Abstract
Pulmonary microvascular endothelial cells (PMVECs) display a rapid angioproliferative phenotype, essential for maintaining homeostasis in steady-state and promoting vascular repair after injury. Although it has long been established that endothelial cytosolic Ca2+ ([Ca2+]i) transients are required for proliferation and angiogenesis, mechanisms underlying such regulation and the transmembrane channels mediating the relevant [Ca2+]i transients remain incompletely understood. In the present study, the functional role of the microvascular endothelial site-specific α1G T-type Ca2+ channel in angiogenesis was examined. PMVECs intrinsically possess an in vitro angiogenic “network formation” capacity. Depleting extracellular Ca2+ abolishes network formation, whereas blockade of vascular endothelial growth factor receptor or nitric oxide synthase has little or no effect, suggesting that the network formation is a [Ca2+]i-dependent process. Blockade of the T-type Ca2+ channel or silencing of α1G, the only voltage-gated Ca2+ channel subtype expressed in PMVECs, disrupts network formation. In contrast, blockade of canonical transient receptor potential (TRP) isoform 4 or TRP vanilloid 4, two other Ca2+ permeable channels expressed in PMVECs, has no effect on network formation. T-type Ca2+ channel blockade also reduces proliferation, cell-matrix adhesion, and migration, three major components of angiogenesis in PMVECs. An in vivo study demonstrated that the mice lacking α1G exhibited a profoundly impaired postinjury cell proliferation in the lungs following lipopolysaccharide challenge. Mechanistically, T-type Ca2+ channel blockade reduces Akt phosphorylation in a dose-dependent manner. Blockade of Akt or its upstream activator, phosphatidylinositol-3-kinase (PI3K), also impairs network formation. Altogether, these findings suggest a novel functional role for the α1G T-type Ca2+ channel to promote the cell’s angiogenic potential via a PI3K-Akt signaling pathway.
Keywords: Akt, angiogenesis, endothelial cell, T-type calcium channel
INTRODUCTION
Angiogenesis plays a critical role in lung vascular integrity, alveolar structure maintenance, and reparative alveolar regeneration to restore gas exchange function following lung injury. Defining the cellular and molecular mechanisms that regulate angiogenesis is essential to develop therapeutic strategies to repair or optimize respiratory capacity in patients with lung diseases. In general, angiogenesis occurs as an orderly cascade of cellular and molecular events that comprise complexes of growth factor-receptor interactions, cell proliferation, migration and reorganization, and cell-cell and cell-matrix interactions.
Although angiogenesis is a fundamental property of endothelium, it is well recognized that not all endothelial cells possess the same angiogenic capacity. Along the pulmonary vascular axis, from arteries to capillaries and veins, lung endothelium displays considerable functional heterogeneity, primarily between extra-alveolar and alveolar septal capillary endothelial cells. Pulmonary microvascular endothelial cells (PMVECs) form the vascular lining of the alveolar-capillary interface, limit fluid filtration into the alveolar airspace and interstitium, and facilitate physiological gas exchange. Conceivably, any disruption of the endothelium in this region must be promptly repaired, a process that likely requires rapid physiologic endothelial “angioproliferative” response to replace lost cells or, in more severe cases, lost vessel structures. Indeed, alveolar capillary endothelium readily undergoes vigorous angioproliferative responses, both in vivo and in vitro (12, 14, 29, 34). Isolated PMVECs characteristically possess intrinsic rapid proliferation and angiogenic capacity when compared with endothelial cells isolated from conduit vessels, e.g., pulmonary artery endothelial cells (PAECs).
Cytosolic Ca2+ ([Ca2+]i) signals are highly conserved and ubiquitous for control of various cellular processes, including motility, proliferation, migration, differentiation, survival, and apoptosis. Strictly regulated [Ca2+]i signals are involved at different phases in the regulation of the complex and multistaged process of angiogenesis (20, 27). Similar to PAECs, PMVECs express canonical transient receptor potential (TRP) isoform 1 and 4 (TRPC1 and TRPC4) that encode for a Ca2+-selective store-operated current (ISOC) (7, 41), of which the conditional activation results in a Ca2+ entry that is endothelial barrier disruptive (40, 41). In addition, PMVECs also possess two novel Ca2+ channels that are not present in extra-alveolar endothelial cells, including the transient receptor potential vanilloid 4 (TRPV4) channel and the α1G-subtype of voltage-gated T-type Ca2+ channel. Activation of these Ca2+ entry pathways independently triggers the events specific to the pulmonary microcirculation. Although TRPV4-mediated Ca2+ entry is an essential determinant for high vascular or airway pressure-induced lung injury, α1G-mediated Ca2+ entry is functionally linked to regulated surface expression of P-selectin in alveolar septal capillary endothelium.
Previous studies from our laboratory and others have demonstrated a potential role of voltage-gated T-type Ca2+ channels in control of cell proliferation in a number of cell types (23, 30). In the present study, we sought to determine whether the endothelial site-specific α1G T-type Ca2+ channel is essentially required for PMVECs to sustain their angiogenic capacity.
MATERIALS AND METHODS
Chemicals and reagents.
The calcium channel blockers employed in this study, namely mibefradil, NNC 55-0396, ML204, and ruthenium red, were purchased from Sigma (St. Louis, MO). l-NNA, Akti-1/2, FR180204, LY294002, LY303511, and wortmannin were purchased from Tocris Bioscience (Bristol, UK). The following primary antibodies, i.e., phospho-Akt (Ser473), phospho-Akt (Thr308), pan-Akt, phospho-ERK (Thr202/Tyr204), and phospho-p38 (Tyr182), all raised in the rabbit, were purchased from Cell Signaling Technology (Danvers, MA). SU5416, l-NAME, and all other chemicals and reagents were purchased from Sigma, unless otherwise stated.
Cell culture.
Rat PMVECs were isolated, cultured, and characterized as previously described (47). Cells were maintained in 100-mm culture dishes in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 μg/ml streptomycin (referred to as “standard media”) in an atmosphere of 5% CO2 in humidified air at 37°C. Cells used in all experiments were below passage 12.
Design and construction of recombinant lentivirus-mediated shRNAs targeting rat α1G, production of recombinant lentivirus, and endothelial cell transduction.
These experiments were performed as we previously described in thorough detail (47). For cell transduction, PMVECs were grown to 20%–40% confluency and subsequently infected with the harvested viral supernatant of the packaging cells.
Animals.
Experimental protocols for mice were approved by the Institutional Animal Care and Use Committee (IACUC) of Medical College of Georgia at Augusta University and the IACUC of the University of Alabama at Birmingham, where the in vivo studies were performed. The α1G-null mutations were originally described by Kim et al. (17). Homozygous α1G-deficient (α1G−/−) mice and their wild-type littermates (α1G+/+) were generated by heterozygote mating. Mice of either sex ranging from 8 to 12 wk in age and 18 to 35 g in body weight were used in the study.
In vitro Matrigel-based network formation assay.
In vitro Matrigel-based network formation assay was performed as described elsewhere. Briefly, the growth factor-reduced Matrigel (Trevigen, Gaithersburg, MD; cat. no. 3433–010–01) was distributed to prechilled Corning Costar 24-well plates (Corning, NY) at 100 μl per well. Matrigel was allowed to jellify at 37°C for at least 30 min. Subconfluent cultured PMVECs were trypsinized, washed twice with phosphate-buffered saline (PBS), resuspended in DMEM at a density of 1 × 105 cells/ml, and seeded on the Matrigel-coated wells with 500 μl/well to reach the final density of 5 × 104 cells/well. Each of the experimental compounds, e.g., SU5416, l-NAME, l-NNA, mibefradil, or NNC 55-0396, etc., was added to the wells at various concentrations, and the plates were then incubated at 37°C for 6 or 18 h. The areas of the tubular network or cells on the culture surface of each well were imaged on a Nikon TS100-F inverted optical microscope with a ×10, 0.30 numerical aperture objective lens at room temperature. Quantitative assessment of network formation was accomplished by measuring the length of the network composed of closed tubular loops or unclosed but continuous cellular cords, as well as the number of closed (complete) tubular loops, using the image processing program ImageJ (Research Services Branch, National Institute of Mental Health Sciences, https://rsb.info.nih.gov/ij/). Results from triplicate wells were expressed as tubular length per field (relative percentage) or number of complete loops per field from 12 to 20 random fields per well. Each assay was replicated at least three times.
Cell proliferation assay.
Subconfluent cultured PMVECs, PMVECs expressing shRNA-targeting rat α1G, or PMVECs expressing scrambled shRNA were trypsinized, washed twice with PBS, resuspended in the standard media, and plated into triplicate wells of Corning Costar six-well culture plates at a density of 2 × 104 cells per well. Cells were then incubated in the standard media in an atmosphere of 5% CO2 in humidified air at 37°C. Every 24 h for 3 days, cells were washed with PBS, harvested with trypsin, and counted with the Cellometer Automated Cell Counter (Nexcelom Bioscience, Lawrence, MA). Each assay was replicated three times.
Cell-matrix adhesion assay.
