Abstract
A functional neurovascular unit (NVU) is central to meeting the brain’s dynamic metabolic needs. Poststroke damage to the NVU within the ipsilateral hemisphere ranges from cell dysfunction to complete cell loss. Thus, understanding poststroke cell-cell communication within the NVU is of critical importance. Loss of coordinated NVU function exacerbates ischemic injury. However, particular cells of the NVU (e.g., astrocytes) and those with ancillary roles (e.g., microglia) also contribute to repair mechanisms. Epidemiological studies support the notion that infarct size and recovery outcomes are heterogeneous and greatly influenced by modifiable and nonmodifiable factors such as sex and the co-morbid condition common to stroke: hypertension. The mechanisms whereby sex and hypertension modulate NVU function are explored, to some extent, in preclinical laboratory studies. We present a review of the NVU in the context of ischemic stroke with a focus on glial contributions to NVU function and dysfunction. We explore the impact of sex and hypertension as modifiable and nonmodifiable risk factors and the underlying cellular mechanisms that may underlie heterogeneous stroke outcomes. Most of the preclinical investigative studies of poststroke NVU dysfunction are carried out primarily in male stroke models lacking underlying co-morbid conditions, which is very different from the human condition. As such, the evolution of translational medicine to target the NVU for improved stroke outcomes remains elusive; however, it is attainable with further research.
Keywords: astrocyte, microglia, neurovascular unit, sex, stroke
INTRODUCTION
Modified from the original phrase “time is money” (Benjamin Franklin in 1748), neurologists and stroke scientists coined the phrase “time is brain,” which speaks to the brain’s currency. Time without blood flow results in irreversible brain damage. It is estimated that an average stroke patient with a blocked large vessel will lose ∼7 miles of axonal fibers, 13.8 billion synapses, and 1.9 million neurons for each hour that ischemia persists (171). Unlike other organ systems, the brain has little capacity to store energy and, therefore, relies on a precisely regulated and steady blood supply for nutrient delivery and waste removal (39). An intricate process, known as neurovascular coupling, fulfills the “on-demand” and diverse cellular metabolic needs resulting from variable levels of neuronal activity simultaneously occurring throughout the brain (182). The neurovascular unit (NVU), having both physical and molecular signaling attributes, is key to carrying out neurovascular coupling (224). In the traditional sense, elements of the NVU are neurons, astrocytes, and vasculature. Brain cells once considered peripheral to the NVU, microglia and oligodendrocytes, may indirectly contribute to neurovascular coupling, as they contribute to NVU homeostasis in health and disease conditions. In contrast to neurovascular coupling, a finely tuned process (recently reviewed in Ref. 92), is autoregulation, a more coarse method of ensuring blood flow. Mechanisms of autoregulation ensure that blood flow is consistent when systemic blood pressure varies (221). These two distinct mechanisms, coarse and fine, highlight the physiological importance and dedication to balancing the brain’s energy supply and demand. Coordinated physiological processes at the NVU maintain brain homeostasis and prevent pathologies such as cognitive impairments. Ischemic stroke, however, severely interrupts both the physical and molecular signaling aspects of the NVU, so that it is either entirely lost in some regions or merely dysfunctional in other regions. In this review we highlight the current knowledge of the effects of ischemic stroke on the NVU, with particular focus on astrocytes and microglia intercellular communication in health and in response to, or mediation of, the deleterious poststroke parenchymal milieu. The heterogeneity of ischemic stroke in terms of risk and severity is due to both modifiable and nonmodifiable factors, such as sex and co-morbid conditions; whenever possible, a discussion of these factors is included.
ISCHEMIC STROKE
Stroke is the fifth-leading cause of death and disability in the United States (21). There are two major types of strokes: ischemic and hemorrhagic. Ischemic stroke is caused by a thrombus or emboli blocking blood flow in the vasculature or by decreased perfusion (132) and is, therefore, a clinical case of the ultimate imbalance between blood flow supply and demand in the brain. Ischemic strokes are by far the most prevalent (~80%); however, hemorrhagic strokes are considerably more deadly, because, with vessel rupture, blood accumulates within the skull and results in sudden and dramatic parenchymal compression and displacement and overwhelming rapid cell death (21). Cell death also occurs with ischemic stroke. Without a continuous source of energy [cerebral blood flow (CBF) ~40 ml·100 mg−1·min−1], the electrical function of neurons is lost (CBF less than ~12 ml·100 g−1·min−1) and cell membrane failure occurs (CBF 6–8 ml·100 g−1·min−1), resulting in cell death from the lack of energy reserves (132). Dying neurons in the ischemic core release cell contents (e.g., ATP, glutamate, and K+), which diffuse into the surrounding parenchyma and initiate a deleterious cascade of events. Peri-infarct regions near the ischemic core are viable, but at-risk, parenchyma (46, 81). Although not ischemic, the peri-infarct region is hypoperfused and subjected to deleterious signals originating from the ischemic core that can result in peri-infarct-spreading depressions, neuronal hyperexcitability, biochemical and molecular changes, and vascular impairments (86). These peri-infarct events exacerbate the supply-and-demand deficit that overwhelms mechanisms of functional hyperemia and severely disrupts neurovascular coupling (165). From a vascular standpoint, therapies to limit ischemic cell death are singular: reperfusion. Reperfusion is the restoration of blood flow to the ischemic brain, and although it may occur spontaneously, more often administration of an antifibrinolytic, such as recombinant tissue plasminogen activator, or endovascular retrieval is needed for best outcomes (188). With continued ischemia (i.e., no reperfusion therapy), the ischemic core will expand to envelop the peri-infarct region. A severe limitation has been that the treatment window is short and finite, however recently expanded (152), and thus not all stroke patients will benefit from this singular treatment option.
Both nonmodifiable (e.g., genetics, age, and sex) and modifiable (e.g., hypertension) risk factors have an impact on stroke incidence, severity, and associated neurological outcomes. Sex differences are well reported in both human and rodent studies of ischemic stroke. Epidemiological findings suggest less infarct risk and severity in premenopausal women than age-matched men (134). However, in women (48–55 yr of age), this protection is reversed following the postmenopausal decline in gonadal sex hormone levels; the average age for stroke is 75 yr in women and 71 yr for men (104). A history of clinical trials investigating the associations between long-term sex hormone treatment and stroke risk or severity conflicts with the abundant preclinical research (13) and laboratory evidence that suggest that estrogen therapy, supplied acutely after stroke, is protective. The stark contrast between clinical and preclinical evidence dampens the enthusiasm to entertain estrogen as a poststroke therapeutic. In 1985, a prospective study of the Framingham Heart Study cohort concluded that women with extended estrogen use for postmenopausal symptoms had an increased relative risk for stroke morbidity (211). In the early 1990s, the Women’s Health Initiative (WHI) began a large-scale investigation into the factors that affect the health of postmenopausal women. Two randomized and double-blind clinical trials emerged from the WHI specifically to investigate the relationship between sex hormone treatments and cardiovascular outcomes but were terminated early due to a harmful risk-to-benefit ratio most notable in ischemic stroke. In 2002, it was concluded that the combined use of estrogen and progesterone by healthy women significantly increased the risk of ischemic stroke, independent of hypertension, by >40% (209). Two years later, a second study that aimed to investigate estrogen alone was terminated due to similar outcomes (83). In both cases, gonadal hormones did not affect stroke severity (evidenced by the Glasgow Coma Scale) or stroke outcomes (83, 209), for better or worse. A more thorough analysis of these studies now indicates a complex role of estrogen replacement in cerebrovascular disease. An initial hypothesis, named the “timing” or “window” hypothesis, which emerged as an explanation of these initial findings, was that estrogen therapy had no effect or was harmful regarding risk following a period of estrogen deficiency. Rather, the benefits of estrogen therapy could be realized when treatment occurred during perimenopause or when initiated soon after menopause (17). However, because the WHI was not designed to test this hypothesis specifically, this interpretation is limited. Nonetheless, the age interval of hormone replacement therapy implementation is now a consideration for further investigation, and preclinical research provides some support of the timing hypothesis. In rodents, treatment with estrogen after 10 wk of estrogen deficiency, somewhat mimicking the WHI clinical trials, resulted in no neuroprotection and also suppressed estrogen’s anti-inflammatory actions, effects that were reversible when estrogen was administered immediately with ovariectomy (190). On the other hand, additional analyses of the WHI study, specifically designed to examine the timing hypothesis, were not supportive. In these studies, the effects of estrogen on WHI clinical outcomes, such as ischemic stroke, did not depend on the time between menopause and first use of the hormone therapy (14, 155).
Despite the findings of clinical investigations, a plethora of preclinical laboratory research has examined the fundamental phenomenon that stroke is less severe in premenopausal women than in men and postmenopausal women. A variety of ischemic stroke models have demonstrated that treatment with either estrogen or progesterone lessens infarct size after stroke when delivered to postmenopausal female or male rodent stroke models (4, 7, 34, 120, 183, 215). Gonadal hormones, in particular estrogen, are implicated in poststroke neuroprotection via multiple mechanisms (4, 105, 127). For example, estrogen promotes cell survival signaling and antioxidant pathways to lessen neuroinflammation (96, 162, 187) and edema (141), which are prominent sources of secondary injury in ischemic stroke. In addition, when administered during reperfusion to increase CBF and reduce infarct injury, estrogen has a vasodilatory effect (129). On the other hand, a treatment regimen of high doses of estrogen in older acyclic females increased infarct size, an outcome that was attributed to the decreased availability of insulin-like growth factor-1 (IGF-1) (174). It is likely that these preclinical findings do not coincide with clinical research for the following reasons. 1) Whereas preclinical studies investigate the effects of sex hormones on stroke severity and injury-related outcomes, they do not examine stroke risk, which was the primary finding of clinical research investigations. Although long-term treatment regimens of estrogen may have a poor risk-to-benefit ratio, the use of sex hormones as an acute treatment of ischemic stroke to lessen brain injury is a more viable option that, when mechanistically understood, should be considered. 2) Most preclinical studies used the surgical removal of ovaries (ovariectomy) as a model of menopause to study postmenopausal ischemic stroke (4); however, only 10% of women enter menopause surgically. Rather, the majority of women enter menopause gradually via the reduction of ovarian function and retention of ovarian tissue, a stark contrast to the ovariectomy model. This limitation can be addressed, while an age-matched experimental design is retained, by using an alternative model such as the 4-vinylcyclohexene diepoxide mouse model of menopause (27, 74, 200, 201).
ASTROCYTE CONTRIBUTIONS TO THE NVU
Over the past nearly five decades, our understanding of astrocyte function has transitioned from trophic, supportive, and metabolic-related to highly complex roles (for a detailed review see Ref. 203). Astrocytes account for 20–40% of all glial cells in the human brain and 10–20% in the rodent brain (189, 203) and are a highly heterogeneous cell population with brain region specificity (203). Diverse intercellular interactions with neurons, microglia, and vascular cells place astrocytes in a dominant position to influence numerous processes across the brain. In rodents, a single (protoplasmic) astrocyte is in contact with 20,000–120,000 synapses (30, 76, 203) and capillaries, allowing a bridge of information from neurons to blood vessels, and vice versa. In addition, extensive gap junction coupling, which forms the astrocytic syncytium, supports the coordination of neuronal networks and cellular responses over greater distances (33, 35). Significant astrocyte functions include synapse formation/elimination (37, 38); synaptic plasticity (148); release of neurotrophic factors; uptake and recycling of neurotransmitters; maintenance of the blood-brain barrier (BBB) (1); CBF regulation (11); and clearance and maintenance of ionic and extracellular neurotransmitters (94), glucose metabolism, and substrate delivery to neurons (125, 128). These functions are, in many instances, accomplished via the action of numerous specialized proteins, including glutamate transporters [e.g., glutamate transporter 1 and glutamate aspartate transporter (166)], K+ channels for the maintenance of extracellular K+ concentrations (e.g., via Kir4.1), and aquaporin 4 (AQP4) for water homeostasis and glymphatic function (131), among others.
Astrocytes are nonexcitable cells. Their functional state (although not exclusive) has been studied by monitoring of intracellular Ca2+ dynamics as well as their effect on neuronal function. The use of genetically encoded Ca2+ indicators [myosin light chain kinase (GCaMP3/6) (177, 197)] has unveiled compartmentalized Ca2+ events in soma, primary processes, and fine processes defined as microdomains (for suggested nomenclature see Ref. 177), but the Ca2+ source and signaling mechanisms underlying activity in subcompartments are poorly understood. Agarwal et al. showed that Ca2+ events within astrocyte microdomains have distinct kinetics and respond to neuromodulators (i.e., norepinephrine) differently from those in soma or larger processes (3). In their study, microdomain Ca2+ events originated from the opening of the mitochondria permeability transition (MPT) pore. Importantly, mitochondrial Ca2+ was associated with signaling pathways involved in astrocyte dysfunction and increased reactive oxygen species (ROS) (3). Agarwal et al. proposed aberrant astrocyte microdomain Ca2+ activity as a potential link to neurodegenerative conditions (3, 111), raising the question of whether these subcompartments constitute an important site for cerebral bioenergetic and redox dysregulation. Astrocyte metabolic activity is critical for neuronal survival; thus, mitochondrial function is of high importance during pathology such as ischemic stroke. Also, astrocyte mitochondrial function is sexually dimorphic, both in the healthy brain and after stroke (65). In the healthy rodent brain, pyruvate dehydrogenase complex activity is higher in females than males (66). In humans, positron emission tomography shows higher glucose metabolism in females than males (217). Ovariectomy and the decline of gonadal hormones diminish mitochondrial function (reviewed in Ref. 66).
