Abstract
Histone deacetylase 4 (Hdac4) is known to control chondrocyte hypertrophy and bone formation. We have previously shown that parathyroid hormone (PTH) regulates many aspects of Hdac4 function in osteoblastic cells in vitro; however, in vivo confirmation was previously precluded by pre-weaning lethality of the Hdac4 deficient mice. To analyze the function of Hdac4 in bone in mature animals, we generated mice with osteoblast lineage-specific knockout of Hdac4 (Hdac4ob−/−) by crossing transgenic mice expressing Cre recombinase under the control of a 2.3kb fragment of the Col1a1 promoter with mice bearing loxP-Hdac4. The Hdac4ob−/− mice survive to adulthood and developed a mild skeletal phenotype. At 12 weeks of age, they had short, irregularly-shaped and stiff tails due to smaller tail vertebrae, with almost no growth plates. The tibial growth plate zone was also thinned and Mmp13 and Sost mRNAs were increased in the distal femurs of Hdac4ob−/− mice. Immunohistochemistry showed that sclerostin was elevated in Hdac4ob−/− mice, suggesting that Hdac4 inhibits its gene and protein expression. To determine the effect of PTH in these mice, hPTH (1–34) or saline were delivered for 14 days with subcutaneously implanted devices in 8-week-old female Hdac4ob−/− and wild type (Hdac4fl/fl) mice. Serum CTX, a marker of bone resorption, was increased in Hdac4ob−/− mice with or without PTH treatment. Tibial cortical BV/TV, Ct.Th, and relative cortical area (RCA) were decreased in Hdac4ob−/− mice but PTH caused no further decrease in Hdac4ob−/− mice. Tibial trabecular BV/TV and thickness were not changed significantly in Hdac4ob−/− mice but decreased with PTH treatment. These results indicate that Hdac4 inhibits bone resorption and has anabolic effects via inhibiting Mmp13 and Sost/sclerostin expression. Hdac4 influences cortical bone mass and thickness and knockout of Hdac4 prevents the catabolic effect of PTH in cortical bone.
Keywords: Catabolic effects of PTH, HDAC4, Osteoblasts, Sclerostin, Cortical bone
INTRODUCTION
Parathyroid hormone (PTH) is an 84-amino acid peptide that regulates calcium and phosphorous concentrations in extracellular fluids. PTH stimulates both catabolic (bone resorption) and anabolic (bone formation) events in the skeleton depending on its dose and the periodicity of its delivery. In humans, continuous elevation of serum PTH levels, as occurs in patients with primary hyperparathyroidism, has a catabolic action on bone, while intermittent daily injection is an approved anabolic therapy for the treatment of osteoporosis. (1) PTH binds to its receptor, PTH1R on osteoblasts, and modulates the expression of key genes that control bone formation, such as Runx2, osterix, collagen type I alpha 1 (Col1a1), and bone sialoprotein (BSP) as well as genes of resorption, such as matrix metalloproteinase-13 (Mmp13) and receptor activator of nuclear factor κ–B ligand (RankL). Zhao et al. showed that collagenolytic degradation was required for PTH’s stimulation of serum calcium, suggesting a role for MMP13 in bone resorption.(2)
Histone deacetylases (HDACs) exert gene regulation in skeletal cells by removing acetyl groups from histones and other proteins, including transcription factors, leading ultimately to condensed chromatin, and suppression of gene transcription. Several HDACs contribute to skeletal development and bone mass maintenance.(3) HDAC4, a class IIa histone deacetylase, is expressed in osteoblasts and prehypertrophic chondrocytes. HDAC4 represses the function of the transcription factors, Runx2 and MEF2C, preventing progression of chondrocytes to hypertrophy. (4,5) HDAC4 also deacetylates Runx2, resulting in repression of its transcriptional activity and its degradation in osteoblasts.(6) Mice with global deletion of Hdac4 (Hdac4−/−) are significantly smaller than wild type mice and die within 7 days after birth.(7) This is mainly due to ectopic ossification of endochondral cartilage (premature hypertrophy of chondrocytes and, thus, mineralization), which prevents expansion of the rib cage and leads to an inability to breathe.(4) Previous work from our laboratory has shown that in osteoblastic cells (UMR 106–01), HDAC4 represses the Mmp13 gene under basal conditions by inhibiting the activity of Runx2. Hdac4−/− mice displayed elevated expression of Mmp13 mRNA and protein levels in hypertrophic chondrocytes and trabecular bone.(8) We also found that HDAC4 interacts with MEF2C at the Mmp13 promoter. PTH causes dissociation of HDAC4 from both Runx2 and MEF2C in osteoblastic cells.(9) Obri et al. have reported that HDAC4 inhibits RankL expression by its actions on MEF2C.(10) However, the role of HDAC4 in osteoblasts in vivo is still not well understood in detail.
Sclerostin, encoded by the Sost gene, has been identified as a negative regulator of bone formation.(11, 12) PTH suppresses SOST expression in vivo and in vitro (UMR 106 cells).(13) Class IIa HDACs (HDAC4 and HDAC5) also suppress SOST expression in osteocytes.(14, 15) On the other hand, Class I HDACs 1, 2 and 3 are required for Sost expression(16) and MEF2A, C, and D positively regulate SOST expression in UMR 106 cells. (17)
In the present study, to define the roles of HDAC4 in osteoblasts/osteocytes and in PTH’s actions on bone, we generated osteoblast-specific knockout of Hdac4 (Hdac4ob−/−) in mice. We demonstrate that HDAC4 influences cortical bone mass and thickness and conditional deletion of Hdac4 in osteoblasts prevents the catabolic effect of PTH in cortical bone. We conclude that HDAC4 inhibits bone resorption and has an anabolic effect via inhibiting Mmp13 and SOST gene expression.
Materials and Methods
Generation of osteoblast-specific Hdac4 knockout mice
All experiments using mice were performed following protocols approved by the New York University Institutional Animal Care and Use Committee (IACUC). Col2.3 1a(I)-Cre and HDAC4 floxed mice (Hdac4fl/fl) were on C57Bl/6 backgrounds. To delete Hdac4 specifically in mature osteoblasts and osteocytes, Hdac4fl/fl mice were crossed with mice bearing the 2.3-kb fragment of the rat α1(I)–collagen promoter fused to Cre. All genotypes were determined using Direct PCR lysis reagent (Viagen Biotech, Los Angeles, CA, USA) and the primers are listed in Table 1. All mice were housed maximally at 5 per cage at 23℃ under standard conditions with a 12-hour light/12-hour dark cycle and free access to water and standard rodent chow.