Corning Costar six-well plates were coated with a uniform layer of 2% Matrigel (Trevigen) at 37°C for 2 h. Subconfluent cultured PMVECs were trypsinized, washed twice with PBS, resuspended in DMEM with 5% FBS at a density of 1 × 105 cells/ml, and plated into triplicate wells with 2 ml/well to achieve the final density of 2 × 105 cells/well. NNC 55-0396 dissolved in distilled water at 2 different concentrations (5 and 10 μmol/l) or distilled water alone was added to the wells. After incubation in an atmosphere of 5% CO2 in humidified air at 37°C for 30 min, the coverslips were gently washed 3 times with ample PBS to remove the nonadherent cells. The remaining adherent cells were fixed with 4% paraformaldehyde and imaged on a Nikon TS100-F inverted optical microscope with a ×10, 0.30 numerical aperture objective at room temperature. The number of adherent cells were quantified using ImageJ as a measure of cell-matrix adhesion strength. Results from triplicate wells were expressed as the relative number of attached cells per field from 10 to 12 random fields per coverslip. Each assay was replicated at least three times.
Scratch wound healing assay.
PMVECs, PMVECs expressing shRNA-targeting rat α1G, or PMVECs expressing scrambled shRNA were cultured in the standard media in 2% Matrigel-coated six-well plates until confluence (but not overgrown). The cells were then washed twice with PBS and incubated overnight in serum-reduced media (DMEM with 2% FBS) before making an incision (scratch) in the cell monolayers with a 200-μl pipette tip. Cells were then washed 3 times with serum-free DMEM, followed by addition of the serum-reduced media along with NNC 55-0396 (5 μmol/l in 1 μl distilled water) or distilled water and incubated in an atmosphere of 5% CO2 in humidified air at 37°C for 18 h. Images were acquired at time 0 and 18 h postscratch on a Nikon TS100-F inverted optical microscope with a ×4, 0.10 numerical aperture objective from the areas where the scratch was created. Quantification of the gap closure was accomplished using ImageJ and presented as the percentage of gap closure, calculated as [(area of original wound − area of unclosed wound at the time of assessment)/area of original wound] ×100%. Each assay was replicated three times.
Western blot analysis.
PMVECs, PMVECs expressing shRNA-targeting rat α1G, or PMVECs expressing scrambled shRNA were grown on six-well plates in the standard media until 70%–90% confluence. Mibefradil or NNC 55-0396 in distilled water at two different concentrations or distilled water alone was added to the wells. After incubation in an atmosphere of 5% CO2 in humidified air at 37°C for 30 min, cells were washed with cold PBS and lysed with radioimmunoprecipitation assay buffer (25 mmol/l Tris, 150 mmol/l NaCl, 1% nonidet P-40, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate, pH 7.6; Pierce Biotechnology, Rockford, IL). Cell lysates were subjected to 10% SDS-PAGE, transferred to nitrocellulose membranes, and blotted with the indicated antibodies, i.e., Akt (1:200), phospho-Akt (Ser473) (1:1,000), phospho-Akt (Thr308) (1:1,000), ERK (1:200), phospho-ERK1/2 (1:1,000), and phospho-p38 (1:1,000), followed by a horseradish peroxidase (HRP)-conjugated secondary antibody. Equal loading of the gel was confirmed by antibody specific for β-actin (1:1,000). Protein bands were visualized with SuperSignal West Dura extended duration substrate (Pierce Biotechnology), and band intensities were quantified as digital densitometry using ImageJ and reported as relative ratios normalized to the average of loading controls. Each assay was replicated three times.
Cellular electrophysiology, data acquisition, and analysis.
Patch-clamp recordings were performed in whole-cell configuration as previously described (36, 42, 48). Briefly, transmembrane currents in single, electrically isolated PMVECs were measured with an EPC-9 amplifier (HEKA Elektronik, Lambrecht/Pfalz, Germany). Data were acquired with PatchMaster software (Version 2 × 69, HEKA Elektronic) and filtered at 2.9 kHz. A two-step voltage protocol, i.e., a 3.25-msec, 80-mV pulse depolarization followed by a 20-msec repolarization of −120 mV, was used to elicit the maximal deactivating tail current (36, 42, 48). The holding potential was set to −90 mV, and the sampling frequency was at 50 kHz. The extracellular solution contained the following (in mmol/l): 2 CaCl2, 110 tetraethylammonium chloride, 10 CsCl, 10 HEPES, and pH was 7.4 adjusted with tetraethylammonium hydroxide. The intracellular solution contained the following (in mmol/l): 130 N-methyl-D-glucamine, 10 EGTA, 5 BAPTA, 10 HEPES, 6 MgCl2, 4 CaCl2, 2 Mg-ATP, and pH was 7.2 adjusted with methane sulfonic acid. All solutions were adjusted to 290–300 mOsm/l with sucrose. Recording pipettes were made of capillary corning glass tubing (Warner Instrument, Hamden, CT), pulled by a two-stage puller (PC-10; Narishige, Tokyo, Japan) and heat-polished with a microforge (MF-200; World Precision Instruments, Sarasota, FL). Pipette resistance was in the range of 3–5 MΩ when filled with our intracellular solution. All of the experiments were performed at room temperature (22°C–25°C). Data are expressed as mean ± SE for the number of cells (n) in which whole-cell recordings were obtained.
Animal protocol for intratracheal LPS-induced lung injury and in vivo cell proliferation assessment.
Mouse intratracheal instillation of lipopolysaccharide (LPS) was performed according to methods previously described elsewhere. Briefly, the mouse was anesthetized and placed on a board in a near-vertical position, and the tongue was withdrawn with a pair of lined forceps. LPS from Escherichia coli (serotype O55:B5; Sigma) at a dose of 3 mg/kg dissolved in 30 μl sterile PBS or 30 μl sterile PBS only was instilled intratracheally via a cannula, followed by 100 μl of air. All experimental mice were killed 72 h after the instillation to complete the in vivo experiment. Three hours before the completion, the mice were intraperitoneally injected with 5-bromodeoxyuridine (BrdU) labeling reagent (Invitrogen, Thermo Fisher Scientific) at a dose of 1 ml/100 g body wt. The incorporation of BrdU was subsequently assessed by BrdU immunohistochemistry.
Immunohistochemistry.
Immunohistochemistry for BrdU was performed as described elsewhere. Briefly, following the euthanization of the above-described experimental mice, lungs were isolated for ex vivo perfusion according to our established protocol (43, 46, 47), and then perfusion-fixed with 4% paraformaldehyde in PBS via vascular perfusion. The lungs were rinsed in Earle's buffered salt solution containing 4% bovine serum albumin, immersed in fixative, embedded in paraffin, and sectioned at 5-μm thickness. The paraffin sections were deparaffinized in xylene and rehydrated in a graded alcohol series. Antigen retrieval was performed using 10 mmol/l sodium citrate (pH 6.0) and microwaved for 8–15 min. Following antigen retrieval, the tissues were incubated with 1 mol/l HCl for 10 min, washed in PBS, and then blocked in 3% H2O2-methanol for 15 min at room temperature to quench the endogenous peroxidase, washed with ddH2O and PBS, and then probed with an anti-BrdU monoclonal antibody (BRD3 antibody, Invitrogen, Thermo Fisher Scientific) diluted in 3% BSA-PBS at a dilution of 1:100 overnight at 4°C in a humidified chamber. Tissues were washed extensively in PBS with Tween 20, and detection was performed using a HRP-conjugated secondary antibody followed by colorimetric detection using a DAB (3,3′-diaminobenzidine) kit. Negative controls were obtained by omission of the anti-BrdU antibody. Tissue was counterstained with Mayer's hematoxylin (Sigma) and dehydrated with ethanol and xylene to prep for mounting. An upright optical microscope (Nikon Eclipse E200) was used for image acquisition (×40 objective).
Morphometric assessment of BrdU-positive cells.
Morphometric analysis was performed to quantitate the number of BrdU-positive cells. Images were visualized in Adobe Photoshop with a grid overlay. The incorporated BrdU volume fraction in the lung for each experiment was determined with a point-counting method. A total of three to six images per lung from three lungs in each treatment group were analyzed. The BrdU volume fraction for each lung was calculated as the ratio of BrdU-positive points relative to total points landing on the nucleus. The volume fraction data were then averaged for each treatment group.
Data analysis.
Numerical data are reported as means ± SE. One-way ANOVA was used to evaluate differences between experimental groups, with a post hoc Tukey’s multiple comparison test as appropriate. Significance was considered when P < 0.05.
RESULTS
Depleting extracellular Ca2+ impairs the network formation capacity of PMVECs.