ASTROCYTE RESPONSES TO ISCHEMIC INJURY AND CONTRIBUTIONS TO RECOVERY
After injury, astrocyte function is dependent on numerous factors, including the type of injury, age, sex, and co-morbid disorders. In stroke, astrocytes have both beneficial and deleterious roles (147). While the brain has little capacity for energy storage, astrocytes store glycogen, giving them an advantage over neurons to become more resistant to ischemic injury (73) and essential players in poststroke recovery. On the other hand, severe ischemia results in mitochondrial dysfunction, energy depletion, and, consequently, dysregulation of astrocyte-mediated brain homeostatic processes, resulting in ionic disequilibrium, membrane depolarization, increased glutamate and Ca2+ release, excitotoxicity (164), oxidative stress, breakdown of the BBB, water dysregulation, and inflammation (49). An interesting study by Hayakawa et al. showed that astrocytes may be able to release, via a cluster of differentiation 38-mediated mechanism, functional extracellular mitochondria particles, which in turn enter neurons and aid in their viability and recovery after stroke (79), increasing the relevance for astrocyte contributions to health and disease. Aberrant astrocyte Ca2+ regulation also follows ischemic injury, contributing to injury exacerbation. Sources of intracellular Ca2+ overload after stroke include N-methyl d-aspartate receptors, voltage-dependent Ca2+ channels, metabotropic receptors, and increased combined activity of the Na+/H+ and Na+/Ca2+ exchangers (reviewed in Ref. 109). Excitotoxic Ca2+ concentrations result in opening of the MPT pore, mitochondrial damage, and release of proapoptotic factors that exacerbate ischemic brain injury (29). Burstein et al. demonstrated mitochondria sex differences in the brain (29). Female mitochondria exhibited reduced Ca2+ capacity and increased propensity for Ca2+-dependent opening of the MPT pore compared with male mitochondria. Both inhibition and genetic deletion of cyclophilin D, a regulator of MPT, eliminated sex differences. In addition, estrogen receptor (ER)-β blockade increased mitochondria Ca2+ capacity, suggesting a functional link between ERβ and cyclophilin D (29). These studies support ERβ signaling as a potential therapeutic target against irreversible mitochondria damage. Using in vivo multiphoton imaging microscopy in a mouse model of ischemia, Rakers et al. demonstrated that peri-infarct depolarizations exacerbate tissue damage via an increase in Ca2+ in both neurons and astrocytes (157). Responses were ameliorated in an inositol trisphosphate type 2 receptor-deficient mouse model, suggesting that astrocyte Ca2+ is a primary contributor to excitotoxicity. In a later study, the same group showed a transient receptor potential vanilloid 4 (TRPV4) channel contribution to astrocytic and neuronal Ca2+ increases along with extracellular glutamate accumulation (156, 157).
TRPV4 channels are Ca2+-permeable nonselective cation channels (150) expressed in multiple cell types and, thus, are involved in a variety of functions, including mechanosensation, systemic tonicity, pain, inflammation, and vascular tone (57, 85, 98, 150, 168, 191, 204). Relevant to the NVU, TRPV4 channel expression has been shown in endothelium, vascular smooth muscle cells, astrocytes, neurons, and microglia cells (20, 88). However, the relative contribution of these channels to neurovascular-related physiological vs. pathological processes, such as ischemia, is poorly understood. In the vasculature, specifically parenchymal arterioles, TRPV4 channels detect and transduce arteriole myogenic responses (e.g., mechanical stress) (48, 102). In cerebral capillary endothelial cells, TRPV4 channels are regulated by the plasma membrane phosphatidylinositol 4,5-biphosphate (PIP2). Interestingly, the presence of PIP2 tonically inhibits TRPV4 channels, resulting in reduced baseline activity. Harraz et al. showed that Gq protein-coupled receptor activation, a step leading to PIP2 depletion, activated TRPV4 channels (77). PIP2-mediated channel regulation is important, because Harraz et al. showed that its presence has opposite effects on the activity of inwardly rectifying K+ (Kir2.1) channels (77, 78, 124). While a physiological role for endothelial Kir channels was proposed to be the spread of a hyperpolarizing current (from capillary to arteriole), resulting in upstream dilation and increases in CBF (124), the impact of PIP2 depletion and, consequently, TRPV4 channel activation on neurovascular-related process remains unclear. To this end, Harraz et al. proposed that TRPV4 channel activation might act as a stop signal to K+ channel-mediated endothelial hyperpolarization (77, 78, 124). These studies highlight the importance of TRPV4-Kir channel regulation in shaping the neurovascular coupling response and its implication in disease conditions.
TRPV4 channels are also expressed in the plasma membrane of astrocytes (19, 20, 119). We demonstrated that increases in intravascular pressure augmented astrocyte Ca2+, partly via a TRPV4-mediated activation (48). Interestingly, not all astrocytes express TRPV4 channels; however, activation of TRPV4-positive cells can trigger intracellular Ca2+ increases in TRPV4-negative astrocytes, a mechanism likely involving ATP/purinergic signaling (176) and amplified by ischemic injury. Under pathological conditions, both increases and decreases in TRPV4 expression/function have been reported. Increased TRPV4 expression and activity were shown in rat hippocampal CA1 region astrocytes following ischemic injury and astrogliosis (31, 178). These studies support the notion that while TRPV4 channels are involved in physiological processes, channel dysregulation via proinflammatory mechanisms may contribute to neurovascular unit dysfunction. To this end, Križaj et al. proposed that Ca2+ overload in retinal ganglion cells results from the enhanced activity of TRPV4, P2X purinoceptor 7, and pannexin channels (110). TRPV4-mediated Ca2+ entry can trigger ATP release (102, 176) and, ultimately, release of proinflammatory signals (e.g., TNF-α and IL-1β) (110). Of more recent interest is the potential of TRPV4 channel antagonists to lessen brain injury by limiting cation influx (to include Ca2+), cell swelling, and deleterious downstream signaling, such as matrix metalloproteinases (MMPs) (88). Future studies addressing the cross talk between vascular and glial cells in the context of hypertension, ischemic injury, and neurovascular unit homeostasis are needed.
Astrocytes near the ischemic infarct undergo functional and structural transformations, referred as reactive gliosis (26, 146, 147). Astrocytes increase expression of the intermediate filament protein glial fibrillary acidic protein (GFAP), an event that is dependent on their relative distance from the insult, used as a biomarker of ischemic damage (159). Astrocytes near the lesion proliferate and form the glial scar, composed of intertwined processes and extracellular matrix (16, 185, 208, 216), requiring ≥2 wk (measured in rodent models) to fully form around the infarct necrotic core. The glial scar demarcates the zone between dead and surviving parenchyma, and while it is thought to play a role in defending against infiltrating peripheral cells (16), recent evidence suggests that the glial scar is not a strong barrier against extracellular fluid entry into the brain (220). Moreover, blood vessels within the infarct core are leaky; therefore, the extracellular fluid leaking into the surrounding tissue likely contains plasma, a highly neurotoxic element (220). Although B lymphocytes and plasma cells remain largely sequestered from healthy tissue (54), products of these cells (e.g., antibodies), as well as MMP, cytokines, and chemokines, leak across the glial scar into the surrounding parenchyma (220). While the barrier properties (to infiltrating extracellular fluid) of the glial scar are unclear, the necrotic and neurotoxic ischemic core will eventually resolve: a stage termed cystic encephalomalacia (139, 220). Farther from the lesion, astrocytes remain in their territory but also undergo morphological changes, including increased gene expression of the intermediate filaments GFAP, vimentin, and nestin (185), changes that are likely to negatively influence NVU cell-cell communication. These findings are significant, considering drug delivery during the stroke recovery period. First, considering the leaky BBB in the weeks and months after stroke, therapeutics that may hasten a return to homeostasis have potential to reach the peri-infarct region. On the other hand, drugs delivered systemically as part of any therapeutic regimen may also leak past the glial scar for unintended effects. However, pharmacokinetic studies to determine the penetration of drugs into the parenchyma are necessary to fully appreciate the benefits or harmful outcomes of a leaky glial scar. In addition to a leaky glial scar, ischemic stroke alters the functional integrity of the BBB, with additional important implications for drug delivery. For example, BBB permeability increases during hypoxia via altered tight junctions, an action mediated by protein kinase C (PKC) isoforms along with zonula occludens-1, occludin, and claudin-5 (123). The redistribution of these proteins allows for increased movement of molecules across the BBB in a size-dependent manner. Importantly, BBB breakdown can result in cerebral edema, worsening stroke outcome. Both astrocytes and cerebral endothelial cells undergo functional changes, which facilitate ionic disturbances and water movement into the brain (207). During ischemia, edema formation is exacerbated by the activation of ion channels and transporters (e.g., Kir4.1, AQP4, Na+-K+-Cl− cotransporter, and Na+/H+ exchanger), impairing osmotic homeostasis (87, 167, 207, 218). Ischemia-induced increases in astrocyte Ca2+ concentrations raise cAMP, which in turn activates AQP4 channels, increasing water permeability and swelling (207). These events are further worsened by ischemia-induced loss of AQP4 polarization (45a, 144). The effects of sex differences on BBB permeability are poorly understood, but evidence supports the idea that estradiol treatment reduces astrocyte swelling and ischemia-induced overexpression of AQP4 (167). Understanding key components of the BBB in health and disease will allow for development of specific strategies that modulate the BBB during the acute phases of stroke, attenuate edema (a deleterious outcome of uncontrolled increased BBB permeability), and improve drug delivery during central nervous system (CNS) injury.
The ability of astrocytes to participate in repair processes is lessened by aging, which increases the state of inflammation and results in more pronounced stroke (36). Proliferation of reactive astrocytes is higher in the aged brain (151), and the neuroprotective function of astrocytes is decreased (36). With aging, the trophic factor IGF-1 declines, an event that is also linked to sex differences (174). Okoreeh et al. determined the importance of astrocyte IGF-1 in middle-aged (10- to 12-mo-old) acyclic female rats (142). They showed that injection of recombinant adeno-associated virus serotype 5 containing the GFAP promoter into the striatum and cortex improved ischemia-induced deleterious outcomes. IGF-1 gene transfer to astrocytes also reduced serum GFAP levels and improved blood pressure, neurological score, and sensory-motor performance (142).
Astrocytes are a target for gonadal hormones (10). Both ERα and ERβ are expressed in astrocytes (5, 67). Studies in primary hypothalamic astrocytes from female rats confirmed the presence of transmembrane G protein-coupled ER (GPER), which is involved in nongenomic signaling (113) and contributes to the neuroprotective effects of 17β-estradiol (192). Estrogen’s neuroprotective actions are well established and extend to the regulation of spine density (72), synaptic number, and synthesis of neurotrophic factors (2, 99, 186). Thus, ischemia-induced increased ERα expression in astrocytes is generally considered neuroprotective (47).
Astrocytes are active participants in inflammatory processes. Astrocytes secrete proinflammatory (IL-6 and IL-1) and anti-inflammatory (IL-10) signals, chemokines [C-C motif ligand 2 (CCL2); C-X-C motif chemokine ligand (CXCL) 1 (CXCL1); CXCL10, also known as interferon-γ-induced protein 10 (IP-10); and CXCL12], and other small-molecule messengers [S100 Ca2+-binding protein B (S100B) and nitric oxide (NO)] (51), which signal to immune cells in physiological and pathological conditions. Within the peri-infarct region, astrocytes are critically important for recovery processes after stroke; however, evidence also suggests that astrocytes release proinflammatory signals that exacerbate tissue damage. Using an in vitro and an in vivo approach, including proteomic profile array analysis (24 h after stroke), Li et al. showed that astrocyte-derived IL-15, which in the periphery plays a role in the maintenance and function of immune cells, including natural killer cells and T lymphocyte (108, 206), induced activation of natural killer cells and CD8+ T lymphocytes (115). IL-15 overexpression in astrocytes increased infiltration of lymphocytes into the brain, exacerbating tissue damage and neurological dysfunction. On the other hand, IL-15 knockdown (using a lentiviral approach) reduced damage and improved neurological outcomes (115). Paralleling the macrophage literature, astrocyte phenotypes, induced by ischemic injury or lipopolysaccharide, are broadly categorized as A1 (neurotoxic) or A2 (neuroprotective) reactive astrocytes (117, 219). Subsequent studies show that A1 astrocytes are highly toxic to a broad array of cell types, including retinal ganglion cells, cortical neurons, embryonic spinal motor neurons, and differentiated oligodendrocytes (117). However, other cell types (preganglionic and γ-mother neurons) were not vulnerable to A1 astrocytes. In addition, A1 astrocytes have a reduced capacity to form robust excitatory synapses due to decreased glypican secretion. In contrast to A1 astrocytes, A2 astrocytes express high levels of neurotrophic factors and cytokines that are useful in the poststroke repair process (219). Using two-photon imaging of male mice subjected to middle cerebral artery occlusion (MCAO model), Fordsmann et al. demonstrated reduced spontaneous Ca2+ activity in the ischemic penumbra in neurons and astrocytes from young (3- to 4-mo-old) mice vs. electrically silent spontaneous Ca2+ activity in neurons and astrocytes from older (18- to 24-mo-old) mice (63). Spontaneous Ca2+ activity was driven by presynaptic and purinergic signaling mechanisms. Importantly, the ischemic penumbra of aged brains was more vulnerable than that of younger animals, in part because of the harmful actions of aged astrocytes under ischemic conditions (63). Delineating between the A1 and A2 phenotypes in a spatiotemporal manner to the ischemic injury and how gonadal hormones may influence these phenotypes is a necessary next step to fully appreciate the complexity of astrocyte-mediated injury and repair processes after stroke.