TABLE 1.
Primer sequences used in genotyping
| Arm1: ATCTGCCCACCAGAGTATGTG |
| Arm2 : AGCTGCAGGAGTTTGTTCTCAACAAG |
| Arm3 : GCTGTCTTGTGGAGAATTGGAG |
Body composition analysis
Male or female Hdac4ob−/− and control (Hdac4fl/fl) mice (10 mice in each group) at 10 or 12 weeks of age were anesthetized by ketamine (100 mg/kg) and xylazine (10 mg/kg) and percentage fat determined using a Lunar PIXImus densitometer (Lunar Corporation, Madison, WI).
Continuous PTH infusion
8-week-old female Hdac4ob−/− and control (Hdac4fl/fl) mice (10 mice in each group) were randomly distributed to vehicle or treatment groups and infused with continuous hPTH (1–34; Bachem) at a dose of 8µg/kg BW/day or vehicle (saline) with Alzet microosmotic pumps (model 1002, Durect, CA, USA) implanted subcutaneously onto the backs at a pumping rate of 0.25 µl/h for 14 days. Some investigators were blinded during allocation, animal handling and endpoint measurements.
Histological Analysis
10-week-old female Hdac4ob−/− and control (Hdac4fl/fl) mice (10 mice in each group) were anesthetized with ketamine and the following tissue samples were retrieved. Tibiae, vertebrae, femurs and tails were fixed in 4% paraformadehyde at 4°C overnight. The samples were then decalcified in 10% EDTA. Paraformaldehyde-fixed paraffin-embedded femurs and tibiae were cut as 5 µm sections, and stained with hematoxylin and eosin (H&E). Immunohistochemical analysis of sclerostin (R&D Systems) was carried out on similar sections. Briefly, deparaffinized and hydrated sections were incubated with Proteinase K Solution (20 µg/ml in TE Buffer, pH 8.0) for 20 min in a water bath at 37°C. After cooling, the sections were rinsed with PBS, the endogenous peroxidase was removed by incubating sections for 15 min at room temperature in 3% H2O2 in methanol. After rinsing sections in a BSA solution (3% in PBS), blocking for 1 hour at room temperature (BSA solution containing 1% Triton and 5% FBS) was performed. Primary antibody diluted in PBS with 3% milk (goat anti-Sclerostin: 1:30) was incubated overnight at 4°C in a humidifying chamber. After three washes in PBS-BSA solution, secondary donkey anti-goat peroxidase (HRP)-conjugated antibody (Santa Cruz Biotechnology) was incubated with the sections for 1 hour at room temperature. Sections were developed using Fast 3′3′-Diaminobenzidine (Sigma-Aldrich), prepared following the manufacturer’s protocol and, after washing with PBS, counterstained with hematoxylin (Sigma-Aldrich) for 1 min. Slides were mounted using Permount mounting medium (Thermo Scientific). Sclerostin-positive osteocytes were manually counted using image J.
TRAP staining of tibial bone sections
Tartrate resistant acid phosphatase (TRAP) staining was performed using the Acid Phosphatase Leukocyte kit (Sigma-Aldrich, St. Louis, MO, USA) following the manufacturer’s protocol after deparaffinization and acetate buffer washing. The staining of surface osteoclasts was quantified using BIOQUANT OSTEO 2015.
Micro-computed tomography (µCT) analysis
The tibiae and vertebrae of mice (female) were fixed in 70% ethanol and prepared for high-resolution µCT (SkyScan 1172). Images were obtained using the following parameters: 60 Kv, 167 µA, pixel size of 9.7 µm, 2000×1332 matrix, 6 averages and a 0.5mm aluminum filter. Images were reconstructed using a thresholding of 0–0.065, beam hardening correction of 40, ring artefact correction of 7, and Gaussian smoothing (factor = 1). The tibial cortical analysis was performed on a region extending 50% of the bone length from the proximal end and extending 62 slices for total cortical cross -sectional bone area (Tt.Ar), cross-sectional marrow area (Ma.Ar), cortical bone (tissue) area (Ct.Ar), polar moment of inertia (MMI) and cortical thickness (Ct.Th). Relative cortical area (RCA) was defined as Ct.Ar/Tt.Ar and represented the relative amount of bone tissue in a given bone area. The trabecular analysis was performed on a region starting 25 slices from the end of the growth plate and extending 258 slices toward the distal tibia. This was examined for trabecular bone mineral density (BMD), bone volume/total volume (BV/TV), trabecular number (Tb.N), trabecular thickness (Tb.Th), and trabecular spacing (Tb.Sp). BMD was calculated as the ratio of bone mineral content (BMC, mg) to the total volume of distal metaphysis analyzed after removing (masking) marrow space grayscale variability. Caudal vertebra 17 cortical analysis was performed on a region of 150 and 70 scan slices from above the caudal growth plate for Hdac4fl/fl and Hdac4ob−/− mice, respectively. Lumbar vertebrae (L4) analysis was performed on the same circular area. The bone parameters were obtained with CtAn Version 1.5 (SkyScan).
Quantitative real-time RT-PCR
Total RNA was obtained from distal femurs using TRIzol reagent (Invitrogen, CA, USA). cDNA was synthesized from 0.1 µg of total RNA using a TaqMan reverse transcription kit (Life Technologies, USA.) with hexamer primers following the protocol described by the manufacturers. Gene expression levels were measured using SYBR Green PCR Reagent (Applied Biosystems). Primer pairs used for quantitative detection of gene expression are listed in Table 2. The quantity of mRNA was calculated by normalizing the Ct (threshold cycle value) of specific genes to the Ct of the housekeeping gene β-actin.
TABLE 2.
Primer sequences for real-time RT-PCR
| Gene | Primers (5’–3’) |
|---|---|
| Mouse Hdac4 | Forward, GGCGAGCACAGAGGTGAAGATG |
| Reverse, GCTGTGCTGTGTCTTCCCAIAC | |
| Mouse Hdac5 | Forward, AAGTTGTTCGCAGATGCCCA |
| Reverse, TTCACCACAGTGGGTTGGTC | |
| Mouse Mmpl3 | Forward, GCCCTGATGTTTCCCATCTA |
| Reverse, TTTTGGGATGCTTAGGGTTG | |
| Mouse Collal | Forward, AGATTGAGAACATCCGCAGCC |
| Reverse, TCCAGTACTCTCCGCTCTTCC | |
| Mouse Sost | Forward, AGCCTTCAGGAATGATGCCAC |
| Reverse, TTTGGCGTCATAGGGATGGT | |
| Mouse Osx | Forward, AGAGGTTCACTCGCTCTGACGA |
| Reverse, TTGCTCAAGTGGTCGCTTCTG | |
| Mouse Runx2 | Forward, CCCAGCCACCTTTACCTACA |
| Reverse, TATGGAGT GCT GCT GGT | |
| Mouse b-Actin | Forward, TCCTCCTGAGCGCAAGTACTCT |
| Reverse, CGGACTCATCGTACTCCTGCTT |
Serum biomarkers
Serum was prepared by allowing the blood to clot for 15–30 min at room temperature followed by centrifugation at 2000 × g for 10 min at 4°C. CTX and P1NP (Immunodiagnostic Systems Inc.) were measured by sandwich ELISAs. All measurements were performed according to the manufacturer’s instructions included with the kits.