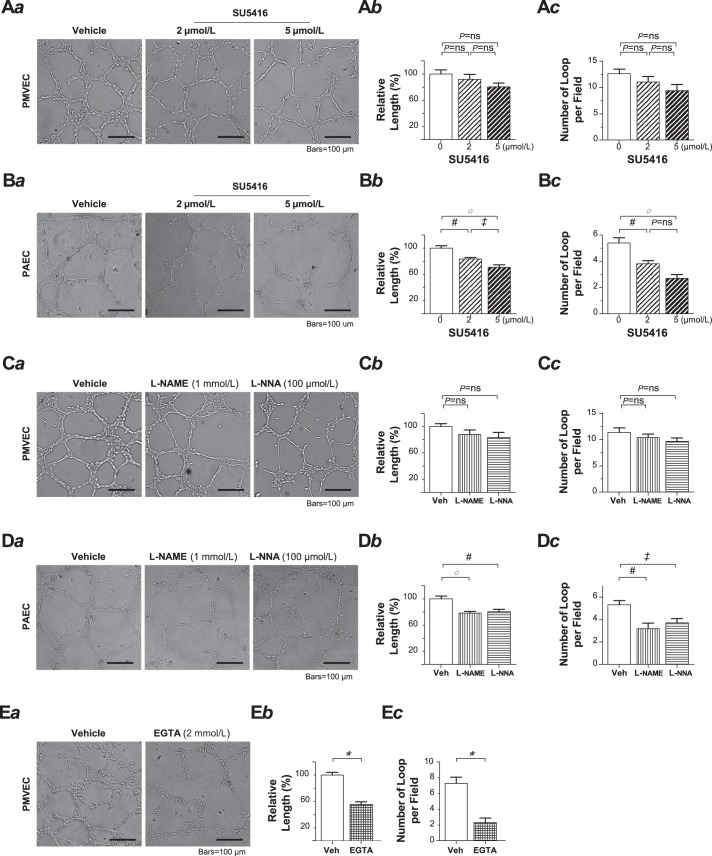
One of the key characteristics of PMVECs is the intrinsic capacity of vigorously forming capillary-like tubular networks in reconstituted basement membrane matrix, e.g., Matrigel. Matrigel network formation assay is a reliable tool to assess angiogenesis in vitro. Since regular Matrigel contains a number of growth factors that may act as angiogenic “stimulators,” we initially examined the angiogenic capacity of PMVECs utilizing a growth factor-reduced Matrigel to minimize the effects of potential extrinsic stimulation. As shown in Fig. 1Aa and Supplemental Video S1 (Supplemental Material for this article is available online at the Journal website), PMVECs retained a stable angiogenic imprint in the growth factor-reduced Matrigel. After cell placement, they initially attached in the first hour and then migrated toward each other over the next few hours to form a well-organized tubular network. Such vigorous network-forming capacity remained virtually unaffected when the PMVECs were exposed to the potent VEGF receptor-2 antagonist SU5416 (2 and 5 μmol/l, Fig. 1A). In contrast, the network formation was markedly reduced in pulmonary artery endothelial cells (PAECs) when the cells were exposed to SU5416 at the same concentrations (Fig. 1B). We next conducted the network formation assay in PMVECs treated with l-NAME and l-NNA, two potent nonselective nitric oxide (NO) synthase (NOS) inhibitors. Note that the chosen l-NAME concentration (1 mmol/l) was more than 2 times that of l-arginine in the medium (~400 μmol/l), sufficient to inhibit NOS. Although these inhibitors remarkably reduced the network formation of PAECs (Fig. 1D), neither l-NAME nor l-NNA exhibited any significant inhibitory effect on the network formation of PMVECs (Fig. 1C). When the assay was performed using a nominally Ca2+-free extracellular solution, i.e., ~6 μmol/l free Ca2+ after extracellular application of Ca2+ chelating agent EGTA, we observed a drastic reduction of network formation in PMVECs within 6 h (Fig. 1E). Note that to avoid the potential impairment of cell adhesion by nominally Ca2+-free extracellular solution that could confound the outcome of the study, regular Ca2+-containing extracellular solution was used initially to allow the cells to attach in the Matrigel during the first hour, and the nominally Ca2+-free extracellular solution was then switched for the rest of the experiment. Also note that we limited the study in the time frame of 6 h for the best performance of the cells under such a Ca2+-free extracellular milieu. These results suggest that the vigorous angiogenic capacity is intrinsic to PMVECs and that the integrity of this capacity occurs in both a VEGF- and NO-independent manner but requires extracellular Ca2+ homeostasis.
Fig. 1.
Pulmonary microvascular endothelial cells (PMVECs) exhibit an in vitro network formation capacity that is independent of VEGF or nitric oxide synthase 3-nitric oxide signaling but requires extracellular Ca2+. A and B: the tubular networks formed by PMVECs (A) and pulmonary artery endothelial cells (PAECs; B) in the absence or presence of SU5416. Representative phase-contrast images (Aa) of the tubular network formed at 18 h after the cells were plated on Matrigel in the absence (left) or presence of SU5416 at the concentration of 2 μmol/l (middle) and 5 μmol/l (right). Relative total tubular length of the network (Ab) including the length of closed and unclosed, but continuous, cellular cords from 10 to 20 random fields from triplicate Matrigel-coated wells in a single assay that was replicated three times. One-way ANOVA (F = 2.2, P = 0.129; Ab). Note that in nontreated (vehicle control) PMVECs, the total tubular length of the network in a given field taken with a ×10, 0.30 numerical aperture objective lens is estimated to be 20–30 mm. One-way ANOVA (F = 2.5, P = 0.095; Ac). One-way ANOVA (F = 22.4, P < 0.0001) with Tukey’s post hoc multiple comparison test (*P < 0.001, #P < 0.01, ‡P < 0.05; Bb). One-way ANOVA (F = 17.7, P < 0.0001) with Tukey’s post hoc multiple comparison test (#P < 0.01, ‡P < 0.05; Bc). C and D: the tubular networks formed by PMVECs (C) and PAECs (D) in the absence or presence of l-NAME or l-NNA. Figure arrangement of a, b, and c in C and D is the same as that in A and B. One-way ANOVA (F = 1.7, P = 0.995; Cb). One-way ANOVA (F = 1.4, P = 0.264; Cc). One-way ANOVA (F = 10.1, P = 0.0005) with Tukey’s post hoc multiple comparison test (*P < 0.001, #P < 0.01; Db). One-way ANOVA (F = 6.8, P = 0.0041) with Tukey’s post hoc multiple comparison test (#P < 0.01, ‡P < 0.05; Dc). E: the tubular networks formed by PMVECs in the absence or presence of extracellular EGTA. Representative phase-contrast images (Ea) of the tubular network formed by PMVECs at 6 h after the cells were plated, relative total tubular length (Eb), and bar graph showing the number of complete loops per field (Ec). *P < 0.0001, Student’s t-test. ns, not significant; Veh, vehicle.
Inhibition of the T-type Ca2+ channel impairs the network formation capacity of PMVECs.
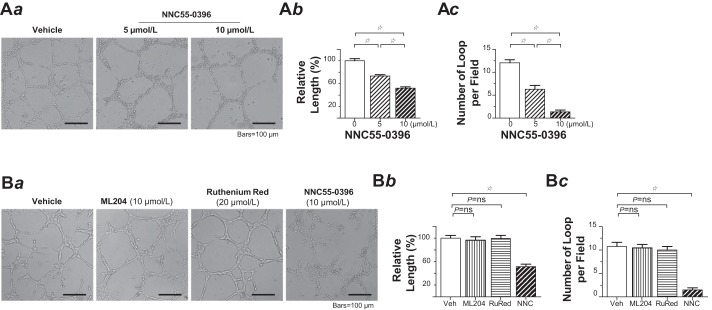
Ca2+ entry from extracellular space and release from intracellular stores represent two principal mechanisms through which [Ca2+]i transients take place upon angiogenic stimulations. Compared with Ca2+ release, Ca2+ entry can support longer lasting [Ca2+]i signals that are critical in mediating the processes of endothelial proliferation, adhesion, migration, and angiogenesis (20, 25). We have previously determined that PMVECs mainly express three distinct Ca2+ entry pathways, namely, canonical transient receptor potential (TRP) isoform 1 and 4 (TRPC1 and TRPC4), that encode for the channel conducting a Ca2+-selective store-operated current (ISOC) (7, 41), TRP vanilloid 4 (TRPV4), and α1G voltage-gated T-type Ca2+ channels (36, 42, 48). We sought to determine whether any of these channels contributes to the PMVEC angiogenic phenotype. Thus, Matrigel network formation assay was performed in PMVECs with each of the pharmacological channel blockers. Blockade of the T-type Ca2+ channel with its highly selective blocker NNC 55-0396 dose-dependently reduced the length and the number of closed tubular loops of the network (Fig. 2A). In stark contrast, pharmacological blockade of TRPC4 or TRPV4 had no effect on network formation (Fig. 2B).
Fig. 2.
T-type Ca2+ channel blockade impaired pulmonary microvascular endothelial cell (PMVEC) network formation capacity. A: the tubular networks formed by PMVECs in the absence or presence of NNC 55-0396. Aa: representative phase-contrast images of the tubular network formed by PMVECs at 18 h after the cells were plated on Matrigel in the absence (left) or presence of NNC 55-0396 at the concentration of 5 μmol/l (middle) and 10 μmol/l (right). Ab: relative total tubular length of the network, including the length of closed and unclosed, but continuous, cellular cords from 10 to 20 random fields from triplicate Matrigel-coated wells in a single assay that was replicated 3 times. One-way ANOVA (F = 69.5, P < 0.0001) with post hoc Tukey’s comparison (☆P < 0.001). Ac: bar graph showing the number of complete loops per field, from 10 to 20 random fields from triplicate Matrigel-coated wells in a single assay that was replicated 3 times. One-way ANOVA (F = 65.2, P < 0.0001) with post hoc Tukey’s multiple comparison test (☆P < 0.001). B: blockade of canonical transient receptor potential (TRP) isoform 4 or TRP vanilloid 4 had no effect on the network formation capacity of PMVECs. Ba: representative phase-contrast images of the tubular network formed by PMVECs at 18 h after the cells were plated on Matrigel in the absence (far left) or presence of, from left to right, ML204, ruthenium red (RuRed), or NNC 55-0396 at each indicated concentration. Bb: relative total tubular length of the network, including the length of closed and unclosed, but continuous, cellular cords from 10 to 20 random fields from triplicate Matrigel-coated wells in a single assay that was replicated 3 times. One-way ANOVA (F = 21.4, P < 0.0001) with post hoc Tukey’s multiple comparison test (☆P < 0.001). Bc: bar graph showing the number of complete loops per field, from 10 to 20 random fields from triplicate Matrigel-coated wells in a single assay that was replicated 3 times. One-way ANOVA (F = 42.8, P < 0.0001) with post hoc Tukey’s multiple comparison test (☆P < 0.001). ns, not significant.
