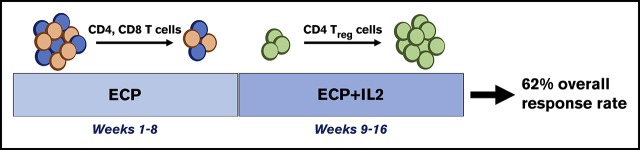
Key Points
ECP and low-dose IL2 have distinct effects on circulating Tcon and Treg cell subsets in SR-cGVHD.
Combined ECP plus low-dose IL2 is safe and provides a therapeutic benefit in >60% of patients with SR-cGVHD.
Abstract
Chronic graft-versus-host disease (cGVHD) affects >50% of hematopoietic stem cell transplant patients. Extracorporeal photopheresis (ECP), an immunomodulatory therapy, provides clinical benefit in steroid-refractory (SR) cGVHD, possibly via regulatory T (Treg) and natural killer (NK) cell expansion. We demonstrated that low-dose interleukin-2 (IL2) led to clinical improvement in SR-cGVHD and stimulated preferential Treg and NK-cell expansion with minimal effect on conventional T (Tcon) cells. We evaluated the effect of ECP (weeks 1-16) plus IL2 (1 × 106 IU/m2, weeks 9-16) in 25 adult patients with SR-cGVHD in a prospective phase 2 trial. Objective responses occurred in 29% and 62% of evaluable patients at weeks 8 (ECP alone) and 16 (ECP plus IL2), respectively. Eight weeks of ECP alone was associated with a marked decline in CD4+ Tcon (P = .03) and CD8+ T cells (P = .0002), with minimal change in Treg cells, Treg:Tcon cell ratio, or NK cells. Adding IL2 induced an increase in Treg cells (P < .05 at weeks 9-16 vs week 8), Treg:Tcon cell ratio (P < .0001 at weeks 9-16 vs week 8), and NK cells (P < .05 at weeks 9-16 vs week 8). Patients responding to ECP alone had significantly fewer CD4+ Tcon and CD8+ T cells at baseline compared with patients who responded after IL2 addition and patients who did not respond; neither Treg nor NK cells were associated with response to ECP alone. Altogether, ECP plus IL2 is safe and effective in patients with SR-cGVHD. ECP and IL2 have distinct immunologic effects, suggesting different therapeutic mechanisms of action. This trial was registered at www.clinicaltrials.gov as #NCT02340676.
Visual Abstract
Introduction
Chronic graft-versus-host disease (cGVHD) is a major cause of morbidity and mortality after allogeneic hematopoietic stem cell transplant (HSCT).1 Corticosteroids are the mainstay of cGVHD therapy despite significant toxicity with long-term use.2 In steroid-refractory (SR) cGVHD, there is no consensus on optimal second-line therapy; nonetheless, advances in the understanding of cGVHD biology have guided use of novel therapies (eg, ibrutinib) that target specific immune cells and signaling pathways.3
Regulatory T (Treg) cells, a subset of CD4+ T cells characterized by constitutive expression of the transcription factor FOXP3 and the high-affinity interleukin-2 (IL2) receptor CD25, are required for self-tolerance.4 In preclinical studies, adoptively transferred Treg cells protected mice from lethal GVHD,5-7 and patients with cGVHD have reduced circulating Treg cells compared with patients without cGVHD.8-10 These observations suggested that increasing Treg cell numbers could provide a therapeutic benefit in cGVHD.
Extracorporeal photopheresis (ECP) is an autologous cellular therapy for SR-cGVHD, capable of facilitating steroid dose reduction and clinical improvement in 30% to 60% of patients.11-15 The anti-inflammatory mechanisms mediating the immunomodulatory effect of ECP are incompletely understood, but studies reported increased Treg and CD56bright natural killer (NK) cells after ECP.16-27
IL2 is a cytokine essential for Treg development, survival, and function.28-30 IL2 treatment expanded Treg cells in both mice and humans.31-33 We demonstrated that daily low-dose IL2 was safe and provided a therapeutic benefit in >50% of patients with SR-cGVHD34,35; treatment was associated with Treg cell expansion in vivo and minimal effect on conventional T (Tcon) cells, and clinical responses correlated with a higher ratio of Treg:Tcon cells. In addition, CD56bright NK cells expanded preferentially with IL2 treatment, consistent with constitutive CD25 expression by this subset.36
We therefore hypothesized that IL2 could enhance the therapeutic efficacy of ECP in SR-cGVHD. We undertook a phase 2 study of sequential treatment with ECP alone for 8 weeks, followed by an additional 8 weeks of ECP plus low-dose IL2 in the same patient. Here, we report clinical and immunologic responses during ECP alone vs ECP plus IL2 in a cohort of 25 adult SR-cGVHD patients.
Methods
Phase 2 clinical protocol
This was a single-arm, single-center, prospective phase 2 trial initiated in 2014 to assess the efficacy and safety of ECP plus daily subcutaneous low-dose IL2 in patients with SR-cGVHD.
Patients meeting the following criteria were included: age ≥18 years; active but clinically stable cGVHD despite prior or current treatment with prednisone (≥0.25 mg/kg per day) for at least 4 weeks; stable prednisone dose without addition or discontinuation of other immunosuppressive medications for at least 4 weeks prior to enrollment; Eastern Cooperative Oncology Group performance status of 0 to 2; and adequate renal, hepatic, pulmonary, and cardiac function. Patients with hepatic or pulmonary dysfunction due to cGVHD were eligible.
Patients were excluded per the following criteria: prednisone >1 mg/kg per day; concurrent use of both a calcineurin inhibitor and sirolimus; history of thrombotic microangiopathy; exposure to ECP or new immunosuppressive medications during the 4 weeks prior to enrollment; donor lymphocyte infusion during the prior 100 days; HIV, hepatitis B, or hepatitis C; active malignancy or infection; and any contraindication to ECP.
