Abstract
Objectives
Multimorbidity of geriatric patients often leads to polypharmacy that increases the risk for drug interactions. Geriatric patients are also more sensitive to adverse drug reactions due to physiological changes resulting from ageing. Hence, the use of medicines should be considered thoroughly. This systematic literature review aimed at identifying and presenting available evidence on the effect of pharmaceutical interventions on geriatric patients, their medications or healthcare costs in a clinical setting in Europe.
Methods
We included all studies on research of pharmaceutical interventions on geriatric inpatients (≥65 years) in Europe since 2001. Database searches were conducted on PubMed, EMBASE, The Cochrane Library and AgeInfo. In addition, the following journals were searched manually: European Journal of Hospital Pharmacy, ‘Krankenhauspharmazie’, ‘Medizinische Monatsschrift für Pharmazeuten’ and ‘Zeitschrift für Gerontologie und Geriatrie’.
Results
Database screening yielded 8058 hits. After deletion of duplicates, screening of title and abstract, 143 full-text articles were analysed and 17 papers were included. Manual searching added four more papers. Included studies were conducted in Belgium, Denmark, England, Ireland, the Netherlands, Sweden and Spain. They demonstrate that pharmaceutical care on wards leads to more appropriate medication use and might reduce outcomes like drug-related readmissions. Intensified pharmaceutical care showed additional effects, even in countries with established pharmaceutical care in hospitals.
Conclusions
This systematic literature review demonstrates that ward-based pharmacists may improve the appropriateness of medications, seamless care and drug safety for geriatric inpatients while being cost effective.
Keywords: Geriatric Medicine, Clinical Pharmacy, Pharmacotherapy, Evidence Based Medicine
Introduction
Rationale
The proportion of elderly people increases rapidly: while the number of persons aged 60 years and above tripled worldwide since 1950, it is estimated to almost triple again by 2050. By that date, at least 22% of the world population will be 60 years or older, with an even higher proportion in developed countries.1 Population estimates for Germany are in line with these numbers.2
The older a patient, the more comorbidities they will have and the more medication they will take.3 4 A German study showed that about 42% of those aged 65 years and above take five or more medicines regularly.5 A cross-sectional study also in Germany revealed that 62% of that age group were multimorbid.4
This combination of multimorbidity and polypharmacy leads to a higher risk of interactions and adverse drug reactions (ADRs).6 7 Furthermore, geriatric patients are often more sensitive to ADRs. This is caused by physiological changes related to ageing, like the decline in renal function and an exaggerated response to CNS-active drugs.8 9
Hence, the use of medicines should be considered carefully when dealing with geriatric patients. The expertise on medications provided by pharmacists could be valuable in this context. A previous systematic review and meta-analysis in the USA revealed better therapeutic outcomes, less hospitalisations, safer medication and better adherence through pharmaceutical interventions (PIs) for geriatric patients across different care settings.10 Two other international systematic reviews demonstrated the positive effect of more appropriate prescribing through pharmaceutical interventions but did not assess hard outcomes like mortality, rehospitalisation or therapeutic outcomes.11 12
A previously conducted systematic literature review about PIs on geriatric patients in the clinical setting in Germany identified only very few studies.13 These were done without control groups and did not include patient-specific outcomes. Due to this meagre data from Germany, we reviewed the situation in Europe to see what has already been proven in other countries to have an impact on the patient’s health.
Objectives
This systematic literature review aimed at identifying and presenting available evidence on the effect of PIs on geriatric patients (≥65 years) in clinical settings in Europe regarding outcomes referring to the patient, its medications or healthcare costs.
Methods
We conducted a search for all studies evaluating PIs on geriatric patients (≥65 years) in Europe that used a control group to analyse the outcomes. The study protocol is available on request.
Literature search strategy
PubMed, EMBASE, CENTRAL (Cochrane Central Register of Controlled Trials) and AgeInfo were searched. The search strategy was designed according to PICOS as illustrated in table 1.
Table 1.
PICOS characteristics
| Population | Geriatric patients |
| Interventions | All interventions by pharmacists in acute care hospitals |
| Comparisons | Control group with standard treatment |
| Outcomes | All kinds of outcomes relating to the patient (therapeutic outcome, quality of life, rehospitalisation and mortality), the medications (drug safety, medication appropriateness and compliance) and costs |
| Study design | Any design containing a control group in addition to the intervention group |
We did not include countries or geographical search terms in the search strategy as we did not want to miss relevant studies that might not have geographical keywords. An example of the search strategy used in PubMed is provided in the online supplementary file.
ejhpharm-2017-001239supp001.docx (103KB, docx)
The full search strategy is available on request. A date limitation was set from 1 January 2001 to 21 April 2016; the search itself was conducted in April 2016. The online archives of the European Journal of Hospital Pharmacy and the German journals ‘Krankenhauspharmazie’, ‘Medizinische Monatsschrift für Pharmazeuten’ and ‘Zeitschrift für Gerontologie und Geriatrie’, as well as the literature included in related systematic literature reviews and the bibliographies of relevant full-text articles were screened.
