Abstract
Background
A recent cluster randomized trial evaluating a multicomponent intervention showed significant reductions in blood pressure in low-income hypertensive subjects in Argentina. The objective of this analysis is to assess the cost-effectiveness of this intervention.
Methods
1,432 hypertensive participants from 18 primary healthcare centers. The intervention included community health worker-led home visits, physician education, and text-messaging. Resource use and quality of life using the EQ-5D-3L were prospectively collected. Study perspective was that of the public healthcare system, and the time horizon was 18 months. Intention-to-treat analysis was used to analyze cost and health outcomes (systolic blood pressure [BP] change and quality adjusted life years[QALYs]). A one GDP per capita per QALY was used as the cost-effectiveness threshold (14,062 US dollars[$]).
Results
Baseline characteristics were similar in the two arms. QALYs significantly increased by 0.06 (95% CI: 0.04 to 0.09) in the intervention group, and systolic BP net difference favored the intervention group: 5.3 mm Hg (95% CI: 0.27 to 10.34). Mean total costs per participant were higher in the intervention arm: $304 in the intervention group and $154 in the control group (adjusted difference of $140.18; 95% CI: $75.41 to $204.94). The incremental cost-effectiveness ratio was $3,299 per QALY (95% CI: 1,635 to 6,099) and $26 per mm Hg of systolic BP (95% CI: 13 to 46). Subgroup analysis showed that the intervention was cost-effective in all pre-specified subgroups (age, gender, cardiovascular risk, and body mass index).
Conclusions
The multicomponent intervention was cost-effective for blood-pressure control among low-income hypertensive patients.
Keywords: Hypertension, cost-effectiveness, primary care, low-income setting
Introduction
Hypertension is among the leading modifiable risk factors for cardiovascular disease and premature death worldwide,(1) and there have been significant advances in prevention and treatment.(2) Nevertheless, increasing prevalence and a high proportion of uncontrolled patients, particularly in low- and middle-income countries (LMIC), indicate that important gaps remain. (3, 4) Globally, almost one in three adults has hypertension, and three out of four live in LMIC (5). Furthermore, only around one in ten hypertensive patients in LMIC have their blood pressure (BP) controlled to <140/90 mm Hg. (3) It is, therefore, a key public health priority to develop and implement effective, affordable, and sustainable hypertension control programs.
The Hypertension Control Program in Argentina (HCPIA) was a cluster randomized trial(6) that evaluated a multicomponent intervention over 18 months among low-income patients with uncontrolled hypertension in Argentina. It showed significant reductions in BP in hypertensive subjects cared for by the national public primary health care system in Argentina. Though substantial reductions in BP of important clinical significance were demonstrated, these benefits should be weighed against costs to determine whether scarce healthcare system resources should be spent on this intervention or better used for other priorities. Previous studies evaluating the cost-effectiveness of hypertension control interventions in LMIC have suggested that some strategies, such as government action to stimulate reductions in the salt content of processed foods or combination treatment for people at high risk for cardiovascular disease, are cost-effective, (7, 8) but none have assessed a multicomponent approach. In order to assess intervention efficiency, we undertook a pre-specified (9) individual patient cost-effectiveness analysis of the multicomponent approach used in the HCPIA trial.
Materials and Methods
The results are reported according to the CHEERS reporting format for economic evaluations (10) and supported by the EQUATOR network. Analyses were conducted following state-of-the-art methods of trial-based economic evaluation.(11) The Institutional Review Boards of Tulane University in the US and Hospital Italiano de Buenos Aires in Argentina approved the study protocol with the planned economic evaluation. Informed consent was signed by all participants during screening. Further trial details have been published previously.(9)
Target population and subgroups
The trial included adult (≥21 years old) patients and family members living in the same household with hypertension and uncontrolled BP (systolic ≥140 mm Hg and/or diastolic ≥90 mm Hg on at least 2 separate visits). In addition to the main analysis, we explored cost-effectiveness in pre-specified subgroups (gender, age, cardiovascular risk, and body mass index).
Setting and location
The trial was conducted in 18 public primary health care centers in poor urban areas of Argentina (in Buenos Aires, Misiones, Tucuman, Corrientes, and Entre Ríos provinces). Cluster randomization was stratified by geographic region, and primary health care centers were randomly assigned to the control or intervention group.
Study perspective
The study perspective was that of the healthcare system.
