 A brief review and comparison of the methods of PEGylation of liposomal particles and their influence on the delivery of RNA.
A brief review and comparison of the methods of PEGylation of liposomal particles and their influence on the delivery of RNA.
Abstract
Gene therapy is a promising approach for personalized medicine, but its application in humans requires development of efficient and safe vehicles. PEGylated liposomes are some of the most suitable delivery systems for nucleic acids because of their stability under physiological conditions and prolonged circulation time, compared to conventional and other types of “stealth” liposomes. In vitro/in vivo activity of PEGylated liposomes is highly dependent on PEG motif abundance. The process of “stealth” coverage formation is a very important parameter for efficient transfection assays and further fate determination of the PEG layer after tissue penetration. In this review, we discuss the latest methods of PEGylated liposome preparation.
Introduction
RNA-interference (RNAi) is a biological process related to mRNA expression inhibition by double-stranded RNAs (dsRNAs). In RNAi processing, short dsRNAs dissociate into single-stranded RNAs, which conjoin with RNA-induced silencing complex (RISC) proteins. Thereafter, the RISC binds complementarily to mRNA and cuts polynucleotide chains, so that the target mRNA expression is inhibited.1 There are two main types of short dsRNAs used for RNAi: endogenous microRNA (miRNA) and artificially synthesized small interfering RNA (siRNA).
A number of RNAi therapeutic candidates have emerged with profiles suggesting that they could become drugs of significant medical importance for diseases like transthyretin amyloidosis, hepatitis B virus infection, solid cancers, and hemophilia.2,3
Despite this robust progress, the current state of RNAi therapeutics development has a particular focus on RNAi delivery and key challenges should be faced to expand therapeutic opportunities.4
Due to the short life span of siRNA in blood serum (about 15 min) and its total negative charge, which hinder siRNA pervasion through the negatively-charged cell membrane, siRNA needs to form nano-sized complexes with carriers for facile intracellular delivery.5 Nanocarriers should exhibit:
• prolonged circulation time;
• effective tissue permeation; and
• a high degree of cellular uptake and successful endosomal escape.
Currently, liposomes are the most investigated siRNA delivery vehicles. Their significant biodegradability and biocompatibility enable their successful entry into clinical investigations. Cationic liposomes are spheroidal nanoparticles with a bilayer structure based on amphiphilic lipids, which resemble the cellular membrane, providing better cellular uptake and fewer side effects, compared to lipid nanoparticles. The latter are spheroidal solid nanoparticles at room and body temperature and loaded with lipophilic compounds, mainly. Thus, cationic liposomes seem to be suitable vectors for siRNA delivery.6–8
“Stealth” liposomes
To obtain “stealth” or sterically stabilized liposomes, modification of the bilayer surface with inert polymers, which control semi-phase processes and prevent interaction of liposomes and blood components, exhibiting the so-called “stealth” properties is used. Improvement of the “stealth” properties of nanoparticles is the key goal of drug delivery, as the efficiency of drug delivery systems is closely related to their circulation time. Prolonged circulation time, low MPS (mononuclear phagocyte system) cellular uptake, low aggregation in blood serum and storage stability are main advantages of “stealth” liposomes.9 The most successful methods of “stealth”-mask creation were achieved by liposomal surface modification with polyacrylamide, polyvinyl alcohol and polycarbohydrates, along with polyethylene glycol (PEG) and its co-polymers.10–12 In the very first preparation strategy liposomes mimicked the red blood cell membrane. This tactic allowed them to co-exist with macrophages in the blood stream. For this purpose the liposomal surface was covered with sterically bulky ganglioside GM1 or phosphatidylinositol (PI), both leading to sufficiently prolonged circulation time.13,14
It was suggested that other flexible hydrophilic polymers can efficiently increase the blood circulation time. In particular, the use of PEG-containing lipids with molar weights of 1900–5000 Da prolonged the circulation time compared to GM1 and PI.15–19 The strategy of application of PEG as a covering polymer for ‘stealth’ liposome formulation is known as PEGylation.20
PEGylated liposomes
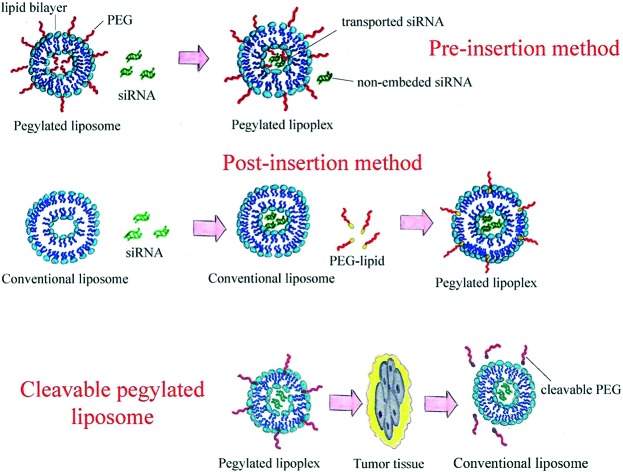
PEGylation can be carried out in two ways: addition of PEG-lipids to lipid composition before liposome formation (pre-insertion method) or mixing of PEG-lipids and liposomal dispersion (post-insertion method).