Statistical analyses
Statistical differences were analyzed either by Student’s t test or by two -way ANOVA using IBM SPSS (v22, Armonk, NY). Results are expressed as mean ± SE and a p < 0.05 was considered significant comparing each of the groups.
Results
Phenotype of osteoblast-specific Hdac4 deficient mice
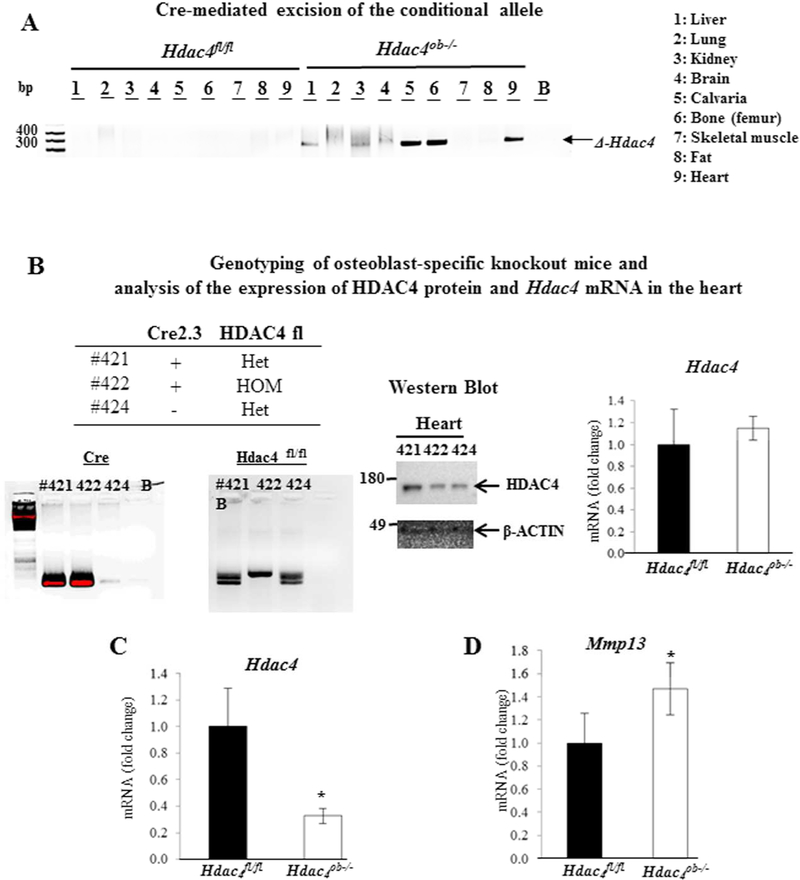
We confirmed the specificity of Hdac4 gene excision in the Col2.3-Cre/Hdac4fl/fl mice by PCR analysis of genomic DNA from different tissues from mature osteoblast lineage-specific, knockout (Hdac4ob−/−), and the control (Hdac4fl/fl) mice. Exon 4 excision (∆-Hdac4) occurred in calvariae and bone from Hdac4ob−/− mice but not in Hdac4fl/fl (Fig 1A). Although the excision also seemed to occur in the heart, we confirmed that HDAC4 protein and Hdac4 mRNA were not affected in the hearts of Hdac4ob−/− mice (Fig. 1B). Hdac4 gene expression levels were approximately decreased by 70% in the distal femur (Fig. 1C) and Mmp13 gene expression was increased by 40% (Fig. 1D). Thus, the establishment of mature osteoblast lineage-specific ablation of HDAC4 was confirmed in Hdac4ob−/− mice.
Figure:1.
(A) Detection of the deletion allele of Hdac4 by PCR in genomic DNA isolated from different tissues using primers arm1 and arm3. (B) Genotyping of Hdac4 mice using primers arm1 and arm2. Analysis of the expression of HDAC4 protein and Hdac4 mRNA in the heart. β-actin was used as a loading control. (C) Hdac4, and (D) Mmp13 levels relative to β-actin were determined in distal femur RNA from 10 week-old Hdac4fl/fl (n = 6) and Hdac4ob−/− mice (n = 8). Data are shown as fold changes compared with levels in Hdac4fl/fl mice. Data shown are means ± SE. *, p< 0.05 by Student’s t test.