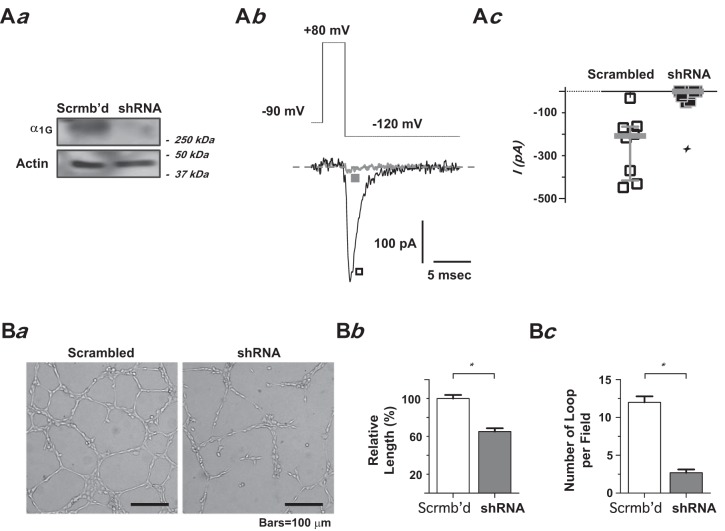
To confirm that the inhibition effect by NNC 55-0396 was indeed due to T-type Ca2+ channel blockade, we utilized an shRNA interfering approach to silence the α1G gene in PMVECs and then examined the effect of α1G-silencing on the cells’ network formation capacity. As described in our previous studies, this lentivirally deliverable shRNA directed against α1G is highly efficient in its delivery and expression (virtually to 100% purity) and highly effective in α1G-knockdown and T-type Ca2+ current elimination in PMVECs (47). The silencing effect on the functional T-type Ca2+ channel was determined in lentivirally-infected PMVECs by examining the deactivating tail-current elicited at the peak of an 80-mV pulse, where the maximally evoked T-type current may be assessed (42, 48). α1G-silencing virtually ablated the functional T-type Ca2+ channel in PMVECs, as evidenced by the near disappearance of the tail current in α1G-knockdown shRNA lentivirally infected PMVECs (Fig. 3A). The T-type current elicited in control cells (infected with scrambled shRNA lentivirus) retained a virtually identical maximal current amplitude as well as I-V relationship when compared with regular PMVECs (data not shown). Consistent with the finding from pharmacological T-type Ca2+ channel blockade, network formation was drastically impaired in stable shRNA transfectant PMVECs in which functional T-type Ca2+ channel was ablated (Fig. 3B). Notably, the extents of both reduction in total tubular length and number of complete loops of the network by α1G-silencing were fairly comparable to those that resulted from using nominally Ca2+-free extracellular solution.
Fig. 3.
α1G-silencing by shRNA impaired pulmonary microvascular endothelial cell (PMVEC) network formation capacity. A: silencing α1G expression by shRNA. Aa: Western blotting analysis of α1G protein levels in PMVECs transduced with shRNA targeting α1G. α1G protein expression was nearly eliminated by shRNA. Ab: representative deactivating tail current (Itail) traces from PMVECs transduced with the shRNA targeting α1G (■) or scrambled control shRNA (□), elicited by repolarization at −120 mV following a short (3.25-ms) test pulse at 80 mV from a holding potential of −90 mV, as schematically illustrated. Ac: scatter plot of individual data points of maximally elicited T-type tail currents from PMVECs transduced with the shRNA targeting α1G (■) or scrambled control shRNA (□) (lines: median with interquartile range). Note the median of Itail amplitude: −208 pA in scrambled control PMVECs vs. 0 pA in shRNA PMVECs (n = 8 each, ✧P < 0.0005, Student’s t-test). B: the tubular networks formed by PMVECs with and without α1G-silencing. Ba: representative phase-contrast images of the tubular network formed by PMVECs at 18 h after the cells were plated on Matrigel: scrambled control PMVECs (left), shRNA PMVECs (right). Bb: relative total tubular length. Bc: bar graph showing the number of complete loops per field, from 10 to 20 random fields from triplicate Matrigel-coated wells in a single assay that was replicated 3 times. *P < 0.0001, Student’s t-test.
Inhibition of the T-type Ca2+ channel reduces PMVEC proliferation, cell-matrix interaction, and migration.
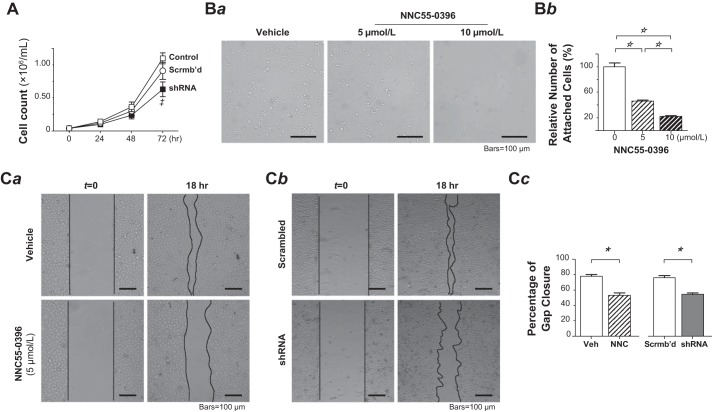
Cell proliferation, adhesion, and migration are the major components of endothelial network formation and angiogenesis (4, 21, 35). Previous studies have demonstrated that PMVECs have higher rates of cell proliferation and migration than PAECs (6, 18, 33, 49) and that Ca2+ entry through the α1G T-type Ca2+ channel regulates the transition of endothelial procoagulant phenotype. We then set out to determine whether the functional T-type Ca2+ channel is involved in cell proliferation in PMVECs. Since prolonged treatment of PMVECs with T-type Ca2+ channel blocker NNC 55-0396 or mibefradil constantly led to cell death (data not shown), we resorted to examining the impact of α1G-silencing on PMVEC proliferation. Silencing the functional T-type Ca2+ channel resulted in a significant reduction in cell proliferation as demonstrated by the decrease in the number of proliferating cells in stable shRNA transfectant PMVECs, e.g., a 31% reduction on day 3 (72 h, shRNA vs. scrambled-shRNA control, P < 0.05, 2-way ANOVA with Bonferroni’s multiple comparisons test; Fig. 4A). Next, we performed cell-matrix adhesion assay in Matrigel-coated substratum to test whether the T-type Ca2+ channel blockade affects cell-matrix adhesion strength. In the presence of NNC 55-0396, the number of PMVECs attached to the substratum within 30 min after cell plating was markedly reduced in a dose-dependent manner, i.e., by 54% and 77% at 5 and 10 μmol/l compared with the nonexposed control, respectively (P < 0.001, one-way ANOVA with post hoc Tukey’s multiple comparison test; Fig. 4B). Further, we employed a wound-healing assay to test if T-type Ca2+ channel blockade in PMVECs impairs cell migration. PMVECs were grown to confluence, a gap was created by scratching the cell monolayer, and then under a serum-starved condition, the process of gap closure, i.e., mostly owing to cell migration, was monitored over an 18-h time frame. As shown in Fig. 4C, the T-type Ca2+ channel blocker NNC 55-0396 (5 μmol/l) or silencing the α1G significantly delayed the gap closure by up to 32% and 28%, respectively (P < 0.0001 each, Student’s t-test). Taken together, α1G T-type Ca2+ channel contributes to all of the major angiogenesis components in PMVECs.
Fig. 4.