MICROGLIA: AN ASSET TO THE NVU
Microglia are the immune cells and resident phagocytes in the brain; their primary essential functions are continuous parenchymal surveillance, maintenance of neural network homeostasis, and response to disease or injury. Microglia are highly ramified (branched) cells with dynamically moving processes, which in turn facilitate their essential functions and cell-cell interactions (194, 212). This ramified morphology is associated with dynamic activities involving process extension and retraction necessary to continuously monitor their specific parenchymal domains. In fact, microglia process extension and retraction rates in the healthy brain are estimated to be 1.47 μm/min (140), a rate necessary for complete brain assessment every few hours. Therefore, the historical functional categorization of ramified microglia as “resting” marginalizes their essential contributions to brain maintenance and homeostasis. Microglia are phagocytes and have an important role in maintaining brain health (i.e., synaptic pruning) (184) and restoration of neuronal function during injury and disease (61, 199). Microglia have a significant role in neurological (43, 184) and behavioral (114) development; they also maintain neuronal networks in the adult brain (95, 205) and work collaboratively with other glia (50). While microglial surveillance functions are vital to maintenance of the complex neural network of glia, neurons, and vasculature, they are rarely considered a key member of the NVU.
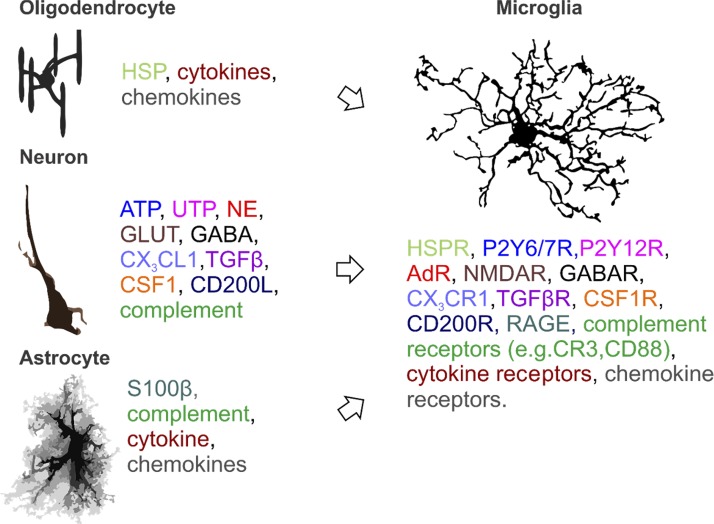
Microglia are intricately linked to their cellular environment, and, rather than being innately driven, much of their activity is heavily influenced by neuronal and glial signaling mediated by the release of small-molecule messengers and soluble factors (212), as summarized in Fig. 1. Hence, the influence of the neighboring environment is an important rationale for studying microglia in vivo or ex vivo, rather than in vitro (71). Microglia are highly responsive to neuronal activity via purines (45, 106, 140), neurotransmitters (62), and chemokines (170), which vary according to the functions of brain regions, sex, and age. Estrogens rapidly modulate neuronal activity. Thus, gonadal hormones may have a significant direct or indirect influence on microglial activity. In the cortex, estrogen potentiates excitatory synapses via increased sensitivity to glutamate through multiple mechanisms, which include altered cellular responsiveness to ionotropic glutamate receptor activation and opioid, dopamine, and GABA receptors (reviewed in Refs. 100, 126, 173), as well as more long-term effects by regulating the brain transcriptome (56).
Fig. 1.
Brain cell-microglia interactions: a nonexhaustive listing of oligodendrocytes (50, 145, 158) and neuronal (198, 212) and astrocytic (116) communication modalities with microglia mediated by small-molecule messengers, soluble factors, cytokines, chemokines (from cells), and associated microglial receptors (color-coded to match ligand and receptor). Such ligand-receptor interactions promote microglial responses, such as changes in morphology (e.g., polarization), increased or decreased process activity, process-directed phagocytosis, synaptic remodeling, and cytokine and chemokine production, which, in turn, elicit a response from responding brain cells. AdR, adenosine receptor; CD200L, cluster of differentiation (CD) 200 ligand; CD200R, CD200 receptor; CSF1, colony-stimulating factor type 1; CSF1R, CSF1 receptor; CX3CL1, C-X3-C motif ligand 1; GABAR, GABA receptor; GLUT, glucose transporter; HSP, heat shock protein; HSPR, HSP receptor; NE, norepinephrine; NMDAR, N-methyl-d-aspartate receptor; P2Y6/7R, pyrimidinergic receptor 6/7; P2Y12R, pyrimidinergic receptor 12; RAGE, receptor for advanced glycation end products; S100B, S100 Ca2+-binding protein B; TGFβ, transforming growth factor-β; TGFβR, TGFβ receptor. [Oligodendrocyte artwork is from Domingues et al. (50), with permission from Frontiers;astrocyte artwork is from Wilhelmsson et al. (210), with permission; copyright (2006) National Academy of Sciences.]
Aging superimposes a progressive and intrinsic change in brain anatomy and physiology (135). As average brain weight decreases, average neuronal and glial numbers decline. In particular, microglial distribution and morphology change (213). Decreased cell numbers and ramification may impair surveillance functions or merely parallel a change in the parenchymal environment, including reduced neuronal activity. With age, microglia expression of immune response signaling receptors, such as major histocompatibility complex II and complement receptor (CR) 3, increases (149). However, it is unclear if these changes, observed in young mice, contribute to, or are a result of, disease pathology.
MICROGLIAL RESPONSES TO ISCHEMIC INJURY AND CONTRIBUTIONS TO RECOVERY
Microglia have an immediate and dynamic response to injury that is dependent on the modality and severity of the injury, such that the response to an acute and robust insult, such as ischemic stroke, is different from the response to chronic neurodegenerative diseases. Such plasticity allows for a more specific and appropriate response from the innate immune system to a variety of infections, diseases, and injury. Similar to the macrophage literature, microglial responses to injury are now grossly classified into two types: M1 and M2. The M1 response is elicited by molecules such as bacterial lipopolysaccharide or the specific T-helper cell 1 cytokines interleukin (IL) 2 and interferon-γ to elicit high-level production of deleterious proinflammatory chemokines (e.g., CXCL8 and CXCL10) and cytokines (e.g., IL-1β, IL-12, and IL-6) and ROS. Such actions aid in amplifying the immune response to injury and are necessary for mounting a defense evolving from a host response to bacteria. On the other hand, the M2 response is elicited by T-helper cell cytokines such as IL-4 and IL-10 and corresponds to tissue recovery, repair, and growth. Over a period that is much debated, microglia transition from the M1 to the M2 response, and IL-4 is reported to play a major role in this transition (223). While the macrophage literature also includes subclassifications of M2, such as M2b (associated with B cell responses) and M2c (associated with macrophage deactivation), these subclassifications have yet to be fully investigated in the context of microglia phenotypes. Studies of the period during which microglia transition between polarities (M1 vs. M2) after stroke and a consensus regarding corresponding functions associated with beneficial or harmful processes to improve stroke outcomes are needed. This critical knowledge will aid in development of targeted therapeutics that optimize neuroinflammatory responses after stroke.
Both age and sex affect microglia M1 vs. M2 polarization. In aged (18-mo-old) mice, microglia appeared less ramified than in young (2-mo-old) mice and lipofuscin deposits within the cells and surrounding tissue were increased (181). Moreover, mRNA expression of the proinflammatory cytokines IL-1β, IL-6, and TNF-α, as well as transforming growth factor-β1 (considered anti-inflammatory), but not IL-10 or IL-12/p40, was increased in microglia from aged mice relative to young mice. Estrogen also modulates microglia-mediated neuroinflammatory proteins via ERs. ER subtypes are classified as intranuclear and membrane-associated. Estrogen binding to intranuclear ERs (e.g., ERα and ERβ) results in protein modulation at the transcriptional level, while binding to membrane-associated ERs, such as GPER-1, will immediately modulate cellular functions and signaling to include an attenuation of neuroinflammatory responses in models of CNS diseases and injury (130, 153, 160, 202). Although all ER subtypes are expressed in the brain, the level of expression of each receptor differs according to brain region (25, 80, 112). Primary microglia, in vitro, express both ERα and ERβ (169), and application of anti-ERα, -ERβ, and -GPER-1 agonists elicits potent anti-inflammatory and neuroprotective responses, whereas antagonists block these responses, in a wide array of brain injury models, including ischemic stroke (121, 169, 222). Progesterone receptor expression on microglia and the role of progesterone-mediated neuroinflammatory responses have been much less investigated.
Microglia phagocytosis, a specific aspect of neuroinflammation, has a strong role in both M1 and M2 phenotypes. However, distinguishing between beneficial and deleterious phagocytosis in vivo is challenging from a methodological standpoint. Phagocytosis is a temporal and step-wise signaling cascade (179) carefully orchestrated to result in the efficient and purposeful internalization and degradation of specific targets (15, 75, 137), which is an asset to the NVU. 1) A signal for phagocytosis is released by affected cells in health or disease situations. The “find-me” (179) signals, such as UTP, ATP, and fractalkine (58, 106, 107, 179), induce a strong and immediate morphological and functional response from adjacent microglia (45, 140). Aberrant find-me signaling results in excessive microglial responses and increased, rather than decreased, injury (122). In a culture model of cell injury, increased signaling via UTP/P2RY6 ligand-receptor interactions resulted in exuberant phagocytosis and ingestion of viable neurons (138). 2) Microglia upregulation of “eat-me” signals (i.e., CR3) mediates interactions with affected cells via microglia surface receptors (101, 179). 3) Microglia engulf debris, evidenced by ball-and-chain morphology and phagosome formation. 4) The internalized debris is degraded, and immune signaling, via cytokine and chemokine release, occurs to amplify phagocytosis. Microglia produce ROS (196) and MMPs during this process as a mechanism to degrade engulfed debris. Any imbalance (deficit or augmentation) of this process, such as aberrant find-me signaling, reduced eat-me signals, or poor recruitment signaling, reduces phagocytosis efficiency. Inefficient, dysfunctional, or excessive phagocytosis can result in engulfment of healthy cells (28), failure to clear or digest debris, and continued ROS and MMP production, for an overall increased neurotoxic milieu (9, 122), and increased injury. Such a scenario eliminates the benefits of phagocytosis (28, 122, 179). Few have addressed whether poststroke microglia phagocytosis differs with sex or sex hormone treatments. In 2016, we showed that the constitutive expression of the phagocytic receptor CR3 differs between males and females and was a first indication that phagocytosis signaling is different and, possibly, a more efficient process in females than males (133). While there is ample literature to describe microglia-related neuroinflammatory responses after stroke (10, 13, 55), considerably more research is necessary to investigate the specifics of microglia phagocytosis in health and disease (42, 44, 225) and the related signaling mechanisms (75, 138) that may differ according to sex and, in females, menopausal status.
ASTROCYTE AND MICROGLIA CROSS TALK
Ischemic injury brings about a profound inflammatory response, and we have described how each of these cells contributes independently to the poststroke inflammatory milieu. Given their robust responses to ischemic injury and close proximities to each other throughout the brain, modalities of astrocyte and microglia cross talk are an important consideration in stroke research and treatment development. For example, therapeutics to modulate microglial responses may affect astrocyte functions or responses. In the face of injury, glia communicate with each other via release of cytokines and small-molecule messengers to promote or propagate microglial or astrocytic actions. As demonstrated in an elegant series of experiments, S100β, a small-molecule messenger released by astrocytes in large quantities after stroke, binds to the receptor for advanced glycated end products and increases concentrations of IL-1β and TNF-α via both NF-κB and activator protein 1 modalities, increasing cyclooxygenase (COX)-2 expression by microglia (23, 24). In neurodegenerative diseases, astrocytes have a role in regulating phagocytosis by releasing factors such as complement 3 (116) and plasminogen activator type 1 (93), as well as monocyte chemoattractant protein-1 and IP-10 (193). In the other direction, from microglia to astrocytes, the combined release of TNF, IL-1α, and complement protein C1q by microglia strongly induces the astrocyte A1 phenotype described above (see astrocyte responses to ischemic injury and contributions to recovery) (117). However, these communication modalities need to be confirmed in the context of ischemic stroke.