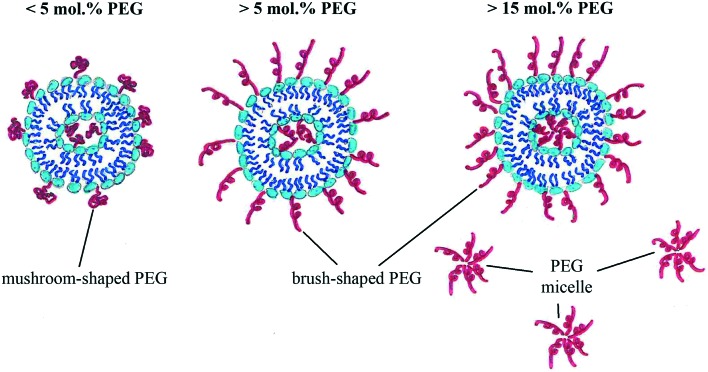
Both the length and coverage density of PEG influence the liposomal PEGylation efficiency. Very short PEG molecules cannot prevent protein absorption and increase the blood circulation time, while very long PEG chains lead to significant decline in transfection activity. Usually PEG molecules with medium length are used for liposome modification. The higher the molar PEG-lipid/lipid composition ratio, the higher the coverage density (Fig. 1):
Fig. 1. PEGylation density – coverage type dependency.
• <5% PEG gives lower than 100% coverage, mushroom-like shape;
• 5–15% PEG gives ∼100% coverage, mushroom- or brush-like shape;
• >15% PEG gives ∼100% coverage, brush-like shape.
Liposome PEGylation increases the circulation time by inhibiting the MPS uptake,21,22 i.e. preventing liposome–macrophage interaction. PEG-liposomes also tend to accumulate in tumor foci.15 Interaction between PEG-liposomes and macrophages depends on two factors: the steric barrier between liposomes and macrophages and binding to blood proteins. In general, liposomes with a brush-like coverage have a more stable steric barrier and prolonged circulation time.23 However, PEGylated liposomes demonstrate remarkably lower cellular uptake and endosomal escape, which reduces the final gene silencing effect.
It is considered that repeated PEG-liposome administration leads to rapid blood clearance, which decreases the therapeutic effect of the drug significantly. After the first administration, activated B-cells produce IgM antibodies. The following administration causes drug–IgM binding, complement system activation and liposome capturing by macrophages.24 This phenomenon is a very fast immune reaction that occurs in 10 min after first intravenous injection.25 Moreover, the spleen seems to be the main producer of anti-PEG IgM.26 It should be noted that secretion of IgM antibodies is dose dependent and that the immune response activation rate is higher when triggered by nucleic acids.27 Nevertheless, the third and later administrations of PEG-liposomes do not cause such a strong effect due to MPS cell saturation.
Pre-insertion PEGylation
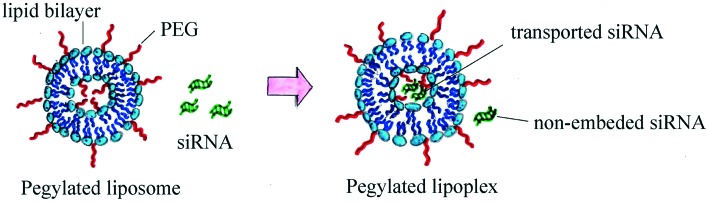
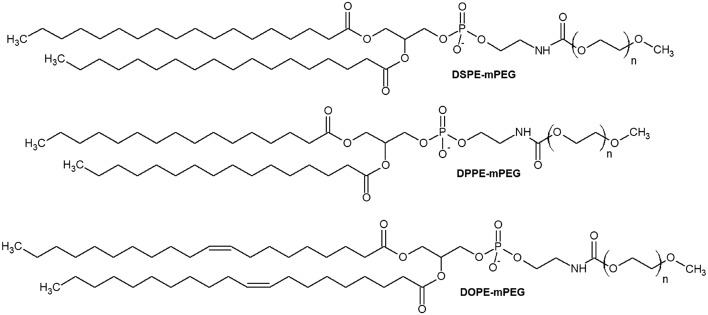
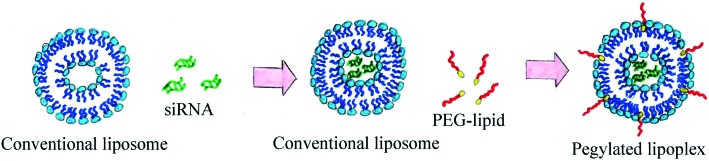
Traditionally, PEGylated liposomes are obtained by the pre-insertion method. Liposomal particles are formed by thin lipid film hydration with the help of sonication.28 Cationic, anionic or neutral lipids are mixed with lipids containing PEGs at their head group on the thin film step.29–31 Then, nucleic acid (NA) is added to form PEGylated lipoplexes (Fig. 2).32,33 This method is suitable for liposomes with low PEG motif density on the periphery (approximately 1%).34 The most commonly used compounds for liposomal PEGylation (Fig. 3) are various phosphatidylethanolamines (PE): DSPE-mPEG,35–37 DPPE-mPEG,38 and DOPE-mPEG,39 which differ in: i) hydrophobic domain; ii) PEG chain length and iii) fatty acid type.
Fig. 2. Scheme of PEGylated lipoplex preparation by the pre-insertion method.