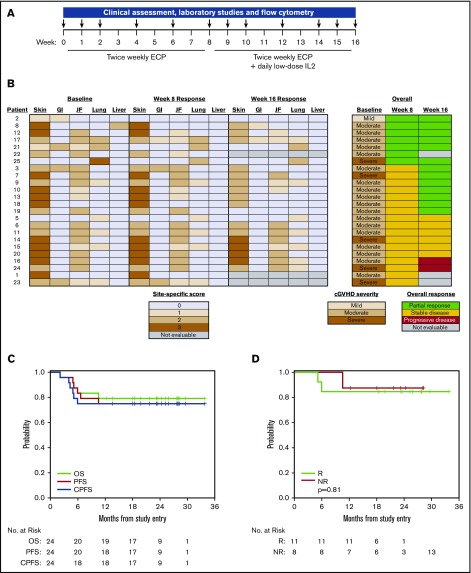
Patients received twice-weekly ECP (Therakos CELLEX, Mallinckrodt Pharmaceuticals) for weeks 1 through 16 and daily low-dose subcutaneous IL2 (1 × 106 IU/m2; Aldesleukin, Prometheus) for week 9 through 16 (Figure 1A). The primary objective was overall response rate (ORR) at week 16 per 2005 National Institutes of Health (NIH) criteria. The secondary objectives were to determine toxicity, immunologic effects, overall survival (OS) and progression-free survival (PFS), nonrelapse mortality, and relapse after starting treatment. The study was powered to detect ≥25% improvement in ORR based on the response rate in our previous IL2 study in SR-cGVHD as a null hypothesis (Ho; P ≤ .5). The target accrual was 25 evaluable patients. With 25 evaluable patients who received ≥1 week of treatment, the study had 85% power with a type I error rate of 5%, based on an exact binomial distribution. The trial was approved by the Dana-Farber/Harvard Cancer Center Institutional Review Board. Written informed consent was obtained prior to enrollment per the Declaration of Helsinki.
Figure 1.
Clinical response, survival, and relapse. (A) Study design. (B) Skin, gastrointestinal (GI), joint/fascia (JF), lung, and liver cGVHD scores at baseline, week 8, and week 16. cGVHD severity at baseline (week 0) and overall responses at week 8 and week 16. (C) OS, PFS, and cGVHD PFS (CPFS) for all patients. (D) OS for responders (R) and nonresponders (NR).
Clinical assessment for response to treatment
Unblinded cGVHD assessments37,38 were performed at baseline and at the end of weeks 8 (ECP alone) and 16 (ECP plus IL2). Laboratory testing and flow cytometry were performed at baseline and at weeks 1, 2, 4, 6, 8, 9, 10, 12, 14, and 16 (Figure 1A). Complete response was resolution of all reversible cGVHD-associated manifestations; partial response (PR) was ≥50% improvement in organ-specific cGVHD without progression at other sites; progressive disease (PD) was ≥25% increase in organ-specific cGVHD; and stable disease (SD) includes patients without complete response, PR, or PD.
Flow cytometry
Briefly, 50 µL of EDTA whole blood was subjected to red blood cell (RBC) lysis using BD PharmLyse (BD Biosciences) and incubated with the following fluorochrome-conjugated monoclonal antibodies: anti-CD3 V450 (UCHT1; BD Biosciences), anti-CD4 allophycocyanin (APC)-H7 (RPA-T4; BD Biosciences), anti-CD8 Pacific Orange (RPA-T8; BioLegend), anti-CD25 phycoerythrin (PE)-Cy7 (M-A251; BD Biosciences), anti-CD127 PE-Cy5 (eBioRDR5; eBioscience), anti-CD45RO fluorescein isothiocyanate (UCHL1; BD Biosciences), and anti-CD62L APC (DREG-56; BD Biosciences) for T-cell subsets; anti-CD56 PE (B159; BD Biosciences), anti-CD3 V450 (UCHT1; BD Biosciences), and anti-CD16 fluorescein isothiocyanate (3G8; BD Biosciences) for natural kill (NK) and NK-T (NKT) cells; and anti-CD19 APC (HIB19; BD Biosciences) for B cells. Flow cytometry was performed using a FACSCanto II (BD Biosciences) and data were analyzed using FACSDiva software (BD Biosciences).
Treg cells were CD3+CD4+CD25mid-highCD127negative-low. Tcon cells were CD3+CD4+CD25negative-lowCD127high. NK and NKT cells were CD56+CD3− and CD56+CD3+, respectively. CD56bright and CD56dim NK cells were CD16−CD3− and CD16+CD3−, respectively. B cells were CD19+CD3−. Naive, effector, effector memory (EM), and central memory T-cell subsets were CD62L+CD45RO−, CD62L−CD45RO−, CD62L−CD45RO+, and CD62L+CD45RO+, respectively.
Statistical analyses
Baseline characteristics were reported descriptively. Fisher's exact test, χ2 test, or Wilcoxon rank-sum test was used for group comparison as appropriate. Immunologic parameters were analyzed descriptively and compared using the Wilcoxon rank-sum test for group comparison and Wilcoxon signed-rank test for paired comparison. OS, PFS, and cGVHD PFS (CPFS) were estimated using the Kaplan-Meier method. Time to events were measured from study enrollment to death (OS), death/disease progression (PFS), or cGVHD progression/death (CPFS), whichever occurred first. All testing was 2-sided at the significance level of 0.05. Multiple comparisons were not adjusted. All analyses were performed using SAS 9.3 (SAS Institute Inc, Cary, NC), and R v2.13.2 (the CRAN project).
Results
Patient characteristics
Twenty-five patients enrolled between April 2015 and April 2017 (Table 1). Median age was 62 years (range, 34-76 years) with 13 female patients (52%). All patients received peripheral blood stem cell grafts; 22 patients (88%) received ≥8 of 8 HLA-matched grafts. Ten patients (40%) had prior acute GVHD. Median time since HSCT was 919 days (range, 177-2842 days). Median time from HSCT to cGVHD onset was 524 days (range, 58-2697 days). Median number of sites of cGVHD involvement was 3 (range, 2-5). Median number of systemic cGVHD therapies prior to enrollment was 2 (range, 1-4), median number of concurrent systemic cGVHD therapies at enrollment was 2 (range, 0-3), and median daily prednisone dose at enrollment was 0.26 mg/kg (range, 0-0.86 mg/kg). A single patient with a history of prednisone-associated psychosis was not on prednisone or other systemic therapies at the time of enrollment. Median follow-up in surviving patients was 24 months (range, 12-34 months).
Table 1.