HYPERTENSION: NEUROVASCULAR UNIT DYSFUNCTION AND RISK FACTOR FOR ISCHEMIC STROKE
Hypertension is the leading modifiable risk factor for ischemic stroke. The American Heart Association defines hypertension as a resting blood pressure that is consistently >120/80 mmHg. By most current estimates, hypertension occurs in ~45% of the population, or ~60% when considering the additional 16% that are undiagnosed and unaware of their hypertensive status (21). The brain’s energy metabolism and CBF are altered by chronic hypertension (64), and this is particularly the case if cerebrovascular perfusion is compromised. Unresolved hypertension results in extensive remodeling of peripheral vessels, along with increased cerebrovascular resistance, resulting in cerebral hypoperfusion (8, 89) and impaired neurovascular coupling (69). The interactive mechanisms driving hypertension-evoked structural and functional changes in the cerebral circulation are complex, poorly understood, and specific to vessel size, while also dependent on the stage and severity of hypertension. The temporal aspect of these injury mechanisms is also poorly understood, and it is possible that injury cascades are set in motion long before functional and structural changes can be observed. Therefore, hypertension can have profound effects on the functional and structural state of the NVU that are “silent” until pathology has progressed extensively. Considering the prevalence of hypertension, when an ischemic stroke happens, it likely does not occur in a “healthy” brain but, rather, in a brain that has been subjected to years of chronic hypertension and the pathobiology summarized here. Therefore, hypertension not only predisposes one to ischemic stroke, but when stroke does occur in a hypertensive individual, this event should be considered a second hit.
Evidence suggests that the combined effects of increases in shear stress, endothelial dysfunction (59, 82), and circulating factors, such as mineralocorticoids (52), angiotensin II (ANG II) (18, 52), and inflammatory signals, play a role in the vascular dysfunction and remodeling observed in hypertension. Chronic increases in intraluminal pressure give rise to a progressive increase in vessel wall thickening (wall hypertrophy) as a way to buffer pressure fluctuations and normalize vascular wall tension (136, 154). While, during physiological laminar flow conditions, vasculature exposure to mechanical forces (e.g., flow, shear stress, and pressure) is stable and protected by signals such as NO, increases in wall stiffening can result in turbulent flow, damage to the microcirculation, and increases in the incidence of stroke and cognitive impairment (12). To this end, hypertension has been shown to increase the production of flow-induced vasoconstricting signals such as 20-hydroxyeicosatetraenoic acid, reduce NO availability, and increase inflammatory signaling via NF-κB (6, 12, 195). Thus, changes in the vessel architecture may be considered a protective mechanism against hyperperfusion, with remodeling occurring in larger vessels first to protect downstream arterioles. However, extensive remodeling of the large intracranial vessels and smaller parenchymal arterioles will eventually limit CBF, for which autoregulation and neurovascular coupling are unable to compensate. Such delineations are important, considering the diversity of NVU structure and function according to vessel size (reviewed in Ref. 92). In addition to biomechanical changes to the NVU, ANG II, a key player in human hypertension, has been shown to cause deleterious changes in multiple cell types of the NVU. In hypothalamic 4B cells, ANG II increased TRPV4 channel trafficking to the membrane and intracellular Ca2+ (172). In parenchymal arteriole myocytes, circulating ANG II modulated TRPV4 channel activity via downstream activation of PKCα bound to A-kinase anchoring protein 150 (191). Activity of the TRPV4 channel was dependent on its distance from A-kinase anchoring protein 150-associated PKCα, an interaction that showed sex differences and, thus, may be hormonally regulated (191). In astrocytes, we showed that chronic ANG II infusion increased TRPV4 channel expression and enhanced TRPV4-mediated currents and astrocyte Ca2+ events (48). Moreover, we also demonstrated that chronic ANG II infusion increased astrogliosis and hippocampus microglia activation (48). These studies support the notion that, in hypertension, cells of the NVU may be subjected to alterations in intracellular Ca2+, likely increasing their vulnerability to ischemia and reducing their recovery capacity after stroke.
Ultimately, the structural and functional changes induced by chronic hypertension predispose an individual to ischemic stroke. Moreover, the hypertension-evoked remodeling of cerebral vessels leads to hypoperfusion and can exacerbate ischemia-driven pathways that disrupt BBB integrity and allow infiltration of inflammatory cells and cytokines into the brain (60, 163, 175). ANG II, a pressor peptide that is dysregulated and overabundant in several forms of hypertension, drives changes in cerebral vascular structure and function (18), including, among others, increased mitochondrial contributions to oxidative stress (53, 103). Evidence suggests that Toll-like receptor (TLR)-4 is a link between ANG II and vascular inflammation. In the aorta, TLR4 expression and cytokine and chemokine production were abolished with anti-TLR4 treatment in ANG II-treated animals (84). The possibility that TLR4 may be a direct link between ANG II and vascular and neural inflammation has been recently reviewed (22).
Hypertension prevalence, pathophysiology, and vascular outcomes are not the same between males and females. Similar to ischemic stroke, premenopausal women stave off the development of hypertension until menopause, at which point hypertension prevalence in women surpasses that in men (21, 118). Multiple mechanisms likely account for these observed sex differences. Much research supports the notion that estrogen plays a large role in modulating the effects of ANG II to increase blood pressure (70, 214) and regulate cerebral circulation (70), as well as to mitigate ANG II-mediated cerebral vascular dysfunction and vascular oxidative stress (32). ANG II stimulates intracellular Ca2+ stores to result in myocyte contractility, contributing to vascular tone. Ca2+ dysregulation occurs in conjunction with ANG II dysregulation, contributing to vascular dysfunction and remodeling (40). Sex differences in cytosolic Ca2+ concentrations, Ca2+ signaling, and Ca2+ mobilization from intracellular stores in rodent hypertension models (68) suggest an additional layer of protection in vascular responses to hypertension in females that is not present in males.
The balance of signals that promote vessel relaxation (e.g., NO) vs. constriction (e.g., COX-derived factors) also favors the female. Estrogen preserves NO production, limits shear stress, and maintains vasodilatory effects in female hypertensive rats; it also has restorative effects in male hypertensive rats (90, 91). By limiting circulating gonadal hormones via orchiectomy, mean arterial blood pressure and impaired NO-mediated endothelial function were reduced in Sprague-Dawley rats fed a high-salt diet, whereas testosterone replacement restored these dysfunctions (143). Vasoconstriction is increased, via the release of COX-derived elements, in hypertensive male vs. female rats (41, 97). In combination, these data suggest that the balance between vasodilation and constriction is favored in females over males. Such positive effects on the vasculature in the female may have a pivotal role in delaying the onset of endothelial dysfunction and vascular remodeling observed in female vs. male models of hypertension.
CONCLUSION
While the incidence of stroke has declined, the prevalence of cognitive impairments is on the rise, increasing the demand for knowledge of the events that lead to dysfunction of the NVU. While much knowledge has been gained about the cellular mechanism underlying NVU function before and after stroke, much more remains to be discovered. This is particularly the case in terms of an understanding of how modifiable and nonmodifiable stroke risk factors influence the risk for stroke, as well as the repair processes following an ischemic insult. The heterogeneous nature of ischemic stroke in humans signals that personalized treatments are necessary for improved outcomes. Without the underlying knowledge to guide personalization, such therapies will remain elusive. Expanding investigations beyond the male healthy rodent stroke model to understand NVU function and dysfunction will broaden our understanding of stroke pathophysiology and mechanisms of recovery that may be exploited for therapy development. Although challenging, such efforts will aid in addressing the gap in translating stroke bench science to bedside treatments.
GRANTS
This work was supported by National Institutes of Health Grants R01 HL-089067 and 1R01 NS-082521-01 (to J. A. Filosa).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
H.W.M. prepared figure; H.W.M. and J.A.F. drafted manuscript; H.W.M. and J.A.F. edited and revised manuscript; H.W.M. and J.A.F. approved final version of manuscript.
REFERENCES
- 1.Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 7: 41–53, 2006. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 2.Acaz-Fonseca E, Sanchez-Gonzalez R, Azcoitia I, Arevalo MA, Garcia-Segura LM. Role of astrocytes in the neuroprotective actions of 17β-estradiol and selective estrogen receptor modulators. Mol Cell Endocrinol 389: 48–57, 2014. doi: 10.1016/j.mce.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal A, Wu PH, Hughes EG, Fukaya M, Tischfield MA, Langseth AJ, Wirtz D, Bergles DE. Transient opening of the mitochondrial permeability transition pore induces microdomain calcium transients in astrocyte processes. Neuron 93: 587–605.e7, 2017. doi: 10.1016/j.neuron.2016.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahnstedt H, McCullough LD, Cipolla MJ. The importance of considering sex differences in translational stroke research. Transl Stroke Res 7: 261–273, 2016. doi: 10.1007/s12975-016-0450-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Bader MD, Malatiali SA, Redzic ZB. Expression of estrogen receptor α and β in rat astrocytes in primary culture: effects of hypoxia and glucose deprivation. Physiol Res 60: 951–960, 2011. [DOI] [PubMed] [Google Scholar]
- 6.Albarrán-Juárez J, Iring A, Wang S, Joseph S, Grimm M, Strilic B, Wettschureck N, Althoff TF, Offermanns S. Piezo1 and Gq/G11 promote endothelial inflammation depending on flow pattern and integrin activation. J Exp Med 215: 2655–2672, 2018. doi: 10.1084/jem.20180483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD. Gender-linked brain injury in experimental stroke. Stroke 29: 159–166, 1998. doi: 10.1161/01.STR.29.1.159. [DOI] [PubMed] [Google Scholar]
- 8.Alosco ML, Gunstad J, Xu X, Clark US, Labbe DR, Riskin-Jones HH, Terrero G, Schwarz NF, Walsh EG, Poppas A, Cohen RA, Sweet LH. The impact of hypertension on cerebral perfusion and cortical thickness in older adults. J Am Soc Hypertens 8: 561–570, 2014. doi: 10.1016/j.jash.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amantea D. Polarizing the immune system towards neuroprotection in brain ischemia. Neural Regen Res 11: 81–82, 2016. doi: 10.4103/1673-5374.169633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arevalo MA, Santos-Galindo M, Acaz-Fonseca E, Azcoitia I, Garcia-Segura LM. Gonadal hormones and the control of reactive gliosis. Horm Behav 63: 216–221, 2013. doi: 10.1016/j.yhbeh.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 11.Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature 468: 232–243, 2010. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Avolio A, Kim MO, Adji A, Gangoda S, Avadhanam B, Tan I, Butlin M. Cerebral haemodynamics: effects of systemic arterial pulsatile function and hypertension. Curr Hypertens Rep 20: 20, 2018. doi: 10.1007/s11906-018-0822-x. [DOI] [PubMed] [Google Scholar]
- 13.Banerjee A, Wang J, Bodhankar S, Vandenbark AA, Murphy SJ, Offner H. Phenotypic changes in immune cell subsets reflect increased infarct volume in male vs. female mice. Transl Stroke Res 4: 554–563, 2013. doi: 10.1007/s12975-013-0268-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banks E, Canfell K. Hormone therapy risks and benefits—the Women’s Health Initiative findings and the postmenopausal estrogen timing hypothesis. Am J Epidemiol 170: 24–28, 2009. doi: 10.1093/aje/kwp113. [DOI] [PubMed] [Google Scholar]