Fig. 3. mPEG-PE lipids: N-(methylpolyoxyethylene oxycarbonyl)-1,2-distearoyl-sn-glycero-3-phosphoethanolamine (DSPE-mPEG), N-(methylpolyoxyethylene oxycarbonyl)-1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine (DPPE-mPEG), and N-(methylpolyoxyethylene oxycarbonyl)-1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-poly(ethylene glycol) (DOPE-mPEG). N – number of oxyethylene –CH2CH2O– units.
It was demonstrated that PEG derivatives with a molecular mass of 2000 Da are the most appropriate for the stabilization of liposomal structures in the bloodstream.40In vivo experiments proved that liposomes modified with PEG2000 are four times more effective in tumor tissue targeting than non-modified liposomes.41 The newest technologies in polymer synthesis (combination of liquid-phase iterative synthesis and selective molecular sieving) give researchers the opportunity to use defined-sequence PEGs.42 PEGs with an exactly defined length and number of oxyethylene units have great biocompatibility and purity.
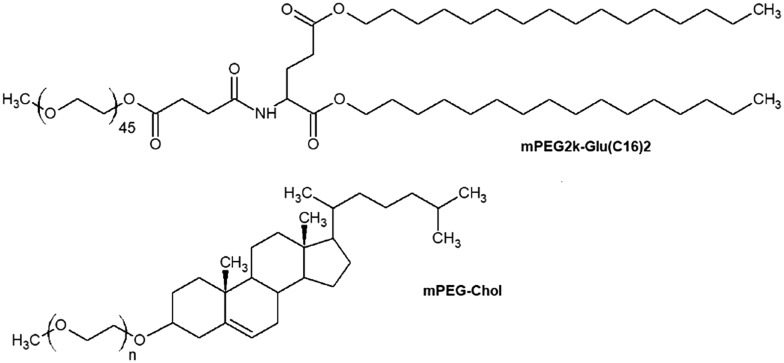
The transfection efficiency and cellular uptake of PEGylated liposomes are affected not only by the PEG chain length but also by the lipid hydrophobic domain nature.43,44 Short aliphatic derivatives easily diffuse from the liposomal surface which results in a decrease of the prolongation effect,45 while asymmetry of the lipophilic part promotes fusion between lipoplexes and the cell membrane.46 Steroids and lipopeptides can also be employed as bases of PEGylated lipids (Fig. 4). For example, PEG-lipopeptides like mPEG2k-Glu(C16)2 have the right balance between low cytotoxicity and good pDNA/siRNA transfection activity.47,48 It was noticed that cholesterol-based PEGylated lipoplexes tend to accumulate mainly in hepatic tumors49 and may have great potential in gene therapy of liver diseases. Liposomes modified with mPEG-Chol (Fig. 4) also demonstrate high dispersion stability compared to PEGylated liposomes which contain PEG-PE lipids.46
Fig. 4. Non-PE PEGylated lipids: mPEG2k-Glu(C16)2 and mPEG-Chol. N – number of oxyethylene –CH2CH2O– units.
To date several drugs based on PEGylated liposomes have been approved by the FDA and already entered the market. They encapsulate and transport doxorubicin,50,51 vincristine52 and daunorubicin.53
When PEG-containing lipids are added during thin lipid film formation, PEG chains become present on both the external and internal membrane parts of the lipid bilayer. PEG motifs present inside the liposome do not form a protective coverage, but hinder siRNA loading into the liposome (Fig. 2). The post-insertion method allows modification of only the outer liposomal bilayer surface.
Post-insertion PEGylation
The post-insertion method (Fig. 5) is more effective in reaching a high level of PEGylation (more than 5% PEG).54 Here, the reaction between a PEG derivative and special hydrophobic anchor or matrix lipids in the outer surface of the liposomal membrane occurs, resulting in the presence of PEGylated conjugates only on the liposomal external lipid bilayer surface.55 This method is applied for biomolecule delivery and liposome-based drug design38,56 due to a suitable ligand arrangement on the outer liposomal surface and a high level of embedding under optimal conditions leading to a decrease in non-specific interactions between ligands and liposomes.
Fig. 5. Scheme of PEGylated lipoplex preparation by the post-insertion method.
Usually, post-insertion is completed after the drug loading step and performed by slow addition of the PEG-lipid solution or PEG micelles to the liposomal dispersion followed by removal of excess PEG derivatives via centrifugation and filtration. Liposomes that are prepared by the post-insertion technique are commonly as stable as the pre-PEGylated ones, but do not have their limitations like low encapsulation rates and weak transfection efficiency.56,57
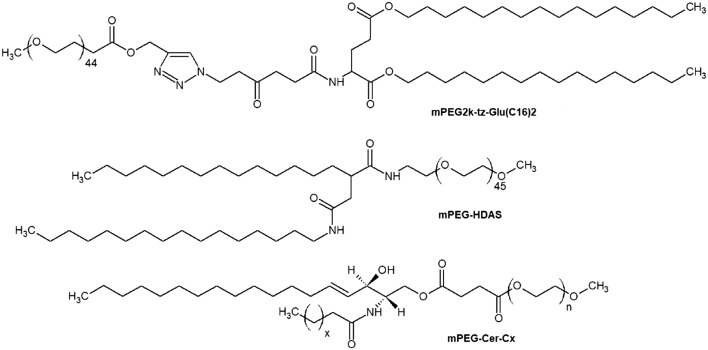
This method does not influence the conventional drug loading technique.57,58 Data from several studies have shown that post-insertion of mPEG-HDAS (Fig. 6) inhibits the complement system activation59 and this lipid penetrates the bilayer significantly higher than mPEG-PEs. PEGylated ceramides (Fig. 6) are also used for post-insertion, showing better transfection efficiency of liposome/siRNA complexes compared to liposomes obtained by the pre-insertion method due to hexagonal phase formation.45,60 The post-insertion technique is a promising approach for the gene delivery of liposomal systems to cancer cells.61,62
Fig. 6. Non-PE PEGylated lipids applied in the post-insertion method: mPEG2k-tz-Glu(C16)2, N-(methylpolyoxyethylene oxyethyl)-2-hexadecylcarbamoylmethyl hexadecanoic amide (mPEG-HDAS), and mPEG-Cer-Cx. N – number of oxyethylene –CH2CH2O– units, x – number of homologous –CH2– groups.