Inclusion and exclusion criteria
Type of participants
We used a broad definition and included all studies with patients 65 years and older in acute care hospitals in Europe.
Type of interventions
We defined a PI as all patient-specific tasks performed by hospital pharmacists with one or more of the following aims: improvement of the therapeutic outcome, quality of life or compliance, increase of medication safety (eg, more appropriate medications and less medication errors) and reduction of rehospitalisation, mortality or costs. Excluded were all interventions regarding smoking cessation, alcohol abuse and weight reduction.
Type of studies
Only studies using a control group to analyse the outcomes were included in the review, that is, randomised controlled trials (RCTs), intervention studies, cohort studies and before-and-after studies. Reviews, editorials, case reports, letters and conference papers were excluded.
Language
Only full-text articles in English and German were included in the review.
Study selection and data extraction
The selection process was carried out following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidance.14 After duplicate removal, two researchers (EH and YH) screened the remaining titles. All abstracts of the selected titles were screened. The included full-text articles were analysed using a self-developed form. We tried to get access to all full-text articles associated to included abstracts. If full-text articles were not accessible online, we contacted the authors at least twice. Information collected with a piloted form included: information on study design, patient groups, details and impact of intervention including principal component. If the published information was unclear, we contacted study authors and asked for clarification. We discussed several full-text articles, before including or excluding them. As the definition of the intervention and assessed outcomes was very broad, data could not simply be combined, and a narrative summary was reported.
Quality assessment
Included studies were assessed using the quality assessment tool for quantitative studies of the Effective Public Health Practice Project (EPHPP).15 The EPHPP tool is considered suitable for systematic reviews with studies of different designs.16 For each included study, one researcher (EH) assessed the study quality. All questions were discussed with the second researcher (YH).
Results
Literature search findings
The search yielded a total number of 8058 citations. After deletion of duplicates, 5889 titles were screened. The screening and selection process is displayed in figure 1. Titles were excluded because they did not match inclusion criteria. The most common reasons for exclusion were: no clinical setting (primary care or care home), languages other than German or English, studies not limited to patients >65 years, studies in countries outside of Europe, not matching the search question at all, purely retrospective, observational studies without control groups and no pharmacist involved in study. A total of 562 abstracts were screened, leaving 143 full-text articles for assessment. Papers/abstracts were excluded due to the following: papers did not investigate geriatric patients or had a broader age range (n=77); full text not available (n=15); no pharmacist involved (n=13); no control group in study design (n=12); and studies were not conducted in a secondary care setting (n=9). This left 17 full-text articles for inclusion. Additionally, a further four papers were identified through hand-searching, so we included a total of 21 papers for qualitative analysis. As some of these papers referred to the same study, 18 studies were included for analysis.
Figure 1.
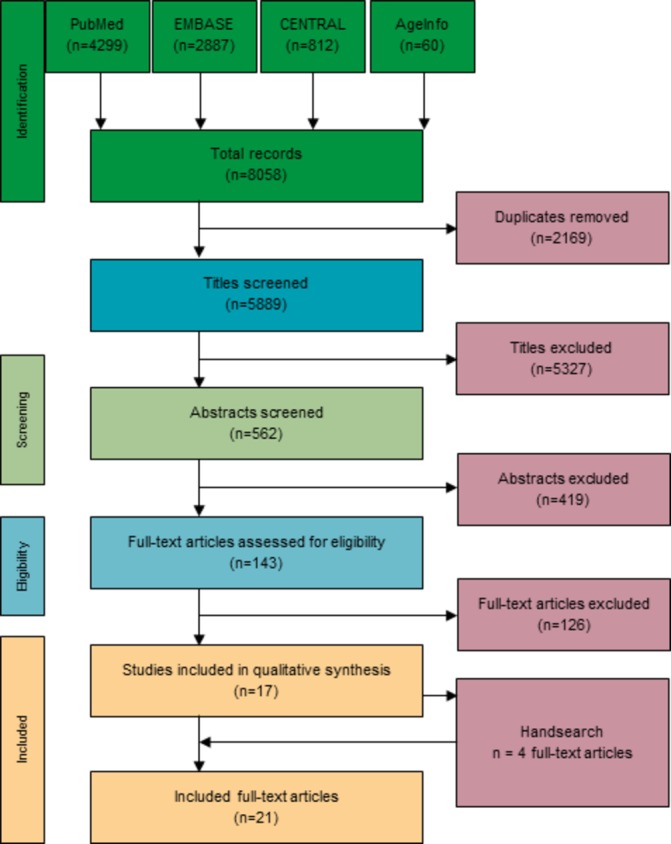
PRISMA flow chart.
Study characteristics
An overview of the study characteristics is presented in table 2 for all included studies. For more details, see the online supplementary file.
Table 2.