Baseline patient characteristics
| N | % | |
|---|---|---|
| Total | 25 | 100 |
| Age, median (range), y | 62 (34, 76) | |
| Patient sex | ||
| Male | 12 | 48 |
| Female | 13 | 52 |
| Donor sex | ||
| Male | 14 | 56 |
| Female | 11 | 44 |
| Primary disease | ||
| AML | 5 | 20 |
| CLL/SLL/PLL | 3 | 12 |
| CML | 1 | 4 |
| ALL | 3 | 12 |
| MDS | 9 | 36 |
| MPD | 2 | 8 |
| MM | 1 | 4 |
| NHL | 1 | 4 |
| ECOG performance status | ||
| 0 | 2 | 8 |
| 1 | 17 | 68 |
| 2 | 5 | 20 |
| Unknown | 1 | 4 |
| Patient CMV serological status | ||
| Negative | 24 | 96 |
| Unknown | 1 | 4 |
| Donor CMV serological status | ||
| Positive | 8 | 32 |
| Conditioning regimen intensity | ||
| Myeloablative | 14 | 56 |
| Nonmyeloablative | 11 | 44 |
| HLA molecular typing (A, B, C, DRB1) | ||
| Matched unrelated | 14 | 56 |
| Matched related | 8 | 32 |
| Mismatch unrelated | 3 | 12 |
| Graft source | ||
| Peripheral blood | 25 | 100 |
| Days from HSCT to study enrollment, median (range) | 919 (177, 2842) | |
| Days from cGVHD onset to study enrollment, median (range) | 524 (58, 2697) | |
| Prior grade I-IV acute GVHD | 10 | 40 |
| No. of cGVHD sites involvement, median (range) | 3 (2, 5) | |
| NIH consensus global cGVHD severity | ||
| Mild | 1 | 4 |
| Moderate | 18 | 72 |
| Severe | 6 | 24 |
| Daily prednisone dose, median (range), mg/kg | 0.26 (0, 0.86)* | |
| No. of prior systemic cGVHD therapies, median (range) | 2 (1, 4) | |
| No. of concurrent systemic cGVHD therapies, median (range) | 2 (0, 3)* | |
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CLL, chronic lymphocytic leukemia; CML, chronic myeloid leukemia; CMV, cytomegalovirus; ECOG, Eastern Cooperative Oncology Group; MDS, myelodysplastic syndrome; MM, multiple myeloma; MPD, myeloproliferative disorder; NHL, non-Hodgkin lymphoma; PLL, prolymphocytic leukemia; SLL, small lymphocytic lymphoma.
A single patient with history of prednisone-associated psychosis was not on prednisone or other systemic therapies at the time of study entry.
Safety and tolerability
One patient withdrew due to adverse effects (AEs) of IL2 (myalgia, fatigue) after 3 weeks of IL2 treatment; among patients who remained on study, none required IL2 dose reduction. There were 4 grade-3 AEs: 1 patient had dehydration, hypotension, and syncope (possibly IL2-related), and 1 patient had ECP-related anemia. Laboratory measures of renal, hepatic, and thyroid function remained within normal limits (data not shown).
One patient with SD after 8 weeks of ECP alone completed 2 weeks of ECP plus IL2 and was subsequently hospitalized for enterococcal bacteremia in the setting of neutropenia; the patient died of complications of infection 12 weeks after discontinuing treatment. A second patient with SD after 8 weeks of ECP alone completed 2 weeks of ECP plus IL2 and died the following week from complications of a pulmonary infection.
Clinical response and survival after ECP alone
Twenty-four enrolled patients were evaluable after 8 weeks of ECP alone; 1 patient received ECP but was found to have only oral and ocular cGVHD and thus was nonevaluable for response per protocol as topical treatment changes were permitted for those sites. Seven patients (29%) had an objective PR and 17 patients (71%) had SD. Response sites included skin (n = 2), GI tract (n = 2), JF (n = 4), lung (n = 1), and liver (n = 1) (Figure 1B). The median daily prednisone dose after 8 weeks of ECP alone was 0.25 mg/kg (range, 0-0.64 mg/kg). The daily prednisone dose was unchanged in 16 patients, decreased in 6 patients, and increased in 1 patient compared with baseline; the median change in prednisone dose from baseline to week 8 was 0% (range, −67% to 300%) (supplemental Figure 1).
Clinical response and survival after ECP plus IL2
Twenty-one patients were evaluable at the end of week 16. Thirteen (62%) had an objective PR, 6 (29%) had SD, and 2 (9%) had PD (Figure 1B). New response sites compared with week 8 included skin (n = 7), GI tract (n = 1), JF (n = 4), and lung (n = 1). The median daily prednisone dose at the end of week 16 was 0.21 mg/kg (range, 0-0.51 mg/kg). Among 20 patients without missing data, the daily prednisone dose was unchanged in 10 patients and decreased in 10 patients compared with week 8; the median change in prednisone dose from week 8 to week 16 was −10% (range, −67% to 0%) (supplemental Figure 1).
Of 17 patients with SD after 8 weeks of ECP alone, 7 had an objective PR, 6 had SD, 2 had PD, and 2 were nonevaluable after addition of IL2. Of 7 patients with a PR after ECP alone, 6 patients maintained a PR; 1 patient discontinued IL2 treatment due to fatigue and myalgias and was thus nonevaluable at week 16. Seven patients with a PR and 4 patients with SD at week 16 continued ECP plus IL2 therapy.
For the entire cohort, the 1-year OS, PFS, and CPFS were 80% (95% confidence interval [CI], 58% to 91%), 76% (95% CI, 54% to 88%), and 76 (95% CI, 54% to 88%), respectively (Figure 1C). Nonrelapse morality and relapse at 1 year were 20% (95% CI, 7% to 38%) and 4% (95% CI, 0.3% to 17%). There was no difference in OS between responders and nonresponders (Figure 1D). Five patients died of cGVHD-related complications. One patient had relapse of primary disease.
Clinical factors associated with response
Age, sex, patient-donor sex-match, conditioning regimen, HLA match, time since HSCT, prior acute GVHD, time from HSCT to cGVHD, and baseline cGVHD severity were not correlated with clinical responses to ECP alone or ECP plus IL2; daily prednisone dose or change in daily prednisone dose were also not associated with clinical responses. Overall, 9 of 9 patients with a pre-HSCT diagnosis of myelodysplastic syndrome (MDS) responded (P = .002 compared with other pre-HSCT diagnoses), including 5 of 7 patients who responded to ECP alone and 4 of 7 patients who responded after addition of IL2 (supplemental Table 1); patients with a pre-HSCT diagnosis of MDS were not significantly different with regard to clinical or transplant-related characteristics (data not shown). Responders at week 16 tended to have received fewer prior cGVHD therapies; 62% of responders received 1 to 2 prior therapies compared with 13% of nonresponders (P = .07).