- 15.Barcia C, Ros CM, Annese V, Carrillo-de Sauvage MA, Ros-Bernal F, Gómez A, Yuste JE, Campuzano CM, de Pablos V, Fernandez-Villalba E, Herrero MT. ROCK/Cdc42-mediated microglial motility and gliapse formation lead to phagocytosis of degenerating dopaminergic neurons in vivo. Sci Rep 2: 809, 2012. doi: 10.1038/srep00809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barreto GE, Sun X, Xu L, Giffard RG. Astrocyte proliferation following stroke in the mouse depends on distance from the infarct. PLoS One 6: e27881, 2011. doi: 10.1371/journal.pone.0027881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barrett-Connor E. Hormones and heart disease in women: the timing hypothesis. Am J Epidemiol 166: 506–510, 2007. doi: 10.1093/aje/kwm214. [DOI] [PubMed] [Google Scholar]
- 18.Baumbach GL, Sigmund CD, Faraci FM. Cerebral arteriolar structure in mice overexpressing human renin and angiotensinogen. Hypertension 41: 50–55, 2003. doi: 10.1161/01.HYP.0000042427.05390.5C. [DOI] [PubMed] [Google Scholar]
- 19.Benfenati V, Amiry-Moghaddam M, Caprini M, Mylonakou MN, Rapisarda C, Ottersen OP, Ferroni S. Expression and functional characterization of transient receptor potential vanilloid-related channel 4 (TRPV4) in rat cortical astrocytes. Neuroscience 148: 876–892, 2007. doi: 10.1016/j.neuroscience.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 20.Benfenati V, Caprini M, Dovizio M, Mylonakou MN, Ferroni S, Ottersen OP, Amiry-Moghaddam M. An aquaporin-4/transient receptor potential vanilloid 4 (AQP4/TRPV4) complex is essential for cell-volume control in astrocytes. Proc Natl Acad Sci USA 108: 2563–2568, 2011. doi: 10.1073/pnas.1012867108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O’Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation 137: e67–e492, 2018. [Erratum in Circulation 137: e493, 2018.] 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 22.Biancardi VC, Bomfim GF, Reis WL, Al-Gassimi S, Nunes KP. The interplay between angiotensin II, TLR4 and hypertension. Pharmacol Res 120: 88–96, 2017. doi: 10.1016/j.phrs.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 23.Bianchi R, Adami C, Giambanco I, Donato R. S100B binding to RAGE in microglia stimulates COX-2 expression. J Leukoc Biol 81: 108–118, 2007. doi: 10.1189/jlb.0306198. [DOI] [PubMed] [Google Scholar]
- 24.Bianchi R, Giambanco I, Donato R. S100B/RAGE-dependent activation of microglia via NF-κB and AP-1 co-regulation of COX-2 expression by S100B, IL-1β and TNF-α. Neurobiol Aging 31: 665–677, 2010. doi: 10.1016/j.neurobiolaging.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 25.Brailoiu E, Dun SL, Brailoiu GC, Mizuo K, Sklar LA, Oprea TI, Prossnitz ER, Dun NJ. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J Endocrinol 193: 311–321, 2007. doi: 10.1677/JOE-07-0017. [DOI] [PubMed] [Google Scholar]
- 26.Brenner M. Role of GFAP in CNS injuries. Neurosci Lett 565: 7–13, 2014. doi: 10.1016/j.neulet.2014.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brooks HL, Pollow DP, Hoyer PB. The VCD mouse model of menopause and perimenopause for the study of sex differences in cardiovascular disease and the metabolic syndrome. Physiology (Bethesda) 31: 250–257, 2016. doi: 10.1152/physiol.00057.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown GC, Neher JJ. Eaten alive! Cell death by primary phagocytosis: “phagoptosis”. Trends Biochem Sci 37: 325–332, 2012. doi: 10.1016/j.tibs.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Burstein SR, Kim HJ, Fels JA, Qian L, Zhang S, Zhou P, Starkov AA, Iadecola C, Manfredi G. Estrogen receptor-β modulates permeability transition in brain mitochondria. Biochim Biophys Acta Bioenerg 1859: 423–433, 2018. doi: 10.1016/j.bbabio.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bushong EA, Martone ME, Jones YZ, Ellisman MH. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci 22: 183–192, 2002. doi: 10.1523/JNEUROSCI.22-01-00183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Butenko O, Dzamba D, Benesova J, Honsa P, Benfenati V, Rusnakova V, Ferroni S, Anderova M. The increased activity of TRPV4 channel in the astrocytes of the adult rat hippocampus after cerebral hypoxia/ischemia. PLoS One 7: e39959, 2012. doi: 10.1371/journal.pone.0039959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Capone C, Anrather J, Milner TA, Iadecola C. Estrous cycle-dependent neurovascular dysfunction induced by angiotensin II in the mouse neocortex. Hypertension 54: 302–307, 2009. doi: 10.1161/HYPERTENSIONAHA.109.133249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Charvériat M, Naus CC, Leybaert L, Sáez JC, Giaume C. Connexin-dependent neuroglial networking as a new therapeutic target. Front Cell Neurosci 11: 174, 2017. doi: 10.3389/fncel.2017.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chauhan A, Moser H, McCullough LD. Sex differences in ischaemic stroke: potential cellular mechanisms. Clin Sci (Lond) 131: 533–552, 2017. doi: 10.1042/CS20160841. [DOI] [PubMed] [Google Scholar]
- 35.Chever O, Dossi E, Pannasch U, Derangeon M, Rouach N. Astroglial networks promote neuronal coordination. Sci Signal 9: ra6, 2016. doi: 10.1126/scisignal.aad3066. [DOI] [PubMed] [Google Scholar]
- 36.Chisholm NC, Sohrabji F. Astrocytic response to cerebral ischemia is influenced by sex differences and impaired by aging. Neurobiol Dis 85: 245–253, 2016. doi: 10.1016/j.nbd.2015.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chung WS, Allen NJ, Eroglu C. Astrocytes control synapse formation, function, and elimination. Cold Spring Harb Perspect Biol 7: a020370, 2015. doi: 10.1101/cshperspect.a020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chung WS, Clarke LE, Wang GX, Stafford BK, Sher A, Chakraborty C, Joung J, Foo LC, Thompson A, Chen C, Smith SJ, Barres BA. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature 504: 394–400, 2013. doi: 10.1038/nature12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cipolla MJ. The Cerebral Circulation. San Rafael, CA: Morgan & Claypool Life Sciences, 2010. [PubMed] [Google Scholar]
- 40.Côrtes SF, Lemos VS, Stoclet JC. Alterations in calcium stores in aortic myocytes from spontaneously hypertensive rats. Hypertension 29: 1322–1328, 1997. doi: 10.1161/01.HYP.29.6.1322. [DOI] [PubMed] [Google Scholar]
- 41.Costa G, Garabito M, Jiménez-Altayó F, Onetti Y, Sabate M, Vila E, Dantas AP. Sex differences in angiotensin II responses contribute to a differential regulation of COX-mediated vascular dysfunction during aging. Exp Gerontol 85: 71–80, 2016. doi: 10.1016/j.exger.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 42.Cotrina ML, Lou N, Tome-Garcia J, Goldman J, Nedergaard M. Direct comparison of microglial dynamics and inflammatory profile in photothrombotic and arterial occlusion evoked stroke. Neuroscience 343: 483–494, 2017. doi: 10.1016/j.neuroscience.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cunningham CL, Martínez-Cerdeño V, Noctor SC. Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J Neurosci 33: 4216–4233, 2013. doi: 10.1523/JNEUROSCI.3441-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dang DD, Saiyin H, Yu Q, Liang WM. Effects of sevoflurane preconditioning on microglia/macrophage dynamics and phagocytosis profile against cerebral ischemia in rats. CNS Neurosci Ther 24: 564–571, 2018. doi: 10.1111/cns.12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci 8: 752–758, 2005. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 45a.de Castro Ribeiro M, Hirt L, Bogousslavsky J, Regli L, Badaut J. Time course of aquaporin expression after transient focal cerebral ischemia in mice. J Neurosci Res 83: 1231–1240, 2006. doi: 10.1002/jnr.20819. [DOI] [PubMed] [Google Scholar]
- 46.del Zoppo GJ, Sharp FR, Heiss WD, Albers GW. Heterogeneity in the penumbra. J Cereb Blood Flow Metab 31: 1836–1851, 2011. doi: 10.1038/jcbfm.2011.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dhandapani KM, Brann DW. Role of astrocytes in estrogen-mediated neuroprotection. Exp Gerontol 42: 70–75, 2007. doi: 10.1016/j.exger.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 48.Diaz JR, Kim KJ, Brands MW, Filosa JA. Augmented astrocyte microdomain Ca2+ dynamics and parenchymal arteriole tone in angiotensin II-infused hypertensive mice. Glia. In press. doi: 10.1002/glia.23564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ding S. Ca2+ signaling in astrocytes and its role in ischemic stroke. Adv Neurobiol 11: 189–211, 2014. doi: 10.1007/978-3-319-08894-5_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Domingues HS, Portugal CC, Socodato R, Relvas JB. Oligodendrocyte, astrocyte, and microglia crosstalk in myelin development, damage, and repair. Front Cell Dev Biol 4: 71, 2016. doi: 10.3389/fcell.2016.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Donato R, Sorci G, Riuzzi F, Arcuri C, Bianchi R, Brozzi F, Tubaro C, Giambanco I. S100B’s double life: intracellular regulator and extracellular signal. Biochim Biophys Acta 1793: 1008–1022, 2009. doi: 10.1016/j.bbamcr.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 52.Dorrance AM, Rupp NC, Nogueira EF. Mineralocorticoid receptor activation causes cerebral vessel remodeling and exacerbates the damage caused by cerebral ischemia. Hypertension 47: 590–595, 2006. doi: 10.1161/01.HYP.0000196945.73586.0d. [DOI] [PubMed] [Google Scholar]
- 53.Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res 102: 488–496, 2008. doi: 10.1161/CIRCRESAHA.107.162800. [DOI] [PubMed] [Google Scholar]
- 54.Doyle KP, Quach LN, Solé M, Axtell RC, Nguyen TV, Soler-Llavina GJ, Jurado S, Han J, Steinman L, Longo FM, Schneider JA, Malenka RC, Buckwalter MS. B-lymphocyte-mediated delayed cognitive impairment following stroke. J Neurosci 35: 2133–2145, 2015. doi: 10.1523/JNEUROSCI.4098-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Drew PD, Chavis JA. Female sex steroids: effects upon microglial cell activation. J Neuroimmunol 111: 77–85, 2000. doi: 10.1016/S0165-5728(00)00386-6. [DOI] [PubMed] [Google Scholar]
- 56.Duclot F, Kabbaj M. The estrous cycle surpasses sex differences in regulating the transcriptome in the rat medial prefrontal cortex and reveals an underlying role of early growth response 1. Genome Biol 16: 256, 2015. doi: 10.1186/s13059-015-0815-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Earley S, Pauyo T, Drapp R, Tavares MJ, Liedtke W, Brayden JE. TRPV4-dependent dilation of peripheral resistance arteries influences arterial pressure. Am J Physiol Heart Circ Physiol 297: H1096–H1102, 2009. doi: 10.1152/ajpheart.00241.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P, Lysiak JJ, Harden TK, Leitinger N, Ravichandran KS. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 461: 282–286, 2009. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Faraci FM, Baumbach GL, Heistad DD. Cerebral circulation: humoral regulation and effects of chronic hypertension. J Am Soc Nephrol 1: 53–57, 1990. [DOI] [PubMed] [Google Scholar]
- 60.Faraco G, Sugiyama Y, Lane D, Garcia-Bonilla L, Chang H, Santisteban MM, Racchumi G, Murphy M, Van Rooijen N, Anrather J, Iadecola C. Perivascular macrophages mediate the neurovascular and cognitive dysfunction associated with hypertension. J Clin Invest 126: 4674–4689, 2016. doi: 10.1172/JCI86950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Filosa JA, Morrison HW, Iddings JA, Du W, Kim KJ. Beyond neurovascular coupling, role of astrocytes in the regulation of vascular tone. Neuroscience 323: 96–109, 2016. doi: 10.1016/j.neuroscience.2015.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fontainhas AM, Wang M, Liang KJ, Chen S, Mettu P, Damani M, Fariss RN, Li W, Wong WT. Microglial morphology and dynamic behavior is regulated by ionotropic glutamatergic and GABAergic neurotransmission. PLoS One 6: e15973, 2011. doi: 10.1371/journal.pone.0015973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fordsmann JC, Murmu RP, Cai C, Brazhe A, Thomsen KJ, Zambach SA, Lonstrup M, Lind BL, Lauritzen M. Spontaneous astrocytic Ca2+ activity abounds in electrically suppressed ischemic penumbra of aged mice. Glia 67: 37–52, 2019. doi: 10.1002/glia.23506. [DOI] [PubMed] [Google Scholar]
- 64.Fujishima M, Ibayashi S, Fujii K, Mori S. Cerebral blood flow and brain function in hypertension. Hypertens Res 18: 111–117, 1995. doi: 10.1291/hypres.18.111. [DOI] [PubMed] [Google Scholar]
- 65.Gaignard P, Fréchou M, Liere P, Thérond P, Schumacher M, Slama A, Guennoun R. Sex differences in brain mitochondrial metabolism: influence of endogenous steroids and stroke. J Neuroendocrinol 30: e12497, 2018. doi: 10.1111/jne.12497. [DOI] [PubMed] [Google Scholar]
- 66.Gaignard P, Savouroux S, Liere P, Pianos A, Thérond P, Schumacher M, Slama A, Guennoun R. Effect of sex differences on brain mitochondrial function and its suppression by ovariectomy and in aged mice. Endocrinology 156: 2893–2904, 2015. doi: 10.1210/en.2014-1913. [DOI] [PubMed] [Google Scholar]
- 67.García-Ovejero D, Veiga S, García-Segura LM, Doncarlos LL. Glial expression of estrogen and androgen receptors after rat brain injury. J Comp Neurol 450: 256–271, 2002. doi: 10.1002/cne.10325. [DOI] [PubMed] [Google Scholar]