Study characteristics sorted by principal component of pharmaceutical intervention
| Year and author | Country | Design | No of patients (total) | Clinical setting | Follow-up period after discharge | Quality of life | Appropriate use | Medication errors | Drug safety—other | Compliance | Rehospitalisation | Mortality | Costs |
| Continuing pharmaceutical care from admission to discharge | |||||||||||||
| Spinewine et al 24 | Belgium | RCT | 203 | Geriatric ward/unit | 1, 3, 12 months | - | x | - | - | - | x | x | - |
| Bergkvist et al 17 | Sweden | HCS | 115 | Internal/general medicine | - | - | - | x | - | - | - | - | - |
| Bergkvist et al 18 | Sweden | CBA | 53 | Internal/general medicine | 2 weeks | - | x | - | - | - | - | - | - |
| Gillespie et al
19
Gillespie et al 20 |
Sweden | RCT | 400 | Internal/general medicine | 12 months | - | x | - | - | - | x | x | x |
| Hellstrom et al 21 | Sweden | CBA | 210 | Internal/general medicine | 3 months | - | x | - | - | - | x | - | - |
| Midlöv et al 22 | Sweden | HCS | 123 | Internal/general medicine | - | - | - | x | - | - | - | - | - |
| Intensified pharmaceutical care on ward | |||||||||||||
| Grimes et al
30
Tallon et al 31 |
Ireland | CBA | 108 | Internal/general medicine | - | - | x | - | - | - | - | - | - |
| Blagburn et al 27 | England | PC | 908 | Geriatric ward/unit | 30 days | - | - | - | - | - | x | - | - |
| Software-based intervention by pharmacist | |||||||||||||
| Gallagher et al
36
O’Sullivan et al 37 |
Ireland | Cluster-RCT | 737 | Internal/general medicine (patients at specialist geriatric services were excluded) | - | - | - | - | X (ADR) | - | - | x | x |
| Interdisciplinary medication review | |||||||||||||
| Lisby et al 34 | Denmark | RCT | 99 | Internal/general medicine | 3 months | x | - | - | - | - | x | x | - |
| Bondesson et al 23 | Sweden | HCS | 201 | Internal/general medicine | - | - | - | - | X (DRP) | - | - | - | - |
| Medication history at admission | |||||||||||||
| Cornu et al 25 | Belgium | UBA | 398 | Geriatric ward/unit | - | - | - | x | - | - | - | - | - |
| Van den Bemt et al 35 | The Netherlands | HCS | 1543 | Internal/general medicine | - | - | - | x | - | - | - | - | - |
| Steurbaut et al 26 | Belgium | UBA | 394 | Geriatric ward/unit | - | - | - | x | - | - | - | - | - |
| Discharge management | |||||||||||||
| López Cabezas et al 32 | Spain | RCT | 134 | Internal/general medicine | 2, 6, 12 months | x | - | - | - | x | x | x | x |
| Nazareth et al 28 | England | RCT | 362 | Geriatric ward/unit | 3, 6 months | x | - | - | - | x | x | x | - |
| Al-Rashed et al 29 | England | PC | 83 | Geriatric ward/unit | 2–3 weeks, 3 months | - | - | - | - | x | x | - | - |
| Intervention regarding the change of parenteral antibiotics to oral antibiotics | |||||||||||||
| del Pozo-Ruiz et al 33 | Spain | HCS | 111 | Geriatric ward/unit | 30 days | - | - | - | x | - | x | - | x |
x=outcome assessed, -=no follow-up/outcome not assessed.
CBA, controlled before–after study; DRP, drug-related problem; HCS, historically controlled study; PC, prospective cohort study; RCT, randomised controlled trial; UBA, uncontrolled before-after study.
The studies were conducted in: Sweden (n=7),17–23 Belgium (n=3),24–26 England (n=3),27–29 Ireland (n=2),30 31 Spain (n=2),32 33 Denmark (n=1)34 and the Netherlands (n=1).35 Most studies were conducted on internal or general medicine wards (n=11),17–23 30 31 34–38 and others were done on geriatric units (n=7).24–29 33
Study design varied greatly: most studies were RCTs (n=6)19 20 24 28 32 34 36 37 with one being a cluster-RCT,37 followed by historically controlled studies (n=5),17 22 23 33 35 controlled before–after studies (n=3),18 21 30 31 prospective cohort studies (n=2)27 29 and uncontrolled before-after studies (n=2).25 26 The sample size ranged from 53 to 1543 patients, with a median of 203 patients and a mean of 354 patients.
Patient characteristics
In all studies, patients were at least 65 years or older, but several studies raised the inclusion age to ≥70 years (n=2),24 34 ≥75 years (n=1)28 and ≥80 years (n=3).19 20 27 While age and admission to the intervention ward were the only inclusion criteria for some studies, most of them had further limitations. Most popular criteria were a minimum number of drugs used,21 23 25 26 28–31 34 35 time spent on ward,23 24 34 36 37 social circumstances outside the hospital, that is, living in a nursing home or at home,17 22 32 admission through emergency department35–37 or language abilities.28 29 Exclusion criteria were, for example, terminal illness,17 18 24 36 37 inclusion during previous admission23 24 30 31 36 37 or short life expectancy.24 34 Only one study defined the medical history/current diagnoses of patient.32 One study only included patients taking specific antibiotics.33
Details of the interventions and outcomes assessed
In accordance with eligibility criteria, all interventions were conducted by a pharmacist. Included studies are described here according to the principal component of their intervention followed by the outcomes assessed for this type of intervention. Most interventions were complex and included multiple components. We identified a total of seven different PIs: continuing pharmaceutical care from admission to discharge, intensified ward-based pharmaceutical care, software-based intervention, interdisciplinary medication review, medication history at admission, discharge management and an intervention regarding the change of parenteral antibiotics to oral antibiotics.
