Supplemental Digital Content is available in the text
Keywords: EcoHIV, fear conditioning, HIV-associated neurocognitive impairment, insulin, intranasal insulin therapy, mouse models, quantitative PCR, radial arm water maze, synaptodendritic injury, working memory
Abstract
Objective:
Almost half of HIV-positive people on antiretroviral therapy have demonstrable mild neurocognitive impairment (HIV-NCI), even when virologically suppressed. Intranasal insulin therapy improves cognition in Alzheimer's disease and diabetes. Here we tested intranasal insulin therapy in a model of HIV-NCI in EcoHIV-infected conventional mice.
Design and methods:
Insulin pharmacokinetics following intranasal administration to mice was determined by ELISA. Mice were inoculated with EcoHIV to cause NCI; 23 days or 3 months after infection they were treated daily for 9 days with intranasal insulin (2.4 IU/mouse) and examined for NCI in behavioral tests and HIV burdens by quantitative PCR. Some animals were tested for hippocampal neuronal integrity by immunostaining and expression of neuronal function-related genes by real time-quantitative PCR. The effect of insulin treatment discontinuation on cognition and neuropathology was also examined.
Results:
Intranasal insulin administration to mice resulted in μIU/ml levels of insulin in cerebrospinal fluid with a half-life of about 2 h, resembling pharmacokinetic parameters of patients receiving 40 IU. Intranasal insulin treatment starting 23 days or 3 months after infection completely reversed NCI in mice. Murine NCI correlated with reductions in hippocampal dendritic arbors and downregulation of neuronal function genes; intranasal insulin reversed these changes coincident with restoration of cognitive acuity, but they returned within 24 h of treatment cessation. Intranasal insulin treatment reduced brain HIV DNA when started 23 but not 90 days after infection.
Conclusion:
Our preclinical studies support the use of intranasal insulin administration for treatment of HIV-NCI and suggest that some dendritic injury in this condition is reversible.
Introduction
Neurocognitive impairments (NCI) in HIV-infected people are collectively known as HIV-associated neurocognitive disorders (HAND) of increasing severity from asymptomatic neurocognitive impairment (ANI), mild neurocognitive disorder (MND), to HIV-associated dementia (HAD) [1]. Chronically HIV-infected people who are virologically suppressed on long-term antiretroviral therapy (ART) rarely progress to HAD but about 50% of them will develop ANI or MND (here referred to as HIV-NCI) [2–4]. Some HIV-NCI patients report difficulties performing daily tasks, but the growing majority have ‘silent’ deficits only apparent in cognitive tests [5,6]. This largely asymptomatic NCI increases a patient's risk of progression to symptomatic disease [7,8] and the severity of NCI manifestations increases with age [3,9,10] suggesting substantial long-term health concerns associated with NCI. There are currently no therapies to prevent NCI progression in virologically suppressed individuals, nor any to address the effects of NCI on long-term management of chronic HIV infection.
HIV-NCI is diagnosed primarily in neuropsychological tests [1,11]. In contrast to HAD, HIV-NCI under ART shows minimal overt neuropathology and the disease is thought to arise from neuronal dysfunction rather than frank neuronal loss [6,12,13]. HIV-NCI includes mild dysfunctions in attention, learning/memory, working memory, executive function, and fine motor skills, in one or multiple domains [1,14], resembling the mild cognitive impairment accompanying aging, type 2 diabetes mellitus (T2DM), and the early prodromal stages of Alzheimer's disease [15–17]. One common determinant in all these conditions is a metabolic dysregulation in the brain, which for aging, obesity, and Alzheimer's disease was at least in part linked in animal models to deficient insulin signaling (reviewed in [6,18]). Intranasal insulin treatment may improve cognitive function in both T2DM and early Alzheimer's disease patients [19–22]. The efficacy of this treatment in HIV-NCI is unknown, but intranasal insulin administration was recently shown to inhibit HIV infection in culture and mitigate feline immunodeficiency virus (FIV)-induced encephalitis in cats [23]. The goal of the present work was to evaluate intranasal insulin therapy in a model of HIV-NCI in conventional mice infected with chimeric HIV, EcoHIV [24,25]. Short-term intranasal insulin therapy reverses hippocampal dendritic injury, gene dysregulation in the brain, and NCI in EcoHIV-infected mice, but these benefits diminish within 24 h of insulin treatment cessation.
Materials and methods
Mice and EcoHIV infection
Animal studies were conducted with the approval of Mount Sinai School of Medicine Institutional Animal Care and Use Committee (protocol IACUC-2014-0124) and were compliant with NIH guidelines. 6–8 week-old C57BL/6J and 129x1/SvJ mice were from the Jackson Laboratory (Farmington, Connecticut, USA). EcoHIV (clone EcoNDK-V5) was prepared and inoculated intraperitoneally at a dose of 2.0 × 106 pg HIV p24 per mouse as described [24,25].
Intranasal insulin delivery and insulin pharmacokinetics
Human insulin (HumulinR, 100 IU/ml; Eli Lilly, Toronto, Ontario, Canada) was administered intranasal at 2.4 IU/mouse as described [26]. For pharmacokinetics measurements, insulin was administered to three to eight mice per group, animals were sacrificed at the indicated time points (Fig. 1) for isolation of plasma, cerebrospinal fluid (CSF), and cortex. Insulin concentrations were determined by human insulin ELISA kit (Alpco, Salem, New Hampshire, USA) [26].
Fig. 1.
Intranasal insulin administration in mouse at 2.4 IU produces therapeutically efficacious pM-level peptide concentrations in mouse brain.
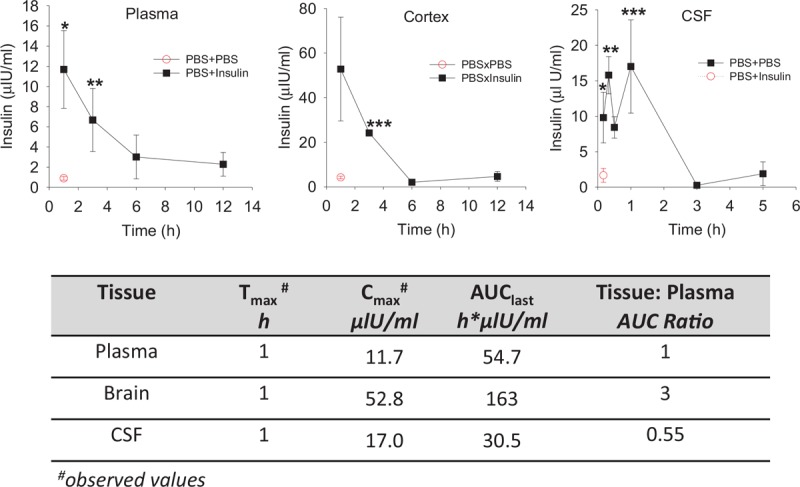
Uninfected mice were treated intranasal with PBS or human insulin at 2.4 IU/mouse (n = 3–8/group) and were sacrificed at the indicated times after insulin administration or 15 min after PBS administration for insulin pharmacokinetics analysis in plasma, cerebrospinal fluid, and cortex. Results are expressed in μIU/ml ± SEM (1 μIU/ml = 0.14 pmol/l). The lower part of Figure shows insulin pharmacokinetic constants for plasma and brain derived from these analyses. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001 vs. PBS × PBS.