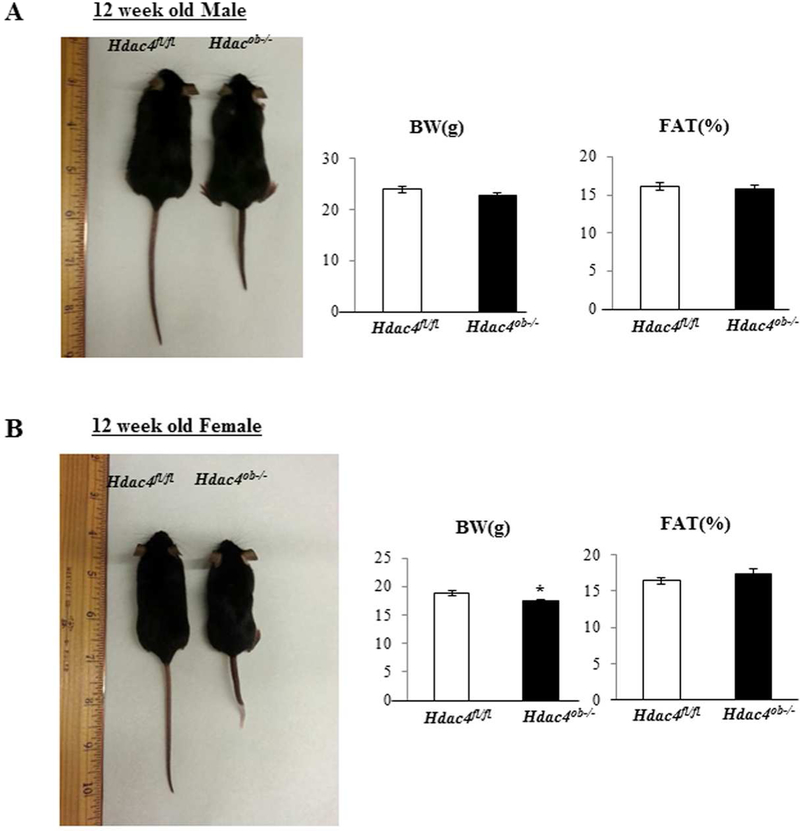
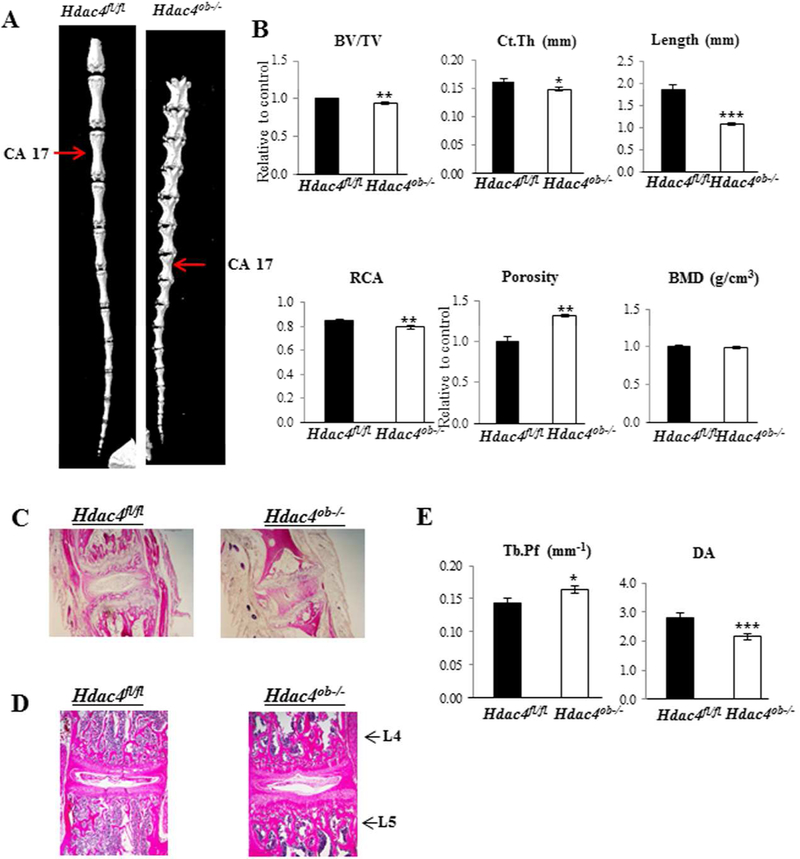
These mice survived to adulthood but showed a mild skeletal phenotype upon gross examination. Female Hdac4 mutant mice were noticeably smaller and weighed around 7% less than Hdac4fl/fl mice but had similar fat percentages (Fig. 2B). There were no significant differences in body weight or fat percentage in male mice (Fig. 2A). Most striking was that all Hdac4ob−/− mice had shorter, irregularly-shaped, and stiff tails at 12 weeks of age (Fig. 2A, B). Representative µCT images of caudal vertebrae from female Hdac4fl/fl and Hdac4ob−/− mice are shown in Fig. 3A. The µCT of caudal vertebra 17 revealed low cortical bone volume (BV/TV), bone area (Ct.Th and RCA), and length compared with Hdac4fl/fl mice. On the other hand, porosity was increased in Hdac4ob−/− mice (Fig. 3B). Hematoxylin and eosin staining of caudal vertebrae showed that the tail phenotype in Hdac4ob−/− mice is due to shorter tail joints, with lesser growth plates and abnormal intervertebral discs (Fig. 3C). The lumbar intervertebral discs also seem to be deformed compared with Hdac4fl/fl mice (Fig. 3D). The µCT of lumbar vertebrae revealed that Hdac4ob−/− mice have greater Tb.Pf and lower DA compared with Hdac4fl/fl mice (Fig. 3E), suggesting that lumbar vertebral trabecular bone of Hdac4ob−/− mice is more disconnected and is weaker than Hdac4fl/fl mice. Overall, these data suggest that Hdac4 is required for caudal and lumbar vertebral bone mass and strength in mice.
Figure. 2. Phenotype of Hdac4ob−/− compared with Hdac4fl/fl mice Twelve-week-old.
(A) Male, (B) Female Hdac4 fl/fl and Hdac4ob−/− mice are shown. Fat percentage was obtained by DXA scan analyses. Hdac4 fl/fl (n=12) and Hdac4ob−/− mice (n=17).
Figure 3. Hdac4ob−/−mice have decreased caudal bone mass and length compared with Hdac4fl/fl mice.
(A) Representative uCT images of the tails are shown. (B) Caudal vertebrae (caudal 17) from 12-week-old female Hdac4fl/fl (n = 6) and Hdac4ob−/− mice (n = 8) were used for µCT analysis. Data shown are means ± SE. *, p< 0.05, **, p< 0.01, ***, p< 0.001 by Student’s t test. Representative images of hematoxylin and eosin stained caudal (C) and lumbar vertebrae (D) are shown. (E) Lumbar vertebrae (L4) from 12-week-old female Hdac4fl/fl (n = 10) and Hdac4ob−/− mice (n = 10) were used for µCT analysis. Data shown are as means ± SE. *, p< 0.05, ***, p< 0.001 by Student’s t test.