Continuing pharmaceutical care from admission to discharge
Multiple studies investigated the effect of continuing pharmaceutical care from admission to discharge.17–22 24 This included medication reconciliation at admission, medication reviews at admission and during the hospital stay, as well as discussion of identified drug-related problems (DRPs) or opportunities for optimisation with the physicians in charge besides patient education. Pharmacists also checked the discharge summary for completeness and correctness regarding the medication,17 18 21 22 or provided oral and written information about the discharge medication to the patients and to the general practitioner (GP).19 20 24 One study added a follow-up telephone call after 2 months.19 20
Four studies tried to improve the medication appropriateness through the intervention using the Medication Appropriateness Index (MAI).18 20 21 24 39 Some studies analysed the MAI score itself,18–21 24 and some analysed the proportion of drugs with at least one inappropriate rating.18 21 The MAI decreased in all studies during the hospital stay, indicating that the medication was more appropriate at discharge compared with admission. A significant reduction of the MAI score was shown in two studies;20 24 two further studies showed a non-significant reduction in MAI score compared with the control group18 21 but one of these significantly reduced the number of drugs with at least one inappropriate rating.21
Other medication appropriateness rating scores used were the Beers criteria,40 ACOVE (Assessing Care of Vulnerable Elders) criteria41 and the STOPP/START (STOPP (Screening Tool of Older Persons’ Potentially inappropriate prescriptions) and START (Screening Tool to Alert doctors to the Right Treatment)) criteria.42 Spinewine et al showed a significant improvement in medication underuse according to ACOVE, while found no significant difference between intervention and control patients regarding the use of drugs that should be avoided in older people (Beers criteria), since this criterion improved from admission to discharge in both groups significantly.24 Another study showed a significant improvement in MAI score and in STOPP/START criteria.20
The Landskrona Integrated Medicines Management project evaluated medication errors at discharge.17 22 It was shown that their basic intervention with continuing pharmaceutical care from admission to discharge decreased the medication errors after discharge by 45%.17 Nonetheless, patients using the ApoDos system, a medication dispensing system in Sweden, still had a high risk of medication errors, presumably due to errors when transferring medicines into the ApoDos system. In the second study, a more collaborative approach with focus on the ApoDos system itself was used,22 as clinicians recorded changes to the medication list directly in the ApoDos system. This change led to a significant reduction in medication errors for ApoDos users.22
The effect on readmission rates was studied by three projects.19–21 24 Two of these studies showed no effect on readmission at different follow-up times: 1, 3 and 12 months24 or only 12 months.19 A possible association with reduced drug-related readmissions was mentioned in one study.21 Although there was no significant reduction in readmissions in general, Gillespie et al demonstrated a correlation between the MAI score and STOPP criteria and drug-related readmissions.20 They also found a reduction in visits to the ED and in all visits to the hospital (ED visits plus readmissions).19
One study found mortality to be significantly reduced at 12 months after discharge, but not at 2 or 6 months after discharge.32 Mortality was non-significantly reduced in another study at 12 months postdischarge.24
One study evaluated the costs of intervention versus the costs for readmissions and visits to the ED in the 12-month follow-up period. Cost savings balanced against the cost of the intervention were US$230 per patient.19
Intensified ward-based pharmaceutical care
Two studies looked at the effect of intensified pharmaceutical care.27 30 31 In the Pharmaceutical Care in Tallaght Hospital (PACT) model, a pharmacist is assigned to a medical team rather than a specific ward. Pharmacists took a leading role in taking the medication history at admission instead of contributing to it and performed discharge medication reconciliation that was not part of standard care. They also had greater freedom to make major changes to the drug chart themselves, cosigned by a medical practitioner, rather than only making minor changes and endorsements.30 31 The main items of this person-centred pharmaceutical care model were medication reconciliation after admission, communication of medication changes to the GP and community pharmacist as well as patient education, assessment of each patient’s need for support, implementation of patient-individual care bundles and follow-up after discharge through a community or hospital pharmacy team.27
The PACT model significantly reduced the MAI score as well as the number of drugs with at least one inappropriate rating during hospital stay.30 31
A possible association with reduced readmissions was mentioned in one study.27
Software-based intervention
A computerised clinical decision support software-based combined with a structured pharmacist review of medication after medication reconciliation allowed the pharmacist to identify DRPs, evaluate the recommendations regarding relevance and prepare a pharmaceutical care plan.36 37
