Abstract
Objective
HIV-infected smokers lose more life years to tobacco use than to HIV infection. The nicotine metabolite ratio (NMR), a biomarker of CYP2A6, represents individual variation in the rate at which nicotine is metabolized and is associated with response to smoking cessation treatments. We evaluated whether HIV-infected smokers metabolize nicotine faster than HIV-uninfected smokers, which may contribute to the disproportionate smoking burden and may have important treatment implications.
Design:
We analyzed baseline data from two clinical trials (NCT01710137; NCT01314001) to compare the NMR in HIV-infected smokers (N=131) to HIV-uninfected smokers (N=199).
Methods
Propensity scores were used to match the groups 2:1 on characteristics that influence NMR: sex, race, body mass index, and smoking rate. Nicotine metabolites were assessed via LC-MS methods and the ratio of 3-hydroxycotinine:cotinine was used to compute the NMR.
Results
HIV-infected smokers had significantly higher NMR (mean=0.47, SEM=0.02) and were more likely to be in the highest NMR quartile compared to HIV-uninfected smokers (mean=0.34, SEM=0.02; ps<0.001).
Conclusions
The higher NMR observed among HIV-infected smokers may partially explain higher smoking rates and lower response to transdermal nicotine therapy. Understanding the mechanisms by which HIV and/or ART contribute to faster nicotine metabolism may guide the use of the NMR to personalize tobacco cessation strategies in this underserved population.
Keywords: Nicotine metabolite ratio, HIV, Tobacco, Biomarkers, Smoking
Introduction
People living with HIV (PLWH) smoke tobacco at higher rates [1–5] and have more difficulty quitting smoking than the general population. HIV-infected smokers lose more life-years to tobacco use than to HIV infection [6, 7]. One plausible mechanism that may underlie smoking behavior among PLWH may be differences in nicotine metabolism rates. Nicotine is primarily metabolized by the cytochrome P450 liver enzyme CYP2A6 into cotinine, which is further metabolized to 3´-hydroxycotinine (3HC) [8]. The ratio of 3HC to cotinine – nicotine metabolite ratio (NMR) – is a well-validated biomarker of nicotine metabolism, and in non-HIV populations higher NMR values (i.e., faster nicotine clearance) are associated with smoking more cigarettes/day, greater nicotine dependence, and more severe nicotine withdrawal symptoms [9]. The NMR can also optimize nicotine dependence treatment [10–12], as shown in a large clinical trial using prospective NMR stratification [13].
The NMR is influenced by biological and environmental factors. Although genetic variation in the CYP2A6 gene affects enzyme activity – and therefore nicotine metabolism ([14]; https://www.pharmvar.org/), African-Americans are more likely to have reduced function variants vs. Caucasians [15] and NMR values parallel these differences. Moreover, higher NMR is associated with being female, older, lower BMI, non-menthol smokers, and greater alcohol consumption [9, 16]. Medications that are substrates, inhibitors, or inducers of CYP2A6 activity may also impact nicotine metabolism. Concentrations of the antiretroviral (ART) efavirenz, which is a substrate of CYP2A6 (among other enzymes), may affect variation in CYP2A6 activity [17].
Although there is limited recent evidence that HIV-infected smokers have higher rates of nicotine metabolism vs. HIV-uninfected smokers [18], the relationship between HIV infection, nicotine metabolism, and CYP2A6 activity warrants investigation in a larger, more representative sample. This study compared NMR values among HIV-infected smokers enrolled in a clinical trial of varenicline for smoking cessation (NCT01710137) to a matched sample of HIV-uninfected smokers who were screened for a large, multi-site smoking cessation clinical trial [13]. We hypothesized that HIV-infected smokers would exhibit faster nicotine metabolism. Understanding whether the NMR is a mechanism underlying tobacco use among PLWH may guide smoking cessation treatment in this population.
Methods
Samples
HIV-infected Participants
Data were from a smoking cessation clinical trial for smokers with HIV (NCT01710137) approved by the University of Pennsylvania IRB. Participants were recruited through Penn’s health system, advertisements, and through a community-based HIV clinic. To be eligible, individuals had to be age ≥18, have a confirmed HIV diagnosis, be treated with ART with HIV viral loads <1000 copies/ml and CD4+ counts >200 cells/mm3, smoke >1 cigarette/day, ALT and AST <2 times upper limit of normal, and creatinine clearance >50 mL/min. Exclusion criteria included history of psychosis or a suicide attempt, current/planned pregnancy, current use of smoking cessation medications, and unstable/untreated alcohol/substance abuse. For this study, the 131/179 enrolled participants who provided blood for NMR analyses were included.