Comparators
The study intervention was a multi-component strategy that included a community health worker (CHW) home-based intervention, physician education, and a text-messaging intervention. CHWs educated, motivated, and facilitated communication between the healthcare system and patients and their families. CHWs received a 2-day interactive training session and onsite field testing and certification in order to facilitate behavioral change in home blood-pressure monitoring, medication adherence, and lifestyle modifications. They were also trained to function as case managers for hypertensive patients and their families by coordinating activities (e.g., physician consultations) and facilitating patient care. They visited participants’ homes monthly during the first 6 months and then every other month throughout the 18-month follow-up. The home-based intervention started with an initial 90-minute home visit where CHWs provided an automatic BP monitor, a pill box to organize medication, educational materials with information regarding hypertension management (adapted from “Your guide to Lowering Blood Pressure”)(12) and a log to record weekly BP values. They also taught the patient and family how to measure BP with the BP monitor, interpret BP values, and provided educational counseling. Subsequent home visits lasted 60 minutes, and CHWs provided tailored counseling to participants and their families on lifestyle modification, home BP monitoring, and medication adherence skills. Subsequent home visits also focused on goal setting, problem solving, social support, and maintaining motivation during challenging situations. If needed, community health workers also helped to schedule appointments with primary care physicians and deliver antihypertensive medications to patients. They also provided feedback to primary care physicians regarding the patients they were visiting. Primary care physicians underwent an online education program on hypertension management focused on treatment for stepped-care management based on clinical guidelines (13, 14). Physicians received monthly lists of patients’ blood-pressure values so that medication adjustments could be made if necessary. Participants also received weekly personalized text-messages to promote lifestyle changes and reminders to reinforce medication adherence. Centers randomized to the control group continued with usual care without any active study intervention.
Time horizon
The trial and the economic evaluation had 18 months of follow-up, and this is the time horizon for this cost-effectiveness analysis.
Discount rate
Due to the short time horizon, no discounting of benefits or costs was included. A supplementary analysis icorporating a 5% discount rate of the 12 to 18 months period did not significantly altered results (see Appendix 5).
Choice of health outcomes
Protocol specified primary outcomes for the economic evaluation were Quality Adjusted Life Years (QALYs) and systolic BP (which was the trial co-primary outcome along with diastolic BP). This paper will focus on QALYs and systolic BP. Results for diastolic BP and hypertension control are presented in the Appendix 1.
Measurement of effectiveness
Analysis was done following the intention-to-treat principle. The EuroQol EQ-5D-3L instrument was administered at baseline and at 18 months, and QALYs were estimated using these two values per patient assuming a linear change between this two time points.(15) The EQ-5D-3L is the most widely used generic preference-based instrument to derive QALYs and has been adapted for Argentina.(16) For health state valuation, Argentinian social values based on the EQ-5D-3L were used.(17)
Estimating resources and costs
The study included two main cost categories from the healthcare perspective: the costs of implementing the intervention itself and the costs associated with the use of health services by individuals in both the intervention and control groups. As recommended in current economic evaluation guidelines, (11) protocol-driven costs (protocol visits, development of educational materials, etc.) were not included.
We included both the fixed and variable costs of implementing the intervention. The fixed costs included the development and maintenance of an on-line platform that contributed to the management of the intervention and generated and sent customized text messages promoting a healthy lifestyle to participants. The variable costs are represented by: (a) training activities for community health workers on the participant intervention; (b) training activities for physicians focused on standard treatment algorithms for stepped-care management based on clinical guidelines; (c) BP monitors for all hypertensive patients for weekly measurement; (d) the number of hours spent by community health workers on education, motivation, social support, and promoting healthcare utilization for participants and their families; (e) the number of hours spent by the community health worker coordinator; and, (f) the number of text messages sent to each participant. The sources of information used to measure and value these items were: the executed trial budget (fixed intervention costs and items a, b, and c of the variable costs), administrative databases generated by the project at the participant level (items d, e, and f), provincial salary records of personnel and official labor market surveys (18) (items e and f), and telephone company prices (item g).
The costs of health care services used by patients were calculated using the utilization rate of each health care resource at the patient level and its associated unit costs in each province. The use of both outpatient and inpatient services (e.g. visits, medications, laboratory studies, hospitalization, etc.) were recorded through specific questionnaires administered at baseline, 6 months, 12 months, and 18 months. If a follow-up questionnaire was missing data on the use of a specific health service, it was assumed that the utilization rate of this service was equal to that reported in the previous questionnaire.
Because the unit costs of health resources differ among the provinces in which the intervention was implemented, public sector unit costs of each province were used to estimate the costs of utilization of health services (19) Thus, the unit costs of health resources are the same for the intervention and control centers within the same province, and they are constant during the intervention period.
Currency, date, conversions
The costs of the development and implementation of the intervention and those associated with the use of health services by individuals were valued in local currency (Argentine pesos; AR$) and converted to US dollars as of July 2017 ($). The conversion rate used in the analysis was 1 US dollar = 17.169 AR$.(20)For international readers interested in valuing costs in International dollars, the PPP conversion factor (Argentinean pesos per international $) was 9.19 in 2016.(21)
Analytical methods
Differences in costs and benefits between study groups, as well as their 95% confidence intervals, were estimated, and incremental cost-effectiveness ratios (ICER) were reported for the different units of health benefit. ICERs express the amount of additional costs needed to achieve an additional unit of health outcome (a healthy year in the case of QALYs, a mm Hg in the case of BP, or an additional patient with controlled hypertension).
Intention-to-treat analyses were performed. For health outcomes, we used a multilevel regression model (STATA, xtmixed command) for the primary analyses, in order to account for the hierarchical (clustered) nature of the data. We undertook data analyses using generalized linear models (GLM), with appropriate family and link components, to account for the potentially non-normally distributed nature of data. This approach is frequently used in and recommended by other studies(22),(23, 24). We additionally tested GLM models with a beta distribution, which showed similar results to the analytical strategy described above. In the present paper, we present results related to the co-primary outcome of the trial (systolic BP change in mm Hg), as well as QALYs. The other trial co-primary outcome (diastolic BP change) and the proportion of hypertension control at 18 months are presented in the Appendix 1.