Tissue collection and EcoHIV burden analysis
Plasma was obtained from blood drawn from retro-orbital sinus of anesthetized mice [27]. CSF (10–15 μl) was collected from cisterna magna essentially as described [28]. Spleens and brains were dissected from animals euthanized by 95% CO2 asphyxiation and processed for HIV burden analysis as described [24].
Behavioral tests
Mice were tested for spatial learning and working memory in radial arm water maze (RAWM) [29] and for contextual associative fear memory by fear conditioning, fold change [30], as previously described [24]. RAWM test consisted of four daily learning trials and a 30 min-delayed retention trial for assessment of working memory [17]. Error averages of the last 3 days of testing were used for statistical analysis. Contextual fold change responses were measured using a SDI Freeze Monitor (San Diego Instruments, San Diego, California, USA) following manufacturer's protocol [24]. Results are presented as mean percentage of total time spent freezing on the training day (baseline) and the contextual fear readout day (context).
Evaluation of hippocampal neuronal integrity by immunofluorescence staining
Mouse perfusion, preparation of frozen brain sections, and immunofluorescence staining of sections were previously described [31]. Antibodies used were rabbit anti-microtubule-associated protein 2 (MAP2) for detection of dendrites (EMD Millipore, Burlington, Massachusetts, USA, 1 : 150) and mouse anti-neuronal nuclear antigen (NeuN) for detection of neuronal nuclei (EMD Millipore, 1 : 150) followed by goat-antirabbit IgG Alexa 488 and donkey-antimouse IgG Alexa 594 (ThermoFisher Scientific, Waltham, Massachusetts, USA, 1 : 150), respectively. Images were captured and fluorescence signals quantified as described [31].
Effect of insulin treatment on expression of neuronal function and metabolism-related genes in the brain
Brain RNA was isolated and reverse-transcribed as described [24,31]. Its complementary DNA (cDNA) was tested for gene expression changes using custom-designed TaqMan Gene Expression Array Plates from ThermoFisher Scientific. Reactions were run in a ThermoFisher Scientific (Applied Biosystems, Waltham, Massachusetts, USA) 7900 instrument according to the manufacturer's protocol.
Pharmacokinetic analysis of intranasal insulin
The pharmacokinetic parameters were calculated using noncompartmental method of software program Phoenix WinNonlin version 6.4 (Certara USA, Inc., Princeton, New Jersey, USA). The maximum plasma concentration (Cmax) and time to Cmax (Tmax) were the observed values. The area under the plasma concentration time curve (AUC) was calculated to the last quantifiable sample (AUClast) by use of the log-linear trapezoidal rule. The brain to plasma partition coefficients were calculated as a ratio of mean AUCs (AUC0−t, brain/AUC0−t, plasma).
Statistical analysis
Differences in EcoHIV burdens and other parameters between control vs. infected and nontreated vs. treated mice were tested by paired Student's t test. Changes in cellular gene expression in brain tissues of infected or drug-treated mice were first normalized to respective uninfected or nondrug-treated controls then compared in t test between infected and drug-treated-infected groups.
Results
Intranasal insulin pharmacokinetics in mice
Figure 1 shows pharmacokinetics evaluation of intranasal insulin delivery (2.4 IU/mouse) in plasma, CSF, and cortex of mock-infected C57BL/6 mice. The insulin pharmacokinetics profiles for plasma and cortex were similar, insulin concentrations reached their respective peaks at 30 min and the peptide cleared to baseline by 6 h. Cortex insulin levels remained more than 10-fold above baseline for 3 h following insulin administration. The CSF insulin profile was irregular with an early peak at 15 min, a Cmax of 17 μIU/ml (2.38 pmol/l) at 1 h, and clearance by 3 h. Insulin Cmax in cortex was substantially higher than in plasma at 53.0 μIU/ml (7.42 pmol/l) vs. 11.7 μIU/ml (1.64 pmol/l), respectively. In addition, overall cortex insulin exposures (measured by area under the curve AUC0−t) were also higher with AUCbrain/AUCplasma ratio of 3 (table in Fig. 1). These results illustrate that intranasal insulin administration in mice at 2.4 IU produces a rapid intracerebral peptide accumulation with therapeutic concentrations lasting 2–3 h.
Intranasal insulin treatment reverses murine HIV-neurocognitive impairment independent of HIV brain burdens
EcoHIV-infected mice manifested cognitive impairment (murine HIV-NCI) 1 month after infection as shown by their failure to learn the hidden platform location in RAWM compared with uninfected mice (Fig. 2a). When treated daily with intranasal insulin for 9 days, infected mice performed similarly in RAWM to untreated or insulin-treated-uninfected mice (Fig. 2a), suggesting that their NCI was reversed. All mouse groups performed equally well in finding the visible platform (right panel in Fig. 2a), indicating that neither EcoHIV infection nor intranasal insulin-affected animal mobility, vision, or motivation. Intranasal insulin treatment had no effect on HIV levels in spleen but significantly reduced HIV brain burdens (Fig. 2b). EcoHIV infection of mice had no effect on brain insulin concentrations compared with mock-infected mice (Fig. 2c). Pharmacokinetics assessment of intranasal insulin delivery after RAWM evaluation indicated preferential peptide accumulation in the brain in both EcoHIV and mock-infected mice (Fig. 2c).
Fig. 2.
Intranasal insulin therapy restores deficient learning/memory in chronically EcoHIV-infected mice.