The reaction between a PEG derivative with a terminal azide group and a hydrophobic alkyne-ended anchor built into the lipid bilayer by a “click” mechanism is an example of the PEGylated liposome formation by the post-injection method by means of chemical bonding.63,64 This reaction of 1,3-dipolar cycloaddition proceeds practically with a quantitative yield and only on the outer surface of the lipid bilayer.61 Sebyakin et al. showed that conjugation of modified PEGs to an azide-containing lipopeptide immersed in the liposomal bilayer leads to mPEG2k-tz-Glu(C16)2 (Fig. 6) formation. It can proceed after liposome formation and loading stages.65 However, this kind of post-insertion of PEGs is poorly covered in the literature, and still the “click”-reaction bonding of various ligands to prepared liposomes is highly applied. In this approach PEG motifs are used as spacers and show good ability for modifications with maleimide, thiol and hydrazone terminal groups.37,66,67 These data promise significant success in the post-insertion method development.
Despite the great transcendence of PEGylated lipoplexes, the use of classical PEG-lipids (both by pre-insertion and post-insertion) brings nanomedicine to the “PEG dilemma” – the balance between prolongation of the blood circulation time and cellular uptake/endosomal escape reduction.68,69 It is known that cationic lipids have two conformations in aqueous solution, they are lamellar and hexagonal phases.70 Liposomes that can rearrange their bilayer into a hexagonal phase after cell internalization exhibit better endosomal escape and transfection efficiency.71–73 Dense PEG coverage creates a steric hindrance for such conversion, inhibiting gene release. Several strategies to overcome the “dilemma” have been put into practice: enhancement of cellular uptake via ligand targeting (glycoproteins and antibodies) and acceleration of endosomal escape via membrane fusion lipids, pH-sensitive cationic lipids (DODAP and DOTAP) that are activated by ionization and cleavable PEG-lipids.68,74 The last one is supposed to turn into a “golden” standard of future PEGylated liposomes.75
Cleavable PEGylated liposomes
The accelerated blood clearance (ABC) phenomenon describes a syndrome, in which the circulation time of the second dose administration of liposomes is significantly shorter than the first one, while their accumulation in the liver and spleen elevates. The immunogenicity of PEGylated liposomes creates a barrier for investigation of liposomal constructions and their clinical use. Antibody production significantly reduces drug effectiveness and safety. Currently, the ABC phenomenon is thought to be related to the anti-PEG IgM, which is produced in the spleen in response to the first injected dose.24,26,76 Then the complement system is activated and the serum factor binds to PEG chains on the liposome periphery selectively. Thereby, the second dose of the administrated drug is captured by Kupffer cells and a circulation time decrease is observed.
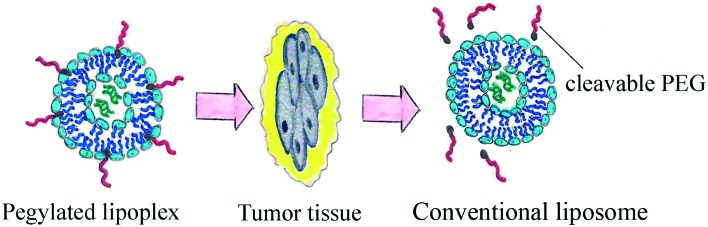
The use of cleavable PEG-lipids was developed as an innovative strategy for the usage of stealth liposomes, due to their properties of removing PEG head groups after cell penetration (Fig. 7). The PEG motif can be attached to the lipid anchor through ester, vinyl ester, hydrazone or disulfide bonds. Synthesis of those kinds of compounds (Fig. 8) is quite simple. It was shown that cleavable PEG derivatives dissociate from the particle periphery after penetration into tumor tissues and the extracellular area. The ABC phenomenon is eliminated or diminished as PEGs are not present in the complement system causing no immune response. The cleavage can be triggered by the temperature or pH level of the surrounding area; the labile bonds of cleavable PEG-lipids are very sensitive to the enzyme action and oxidation process.75 PEG derivative cleavage does not cause the emergence of liposomal membrane damage; thus, cleavable PEG-lipids favorably differ from non-cleavable ones. It has been shown that the distribution in the liver and spleen and the blood clearance speed of cleavable PEG-liposomes after repeated administration remained at a constant level.77 The cleavage process occurs very selectively in diseased tissue due to its unusual properties. For example, the extracellular area of solid tumors has a lower pH value than other body media or hyperthermia is induced in inflamed tissues caused by pyrogen action.