T-type Ca2+ channel blockade or α1G-silencing by shRNA reduced pulmonary microvascular endothelial cell (PMVEC) proliferation, cell-matrix interaction, and migration. A: effect of α1G-gene silencing on cell proliferation of PMVECs. Cell growth was observed over a 3-day period in normal (□), scrambled-shRNA-transduced (○), and α1G-shRNA-transduced (■) PMVECs (n = 3 each). The α1G-shRNA transduction reduced the proliferation of PMVECs by 31% at day 3. ‡P < 0.05 relative to scrambled-shRNA-transduced group at the same time point. Two-way ANOVA with Bonferroni’s multiple comparisons test. B: effect of T-type Ca2+ channel blockade on cell-matrix interaction of PMVECs. Ba: representative images of adherent PMVECs remaining on the Matrigel-coated substratum after rinsing with PBS following cell seeding and subsequent 30-min incubation in the absence (left) or presence of NNC 55-0396 at the concentration of 5 (middle) and 10 (right) μmol/l. Images acquired under an inverted light microscope with a ×10, 0.30 numerical aperture objective. Bb: cell-matrix adhesion strength is estimated as relative number of attached cells in treated PMVECs relative to untreated control PMVECs (mean ± SE), obtained from 10 to 12 random fields from triplicate Matrigel-coated substratum in a single assay that was replicated 3 times. NNC 55-0396 dose-dependently reduced the number of PMVECs attached to the substratum. One-way ANOVA (F = 114, P < 0.0001) with post hoc Tukey’s multiple comparison test (☆P < 0.001). C: effect of T-type Ca2+ channel blockade or shRNA α1G suppression on cell migration of PMVECs. Ca: representative images of monolayer PMVECs in serum-starved media at 0 and 18 h postscratch in the absence (vehicle control) or presence of NNC 55-0396 (5 μmol/l) during the 18-h interval. Cb: representative images of cell monolayers of scrambled-RNA-transduced PMVECs and α1G-shRNA-transduced PMVECs in serum-starved media at 0 and 18 h postscratch. The images were acquired on a Nikon TS100-F inverted optical microscope with a ×10, 0.30 numerical aperture objective. The lines indicated the boundary of scratch or frontier of gap closure. Cell migration was assessed by area recovery of the scratched gap, measured at time 0 and 18 h in every group. The percentage recovery (closure) of initial scratch area was compared. Cc: quantification results (mean ± SEM) of wound closure from triplicate wells of each cell group in a single assay that was replicated 3 times (*P < 0.0001, Student’s t-test).
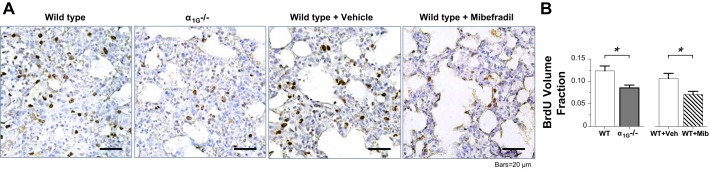
Blockade or knockout of α1G reduces postinjury proliferation in the lung.
The formation of new blood vessels through de novo angiogenesis is an essential process in developmental biology, postnatal vascular homeostasis and wound healing, as well as in many disease pathogeneses. To explore whether the endothelial α1G T-type Ca2+ channel plays an active role in vivo in endothelial regeneration or vascular repair after inflammatory vascular injury, we conducted the study utilizing a murine model of LPS-induced acute lung injury, in which the lung injury was produced in mice by intratracheal LPS challenge, lung tissue specimen was collected at 72 h after the challenge, and postinjury cell proliferation was subsequently assessed with BrdU immunohistochemistry in the lung sections. We observed a dramatic reduction in postinjury cell proliferation, i.e., BrdU incorporation, likely within lung microvascular segments from the α1G-knockout mice or wild-type mice systemically treated with T-type Ca2+ channel blocker before and after LPS challenge as compared with the lungs from their untreated wild-type littermates (Fig. 5A). To verify this subjective assessment, we utilized a morphometric approach to determine a volume fraction for BrdU incorporation in the lung tissue in each group. The results of this quantitative assessment (Fig. 5B) explicitly show that the BrdU volume fraction is selectively decreased in lungs from the α1G-knockout mice or wild-type mice systemically treated with the T-type Ca2+ channel blocker (P < 0.0001 each, Student’s t-test). These results provide in vivo clues that overall postinjury proliferation is compromised in lungs lacking α1G or in the presence of T-type Ca2+ channel blockade.
Fig. 5.
T-type channel blockade or α1G knockout reduced postinjury proliferation in the lung. A: representative micrographs of immunohistochemistry for 5-bromodeoxyuridine (BrdU) in tissue sections from lungs of wild-type (α1G+/+) mice, α1G-knockout (α1G−/−) mice, and wild-type mice with vehicle or mibefradil intraperitoneal injection, collected 72 h after the mice were intratracheally instilled with LPS. Note that mibefradil (5 mg/kg) was administered intraperitoneally once a day for 3 doses, with the first dose given 1 day before LPS challenge. BrdU, used to identify proliferating cells, was intraperitoneally injected to the mice 3 h before euthanization and lung collection. Lungs from the α1G-knockout mice or the wild-type mice treated with mibefradil displayed a considerable reduction of immunoreactivity to BrdU. B: morphometric assessment of BrdU immunoreactivity with a point-counting strategy, yielding a measure of the BrdU volume fraction, calculated as the ratio of BrdU positive points relative to total points landing on the nucleus. The volume fraction data were then averaged for each treatment group. BrdU volume fraction (means ± SE) is significantly lower in lungs from the α1G-knockout mice or the wild-type mice treated with mibefradil as compared with the lungs from the wild-type mice or the wild-type mice treated with vehicle alone. Four mice in each group; *P < 0.0001, Student’s t-test. Veh, vehicle; WT, wild-type.
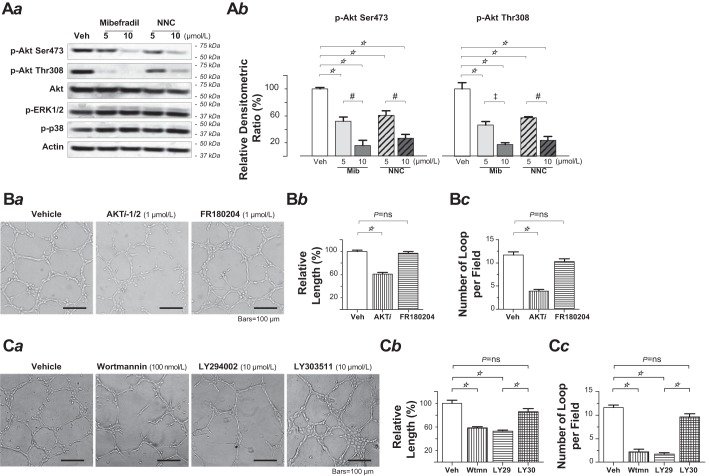
T-type Ca2+ channel blockade decreased Akt phosphorylation in PMVECs.
We then wanted to resolve the possible mechanisms for the angiogenic compromise by the T-type Ca2+ channel inhibition. The phosphatidylinositol-3 kinase (PI3K)-Akt signaling axis is a crucial signaling system in endothelial cells, as the axis is activated by many angiogenic growth factors, and it in turn regulates a variety of downstream target molecules that are potentially involved in angiogenesis (1, 9, 22, 24). Akt, also named protein kinase B, is a serine-threonine kinase that plays a crucial role in endothelial cell survival and angiogenesis (1, 9, 22, 24). Akt is activated downstream of PI3K by phosphorylation of its two regulatory residues, threonine 308 (Thr308) and serine 473 (Ser473), and maximal Akt activity is dependent on the phosphorylation status of both residues (3). Indeed, prominent Akt phosphorylation at both Thr308 and Ser473 was detected in PMVECs under a nonstimulated condition. Exposure of PMVECs to the T-type Ca2+ channel blocker mibefradil or NNC 55-0396 (5 μmol/l or 10 μmol/l each) did not alter the level of total Akt protein but markedly reduced Akt phosphorylation on both sites in what appears to be a dose-dependent manner (Fig. 6A). These effects by T-type Ca2+ channel blockade in PMVECs were likely specific for Akt because, in contrast, no change in ERK or p38 kinase phosphorylation was observed following exposure to either T-type Ca2+ channel blocker (Fig. 6Aa).
Fig. 6.
T-type Ca2+ channel blockade decreased Akt phosphorylation in pulmonary microvascular endothelial cells (PMVECs). A: Western blot analysis of phospho-Akt (Ser473), phospho-Akt (Thr308), total Akt, phospho-ERK1/2 (Thr202/Tyr204), and phospho-p38 level in PMVECs following a 6-h exposure to vehicle alone (control), mibefradil, or NNC 55-0396 at the indicated concentrations (5 or 10 μmol/l for each blocker). Whole cell lysates from the PMVECs were separated by SDS-PAGE and immunoblotted with the indicated antibodies. Aa: representative Western blot from three independent experiments. Ab: relative densitometric ratios (mean ± SE) of phospho-Akt (Ser473) and phospho-Akt (Thr308), calculated from the ratio of the adjusted band intensity of each individual protein (to its correspondent loading control) to the adjusted band intensity of the same protein in control cell group that was only with vehicle exposure. Replicated three times. One-way ANOVA (F = 30.4, P < 0.0001 for p-Akt Ser473; F = 32.6, P < 0.0001 for p-Akt Thr308) with Tukey’s post hoc multiple comparison test (☆P < 0.001, #P < 0.01, ‡P < 0.05). Both mibefradil and NNC 55-0396 dose-dependently decreased the level of phospho-Akt (Ser473) and phospho-Akt (Thr308). B: Akt inhibition impaired the network formation capacity of PMVECs. Ba: representative phase-contrast images of the tubular network formed by PMVECs at 18 h after the cells were plated on Matrigel in the absence (left) or presence of AKTi-1/2 (middle) and FR180204 (right). Bb: relative total tubular length of the network, including the length of closed and unclosed, but continuous, cellular cords from 10 to 20 random fields from triplicate Matrigel-coated wells in a single assay that was replicated 3 times. One-way ANOVA (F = 57.7, P < 0.0001) with post hoc Tukey’s comparison (☆P < 0.001). Bc: bar graph showing the number of complete loops per field, from 10 to 20 random fields from triplicate Matrigel-coated wells in a single assay that was replicated 3 times. One-way ANOVA (F = 56.2, P < 0.0001) with post hoc Tukey’s multiple comparison test (☆P < 0.001). C: phosphatidylinositol 3-kinase inhibition impaired the network formation capacity of PMVECs. Ca: representative phase-contrast images of the tubular network formed by PMVECs at 18 h after the cells were plated on Matrigel in the absence (far left) or presence of, from left to right, wortmannin, LY294002, and LY303511. Cb: relative total tubular length of the network, including the length of closed and unclosed, but continuous, cellular cords from 10 to 20 random fields from triplicate Matrigel-coated wells in a single assay that was replicated 3 times. One-way ANOVA (F = 31.8, P < 0.0001) with post hoc Tukey’s comparison (☆P < 0.001). Cc: bar graph showing the number of complete loops per field, from 10 to 20 random fields from triplicate Matrigel-coated wells in a single assay that was replicated 3 times. One-way ANOVA (F = 88.4, P < 0.0001) with post hoc Tukey’s multiple comparison test (☆P < 0.001). ns, not significant; Veh, vehicle; Wtmn, wortmannin.