HIV-uninfected Participants
Treatment-seeking smokers responded to advertisements for a smoking cessation clinical trial (NCT01314001). Smokers ages 18–65 who reported smoking ≥10 cigarettes/day for the past 6 months were included. Participants provided written informed consent. Those eligible provided a blood sample for NMR determination. See Supplemental Material for exclusion criteria. The study protocol, including NMR determination, has been described elsewhere [16, 19].
Measures
Demographic and Smoking-related Measures (Both Samples)
Demographic information was ascertained. Smoking-related data (e.g., current smoking rate, number of previous quit attempts), the Fagerström Test for Cigarette Dependence (FTCD; [20]), and a breath carbon monoxide (CO) measure were collected at baseline while individuals were still smoking (i.e., prior to the quit attempt). See Supplemental Material for subjective measures.
Nicotine metabolites (Both Samples)
Blood samples (10 ml) were collected at the eligibility visit and frozen until cotinine and 3′-hydroxycotinine (3-HC) were assessed by liquid chromatography-tandem mass spectrometry (LC-MS) [21, 22]. The continuous measure of the NMR is the ratio of 3-HC:cotinine. Participants were categorized by NMR quartiles (e.g., lowest quartile equals 25% of people with the slowest nicotine metabolism, vs. highest quartile equals 25% of people with the fastest nicotine metabolism) [10]. The NMR quartile means, medians, upper and lower limits, and sample size were: (1) 0.18, 0.20 (<0.2591), 142; (2) 0.30, 0.30 (0.2592–0.3519) 142; (3) 0.40, 0.39 (0.352–0.466), 142; and (4) 0.63, 0.56 (>0.466), 142 [10].
HIV-related Health Outcomes (HIV-infected Sample Only)
Disease-related characteristics, including current HIV viral load and CD4+ count (within the past 6 months), were collected from medical records. Mode of HIV acquisition and current ART medications and adherence were collected through self-report. Because efavirenz is a substrate of CYP2A6 [23] and because liver diseases, such as hepatitis [24], may induce CYP2A6, exploratory analyses excluded those taking efavirenz and those with a history of hepatitis.
Data Analysis
Descriptive statistics were used to characterize the two samples and Pearson r and t-tests were used to examine univariate correlates of NMR (Supplemental Table 1). Propensity score matching was utilized to create a matched sample of HIV-uninfected smokers using variables associated with NMR (i.e., sex, age, race, body mass index (BMI), and smoking rate) and characteristics that differed between the groups (i.e., education). Subjects were matched using a 2:1 ratio [25, 26] and calipers equal to 0.2 [27]. A mixed model using the matched sample of 131 HIV-infected and 199 HIV-uninfected smokers (n=330) evaluated the effect of HIV on NMR with matching weight as a random effect. Thus, we had 80% power using a conservative α=0.01 to detect an effect size of d=0.39 between groups. Multinomial logistic regression was used to evaluate the relative risk of HIV-infected individuals being in the highest NMR quartile (fastest metabolizers), vs. the lowest NMR quartile (slowest metabolizers). See Supplemental Material for analysis of subjective measures.
Results
Sample Characteristics
Demographic and smoking-related characteristics are presented in Table 1. The matched samples did not differ on most characteristics except that the HIV-infected sample smoked fewer cigarettes per day and a greater proportion of the HIV-infected sample reported an annual income less than $35k.
Table 1.