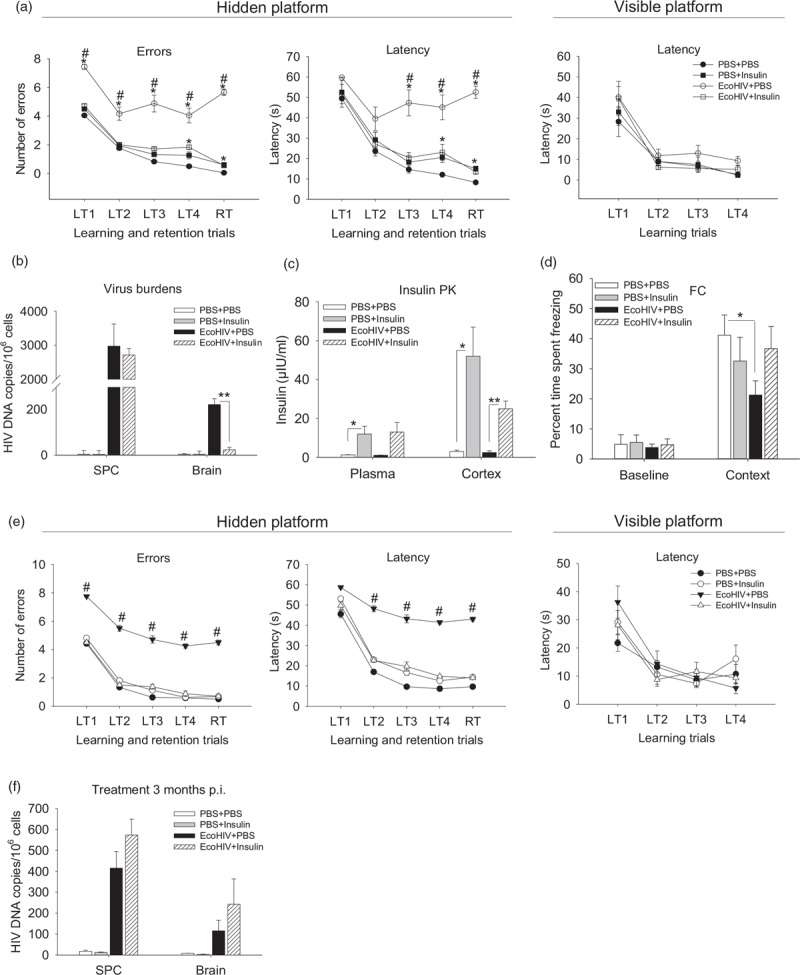
(a–d) Effect of intranasal insulin treatment on EcoHIV-induced spatial learning/memory 1 month after infection. Mice were EcoHIV or mock (PBS) infected for 21 days, the animals were then acclimated to intranasal delivery with daily intranasal PBS administration (24 μl/mouse) for 5 days followed by daily intranasal insulin or intranasal vehicle (PBS) treatment for 9 days. The treatment groups (10 animals each) were PBS + PBS, PBS + insulin, EcoHIV + PBS, and EcoHIV + insulin. Mice were tested in radial arm water maze during days 2–9 of treatment and sacrificed on the last day of treatment (34 days after infection) for intranasal insulin pharmacokinetics test, tissue collection, and analysis. (a) Results of radial arm water maze behavioral test showing number of errors made (left panel) and time spent (latency, middle panel) in finding hidden platform. Right panel: time spent (latency) in finding visible platform. ∗P < 0.04, #P < 0.01. (b) EcoHIV burdens in spleen cells (SPC) and brain measured by quantitative PCR. ∗∗P < 0.0003. (c) Insulin pharmacokinetics measured after completion of radial arm water maze testing (n = 3/pharmacokinetics group). ∗P < 0.01, ∗∗P < 0.001. (d) Effect of intranasal insulin treatment on EcoHIV-induced fear-associative memory 1 month after infection. The experiment was repeated as described above using 129x1/SvJ mice and the animals were subjected to contextual fold change test during intranasal insulin treatment. The results are expressed as percentage mean time spent freezing and shown for mice tested prior to fear conditioning with paired sound-shock stimuli (baseline) and for conditioned mice placed 24 h later in chamber used for conditioning (context); ∗P < 0.05. (e) and (f) Effects of insulin upon neurocognitive impairment and virus burden in mice in long-term chronically EcoHIV-infected mice. The experiment described under part (a) was repeated except that intranasal-insulin treatment and radial arm water maze test were conducted 3 months after EcoHIV infection. The figure shows averaged results of last training trial and retention trial. ∗P < 0.05, #P < 0.05. (f) SPC and brain EcoHIV burdens measured by quantitative PCR. The differences between EcoHIV + PBS and EcoHIV + insulin were not significant: (P = 0.11 for spleen cells and P = 0.28 for brain).
To confirm insulin effects on cognition, groups of mice were subjected to intranasal insulin treatment and evaluated for a contextual associative fear responses [30] by fold change (Fig. 2d). Infected conditioned mice spent less time exhibiting the threat-induced freezing response compared with control mice when placed in the fear conditioning chamber the next day (context panel in Fig. 2d), indicating impairment in recall of the conditioned response. Intranasal insulin treatment returned the freezing response time of infected mice to that of uninfected animals (Fig. 2d). Finally, we inquired whether EcoHIV-infected mice remain sensitive to the beneficial effects of insulin 3 months after infection (Fig. 2e). The RAWM measures of murine NCI were similar 1 and 3 months after HIV infection, confirming the chronic nature of HIV-NCI in mice [24]. This chronic NCI was also fully reversible with 9 days of intranasal insulin treatment (Fig. 2a and e). However, in contrast to early HIV-NCI in mice, HIV DNA brain levels were not significantly affected by insulin treatment 3 months after infection (Fig. 2f). Direct evaluation of insulin effects on EcoHIV replication in primary mouse macrophages in culture (see Figure in Supplemental Digital Content) indicated that insulin can inhibit HIV replication but only at IU/ml concentrations, which were about 100-fold higher than those attained in the brain after intranasal insulin (Fig. 1). These results suggest that intranasal insulin therapy is equally effective in reversing early and chronic HIV-NCI in EcoHIV-infected mice but that the effect of insulin on cognition in long-term-infected animals is independent of HIV DNA levels in brain.
Intranasal insulin treatment reverses hippocampal dendritic injury and normalizes expression of selected brain function genes in murine HIV-neurocognitive impairment
The spatial memory deficit in EcoHIV-infected mice (Fig. 2a) points to the hippocampus as the primary anatomical substrate of this dysfunction [17,29]. We tested hippocampal neuronal integrity in HIV-NCI mice by staining brain sections for the neuronal dendrite marker MAP2 [32,33] and neuronal cell body marker NeuN (Fig. 3a). We found a significant reduction in MAP2 staining in the CA1 and CA3 regions of the hippocampus of infected mice compared with controls (Fig. 3a and b), coinciding in real time with NCI in these animals shown in Fig. 2a. Insulin treatment of infected mice restored MAP2 stained dendrites to control levels (Fig. 3a and b), coinciding with restoration of spatial memory (Fig. 2a). In contrast, neuronal cell bodies visualized by NeuN costaining remained unaltered under all conditions tested, suggesting that EcoHIV infection does not cause hippocampal neuronal loss (Fig. 3a and b), similar to the normal HIV neuropathology in HIV-infected patients on ART [13]. These results suggest that EcoHIV infection disrupts neuronal dendritic integrity in the hippocampus and that this process is reversible by intranasal insulin treatment.
Fig. 3.