Complete blood count parameters
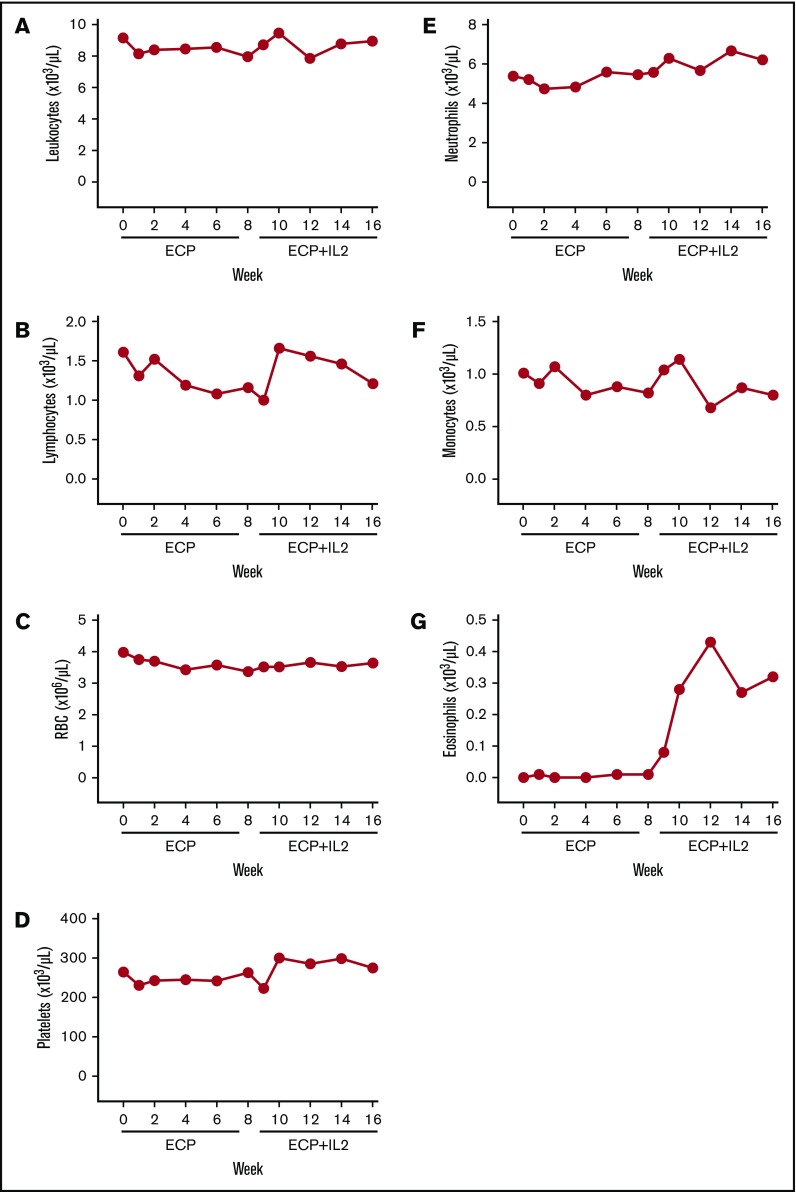
Leukocyte counts were stable during the 16-week trial (Figure 2A). Lymphocyte counts declined during ECP alone from a median of 1610 cells per microliter (interquartile range [IQR], 1210-2630) at baseline to 1160 cells per microliter (IQR, 800-1460) at week 8 (P = .0002); counts returned to baseline after 2 weeks of IL2 treatment (median, 1660 cells per microliter; IQR, 1152-2513 at week 10; P = .45 vs baseline) and remained stable for the following 6 weeks (Figure 2B). Platelet counts increased slightly at week 16 vs baseline (P = .02) (Figure 2D). RBC, neutrophil, and monocyte counts were stable (Figure 2C,E-F). Consistent with previous studies,34,35,39 asymptomatic eosinophilia occurred with IL2 from a median of 10 cells per microliter (IQR, 0-70) at week 8 to a peak of 430 cells per microliter (IQR, 200-1020) at week 12 (P = .03) and a plateau at week 16 of 320 cells per microliter (IQR, 160-680) (Figure 2G).
Figure 2.
Complete blood counts. Median absolute numbers of peripheral blood leukocytes (A), lymphocytes (B), RBCs (C), platelets (D), neutrophils (E), monocytes (F), and eosinophils (G).
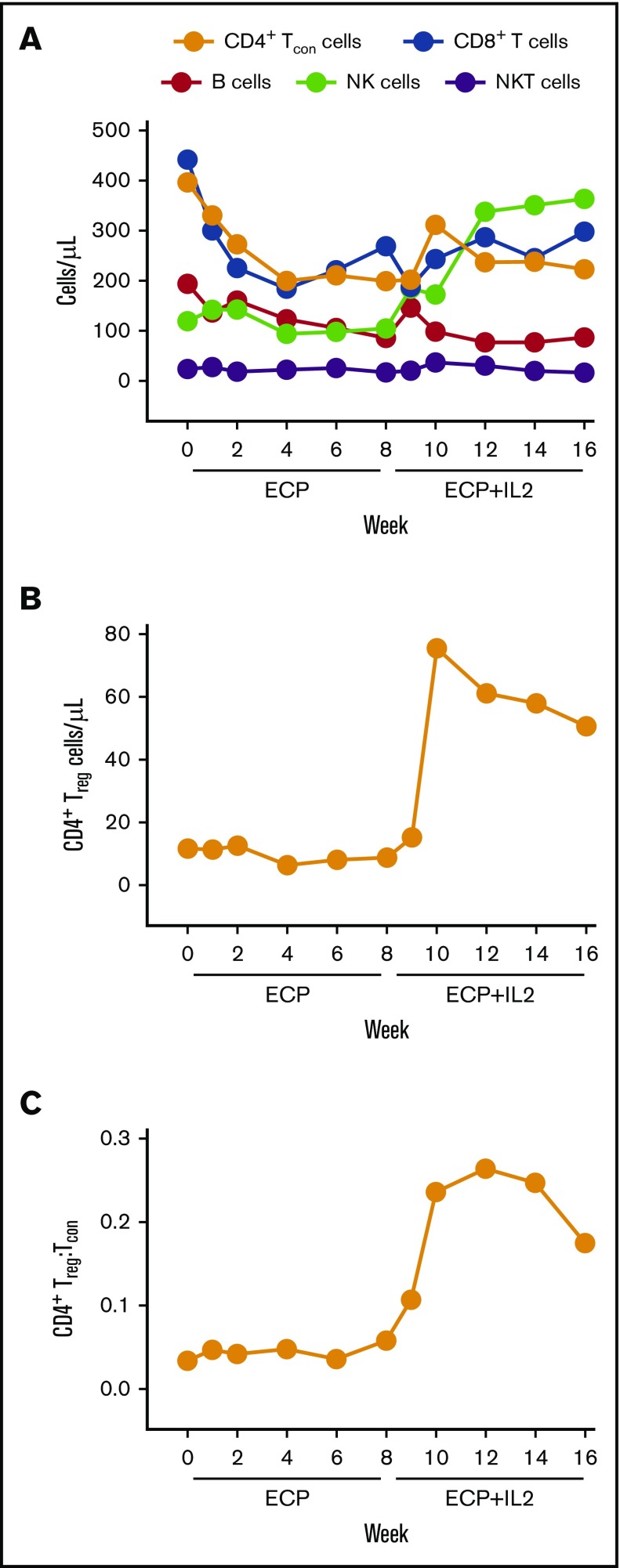
Effect of ECP alone on lymphocyte subsets
Total CD4+ and CD8+ T-cell counts decreased significantly during the first 4 weeks of ECP alone. CD19+ B cells decreased steadily over the entire 8-week course of ECP alone (P < .05 compared with baseline at weeks 1, 4, and 8). There was minimal change in NK and NKT cell counts (Figure 3A); among NK cells, there was no significant change in CD56bright or CD56dim subsets (supplemental Figure 2). The median total CD4+ Tcon cell count was 397 cells per microliter (IQR, 191-487) at baseline, 200 cells per microliter (IQR, 108-410) at week 4 (P = .003 vs baseline), and 200 cells per microliter (IQR, 128-416) at week 8 (P = .03 vs baseline); decrease in the number of effector and EM subsets accounted for most of the reduction (supplemental Figure 3A). The median total CD4+ Treg cell count was 12 cells per microliter (IQR, 6-18) at baseline, 6 cells per microliter (IQR, 4-9) at week 4 (P = .024 vs baseline), and 9 cells per microliter (IQR, 5-18) at week 8 (P = .50 vs baseline) (Figure 3B). The Treg:Tcon cell ratio did not change significantly with ECP alone (Figure 3C). The median total CD8+ T-cell count decreased from 442 cells per microliter (IQR, 145-914) at baseline to 185 cells per microliter (IQR, 60-571) at week 4 (P = .001 vs baseline) and 269 cells per microliter (IQR, 98-467) at week 8 (P = .0002 vs baseline) (Figure 3A); decrease in the effector subset accounted for the majority of the reduction in CD8+ T cells (supplemental Figure 3B).