For the primary analysis, all intervention costs were assigned to intervention groups using an intention-to-treat approach. Healthcare costs were retrieved at baseline and 18 months and included ambulatory and inpatient costs, laboratory and other diagnostic tests, and medications. Missing values for health and resource use outcomes were low (less than 7%) and not associated with the main covariables and, thus, were assumed to be missing at random. A complete case sensitivity analysis, including imputation of the missing values using the multiple imputation method, did not meaningfully change the final results. (see Appendix 4)
In order to define if an intervention is cost-effective, it is necessary to establish a decision rule, defined as a willingness-to-pay value for the outcome of interest that will be used as a threshold. Despite previous use and recommendations of higher thresholds, such as the World Health Organization’s 3 GDP per DALY recommendation,(25) we adopted a more stringent threshold consistent with recent studies: one GDP per capita per QALY(26–28). That is, if for a given intervention the ICER is above this threshold, it will be deemed too expensive and thus not cost-effective, whereas if the ICER lies below this threshold, the intervention will be judged cost-effective. The GDP per capita for Argentina used was 14,062 US dollars or 241,430 Argentine pesos (2017 values). (29)
To incorporate uncertainty into the main outcome of the economic evaluation (the ICER), we estimated the ICER 95% credible interval (CI) through non-parametric bootstrapping (30–32). Bootstrapping was conducted in cost and effectiveness simultaneously by resampling without replenishment from the original database. In each of the 1,000 iterations, the statistical means of cost and effectiveness for each group (intervention and control) were estimated, allowing the calculation of the 1,000 corresponding ICERs. The 95% CI of this variable was estimated by the percentile method.
Additionally, cost-effectiveness acceptability curves were used to graphically report the uncertainty around the main results and show the probability of the intervention being cost-effective at different willingness to pay values per unit of health benefit.
Heterogeneity was explored by evaluating whether cost-effectiveness differed in pre-specified subgroups (age, gender, history of cardiovascular disease, and body mass index).
In order to explore a potential dose-response relationship, we additionally explored whether the intervention differed in cost-effectiveness in patients with greater adherence to the intervention. This was done by stratifying patients in the intervention group by Morisky Medication Adherence Score,(33) number of text messages received, and number of home visits.
Results
A total of 1,432 hypertensive subjects were recruited from June 2013 to April 2015: 743 from 9 intervention centers and 689 from 9 control centers. Community health workers completed 92.8% (8,272/8,916) of planned home-based intervention visits. Baseline characteristics, including quality of life and resource use, were balanced between groups (Table 1).
Table 1.
Baseline Characteristics of HCPIA trial participants
| Intervention group | Control group | P value | ||
|---|---|---|---|---|
| (n = 743) | (n = 689) | |||
| Sociodemographic factors | ||||
| Age, years | Mean (SD) | 56.1 (13.6) | 55.5 (13.0) | 0.45 |
| Female | % (IC) | 53.02 (49.43–56.62) | 54.86 (51.14–58.59) | 0.53 |
| Behaviors | ||||
| Smoking status: Current smoker | % (IC) | 19.51 (16.66–22.37) | 19.59 (16.62–22.56) | 0.99 |
| Weekly alcohol intake, n (%) | % (IC) | 33.38 (29.97–36.78) | 31.19 (26.75–33.62) | 0.19 |
| Physical activity: METS/week | Mean (SD) | 21.8 (44.1) | 24.2 (59.7) | 0.30 |
| Physical measurements | ||||
| SBP (mmHg) | Mean (SD) | 151.7 (16.8) | 149.8 (15.5) | 0.03 |
| DBP (mmHg) | Mean (SD) | 92.2 (12.2) | 90.1 (12.9) | 0.002 |
| BMI (kg/m2) | Mean (SD) | 31.8 (6.6) | 31.5 (6.5) | 0.36 |
| Cardiovascular risk | ||||
| High risk >20% | % (IC) | 0.62 (0.58; 0.65) | 0.58 (0.54; 0.61) | 0.13 |
| Quality of life | ||||
| EQ5D | Mean (SD) | 0.819 (0.22) | 0.789 (0.23) | 0.01 |
| EQ5D VAS | Mean (SD) | 75.30 (18.09) | 74.05 (17.84) | 0.19 |
| Health service use (previous 6 months) | ||||
| Hospitalization | % (IC) | 8.94 (6.88–11.01) | 7.98 (5.95–10.01) | 0.51 |
| Hospitalization LOS in General room | Mean (SD) | 4.19 (3.69) | 4.78 (9.54) | 0.67 |
| Hospitalization LOS in ICU | Mean (SD) | 1.19 (2.40) | 1.23 (2.85) | 0.95 |
| Visit to a general ward | % (IC) | 31.61 (28.25–34.98) | 31.98 (28.48–35.47) | 0.89 |
| Outpatient care and testing | % (IC) | 59.26 (55.70–62.83) | 56.98 (53.27–60.68) | 0.43 |
| Antihypertensive medications | % (IC) | 92.01 (89.95–94.08) | 89.34 (86.89–91.78) | 0.10 |
SBP: Systolic Blood Pressure; DBP: Diastolic Blood Pressure; BMI: Body Mass Index; EQ5D: EuroQol 5D; EQ5D VAS: EuroQol 5D Visual Analogue Scale; ICU: Intensive Care or Conoray Unit; LOS:length of stay
During 18 months of follow-up, the intervention group accumulated 1.29 QALYs (95% CI: 1.28 to 1.31), while the control group had 1.23 (95% CI: 1.21 to 1.25), for a 0.060 crude QALY gain. When adjusting for baseline utility and other covariables, the adjusted gain in QALYs was 0.042 (95% CI: 0.007 to 0.077, p= 0.017). More details of quality of life results are shown in Table 2. Net difference in systolic and diastolic BP were −5.3 mm Hg (95% CI: −10.34, −0.27; p<0.001) and −5.12 mm Hg (95% CI: −8.88, −1.36; p<0.001), respectively, both favoring the intervention. The net increase in the proportion of controlled hypertension was 20.6% (95% CI: 15.4 to 25.9%; p<0.001). Further results related to hypertension control and diastolic blood pressure are presented in the Appendix 1 and elsewhere.(6)
Table 2.