Intranasal insulin treatment reverses hippocampal dendritic injury and normalizes expression of selected brain function genes in EcoHIV-infected mice with neurocognitive impairment.
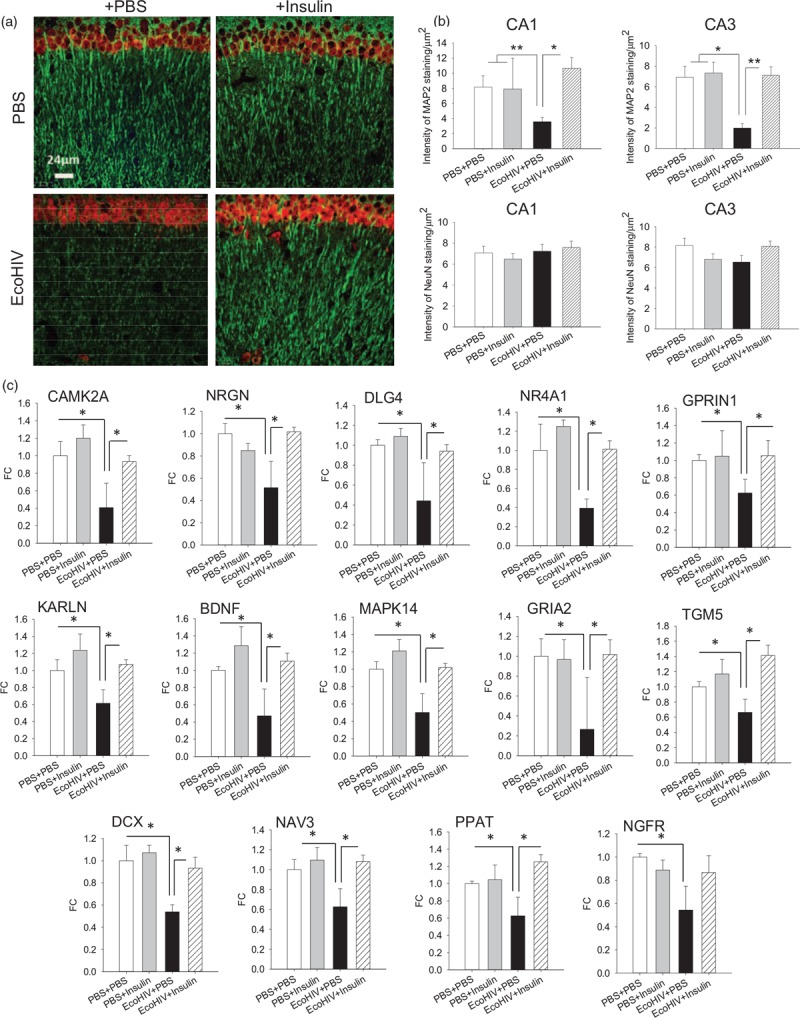
Brain specimens were from mice (three per variable) that completed behavioral testing in experiment shown in Fig. 2(a–c). (a) Representative confocal microscope images from the CA1 region of the hippocampus showing detection of neuronal dendritic [microtubule-associated protein 2 (MAP2), in green] and nuclear [neuronal nuclear antigen (NeuN), in red] proteins; scale bar = 24 μm. (b) Quantification of MAP2 and NeuN staining in the CA1 and CA3 regions of the hippocampus; ∗P < 0.01, ∗∗P < 0.005. (c) Expression of selected brain function-related genes in brain tissues. Total brain RNA was isolated and reverse-transcribed to complementary DNA (cDNA) as previously described [34]. The cDNAs were evaluated on custom TaqMan Array 96-Well Plates (ThermoFisher Scientific) according to manufacturer's instructions. The 88 genes represented in the arrays (Table S1) were chosen according to their published functions in synaptic plasticity, neuronal function, and brain metabolism. Fold change in gene expression relative to control (PBS intraperitoneally + PBS intranasal); ∗P < 0.05.
To confirm these observations, mouse brain tissues were evaluated by custom quantitative PCR arrays for expression of 88 genes selected for their roles in synaptic plasticity, neuronal function, and brain metabolism (some of these genes were previously identified by us and others in the genome-wide microarray analyses of brain tissues from HAND patients [34,35]). Fourteen genes were significantly downmodulated (P < 0.05) by a factor of 1.5–3.8 in EcoHIV-infected mice compared with uninfected controls, and their downmodulation was fully reversed by intranasal insulin treatment (Fig. 3c and Table in Supplemental Digital Content). Ten significantly downregulated genes, CAMK2A, calcium/calmodulin-dependent protein kinase II, NRGN, neurogranin, DLG4, NR4A1, GPRIN1, KALRN, NGFR, BDNF, brain-derived neurotrophic factor, MAPK14, and GRIA2 are implicated in synaptic plasticity, dendrite biology, and neuronal signal transmission, two in neurogenesis (DCX, doublecortin, and NAV3, neuron navigator 3), and two (PPAT, phosphoribosyl pyrophosphate amidotransferase, and TMG5, transglutaminase 5) in glutamine and energy metabolism (Fig. 3c). Most of the other genes on the array showed a trend to below-normal expression in EcoHIV-infected mice and to normalization by insulin treatment. For complete array results and full names and functions of the significantly altered genes, see Table in Supplemental Digital Content.
The benefits of intranasal insulin treatment in murine HIV-neurocognitive impairment diminish rapidly upon treatment discontinuation
The contrast between the short half-life of intranasal-delivered insulin in mouse brain (Fig. 1) and the effectiveness of intranasal insulin in ameliorating HIV-NCI (Figs. 2 and 3), prompted us to investigate the durability of insulin effects on cognition. To this end, we discontinued intranasal insulin treatment in parallel groups of uninfected and EcoHIV-infected mice on the third day of cognitive evaluation in RAWM (Fig. 4a and b and blue columns in Fig. 4c). RAWM evaluation proceeded until completion, mice were tested for virus burden (Fig. 4c) and hippocampal dendrite integrity (Fig. 4d and e). The standard last 3-day average representation of RAWM results (Fig. 4a) shows that treatment cessation in infected animals dramatically worsened their performance in the maze compared with infected, continually treated animals. A plot of daily retention trial (working memory) data for all animal groups (Fig. 4b) revealed that intranasal insulin began to restore working memory in infected mice on the first day of testing, reached statistical significance on the second day, and maximum effect by day 5. However, discontinuation of insulin treatment in infected mice resulted in a complete return of working memory impairment within 24 h (Fig. 4b, blue columns). Reproducing data shown in Fig. 2, untreated infected mice tested 1 month after infection had detectable HIV DNA in the brain that was reduced following intranasal insulin by more than 90%; insulin had no effect on levels of splenic virus (Fig. 4c). Discontinuation of insulin treatment restored HIV brain burdens to close to pretreatment levels and significantly increased peripheral virus burdens compared with untreated mice (Fig. 4c). Analysis of hippocampal dendrite integrity revealed a similar pattern of efficacy (Fig. 4d and e), with insulin restoring dendrite MAP2 integrity in the CA1 and CA3 regions of the hippocampus to levels similar to uninfected mice. Cessation of insulin treatment resulted in a return of dendritic damage with MAP2 staining at levels seen in infected untreated mice. These results confirm the beneficial effects of intranasal insulin on cognition and hippocampal dendrite integrity in EcoHIV-infected mice (Figs. 2 and 3) and show that treatment discontinuation rapidly reverses these benefits.