HDAC4 regulates body weight and length of growth plates of long bones
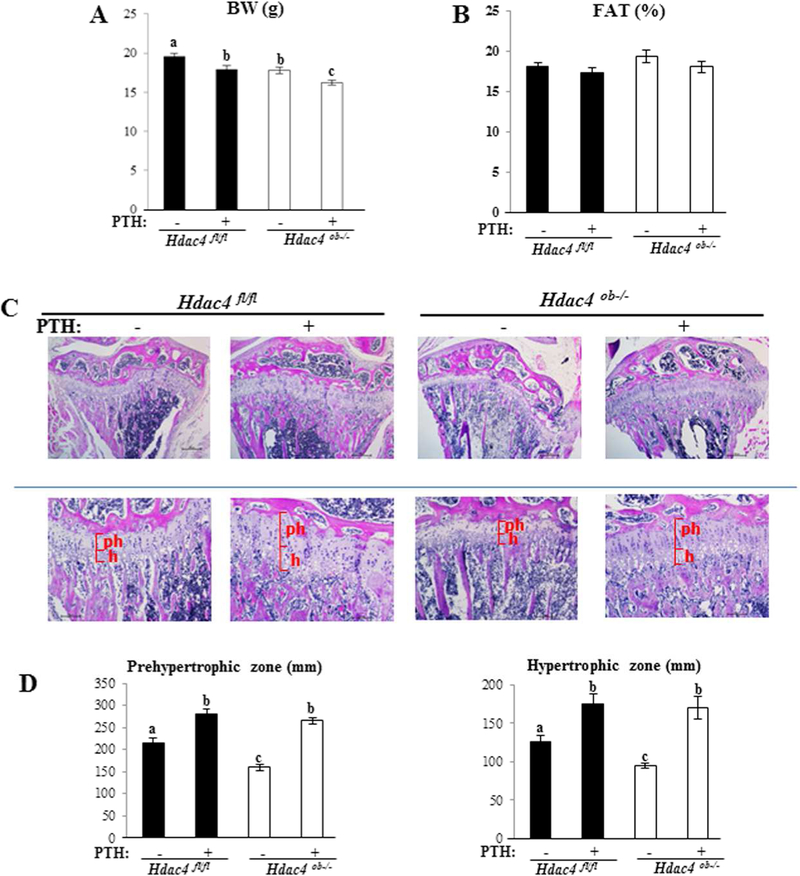
To determine the role of HDAC4 in the catabolic action of PTH, 8-week-old female Hdac4ob−/− or Hdac4fl/fl mice were treated with continuous infusion of PTH for 2 weeks. Body weight was significantly decreased in Hdac4ob−/− compared to Hdac4fl/fl mice. PTH treatment induced significant body weight loss (approximately 10%) from baseline in both Hdac4fl/fl and Hdac4ob−/− mice (Figure 4A). There was no significant change in fat percentage between control and PTH-infused Hdac4ob−/− or Hdac4fl/fl mice (Figure 4B). Histological examination of tibiae showed significantly shorter tibial prehypertrophic and hypertrophic growth plate zones in Hdac4ob−/− compared to Hdac4fl/fl mice. On the other hand, continuous PTH treatment (both Hdac4fl/fl and Hdac4ob−/− mice) caused lengthening of both zones (Fig. 4C, D).
Figure 4. Body weight and growth plate histological examination of Hdac4fl/fl and Hdac4ob−/−mice after continuous PTH treatment.
(A) Body weight and (B) Fat % by DXA in 10-week-old female Hdac4fl/fl and Hdac4ob−/− mice treated with continuous PTH or vehicle, (Hdac4 fl/fl; n=12, Hdac4 fl/fl with PTH; n=13, Hdac4ob−/−; n=11, Hdac4ob−/−; with PTH; n=12). (C) Representative hematoxylin and eosin stained sections of the growth plate of the tibiae are shown. (D) Morphometric analysis of the length of the prehypertrophic (ph), and hypertrophic (h) zones in female mice. Hdac4 fl/fl; n=5, Hdac4 fl/fl with PTH; n=5, Hdac4ob−/−; n=6, Hdac4ob−/−; with PTH; n=6. Data shown are mean ± SE. Different letters indicate p<0.05 vs. one another. The images were quantified using Image J software.
HDAC4 is required for normal accrual of cortical bone mass and for PTH’s catabolic effects on cortical bone
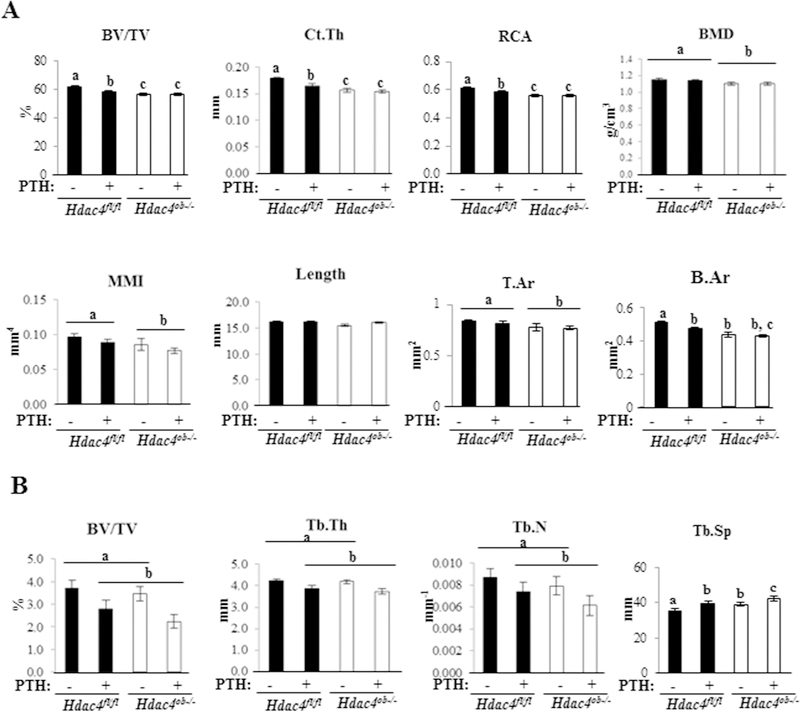
We performed µCT analysis to determine the tibial cortical structure. Hdac4ob−/− mice exhibited decreased cortical bone volume (BV/TV), cortical thickness (Ct.Th), relative cortical area (RCA), T.Ar, B.Ar, BMD and MMI compared to Hdac4fl/fl mice. Continuous PTH treatment significantly reduced cortical BV/TV, Ct.Th, and RCA in wild-type animals. In contrast, Hdac4ob−/− mice were protected from the PTH-induced decrease in BV/TV, bone area and architecture (Figure 5A). These results indicate that HDAC4 is involved in maintenance of cortical bone mass and thickness and genetic deletion of Hdac4 in osteoblastic cells prevents the catabolic effect of PTH in cortical bone.
Figure 5. Hdac4ob−/− mice have decreased cortical bone but not trabecular bone and PTH is unable to cause a catabolic effect in cortical bone while this is retained in trabecular bone.
(A) Tibiae from female 10-week-old female Hdac4 fl/fl and Hdac4ob−/− mice treated with continuous PTH for 14 days or vehicle were used for cortical or trabecular (B) µCT analysis. (Hdac4 fl/fl; n=9, Hdac4 fl/fl with PTH; n=11, Hdac4ob−/−; n=9, Hdac4ob−/−; with PTH; n=9). Data shown are means ± SE. Different letters indicate p<0.05 vs. one another.