Fig. 7. PEG cleavage after tumor tissue penetration.
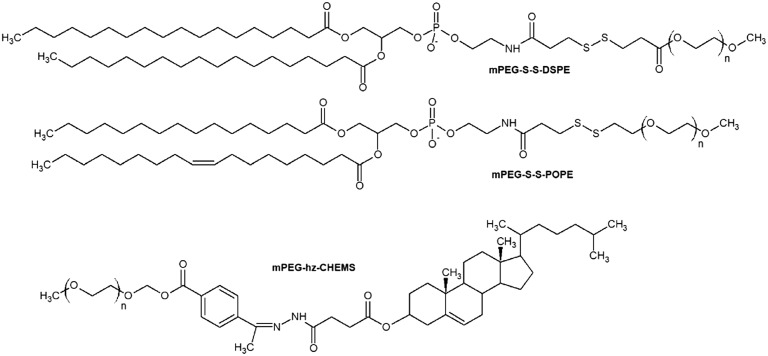
Fig. 8. Cleavable PEGylated lipids: mPEG-S-S-DSPE, mPEG-S-S-POPE, mPEG-hz-CHEMS. N – number of oxyethylene –CH2CH2O– units.
The higher the PEG cleavage degree, the less severe the occurrence of the ABC phenomenon. Therefore, mPEG-hz-CHEMS shows a lower ABC compared to mPEG-Chol and mPEG-DSPE. Disulfide bonds in mPEG-S-S-DSPE66 and mPEG-S-S-POPE78 lipids are destroyed in the presence of proteolytic enzymes (glutathione)/cysteine. Thus unshielded lipoplexes can escape from endosomes rapidly and release the drug fully until it would be disrupted in lysosomes.
Conclusion
To reach a higher transfection effect, RNAs have to be packed into cationic liposomes. PEGylation increases the circulation time and tissue permeability of cationic liposomes. PEG-modified liposomes are able to deliver siRNA. There are two methods of PEG-based modification: pre- and post-insertion; the latter is preferable for considerable addition of PEG derivatives and helps to load bigger doses of the transferred material. PEGylation decreases the cellular uptake of liposomes and endosomal escape, the so-called “PEG dilemma”. To solve these problems, cleavable PEGylated liposomes were elaborated. Further investigations of the immunogenic properties of PEG coating, its density, the presence of additional functional groups, dosage frequency, etc. should be carried out.
Conflicts of interest
There are no conflicts of interest to declare.
Acknowledgments
This work was supported by the Russian Science Foundation (grant number 17-74-10111) and President's Grant for Young Scientists (MK-2003.2019.4) in part of “Post-insertion PEGylation”.
References
- Fire A., Xu S., Montgomery M. K., Kostas S. A., Driver S. E., Mello C. C. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- Khaitov M. R., Shilovskiy I. P., Nikonova A. A., Shershakova N. N., Kamyshnikov O. Y., Babakhin A. A., Hum. Gene Ther., 2014, 257 , 642 –650 , , Available from: http://www.ncbi.nlm.nih.gov/pubmed/24655063 . [DOI] [PubMed] [Google Scholar]
- Ashfaq U. A., Yousaf M. Z., Aslam M., Ejaz R., Jahan S., Ullah O., Virol J., 2011, 8 , 276 –282 , , Available from: http://virologyj.biomedcentral.com/articles/10.1186/1743-422X-8-276 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haussecker D., J. Controlled Release, 2014, 195 , 49 –54 , , Available from: 10.1016/j.jconrel.2014.07.056 . [DOI] [PubMed] [Google Scholar]
- Hoerter J. A. H., Walter N. G., RNA, 2007, 13 , 1887 –1893 , , Available from: http://www.rnajournal.org/cgi/doi/10.1261/rna.602307 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spagnou S., Miller A. D., Keller M. Biochemistry. 2004;43:13348–13356. doi: 10.1021/bi048950a. [DOI] [PubMed] [Google Scholar]
- Koloskova O. O., Nosova A. S., Sebyakin Y. L., Ilyukhina A. A., Shilovskiy I. P., Khaitov M. R. Russ. J. Biopharm. 2017;9(5):3–10. [Google Scholar]
- Turetskiy E. A., Koloskova O. O., Nosova A. S., Shilovskiy I. P., Sebyakin Y. L., Khaitov M. R. Biomed. Khim. 2017;63(5):472–475. doi: 10.18097/PBMC20176305472. [DOI] [PubMed] [Google Scholar]
- Reibel A. T., Müller S. S., Pektor S., Miederer M., Frey H., Roesch F. Biomacromolecules. 2015;16(3):842–851. doi: 10.1021/bm5017332. [DOI] [PubMed] [Google Scholar]
- Owens D. E., Peppas N. A. Int. J. Pharm. 2006;307(1):93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Zalipsky S. and Harris J. M., Introduction to Chemistry and Biological Applications of Poly (ethylene glycol). In: In Poly(ethylene glycol), 1997, pp. 1–13, Available from: http://scholar.google.com/scholar?hl=en&btnG=Search&q=intitle:Introduction+to+Chemistry+and+Biological+Applications+of+Poly(ethylene+glycol)#1. [Google Scholar]
- Veronese F. M., Pasut G. Drug Discovery Today. 2005;10(21):1451–1458. doi: 10.1016/S1359-6446(05)03575-0. [DOI] [PubMed] [Google Scholar]
- Allen T. M., Chonn A. FEBS Lett. 1987;223(1):42–46. doi: 10.1016/0014-5793(87)80506-9. [DOI] [PubMed] [Google Scholar]