Demographic Characteristics and Smoking-related Variables
| Variable | HIV- (full sample; n=1,807) | HIV- (matched sample; n=199) | HIV+ (n=131) | Total Matched Sample (N=330) | p-value b |
|---|---|---|---|---|---|
| Demographic variables | |||||
| Female sex (n, %) | 801, 44%a | 51, 26% | 39, 30% | 90, 27% | 0.41 |
| African American race (n, %) | 608, 34% a | 143, 72% | 103, 79% | 246, 75% | 0.17 |
| Education HS/GED or less (n, %) | 545, 30% a | 112, 56% | 79, 60% | 191, 58% | 0.47 |
| Income ≤ $35,000 (n, %) | 814, 46% a | 124, 64% | 113, 86% | 237, 72% | <0.001 |
| Age | 45.4 (11.3) a | 47.6 (10.2) | 48.0 (9.9) | 47.8 (10.0) | 0.73 |
| Body Mass Index | 28.7 (6.3) a | 28.2 (6.1) | 27.9 (7.3) | 28.1 (6.6) | 0.72 |
| Smoking-related variables | |||||
| Cigarettes per day | 18.5 (7.4) a | 14.9 (6.2) | 12.7 (7.3) | 14.1 (6.8) | 0.003 |
| Nicotine Dependence | 5.2 (2.0) a | 5.0 (1.9) | 4.7 (2.0) | 4.8 (1.9) | 0.18 |
| Cotinine, ng/ml | 245.9 (116.8) a | 255.7 (127) | 174.9 (127.1) | 223.6 (132.9) | <0.001 |
| 3-HC, ng/ml | 89.4 (57.5) a | 80.1 (57.7) | 73.1 (59.3) | 77.9 (58.4) | 0.23 |
| NMR, ng/ml | 0.38 (0.21) a | 0.34 (0.23) | 0.47 (0.3) | 0.39 (0.26) | <0.001 |
| Characteristics for HIV sample only | |||||
| ART adherence past 2 weeks (%, range) | 99%, 86–100% | ||||
| % Undetectable Viral Load (<50 copies/ml; n, %) | 105 (80%) | ||||
| Plasma CD4 Count (cells/mm3) | 714.4 (341.6) | ||||
| Mode of HIV transmission (n, % sex) | 121, 92% | ||||
| History of HCV (n, %) | 12, 9% | ||||
Note: Unless otherwise noted, values are mean (SD); BMI = Body Mass Index.
p<0.05 when comparing full sample to HIV-infected sample;
p-value is for the comparison between the matched HIV-infected and –uninfected samples
Group differences in NMR by HIV status
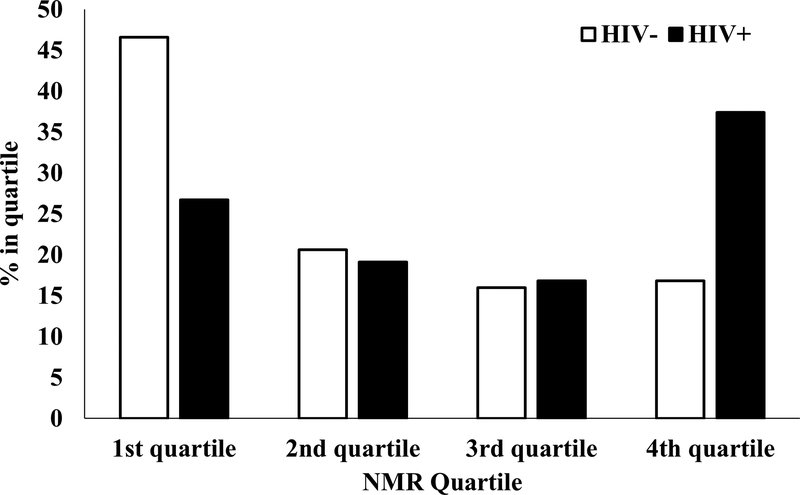
HIV-infected smokers had significantly higher NMR vs. HIV-uninfected smokers (β=0.13, 95% CI: 0.07,0.19, p<0.001, d=0.48). The groups also differed when comparing the 1st quartile to the 4th quartile. HIV-infected smokers were significantly more likely to be in the 4th quartile (i.e., fastest metabolizers), relative to HIV-uninfected smokers (RR=3.1, 95% CI: 1.7,5.5, p<0.001; Figure 1). When individuals taking efavirenz (n=22) or those with a history of hepatitis (n=12) were removed from analyses, results were not meaningfully different.
Figure 1. NMR Quartile by HIV Status in Matched Sample.
There were significantly more HIV-infected smokers (35%) characterized as fastest metabolizers (4th quartile) compared to HIV-uninfected smokers (17%). In contrast, 47% of HIV-uninfected smokers were characterized as the slowest metabolizers compared to 29% of HIV-infected smokers.
Discussion
PLWH lose more life-years to tobacco use than to HIV infection [6], highlighting the need to better understand the mechanisms that underlie high smoking rates in this population. We compared NMR values – a well-validated index of nicotine metabolism – in a relatively large sample of HIV-infected smokers to a matched sample of HIV-uninfected smokers. This is the first study to directly compare the NMR among PLWH to the general population of treatment-seeking smokers.