Quality of Life measures and patient-based outcomes at baseline and 18 months.
| Intervention group | Control group | |
|---|---|---|
| QoL (EQ-5D) score, mean (SD) | ||
| Baseline | 0.819 (0.80–0.84) | 0.789 (0.77–0.81) |
| 18 months | 0.904 (0.89–0.92) | 0.843 (0.83–0.86) |
| Δ pre and post intervention | 0.084 | 0.053 |
| 18 month crude QALYs, mean (95% CI) | 1.295 (1.28–1.31) | 1.229 (1.21–1.25) |
| Difference | 0.067 (0.04–0.09) | |
| 18 month adjusted QALYs, mean (95% CI) | ||
| Difference adjusted for baseline utility | 0.040 (0.006–0.074) | |
| Difference adjusted for baseline utility and other variables* | 0.042 (0.007–0.077) | |
| 18 month SBP net difference from baseline, mmHg (95% CI) | ||
| Crude difference | −6.316 (−12.18, −0.45) | |
| Adjusted difference* | −5.302 (−10.34, −0.265) | |
Notes:
Adjusted for sex, age, SBP, history CVD and history of hypercholesterolemia at baseline.
QoL: Quality of Life; QALYs: Quality-Adjusted Life Years; EQ-5D: EuroQol 5D; SBP: Systolic Blood Pressure
Table 3 shows intervention and non-intervention costs related to health service resource use at 18 months. Taking all costs into account, there was a significantly increased total mean cost in the intervention arm, with an increase of US $150.54 (95% CI: US $92.16 to US $208.92) [AR $2,584.62; 95% CI: AR $1,582.29 to AR $3,586.95] per patient, or US $8.36 [AR $143.59] per patient per month. Patients received a median number of 12 home visits (IQR=11–12) and a median of 85 text messages during the intervention (IQR=81–85). Mean intervention 18 month costs were US $108.02 per patient (95% CI: US $59.05 to US $117.74) [AR $1,854.60; 95% CI: AR $1,013.83 to AR $2,021.48)]. Average health service use and costs not related to the intervention were similar between the two groups: US $196.26 (95% CI: US $0.00 to US $1,193.35) [AR $3,369.59; 95% CI: AR $0.00 to AR $20,488.63)] and US $153.58 (95% CI: US $0.00 to US $1,197.11) [AR $2,636.82; 95% CI: AR $0.00 to AR $20,553.18)] for the intervention and control groups, respectively.
Table 3.
Intervention and health services costs at 18 months.