- Gabizon A., Price D. C., Huberty J., Bresalier R. S. Cancer Res. 1990;50(19):6371–6378. [PubMed] [Google Scholar]
- Papahadjopoulos D., Allen T. M., Gabizon A., Mayhew E., Matthay K., Huang S. K., Proc. Natl. Acad. Sci. U. S. A., 1991, 8824 , 11460 –11464 , , Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=53155&tool=pmcentrez&rendertype=abstract . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klibanov A. L., Maruyama K., Torchilin V. P., Huang L. FEBS Lett. 1990;268(1):235–237. doi: 10.1016/0014-5793(90)81016-h. [DOI] [PubMed] [Google Scholar]
- Blume G., Cevc G., Biochim. Biophys. Acta, 1990, 10291 , 91 –97 , , Available from: http://linkinghub.elsevier.com/retrieve/pii/000527369090440Y . [DOI] [PubMed] [Google Scholar]
- Mori A., Klibanov A. L., Torchilin V. P., Huang L. FEBS Lett. 1991;284(2):263–266. doi: 10.1016/0014-5793(91)80699-4. [DOI] [PubMed] [Google Scholar]
- Allen T. M., Hansen C., Martin F., Redemann C., Yau-Young A. Biochim. Biophys. Acta, Biomembr. 1991;1066(1):29–36. doi: 10.1016/0005-2736(91)90246-5. [DOI] [PubMed] [Google Scholar]
- Nosova A. S., Koloskova O. O., Sebyakin Y. L., Khaitov M. R. Russ. J. Biopharm. 2018;10:3–9. [Google Scholar]
- Hubbell J. A., Massia S. P., Desai N. P., Drumheller P. D., Nat. Biotechnol., 1991, 96 , 568 –572 , , Available from: http://www.nature.com/doifinder/10.1038/nbt0691-568 . [DOI] [PubMed] [Google Scholar]
- Hong B., Brockenbrough J. S., Wu P., Aris J. P. Mol. Cell. Biol. 1997;17(1):378–388. doi: 10.1128/mcb.17.1.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majzoub R. N., Ewert K. K., Jacovetty E. L., Carragher B., Potter C. S., Li Y. Langmuir. 2015;31(25):7073–7083. doi: 10.1021/acs.langmuir.5b00993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida T., Ichihara M., Wang X. Y., Yamamoto K., Kimura J., Majima E. J. Controlled Release. 2006;112(1):15–25. doi: 10.1016/j.jconrel.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Hadjidemetriou M., Al-Ahmady Z., Kostarelos K. Nanoscale. 2016;8(13):6948–6957. doi: 10.1039/c5nr09158f. [DOI] [PubMed] [Google Scholar]
- Ishida T., Ichihara M., Wang X., Kiwada H. J. Controlled Release. 2006;115(3):243–250. doi: 10.1016/j.jconrel.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Shiraishi K., Kawano K., Maitani Y., Aoshi T., Ishii K. J., Sanada Y., J. Controlled Release, 2016, 234 , 59 –67 , , Available from: 10.1016/j.jconrel.2016.05.010 . [DOI] [PubMed] [Google Scholar]
- Torchilin V. P. and Weissing V., Liposomes: a practical approach, 2003, p. 424. [Google Scholar]
- Kapoor M., Burgess D. J., Int. J. Pharm., 2012, 4321–2 , 80 –90 , , Available from: 10.1016/j.ijpharm.2012.04.058 . [DOI] [PubMed] [Google Scholar]
- Akinc A., Zumbuehl A., Goldberg M., Leshchiner E. S., Busini V., Hossain N. Nat. Biotechnol. 2008;26(5):561–569. doi: 10.1038/nbt1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouxsein N. F., McAllister C. S., Ewert K. K., Samuel C. E., Safinya C. R. Biochemistry. 2007;46(16):4785–4792. doi: 10.1021/bi062138l. [DOI] [PubMed] [Google Scholar]
- Halder J., Kamat A. A., Landen C. N., Han L. Y., Lutgendorf S. K., Lin Y. G. Clin. Cancer Res. 2006;12(16):4916–4924. doi: 10.1158/1078-0432.CCR-06-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead K. A., Dorkin J. R., Vegas A. J., Chang P. H., Veiseh O., Matthews J., Nat. Commun., 2014, 5 , 1 –10 , , Available from: 10.1038/ncomms5277 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehring V., Schaeper U., Ahrens K., Santel A., Keil O., Eisermann M., Mol. Ther., 2014, 224 , 811 –820 , , Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3982492&tool=pmcentrez&rendertype=abstract . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saw P. E., Park J., Lee E., Ahn S., Lee J., Kim H. Theranostics. 2015;5(7):9–14. doi: 10.7150/thno.10732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H., Abu A. S., Nishio M., Tanaka M., Ando H., Kiwada H., J. Controlled Release, 2015, 220 , 406 –413 , , Available from: 10.1016/j.jconrel.2015.11.002 . [DOI] [PubMed] [Google Scholar]