This intervention led to a significant reduction in proportion of patients experiencing an ADR (13.9% vs 20.7%) with the number needed to treat to avoid one ADR during hospital stay being 14 (95% CI 8 to 68).37
Mortality during the hospital stay was evaluated as a secondary outcome but did not show any significant differences between control and intervention.37
The intervention was cheaper as well as more effective than usual care in this study.36
Interdisciplinary medication review
Lisby et al intervened through an interdisciplinary systematic medication review by a clinical pharmacist and a clinical pharmacologist after admission.34 DRPs were described in a note with recommendation for changes.34 There was no effect shown on quality of life, rehospitalisation or mortality.34
Pharmacists in another study performed a medication review with an ADR-reporting form to identify potential DRPs and discussed those with an interdisciplinary team, consisting of physicians, nurses, care providers, the clinical pharmacist and paramedics.23 This study found significantly fewer DRPs in the intervention group than in the control group, and the unidentified DRPs were less clinically significant in the intervention group.23
Medication history at admission
Several studies examined the medication history at admission taken by pharmacists, or pharmacy technicians under the surveillance of pharmacists.25 26 35
These studies showed that pharmacists identified significantly more medications than physicians25 26 and created less unintentional medication discrepancies.25 35
Discharge management
Three studies involved discharge counselling by the pharmacist and follow-up via telephone32 or a home visit postdischarge.28 29 Nazareth et al also gave a copy of the discharge plan to involved carers, the community pharmacist and GP.28
The quality of life or general well-being of the patients was examined by three studies, but no difference was demonstrated between intervention and control patients.28 32 34
The studies showed varying results regarding the compliance of patients. Al-Rashed et al showed a significantly better compliance at the visits 2–3 weeks and 3 months postdischarge;29 López-Cabezas et al showed a significant improvement at 2 and 6 months, but not at 12 months postdischarge.32 In the third study, there was no difference between the control and intervention group regarding compliance (follow-up 3 and 6 months postdischarge).28
One study showed no effect on readmissions at 3 and 6 months follow-up.28 Additional support at and after discharge in two further studies resulted in significantly lower readmission rates at 3 and 6 months,29 and at 2 and 6 months respectively,32 but non-significantly lower rates after 12 months.32
The effect on mortality varied. One study showed no significant effect on mortality during hospital stay as well as 3 and 6 months postdischarge.28 Another study found significantly fewer deaths in the intervention group than in the control group at 12-month follow-up.32
One study evaluated the costs of intervention compared with the costs for resulting hospital readmissions, which led to a difference of €578 per patient favourable to the intervention group.32
Intervention regarding the change of parenteral antibiotics to oral antibiotics
One study examined an intervention by pharmacists who recommended switching from intravenous to oral treatment where appropriate on day 3 of intravenous treatment with levofloxacin or ciprofloxacin.33
There were significant reductions in the costs of therapy and the duration of intravenous treatment but not in the total duration of treatment.33
Results of the quality assessment
The result of the quality assessment is presented in table 3. Most of the studies have a moderate (n=9)19–21 24–26 28 29 34 36 37 or a weak quality rating (n=8)17 18 22 23 27 32 33 35 and only one a strong quality rating.30 31
Table 3.
Quality assessment
| Year and author | Country | study design | Selection bias | Study design | Confounders | Blinding | Data collection methods | Withdrawals and dropouts | Global rating |
| Al-Rashed et al 29 | England | PC | + | + | ++ | + | − | ++ | + |
| Bergkvist et al 17 | Sweden | HCS | + | − | ++ | + | − | NA | − |
| Bergkvist et al 18 | Sweden | CBA | + | + | − | − | ++ | ++ | − |
| Blagburn et al 27 | England | PC | + | + | − | + | − | NA | − |
| Bondesson et al 23 | Sweden | HCS | ++ | − | ++ | + | − | + | − |
| Cornu et al 25 | Belgium | UBA | + | + | ++ | + | − | NA | + |
| del Pozo-Ruiz et al 33 | Spain | HCS | + | − | ++ | + | − | NA | − |
| Gillespie et al
19
Gillespie et al 20 |
Sweden | RCT | ++ | ++ | ++ | + | − | ++ | + |
| Gallagher et al
36
O’Sullivan et al 37 |
Ireland | Cluster-RCT | ++ | ++ | ++ | + | − | ++ | + |
| Grimes et al
30
Tallon et al 31 |
Ireland | CBA | + | + | ++ | + | ++ | NA | ++ |
| Hellstrom et al 21 | Sweden | CBA | + | + | ++ | + | ++ | NA | + |
| López Cabezas et al 32 | Spain | RCT | + | ++ | ++ | + | − | − | − |
| Lisby et al 34 | Denmark | RCT | + | ++ | ++ | + | − | + | + |
| Midlöv et al 22 | Sweden | HCS | + | − | − | + | − | NA | − |
| Nazareth et al 28 | England | RCT | + | ++ | ++ | + | + | + | + |
| Spinewine et al 24 | Belgium | RCT | ++ | ++ | ++ | + | + | ++ | + |
| Steurbaut et al 26 | Belgium | UBA | ++ | + | ++ | + | − | NA | + |
| van den Bemt et al 35 | The Netherlands | HCS | + | − | ++ | + | − | NA | − |
++Strong.
+Moderate.
−Weak.
CBA, controlled before-after study; HCS, historically controlled study; NA, not applicable; PC, prospective cohort study; RCT, randomised controlled trial; UBA, uncontrolled before–after study.