Fig. 4.
Discontinuation of intranasal insulin therapy in EcoHIV-infected mice with neurocognitive impairment eliminates beneficial effects of insulin on hippocampal dendritic injury and neurocognitive impairment.

Mice were infected and treated with intranasal insulin as described in Fig. 2(a–c) except that the groups (PBS + insulin) and (EcoHIV + insulin) contained 16 mice each. Insulin administration was discontinued in eight mice each in these groups from day 3 of radial arm water maze testing until experiment completion (blue bars in Fig. 2b) and the new groups were labeled (PBS + insulin/disc) and (EcoHIV + insulin/disc). (a) Standard presentation of radial arm water maze results in this experiment showing errors (left panel), latency (middle panel), and visible platform latency (right panel) averaged for the last 3 days of the maze testing. ∗P < 0.001, #P < 0.002. (b) Daily radial arm water maze retention trial results showing errors in finding hidden platform; red arrows indicate when insulin treatment was discontinued; blue-hued columns represent insulin-discontinued mouse groups. ∗P < 0.001, #P < 0.002. (c) Lymphoid and brain HIV burdens. ∗P < 0.001, #P < 0.002. (d) Representative confocal microscope images from the CA1 region of the hippocampus showing detection of neuronal dendritic (MAP2, in green) and nuclear (NeuN, in red) markers; scale bar = 45 μm. (e) Quantification of MAP2 staining in the CA1 and CA3 regions of the hippocampus; ∗P < 0.01, ∗∗P < 0.005.
Discussion
Our findings demonstrate that intranasal insulin treatment reverses cognitive impairment in conventional EcoHIV-infected mice. The behavioral defects and physiological changes in brains of these mice may form a useful model of HIV-NCI in HIV-positive people on ART. Chronically EcoHIV-infected mice have low lymphoid and brain HIV burdens and remain immunocompetent, in part due to control of HIV replication by the host immune system [24,31,36,37]. Like chronically HIV-infected patients on ART [38], despite virus control, infected mice develop mild abnormalities in gut, lung, and nervous system including chronic, ART-independent HIV-NCI [24,25,31,36,39–42]. Human HIV-NCI is a life-long condition that cannot be prevented even when ART is initiated several months after primary HIV infection [43]. Although animal models cannot fully replicate human disease, our results in EcoHIV-infected mice suggest that intranasal insulin delivery could be an effective treatment for HIV-NCI. This conclusion is based on several findings in the present work.
Starting with intranasal insulin administration, both peak central nervous system (CNS) concentrations and half-life of 2.4 IU intranasal-delivered insulin in mice resembled the intranasal-insulin pharmacokinetics profile in humans receiving 40 IU [44]. Separately, we have shown that intranasal insulin at this dose increases energy metabolism in mouse brain but not plasma glucose levels [26], affirming the reported safety profile of short-term intranasal insulin administration in people [45]. Because 40 IU insulin shows reproducible therapeutic efficacy in clinical trials of Alzheimer's disease and T2DM patients [19–22], we have achieved therapeutic doses of insulin in mouse CNS. As in animal models of nonviral cognitive diseases [46–48], short-term intranasal insulin treatment here was effective in reversing murine HIV-NCI. However, as in the SAMP8 mouse model of aging/Alzheimer's disease [48], the beneficial effect of a single intranasal insulin administration on cognition in infected mice lasted only about 24 h, possibly due to combined limitations of short half-life of intranasal-delivered insulin (Fig. 1 and [44]) and transient nature of insulin signaling [49]. This suggests that long-term intranasal insulin administration will be essential for the preservation of cognitive function [50] and implies prospect of life-long adjunct intranasal insulin therapy to mitigate progression of NCI with aging [3,10]. Importantly, this is feasible, however, further studies are imperative to determine potential side effects of such therapy [45,51] and complications that may arise from patient noncompliance and other treatments.
Second, we show that intranasal insulin treatment reversed impairment in all three cognitive abilities tested in EcoHIV-infected mice, visuospatial learning, working memory, and contextual (explicit) memory. Working memory is a prefrontal cortex (PFC) executive function [52,53] required for spatial learning in RAWM [17,29], whereas both visuospatial and explicit memory require functional integrity of the hippocampus and entorhinal cortex in the medial temporal lobe [54,55]. Thus, mouse HIV-NCI resembles human HIV-NCI in the putative diffuse injury to the anatomical structures and neuronal networks spanning the PFC, striatum, and medial temporal lobe, a feature distinguishing mild NCI from the characteristic severe white matter injury and motor dysfunction of HIV dementia [3,12,56]. The cognitive abilities measured in EcoHIV-infected mice are among seven cognitive domains evaluated in standardized neuropsychological tests for NCI diagnosis [1] and they are frequently abnormal in clinically asymptomatic NCI individuals (reviewed in [14,57,58]). Declines in working and explicit memory also serve as indicators of HIV-NCI progression, particularly with aging [3,9,10]. The beneficial effects of intranasal insulin on these functions in EcoHIV-infected mice, including in older animals (Fig. 2e), indicate that this treatment could mitigate some of the major cognitive deficits in patients with HIV-NCI and potentially lessen the effect of aging on NCI severity.
Third, our results link memory impairment in EcoHIV-infected mice to downregulation of selected neuronal function genes and hippocampal dendritic dearborization without apparent neuronal loss in the region. The joint onset of these changes, their joint reversal by intranasal insulin, and their joint reappearance with insulin withdrawal indicate a strong causal relationship between the hippocampal injury and memory defects in this model. Synaptic but not dendritic injury and impaired memory were observed in transgenic mice expressing Tat in astrocytes [59] suggesting that Tat produced by EcoHIV-infected cells [36] contributes to murine NCI observed here. Synaptodendritic injury, rather than neurodegeneration, is considered the primary defect responsible for NCI in HIV infection [12,60], but it was first documented in brain tissues from AIDS patients with active HIV replication [61,62]. Neuropathological studies in successfully HIV-suppressed individuals with NCI are rare [63]. However, a consensus is building that these individuals have limited HIV-specific brain abnormalities including minimal HIV-related neuroinflammation [6,13,63] and that their NCI correlates with synaptodendritic injury, best represented by reduced combined scores of MAP2 and synaptic marker synaptophysin [64]. Considering our findings here and elsewhere on immunocompetence and unremarkable brain histopathology in chronically EcoHIV-infected mice [24,36,65], we propose that this model mimics neuronal injury aspects of NCI in HIV suppressed people, particularly patients with visuospatial and verbal memory deficits and nonnecrotic functional changes in the hippocampus [66,67].