| Intervention group | Control group | Intervention group vs control
group |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cost per patient (USD) | Cost per patient (USD) | Cost difference per patient
(USD) |
|||||||||
| Costs | Use (%) | Mean | 95% CI | Use (%) | Mean | 95% CI | Mean | 95% CI | |||
| Intervention | |||||||||||
| Platform development and maintenence | 100 | 6.87 | - | - | - | - | - | - | - | - | - |
| Training workshops | 100 | 4.02 | - | - | - | - | - | - | - | - | - |
| Patient educational materials | 100 | 6.12 | - | - | - | - | - | - | - | - | - |
| Self monitoring blood pressure | 100 | 18.29 | - | - | - | - | - | - | - | - | - |
| Community health workers visits | 100 | 61.27 | 17.47 | 69.89 | - | - | - | - | 61.27*** | 60.33 | 62.21 |
| Field work coordination | 100 | 3.89 | 2.58 | 6.44 | - | - | - | - | 3.89*** | 3.82 | 3.97 |
| Text messages (SMS) | 100 | 7.56 | 0 | 8.66 | - | - | - | - | 7.56*** | 7.39 | 7.74 |
| Sub-total | - | 108.02 | 59.05 | 117.74 | - | - | - | - | 108.02*** | 107.01 | 109.04 |
| Health services | |||||||||||
| Hospitalization within 6 months | 13.58 | 5.47 | 0 | 46.48 | 13.58 | 3.77 | 0 | 44.90 | 1.69** | 0.31 | 3.08 |
| Hospitalization LOS in general ward**** | 3.36 | 41.06 | 0 | 526.03 | 2.9 | 28.6 | 0 | 440.78 | 12.46 | -13.49 | 38.40 |
| Hospitalization in CU or ICU**** | 0.27 | 5.69 | 0 | 0.00 | 0.58 | 26.21 | 0 | 0 | −20.52 | −48.72 | 7.68 |
| Outpatient care and testing | 51.84 | 29.82 | 0 | 70.24 | 37.35 | 17.09 | 0 | 70.24 | 12.73*** | 9.68 | 15.80 |
| Antihypertensive medications | 88.72 | 118.14 | 0 | 744.64 | 83.64 | 82.57 | 0 | 620.43 | 35.57* | −5.30 | 76.43 |
| Sub-total | - | 196.26 | 0 | 1,193.35 | - | 153.74 | 0 | 1,197.11 | 42.52 | −15.80 | 100.85 |
| Total | - | 304.28 | 72.24 | 1,302.11 | - | 153.74 | 0 | 1,197.11 | 150.54*** | 92.16 | 208.92 |
ICERs were US $3,299.42 per QALY (95% CI: US $1,635.42 to US $6,099.06) [AR $56,647.74; 95% CI: AR $28,078.53 to AR $104,714.76] and US $26.43 per mm Hg systolic blood pressure reduction (95% CI: US $13.08 to US $45.91) [AR $453.78; 95% CI: AR $224.57 to AR $788.23]. Table 4 presents the differences in outcomes and total costs between groups adjusted for main individual characteristics, ICERs, and the uncertainty surrounding these estimates. See additional cost-effectiveness results for diastolic blood pressure and hypertension control in the Appendix.
Table 4.
Cost-effectiveness of the multicomponent intervention
| Variable | Intervention group vs control group* |
95% CI | ICER | 95% CI** |
|---|---|---|---|---|
| QALY difference | 0.042 | (0.01 to 0.08) | 3299.42 | (1635.42 to 6099.06) |
| SBP difference, mm Hg | −5.30 | (−10.34 to −0.27) | 26.43 | (13.08 to 45.91) |
| HTN control difference, % | 19% | (5% to 33%) | 721.42 | (352.12 to 1087.60)) |
| Total cost difference, $ | 140.18 | (75.41 to 204.94) | - | - |
Notes:
Adjusted for sex, age, SBP, history of CVD, and history of hypercholesterolemia at baseline.
The 95% confidence intervals were estimated using the bootstrap method based with 1000 replications.
SBP: Systolic Blood Pressure; QALY: Quality-Adjusted Life Year; HTN: Hypertension
Parameter estimates and global uncertainty are additionally presented as cost-effectiveness scatterplots and acceptability curves, which depict the probability of this multicomponent intervention being cost-effective at different willingness to pay values per unit of QALYs or mm Hg of systolic blood pressure reduction (See Figure 1). There is a 100% certainty that the intervention would be cost-effective at a willingness to pay per QALY of 1 GDP per capita (see Appendix 3 for acceptability curves for diastolic blood pressure and hypertension control).
Fig. 1.
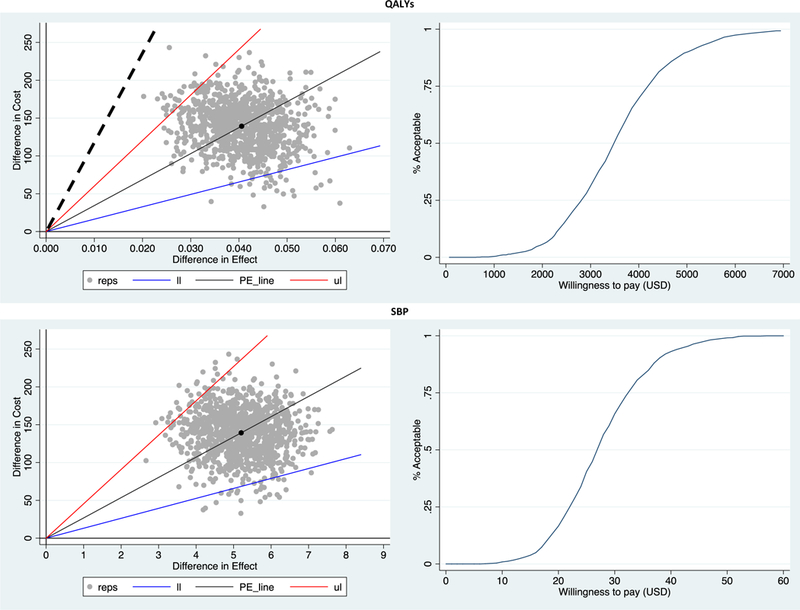
Scatterplots and cost-effectiveness acceptability curves depicting the uncertainty around main study results.
In pre-specified subgroup analyses the intervention was cost-effective in all subgroups. Though results were less precise when analyzing subgroups, they showed the intervention was somewhat more cost-effective in younger ages (ICER: US $2,156.19 [AR $37,019.63] per QALY), in women (ICER: US $3,292.60 [AR $56,530.65] per QALY), in subjects with higher cardiovascular risk (ICER: US $2,726.09 [AR $46,804.24] per QALY), and in those with high body mass index (ICER: US $1,796.17 [AR $30,838.44]). The intervention was also somewhat more cost-effective in those with a lower Morisky adherence score (see Table 5). The results of cost-effectiveness by number of text messages and home visits received suggested the intervention was more cost-effective in those groups with a less intense intervention dose (see Appendix 2 for results).