Discussion
This systematic literature review showed that pharmacists can have a marked positive effect for geriatric inpatients, in particular regarding more appropriate use of medications, fewer medication errors and other drug-related outcomes like ADRs and the number of DRPs. All studies evaluating costs favoured the intervention. Hard outcomes such as quality of life, mortality, compliance and readmissions presented variable results.
The positive correlation between PIs in older patients and appropriate prescribing is in accordance with other previously conducted systematic reviews and meta-analyses that reviewed specifically studies that evaluated appropriate prescribing.11 12 Both studies conclude that there is a significant reduction in MAI through pharmacists’ interventions, but it is unclear to what extent the MAI reduction influences the risk of harm. One review included secondary outcomes like number of hospital admissions, medication-related problems, medication adherence and quality of life, but the effect of the interventions was unclear for these outcomes comparable to our results.11
As outcomes like quality of life, mortality, readmissions and compliance depend on many influencing factors, the effect of PIs might be minimal and thus not significantly detectable in studies mostly powered for medication-related outcomes. Regarding readmissions, it might be necessary to differentiate between drug-related readmissions and non-drug-related readmissions. This was not done in most of the studies included in this review. Nevertheless, one study showed a positive correlation of MAI and STOPP scores with drug-related readmissions,20 and one study showed a non-significant reduction in drug-related readmissions.21 The effect of PIs in hospital decreased over time. No study showed a prolonged effect on readmissions after 12 months, whereas there was a positive effect for some shorter follow-up times. This might be a result of further changes to the medications unrelated to the first admission.
None of the interventions is favourable regarding the hard outcomes reported. In our opinion, continuing pharmaceutical care from admission to discharge should be evaluated further. This intervention includes a medication history at admission and discharge management by the pharmacist and can include other interventions like interdisciplinary medication reviews during time on ward and recommendations for switching intravenous to oral treatment to per os.
Limitations
Despite the rigorous and systematic search process, it cannot be ruled out that some relevant studies have been inadvertently excluded. We did not search grey literature and conference publications and included only German and English literature. Some additional useful information might have been missed as descriptive studies were excluded. Marked differences between the studies regarding their standard treatment, intervention, studied outcomes, duration of follow-up, health system or setting made it difficult to extrapolate the findings of specific studies to other countries or clinics. Besides the diverse health systems in different European countries, there are also different settings in the studies like special geriatric clinics versus interventions in older persons in general internal medicine departments. With the interventions and outcomes researched being so diverse, a meta-analysis was not conducted. In the future, more detailed reporting of the interventions and the standard care would be helpful to assess and replicate interventions.
Conclusion
Pharmacists may improve the appropriateness of medications, seamless care and drug safety for geriatric inpatients while being cost-effective. More research is required to see if these results also apply in other countries with different healthcare settings and to determine the effect on hard outcomes such as quality of life, mortality, compliance and readmissions. More complex interventions like pharmaceutical care from admission to discharge is favourable as this kind of intervention can reduce medication errors at admission through medication history as well as improve the appropriateness of prescribing and might reduce drug-related readmissions through discharge management.
Key messages.
What is already known on this subject
Geriatric patients are often multimorbid and take many medicines.
Geriatric patients are more prone to adverse drug reactions.
Adverse drug reactions have considerable impact on healthcare costs.
What this study adds
Pharmacists on wards can have a positive effect on drug-related outcomes for geriatric inpatients.
The effect of pharmacists on wards regarding hard outcomes such as quality of life, mortality, compliance and readmissions showed variable results.
Acknowledgments
We thank Craig Rore for comments and language editing of the manuscript. This research was supported within the interprofessional PhD-program Clinical Pharmacy, LMU Munich.