The NMR reflects genetic and environmental sources of variation in nicotine metabolism including race, sex, smoking rate, and BMI [16]. The HIV-infected smokers in this study were predominantly African American and male, had an average BMI in the overweight range, and smoked fewer cigarettes per day than the HIV-uninfected sample indicating it was plausible to expect that their NMRs would be lower than in the general population of smokers. Our data indicate the opposite – that, compared to the matched sample, HIV-infected smokers have significantly higher NMR values and were more likely to be characterized as fast metabolizers (4th quartile), reflecting faster nicotine metabolism. Even after removing individuals taking efavirenz, an ART that plausibly might increase CYP2A6 activity, the group difference remained significant. Nevertheless, demographic and smoking-related variables do not capture all of the variance in NMR and future studies should include CYP2A6 genotypes as markers of CYP2A6 activity [28].
CYP2A6 activity may also play a role in mediating HIV-1 pathogenesis by inducing reactive oxygen species (ROS), a marker and mediator of oxidative stress [29–31]. CYP enzymes (including 2A6) are expressed in monocytes [32], which play a role in the neurodegenerative effects of HIV [33]. If HIV-infected smokers have increased CYP2A6 activity, tobacco use and HIV may interact to increase oxidative stress [32]. Evidence that HIV-infected smokers (not on ART) may have higher viral loads vs. HIV-infected non-smokers was supported by in vitro models demonstrating increased viral replication in HIV-infected macrophages treated with cigarette smoke condensate [34]. Thus, CYP2A6 activity, faster nicotine metabolism, and smoking may synergistically contribute to HIV-1 pathogenesis and sequelae such as HAND.
These data raise several important questions. HIV itself may induce CYP2A6, thereby increasing the NMR, as the enzyme can be induced by inflammation and is found at higher levels in livers of hepatitis patients [24, 35–37]. Although our results were unchanged after excluding those with a history of hepatitis, it is possible that HIV-1 infection increases nicotine metabolism. Confirmation would require evaluating the NMR pre- and post-infection, which is challenging. Alternatively, although the higher NMRs persisted after excluding individuals taking efavirenz, one drug that is a known CYP2A6 substrate, other ARTs may influence CYP2A6 activity. The mechanisms through which HIV may increase CYP2A6 activity and nicotine metabolism warrant investigation.
Recent studies have investigated the mechanisms underlying the effects of the NMR on smoking behavior in the general population. Among abstinent smokers, faster nicotine metabolism was associated with increased cigarette craving, subjective nicotine reward, and physiological responses (e.g., increased heart rate) in response to intravenous nicotine [38]. Neuroimaging studies also suggest that NMR plays a role in brain responses to tobacco use. Slow metabolizers had reduced nicotinic receptor availability following overnight abstinence, vs. fast metabolizers [39] and fast metabolizers exhibit increased smoking cue-related brain activity [40, 41]. Therefore, the higher NMR among PLWH in the present study may contribute to increased nicotine reinforcement, nicotinic receptor availability, and cue-related neural responses among HIV-infected smokers. These data imply that HIV-infected smokers are strong candidates for varenicline, which has been shown to be more effective for fast metabolizers [13].
Several limitations warrant mention. First, these data were cross-sectional and mechanistic, prospective studies are needed to investigate the temporal relationship between HIV infection, ART use, and increased nicotine metabolism. Second, although none of the HIV-uninfected smokers self-reported an HIV diagnosis or taking ART, we did not have biological confirmation. Because the prevalence of HIV in the cities where the HIV-uninfected sample was recruited is <2% (Philadelphia, Toronto, Buffalo, and Houston), it is unlikely we misclassified HIV status [42–45]. Lastly, NMR may be elevated via alteration in UGT enzymes which glucuronidate cotinine and 3HC, including UGT2B7 which glucuronidates both 3HC and efavirenz [17, 46].
Conclusions
These findings suggest that HIV-infected smokers metabolize nicotine faster than HIV-uninfected smokers, even after controlling for relevant demographic and behavioral factors. Understanding the mechanisms that contribute to faster nicotine metabolism among PLWH is necessary to understand tobacco’s role in undermining clinical outcomes in HIV, and identifying novel therapeutic interventions.
Supplementary Material
Acknowledgments
We would like to thank Maria Novalen and Paul Sanborn for their technical assistance with sample collection the LC-MS/MS analyses.