Figure 3.
Immunologic effects on lymphocytes. (A-B) Median absolute numbers of peripheral blood lymphocyte subsets, including CD4+ Tcon cells, CD8+ T cells, B cells, NK cells, NKT cells, and Treg cells. (C) CD4+ Treg:Tcon ratio. Lymphocyte subsets were identified by flow cytometry using specific antibodies described in “Methods.”
Effect of ECP plus IL2 on lymphocyte subsets
With the addition of IL2, there was a significant increase in Treg cells from 9 cells per microliter (IQR, 5-18) at week 8 to a peak of 76 cells per microliter (IQR, 23-222) at week 10 (approximately eightfold increase, P < .0001 vs week 8) and a plateau of 51 cells per microliter (IQR, 33-75) at week 16 (approximately fivefold increase, P < .0001 vs week 8) (Figure 3B); EM cells comprised the majority of the increase in Treg cells, most notably at weeks 10 through 16 (supplemental Figure 3C). The Treg:Tcon cell ratio increased from 0.06 (IQR, 0.03-0.09) at week 8 to a peak of 0.26 (IQR, 0.14-0.39) at week 12 (P < .0001 vs week 8) and a plateau of 0.18 (IQR, 0.14-0.35) at week 16 (P < .0001 vs week 8) (Figure 3C). NK cells increased from 105 cells per microliter (IQR, 76-159) at week 8 to a peak of 358 cells per microliter (IQR, 237-466) at week 12 (P < .0001 vs week 8) and a plateau of 364 cells per microliter (IQR, 201-575) at week 16 (P < .0001 vs week 8) (Figure 3A). There was minimal change in CD4+ Tcon, CD8+ T, B, or NKT cells (Figure 3A).
Immunologic parameters associated with clinical response
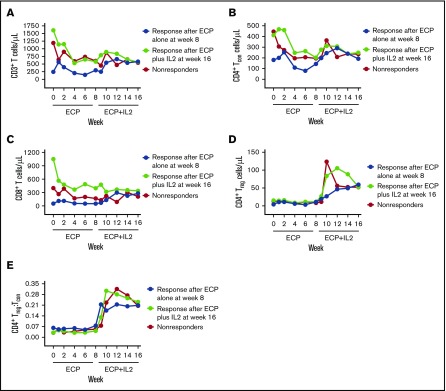
Patients with a PR after 8 weeks of ECP alone had lower baseline numbers of CD3+ T cells compared with patients who responded after ECP plus IL2 and compared with patients who did not respond to either treatment (median, 246 vs 1605 vs 1192 cells per microliter for ECP responders vs ECP plus IL2 responders vs nonresponders, respectively; P = .04) (Figure 4A). ECP responders also had lower baseline numbers of CD4+ Tcon cells (median, 181 vs 413 vs 446 cells per microliter for ECP responders vs ECP plus IL2 responders vs nonresponders, respectively; P = .05) and CD8+ T cells (median, 52 vs 1051 vs 399 cells per microliter for ECP responders vs ECP plus IL2 responders vs nonresponders, respectively; P = .03) (Figure 4B-C). Treg cell numbers and the Treg:Tcon cell ratio were not significantly different at any time point among patients who responded to ECP alone, patients who responded after ECP plus IL2, and patients who did not respond (Figure 4D-E). However, patients who responded after ECP plus IL2 tended to have a greater increase in Treg cell numbers at week 9 (median increment, 22.1 cells per microliter) vs responders to ECP alone (median increment, 6.3 cells per microliter) and vs patients who did not respond to either treatment (median increment, 1.7 cells per microliter) (P = .06) (Figure 4D).
Figure 4.
Changes in T-lymphocyte populations among responders and nonresponders. Median absolute numbers of peripheral blood CD3+ T cells (A), CD4+ Tcon cells (B), CD8+ T cells (C), and CD4+ Treg cells (D), and the CD4+ Treg:Tcon ratio (E) in patients who responded to ECP alone at week 8 (blue), patients who responded after ECP plus IL2 at week 16 (green), and patients who did not respond (red).
Discussion
We conducted a phase 2 clinical trial to assess the impact of adding IL2 to ECP in SR-cGVHD. The therapeutic effect of both ECP and IL2 was reportedly associated with Treg cell expansion; in addition, IL2 treatment led to increased CD56bright NK cells, which has also been associated with ECP. Thus, we hypothesized that adding IL2 could improve response in ECP-treated SR-cGVHD patients via a combined immunomodulatory effect on Treg cell and CD56bright NK cells. Our sequential treatment trial design allowed for evaluation of the clinical and immunologic impact of ECP alone and ECP plus IL2 in the same patient; doses of prednisone and other immunosuppressive medications were relatively stable, which minimized potential confounding effects during the trial. Our study was limited by sample size, clinical heterogeneity, and differences in treatment prior to enrollment, which are often unavoidable in a phase 2 trial. In addition, our study did not definitively answer the question of whether combined ECP plus IL2 is superior or equivalent to treatment with either therapy alone. Nonetheless, the 16-week course of treatment was safe, well tolerated, and effective in the majority of treated patients; an objective PR was observed in 13 of 21 evaluable patients (62%) who completed 8 weeks of ECP alone followed by 8 weeks of ECP plus IL2.