Footnotes
Contributors: EH and YH conceived the idea for conducting the review. EH conducted the database searches. All authors were involved in the selection process as outlined in the PRISMA flow chart. The final manuscript was written and checked by all authors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. United Nations. Department of Economic. World population ageing 2009: United Nations Publications, 2010. [Google Scholar]
- 2. Statistisches Bundesamt. Bevölkerung Deutschlands bis 2016 - 13. koordinierte Bevölkerungsvorausberechnung. Wiesbaden: Statistisches Bundesamt, 2015. [Google Scholar]
- 3. Schwabe U, Paffrath D. Arzneiverordnungs-Report. Berlin, Heidelberg: Springer, 2015. [Google Scholar]
- 4. van den Bussche H, Schaefer I, Koller D, et al. Multimorbidity in the German Elderly Population - Part 1: prevalence in Ambulatory Medical Care. Z Allgemeinmed 2012;88:365–71. 10.3238/zfa.2012.365-371 [DOI] [Google Scholar]
- 5. Thürmann PA, Holt-Noreiks S, Nink K, et al. ; Arzneimittelversorgung älterer Patienten In: Günster C, Klose J, Schmacke N, eds Versorgung-Report 2012. Stuttgart, 2012:111–30. [Google Scholar]
- 6. Field TS, Gurwitz JH, Harrold LR, et al. Risk factors for adverse drug events among older adults in the ambulatory setting. J Am Geriatr Soc 2004;52:1349–54. 10.1111/j.1532-5415.2004.52367.x [DOI] [PubMed] [Google Scholar]
- 7. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf 2014;13:57–65. 10.1517/14740338.2013.827660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bowie MW, Slattum PW. Pharmacodynamics in older adults: a review. Am J Geriatr Pharmacother 2007;5:263–303. 10.1016/j.amjopharm.2007.10.001 [DOI] [PubMed] [Google Scholar]
- 9. Delafuente JC. Pharmacokinetic and pharmacodynamic alterations in the geriatric patient. Consult Pharm 2008;23:324–34. 10.4140/TCP.n.2008.324 [DOI] [PubMed] [Google Scholar]
- 10. Lee JK, Slack MK, Martin J, et al. Geriatric patient care by U.S. pharmacists in healthcare teams: systematic review and meta-analyses. J Am Geriatr Soc 2013;61:1119–27. 10.1111/jgs.12323 [DOI] [PubMed] [Google Scholar]
- 11. Cooper JA, Cadogan CA, Patterson SM, et al. Interventions to improve the appropriate use of polypharmacy in older people: a Cochrane systematic review. BMJ Open 2015;5:e009235 10.1136/bmjopen-2015-009235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Walsh KA, O’Riordan D, Kearney PM, et al. Improving the appropriateness of prescribing in older patients: a systematic review and meta-analysis of pharmacists' interventions in secondary care. Age Ageing 2016;45:201–9. 10.1093/ageing/afv190 [DOI] [PubMed] [Google Scholar]
- 13. Hartel E, Hopf Y, Drey M. Krankenhausapotheker in der Geriatrie – ein systematisches Review. Z Gerontol Geriatr 2016;49:S117. [Google Scholar]
- 14. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thomas BH, Ciliska D, Dobbins M, et al. A process for systematically reviewing the literature: providing the research evidence for public health nursing interventions. Worldviews Evid Based Nurs 2004;1:176–84. 10.1111/j.1524-475X.2004.04006.x [DOI] [PubMed] [Google Scholar]
- 16. Deeks JJ, Dinnes J, D’Amico R, et al. Evaluating non-randomised intervention studies. Health Technol Assess 2003;7:1–173. iii-x 10.3310/hta7270 [DOI] [PubMed] [Google Scholar]
- 17. Bergkvist A, Midlöv P, Höglund P, et al. Improved quality in the hospital discharge summary reduces medication errors--LIMM: landskrona Integrated Medicines Management. Eur J Clin Pharmacol 2009;65:1037–46. 10.1007/s00228-009-0680-1 [DOI] [PubMed] [Google Scholar]
- 18. Bergkvist A, Midlöv P, Höglund P, et al. A multi-intervention approach on drug therapy can lead to a more appropriate drug use in the elderly. LIMM-Landskrona Integrated Medicines Management. J Eval Clin Pract 2009;15:660–7. 10.1111/j.1365-2753.2008.01080.x [DOI] [PubMed] [Google Scholar]
- 19. Gillespie U, Alassaad A, Henrohn D, et al. A comprehensive pharmacist intervention to reduce morbidity in patients 80 years or older: a randomized controlled trial. Arch Intern Med 2009;169:894–900. 10.1001/archinternmed.2009.71 [DOI] [PubMed] [Google Scholar]
- 20. Gillespie U, Alassaad A, Hammarlund-Udenaes M, et al. Effects of pharmacists' interventions on appropriateness of prescribing and evaluation of the instruments' (MAI, STOPP and STARTs') ability to predict hospitalization--analyses from a randomized controlled trial. PLoS One 2013;8:e62401 10.1371/journal.pone.0062401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hellström LM, Bondesson A, Höglund P, et al. Impact of the Lund Integrated Medicines Management (LIMM) model on medication appropriateness and drug-related hospital revisits. Eur J Clin Pharmacol 2011;67:741–52. 10.1007/s00228-010-0982-3 [DOI] [PubMed] [Google Scholar]
- 22. Midlöv P, Bahrani L, Seyfali M, et al. The effect of medication reconciliation in elderly patients at hospital discharge. Int J Clin Pharm 2012;34:113–9. 10.1007/s11096-011-9599-6 [DOI] [PubMed] [Google Scholar]