Role of Funding Sources
This research was supported by grants from the National Institutes of Health (R01 DA033681; K24 DA045244; R01 DA042682; R01 DA044906; and U01 DA020830) and through core services and support from the Penn Center for AIDS Research (P30 AI045008) and the Penn Mental Health AIDS Research Center (P30 MH097488). The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH).
Footnotes
Conflicts of Interest: Dr. Schnoll receives medication and placebo free of charge from Pfizer for clinical trials and has provided consultation to Pfizer, GlaxoSmithKline, and Curaleaf. Dr. Gross serves on a Pfizer Data and Safety Monitoring Board for a drug unrelated to smoking or HIV. Dr. Ashare has an investigator-initiated grant from Novo Nordisk for a drug unrelated to the current study. Dr. Tyndale has consulted for Quinn Emmanual, Apotex and Ethismos.
References
- 1.Crothers K, Griffith TA, McGinnis KA, Rodriguez-Barradas MC, Leaf DA, Weissman S, et al. The impact of cigarette smoking on mortality, quality of life, and comorbid illness among HIV-positive veterans. J Gen Intern Med 2005; 20(12):1142–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feldman JG, Minkoff H, Schneider MF, Gange SJ, Cohen M, Watts DH, et al. Association of cigarette smoking with HIV prognosis among women in the HAART era: a report from the women’s interagency HIV study. Am J Public Health 2006; 96(6):1060–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Webb MS, Vanable PA, Carey MP, Blair DC. Cigarette smoking among HIV+ men and women: examining health, substance use, and psychosocial correlates across the smoking spectrum. J Behav Med 2007; 30(5):371–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collins RL, Kanouse DE, Gifford AL, Senterfitt JW, Schuster MA, McCaffrey DF, et al. Changes in health-promoting behavior following diagnosis with HIV: prevalence and correlates in a national probability sample. Health Psychol 2001; 20(5):351–360. [PubMed] [Google Scholar]
- 5.Burkhalter JE, Springer CM, Chhabra R, Ostroff JS, Rapkin BD. Tobacco use and readiness to quit smoking in low-income HIV-infected persons. Nicotine Tob Res 2005; 7(4):511–522. [DOI] [PubMed] [Google Scholar]
- 6.Helleberg M, Afzal S, Kronborg G, Larsen CS, Pedersen G, Pedersen C, et al. Mortality attributable to smoking among HIV-1-infected individuals: a nationwide, population-based cohort study. Clin Infect Dis 2013; 56(5):727–734. [DOI] [PubMed] [Google Scholar]
- 7.Reddy KP, Parker RA, Losina E, Baggett TP, Paltiel AD, Rigotti NA, et al. Impact of Cigarette Smoking and Smoking Cessation on Life Expectancy Among People With HIV: A US-Based Modeling Study. J Infect Dis 2016; 214(11):1672–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hukkanen J, Jacob P 3rd, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev 2005; 57(1):79–115. [DOI] [PubMed] [Google Scholar]
- 9.Allenby CE, Boylan KA, Lerman C, Falcone M. Precision Medicine for Tobacco Dependence: Development and Validation of the Nicotine Metabolite Ratio. J Neuroimmune Pharmacol 2016; 11(3):471–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schnoll RA, Patterson F, Wileyto EP, Tyndale RF, Benowitz N, Lerman C. Nicotine metabolic rate predicts successful smoking cessation with transdermal nicotine: a validation study. Pharmacol Biochem Behav 2009; 92(1):6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaufmann A, Hitsman B, Goelz PM, Veluz-Wilkins A, Blazekovic S, Powers L, et al. Rate of nicotine metabolism and smoking cessation outcomes in a community-based sample of treatment-seeking smokers. Addict Behav 2015; 51:93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lerman C, Tyndale R, Patterson F, Wileyto EP, Shields PG, Pinto A, et al. Nicotine metabolite ratio predicts efficacy of transdermal nicotine for smoking cessation. Clin Pharmacol Ther 2006; 79(6):600–608. [DOI] [PubMed] [Google Scholar]