Continuous PTH treatment decreased trabecular bone mass; Hdac4 deficiency did not attenuate this decrease in trabecular bone
We observed that continuous PTH treatment significantly decreased tibial trabecular BV/TV, trabecular thickness (Tb.Th), and trabecular number (Tb.N) in Hdac4fl/fl and Hdac4ob−/− mice. On the other hand, there were no significant differences comparing Hdac4ob−/− with Hdac4fl/fl mice and Hdac4 deficiency did not attenuate the PTH catabolic action in trabecular bone (Figure 5B). Trabecular separation (Tb. Sp) was significantly increased in Hdac4ob−/− mice and was further increased by PTH treatment in the knockout mice. These data suggest that continuous PTH increases trabecular bone loss and HDAC4 deletion does not prevent the catabolic effect of PTH in trabecular bone.
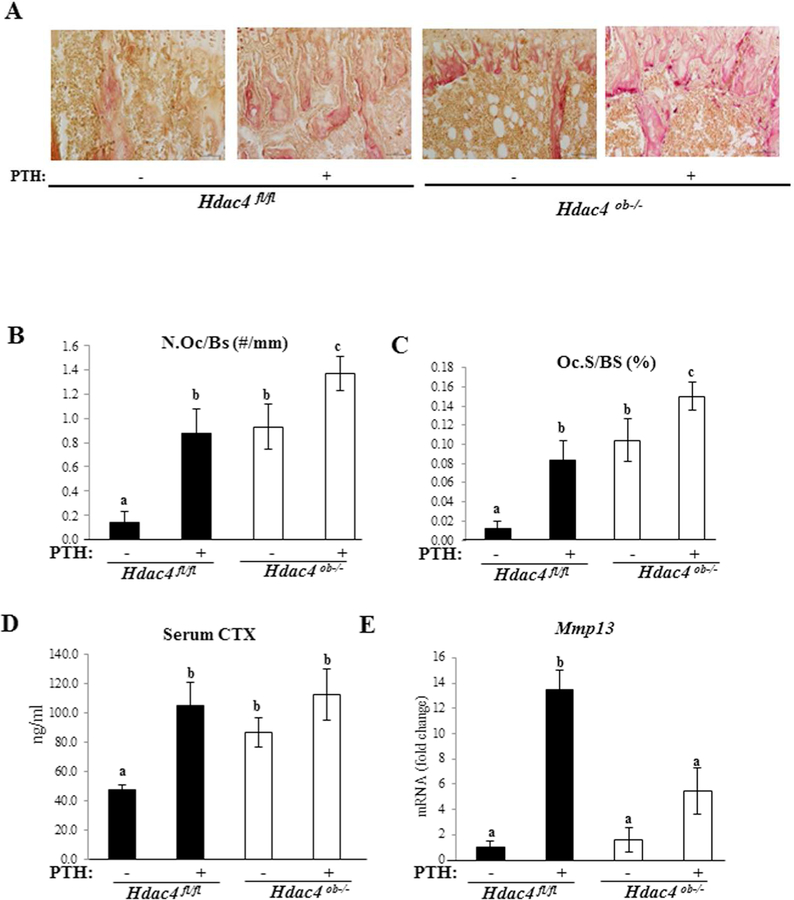
HDAC4 counteracts bone resorption
To determine the role of HDAC4 in bone resorption, we examined TRAP staining, a marker of differentiated osteoclasts in Hdac4fl/fl and Hdac4ob−/− mice (Fig. 6A). Quantitative analysis, osteoclast numbers (N.Oc/BS, n/mm) and osteoclast surface (Oc.S/BS, %) relative to bone surface were higher in Hdac4ob−/− mice compared to Hdac4fl/fl mice. Continuous PTH treatment increased these indices of osteoclastic function in both Hdac4fl/fl and Hdac4ob−/− groups (Fig. 6B, C). Serum CTX levels, a circulating bone resorption marker, were significantly increased in Hdac4ob−/− without PTH treatment compared with Hdac4fl/fl mice (Fig. 6D) and remained elevated with PTH treatment. Utilizing real time RT-qPCR, we observed that the expression levels of Mmp13 were higher in the PTH-treated wild-type mice than in the vehicle control (Fig. 6E). In Hdac4ob−/− mice, the expression tended to increase compared with vehicle-treated mice but did not achieve statistical significance. Interestingly, Mmp13 gene expression was lower in PTH-treated Hdac4ob−/− mice compared to PTH-treated Hdac4fl/fl mice. These results suggest that HDAC4 counteracts both basal and PTH-induced bone resorption. Nevertheless, HDAC4 is necessary for inducing Mmp13 gene expression by continuous PTH. It should be noted that RankL and Opg mRNA levels were not significantly different across the groups at the time the mice were killed.
Figure 6. Osteoclast activity is increased in Hdac4ob−/− mice.
(A) TRAP staining of tibiae of female 10-week-old Hdac4fl/fl and Hdac4ob−/− mice treated with PTH or vehicle is shown. 20X. (B) Quantification of osteoclast number and (C) osteoclast surface % in the animals in (A). Data shown as means ± SE. Different letters indicate p<0.05 vs. one another. (Hdac4 fl/fl; n=4, Hdac4 fl/fl with PTH; n=5, Hdac4ob−/−; n=−5, Hdac4ob−/−; with PTH; n=4). (D) Serum CTX levels (Hdac4 fl/fl; n=9, Hdac4 fl/fl with PTH; n=10, Hdac4ob−/−; n=10, Hdac4ob−/− with PTH; n=10), data shown as means ± SE and different letters indicate p<0.05 vs. one another. (E) The relative levels of Mmp13 mRNA normalized to β-actin (Hdac4 fl/fl; n=7, Hdac4 fl/fl with PTH; n=7, Hdac4ob−/−; n=6, Hdac4ob−/− with PTH; n=8). Total RNA was extracted from distal femurs of 10-week-old females and measured using real time RT-PCR. Data are shown as fold change to vehicle-treated Hdac4fl/fl and as means ± SE. Different letters indicate p<0.05 vs. one another.