The majority of patients enrolled in our study had moderate SR-cGVHD with 1 to 4 prior systemic therapies. These features of our cohort could have contributed to the high ORR , and it is conceivable that the observed response rate would have been lower in a cohort that was enriched for patients with severe SR-cGVHD. However, the 16-week duration of treatment was also relatively short and it is likely that additional patients, including those with severe disease, would have responded with continued treatment up to 24 weeks.
Twenty-nine percent of evaluable patients showed a PR after 8 weeks of ECP alone. The design of our study did not allow for assessment of the efficacy of ECP alone beyond 8 weeks, but it is possible that patients who responded during treatment with ECP plus IL-2 would have achieved a PR with extended ECP monotherapy. However, the response rate to ECP alone in our study is within the range reported in a recent analysis of prior studies of ECP in adults with SR-cGVHD that documented ORRs of 30% to 100%.40 The 8-week period of treatment with ECP alone is within the range of reported median times to response (4-24 weeks).13,14,25,41,42 However, it is difficult to directly compare prior reports with our study, given clinical and trial design heterogeneity.
Seven of 17 patients (41%) with SD after 8 weeks of ECP alone had a new PR after addition of IL2. This is similar to the response observed in our prior phase 1 study of IL2 alone (52%), which also measured clinical efficacy after 8 weeks of treatment.34 In our prior phase 2 study of IL2 alone, which evaluated a patient cohort earlier in the course of SR-cGVHD (ie, ≤2 prior failed therapies), 61% of patients achieved a PR after 12 weeks of treatment. Compared with our current study, patients in our prior phase 2 study of IL2 alone were younger (median, age 62 vs 51 years) and had shorter times from HSCT to start of therapy (median, 919 vs 616 days) and from cGVHD onset to start of therapy (median, 524 vs317 days); all of these factors were statistically significant predictors of response to IL2 in multivariable analysis (ie, younger patients with shorter times to start of IL2 therapy were more likely to have a PR after 12 weeks of treatment). Thus, it is possible that both treatment duration and clinical factors could have predicted a lower response rate to IL2 alone in the current cohort, and the reported week 16 responses in our trial of ECP plus IL2 may indicate clinical benefit beyond that seen with IL2 alone. However, it is not possible to definitively attribute week 16 responses in the current study to ECP plus IL2. This would need evaluation in a prospective randomized trial including ECP-alone and IL2-alone arms.
Unexpectedly, the predominant and consistent immunologic effect of ECP alone was a selective reduction in circulating T and B lymphocytes. The percentage of lymphopenic patients increased from 54% at baseline to 87% after 8 weeks of ECP alone; we did not observe an increase in opportunistic infections or malignant relapse, consistent with previous ECP studies.43 The decrease in lymphocytes could simply be the result of lymphocyte apoptosis caused directly by ECP.44,45 The progressive decrease in circulating lymphocytes during ECP could also involve reduction in cytokines required for proliferation/survival (eg, IL2, IL-7, or IL-15), increased expression of proapoptotic receptors (eg, Fas), decreased egress from lymph nodes (eg, via S1P1, CCR7, CXCR4, or CXCR5 signaling alterations), and/or increased vascular adhesion and extravasation. Exploration of these potential mechanisms could uncover novel therapeutic approaches in SR-cGVHD.
Contrary to prior studies,26,27 we did not observe a change in total NK cells or in CD56bright or CD56dim subsets during treatment with ECP alone. This discrepancy could potentially be explained by significantly longer duration of ECP treatment in prior studies; the patients described by Alcindor et al received ECP for a median time of 9 months compared with 4 months in the study presented here.26 Treatment with ECP alone was also not associated with an increase in Treg cells, which has been previously reported.16,18-21,23-25 There are several potential explanations for this discrepancy. First, it is possible that Treg cell expansion occurs over a longer course of ECP. Second, clinical heterogeneity may account for differences. Two studies included both pediatric and adult patients with acute GVHD and cGVHD,20,23 and 4 studies reported results from patients receiving ECP for solid organ transplant rejection.16,18,19,21 Third, dose modification of prednisone and other immunosuppressive drugs, which could affect Treg cell homeostasis independently,46-48 was limited during the 16-week course of our study.
The order of treatments in our study (ie, ECP alone followed by ECP plus IL2) could have affected ORRs or the observed immunological effects on peripheral blood lymphocytes. Interestingly, responders to ECP alone tended to have blunted Treg cell responses to IL2, which may indicate that (1) ECP responders comprise a distinct subset of SR-cGVHD patients or (2) ECP alone for 8 weeks diminishes subsequent immunologic responses to IL2. It is unclear whether reversing the order of treatment (ie, IL2 alone followed by ECP plus IL2) or prolonging treatment with a single therapy would alter ORRs, responses in particular patients, or the immunologic effects on lymphocyte numbers. A larger study of cGVHD patients including additional treatment groups would be required to address this possibility.
The T-cell count at baseline was a significant predictor of response to ECP alone; specifically, ECP responders had fewer CD4+ Tcon and CD8+ T cells compared with patients who responded after IL2 addition and compared with nonresponders. The biological explanation for this association is unclear, but it is possible that low numbers of circulating T cells mark a subset of patients with unique cGVHD pathophysiology that is responsive to ECP. Higher baseline CD4+ Tcon cells could pose a more substantial barrier to the ECP-associated decrease in Tcon cells and the IL2-mediated increase in Treg:Tcon cell ratio. Alternatively, higher T-cell numbers in patients who did not respond after 8 weeks of ECP could simply indicate more severe or refractory disease, though disease severity was not a significant predictor of response in our statistical analyses. Regardless of the biological explanation for this association, it is clear that nonresponders after ECP required a longer course of treatment or additional immunosuppressive therapies; defining a threshold number of T cells that separates responders from nonresponders could help in choosing different or more intensive therapies beyond ECP for patients with SR-cGVHD.
In summary, 8 weeks of ECP alone followed by 8 weeks of ECP plus IL2 was safe and well tolerated, and generated objective clinical responses in 62% of adult patients with advanced SR-cGVHD. ECP alone was associated with a decrease in circulating CD4+ and CD8+ T cells, and lower baseline T-cell counts correlated with response. As observed previously, IL2 caused a preferential increase in Treg cells and the Treg:Tcon cell ratio. Among patients who responded after addition of IL2, there was a trend toward greater Treg increment vs patients who responded to ECP alone and patients who did not respond during the 16-week trial. Taken together, our data indicate that ECP and IL2 have distinct effects on peripheral blood lymphocyte populations, suggesting different mechanisms of action; additional studies to identify correlations between disease characteristics, immunologic parameters, and response to ECP with or without IL2 are warranted.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank Susan Stephenson for assistance with collecting study data.