- 68.Giachini FR, Lima VV, Filgueira FP, Dorrance AM, Carvalho MH, Fortes ZB, Webb RC, Tostes RC. STIM1/Orai1 contributes to sex differences in vascular responses to calcium in spontaneously hypertensive rats. Clin Sci (Lond) 122: 215–226, 2012. doi: 10.1042/CS20110312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol (1985) 100: 328–335, 2006. doi: 10.1152/japplphysiol.00966.2005. [DOI] [PubMed] [Google Scholar]
- 70.Girouard H, Lessard A, Capone C, Milner TA, Iadecola C. The neurovascular dysfunction induced by angiotensin II in the mouse neocortex is sexually dimorphic. Am J Physiol Heart Circ Physiol 294: H156–H163, 2008. doi: 10.1152/ajpheart.01137.2007. [DOI] [PubMed] [Google Scholar]
- 71.Gosselin D, Skola D, Coufal NG, Holtman IR, Schlachetzki JCM, Sajti E, Jaeger BN, O’Connor C, Fitzpatrick C, Pasillas MP, Pena M, Adair A, Gonda DD, Levy ML, Ransohoff RM, Gage FH, Glass CK. An environment-dependent transcriptional network specifies human microglia identity. Science 356: eaal322, 2017. doi: 10.1126/science.aal3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci 10: 1286–1291, 1990. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gürer G, Gursoy-Ozdemir Y, Erdemli E, Can A, Dalkara T. Astrocytes are more resistant to focal cerebral ischemia than neurons and die by a delayed necrosis. Brain Pathol 19: 630–641, 2009. doi: 10.1111/j.1750-3639.2008.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Haas JR, Christian PJ, Hoyer PB. Effects of impending ovarian failure induced by 4-vinylcyclohexene diepoxide on fertility in C57BL/6 female mice. Comp Med 57: 443–449, 2007. [PubMed] [Google Scholar]
- 75.Hadas S, Spira M, Hanisch UK, Reichert F, Rotshenker S. Complement receptor-3 negatively regulates the phagocytosis of degenerated myelin through tyrosine kinase Syk and cofilin. J Neuroinflammation 9: 166, 2012. doi: 10.1186/1742-2094-9-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Halassa MM, Fellin T, Takano H, Dong JH, Haydon PG. Synaptic islands defined by the territory of a single astrocyte. J Neurosci 27: 6473–6477, 2007. doi: 10.1523/JNEUROSCI.1419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Harraz OF, Longden TA, Dabertrand F, Hill-Eubanks D, Nelson MT. Endothelial GqPCR activity controls capillary electrical signaling and brain blood flow through PIP2 depletion. Proc Natl Acad Sci USA 115: E3569–E3577, 2018. doi: 10.1073/pnas.1800201115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Harraz OF, Longden TA, Hill-Eubanks D, Nelson MT. PIP2 depletion promotes TRPV4 channel activity in mouse brain capillary endothelial cells. eLife 7: e38689, 2018. doi: 10.7554/eLife.38689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hayakawa K, Esposito E, Wang X, Terasaki Y, Liu Y, Xing C, Ji X, Lo EH. Transfer of mitochondria from astrocytes to neurons after stroke. Nature 535: 551–555, 2016. [Erratum in Nature 539: 123, 2016. 10.1038/nature19805. 27629516.] 10.1038/nature18928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hazell GG, Yao ST, Roper JA, Prossnitz ER, O’Carroll AM, Lolait SJ. Localisation of GPR30, a novel G protein-coupled oestrogen receptor, suggests multiple functions in rodent brain and peripheral tissues. J Endocrinol 202: 223–236, 2009. doi: 10.1677/JOE-09-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Heiss WD, Graf R. The ischemic penumbra. Curr Opin Neurol 7: 11–19, 1994. doi: 10.1097/00019052-199402000-00004. [DOI] [PubMed] [Google Scholar]
- 82.Heistad DD, Baumbach GL. Cerebral vascular changes during chronic hypertension: good guys and bad guys. J Hypertens Suppl 10, Suppl 7: S71–S75, 1992. doi: 10.1097/00004872-199212000-00008. [DOI] [PubMed] [Google Scholar]
- 83.Hendrix SL, Wassertheil-Smoller S, Johnson KC, Howard BV, Kooperberg C, Rossouw JE, Trevisan M, Aragaki A, Baird AE, Bray PF, Buring JE, Criqui MH, Herrington D, Lynch JK, Rapp SR, Torner J; WHI Investigators . Effects of conjugated equine estrogen on stroke in the Women’s Health Initiative. Circulation 113: 2425–2434, 2006. doi: 10.1161/CIRCULATIONAHA.105.594077. [DOI] [PubMed] [Google Scholar]
- 84.Hernanz R, Martínez-Revelles S, Palacios R, Martín A, Cachofeiro V, Aguado A, García-Redondo L, Barrús MT, de Batista PR, Briones AM, Salaices M, Alonso MJ. Toll-like receptor 4 contributes to vascular remodelling and endothelial dysfunction in angiotensin II-induced hypertension. Br J Pharmacol 172: 3159–3176, 2015. doi: 10.1111/bph.13117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hill-Eubanks DC, Gonzales AL, Sonkusare SK, Nelson MT. Vascular TRP channels: performing under pressure and going with the flow. Physiology (Bethesda) 29: 343–360, 2014. doi: 10.1152/physiol.00009.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hinzman JM, DiNapoli VA, Mahoney EJ, Gerhardt GA, Hartings JA. Spreading depolarizations mediate excitotoxicity in the development of acute cortical lesions. Exp Neurol 267: 243–253, 2015. doi: 10.1016/j.expneurol.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 87.Hirt L, Fukuda AM, Ambadipudi K, Rashid F, Binder D, Verkman A, Ashwal S, Obenaus A, Badaut J. Improved long-term outcome after transient cerebral ischemia in aquaporin-4 knockout mice. J Cereb Blood Flow Metab 37: 277–290, 2017. doi: 10.1177/0271678X15623290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hoshi Y, Okabe K, Shibasaki K, Funatsu T, Matsuki N, Ikegaya Y, Koyama R. Ischemic brain injury leads to brain edema via hyperthermia-induced TRPV4 activation. J Neurosci 38: 5700–5709, 2018. doi: 10.1523/JNEUROSCI.2888-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hu X, De Silva TM, Chen J, Faraci FM. Cerebral vascular disease and neurovascular injury in ischemic stroke. Circ Res 120: 449–471, 2017. doi: 10.1161/CIRCRESAHA.116.308427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huang A, Sun D, Kaley G, Koller A. Estrogen preserves regulation of shear stress by nitric oxide in arterioles of female hypertensive rats. Hypertension 31: 309–314, 1998. doi: 10.1161/01.HYP.31.1.309. [DOI] [PubMed] [Google Scholar]
- 91.Huang A, Sun D, Koller A, Kaley G. 17β-Estradiol restores endothelial nitric oxide release to shear stress in arterioles of male hypertensive rats. Circulation 101: 94–100, 2000. doi: 10.1161/01.CIR.101.1.94. [DOI] [PubMed] [Google Scholar]
- 92.Iadecola C. The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron 96: 17–42, 2017. doi: 10.1016/j.neuron.2017.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jeon H, Kim JH, Kim JH, Lee WH, Lee MS, Suk K. Plasminogen activator inhibitor type 1 regulates microglial motility and phagocytic activity. J Neuroinflammation 9: 149, 2012. doi: 10.1186/1742-2094-9-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jessen NA, Munk AS, Lundgaard I, Nedergaard M. The glymphatic system: a beginner’s guide. Neurochem Res 40: 2583–2599, 2015. doi: 10.1007/s11064-015-1581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ji K, Akgul G, Wollmuth LP, Tsirka SE. Microglia actively regulate the number of functional synapses. PLoS One 8: e56293, 2013. doi: 10.1371/journal.pone.0056293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Johnson AB, Sohrabji F. Estrogen’s effects on central and circulating immune cells vary with reproductive age. Neurobiol Aging 26: 1365–1374, 2005. doi: 10.1016/j.neurobiolaging.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 97.Kähönen M, Tolvanen JP, Sallinen K, Wu X, Pörsti I. Influence of gender on control of arterial tone in experimental hypertension. Am J Physiol Heart Circ Physiol 275: H15–H22, 1998. [DOI] [PubMed] [Google Scholar]
- 98.Kanju P, Liedtke W. Pleiotropic function of TRPV4 ion channels in the central nervous system. Exp Physiol 101: 1472–1476, 2016. doi: 10.1113/EP085790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Karki P, Smith K, Johnson J Jr, Lee E. Astrocyte-derived growth factors and estrogen neuroprotection: role of transforming growth factor-α in estrogen-induced upregulation of glutamate transporters in astrocytes. Mol Cell Endocrinol 389: 58–64, 2014. doi: 10.1016/j.mce.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kelly MJ, Rønnekleiv OK. Control of CNS neuronal excitability by estrogens via membrane-initiated signaling. Mol Cell Endocrinol 308: 17–25, 2009. doi: 10.1016/j.mce.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev 91: 461–553, 2011. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- 102.Kim KJ, Iddings JA, Stern JE, Blanco VM, Croom D, Kirov SA, Filosa JA. Astrocyte contributions to flow/pressure-evoked parenchymal arteriole vasoconstriction. J Neurosci 35: 8245–8257, 2015. doi: 10.1523/JNEUROSCI.4486-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kimura S, Zhang GX, Nishiyama A, Shokoji T, Yao L, Fan YY, Rahman M, Abe Y. Mitochondria-derived reactive oxygen species and vascular MAP kinases: comparison of angiotensin II and diazoxide. Hypertension 45: 438–444, 2005. doi: 10.1161/01.HYP.0000157169.27818.ae. [DOI] [PubMed] [Google Scholar]
- 104.Kissela BM, Khoury JC, Alwell K, Moomaw CJ, Woo D, Adeoye O, Flaherty ML, Khatri P, Ferioli S, De Los Rios La Rosa F, Broderick JP, Kleindorfer DO. Age at stroke: temporal trends in stroke incidence in a large, biracial population. Neurology 79: 1781–1787, 2012. doi: 10.1212/WNL.0b013e318270401d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Koellhoffer EC, McCullough LD. The effects of estrogen in ischemic stroke. Transl Stroke Res 4: 390–401, 2013. doi: 10.1007/s12975-012-0230-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Koizumi S, Ohsawa K, Inoue K, Kohsaka S. Purinergic receptors in microglia: functional modal shifts of microglia mediated by P2 and P1 receptors. Glia 61: 47–54, 2013. doi: 10.1002/glia.22358. [DOI] [PubMed] [Google Scholar]
- 107.Koizumi S, Shigemoto-Mogami Y, Nasu-Tada K, Shinozaki Y, Ohsawa K, Tsuda M, Joshi BV, Jacobson KA, Kohsaka S, Inoue K. UDP acting at P2Y6 receptors is a mediator of microglial phagocytosis. Nature 446: 1091–1095, 2007. doi: 10.1038/nature05704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kokaji AI, Hockley DL, Kane KP. IL-15 transpresentation augments CD8+ T cell activation and is required for optimal recall responses by central memory CD8+ T cells. J Immunol 180: 4391–4401, 2008. doi: 10.4049/jimmunol.180.7.4391. [DOI] [PubMed] [Google Scholar]
- 109.Kostandy BB. The role of glutamate in neuronal ischemic injury: the role of spark in fire. Neurol Sci 33: 223–237, 2012. doi: 10.1007/s10072-011-0828-5. [DOI] [PubMed] [Google Scholar]
- 110.Križaj D, Ryskamp DA, Tian N, Tezel G, Mitchell CH, Slepak VZ, Shestopalov VI. From mechanosensitivity to inflammatory responses: new players in the pathology of glaucoma. Curr Eye Res 39: 105–119, 2014. doi: 10.3109/02713683.2013.836541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kuchibhotla KV, Lattarulo CR, Hyman BT, Bacskai BJ. Synchronous hyperactivity and intercellular calcium waves in astrocytes in Alzheimer mice. Science 323: 1211–1215, 2009. doi: 10.1126/science.1169096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kuiper GG, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors α and β. Endocrinology 138: 863–870, 1997. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 113.Kuo J, Hamid N, Bondar G, Prossnitz ER, Micevych P. Membrane estrogen receptors stimulate intracellular calcium release and progesterone synthesis in hypothalamic astrocytes. J Neurosci 30: 12950–12957, 2010. doi: 10.1523/JNEUROSCI.1158-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lenz KM, Nugent BM, Haliyur R, McCarthy MM. Microglia are essential to masculinization of brain and behavior. J Neurosci 33: 2761–2772, 2013. doi: 10.1523/JNEUROSCI.1268-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li M, Li Z, Yao Y, Jin WN, Wood K, Liu Q, Shi FD, Hao J. Astrocyte-derived interleukin-15 exacerbates ischemic brain injury via propagation of cellular immunity. Proc Natl Acad Sci USA 114: E396–E405, 2017. doi: 10.1073/pnas.1612930114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lian H, Litvinchuk A, Chiang AC, Aithmitti N, Jankowsky JL, Zheng H. Astrocyte-microglia cross talk through complement activation modulates amyloid pathology in mouse models of Alzheimer’s disease. J Neurosci 36: 577–589, 2016. doi: 10.1523/JNEUROSCI.2117-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Münch AE, Chung WS, Peterson TC, Wilton DK, Frouin A, Napier BA, Panicker N, Kumar M, Buckwalter MS, Rowitch DH, Dawson VL, Dawson TM, Stevens B, Barres BA. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541: 481–487, 2017. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lima R, Wofford M, Reckelhoff JF. Hypertension in postmenopausal women. Curr Hypertens Rep 14: 254–260, 2012. doi: 10.1007/s11906-012-0260-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lipski J, Park TI, Li D, Lee SC, Trevarton AJ, Chung KK, Freestone PS, Bai JZ. Involvement of TRP-like channels in the acute ischemic response of hippocampal CA1 neurons in brain slices. Brain Res 1077: 187–199, 2006. doi: 10.1016/j.brainres.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 120.Liu R, Wang X, Liu Q, Yang SH, Simpkins JW. Dose dependence and therapeutic window for the neuroprotective effects of 17β-estradiol when administered after cerebral ischemia. Neurosci Lett 415: 237–241, 2007. doi: 10.1016/j.neulet.2007.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu X, Fan XL, Zhao Y, Luo GR, Li XP, Li R, Le WD. Estrogen provides neuroprotection against activated microglia-induced dopaminergic neuronal injury through both estrogen receptor-α and estrogen receptor-β in microglia. J Neurosci Res 81: 653–665, 2005. doi: 10.1002/jnr.20583. [DOI] [PubMed] [Google Scholar]