- 23. Bondesson A, Eriksson T, Kragh A, et al. In-hospital medication reviews reduce unidentified drug-related problems. Eur J Clin Pharmacol 2013;69:647–55. 10.1007/s00228-012-1368-5 [DOI] [PubMed] [Google Scholar]
- 24. Spinewine A, Swine C, Dhillon S, et al. Effect of a collaborative approach on the quality of prescribing for geriatric inpatients: a randomized, controlled trial. J Am Geriatr Soc 2007;55:658–65. 10.1111/j.1532-5415.2007.01132.x [DOI] [PubMed] [Google Scholar]
- 25. Cornu P, Steurbaut S, Leysen T, et al. Effect of medication reconciliation at hospital admission on medication discrepancies during hospitalization and at discharge for geriatric patients. Ann Pharmacother 2012;46:484–94. 10.1345/aph.1Q594 [DOI] [PubMed] [Google Scholar]
- 26. Steurbaut S, Leemans L, Leysen T, et al. Medication history reconciliation by clinical pharmacists in elderly inpatients admitted from home or a nursing home. Ann Pharmacother 2010;44:1596–603. 10.1345/aph.1P192 [DOI] [PubMed] [Google Scholar]
- 27. Blagburn J, Kelly-Fatemi B, Akhter N, et al. Person-centred pharmaceutical care reduces emergency readmissions. Eur J Hosp Pharm Sci Pract 2016;23:80–5. 10.1136/ejhpharm-2015-000736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nazareth I, Burton A, Shulman S, et al. A pharmacy discharge plan for hospitalized elderly patients--a randomized controlled trial. Age Ageing 2001;30:33–40. 10.1093/ageing/30.1.33 [DOI] [PubMed] [Google Scholar]
- 29. Al-Rashed SA, Wright DJ, Roebuck N, et al. The value of inpatient pharmaceutical counselling to elderly patients prior to discharge. Br J Clin Pharmacol 2002;54:657–64. 10.1046/j.1365-2125.2002.01707.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Grimes TC, Deasy E, Allen A, et al. Collaborative pharmaceutical care in an irish hospital: uncontrolled before-after study. BMJ Qual Saf 2014;23:574–83. 10.1136/bmjqs-2013-002188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tallon M, Barragry J, Allen A, et al. Impact of the Collaborative Pharmaceutical Care at Tallaght Hospital (PACT) model on medication appropriateness of older patients. European Journal of Hospital Pharmacy 2016;23:16–21. 10.1136/ejhpharm-2014-000511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. López Cabezas C, Falces Salvador C, Cubí Quadrada D, et al. Randomized clinical trial of a postdischarge pharmaceutical care program vs regular follow-up in patients with heart failure. Farm Hosp 2006;30:328–42. 10.1016/S1130-6343(06)74004-1 [DOI] [PubMed] [Google Scholar]
- 33. del Pozo-Ruiz JJ, Martín-Pérez E, Malafarina V. Pharmacoeconomic and clinical aspect of a sequential intravenous to oral therapy plan in an acute geriatric ward. Eur Geriatr Med 2016;7:70–6. 10.1016/j.eurger.2015.10.009 [DOI] [Google Scholar]
- 34. Lisby M, Thomsen A, Nielsen LP, et al. The effect of systematic medication review in elderly patients admitted to an acute ward of internal medicine. Basic Clin Pharmacol Toxicol 2010;106:422–7. 10.1111/j.1742-7843.2009.00511.x [DOI] [PubMed] [Google Scholar]
- 35. van den Bemt PM, van der Schrieck-de Loos EM, van der Linden C, et al. Effect of medication reconciliation on unintentional medication discrepancies in acute hospital admissions of elderly adults: a multicenter study. J Am Geriatr Soc 2013;61:1262–8. 10.1111/jgs.12380 [DOI] [PubMed] [Google Scholar]
- 36. Gallagher J, O’Sullivan D, McCarthy S, et al. Structured pharmacist review of medication in older hospitalised patients: a Cost-Effectiveness analysis. Drugs Aging 2016;33:285–94. 10.1007/s40266-016-0348-3 [DOI] [PubMed] [Google Scholar]
- 37. O’Sullivan D, O’Mahony D, O’Connor MN, et al. Prevention of adverse drug reactions in Hospitalised Older Patients using a Software-Supported Structured Pharmacist intervention: a Cluster Randomised Controlled Trial. Drugs Aging 2016;33:63–73. 10.1007/s40266-015-0329-y [DOI] [PubMed] [Google Scholar]
- 38. López Cabezas C, Falces Salvador C, Cubí Quadrada D, et al. Randomized clinical trial of a postdischarge pharmaceutical care program vs regular follow-up in patients with heart failure. Farm Hosp 2006;30:328–42. 10.1016/S1130-6343(06)74004-1 [DOI] [PubMed] [Google Scholar]
- 39. Hanlon JT, Schmader KE, Samsa GP, et al. A method for assessing drug therapy appropriateness. J Clin Epidemiol 1992;45:1045–51. 10.1016/0895-4356(92)90144-C [DOI] [PubMed] [Google Scholar]
- 40. Beers MH. Explicit criteria for determining potentially inappropriate medication use by the elderly. an update. Arch Intern Med 1997;157:1531–6. 10.1001/archinte.1997.00440350031003 [DOI] [PubMed] [Google Scholar]
- 41. Wenger NS, Shekelle PG. Assessing care of vulnerable elders: acove project overview. Ann Intern Med 2001;135(8 Pt 2):642–6. 10.7326/0003-4819-135-8_Part_2-200110161-00002 [DOI] [PubMed] [Google Scholar]
- 42. Gallagher P, Ryan C, Byrne S, et al. STOPP (Screening Tool of Older person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther 2008;46:72–83. 10.5414/CPP46072 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ejhpharm-2017-001239supp001.docx (103KB, docx)