Table 5.
Subgroup analysis.
| Adjusted Cost Difference for Intervention vs. Control* |
Adjusted QALY Difference for Intervention vs. Control* |
Adjusted SBP Difference for Intervention vs. Control* |
||||||
|---|---|---|---|---|---|---|---|---|
| N | Mean (95% CI) | Mean (95% CI) | ICER | 95%CI** | Mean (95% CI) | ICER | 95%CI** | |
| Age | ||||||||
| ≥60 years | 558 | 227.93 (105.87, 349.99) | 0.037 (−0.01, 0.08) | 6160.27 | (2862.48 to 16543.90) | −5.02 (−9.48, −0.57) | 45.40 | (20.77 to 99.53) |
| <60 years | 874 | 90.56 (10.88, 170.25) | 0.042 (0.01, 0.08) | 2156.19 | (352.09 to 4669.80) | −5.11 ( −10.57, 0.34) | 17.72 | (2.98 to 35.88) |
| Sex | ||||||||
| Men | 660 | 110.55 (13.05, 208.06) | 0.037 (0.01, 0.07) | 2987.84 | (242.03 to 7636.44) | −3.44 (−9.08, 2.18) | 32.14 | (1.75 to 99.95) |
| Women | 772 | 164.63 (71.85, 257.40) | 0.05 (0.01, 0.09) | 3292.60 | (1978.58 to 8162.88) | −6.41 (−11.40, −1.43) | 25.68 | (13.55 to 44.98) |
| Cardiovascular risk | ||||||||
| High >20 | 857 | 125.40 (31.17, 219.62) | 0.046 (0.01, 0.09) | 2726.09 | (993.01 to 6106.72) | −6.59 ( −13.36, 0.16) | 19.03 | (8.27 to 49.69) |
| Not high <20 | 575 | 152.64 (61.33, 243.94) | 0.033 (0.01, 0.06) | 4625.45 | (1977.64 to 10674.81) | −5.42 ( −10.69, −0.15) | 28.16 | (15.69 to 73.69) |
| Body mass index | ||||||||
| ≥30 kg/m2 | 798 | 84.42 (−6.12, 174.95) | 0.047 (0.01, 0.09) | 1796.17 | (−206.97 to 4250.66) | −4.59 (−10.38, 1.18) | 18.39 | (−1.79 to 50.40) |
| <30 kg/m2 | 634 | 210.33 (117.85, 302.80) | 0.035 (0.01, 0.07) | 6009.43 | (3273.01 to 17477.13) | −5.65 (−10.08, −1.24) | 37.23 | (20.49 to 68.16) |
| Morisky score | ||||||||
| Low adherence (0–5) | 358 | 83.22 (−57.97, 224.40) | 0.05 (0.001–0.10) | 1664.40 | (−1223.41 to 6095.28) | −4.77 (−10.03, 0.47) | 17.45 | (−13.09 to 54.68) |
| Intermediate (6–7) | 406 | 162.13 (21.26, 303.01) | 0.07 (0.015–0.12) | 2316.14 | (565.43 to 11989.43) | −8.60 (−13.88, −3.32) | 18.85 | (3.95 to 55.15) |
| High adherence (8) | 420 | 147.03 (87.84, 206.21) | 0.02 (−0.01–0.06) | 7351.50 | (1789.59 to 25628) | −3.81 ( −9.90, 2.28) | 38.59 | (13.55 to 120.21) |
Discussion
The present study is, to our knowledge, the first randomized cluster trial-based economic evaluation to assess a multicomponent intervention led by community health workers, including complementary physician education and a mobile health intervention, in a low-resource setting in LMICs. This trial among low-income hypertensive patients receiving care from public primary health care centers showed that the intervention, over an 18 month time horizon, was more effective than usual care in reducing blood pressure and increasing healthy years of life. Adherence to the community health worker-led intervention, including home visits, was very high in our study. In Argentina, community health workers are integrated into the primary care team, so this facilitated their recruitment and training for this trial. The public primary care network also has clinics located in low-resource settings and provides medications for chronic diseases free of charge, so the infrastructure is in place to provide access to care for low-income hypertensive participants. The intervention was more costly than usual care (US $8.36 [AR $143.59] per patient per month) and was shown to be a cost-effective strategy when a one GDP per QALY threshold was used. The main results were robust, as our analysis showed that when using a one GDP per QALY threshold, this intervention had a 100% probability of being cost-effective.