HDAC4 exerts an anabolic effect
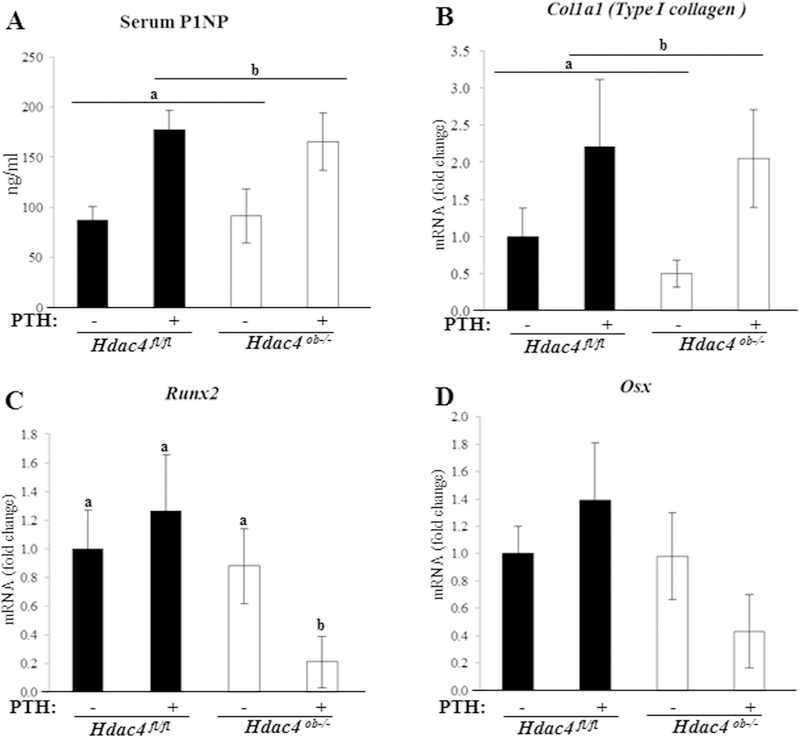
Next, to determine the role of HDAC4 in possible anabolic effects on bone, we investigated biomarkers of bone formation: serum P1NP and Col1a1 (Type I collagen) mRNA levels. As shown in Fig.7A, PTH-treated mice responded with a significant increase in serum P1NP levels compared to vehicle controls. Next, we observed Col1a1 was increased in the PTH-treated mice (Fig.7B). On the other hand, in Hdac4ob−/− mice, serum P1NP and Col1a1 (Type I collagen) levels were not changed compared to Hdac4fl/fl mice (Fig. 7A, B). Runx2 is an essential regulator for osteoblastic function, and regulates many osteoblastic genes, including Col1a1 and Mmp13.(18, 19) Interestingly, Runx2 expression did not change in Hdac4ob−/− mice, however it was decreased in the PTH-treated Hdac4ob−/− mice (Fig.7C). Osterix (OSX) acts downstream of RUNX2 during bone development, and interacts with RUNX2 to coordinately induce the expression of the Col1a1 gene.(20) Fig 7D demonstrated that Osx expression has a similar pattern to Runx2 although the differences were not significant. These results suggest that HDAC4 is required to maintain Runx2 expression after continuous PTH treatment. RUNX2 and OSX have been shown to regulate the SOST promoter in human cells.(21)
Figure 7. Bone formation markers were increased by PTH in Hdac4 fl/fl and Hdac4ob−/− mice while Runx2 decreased.
Ten-week-old female Hdac4 fl/fl and Hdac4 ob−/− mice were examined after treatment with PTH or vehicle. (A) Serum P1NP levels. n=10 in all groups. Data are shown as are means ± SE. (B) Type I collagen mRNA, (Hdac4 fl/fl; n=10, Hdac4 fl/fl with PTH; n=10, Hdac4ob−/−; n=10, Hdac4ob−/− with PTH; n=11), (C) Runx2 mRNA(Hdac4fl/fl; n=8, Hdac4fl/fl with PTH; n=10, Hdac4ob−/−; n=10, Hdac4ob−/− with PTH; n=11), (D) Osx mRNA(Hdac4fl/fl; n=8, Hdac4fl/fl with PTH; n=10, Hdac4ob−/−; n=10, Hdac4ob−/− with PTH; n=11), were measured using real time RT-PCR. The relative levels of mRNAs were normalized to β-actin. Data are shown as fold change to vehicle-treated Hdac4fl/fl and as means ± SE.
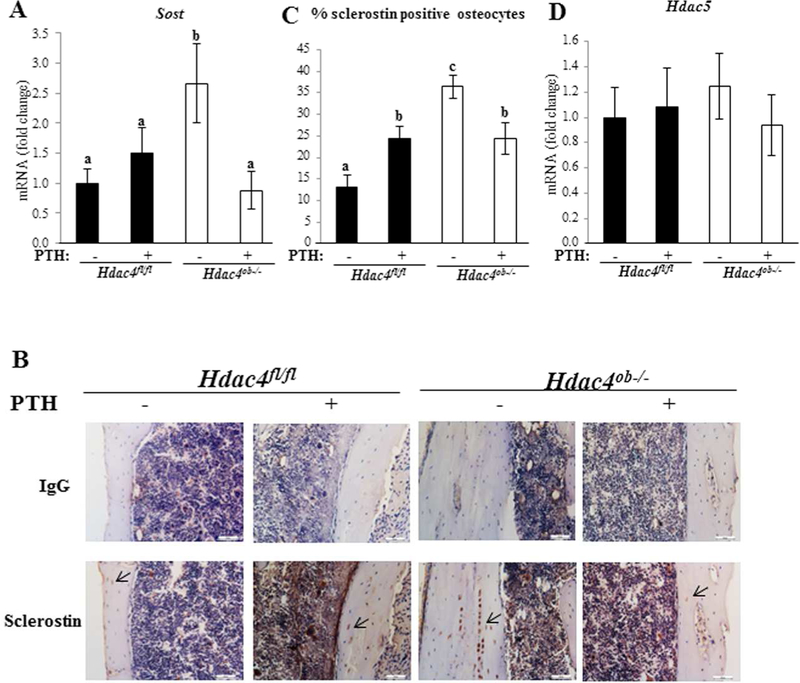
Continuous PTH treatment did not affect Sost mRNA levels in the Hdac4fl/fl mice (Fig 8a). In contrast, Sost mRNA was highly elevated in Hdac4ob−/− mice compared with Hdac4fl/fl mice. Surprisingly, deletion of HDAC4 in osteoblast lineage cells resulted in a significant inhibition of Sost expression by PTH. Sclerostin, a protein encoded by the Sost gene and expressed by osteocytes, inhibits osteoblastic bone formation.(12, 21) To confirm protein levels of sclerostin, we performed immunohistochemistry (IHC) and found that staining for sclerostin was stronger and the percentage of sclerostin-ositive osteocytes in Hdac4ob−/− mice was increased compared to Hdac4fl/fl mice and in these mice, PTH treatment caused a decrease in sclerostin staining (Fig. 8B, C). These results indicate that HDAC4 has an anabolic effect, likely at least in part via inhibition of Sost mRNA and sclerostin protein levels, and this is attenuated by continuous PTH in the absence of HDAC4. Since we have found that HDAC4 and HDAC5 co-immunoprecipitate (data not shown) and have been shown to co-regulate Sost in osteocytic cells(15), we analyzed Hdac5 expression but it did not change in Hdac4ob−/− mice with and without PTH (Fig. 8D).