PI3K or Akt inhibition impaired the network formation capacity of PMVECs.
Now that inhibition of the endothelial α1G T-type Ca2+ channel impairs the cell’s angiogenic potential and, in the meantime, decreases Akt activity by reducing the phosphorylation at its regulatory sites Thr308 and Ser473, direct inhibition of Akt or its upstream activator PI3K should also disrupt in vitro network formation of PMVECs. In the study utilizing Matrigel network formation assay, application of the Akt inhibitor Akti-1/2 (1 μmol/l) to PMVECs significantly reduced network formation (Fig. 6B). Application of each of the 2 PI3K inhibitors, i.e., LY294002 (10 μmol/l) and wortmannin (100 nmol/l), also significantly reduced network formation, whereas LY303511, a close structural analog of LY294002, which does not inhibit PI3K-dependent phosphorylation of Akt, failed to do so (Fig. 6C). By the same token, because we found no effect of T-type Ca2+ channel inhibition on levels of ERK phosphorylation in PMVECs, the inhibition of ERK should not disrupt the cells’ in vitro network formation. Indeed, application of the selective ERK inhibitor FR180204 (1 μmol/l) to PMVECs did not affect the cells in vitro network formation (Fig. 6B). Altogether, these results suggest that, in PMVECs, basal α1G-Ca2+ and PI3K-Akt signaling axis may interact intimately to control the cell’s proangiogenic phenotype.
DISCUSSION
This study provides compelling evidence for a novel functional role of the endothelial α1G T-type Ca2+ channel in angiogenesis.
Matrigel is among the most popular in vitro angiogenesis assays that utilize extracellular matrix substitutes to induce capillary-like network formation from endothelial cells. In Matrigel matrix, endothelial cells proliferate and migrate to form a capillary-like tubular network, representing a preliminary step of neovessel formation. Regular Matrigel has biological activity largely dependent on its composition in growth factors (15, 19). To assess the “intrinsic” angiogenic capacity of PMVECs, we intended to use a growth factor-reduced Matrigel matrix preparation that has effectively reduced the level of assorted growth factors. PMVECs displayed a vigorous ability to rapidly form a capillary-like network in the growth factor-reduced Matrigel. Vascular endothelial growth factor (VEGF) is an important mediator that is widely recognized to promote angiogenesis (8). Yet, strikingly, we observed that the network formation efficiency of PMVECs remained remarkably intact when the cells were treated with a highly potent VEGF receptor-2 inhibitor, SU5416. Endothelium-derived vasoactive autocoid nitric oxide (NO), generated upon the stimulation of VEGF and other growth factors or neurohumoral inflammatory mediators, also promotes angiogenesis (2, 5, 28, 39, 45). It is intriguing that antagonizing nitric oxide synthase (NOS) with l-NAME or l-NNA barely interfered in vitro network formation of PMVECs, whereas it rather dramatically reduced the network formation of PAECs. VEGF is widely recognized for its functional role in promoting angiogenesis (8). These findings provide unequivocal evidence that PMVECs display a unique proangiogenic phenotype with an intrinsic capacity to readily launch on “angiogenesis” independent of VEGF or endothelial NOS (NOS3)-NO signaling systems.
We found that depletion of extracellular free Ca2+, i.e., when PMVEC extracellular Ca2+ was chelated with EGTA to a low micromolar level, abolished the network formation. This result emphasizes that extracellular Ca2+ homeostasis is an imperative need for the cells to sustain their angiogenic activity. In steady-state, cytosolic free Ca2+ ([Ca2+]i) is maintained at the level of nearly 10−7 mol/l, lower than the extracellular by a factor of 10−4. This immense concentration gradient is achieved by means of meticulously regulated Ca2+ entry through a collection of Ca2+-permeable channels and active mechanisms that remove Ca2+ from the cytosol, e.g., the plasma membrane Ca2+-ATPase, the Na+-Ca2+ exchanger, and the sarco-endoplasmic reticulum Ca2+-ATPase. Although transient increases in [Ca2+]i could still occur through Ca2+ release from the intracellular stores when extracellular Ca2+ is temporally depleted, prolonged cellular activities, as in the case of angiogenesis, most likely rely on Ca2+ entry that would not be possible in the absence of extracellular Ca2+ homeostasis.
Lung endothelial cell Ca2+ entry occurs through a variety of Ca2+-permeable channels that respond to different stimuli and couple to different cellular responses. PMVECs express canonical transient receptor potential (TRP) isoform 1 and 4 (TRPC1 and TRPC4) that encode for a Ca2+-selective ISOC (7, 41). Ca2+ entry resulting from activation of ISOC leads specifically to endothelial barrier disruption (40, 41). In addition, TRP vanilloid 4 (TRPV4) and α1G voltage-gated T-type Ca2+ channels represent two novel Ca2+ entry pathways in the alveolar septal capillary endothelium (42, 48). Ca2+ entry elicited by activation of TRPV4 results in selective impairment of endothelial barrier integrity, whereas Ca2+ entry elicited by activation of α1G T-type Ca2+ channel specifically results in P-selectin surface expression in alveolar capillary endothelium (43, 46).
The present study demonstrates for the first time a novel functional role for the α1G T-type Ca2+ channel in angiogenesis. Pharmacological T-type Ca2+ channel blockade and α1G gene silencing both blocked the network formation of PMVECs in vitro. The implication of Ca2+ entry pathway in network formation appears to be α1G-specific and does not imply the involvement of any other Ca2+ entry pathways, as, first, neither TRPC4 nor TRPV4 blockade affected the network formation of PMVECs, and second, the observations also draw a closer parallel in the reduction of the network formation between the cause of nominally Ca2+-free medium and the cause of α1G-silencing. It is worth noting that there appeared to be some “residual” network formation under both conditions, i.e., the condition of virtually complete α1G-silencing and the condition of nominally Ca2+-free extracellular environment. A rational interpretation of this discrepancy is the unbridgeable difference between the realistically achieved level of α1G-silencing and the absolute α1G-knockdown and the difference between nominal and absolute Ca2+-free extracellular environment. We reason that it is the absolute α1G-knockdown or absolute Ca2+-free extracellular environment that is required to abolish the network formation in PMVECs.
Disruption of the network formation in vitro was not alone the consequence of T-type Ca2+ channel blockade, as cell proliferation, cell-matrix adhesion, and cell migration were all markedly reduced by T-type Ca2+ channel blockade or α1G silencing. Thus, T-type Ca2+ channel blockade evidently disrupts multiple components of the PMVEC angiogenic activities. We also examined cell proliferation in vivo using a mouse model of LPS-induced lung injury. Postinjury cell proliferation, assessed with BrdU immunohistochemistry in lung sections, was dramatically reduced in lungs from α1G-knockout mice or wild-type mice systemically treated with T-type Ca2+ channel blocker compared with the lungs from their untreated wild-type littermates. Postinjury proliferation occurs in several different cell types, including endothelial cells, epithelial cells, alveolar macrophages, and fibroblasts. As BrdU immunohistochemistry does not exclude proliferating cells of nonendothelial origins from the whole picture of proliferation, these results need to be interpreted with caution. Nonetheless, the data may suggest an active role for α1G in in vivo tissue regeneration and repair during recovery from acute lung injury.
An important biophysical property of the T‐type Ca2+ channels is that all three channel isoforms, namely α1G, α1H, and α1I, exhibit a tiny yet distinctly evident window current. That is, there exists a voltage range where a fraction of channels can activate but not completely inactivate and therefore remain open and constantly conduct Ca2+ entry, i.e., “window” T‐type Ca2+ current. Superimposition of the voltage-dependent activation and inactivation curves predicts that the voltage range of the endothelial cell window α1G T-type Ca2+ current is between −60 and −30 mV (42, 48). Such voltage range sandwiches perfectly into the two sets of resting membrane potential found in most endothelial cells, −70 to −60 mV and −40 to −10 mV (26), and α1G-mediated Ca2+ entry may take place readily in the form of window current as membrane potential fluctuations. Thus, α1G may represent a novel background Ca2+ entry pathway in PMVECs under basal conditions as well as during environmental and physiological stimulations, necessary for cells to sustain a proangiogenic phenotype.