This work was supported by National Institutes of Health, National Cancer Institute grants R01CA183560, R01CA183559, and P01CA142106 and the Ted and Eileen Pasquarello Research Fund.
Authorship
Contribution: R.B., H.T.K., W.J.S., R.J.S., and J.K. conceived and designed the study; J.K. and W.J.S. wrote the protocol; J.K. is the study principal investigator; S.N., E.P.A., P.A., C.S.C., V.T.H., J.H.A., and R.J.S. and J.K. enrolled patients to study and took direct care of patients; R.B., H.T.K., S.J.P., N.V.M., E.H., C.G.R., M.J.F., J.W., T.K., J.R., and J.K. collected and assembled data; R.B., H.T.K., J.R., and J.K. analyzed and interpreted data; H.T.K. performed statistical analysis; R.B., H.T.K., and J.K. generated figures and wrote the manuscript; and all authors reviewed and edited the manuscript.
Conflict-of-interest disclosure: J.K. reports research funding from Prometheus Laboratories Inc, Miltenyi Biotec, and Millennium Pharmaceuticals; consulting fees from Amgen, Equillium, and Fortress Biotech; and advisory board fees from Takeda and Kadmon. The remaining authors declare no competing financial interests.
Correspondence: John Koreth, Dana-Farber Cancer Institute, D2029, 450 Brookline Ave, Boston, MA 02215; e-mail: john_koreth@dfci.harvard.edu.
References
- 1.Socié G, Ritz J. Current issues in chronic graft-versus-host disease. Blood. 2014;124(3):374-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolff D, Gerbitz A, Ayuk F, et al. . Consensus conference on clinical practice in chronic graft-versus-host disease (GVHD): first-line and topical treatment of chronic GVHD. Biol Blood Marrow Transplant. 2010;16(12):1611-1628. [DOI] [PubMed] [Google Scholar]
- 3.Cutler CS, Koreth J, Ritz J. Mechanistic approaches for the prevention and treatment of chronic GVHD. Blood. 2017;129(1):22-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10(7):490-500. [DOI] [PubMed] [Google Scholar]
- 5.Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002;99(10):3493-3499. [DOI] [PubMed] [Google Scholar]
- 6.Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med. 2002;196(3):389-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edinger M, Hoffmann P, Ermann J, et al. . CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. 2003;9(9):1144-1150. [DOI] [PubMed] [Google Scholar]
- 8.Sanchez J, Casaño J, Alvarez MA, et al. . Kinetic of regulatory CD25high and activated CD134+ (OX40) T lymphocytes during acute and chronic graft-versus-host disease after allogeneic bone marrow transplantation. Br J Haematol. 2004;126(5):697-703. [DOI] [PubMed] [Google Scholar]
- 9.Zorn E, Kim HT, Lee SJ, et al. . Reduced frequency of FOXP3+ CD4+CD25+ regulatory T cells in patients with chronic graft-versus-host disease. Blood. 2005;106(8):2903-2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsuoka K, Kim HT, McDonough S, et al. . Altered regulatory T cell homeostasis in patients with CD4+ lymphopenia following allogeneic hematopoietic stem cell transplantation. J Clin Invest. 2010;120(5):1479-1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greinix HT, Volc-Platzer B, Rabitsch W, et al. . Successful use of extracorporeal photochemotherapy in the treatment of severe acute and chronic graft-versus-host disease. Blood. 1998;92(9):3098-3104. [PubMed] [Google Scholar]
- 12.Foss FM, DiVenuti GM, Chin K, et al. . Prospective study of extracorporeal photopheresis in steroid-refractory or steroid-resistant extensive chronic graft-versus-host disease: analysis of response and survival incorporating prognostic factors. Bone Marrow Transplant. 2005;35(12):1187-1193. [DOI] [PubMed] [Google Scholar]
- 13.Couriel DR, Hosing C, Saliba R, et al. . Extracorporeal photochemotherapy for the treatment of steroid-resistant chronic GVHD. Blood. 2006;107(8):3074-3080. [DOI] [PubMed] [Google Scholar]
- 14.Flowers ME, Apperley JF, van Besien K, et al. . A multicenter prospective phase 2 randomized study of extracorporeal photopheresis for treatment of chronic graft-versus-host disease. Blood. 2008;112(7):2667-2674. [DOI] [PubMed] [Google Scholar]
- 15.Jagasia M, Greinix HT, Scheid C, et al. . A randomized controlled study of extracorporeal photopheresis (ECP) therapy with UVADEX (R) (Methoxsalen) for National Institutes of Health (NIH) graded moderate to severe chronic graft-vs-host disease (cGVHD) [abstract]. Blood. 2017;130(suppl 1). Abstract 1970. [Google Scholar]
- 16.Lamioni A, Parisi F, Isacchi G, et al. . The immunological effects of extracorporeal photopheresis unraveled: induction of tolerogenic dendritic cells in vitro and regulatory T cells in vivo. Transplantation. 2005;79(7):846-850. [DOI] [PubMed] [Google Scholar]
- 17.Maeda A, Schwarz A, Kernebeck K, et al. . Intravenous infusion of syngeneic apoptotic cells by photopheresis induces antigen-specific regulatory T cells. J Immunol. 2005;174(10):5968-5976. [DOI] [PubMed] [Google Scholar]
- 18.Meloni F, Cascina A, Miserere S, Perotti C, Vitulo P, Fietta AM. Peripheral CD4(+)CD25(+) TREG cell counts and the response to extracorporeal photopheresis in lung transplant recipients. Transplant Proc. 2007;39(1):213-217. [DOI] [PubMed] [Google Scholar]
- 19.Lamioni A, Carsetti R, Legato A, et al. . Induction of regulatory T cells after prophylactic treatment with photopheresis in renal transplant recipients. Transplantation. 2007;83(10):1393-1396. [DOI] [PubMed] [Google Scholar]
- 20.Biagi E, Di Biaso I, Leoni V, et al. . Extracorporeal photochemotherapy is accompanied by increasing levels of circulating CD4+CD25+GITR+Foxp3+CD62L+ functional regulatory T-cells in patients with graft-versus-host disease. Transplantation. 2007;84(1):31-39. [DOI] [PubMed] [Google Scholar]
- 21.George JF, Gooden CW, Guo L, Kirklin JK. Role for CD4(+)CD25(+) T cells in inhibition of graft rejection by extracorporeal photopheresis [published correction appears in J Heart Lung Transplant. 2008;27(10):1190]. J Heart Lung Transplant. 2008;27(6):616-622. [DOI] [PubMed] [Google Scholar]