- Yang Y., Yang Y., Xie X., Cai X., Zhang H., Gong W., Biomaterials, 2014, 3514 , 4368 –4381 , , Available from: 10.1016/j.biomaterials.2014.01.076 . [DOI] [PubMed] [Google Scholar]
- Sou K., Endo T., Takeoka S., Tsuchida E. Bioconjugate Chem. 2000;11:372–379. doi: 10.1021/bc990135y. [DOI] [PubMed] [Google Scholar]
- Majzoub R. N., Chan C. L., Ewert K. K., Silva B. F. B., Liang K. S., Jacovetty E. L. Biomaterials. 2014;35(18):4996–5005. doi: 10.1016/j.biomaterials.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzi D., Colapicchioni V., Caracciolo G., Piovesana S., Capriotti A. L., Palchetti S., Nanoscale, 2014, 65 , 2782 –2792 , , Available from: http://www.ncbi.nlm.nih.gov/pubmed/24463404 . [DOI] [PubMed] [Google Scholar]
- Tang J., Kuai R., Yuan W., Drake L., Moon J. J., Schwendeman A., Nanomedicine, 2017, 136 , 1869 –1878 , , Available from: 10.1016/j.nano.2017.04.009 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong R., Liu R., Gaffney P. R. J., Schaepertoens M., Marchetti P., Williams C. M., Nat. Chem., 2018, 112 , 136 –145 , , Available from: 10.1038/s41557-018-0169-6 . [DOI] [PubMed] [Google Scholar]
- Xing H., Li J., Xu W., Hwang K., Wu P., Yin Q., ChemBioChem, 2016, 1712 , 1111 –1117 , , Available from: http://doi.wiley.com/10.1002/cbic.201600092 . [DOI] [PubMed] [Google Scholar]
- Puchkov P. A., Kartashova I. A., Shmendel E. V., Luneva A. S., Morozova N. G., Zenkova M. A., Bioorg. Med. Chem. Lett., 2017, 2715 , 3284 –3288 , , Available from: 10.1016/j.bmcl.2017.06.026 . [DOI] [PubMed] [Google Scholar]
- Song L. Y., Ahkong Q. F., Rong Q., Wang Z., Ansell S., Hope M. J. Biochim. Biophys. Acta, Biomembr. 2002;1558(1):1–13. doi: 10.1016/s0005-2736(01)00399-6. [DOI] [PubMed] [Google Scholar]
- Zhi D., Zhang S., Wang B., Zhao Y., Yang B., Yu S. Bioconjugate Chem. 2010;21(4):563–577. doi: 10.1021/bc900393r. [DOI] [PubMed] [Google Scholar]
- Koloskova O. O., Nikonova A. A., Budanova U. A., Shilovskiy I. P., Kofiadi I. A., Ivanov A. V., Eur. J. Pharm. Biopharm., 2016, 102 , 159 –167 , , Available from: 10.1016/j.ejpb.2016.03.014 . [DOI] [PubMed] [Google Scholar]
- Koloskova O. O., Gileva A. M., Drozdova M. G., Grechihina M. V., Suzina N. E., Budanova U. A., Colloids Surf., B, 2018, 167 , 328 –336 , , Available from: 10.1016/j.colsurfb.2018.04.003 . [DOI] [PubMed] [Google Scholar]
- Hattori Y., Machida Y., Honda M., Takeuchi N., Yoshiike Y., Ohno H., J. Liposome Res., 2016, 274 , 264 –273 , , Available from: http://www.ncbi.nlm.nih.gov/pubmed/27345333 . [DOI] [PubMed] [Google Scholar]
- Hensley M. L., Hoppe B., Leon L., Sabbatini P., Aghajanian C., Chi D., Gynecol. Oncol., 2001, 823 , 464 –469 , , Available from: http://www.ncbi.nlm.nih.gov/pubmed/11520141 . [DOI] [PubMed] [Google Scholar]
- Wibroe P. P., Ahmadvand D., Ali M., Yaghmur A., Moghimi S. M., J. Controlled Release, 2016, 221 , 1 –8 , , Available from: 10.1016/j.jconrel.2015.11.021 . [DOI] [PubMed] [Google Scholar]
- Sarris A. H., Hagemeister F., Romaguera J., Rodriguez M. A., Mclaughlin P., Tsimberidou A. M. Ann. Oncol. 2000;11:69–72. doi: 10.1023/a:1008348010437. [DOI] [PubMed] [Google Scholar]
- Allen T. M., Cullis P. R., Adv. Drug Delivery Rev., 2013, 651 , 36 –48 , , Available from: 10.1016/j.addr.2012.09.037 . [DOI] [PubMed] [Google Scholar]
- Uster P. S., Allen T. M., Daniel B. E., Mendez C. J., Newman M. S., Zhu G. Z. FEBS Lett. 1996;386(2–3):243–246. doi: 10.1016/0014-5793(96)00452-8. [DOI] [PubMed] [Google Scholar]
- Nobs L., Buchegger F., Gurny R., Allémann E. J. Pharm. Sci. 2004;93(8):1980–1992. doi: 10.1002/jps.20098. [DOI] [PubMed] [Google Scholar]
- Awasthi V. D., Garcia D., Klipper R., Goins B. A., Phillips W. T., J. Pharmacol. Exp. Ther., 2004, 3091 , 241 –248 , , Available from: http://www.ncbi.nlm.nih.gov/pubmed/14718581%5Cnhttp://jpet.aspetjournals.org/content/309/1/241.full.pdf . [DOI] [PubMed] [Google Scholar]