In recent studies, initial T-type Ca2+ channel blockade and its resultant suppression of cell proliferation were linked together with suppressed PI3K-Akt signaling in certain cancer cells (10, 38). In the present study, PMVECs were revealed to have a considerable level of Akt phosphorylation at both Thr308 and Ser473 residues under resting conditions, indicating bustling basal Akt activity. Pharmacological inhibition of Akt disrupts in vitro network formation of PMVECs. Inhibition of PI3K, the upstream regulator of Akt in the PI3K-Akt signaling pathway, also disrupts network formation. In contrast, inhibition of ERK or p38 kinases did not affect network formation, although the basal level of ERK and p38 kinase phosphorylation was also evident in nonstimulated PMVECs. These results indicate that Akt activity is a critical determinant of the intrinsic angiogenic capacity of PMVECs.
Increase in [Ca2+]i is essential for activation of the PI3K-Akt cascade as well as Akt phosphorylation in a number of cell systems (11, 16, 32, 37, 44). Here, we observed that blockade of the T-type Ca2+ channel decreased Akt phosphorylation at both Thr308 and Ser473 residues in a dose-dependent manner, an effect without interfering Akt total protein amount. Since the complete Akt activity depends on the phosphorylation level at both of the two residues as well as on its total protein concentration (37), these results demonstrate a direct and specific link between the functional α1G, conceivably through its window T-type Ca2+ current, to the basal Akt activity and then to the angiogenic potential of the cells. Consistent with this notion, blockade of the T-type Ca2+ channel in PMVECs did not alter the phosphorylation of ERK and p38 kinase, whose inhibition had no effect on network formation of the cells. A possible mechanism that links the α1G-mediated Ca2+ current to the basal phosphorylation status of Akt is through Ca2+/calmodulin (CaM)‐dependent protein kinases (CaMKs). Many cellular Ca2+-dependent signaling cascades utilize CaM as the intracellular Ca2+ receptor. Ca2+/CaM binds and activates a plethora of enzymes, including Ca2+/CaMKs. Studies have shown that CaMKs participate in the activation of multiple targets, including Akt and PI3K (13, 31, 44). We examined the phosphorylation level of CaMK2, one of the CaMKs expressed in PMVECs, but found no change of its phosphorylation level after application of T-type Ca2+ channel blockers (data not shown). Considering that there still exist numerous Ca2+-dependent protein kinases in PMVECs, identifying the actual Ca2+-dependent protein kinase of the α1G channel will be a challenging task in the future.
In conclusion, we have demonstrated for the first time that voltage-gated T-type Ca2+ channels play an important role in angiogenesis in pulmonary microvascular endothelial cells. Our work supports a novel and physiologically relevant paradigm in lung endothelial biology, in which the endothelial site-specific α1G T-type Ca2+ channel in the nonstimulated plasma membrane functions as a background Ca2+ entry pathway that couples a PI3K-Akt signaling cascade and constitutive Akt phosphorylation leading to a proangiogenic endothelial phenotype. We anticipate that modulators of the α1G-dependent Ca2+ signaling may have the therapeutic potential to optimize endothelial angiogenic capacity under physiological conditions or during pathological disease processes.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-66299 and start-up funds from the Department of Anesthesiology and Perioperative Medicine, University of Alabama at Birmingham (to S. Wu).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Z.Z. and S.W. conceived and designed research; Z.Z., H.C., P.X., C.A.D., and M.F.A. performed experiments; Z.Z., H.C., P.X., and S.W. analyzed data; Z.Z., J.A.C.K., and S.W. interpreted results of experiments; Z.Z., H.C., and S.W. prepared figures; Z.Z. and S.W. drafted manuscript; S.W. edited and revised manuscript; Z.Z., H.C., P.X., C.A.D., J.A.C.K., M.F.A., and S.W. approved final version of manuscript.
Supplemental Data
PMVECs rapidly form capillary-like tubular networks in Matrigel - .avi (12 MB)
ACKNOWLEDGMENTS
The authors thank Prof. Hee-Sup Shin (Center for Cognition and Sociality, Institute for Basic Science, Daejeon, Republic of Korea) for kindly providing α1G−/− mice. The authors thank Tim Kurtz (Medical College of Georgia, Augusta University, Augusta, GA) for excellent technical assistance.
REFERENCES
- 1.Ackah E, Yu J, Zoellner S, Iwakiri Y, Skurk C, Shibata R, Ouchi N, Easton RM, Galasso G, Birnbaum MJ, Walsh K, Sessa WC. Akt1/protein kinase Balpha is critical for ischemic and VEGF-mediated angiogenesis. J Clin Invest 115: 2119–2127, 2005. doi: 10.1172/JCI24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Ani B, Hewett PW, Ahmed S, Cudmore M, Fujisawa T, Ahmad S, Ahmed A. The release of nitric oxide from S-nitrosothiols promotes angiogenesis. PLoS One 1: e25, 2006. doi: 10.1371/journal.pone.0000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J 15: 6541–6551, 1996. doi: 10.1002/j.1460-2075.1996.tb01045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ausprunk DH, Folkman J. Migration and proliferation of endothelial cells in preformed and newly formed blood vessels during tumor angiogenesis. Microvasc Res 14: 53–65, 1977. doi: 10.1016/0026-2862(77)90141-8. [DOI] [PubMed] [Google Scholar]
- 5.Bussolati B, Dunk C, Grohman M, Kontos CD, Mason J, Ahmed A. Vascular endothelial growth factor receptor-1 modulates vascular endothelial growth factor-mediated angiogenesis via nitric oxide. Am J Pathol 159: 993–1008, 2001. doi: 10.1016/S0002-9440(10)61775-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cioffi DL, Moore TM, Schaack J, Creighton JR, Cooper DM, Stevens T. Dominant regulation of interendothelial cell gap formation by calcium-inhibited type 6 adenylyl cyclase. J Cell Biol 157: 1267–1278, 2002. doi: 10.1083/jcb.200204022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cioffi DL, Wu S, Chen H, Alexeyev M, St Croix CM, Pitt BR, Uhlig S, Stevens T. Orai1 determines calcium selectivity of an endogenous TRPC heterotetramer channel. Circ Res 110: 1435–1444, 2012. doi: 10.1161/CIRCRESAHA.112.269506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coultas L, Chawengsaksophak K, Rossant J. Endothelial cells and VEGF in vascular development. Nature 438: 937–945, 2005. doi: 10.1038/nature04479. [DOI] [PubMed] [Google Scholar]
- 9.Downward J. PI 3-kinase, Akt and cell survival. Semin Cell Dev Biol 15: 177–182, 2004. doi: 10.1016/j.semcdb.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Dziegielewska B, Casarez EV, Yang WZ, Gray LS, Dziegielewski J, Slack-Davis JK. T-type Ca2+ channel inhibition sensitizes ovarian cancer to carboplatin. Mol Cancer Ther 15: 460–470, 2016. doi: 10.1158/1535-7163.MCT-15-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farnsworth CL, Freshney NW, Rosen LB, Ghosh A, Greenberg ME, Feig LA. Calcium activation of Ras mediated by neuronal exchange factor Ras-GRF. Nature 376: 524–527, 1995. doi: 10.1038/376524a0. [DOI] [PubMed] [Google Scholar]
- 12.Gargett CE, Rogers PA. Human endometrial angiogenesis. Reproduction 121: 181–186, 2001. doi: 10.1530/rep.0.1210181. [DOI] [PubMed] [Google Scholar]
- 13.Gocher AM, Azabdaftari G, Euscher LM, Dai S, Karacosta LG, Franke TF, Edelman AM. Akt activation by Ca2+/calmodulin-dependent protein kinase kinase 2 (CaMKK2) in ovarian cancer cells. J Biol Chem 292: 14188–14204, 2017. doi: 10.1074/jbc.M117.778464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hudlicka O, Brown M, Egginton S. Angiogenesis in skeletal and cardiac muscle. Physiol Rev 72: 369–417, 1992. doi: 10.1152/physrev.1992.72.2.369. [DOI] [PubMed] [Google Scholar]
- 15.Hughes CS, Postovit LM, Lajoie GA. Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics 10: 1886–1890, 2010. doi: 10.1002/pmic.200900758. [DOI] [PubMed] [Google Scholar]
- 16.Joyal JL, Burks DJ, Pons S, Matter WF, Vlahos CJ, White MF, Sacks DB. Calmodulin activates phosphatidylinositol 3-kinase. J Biol Chem 272: 28183–28186, 1997. doi: 10.1074/jbc.272.45.28183. [DOI] [PubMed] [Google Scholar]
- 17.Kim D, Song I, Keum S, Lee T, Jeong MJ, Kim SS, McEnery MW, Shin HS. Lack of the burst firing of thalamocortical relay neurons and resistance to absence seizures in mice lacking α(1G) T-type Ca(2+) channels. Neuron 31: 35–45, 2001. doi: 10.1016/S0896-6273(01)00343-9. [DOI] [PubMed] [Google Scholar]