The mechanisms responsible for the neuronal injury observed here are unknown. The restoration of dendritic arbors and memory after insulin treatment indicates that relevant neurons survive and can return to full functionality, similar to reversal of memory and synaptic deficits in amyloid beta precursor protein (APP) transgenic mice treated with insulin-like growth factor-2 (IGF-2) [68] and transient loss of MAP2 dendritic staining after moderate traumatic brain injury [69]. Common to the three systems are instability of hippocampal dendritic arbors and synaptic termini in response to pathogenic stimuli and dendritic regrowth by surviving neurons either after cessation of the stimulus [69] or upon empirical restorative treatment ([68] and this work). The speed of dendritic changes is illustrated in the brain trauma study, where hippocampal MAP2 staining declined 4 h after brain impact and recovered partially by 24 h [69]. The proposed mechanism of dendritic structural stability includes reversible MAP2-microtubule association under the control of mitogen-activated protein kinase/extracellular signal-regulated kinase (MEK-ERK) and CaMKII pathways [70]. Notably, CaMKII and its regulator NRGN [71] are transcriptionally downregulated in EcoHIV infection here, as is the neurotrophin BDNF that enhances dendrite formation [72], and they are restored to normal expression with insulin treatment. Although further similarities between EcoHIV model and HIV-NCI in patients on ART must be established, mechanistic studies on the reversible destabilization of hippocampal dendritic arbors by HIV in this model may lead to long-acting treatment of human HIV-NCI.
Finally, our results open avenues for better understanding of beneficial effects of insulin on HIV-NCI in clinically asymptomatic people. The brain is an insulin-sensitive organ [51] and multiple studies in animals and humans suggest a role for insulin and IGF in maintaining neuronal plasticity and cognitive function (reviewed in [18,51,73]). We have recently shown that murine NCI caused by EcoHIV (but not virus replication) can be prevented by treatment with the glutaminase antagonist 6-diazo-5-oxo-l-norleucine [25]. This finding, and downmodulation of the PPAT and TMG5 metabolism-related genes here, suggest that EcoHIV-infected mice demonstrate some of the metabolic dysregulation characterizing human HAND [6,74], possibly explaining some benefits of insulin treatment. The question of potential development of insulin resistance [74] upon long-term intranasal insulin treatment can also be experimentally addressed. Finally, the recent FIV study [23] and the present work indicate that insulin can affect FIV and HIV infection in the brain of their respective hosts. Reduction in HIV brain burdens or expression would mitigate the pathogenic stimulus for cognitive impairment. Exploration of these insulin effects may lead to new antiviral strategies that will also mitigate NCI.
Acknowledgements
We thank Dr O. Arancio, Columbia University, for help in introducing mouse behavioral tests to our laboratory and Ms I. Totillo for help in article preparation. We also recognize the role of Heather Thomas, MBA, as Program Manager of the Johns Hopkins NIMH Center, and support from the JHU Center for AIDS Research.
Author contributions: B.-H.K. performed investigations and formal analysis, and drafted the original article; J.K. contributed animal behavior methodology and performed investigations; A.B. performed investigations and formal analysis; E.H., H.H., M.T.N., and R.R. performed investigations; M.J.P. performed formal analysis and edited the article; N.J.H. edited the article; J.C.M. and B.S.S. conceptualized the project and edited the article; D.J.V. conceptualized and supervised the project, performed formal analysis, and wrote the final article.
The current work was supported by P01MH105280 (J.C.M., N.J.H., B.S.S., D.J.V.); R01DA037611 (D.J.V.); R01MH104145 (D.J.V.), P30MH075673 (J.C.M., N.J.H., B.S.S.); R01NS094146 (M.J.P., D.J.V.); and P30AI094189-06 (JHU Center for AIDS Research).
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
References
- 1.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 2007; 69:1789–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harezlak J, Buchthal S, Taylor M, Schifitto G, Zhong J, Daar E, et al. Persistence of HIV-associated cognitive impairment, inflammation, and neuronal injury in era of highly active antiretroviral treatment. AIDS 2011; 25:625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol 2011; 17:3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robertson KR, Smurzynski M, Parsons TD, Wu K, Bosch RJ, Wu J, et al. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS 2007; 21:1915–1921. [DOI] [PubMed] [Google Scholar]
- 5.Gates TM, Cysique LA. The chronicity of HIV infection should drive the research strategy of NeuroHIV treatment studies: a critical review. CNS Drugs 2016; 30:53–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saylor D, Dickens AM, Sacktor N, Haughey N, Slusher B, Pletnikov M, et al. HIV-associated neurocognitive disorder – pathogenesis and prospects for treatment. Nat Rev Neurol 2016; 12:234–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grant I, Franklin DR, Jr, Deutsch R, Woods SP, Vaida F, Ellis RJ, et al. Asymptomatic HIV-associated neurocognitive impairment increases risk for symptomatic decline. Neurology 2014; 82:2055–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sacktor N, Skolasky RL, Seaberg E, Munro C, Becker JT, Martin E, et al. Prevalence of HIV-associated neurocognitive disorders in the Multicenter AIDS Cohort Study. Neurology 2016; 86:334–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brouillette M-J, Yuen T, Fellows LK, Cysique LA, Heaton RK, Mayo NE. Identifying neurocognitive decline at 36 months among HIV-positive participants in the CHARTER Cohort using group-based trajectory analysis. PLoS One 2016; 11:e0155766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodkin K, Miller EN, Cox C, Reynolds S, Becker JT, Martin E, et al. Effect of ageing on neurocognitive function by stage of HIV infection: evidence from the Multicenter AIDS Cohort Study. Lancet HIV 2017; 4:e411–e422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valcour V, Sithinamsuwan P, Letendre S, Ances B. Pathogenesis of HIV in the central nervous system. Curr HIV/AIDS Rep 2011; 8:54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci 2007; 8:33–44. [DOI] [PubMed] [Google Scholar]
