Key Points
Collaboration among clinical experts, behavioral research methodologists, and patients can yield valuable symptom-assessment tools.
Patients with ECD possess widely varied and unappreciated symptomatology.
Abstract
Erdheim-Chester disease (ECD) is an ultra-rare hematologic neoplasm characterized by somatic mutations of the MAPK pathway and by accumulation of lesional histiocytes within tissues. Clinical phenotypes and sites of disease involvement are heterogenous in ECD, and no tool exists for systematic and comprehensive assessment of ECD symptomatology. We describe a collaborative effort among ECD specialists, patient-reported outcome (PRO) methodologists, and ECD patients to develop the Erdheim-Chester Disease Symptom Scale (ECD-SS): a symptom inventory for clinical ECD care and evaluation of ECD therapies. Methodologically rigorous focus groups led to the identification of 63 ECD symptoms in 6 categories, incorporated into the ECD-SS with respect to both severity and frequency. Among 50 ECD patients participating in a prospective registry study completing the ECD-SS, 46 (92%) reported neurological/psychological symptoms, 29 (58%) reported pain, and at least one-half reported mood symptoms, memory problems, or fatigue. Symptoms were highly frequent or almost constant regardless of their severity. The ECD-SS is a rigorously developed, patient-centered tool that demonstrates the wide and previously unappreciated burden of symptomatology experienced by ECD patients. Further studies will refine the symptom inventory and define its psychometric properties and role in clinical care and investigation in the context of ECD.
Visual Abstract
Introduction
Erdheim-Chester disease (ECD) is an inflammatory hematologic neoplasm, marked by recurrent somatic mutations in the MAPK pathway whereby histiocytes accumulate in tissues and cause illness.1 ECD lesions can infiltrate any organ system2,3 and cause pain, organ dysfunction, and disfigurement from tumoral infiltration. Additionally, clinical disease is mediated by cytokine elevations and perturbations,2,4,5 causing constitutional symptoms of fever, sweats, and fatigue. Despite the array of symptoms that characterize ECD, there are no standardized disease-specific assessments to measure symptom burden within this heterogeneous population. Patient-reported outcomes (PROs) are data elements directly reported by patients about symptoms and quality of life. There is increasing evidence that rigorous collection and analysis of PROs improves patient-centered care, especially for rare diseases.6-8 To create an informative PRO tool across the phenotypic spectrum of ECD, both for clinical care and for evaluating ECD therapies in clinical trials, we organized a collaboration among ECD clinicians, PRO methodologists, and ECD patients. Here, we describe the process of establishing the content validity of the Erdheim-Chester Disease Symptom Scale (ECD-SS) using state-of-the-art focus-group methodology, and we present the first prospective data on frequency and severity of symptoms in a cohort of ECD patients.
Methods
Institutional review board (IRB) approval was obtained to conduct focus groups and to analyze transcripts of audio-recorded data. The discussions described herein took place at the Patient and Family Gathering hosted by the Erdheim-Chester Disease Global Alliance on 10 October 2015 during the Fourth Annual International ECD Medical Symposium in Houston, TX. Because focus-group data were collected anonymously and without protected health information, the requirement for written informed consent was waived. ECD patients or caregivers could partake in the discussions.
The format (setting, duration), content (wording of questions and prompts), and execution (sequence of discussions, techniques for facilitation) of focus groups was designed by the Patient-Reported Outcomes, Community-Engagement and Language (PRO-CEL) Core at Memorial Sloan Kettering Cancer Center based on methodological best practices for PRO measure design.9,10 Focus groups (conducted by E.L.D. and J.J.B.) with 7 to 9 participants each were planned to last 60 to 90 minutes. Focus-group discussions were iterative in their design and execution with 3 phases as follows.
Concept elicitation: phase 1
Facilitators asked open-ended questions about ECD symptoms, allowing participants to describe their experiences fully and without interruption. Participants were also asked their opinions about how ECD symptoms should best be measured. Facilitators interpreted and reflected responses to the group to generate a list of specific symptoms from the discussion.
Integration of expert suggestions: phase 2
Prior to the focus groups, clinicians with expertise in ECD (E.L.D., D.M.H., O.A.-W., F.J.) created a list of symptoms synthesized from ECD literature, guidelines,11 and clinical experience. This proposed symptom list was presented to participants, who were invited to reflect upon the items for inclusion in or exclusion from the list created in phase 1.