Approximately 80% of all cardiovascular mortality occurs in LMICs, where the greatest burden of hypertension is observed.(3, 34) Despite the availability of affordable antihypertensive medications and lifestyle interventions, the proportion of hypertension control remains low.(3, 35–37) The results of this study show that a multicomponent intervention led by community health workers could be a cost-effective strategy to improve hypertension control in LMICs. If scaled-up, it could result in a significant reduction in uncontrolled hypertension and related cardiovascular disease by overcoming key barriers that are currently a major obstacle for hypertension control both in LMICs and in underserved populations in high-income countries.(38)
This is the first economic evaluation of a multicomponent community health worker-led intervention similar to ours in a LMIC setting. Our results show that the HCPIA intervention had similar or better cost-effectiveness than several strategies for hypertension control tested in low resource settings, such as multidrug or polypill therapies in high cardiovascular risk populations, community health workers to improve medication adherence, and clinical decision support systems. Table A3 shows our results compared to results of other relevant studies that used QALYs, disability-adjusted life years (DALYs), or blood pressure change as health outcomes.
Our study has some limitations. First, the time horizon was short relative to the relevant decision-making timeframe. This is a common limitation of trial-based economic evaluations. Second, our study was done from the healthcare perspective, so we did not include other societal costs, such as productivity costs, transportation costs, and other direct non-medical costs, which is increasingly recommended. (39) Third, though the study was undertaken in a real-life setting, and protocol related costs were not included it was an experimental trial. This is a well-known limitation of trial-based economic evaluations. (40)
In conclusion, our study found that among low-income patients with uncontrolled hypertension in Argentina, a multicomponent intervention led by community health workers was cost-effective. The intervention significantly improved blood pressure control and quality of life over an 18-month intervention at reasonable incremental costs.
Supplementary Material
Highlights.
-
What is already known about the topic?
Multicomponent interventions, including community health worker and mobile health strategies, to control hypertension have been shown to be effective in some settings and to be cost-effective in high-income countries. No previous economic evaluation based on a rigorous randomized trial in low-income settings has been reported.
-
What does the paper add to existing knowledge?
This economic evaluation based on a cluster randomised trial in a low-income setting gives a detailed account of the effects and costs of implementation of a multicomponent intervention. The intervention significantly increased years of healthy life (QALYs) and improved blood pressure at small additional costs to the healthcare system, confirming its cost-effectiveness in this setting. The robust results, which demonstrated consistent evidence of cost-effectiveness regardless of methodologic assumptions and across subgroups of age, gender, cardiovascular risk, and body mass index, leave little uncertainty about the cost-effectivness of the intervention in this setting.
Acknowledgements:
Andrea Manca, from the centre for health economics (CHE) of the University of York, for his statistical advice. Daniel Comandé, librarian, IECS, for his help with the literature search strategy.
Funding: This study was supported by the National Heart, Lung, and Blood Institute, and partially by the National Institute of General Medical Sciences. None of the funding agencies contributed to the design of the trial, the collection or analysis of the data, the writing of the manuscript, or the decision to submit the manuscript for publication.
Funding, COI.
The Hypertension Control Program in Argentina was funded by the National Heart, Lung, and Blood Institute, National Institutes of Health (U01HL114197) under the Global Alliance for Chronic Diseases programme, and partially by the National Institute of General Medical Sciences. None of the funding agencies contributed to the design of the trial, the collection or analysis of the data, the writing of the manuscript, or the decision to submit any of the related manuscripts for publication.
References
- 1.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. The Lancet. 380: 2095–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reklaitiene R, Tamosiunas A, Virviciute D, et al. Trends in prevalence, awareness, treatment, and control of hypertension, and the risk of mortality among middle-aged Lithuanian urban population in 1983–2009. BMC Cardiovascular Disorders. 2012; 12: 68–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mills KT, Bundy JD, Kelly TN, et al. Global Disparities of Hypertension Prevalence and Control: A Systematic Analysis of Population-Based Studies From 90 Countries. Circulation. 2016; 134: 441–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pereira M, Lunet N, Azevedo A, et al. Differences in prevalence, awareness, treatment and control of hypertension between developing and developed countries. Journal of hypertension. 2009; 27: 963–75. [DOI] [PubMed] [Google Scholar]
- 5.Merai R, Siegel C, Rakotz M, et al. CDC Grand Rounds: A Public Health Approach to Detect and Control Hypertension. MMWR Morbidity and mortality weekly report. 2016; 65: 1261–64. [DOI] [PubMed] [Google Scholar]
- 6.He J, Irazola V, Mills KT, et al. Effect of a community health worker–led multicomponent intervention on blood pressure control in low-income patients in argentina: A randomized clinical trial. JAMA. 2017; 318: 1016–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murray CJ, Lauer JA, Hutubessy RC, et al. Effectiveness and costs of interventions to lower systolic blood pressure and cholesterol: a global and regional analysis on reduction of cardiovascular-disease risk. Lancet (London, England). 2003; 361: 717–25. [DOI] [PubMed] [Google Scholar]
- 8.Rubinstein A, García Martí S, Souto A, et al. Generalized cost-effectiveness analysis of a package of interventions to reduce cardiovascular disease in Buenos Aires, Argentina. Cost Effectiveness and Resource Allocation : C/E. 2009; 7: 10–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mills KT, Rubinstein A, Irazola V, et al. Comprehensive approach for hypertension control in low-income populations: rationale and study design for the hypertension control program in Argentina. The American journal of the medical sciences. 2014; 348: 139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drummond MF, Sculpher MJ, Claxton K, et al. Methods for the economic evaluation of health care programmes. Oxfordshire: Oxford University Press, 2015. [Google Scholar]
- 11.Ramsey SD, Willke RJ, Glick H, et al. Cost-effectiveness analysis alongside clinical trials II-An ISPOR Good Research Practices Task Force report. Value in health : the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2015; 18: 161–72. [DOI] [PubMed] [Google Scholar]
- 12.US Department of Health and Human Services. Your Guide to Lowering Blood Pressure. 2014. [Google Scholar]
- 13.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. Jama. 2003; 289: 2560–72. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez RA, Ayala M, Baglivo H, et al. Latin American guidelines on hypertension. Latin American Expert Group. Journal of hypertension. 2009; 27: 905–22. [DOI] [PubMed] [Google Scholar]
- 15.Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health economics. 2005; 14: 487–96. [DOI] [PubMed] [Google Scholar]
- 16.Rasanen P, Roine E, Sintonen H, et al. Use of quality-adjusted life years for the estimation of effectiveness of health care: A systematic literature review. International journal of technology assessment in health care. 2006; 22: 235–41. [DOI] [PubMed] [Google Scholar]
- 17.Augustovski FA, Irazola VE, Velazquez AP, et al. Argentine valuation of the EQ-5D health states. Value in health : the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2009; 12: 587–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Instituto Nacional de Estadística y Censos (INDEC). Informes Técnicos. Indice de Salarios 2017. [Google Scholar]
- 19.Unit Costs Database. Institute for Clinical Effectiveness and Health Policy (IECS).