- 13.Lerman C, Schnoll RA, Hawk LW Jr., Cinciripini P, George TP, Wileyto EP, et al. Use of the nicotine metabolite ratio as a genetically informed biomarker of response to nicotine patch or varenicline for smoking cessation: a randomised, double-blind placebo-controlled trial. Lancet Respir Med 2015; 3(2):131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piliguian M, Zhu AZ, Zhou Q, Benowitz NL, Ahluwalia JS, Sanderson Cox L, et al. Novel CYP2A6 variants identified in African Americans are associated with slow nicotine metabolism in vitro and in vivo. Pharmacogenet Genomics 2014; 24(2):118–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu AZ, Zhou Q, Cox LS, Ahluwalia JS, Benowitz NL, Tyndale RF. Variation in trans-3’-hydroxycotinine glucuronidation does not alter the nicotine metabolite ratio or nicotine intake. PLoS One 2013; 8(8):e70938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chenoweth MJ, Novalen M, Hawk LW Jr., Schnoll RA, George TP, Cinciripini PM, et al. Known and novel sources of variability in the nicotine metabolite ratio in a large sample of treatment-seeking smokers. Cancer Epidemiol Biomarkers Prev 2014; 23(9):1773–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDonagh EM, Lau JL, Alvarellos ML, Altman RB, Klein TE. PharmGKB summary: Efavirenz pathway, pharmacokinetics. Pharmacogenet Genomics 2015; 25(7):363–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Earla R, Ande A, McArthur C, Kumar A, Kumar S. Enhanced nicotine metabolism in HIV-1-positive smokers compared with HIV-negative smokers: simultaneous determination of nicotine and its four metabolites in their plasma using a simple and sensitive electrospray ionization liquid chromatography-tandem mass spectrometry technique. Drug Metab Dispos 2014; 42(2):282–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schnoll RA, George TP, Hawk L, Cinciripini P, Wileyto P, Tyndale RF. The relationship between the nicotine metabolite ratio and three self-report measures of nicotine dependence across sex and race. Psychopharmacology (Berl) 2014; 231(12):2515–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fagerstrom K Determinants of tobacco use and renaming the FTND to the Fagerstrom Test for Cigarette Dependence. Nicotine Tob Res 2012; 14(1):75–78. [DOI] [PubMed] [Google Scholar]
- 21.St Helen G, Jacob P 3rd, Benowitz NL. Stability of the nicotine metabolite ratio in smokers of progressively reduced nicotine content cigarettes. Nicotine Tob Res 2013; 15(11):1939–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanner JA, Novalen M, Jatlow P, Huestis MA, Murphy SE, Kaprio J, et al. Nicotine metabolite ratio (3-hydroxycotinine/cotinine) in plasma and urine by different analytical methods and laboratories: implications for clinical implementation. Cancer Epidemiol Biomarkers Prev 2015; 24(8):1239–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mutlib AE, Chen H, Nemeth GA, Markwalder JA, Seitz SP, Gan LS, et al. Identification and characterization of efavirenz metabolites by liquid chromatography/mass spectrometry and high field NMR: species differences in the metabolism of efavirenz. Drug Metab Dispos 1999; 27(11):1319–1333. [PubMed] [Google Scholar]
- 24.Kirby GM, Batist G, Alpert L, Lamoureux E, Cameron RG, Alaoui-Jamali MA. Overexpression of cytochrome P-450 isoforms involved in aflatoxin B1 bioactivation in human liver with cirrhosis and hepatitis. Toxicol Pathol 1996; 24(4):458–467. [DOI] [PubMed] [Google Scholar]
- 25.Rassen JA, Shelat AA, Myers J, Glynn RJ, Rothman KJ, Schneeweiss S. One-to-many propensity score matching in cohort studies. Pharmacoepidemiol Drug Saf 2012; 21 Suppl 2:69–80. [DOI] [PubMed] [Google Scholar]
- 26.Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res 2011; 46(3):399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat 2011; 10(2):150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanner J-A, Prasad B, Claw KG, Stapleton P, Chaudhry A, Schuetz EG, et al. Predictors of Variation in CYP2A6 mRNA, Protein, and Enzyme Activity in a Human Liver Bank: Influence of Genetic and Nongenetic Factors. The Journal of Pharmacology and Experimental Therapeutics 2017; 360(1):129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ande A, McArthur C, Kumar A, Kumar S. Tobacco smoking effect on HIV-1 pathogenesis: role of cytochrome P450 isozymes. Expert Opin Drug Metab Toxicol 2013; 9(11):1453–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin M, Kumar A, Kumar S. Ethanol-mediated regulation of cytochrome P450 2A6 expression in monocytes: role of oxidative stress-mediated PKC/MEK/Nrf2 pathway. PLoS One 2012; 7(4):e35505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.A’edah A-B, Jukka H, Risto J, Minna R-R, Hannu R, Matti AL. Function and Regulation of the Cyp2a5/CYP2A6 Genes in Response to Toxic Insults in the Liver. Current Drug Metabolism 2013; 14(1):137–150. [PubMed] [Google Scholar]