Refinement: phase 3
Participants reviewed and appraised the aggregated list of participant- and clinician-generated symptoms to refine and reconsider the entire inventory. Participants were invited to share further thoughts about the discussion, and, in the process, to further add, remove, or modify symptom items. Aspects of symptomatology (eg, frequency, severity) most relevant to measurement were discussed. At the conclusion of the discussion, there was consensus that all participants’ symptoms were represented on the list and that, conversely, there were no extraneous items on the list (ie, applicable to no one in the group). Transcripts of the discussion were subsequently reviewed by the PRO methodologists to develop the ECD-SS.
Pilot data collection and analysis
The ECD-SS was administered to participants in a prospective, IRB-approved ECD registry study (NCT03329274). All patients provided informed consent to the study per the Declaration of Helsinki. Participants completed the ECD-SS via a secure web-based platform implemented frequently in PRO research. Symptom frequency and severity were analyzed descriptively using SAS version 9.4 (Cary, NC).
Results
Focus groups and ECD-SS
Three focus-group discussions were conducted, each with 8 participants. Of the 24 participants, 16 were ECD patients and 8 were caregivers. Each focus-group discussion was 45 to 60 minutes in length.
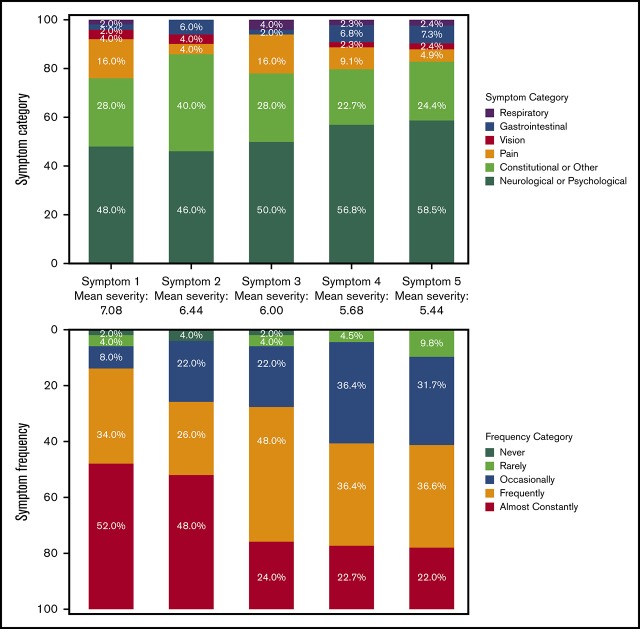
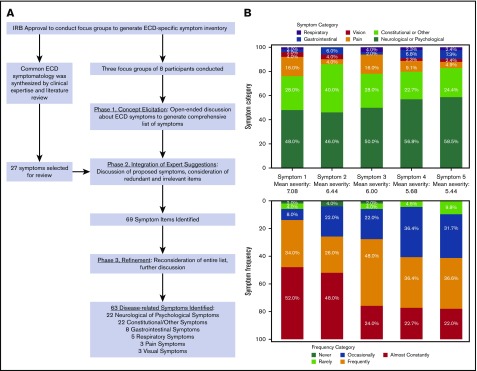
The process of generating the ECD-SS symptom inventory is schematized in Figure 1. Six categories of symptoms were identified: neurologic or psychological, gastrointestinal, pain-related, vision-related, respiratory or breathing, and a broad category of constitutional and other symptoms. Following phase 2 of the discussions, 69 symptoms were identified. Six symptoms were removed during refinement, yielding 63 ECD symptoms.
Figure 1.
ECD-SS. (A) The collaborative process of generating the ECD-SS symptom inventory. (B) The distributions of symptom categories and frequencies are presented by each level of symptom severity. Mean symptom severity is presented between each stacked bar chart.
The scale was designed to measure both symptom frequency and severity. ECD-SS respondents check off all experienced ECD symptoms and rank the severity of their 5 most severe symptoms. Each symptom is rated on a numeric severity scale from 0 to 10. Frequency is rated for each symptom on a 5-point Likert scale (never, rarely, occasionally, frequently, almost constantly).
ECD symptoms
We present ECD-SS data from 50 ECD patients, a separate cohort from focus-group participants; patient characteristics and reported symptoms are summarized in Table 1. Sixty-two of 63 symptoms in the inventory were endorsed by a least 1 participant. Neurologic or psychologic symptoms were reported by 92%, with 50% or more of participants reporting depression or sadness, stress/anxiety, or memory problems, and 72% reporting fatigue or sleepiness. Aching bones or joints was reported by 56%, generalized pain by 36%, and aching muscles by 28%. Symptoms related to balance and dexterity, as well as those related to cognition were frequently reported. The distribution of symptom categories and their frequency, according to symptom severity, is presented in Figure 1. For the most severe symptom reported, the mean severity was 7.08 (standard deviation, 2.02). This most severe symptom reported was neurologic/psychological for 24 (48%) of participants, constitutional/other for 14 (28%), gastrointestinal for 1 (2%), pain for 8 (16%), visual for 2 (4%), and respiratory for 1 (2%). Symptoms were reported to be highly frequent, irrespective of severity: the most severe symptom was reported to be either frequent or almost constant for 43 participants (86%) and was frequent or almost constant for 29 (58%) for the least severe symptom. Of note, 37 participants (74%) were undergoing active treatment at the time of completing the ECD-SS.