- 122.Loane DJ, Kumar A. Microglia in the TBI brain: the good, the bad, and the dysregulated. Exp Neurol 275: 316–327, 2016. doi: 10.1016/j.expneurol.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lochhead JJ, Ronaldson PT, Davis TP. Hypoxic stress and inflammatory pain disrupt blood-brain barrier tight junctions: implications for drug delivery to the central nervous system. AAPS J 19: 910–920, 2017. doi: 10.1208/s12248-017-0076-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Longden TA, Dabertrand F, Koide M, Gonzales AL, Tykocki NR, Brayden JE, Hill-Eubanks D, Nelson MT. Capillary K+-sensing initiates retrograde hyperpolarization to increase local cerebral blood flow. Nat Neurosci 20: 717–726, 2017. doi: 10.1038/nn.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Magistretti PJ, Allaman I. A cellular perspective on brain energy metabolism and functional imaging. Neuron 86: 883–901, 2015. doi: 10.1016/j.neuron.2015.03.035. [DOI] [PubMed] [Google Scholar]
- 126.Malyala A, Kelly MJ, Rønnekleiv OK. Estrogen modulation of hypothalamic neurons: activation of multiple signaling pathways and gene expression changes. Steroids 70: 397–406, 2005. doi: 10.1016/j.steroids.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 127.Manwani B, Bentivegna K, Benashski SE, Venna VR, Xu Y, Arnold AP, McCullough LD. Sex differences in ischemic stroke sensitivity are influenced by gonadal hormones, not by sex chromosome complement. J Cereb Blood Flow Metab 35: 221–229, 2015. doi: 10.1038/jcbfm.2014.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Marina N, Turovsky E, Christie IN, Hosford PS, Hadjihambi A, Korsak A, Ang R, Mastitskaya S, Sheikhbahaei S, Theparambil SM, Gourine AV. Brain metabolic sensing and metabolic signaling at the level of an astrocyte. Glia 66: 1185–1199, 2018. doi: 10.1002/glia.23283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.McCullough LD, Alkayed NJ, Traystman RJ, Williams MJ, Hurn PD. Postischemic estrogen reduces hypoperfusion and secondary ischemia after experimental stroke. Stroke 32: 796–802, 2001. doi: 10.1161/01.STR.32.3.796. [DOI] [PubMed] [Google Scholar]
- 130.Merchenthaler I, Lane MV, Numan S, Dellovade TL. Distribution of estrogen receptor-α and -β in the mouse central nervous system: in vivo autoradiographic and immunocytochemical analyses. J Comp Neurol 473: 270–291, 2004. doi: 10.1002/cne.20128. [DOI] [PubMed] [Google Scholar]
- 131.Mestre H, Kostrikov S, Mehta RI, Nedergaard M. Perivascular spaces, glymphatic dysfunction, and small vessel disease. Clin Sci (Lond) 131: 2257–2274, 2017. doi: 10.1042/CS20160381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mohr JP, Albers GW, Amarenco P, Babikian VL, Biller J, Brey RL, Coull B, Easton JD, Gomez CR, Helgason CM, Kase CS, Pullicino PM, Turpie AG. American Heart Association Prevention Conference. IV. Prevention and rehabilitation of stroke. etiology of stroke. Stroke 28: 1501–1506, 1997. [DOI] [PubMed] [Google Scholar]
- 133.Morrison HW, Filosa JA. Sex differences in astrocyte and microglia responses immediately following middle cerebral artery occlusion in adult mice. Neuroscience 339: 85–99, 2016. doi: 10.1016/j.neuroscience.2016.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB; Writing Group Members; American Heart Association Statistics Committee; Stroke Statistics Subcommittee . Executive summary: heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation 133: 447–454, 2016. doi: 10.1161/CIR.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 135.Mrak RE, Griffin ST, Graham DI. Aging-associated changes in human brain. J Neuropathol Exp Neurol 56: 1269–1275, 1997. doi: 10.1097/00005072-199712000-00001. [DOI] [PubMed] [Google Scholar]
- 136.Mulvany MJ, Baumbach GL, Aalkjaer C, Heagerty AM, Korsgaard N, Schiffrin EL, Heistad DD. Vascular remodeling. Hypertension 28: 505–506, 1996. [PubMed] [Google Scholar]
- 137.Murphy DA, Courtneidge SA. The “ins” and “outs” of podosomes and invadopodia: characteristics, formation and function. Nat Rev Mol Cell Biol 12: 413–426, 2011. doi: 10.1038/nrm3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Neher JJ, Neniskyte U, Hornik T, Brown GC. Inhibition of UDP/P2Y6 purinergic signaling prevents phagocytosis of viable neurons by activated microglia in vitro and in vivo. Glia 62: 1463–1475, 2014. doi: 10.1002/glia.22693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nguyen TV, Frye JB, Zbesko JC, Stepanovic K, Hayes M, Urzua A, Serrano G, Beach TG, Doyle KP. Multiplex immunoassay characterization and species comparison of inflammation in acute and non-acute ischemic infarcts in human and mouse brain tissue. Acta Neuropathol Commun 4: 100, 2016. [Erratum in Acta Neuropathol Commun 4: 104, 2016.] 10.1186/s40478-016-0371-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308: 1314–1318, 2005. doi: 10.1126/science.1110647. . [DOI] [PubMed] [Google Scholar]
- 141.O’Donnell ME, Lam TI, Tran LQ, Foroutan S, Anderson SE. Estradiol reduces activity of the blood-brain barrier Na-K-Cl cotransporter and decreases edema formation in permanent middle cerebral artery occlusion. J Cereb Blood Flow Metab 26: 1234–1249, 2006. doi: 10.1038/sj.jcbfm.9600278. [DOI] [PubMed] [Google Scholar]
- 142.Okoreeh AK, Bake S, Sohrabji F. Astrocyte-specific insulin-like growth factor-1 gene transfer in aging female rats improves stroke outcomes. Glia 65: 1043–1058, 2017. doi: 10.1002/glia.23142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Oloyo AK, Sofola OA, Anigbogu CN. Orchidectomy attenuates impaired endothelial effects of a high-salt diet in Sprague-Dawley rats. Can J Physiol Pharmacol 89: 295–304, 2011. doi: 10.1139/y11-023. [DOI] [PubMed] [Google Scholar]
- 144.Papadopoulos MC, Verkman AS. Aquaporin-4 and brain edema. Pediatr Nephrol 22: 778–784, 2007. doi: 10.1007/s00467-006-0411-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Peferoen L, Kipp M, van der Valk P, van Noort JM, Amor S. Oligodendrocyte-microglia cross-talk in the central nervous system. Immunology 141: 302–313, 2014. doi: 10.1111/imm.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia 50: 427–434, 2005. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- 147.Pekny M, Pekna M, Messing A, Steinhäuser C, Lee JM, Parpura V, Hol EM, Sofroniew MV, Verkhratsky A. Astrocytes: a central element in neurological diseases. Acta Neuropathol 131: 323–345, 2016. doi: 10.1007/s00401-015-1513-1. [DOI] [PubMed] [Google Scholar]
- 148.Perez-Alvarez A, Navarrete M, Covelo A, Martin ED, Araque A. Structural and functional plasticity of astrocyte processes and dendritic spine interactions. J Neurosci 34: 12738–12744, 2014. doi: 10.1523/JNEUROSCI.2401-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Perry VH, Matyszak MK, Fearn S. Altered antigen expression of microglia in the aged rodent CNS. Glia 7: 60–67, 1993. doi: 10.1002/glia.440070111. [DOI] [PubMed] [Google Scholar]
- 150.Plant TD, Strotmann R. Trpv4. Handb Exp Pharmacol 179: 189–205, 2007. doi: 10.1007/978-3-540-34891-7_11. [DOI] [PubMed] [Google Scholar]
- 151.Popa-Wagner A, Badan I, Walker L, Groppa S, Patrana N, Kessler C. Accelerated infarct development, cytogenesis and apoptosis following transient cerebral ischemia in aged rats. Acta Neuropathol 113: 277–293, 2007. doi: 10.1007/s00401-006-0164-7. [DOI] [PubMed] [Google Scholar]
- 152.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL; American Heart Association Stroke Council . 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 49: e46–e110, 2018. [Erratum in Stroke 49: e138, 2018. 10.1161/STR.0000000000000163. 29483362] 10.1161/STR.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 153.Pozzi S, Benedusi V, Maggi A, Vegeto E. Estrogen action in neuroprotection and brain inflammation. Ann NY Acad Sci 1089: 302–323, 2006. doi: 10.1196/annals.1386.035. [DOI] [PubMed] [Google Scholar]
- 154.Prado CM, Ramos SG, Alves-Filho JC, Elias J Jr, Cunha FQ, Rossi MA. Turbulent flow/low wall shear stress and stretch differentially affect aorta remodeling in rats. J Hypertens 24: 503–515, 2006. doi: 10.1097/01.hjh.0000209987.51606.23. [DOI] [PubMed] [Google Scholar]
- 155.Prentice RL, Manson JE, Langer RD, Anderson GL, Pettinger M, Jackson RD, Johnson KC, Kuller LH, Lane DS, Wactawski-Wende J, Brzyski R, Allison M, Ockene J, Sarto G, Rossouw JE. Benefits and risks of postmenopausal hormone therapy when it is initiated soon after menopause. Am J Epidemiol 170: 12–23, 2009. doi: 10.1093/aje/kwp115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rakers C, Petzold GC. Astrocytic calcium release mediates peri-infarct depolarizations in a rodent stroke model. J Clin Invest 127: 511–516, 2017. doi: 10.1172/JCI89354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rakers C, Schmid M, Petzold GC. TRPV4 channels contribute to calcium transients in astrocytes and neurons during peri-infarct depolarizations in a stroke model. Glia 65: 1550–1561, 2017. doi: 10.1002/glia.23183. [DOI] [PubMed] [Google Scholar]
- 158.Ramesh G, Benge S, Pahar B, Philipp MT. A possible role for inflammation in mediating apoptosis of oligodendrocytes as induced by the Lyme disease spirochete Borrelia burgdorferi. J Neuroinflammation 9: 72, 2012. doi: 10.1186/1742-2094-9-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ren C, Kobeissy F, Alawieh A, Li N, Li N, Zibara K, Zoltewicz S, Guingab-Cagmat J, Larner SF, Ding Y, Hayes RL, Ji X, Mondello S. Assessment of serum UCH-L1 and GFAP in acute stroke patients. Sci Rep 6: 24588, 2016. doi: 10.1038/srep24588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 307: 1625–1630, 2005. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 162.Ritzel RM, Capozzi LA, McCullough LD. Sex, stroke, and inflammation: the potential for estrogen-mediated immunoprotection in stroke. Horm Behav 63: 238–253, 2013. doi: 10.1016/j.yhbeh.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rosenberg GA. Extracellular matrix inflammation in vascular cognitive impairment and dementia. Clin Sci (Lond) 131: 425–437, 2017. doi: 10.1042/CS20160604. [DOI] [PubMed] [Google Scholar]
- 164.Rosenberg PA, Aizenman E. Hundred-fold increase in neuronal vulnerability to glutamate toxicity in astrocyte-poor cultures of rat cerebral cortex. Neurosci Lett 103: 162–168, 1989. doi: 10.1016/0304-3940(89)90569-7. [DOI] [PubMed] [Google Scholar]
- 165.Rossi DJ, Brady JD, Mohr C. Astrocyte metabolism and signaling during brain ischemia. Nat Neurosci 10: 1377–1386, 2007. doi: 10.1038/nn2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron 16: 675–686, 1996. doi: 10.1016/S0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 167.Rutkowsky JM, Wallace BK, Wise PM, O’Donnell ME. Effects of estradiol on ischemic factor-induced astrocyte swelling and AQP4 protein abundance. Am J Physiol Cell Physiol 301: C204–C212, 2011. doi: 10.1152/ajpcell.00399.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ryskamp DA, Iuso A, Križaj D. TRPV4 links inflammatory signaling and neuroglial swelling. Channels (Austin) 9: 70–72, 2015. doi: 10.1080/19336950.2015.1017998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Saijo K, Collier JG, Li AC, Katzenellenbogen JA, Glass CK. An ADIOL-ERβ-CtBP transrepression pathway negatively regulates microglia-mediated inflammation. Cell 145: 584–595, 2011. doi: 10.1016/j.cell.2011.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Saijo K, Glass CK. Microglial cell origin and phenotypes in health and disease. Nat Rev Immunol 11: 775–787, 2011. doi: 10.1038/nri3086. [DOI] [PubMed] [Google Scholar]
- 171.Saver JL. Time is brain—quantified. Stroke 37: 263–266, 2006. doi: 10.1161/01.STR.0000196957.55928.ab. [DOI] [PubMed] [Google Scholar]
- 172.Saxena A, Bachelor M, Park YH, Carreno FR, Nedungadi TP, Cunningham JT. Angiotensin II induces membrane trafficking of natively expressed transient receptor potential vanilloid type 4 channels in hypothalamic 4B cells. Am J Physiol Regul Integr Comp Physiol 307: R945–R955, 2014. doi: 10.1152/ajpregu.00224.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sellers K, Raval P, Srivastava DP. Molecular signature of rapid estrogen regulation of synaptic connectivity and cognition. Front Neuroendocrinol 36: 72–89, 2015. doi: 10.1016/j.yfrne.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 174.Selvamani A, Sohrabji F. The neurotoxic effects of estrogen on ischemic stroke in older female rats is associated with age-dependent loss of insulin-like growth factor-1. J Neurosci 30: 6852–6861, 2010. doi: 10.1523/JNEUROSCI.0761-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Setiadi A, Korim WS, Elsaafien K, Yao ST. The role of the blood-brain barrier in hypertension. Exp Physiol 103: 337–342, 2018. doi: 10.1113/EP086434. [DOI] [PubMed] [Google Scholar]