- 32.Jin M, Earla R, Shah A, Earla RL, Gupte R, Mitra AK, et al. A LC-MS/MS method for concurrent determination of nicotine metabolites and role of CYP2A6 in nicotine metabolism in U937 macrophages: implications in oxidative stress in HIV + smokers. J Neuroimmune Pharmacol 2012; 7(1):289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yadav A, Collman RG. CNS inflammation and macrophage/microglial biology associated with HIV-1 infection. J Neuroimmune Pharmacol 2009; 4(4):430–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ande A, McArthur C, Ayuk L, Awasom C, Achu PN, Njinda A, et al. Effect of mild-to-moderate smoking on viral load, cytokines, oxidative stress, and cytochrome P450 enzymes in HIV-infected individuals. PLoS One 2015; 10(4):e0122402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gilmore WJ, Hartmann G, Piquette-Miller M, Marriott J, Kirby GM. Effects of lipopolysaccharide-stimulated inflammation and pyrazole-mediated hepatocellular injury on mouse hepatic Cyp2a5 expression. Toxicology 2003; 184(2–3):211–226. [DOI] [PubMed] [Google Scholar]
- 36.Nichols KD, Kirby GM. Microarray analysis of hepatic gene expression in pyrazole-mediated hepatotoxicity: identification of potential stimuli of Cyp2a5 induction. Biochem Pharmacol 2008; 75(2):538–551. [DOI] [PubMed] [Google Scholar]
- 37.Gilmore WJ, Kirby GM. Endoplasmic reticulum stress due to altered cellular redox status positively regulates murine hepatic CYP2A5 expression. J Pharmacol Exp Ther 2004; 308(2):600–608. [DOI] [PubMed] [Google Scholar]
- 38.Sofuoglu M, Herman AI, Nadim H, Jatlow P. Rapid nicotine clearance is associated with greater reward and heart rate increases from intravenous nicotine. Neuropsychopharmacology 2012; 37(6):1509–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dubroff JG, Doot RK, Falcone M, Schnoll RA, Ray R, Tyndale RF, et al. Decreased Nicotinic Receptor Availability in Smokers with Slow Rates of Nicotine Metabolism. J Nucl Med 2015; 56(11):1724–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang DW, Hello B, Mroziewicz M, Fellows LK, Tyndale RF, Dagher A. Genetic variation in CYP2A6 predicts neural reactivity to smoking cues as measured using fMRI. Neuroimage 2012; 60(4):2136–2143. [DOI] [PubMed] [Google Scholar]
- 41.Falcone M, Cao W, Bernardo L, Tyndale RF, Loughead J, Lerman C. Brain Responses to Smoking Cues Differ Based on Nicotine Metabolism Rate. Biol Psychiatry 2016; 80(3):190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Houston Health Department, HIV Surveillance Program. HIV Infection in Houston: An Epidemiologic Profile 2010–2014 Houston, Texas; 2015. [Google Scholar]
- 43.HIV/AIDS Annual Surveillance Report. Bureau of HIV/AIDS Epidemiology (BHAE), AIDS Institute, NY State Department of Health. Available at: http://www.health.ny.gov/diseases/aids/general/statistics/annual/index.htm
- 44.Public Health Agency of Canada. Summary: Estimates of HIV incidence, prevalence and proportion undiagnosed in Canada, 2014, Public Health Agency of Canada, Ottawa, 2015. https://www.canada.ca/en/public-health/services/publications/diseases-conditions/summary-estimates-hiv-incidence-prevalence-proportion-undiagnosed-canada-2014.html.
- 45.Philadelphia Department of Public Health, AIDS Activities Coordinating Office Surveillance Report, 2014. Philadelphia, PA: City of Philadelphia; September 2015. In. [Google Scholar]
- 46.Wassenaar CA, Conti DV, Das S, Chen P, Cook EH, Ratain MJ, et al. UGT1A and UGT2B genetic variation alters nicotine and nitrosamine glucuronidation in european and african american smokers. Cancer Epidemiol Biomarkers Prev 2015; 24(1):94–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.