- 20.Banco Central de la República Argentina. Publicaciones y estadísticas. Tipos de cambio.
- 21.World Bank. International Comparison Program database.
- 22.Grieve R, Nixon R, Thompson SG, et al. Using multilevel models for assessing the variability of multinational resource use and cost data. Health economics. 2005; 14: 185–96. [DOI] [PubMed] [Google Scholar]
- 23.Ng ES, Diaz-Ordaz K, Grieve R, et al. Multilevel models for cost-effectiveness analyses that use cluster randomised trial data: An approach to model choice. Statistical methods in medical research. 2016; 25: 2036–52. [DOI] [PubMed] [Google Scholar]
- 24.Hunger M, Doring A, Holle R. Longitudinal beta regression models for analyzing health-related quality of life scores over time. BMC medical research methodology. 2012; 12: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sachs JD. Macroeconomics and health: investing in health for economic development. Revista Panamericana de Salud Pública. 2002; 12: 143–44. [Google Scholar]
- 26.Eichler HG, Kong SX, Gerth WC, et al. Use of cost-effectiveness analysis in health-care resource allocation decision-making: how are cost-effectiveness thresholds expected to emerge? Value in health : the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2004; 7: 518–28. [DOI] [PubMed] [Google Scholar]
- 27.Shillcutt SD, Walker DG, Goodman CA, et al. Cost effectiveness in low- and middle-income countries: a review of the debates surrounding decision rules. PharmacoEconomics. 2009; 27: 903–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woods B, Revill P, Sculpher M, et al. Country-Level Cost-Effectiveness Thresholds: Initial Estimates and the Need for Further Research. Value in Health. 2016; 19: 929–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.International Monetary Found. World Economic Outlook.
- 30.Glick HA, Doshi JA, Sonnad SS, et al. Economic evaluation in clinical trials. Oxforshire: Oxford University Press, 2007. [Google Scholar]
- 31.Briggs AH, Wonderling DE, Mooney CZ. Pulling cost-effectiveness analysis up by its bootstraps: a non-parametric approach to confidence interval estimation. Health economics. 1997; 6: 327–40. [DOI] [PubMed] [Google Scholar]
- 32.Nixon RM, Wonderling D, Grieve RD. Non-parametric methods for cost-effectiveness analysis: the central limit theorem and the bootstrap compared. Health economics. 2010; 19: 316–33. [DOI] [PubMed] [Google Scholar]
- 33.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Medical care. 1986; 24: 67–74. [DOI] [PubMed] [Google Scholar]
- 34.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet (London, England). 2012; 380: 2095–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. The Lancet. 2016; 387: 957–67. [DOI] [PubMed] [Google Scholar]
- 36.Gu D, He J, Coxson PG, et al. The Cost-Effectiveness of Low-Cost Essential Antihypertensive Medicines for Hypertension Control in China: A Modelling Study. PLoS medicine. 2015; 12: e1001860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steinberg D, Bennett GG, Svetkey L. The DASH Diet, 20 Years Later. Jama. 2017; 317: 1529–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Institute of Medicine (US) Committee on Public Health Priorities to Reduce and Control Hypertension. A Population-Based Policy and Systems Change Approach to Prevent and Control Hypertension. Washington, DC: National Academies Press (US), 2010. [PubMed] [Google Scholar]
- 39.Sanders GD, Neumann PJ, Basu A, et al. Recommendations for Conduct, Methodological Practices, and Reporting of Cost-effectiveness Analyses: Second Panel on Cost-Effectiveness in Health and Medicine. Jama. 2016; 316: 1093–103. [DOI] [PubMed] [Google Scholar]
- 40.Sculpher MJ, Claxton K, Drummond M, et al. Whither trial-based economic evaluation for health care decision making? Health economics. 2006; 15: 677–87. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