- 13.Gelman BB. Neuropathology of HAND with suppressive antiretroviral therapy: encephalitis and neurodegeneration reconsidered. Curr HIV/AIDS Rep 2015; 12:272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woods SP, Moore DJ, Weber E, Grant I. Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychol Rev 2009; 19:152–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Langa KM, Levine DA. The diagnosis and management of mild cognitive impairment: a clinical review. JAMA 2014; 312:2551–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biessels GJ, Despa F. Cognitive decline and dementia in diabetes mellitus: mechanisms and clinical implications. Nat Rev Endocrinol 2018; 14:591–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puzzo D, Lee L, Palmeri A, Calabrese G, Arancio O. Behavioral assays with mouse models of Alzheimer's disease: practical considerations and guidelines. Biochem Pharmacol 2014; 88:450–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calvo-Ochoa E, Arias C. Cellular and metabolic alterations in the hippocampus caused by insulin signalling dysfunction and its association with cognitive impairment during aging and Alzheimer's disease: studies in animal models. Diabetes Metab Res Rev 2015; 31:1–13. [DOI] [PubMed] [Google Scholar]
- 19.Craft S, Baker LD, Montine TJ, Minoshima S, Watson GS, Claxton A, et al. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol 2012; 69:29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freiherr J, Hallschmid M, Frey WH, 2nd, Brünner YF, Chapman CD, Hölscher C, et al. Intranasal insulin as a treatment for Alzheimer's disease: a review of basic research and clinical evidence. CNS Drugs 2013; 27:505–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Novak V, Milberg W, Hao Y, Munshi M, Novak P, Galica A, et al. Enhancement of vasoreactivity and cognition by intranasal insulin in type 2 diabetes. Diabetes Care 2014; 37:751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ott V, Benedict C, Schultes B, Born J, Hallschmid M. Intranasal administration of insulin to the brain impacts cognitive function and peripheral metabolism. Diabetes Obes Metab 2012; 14:214–221. [DOI] [PubMed] [Google Scholar]
- 23.Mamik MK, Asahchop EL, Chan WF, Zhu Y, Branton WG, McKenzie BA, et al. Insulin treatment prevents neuroinflammation and neuronal injury with restored neurobehavioral function in models of HIV/AIDS neurodegeneration. J Neurosci 2016; 36:10683–10695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gu CJ, Borjabad A, Hadas E, Kelschenbach J, Kim BH, Chao W, et al. EcoHIV infection of mice establishes latent viral reservoirs in T cells and active viral reservoirs in macrophages that are sufficient for induction of neurocognitive impairment. PLoS Pathog 2018; 14:e1007061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nedelcovych MT, Tenora L, Kim B-H, Kelschenbach J, Chao W, Hadas E, et al. N-(pivaloyloxy)alkoxy-carbonyl prodrugs of the glutamine antagonist 6-diazo-5-oxo-l-norleucine (DON) as a potential treatment for HIV associated neurocognitive disorders. J Med Chem 2017; 60:7186–7198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nedelcovych MT, Gadiano AJ, Wu Y, Manning AA, Thomas AG, Khuder SS, et al. Pharmacokinetics of intranasal versus subcutaneous insulin in the mouse. ACS Chem Neurosci 2018; 9:809–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fried JH, Worth DB, Brice AK, Hankenson FC. Type, duration, and incidence of pathologic findings after retroorbital bleeding of mice by experienced and novice personnel. J Am Assoc Lab Anim Sci 2015; 54:317–327. [PMC free article] [PubMed] [Google Scholar]
- 28.Liu L, Duff K. A technique for serial collection of cerebrospinal fluid from the cisterna magna in mouse. J Vis Exp 2008; 21:960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diamond DM, Park CR, Heman KL, Rose GM. Exposing rats to a predator impairs spatial working memory in the radial arm water maze. Hippocampus 1999; 9:542–552. [DOI] [PubMed] [Google Scholar]
- 30.LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol 2003; 23:727–738. [DOI] [PubMed] [Google Scholar]
- 31.He H, Sharer LR, Chao W, Gu CJ, Borjabad A, Hadas E, et al. Enhanced human immunodeficiency virus type 1 expression and neuropathogenesis in knockout mice lacking type I interferon responses. J Neuropathol Exp Neurol 2014; 73:59–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harada A, Teng J, Takei Y, Oguchi K, Hirokawa N. MAP2 is required for dendrite elongation, PKA anchoring in dendrites, and proper PKA signal transduction. J Cell Biol 2002; 158:54154–54159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schartz ND, Herr SA, Madsen L, Butts SJ, Torres C, Mendez LB, et al. Spatiotemporal profile of Map2 and microglial changes in the hippocampal CA1 region following pilocarpine-induced status epilepticus. Sci Rep 2016; 6:24988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borjabad A, Morgello S, Chao W, Kim S-Y, Brooks AI, Murray J, et al. Significant effects of antiretroviral therapy on global gene expression in brain tissues of patients with HIV-1-associated neurocognitive disorders. PLoS Pathog 2011; 7:e1002213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gelman BB, Chen T, Lisinicchia JG, Soukup VM, Carmical JR, Starkey JM, et al. The National NeuroAIDS Tissue Consortium brain gene array: two types of HIV-associated neurocognitive impairment. PLoS One 2012; 7:e46178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Potash MJ, Chao W, Bentsman G, Paris N, Saini M, Nitkiewicz J, et al. A mouse model for study of systemic HIV-1 infection, antiviral immune responses, and neuroinvasiveness. Proc Natl Acad Sci U S A 2005; 102:3760–3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelschenbach J, Borjabad A, Chao W, Gu C-J, He H, Bentsman G, et al. Replication of chimeric HIV in mouse brain induces HAND-like pathology and cognitive disease independent of HIV envelope glycoprotein. J Neurovirol 2012; 18 Suppl. 1:S53. [Google Scholar]
- 38.Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet 2013; 382:1525–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones LD, Jackson JW, Maggirwar SB. Modeling HIV-1 induced neuroinflammation in mice: role of platelets in mediating blood-brain barrier dysfunction. PLoS One 2016; 11:e0151702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olson KE, Bade AN, Namminga KL, Potash MJ, Mosley RL, Poluektova LY, et al. Persistent EcoHIV infection induces nigral degeneration in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-intoxicated mice. J Neurovirol 2018; 24:398–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Geraghty P, Hadas E, Kim BH, Dabo AJ, Volsky DJ, Foronjy R. HIV infection model of chronic obstructive pulmonary disease in mice. Am J Physiol Lung Cell Mol Physiol 2017; 312:L500–L509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sindberg GM, Sharma U, Banerjee S, Anand V, Dutta R, Gu CJ, et al. An infectious murine model for studying the systemic effects of opioids on early HIV pathogenesis in the gut. J Neuroimmune Pharmacol 2015; 10:74–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kore I, Ananworanich J, Valcour V, Fletcher JLK, Chalermchai T, Paul R, et al. Neuropsychological impairment in acute HIV and the effect of immediate antiretroviral therapy. J Acquir Immune Defic Syndr 2015; 70:393–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci 2002; 5:514–516. [DOI] [PubMed] [Google Scholar]