- 18.King J, Hamil T, Creighton J, Wu S, Bhat P, McDonald F, Stevens T. Structural and functional characteristics of lung macro- and microvascular endothelial cell phenotypes. Microvasc Res 67: 139–151, 2004. doi: 10.1016/j.mvr.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 19.Kleinman HK, Martin GR. Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol 15: 378–386, 2005. doi: 10.1016/j.semcancer.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Kohn EC, Alessandro R, Spoonster J, Wersto RP, Liotta LA. Angiogenesis: role of calcium-mediated signal transduction. Proc Natl Acad Sci USA 92: 1307–1311, 1995. doi: 10.1073/pnas.92.5.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lamalice L, Le Boeuf F, Huot J. Endothelial cell migration during angiogenesis. Circ Res 100: 782–794, 2007. doi: 10.1161/01.RES.0000259593.07661.1e. [DOI] [PubMed] [Google Scholar]
- 22.Lee MY, Luciano AK, Ackah E, Rodriguez-Vita J, Bancroft TA, Eichmann A, Simons M, Kyriakides TR, Morales-Ruiz M, Sessa WC. Endothelial Akt1 mediates angiogenesis by phosphorylating multiple angiogenic substrates. Proc Natl Acad Sci USA 111: 12865–12870, 2014. doi: 10.1073/pnas.1408472111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu F, Chen H, Zhou C, Liu S, Guo M, Chen P, Zhuang H, Xie D, Wu S. T-type Ca2+ channel expression in human esophageal carcinomas: a functional role in proliferation. Cell Calcium 43: 49–58, 2008. doi: 10.1016/j.ceca.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manning BD, Toker A. AKT/PKB signaling: navigating the network. Cell 169: 381–405, 2017. doi: 10.1016/j.cell.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munaron L. Calcium signalling and control of cell proliferation by tyrosine kinase receptors (review). Int J Mol Med 10: 671–676, 2002. [PubMed] [Google Scholar]
- 26.Nilius B, Droogmans G. Ion channels and their functional role in vascular endothelium. Physiol Rev 81: 1415–1459, 2001. doi: 10.1152/physrev.2001.81.4.1415. [DOI] [PubMed] [Google Scholar]
- 27.Patton AM, Kassis J, Doong H, Kohn EC. Calcium as a molecular target in angiogenesis. Curr Pharm Des 9: 543–551, 2003. doi: 10.2174/1381612033391559. [DOI] [PubMed] [Google Scholar]
- 28.Pyriochou A, Beis D, Koika V, Potytarchou C, Papadimitriou E, Zhou Z, Papapetropoulos A. Soluble guanylyl cyclase activation promotes angiogenesis. J Pharmacol Exp Ther 319: 663–671, 2006. doi: 10.1124/jpet.106.108878. [DOI] [PubMed] [Google Scholar]
- 29.Reynolds LP, Redmer DA. Expression of the angiogenic factors, basic fibroblast growth factor and vascular endothelial growth factor, in the ovary. J Anim Sci 76: 1671–1681, 1998. doi: 10.2527/1998.7661671x. [DOI] [PubMed] [Google Scholar]
- 30.Rodman DM, Reese K, Harral J, Fouty B, Wu S, West J, Hoedt-Miller M, Tada Y, Li KX, Cool C, Fagan K, Cribbs L. Low-voltage-activated (T-type) calcium channels control proliferation of human pulmonary artery myocytes. Circ Res 96: 864–872, 2005. doi: 10.1161/01.RES.0000163066.07472.ff. [DOI] [PubMed] [Google Scholar]
- 31.Rotfeld H, Hillman P, Ickowicz D, Breitbart H. PKA and CaMKII mediate PI3K activation in bovine sperm by inhibition of the PKC/PP1 cascade. Reproduction 147: 347–356, 2014. doi: 10.1530/REP-13-0560. [DOI] [PubMed] [Google Scholar]
- 32.Soderling TR. The Ca-calmodulin-dependent protein kinase cascade. Trends Biochem Sci 24: 232–236, 1999. doi: 10.1016/S0968-0004(99)01383-3. [DOI] [PubMed] [Google Scholar]
- 33.Solodushko V, Fouty B. Proproliferative phenotype of pulmonary microvascular endothelial cells. Am J Physiol Lung Cell Mol Physiol 292: L671–L677, 2007. doi: 10.1152/ajplung.00304.2006. [DOI] [PubMed] [Google Scholar]
- 34.Sypniewska G, Björntorp P. Increased DNA synthesis in adipocytes and capillary endothelium in rat adipose tissue during overfeeding. Eur J Clin Invest 17: 202–207, 1987. doi: 10.1111/j.1365-2362.1987.tb01236.x. [DOI] [PubMed] [Google Scholar]
- 35.Tahergorabi Z, Khazaei M. A review on angiogenesis and its assays. Iran J Basic Med Sci 15: 1110–1126, 2012. [PMC free article] [PubMed] [Google Scholar]
- 36.Townsley MI, King JA, Alvarez DF. Ca2+ channels and pulmonary endothelial permeability: insights from study of intact lung and chronic pulmonary hypertension. Microcirculation 13: 725–739, 2006. doi: 10.1080/10739680600930362. [DOI] [PubMed] [Google Scholar]
- 37.Vaillant AR, Mazzoni I, Tudan C, Boudreau M, Kaplan DR, Miller FD. Depolarization and neurotrophins converge on the phosphatidylinositol 3-kinase-Akt pathway to synergistically regulate neuronal survival. J Cell Biol 146: 955–966, 1999. doi: 10.1083/jcb.146.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valerie NC, Dziegielewska B, Hosing AS, Augustin E, Gray LS, Brautigan DL, Larner JM, Dziegielewski J. Inhibition of T-type calcium channels disrupts Akt signaling and promotes apoptosis in glioblastoma cells. Biochem Pharmacol 85: 888–897, 2013. doi: 10.1016/j.bcp.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 39.Verma S, Wang CH, Li SH, Dumont AS, Fedak PW, Badiwala MV, Dhillon B, Weisel RD, Li RK, Mickle DA, Stewart DJ. A self-fulfilling prophecy: C-reactive protein attenuates nitric oxide production and inhibits angiogenesis. Circulation 106: 913–919, 2002. doi: 10.1161/01.CIR.0000029802.88087.5E. [DOI] [PubMed] [Google Scholar]
- 40.Wu S, Chen H, Alexeyev MF, King JA, Moore TM, Stevens T, Balczon RD. Microtubule motors regulate ISOC activation necessary to increase endothelial cell permeability. J Biol Chem 282: 34801–34808, 2007. doi: 10.1074/jbc.M704522200. [DOI] [PubMed] [Google Scholar]
- 41.Wu S, Cioffi EA, Alvarez D, Sayner SL, Chen H, Cioffi DL, King J, Creighton JR, Townsley M, Goodman SR, Stevens T. Essential role of a Ca2+-selective, store-operated current (ISOC) in endothelial cell permeability: determinants of the vascular leak site. Circ Res 96: 856–863, 2005. doi: 10.1161/01.RES.0000163632.67282.1f. [DOI] [PubMed] [Google Scholar]
- 42.Wu S, Haynes J Jr, Taylor JT, Obiako BO, Stubbs JR, Li M, Stevens T. Cav3.1 (α1G) T-type Ca2+ channels mediate vaso-occlusion of sickled erythrocytes in lung microcirculation. Circ Res 93: 346–353, 2003. doi: 10.1161/01.RES.0000087148.75363.8F. [DOI] [PubMed] [Google Scholar]
- 43.Wu S, Jian MY, Xu YC, Zhou C, Al-Mehdi AB, Liedtke W, Shin HS, Townsley MI. Ca2+ entry via α1G and TRPV4 channels differentially regulates surface expression of P-selectin and barrier integrity in pulmonary capillary endothelium. Am J Physiol Lung Cell Mol Physiol 297: L650–L657, 2009. doi: 10.1152/ajplung.00015.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yano S, Tokumitsu H, Soderling TR. Calcium promotes cell survival through CaM-K kinase activation of the protein-kinase-B pathway. Nature 396: 584–587, 1998. doi: 10.1038/25147. [DOI] [PubMed] [Google Scholar]
- 45.Zhang R, Wang L, Zhang L, Chen J, Zhu Z, Zhang Z, Chopp M. Nitric oxide enhances angiogenesis via the synthesis of vascular endothelial growth factor and cGMP after stroke in the rat. Circ Res 92: 308–313, 2003. doi: 10.1161/01.RES.0000056757.93432.8C. [DOI] [PubMed] [Google Scholar]
- 46.Zhou C, Chen H, King JA, Sellak H, Kuebler WM, Yin J, Townsley MI, Shin HS, Wu S. α1G T-type calcium channel selectively regulates P-selectin surface expression in pulmonary capillary endothelium. Am J Physiol Lung Cell Mol Physiol 299: L86–L97, 2010. doi: 10.1152/ajplung.00331.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou C, Chen H, Lu F, Sellak H, Daigle JA, Alexeyev MF, Xi Y, Ju J, van Mourik JA, Wu S. Cav3.1 (α1G) controls von Willebrand factor secretion in rat pulmonary microvascular endothelial cells. Am J Physiol Lung Cell Mol Physiol 292: L833–L844, 2007. doi: 10.1152/ajplung.00377.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou C, Wu S. T-type calcium channels in pulmonary vascular endothelium. Microcirculation 13: 645–656, 2006. doi: 10.1080/10739680600930289. [DOI] [PubMed] [Google Scholar]
- 49.Zhu B, Strada S, Stevens T. Cyclic GMP-specific phosphodiesterase 5 regulates growth and apoptosis in pulmonary endothelial cells. Am J Physiol Lung Cell Mol Physiol 289: L196–L206, 2005. doi: 10.1152/ajplung.00433.2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PMVECs rapidly form capillary-like tubular networks in Matrigel - .avi (12 MB)