Table 1.
Clinical characteristics and prevalence of symptoms as reported on the ECD-SS
| Characteristic | N* | %* |
|---|---|---|
| Age, y | 56.1 (median) | 18-77 (range) |
| Sex | ||
| Male | 28 | 56 |
| Female | 21 | 42 |
| Unknown | 1 | 2 |
| Race | ||
| White | 46 | 92 |
| African American | 1 | 2 |
| Asian | 2 | 4 |
| Unknown | 1 | 2 |
| Self-reported disease locations | ||
| Bone | 36 | 72 |
| Skin | 8 | 16 |
| Brain | 23 | 46 |
| Lungs | 8 | 16 |
| Heart | 14 | 28 |
| Kidneys | 23 | 46 |
| Eyes | 16 | 32 |
| Spine | 9 | 18 |
| Treatment at time of completing ECD-SS | ||
| Targeted therapy† | 27 | 54 |
| Conventional therapy‡ | 9 | 18 |
| Targeted and conventional therapy | 1 | 2 |
| No current treatment | 9 | 18 |
| Unknown | 4 | 8 |
| Neurologic or psychological symptoms | ||
| Memory problems (forgetfulness, repeating questions or statements) | 26 | 52 |
| Depression or sadness | 25 | 50 |
| Stress/anxiety | 25 | 50 |
| Trouble with balance or walking | 25 | 50 |
| Short-tempered | 21 | 42 |
| Discouragement | 19 | 38 |
| Weakness of the arms or legs | 18 | 36 |
| Mood swings | 17 | 34 |
| Trouble with dexterity/coordination | 17 | 34 |
| Difficulty concentrating or paying attention | 17 | 34 |
| Numbness or tingling in hands or feet | 15 | 30 |
| Ringing in the ears (tinnitus) | 14 | 28 |
| Speech difficulties | 13 | 26 |
| Choking (while eating or drinking) | 11 | 22 |
| Difficulty swallowing | 11 | 22 |
| Dizziness | 11 | 22 |
| Head rush or light headedness or spinning sensation (vertigo) | 10 | 20 |
| Headache | 8 | 16 |
| Inappropriate crying | 3 | 6 |
| Inappropriate behavior | 3 | 6 |
| Personality changes | 2 | 4 |
| Inappropriate laughter | 1 | 2 |
| Constitutional or other symptoms | ||
| Fatigue or sleepiness | 36 | 72 |
| Decreased sexual interest | 16 | 32 |
| Frequent or excessive urination | 15 | 30 |
| Insomnia or difficulty sleeping | 14 | 28 |
| Sudden urge to urinate | 13 | 26 |
| Night sweats | 12 | 24 |
| Inability to sleep due to pain | 11 | 22 |
| Itchy skin | 11 | 22 |
| Rash or skin problems | 11 | 22 |
| Frequent napping | 10 | 20 |
| Swelling of the arms or legs (edema) | 10 | 20 |
| Inability to drive | 8 | 16 |
| Hot flashes | 7 | 14 |
| Inability to sleep lying down | 7 | 14 |
| Problems tasting food | 4 | 8 |
| Changes in smell | 3 | 6 |
| Pounding or racing heart (palpitations) | 3 | 6 |
| Other problem that was not listed or I do not have any symptoms. | 3 | 6 |
| Fever | 2 | 4 |
| Hyperhidrosis (excessive sweating) | 2 | 4 |
| Urinary incontinence | 2 | 4 |
| Urinary tract pain | 1 | 2 |
| Gastrointestinal symptoms | ||
| Dry mouth | 15 | 30 |
| Diarrhea | 13 | 26 |
| Abdominal pain | 10 | 20 |
| Decreased appetite | 7 | 14 |
| Nausea | 7 | 14 |
| Dental problems | 3 | 6 |
| Ulcers or other stomach problems | 1 | 2 |
| Vomiting | 0 | 0 |
| Pain | ||
| Aching bones or joints | 28 | 56 |
| Pain | 18 | 36 |
| Aching muscles | 14 | 28 |
| Visual symptoms | ||
| Blurred vision | 11 | 22 |
| Changes in vision | 6 | 12 |
| Double vision | 5 | 10 |
| Respiratory symptoms | ||
| Cough | 9 | 18 |
| Shortness of breath (in general) | 7 | 14 |
| Trouble breathing at night | 3 | 6 |
| Trouble breathing/shortness of breath (in general) | 1 | 2 |
| Hypoxia (air hunger from low oxygen) | 1 | 2 |
| Most common in patients on no treatment (N = 9) | ||
| Stress/anxiety | 8 | 89 |
| Fatigue or sleepiness | 7 | 78 |
| Memory problems | 7 | 78 |
| Depression or sadness | 6 | 67 |
| Aching bones or joints | 6 | 67 |
| Most common in patients on conventional therapy (N = 9) | ||
| Short-tempered | 5 | 56 |
| Difficulty concentrating or paying attention | 5 | 56 |
| Aching bones or joints | 5 | 56 |
| Pain | 5 | 56 |
| Fatigue or sleepiness | 5 | 56 |
| Most common in patients on targeted therapy (N = 27) | ||
| Fatigue or sleepiness | 19 | 70 |
| Depression or sadness | 13 | 48 |
| Trouble with balance or walking | 13 | 48 |
| Memory problems | 13 | 48 |
| Aching bones or joints | 13 | 48 |