- 22.Gatza E, Rogers CE, Clouthier SG, et al. . Extracorporeal photopheresis reverses experimental graft-versus-host disease through regulatory T cells. Blood. 2008;112(4):1515-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Biaso I, Di Maio L, Bugarin C, et al. . Regulatory T cells and extracorporeal photochemotherapy: correlation with clinical response and decreased frequency of proinflammatory T cells. Transplantation. 2009;87(9):1422-1425. [DOI] [PubMed] [Google Scholar]
- 24.Schmitt S, Johnson TS, Karakhanova S, Näher H, Mahnke K, Enk AH. Extracorporeal photophoresis augments function of CD4+CD25+FoxP3+ regulatory T cells by triggering adenosine production. Transplantation. 2009;88(3):411-416. [DOI] [PubMed] [Google Scholar]
- 25.Tsirigotis P, Kapsimalli V, Baltadakis I, et al. . Extracorporeal photopheresis in refractory chronic graft-versus-host disease: the influence on peripheral blood T cell subpopulations. A study by the Hellenic Association of Hematology. Transfus Apheresis Sci. 2012;46(2):181-188. [DOI] [PubMed] [Google Scholar]
- 26.Alcindor T, Gorgun G, Miller KB, et al. . Immunomodulatory effects of extracorporeal photochemotherapy in patients with extensive chronic graft-versus-host disease. Blood. 2001;98(5):1622-1625. [DOI] [PubMed] [Google Scholar]
- 27.Zhu L, Couriel DR, Chang CH. The effect of extracorporeal photopheresis on T cell response in chronic graft-versus-host disease. Leuk Lymphoma. 2016;57(2):376-384. [DOI] [PubMed] [Google Scholar]
- 28.Furtado GC, Curotto de Lafaille MA, Kutchukhidze N, Lafaille JJ. Interleukin 2 signaling is required for CD4(+) regulatory T cell function. J Exp Med. 2002;196(6):851-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells [published correction appears in Nat Immunol. 2006;7(4):427]. Nat Immunol. 2005;6(11):1142-1151. [DOI] [PubMed] [Google Scholar]
- 30.Chinen T, Kannan AK, Levine AG, et al. . An essential role for the IL-2 receptor in Treg cell function. Nat Immunol. 2016;17(11):1322-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang H, Chua KS, Guimond M, et al. . Lymphopenia and interleukin-2 therapy alter homeostasis of CD4+CD25+ regulatory T cells. Nat Med. 2005;11(11):1238-1243. [DOI] [PubMed] [Google Scholar]
- 32.Ahmadzadeh M, Rosenberg SA. IL-2 administration increases CD4+ CD25(hi) Foxp3+ regulatory T cells in cancer patients. Blood. 2006;107(6):2409-2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zorn E, Nelson EA, Mohseni M, et al. . IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood. 2006;108(5):1571-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koreth J, Matsuoka K, Kim HT, et al. . Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med. 2011;365(22):2055-2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koreth J, Kim HT, Jones KT, et al. . Efficacy, durability, and response predictors of low-dose interleukin-2 therapy for chronic graft-versus-host disease. Blood. 2016;128(1):130-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirakawa M, Matos TR, Liu H, et al. . Low-dose IL-2 selectively activates subsets of CD4+ Tregs and NK cells. JCI Insight. 2016;1(18):e89278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pavletic SZ, Martin P, Lee SJ, et al. ; Response Criteria Working Group. Measuring therapeutic response in chronic graft-versus-host disease: National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: IV. Response Criteria Working Group report. Biol Blood Marrow Transplant. 2006;12(3):252-266. [DOI] [PubMed] [Google Scholar]
- 38.Arai S, Jagasia M, Storer B, et al. . Global and organ-specific chronic graft-versus-host disease severity according to the 2005 NIH Consensus Criteria. Blood. 2011;118(15):4242-4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Gool F, Molofsky AB, Morar MM, et al. . Interleukin-5-producing group 2 innate lymphoid cells control eosinophilia induced by interleukin-2 therapy. Blood. 2014;124(24):3572-3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alfred A, Taylor PC, Dignan F, et al. . The role of extracorporeal photopheresis in the management of cutaneous T-cell lymphoma, graft-versus-host disease and organ transplant rejection: a consensus statement update from the UK Photopheresis Society. Br J Haematol. 2017;177(2):287-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bisaccia E, Palangio M, Gonzalez J, et al. . Treatment of extensive chronic graft-versus-host disease with extracorporeal photochemotherapy. J Clin Apher. 2006;21(3):181-187. [DOI] [PubMed] [Google Scholar]
- 42.Del Fante C, Scudeller L, Viarengo G, Bernasconi P, Perotti C. Response and survival of patients with chronic graft-versus-host disease treated by extracorporeal photochemotherapy: a retrospective study according to classical and National Institutes of Health classifications. Transfusion. 2012;52(9):2007-2015. [DOI] [PubMed] [Google Scholar]
- 43.Radojcic V, Pletneva MA, Couriel DR. The role of extracorporeal photopheresis in chronic graft-versus-host disease. Transfus Apheresis Sci. 2015;52(2):157-161. [DOI] [PubMed] [Google Scholar]
- 44.Yoo EK, Rook AH, Elenitsas R, Gasparro FP, Vowels BR. Apoptosis induction of ultraviolet light A and photochemotherapy in cutaneous T-cell lymphoma: relevance to mechanism of therapeutic action. J Invest Dermatol. 1996;107(2):235-242. [DOI] [PubMed] [Google Scholar]
- 45.Enomoto DN, Schellekens PT, Yong SL, ten Berge IJ, Mekkes JR, Bos JD. Extracorporeal photochemotherapy (photopheresis) induces apoptosis in lymphocytes: a possible mechanism of action of PUVA therapy. Photochem Photobiol. 1997;65(1):177-180. [DOI] [PubMed] [Google Scholar]
- 46.Karagiannidis C, Akdis M, Holopainen P, et al. . Glucocorticoids upregulate FOXP3 expression and regulatory T cells in asthma. J Allergy Clin Immunol. 2004;114(6):1425-1433. [DOI] [PubMed] [Google Scholar]
- 47.Suárez A, López P, Gómez J, Gutiérrez C. Enrichment of CD4+ CD25high T cell population in patients with systemic lupus erythematosus treated with glucocorticoids. Ann Rheum Dis. 2006;65(11):1512-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Azab NA, Bassyouni IH, Emad Y, Abd El-Wahab GA, Hamdy G, Mashahit MA. CD4+CD25+ regulatory T cells (TREG) in systemic lupus erythematosus (SLE) patients: the possible influence of treatment with corticosteroids. Clin Immunol. 2008;127(2):151-157. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.