited 40 years later. Isr J Psychiatry Relat Sci 47: 56–63, 2010. [PMC free article] [PubMed] [Google Scholar]
- 12.Paolucci EM, Loukov D, Bowdish DME, Heisz JJ. Exercise reduces depression and inflammation but intensity matters. Biol Psychol 133: 79–84, 2018. doi: 10.1016/j.biopsycho.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 13.Ruas JL, White JP, Rao RR, Kleiner S, Brannan KT, Harrison BC, Greene NP, Wu J, Estall JL, Irving BA, Lanza IR, Rasbach KA, Okutsu M, Nair KS, Yan Z, Leinwand LA, Spiegelman BM. A PGC-1α isoform induced by resistance training regulates skeletal muscle hypertrophy. Cell 151: 1319–1331, 2012. doi: 10.1016/j.cell.2012.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 163: 1905–1917, 2006. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 15.Schlittler M, Goiny M, Agudelo LZ, Venckunas T, Brazaitis M, Skurvydas A, Kamandulis S, Ruas JL, Erhardt S, Westerblad H, Andersson DC. Endurance exercise increases skeletal muscle kynurenine aminotransferases and plasma kynurenic acid in humans. Am J Physiol Cell Physiol 310: C836–C840, 2016. doi: 10.1152/ajpcell.00053.2016. [DOI] [PubMed] [Google Scholar]
- 16.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protocols 3: 1101–1108, 2008. [DOI] [PubMed] [Google Scholar]
- 17.Sheikh JI, Yesavage J. Geriatric Depression Scale (GDS): recent evidence and development of a short version. Clin Gerontol 5: 165–173, 1986. doi: 10.1300/J018v05n01_09. [DOI] [Google Scholar]
- 18.World Health Organization. Global Health Estimates 2015: Disease Burden by Cause, Age, Sex, by Country and by Region, 2000–2015. New York: World Health Organization, 2016. [Google Scholar]