Columns 2 and 3 represent number and percent of patients, respectively, except as noted in row 1.
Vemurafenib, dabrafenib, cobimetinib, trametinib, or dual BRAF/MEK therapy.
Corticosteroids, interferon, anakinra, tocilizumab, methotrexate.
Discussion
We describe here the collaborative and patient-centered methodologic process of developing a comprehensive symptom inventory for ECD. The disease symptomatology captured from the ECD-SS suggests an extensive burden of symptoms in ECD patients, exceeding that which has been described in the literature.11-14 All symptoms on the inventory except for 1 were endorsed by at least 1 of the 50 ECD patients completing the assessment, supporting the content validity of the ECD-SS items as resonant with real-world patient experience. These pilot data further illustrated that frequent symptoms such as impaired cognition, psychological distress, pain, and constitutional symptoms are those that do not have a correlate on imaging scans. Moreover, these symptoms are present in a largely treated population, suggesting inadequate supportive management even in the setting of controlled disease. Therefore, these symptoms are not measured, or evaluated with respect to therapeutic response, by traditional radiologic assessments. This highlights an important gap, and opportunity for meaningful improvement, within the clinical assessment of ECD patients. A limitation of this kind of assessment, particularly when performed cross-sectionally, is that disease-related symptoms are not distinguished from treatment side effects. Further study of the ECD-SS will involve investigation of its psychometric properties, including methods such as internal consistency, factor analysis, and principal components analysis, which could provide quantitative insights into reconsidering the symptom categories and refining the inventory by removing redundant items. Other avenues of future inquiry will involve methods of composite scoring and of changes in symptom scores across time points. Continued implementation and evaluation of the ECD-SS within clinical care and investigational studies will help to define its role in comprehensive ECD assessment.
Acknowledgments
This work was supported by funding from the Erdheim-Chester Disease Global Alliance, the Frame Fund, and the Joy Family West Foundation. This research was also funded in part through National Institutes of Health, National Cancer Institute Cancer Center Support grant P30 CA008748.
Authorship
Contribution: E.L.D., J.J.B., T.M.A., F.J., K.B., and J.C. collected the data; E.L.D., A.S.R., J.J.B., E.S., A.J.A., R.R., K.B., J.C., J.J.M., T.M.A., and K.S.P. analyzed and interpreted the data; E.L.D., A.S.R., J.J.B., E.S., A.J.A., D.M.H., O.A.-W., R.R., F.J., K.B., J.C., J.J.M., T.M.A., and K.S.P. wrote the manuscript; and all authors approved the final manuscript.
Conflict-of-interest disclosure: D.M.H. reports personal fees from Atara Biotherapeutics, Chugai Pharma, Boehringer Ingelheim, AstraZeneca, Pfizer, Bayer, Debiopharm Group, and Genetech; and grants from AstraZeneca, Puma Biotechnology, and Loxo Oncology, outside of the submitted work. O.A.-W. reports grants from the National Cancer Institute, National Institutes of Health; grants and personal fees from H3B Biomedicine and Foundation Medicine Inc; and personal fees from Merck, outside of the submitted work. R.R. reports personal fees from Incyte, outside of the submitted work. F.J. reports research support from Novartis, Genentech, BioMed Valley Discoveries, Plexxikon, Deciphera, Piqur, Symphogen, Bayer, FujiFilm Corporation, and Upsher-Smith Laboratories; is on the scientific advisory boards of IFM Therapeutics, Synlogic, Guardant Health, and Deciphera; is a paid consultant for Trovagene and Immunomet; and has ownership interests in Trovagene; all are outside of the submitted work. K.S.P. reports stock ownership in Johnson & Johnson, Viking Therapeutics, Catalyst Biotech, and Pfizer, outside of the submitted work. The remaining authors declare no competing financial interests.