- 176.Shibasaki K, Ikenaka K, Tamalu F, Tominaga M, Ishizaki Y. A novel subtype of astrocytes expressing TRPV4 (transient receptor potential vanilloid 4) regulates neuronal excitability via release of gliotransmitters. J Biol Chem 289: 14470–14480, 2014. doi: 10.1074/jbc.M114.557132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Shigetomi E, Bushong EA, Haustein MD, Tong X, Jackson-Weaver O, Kracun S, Xu J, Sofroniew MV, Ellisman MH, Khakh BS. Imaging calcium microdomains within entire astrocyte territories and endfeet with GCaMPs expressed using adeno-associated viruses. J Gen Physiol 141: 633–647, 2013. doi: 10.1085/jgp.201210949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Shirakawa H, Nakagawa T, Kaneko S. [Pathophysiological roles of transient receptor potential channels in glial cells]. Yakugaku Zasshi 130: 281–287, 2010. doi: 10.1248/yakushi.130.281. [DOI] [PubMed] [Google Scholar]
- 179.Sierra A, Abiega O, Shahraz A, Neumann H. Janus-faced microglia: beneficial and detrimental consequences of microglial phagocytosis. Front Cell Neurosci 7: 6, 2013. doi: 10.3389/fncel.2013.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sierra A, Gottfried-Blackmore AC, McEwen BS, Bulloch K. Microglia derived from aging mice exhibit an altered inflammatory profile. Glia 55: 412–424, 2007. doi: 10.1002/glia.20468. [DOI] [PubMed] [Google Scholar]
- 182.Silva AC, Lee SP, Iadecola C, Kim SG. Early temporal characteristics of cerebral blood flow and deoxyhemoglobin changes during somatosensory stimulation. J Cereb Blood Flow Metab 20: 201–206, 2000. doi: 10.1097/00004647-200001000-00025. [DOI] [PubMed] [Google Scholar]
- 183.Simpkins JW, Rajakumar G, Zhang YQ, Simpkins CE, Greenwald D, Yu CJ, Bodor N, Day AL. Estrogens may reduce mortality and ischemic damage caused by middle cerebral artery occlusion in the female rat. J Neurosurg 87: 724–730, 1997. doi: 10.3171/jns.1997.87.5.0724. [DOI] [PubMed] [Google Scholar]
- 184.Šišková Z, Tremblay ME. Microglia and synapse: interactions in health and neurodegeneration. Neural Plast 2013: 1–10, 2013. doi: 10.1155/2013/425845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci 32: 638–647, 2009. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sohrabji F, Miranda RC, Toran-Allerand CD. Identification of a putative estrogen response element in the gene encoding brain-derived neurotrophic factor. Proc Natl Acad Sci USA 92: 11110–11114, 1995. doi: 10.1073/pnas.92.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sohrabji F, Williams M. Stroke neuroprotection: oestrogen and insulin-like growth factor-1 interactions and the role of microglia. J Neuroendocrinol 25: 1173–1181, 2013. doi: 10.1111/jne.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Stapf C, Mohr JP. Ischemic stroke therapy. Annu Rev Med 53: 453–475, 2002. doi: 10.1146/annurev.med.53.082901.104106. [DOI] [PubMed] [Google Scholar]
- 189.Sun W, Cornwell A, Li J, Peng S, Osorio MJ, Aalling N, Wang S, Benraiss A, Lou N, Goldman SA, Nedergaard M. SOX9 is an astrocyte-specific nuclear marker in the adult brain outside the neurogenic regions. J Neurosci 37: 4493–4507, 2017. doi: 10.1523/JNEUROSCI.3199-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Suzuki S, Brown CM, Dela Cruz CD, Yang E, Bridwell DA, Wise PM. Timing of estrogen therapy after ovariectomy dictates the efficacy of its neuroprotective and antiinflammatory actions. Proc Natl Acad Sci USA 104: 6013–6018, 2007. doi: 10.1073/pnas.0610394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Tajada S, Moreno CM, O’Dwyer S, Woods S, Sato D, Navedo MF, Santana LF. Distance constraints on activation of TRPV4 channels by AKAP150-bound PKCα in arterial myocytes. J Gen Physiol 149: 639–659, 2017. doi: 10.1085/jgp.201611709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Tang H, Zhang Q, Yang L, Dong Y, Khan M, Yang F, Brann DW, Wang R. Reprint of “GPR30 mediates estrogen rapid signaling and neuroprotection”. Mol Cell Endocrinol 389: 92–98, 2014. doi: 10.1016/j.mce.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 193.Tanuma N, Sakuma H, Sasaki A, Matsumoto Y. Chemokine expression by astrocytes plays a role in microglia/macrophage activation and subsequent neurodegeneration in secondary progressive multiple sclerosis. Acta Neuropathol 112: 195–204, 2006. doi: 10.1007/s00401-006-0083-7. [DOI] [PubMed] [Google Scholar]
- 194.Tay TL, Savage JC, Hui CW, Bisht K, Tremblay ME. Microglia across the lifespan: from origin to function in brain development, plasticity and cognition. J Physiol 595: 1929–1945, 2017. doi: 10.1113/JP272134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Thorin-Trescases N, de Montgolfier O, Pinçon A, Raignault A, Caland L, Labbé P, Thorin E. Impact of pulse pressure on cerebrovascular events leading to age-related cognitive decline. Am J Physiol Heart Circ Physiol 314: H1214–H1224, 2018. doi: 10.1152/ajpheart.00637.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Tian DS, Li CY, Qin C, Murugan M, Wu LJ, Liu JL. Deficiency in the voltage-gated proton channel Hv1 increases M2 polarization of microglia and attenuates brain damage from photothrombotic ischemic stroke. J Neurochem 139: 96–105, 2016. doi: 10.1111/jnc.13751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Tong X, Shigetomi E, Looger LL, Khakh BS. Genetically encoded calcium indicators and astrocyte calcium microdomains. Neuroscientist 19: 274–291, 2013. doi: 10.1177/1073858412468794. [DOI] [PubMed] [Google Scholar]
- 198.Tremblay ME, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS Biol 8: e1000527, 2010. doi: 10.1371/journal.pbio.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Tremblay ME, Stevens B, Sierra A, Wake H, Bessis A, Nimmerjahn A. The role of microglia in the healthy brain. J Neurosci 31: 16064–16069, 2011. doi: 10.1523/JNEUROSCI.4158-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Van Kempen TA, Gorecka J, Gonzalez AD, Soeda F, Milner TA, Waters EM. Characterization of neural estrogen signaling and neurotrophic changes in the accelerated ovarian failure mouse model of menopause. Endocrinology 155: 3610–3623, 2014. doi: 10.1210/en.2014-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Van Kempen TA, Milner TA, Waters EM. Accelerated ovarian failure: a novel, chemically induced animal model of menopause. Brain Res 1379: 176–187, 2011. doi: 10.1016/j.brainres.2010.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Vegeto E, Belcredito S, Ghisletti S, Meda C, Etteri S, Maggi A. The endogenous estrogen status regulates microglia reactivity in animal models of neuroinflammation. Endocrinology 147: 2263–2272, 2006. doi: 10.1210/en.2005-1330. [DOI] [PubMed] [Google Scholar]
- 203.Verkhratsky A, Nedergaard M. Physiology of astroglia. Physiol Rev 98: 239–389, 2018. doi: 10.1152/physrev.00042.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Vriens J, Janssens A, Prenen J, Nilius B, Wondergem R. TRPV channels and modulation by hepatocyte growth factor/scatter factor in human hepatoblastoma (HepG2) cells. Cell Calcium 36: 19–28, 2004. doi: 10.1016/j.ceca.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 205.Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci 29: 3974–3980, 2009. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Waldmann TA, Tagaya Y. The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu Rev Immunol 17: 19–49, 1999. doi: 10.1146/annurev.immunol.17.1.19. [DOI] [PubMed] [Google Scholar]
- 207.Wang YF, Parpura V. Central role of maladapted astrocytic plasticity in ischemic brain edema formation. Front Cell Neurosci 10: 129, 2016. doi: 10.3389/fncel.2016.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Wanner IB, Anderson MA, Song B, Levine J, Fernandez A, Gray-Thompson Z, Ao Y, Sofroniew MV. Glial scar borders are formed by newly proliferated, elongated astrocytes that interact to corral inflammatory and fibrotic cells via STAT3-dependent mechanisms after spinal cord injury. J Neurosci 33: 12870–12886, 2013. doi: 10.1523/JNEUROSCI.2121-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wassertheil-Smoller S, Hendrix SL, Limacher M, Heiss G, Kooperberg C, Baird A, Kotchen T, Curb JD, Black H, Rossouw JE, Aragaki A, Safford M, Stein E, Laowattana S, Mysiw WJ; WHI Investigators . Effect of estrogen plus progestin on stroke in postmenopausal women: the Women’s Health Initiative: a randomized trial. JAMA 289: 2673–2684, 2003. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- 210.Wilhelmsson U, Bushong EA, Price DL, Smarr BL, Phung V, Terada M, Ellisman MH, Pekny M. Redefining the concept of reactive astrocytes as cells that remain within their unique domains upon reaction to injury. Proc Natl Acad Sci USA 103: 17513–17518, 2006. doi: 10.1073/pnas.0602841103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Wilson PW, Garrison RJ, Castelli WP. Postmenopausal estrogen use, cigarette smoking, and cardiovascular morbidity in women over 50. The Framingham Study. N Engl J Med 313: 1038–1043, 1985. doi: 10.1056/NEJM198510243131702. [DOI] [PubMed] [Google Scholar]
- 212.Wohleb ES. Neuron-microglia interactions in mental health disorders: “for better, and for worse”. Front Immunol 7: 544, 2016. doi: 10.3389/fimmu.2016.00544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Wong WT. Microglial aging in the healthy CNS: phenotypes, drivers, and rejuvenation. Front Cell Neurosci 7: 22, 2013. doi: 10.3389/fncel.2013.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Xue B, Pamidimukkala J, Lubahn DB, Hay M. Estrogen receptor-α mediates estrogen protection from angiotensin II-induced hypertension in conscious female mice. Am J Physiol Heart Circ Physiol 292: H1770–H1776, 2007. doi: 10.1152/ajpheart.01011.2005. [DOI] [PubMed] [Google Scholar]
- 215.Yang SH, Shi J, Day AL, Simpkins JW. Estradiol exerts neuroprotective effects when administered after ischemic insult. Stroke 31: 745–750, 2000. doi: 10.1161/01.STR.31.3.745. [DOI] [PubMed] [Google Scholar]
- 216.Yasuda Y, Tateishi N, Shimoda T, Satoh S, Ogitani E, Fujita S. Relationship between S100β and GFAP expression in astrocytes during infarction and glial scar formation after mild transient ischemia. Brain Res 1021: 20–31, 2004. doi: 10.1016/j.brainres.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 217.Yoshizawa H, Gazes Y, Stern Y, Miyata Y, Uchiyama S. Characterizing the normative profile of 18F-FDG PET brain imaging: sex difference, aging effect, and cognitive reserve. Psychiatry Res 221: 78–85, 2014. doi: 10.1016/j.pscychresns.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 218.Yuen N, Lam TI, Wallace BK, Klug NR, Anderson SE, O’Donnell ME. Ischemic factor-induced increases in cerebral microvascular endothelial cell Na/H exchange activity and abundance: evidence for involvement of ERK1/2 MAP kinase. Am J Physiol Cell Physiol 306: C931–C942, 2014. doi: 10.1152/ajpcell.00021.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, Barres BA. Genomic analysis of reactive astrogliosis. J Neurosci 32: 6391–6410, 2012. doi: 10.1523/JNEUROSCI.6221-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Zbesko JC, Nguyen TV, Yang T, Frye JB, Hussain O, Hayes M, Chung A, Day WA, Stepanovic K, Krumberger M, Mona J, Longo FM, Doyle KP. Glial scars are permeable to the neurotoxic environment of chronic stroke infarcts. Neurobiol Dis 112: 63–78, 2018. doi: 10.1016/j.nbd.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Zhang R, Zuckerman JH, Iwasaki K, Wilson TE, Crandall CG, Levine BD. Autonomic neural control of dynamic cerebral autoregulation in humans. Circulation 106: 1814–1820, 2002. doi: 10.1161/01.CIR.0000031798.07790.FE. [DOI] [PubMed] [Google Scholar]
- 222.Zhao TZ, Ding Q, Hu J, He SM, Shi F, Ma LT. GPER expressed on microglia mediates the anti-inflammatory effect of estradiol in ischemic stroke. Brain Behav 6: e00449, 2016. doi: 10.1002/brb3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Zhao X, Wang H, Sun G, Zhang J, Edwards NJ, Aronowski J. Neuronal interleukin-4 as a modulator of microglial pathways and ischemic brain damage. J Neurosci 35: 11281–11291, 2015. doi: 10.1523/JNEUROSCI.1685-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci 6: 43–50, 2003. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]
- 225.Zrzavy T, Machado-Santos J, Christine S, Baumgartner C, Weiner HL, Butovsky O, Lassmann H. Dominant role of microglial and macrophage innate immune responses in human ischemic infarcts. Brain Pathol 28: 791–805, 2018. doi: 10.1111/bpa.12583. [DOI] [PMC free article] [PubMed] [Google Scholar]