- 45.Kupila A, Sipila J, Keskinen P, Simell T, Knip M, Pulkki K, et al. Intranasally administered insulin intended for prevention of type 1 diabetes – a safety study in healthy adults. Diabetes Metab Res Rev 2003; 19:415–420. [DOI] [PubMed] [Google Scholar]
- 46.Chen Y, Zhao Y, Dai C-L, Liang Z, Run X, Iqbal K, et al. Intranasal insulin restores insulin signaling, increases synaptic proteins, and reduces Aβ level and microglia activation in the brains of 3xTg-AD mice. Exp Neurol 2014; 261:610–619. [DOI] [PubMed] [Google Scholar]
- 47.Marks DR, Tucker K, Cavallin MA, Mast TG, Fadool DA. Awake intranasal insulin delivery modifies protein complexes and alters memory, anxiety, and olfactory behaviors. J Neurosci 2009; 29:6734–6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salameh TS, Bullock KM, Hujoel IA, Niehoff ML, Wolden-Hanson T, Kim J, et al. Central nervous system delivery of intranasal insulin: mechanisms of uptake and effects on cognition. J Alzheimers Dis 2015; 47:715–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krüger M, Kratchmarova I, Blagoev B, Tseng Y-H, Kahn CR, Mann M. Dissection of the insulin signaling pathway via quantitative phosphoproteomics. Proc Natl Acad Sci U S A 2008; 105:2451–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chapman CD, Schiöth HB, Grillo CA, Benedict C. Intranasal insulin in Alzheimer's disease: food for thought. Neuropharmacology 2018; 136 (Pt B):196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heni M, Kullmann S, Preissl H, Fritsche A, Haring H-U. Impaired insulin action in the human brain: causes and metabolic consequences. Nat Rev Endocrinol 2015; 11:701–711. [DOI] [PubMed] [Google Scholar]
- 52.Baddeley A. Working memory: theories, models, and controversies. Annu Rev Psychol 2012; 63:1–29. [DOI] [PubMed] [Google Scholar]
- 53.Bizon JL, Foster TC, Alexander GE, Glisky EL. Characterizing cognitive aging of working memory and executive function in animal models. Front Aging Neurosci 2012; 4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dickerson BC, Eichenbaum H. The episodic memory system: neurocircuitry and disorders. Neuropsychopharmacology 2010; 35:86–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tsutsui K-I, Oyama K, Nakamura S, Iijima T. Comparative overview of visuospatial working memory in monkeys and rats. Front Syst Neurosci 2016; 10:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sacktor N, Robertson K. Evolving clinical phenotypes in HIV-associated neurocognitive disorders. Curr Opin HIV AIDS 2014; 9:517–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carroll A, Brew B. HIV-associated neurocognitive disorders: recent advances in pathogenesis, biomarkers, and treatment. F1000Res 2017; 6:312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walker KA, Brown GG. HIV-associated executive dysfunction in the era of modern antiretroviral therapy: a systematic review and meta-analysis. J Clin Exp Neuropsychol 2018; 40:357–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fitting S, Ignatowska-Jankowska BM, Bull C, Skoff RP, Lichtman AH, Wise LE, et al. Synaptic dysfunction in the hippocampus accompanies learning and memory deficits in human immunodeficiency virus type-1 Tat transgenic mice. Biol Psychiatry 2013; 73:443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ru W, Tang S-J. HIV-associated synaptic degeneration. Mol Brain 2017; 10:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Everall IP, Heaton RK, Marcotte TD, Ellis RJ, McCutchan JA, Atkinson JH, et al. Cortical synaptic density is reduced in mild to moderate human immunodeficiency virus neurocognitive disorder. Brain Pathol 1999; 2:209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Masliah E, Heaton RK, Marcotte TD, Ellis RJ, Wiley CA, Mallory M, et al. Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. HNRC Group. The HIV Neurobehavioral Research Center. Ann Neurol 1997; 42:963–972. [DOI] [PubMed] [Google Scholar]
- 63.Scaravilli F, Bazille C, Gray F. Neuropathologic contributions to understanding AIDS and the central nervous system. Brain Pathol 2007; 17:197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Levine AJ, Soontornniyomkij V, Achim CL, Masliah E, Gelman BB, Sinsheimer JS, et al. Multilevel analysis of neuropathogenesis of neurocognitive impairment in HIV. J Neurovirol 2016; 22:431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.He H, Gu C-J, Chao W, Borjabad A, Ichiyama K, Do M, et al. Reduced control of HIV infection and viral neuropathogenesis in knock-out mice lacking type I interferon responses. J Neurovirol 2012; 18 Suppl. 1:S45–S46. [Google Scholar]
- 66.Maki PM, Cohen MH, Weber K, Little DM, Fornelli D, Rubin LH, et al. Impairments in memory and hippocampal function in HIV-positive vs. HIV-negative women: a preliminary study. Neurology 2009; 72:1661–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang M, Wang Q, Ding H, Shang H. Association of hippocampal magnetic resonance imaging with learning and memory deficits in HIV-1-seropositive patients. J Acquir Immune Defic Syndr 2015; 70:436–443. [DOI] [PubMed] [Google Scholar]
- 68.Pascual-Lucas M, Viana da Silva S, Di Scala M, Garcia-Barroso C, González-Aseguinolaza G, Mulle C, et al. Insulin-like growth factor 2 reverses memory and synaptic deficits in APP transgenic mice. EMBO Mol Med 2014; 6:1246–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huh JW, Raghupathi R, Laurer HL, Helfaer MA, Saatman KE. Transient loss of microtubule-associated protein 2 immunoreactivity after moderate brain injury in mice. J Neurotrauma 2003; 20:975–984. [DOI] [PubMed] [Google Scholar]
- 70.Vaillant AR, Zanassi P, Walsh GS, Aumont A, Alonso A, Miller FD. Signaling mechanisms underlying reversible, activity-dependent dendrite formation. Neuron 2002; 34:985–998. [DOI] [PubMed] [Google Scholar]
- 71.Shukla PK, Tang L, Wang ZJ. Phosphorylation of neurogranin, protein kinase C, and Ca2+/calmodulin dependent protein kinase II in opioid tolerance and dependence. Neurosci Lett 2006; 404:266–269. [DOI] [PubMed] [Google Scholar]
- 72.McAllister AK, Katz LC, Lo DC. Neurotrophins and synaptic plasticity. Annu Rev Neurosci 1999; 22:295–318. [DOI] [PubMed] [Google Scholar]
- 73.Ferrario CR, Reagan LP. Insulin-mediated synaptic plasticity in the CNS: anatomical, functional and temporal contexts. Neuropharmacology 2017; 136 (Pt B):182–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vazquez-Santiago FJ, Noel RJ, Jr, Porter JT, Rivera-Amill V. Glutamate metabolism and HIV-associated neurocognitive disorders. J Neurovirol 2014; 20:315–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