Correspondence: Eli L. Diamond, Department of Neurology, Memorial Sloan Kettering Cancer Center, 160 East 53rd St, 2nd Floor Neurology, New York, NY 10022; e-mail: diamone1@mskcc.org.
References
- 1.Haroche J, Cohen-Aubart F, Rollins BJ, et al. Histiocytoses: emerging neoplasia behind inflammation. Lancet Oncol. 2017;18(2):e113-e125. [DOI] [PubMed] [Google Scholar]
- 2.Arnaud L, Gorochov G, Charlotte F, et al. Systemic perturbation of cytokine and chemokine networks in Erdheim-Chester disease: a single-center series of 37 patients. Blood. 2011;117(10):2783-2790. [DOI] [PubMed] [Google Scholar]
- 3.Dagna L, Corti A, Langheim S, et al. Tumor necrosis factor α as a master regulator of inflammation in Erdheim-Chester disease: rationale for the treatment of patients with infliximab. J Clin Oncol. 2012;30(28):e286-e290. [DOI] [PubMed] [Google Scholar]
- 4.Aouba A, Terrier B, Vasiliu V, et al. Dramatic clinical efficacy of cladribine in Rosai-Dorfman disease and evolution of the cytokine profile: towards a new therapeutic approach. Haematologica. 2006;91(suppl 12):ECR52. [PubMed] [Google Scholar]
- 5.Morimoto A, Oh Y, Nakamura S, et al. ; Japan Langerhans Cell Histiocytosis Study Group. Inflammatory serum cytokines and chemokines increase associated with the disease extent in pediatric Langerhans cell histiocytosis. Cytokine. 2017;97:73-79. [DOI] [PubMed] [Google Scholar]
- 6.Pakhomov SV, Jacobsen SJ, Chute CG, Roger VL. Agreement between patient-reported symptoms and their documentation in the medical record. Am J Manag Care. 2008;14(8):530-539. [PMC free article] [PubMed] [Google Scholar]
- 7.Basch E. Patient-reported outcomes - harnessing patients’ voices to improve clinical care. N Engl J Med. 2017;376(2):105-108. [DOI] [PubMed] [Google Scholar]
- 8.Kotronoulas G, Kearney N, Maguire R, et al. What is the value of the routine use of patient-reported outcome measures toward improvement of patient outcomes, processes of care, and health service outcomes in cancer care? A systematic review of controlled trials. J Clin Oncol. 2014;32(14):1480-1501. [DOI] [PubMed] [Google Scholar]
- 9.Patrick DL, Burke LB, Gwaltney CJ, et al. Content validity–establishing and reporting the evidence in newly developed patient-reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO good research practices task force report: part 1–eliciting concepts for a new PRO instrument. Value Health. 2011;14(8):967-977. [DOI] [PubMed] [Google Scholar]
- 10.Patrick DL, Burke LB, Gwaltney CJ, et al. Content validity–establishing and reporting the evidence in newly developed patient-reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO Good Research Practices Task Force report: part 2–assessing respondent understanding. Value Health. 2011;14(8):978-988. [DOI] [PubMed] [Google Scholar]
- 11.Diamond EL, Dagna L, Hyman DM, et al. Consensus guidelines for the diagnosis and clinical management of Erdheim-Chester disease. Blood. 2014;124(4):483-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haroche J, Arnaud L, Cohen-Aubart F, et al. Erdheim-Chester disease. Rheum Dis Clin North Am. 2013;39(2):299-311. [DOI] [PubMed] [Google Scholar]
- 13.Munoz J, Janku F, Cohen PR, Kurzrock R. Erdheim-Chester disease: characteristics and management. Mayo Clin Proc. 2014;89(7):985-996. [DOI] [PubMed] [Google Scholar]
- 14.Mazor RD, Manevich-Mazor M, Shoenfeld Y. Erdheim-Chester disease: a comprehensive review of the literature. Orphanet J Rare Dis. 2013;8(1):137. [DOI] [PMC free article] [PubMed] [Google Scholar]