- Visser C. C., Stevanović S., Voorwinden L. H., Van Bloois L., Gaillard P. J., Danhof M. Eur. J. Pharm. Sci. 2005;25(2–3):299–305. doi: 10.1016/j.ejps.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Steenpaß T., Lung A., Schubert R. Biochim. Biophys. Acta, Biomembr. 2006;1758(1):20–28. doi: 10.1016/j.bbamem.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Nag O. K., Yadav V. R., Hedrick A., Awasthi V., Int. J. Pharm., 2013, 4461–2 , 119 –129 , , Available from: 10.1016/j.ijpharm.2013.02.026 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira A. C. N., Raemdonck K., Martens T., Rombouts K., Simon-Vazquez R., Botelho C., Acta Biomater., 2015, 25 , 216 –229 , , Available from: 10.1016/j.actbio.2015.07.032 . [DOI] [PubMed] [Google Scholar]
- Golkar N., Tamaddon A. M., Samani S. M., J. Liposome Res., 2015, 262 , 113 –125 , , Available from: 10.3109/08982104.2015.1048692 . [DOI] [PubMed] [Google Scholar]
- Belletti D., Tosi G., Forni F., Lagreca I., Barozzi P., Pederzoli F., Eur. J. Pharm. Biopharm., 2016, 99 , 7 –17 , , Available from: 10.1016/j.ejpb.2015.11.007 . [DOI] [PubMed] [Google Scholar]
- Hassane F. S., Frisch B., Schuber F. Bioconjugate Chem. 2006;17(3):849–854. doi: 10.1021/bc050308l. [DOI] [PubMed] [Google Scholar]
- Kumar A., Erasquin U. J., Qin G., Li K., Cai C. Chem. Commun. 2010;46(31):5746–5748. doi: 10.1039/c0cc00784f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koloskova O. O., Borodin Y. G., Budanova U. A., Sebyakin Y. L. Russ. J. Biopharm. 2010;2(6):16–20. [Google Scholar]
- Mei L., Fu L., Shi K., Zhang Q., Liu Y., Tang J., Int. J. Pharm., 2014, 4681–2 , 26 –38 , , Available from: 10.1016/j.ijpharm.2014.04.008 . [DOI] [PubMed] [Google Scholar]
- Ewert K. K., Kotamraju V. R., Majzoub R. N., Steffes V. M., Wonder E. A., Teesalu T., Bioorg. Med. Chem. Lett., 2016, 266 , 1618 –1623 , , Available from: 10.1016/j.bmcl.2016.01.079 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y., Tian J., Chen X., Biomaterials, 2016, 79 , 56 –68 , , Available from: 10.1016/j.biomaterials.2015.11.056 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechanteur A., Furst T., Evrard B., Delvenne P., Hubert P., Piel G., Eur. J. Pharm. Sci., 2016, 93 , 493 –503 , , Available from: 10.1016/j.ejps.2016.08.058 . [DOI] [PubMed] [Google Scholar]
- Smisterova J., Wagenaar A., Stuart M. C. A., Polushkin E., Brinke G., Hulst R. J. Biol. Chem. 2001;276(50):47615–47622. doi: 10.1074/jbc.M106199200. [DOI] [PubMed] [Google Scholar]
- Dan N., Danino D., Adv. Colloid Interface Sci., 2014, 205 , 230 –239 , , Available from: 10.1016/j.cis.2014.01.013 . [DOI] [PubMed] [Google Scholar]
- Koltover I., Salditt T., Ra J. O. Science. 1998;281:78–81. doi: 10.1126/science.281.5373.78. [DOI] [PubMed] [Google Scholar]
- Rädler J. O., Ra J. O., Koltover I., Salditt T., Safinya C. R. Science. 1997;275:810–814. doi: 10.1126/science.275.5301.810. [DOI] [PubMed] [Google Scholar]
- Hatakeyama H., Akita H., Harashima H., Biol. Pharm. Bull., 2013, 366 , 892 –899 , , Available from: http://jlc.jst.go.jp/DN/JST.JSTAGE/bpb/b13-00059?lang=en&from=CrossRef&type=abstract . [DOI] [PubMed] [Google Scholar]
- Fang Y., Xue J., Gao S., Lu A., Yang D., Jiang H., Drug Delivery, 2017, 24sup 1 , 22 –32 , , Available from: 10.1080/10717544.2017.1388451 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida T., Harada M., Xin Y. W., Ichihara M., Irimura K., Kiwada H. J. Controlled Release. 2005;105(3):305–317. doi: 10.1016/j.jconrel.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Xu H., Wang K. Q., Deng Y. H., Chen D. W., Biomaterials, 2010, 3117 , 4757 –4763 , , Available from: 10.1016/j.biomaterials.2010.02.049 . [DOI] [PubMed] [Google Scholar]
- Kulkarni P. S., Haldar M. K., Nahire R. R., Katti P., Ambre A. H., Muhonen W. W. Mol. Pharmaceutics. 2014;11:2390–2399. doi: 10.1021/mp500108p. [DOI] [PMC free article] [PubMed] [Google Scholar]