Figure 8. Increased expression of Sost/sclerostin in Hdac4ob−/− mice Sost.
mRNA (A) was measured using real time RT-PCR. The relative levels of mRNAs were normalized to β-actin. Data are shown as means ± SE for –fold change to vehicle-treated Hdac4fl/fl. (Hdac4 fl/fl; n= 9, Hdac4 fl/fl with PTH; n= 9, Hdac4ob−/−; n= 9, Hdac4ob−/− with PTH; n=11). (B) Immunohistochemistry for sclerostin. Representative longitudinal sections of the cortical region of the tibiae. (C) Ratio of number of sclerostin-ositive osteocytes to total number of osteocytes were quantified in lacunae of tibial cortical bones using image J (Hdac4 fl/fl; n=4, Hdac4 fl/fl with PTH; n=4, Hdac4ob−/−; n=8, Hdac4ob−/− with PTH; n=5). Data shown as means ± SE. Different letters indicate p<0.05 vs. one another. Hdac5 mRNA (D) was measured using real time RT-PCR (Hdac4 fl/fl; n= 8, Hdac4 fl/fl with PTH; n= 8, Hdac4ob−/−; n= 8, Hdac4ob−/−; with PTH; n=7). Data are shown as fold change to vehicle-treated Hdac4fl/fl and as means ± SE.
Discussion
Here, we describe the conditional deletion of Hdac4 by using the Cre-loxP system to analyze the function of HDAC4 in differentiated mature osteoblasts and osteocytes and its role in PTH’s catabolic action on bone. Both continuous and intermittent PTH increase bone turnover in trabecular and cortical bones. However, chronic PTH elevation is associated with excess production of osteoclasts coupled to increased osteoblasts with a negative balance between bone formation and resorption, consequently resulting in a final net bone loss. Primary hyperparathyroidism and continuous PTH treatment cause cortical bone loss by enhancing endosteal resorption through stimulation of osteoclast formation and activity.(23, 24) PTH also stimulates both the resorption and the formation of trabecular bone(24, 25, 26, 27) and severe chronic elevation of PTH levels may lead to trabecular bone loss.(24) In agreement with previous studies, our data showed that continuous PTH induced both cortical and trabecular bone loss, and increased osteoclast number and activity but continuous PTH also stimulated bone formation markers, serum P1NP levels and Type I collagen mRNA.
Our µCT analyses showed that Hdac4ob−/− mice have significantly reduced cortical thickness and bone area, and no difference was seen in trabecular bone. Cortical bone, which represents more than 80% of skeletal mass, provides important mechanical support.(28, 29) Bone growth in width is achieved through periosteal apposition from the action of periosteal osteoblasts(30), and resorption at the endosteal surface. (31) The balance between periosteal bone apposition and endosteal bone resorption is important for advancing bone growth; producing greater cortical bone diameter and thickness. We suggest that HDAC4 in mature osteoblasts plays an important role in the maintenance of cortical bone. Interestingly, Hdac4ob−/− mice showed attenuated PTH catabolic actions in cortical bone, but not in trabecular bone. Elevated PTH levels in humans are associated with a lower bone mass at all skeletal sites, but particularly at sites containing predominantly cortical bone.(32) In fact, both hyperparathyroidism and continuous PTH treatment usually produce a modest increase in trabecular bone.(27, 33)
The findings described in this report demonstrate that HDAC4 in mature osteoblasts functions as a repressor of basal bone resorption but is required for the bone resorptive effects of continuous PTH. We also showed that HDAC4 contributes to blocking the antianabolic effect of Sost/sclerostin expression; on the other hand, continuous PTH is able to decrease Sost/sclerostin expression in the absence of HDAC4. It has been shown that Sost transcription is controlled mainly via its proximal promoter and distal enhancer (evolutionarily conserved region 5, ECR5).(34) RUNX2 and osterix (OSX) bind to the SOST promoter and have been shown to positively regulate SOST expression in human cells.(21) The distal enhancer, ECR5, is a 255-bp fragment within the 52kb VB region of the Sost gene. It has been shown that ECR5 is essential for Sost expression in mouse osteocytes(35) and Mef2c is involved in both basal and PTH-decreased Sost expression through the MEF2 binding site within the ECR5 element.(17, 35) Wein et al. have shown that HDAC5 binds to the Sost enhancer and inhibits the function of Mef2c in osteocytes.(15) The same researchers have reported that HDAC4/5 are required for PTH repression of Sost through effects on Mef2c binding to the Sost enhancer in osteocytes.(36) They showed that Sost mRNA was increased in mice lacking Hdac5, but not in mice with Hdac4 deletion from osteocytes using DMP1-Cre, but deletion of both Hdac4 and Hdac5 showed a greater increase than Hdac5 single knockout. The double knockout mice showed abolition of PTH-induced Sost-regulation. In addition, Baertschi et al. have shown that PTH leads to a rapid and strong nuclear localization of HDAC5 and an increase in nuclear HDAC4 in UMR 106 cells.(16) The decrease in Sost mRNA after PTH treatment in the Hdac4ob−/− mice may be due to unimpeded translocation of HDAC5 into nuclei which does not appear to occur in the wild type mice. Here, we showed that Sost/sclerostin expression was increased in mice with Hdac4 deletion from mature osteoblasts and this was attenuated by continuous PTH.
Col2.3-Cre is known to be active in the mature osteoblast lineage (osteoblasts and osteocytes).(37) Mef2c and sclerostin co-localize in osteoblastic cells, UMR 106 cells, and mouse osteocytes.(17) It has been reported that the SOST promoter is required for high levels of osteocyte specific expression, although ECR5 is sufficient to induce expression in osteocytes.(35) It seems that both promoter and enhancer are necessary for Sost gene regulation. Previously we reported in UMR 106–01 cells that, for the Mmp13 promoter, Runx2 associates with the RD site and c-Fos and c-Jun together with Mef2c associate with the AP-1 site. Under basal conditions, HDAC4 inhibits Mmp13 expression through interaction with Runx2, and PTH induces Mmp13 transcription by regulating the dissociation of HDAC4 from Runx2.(8, 38) HDAC5, Runx2 and Mef2c may be involved in the regulation of Sost expression in Hdac4ob−/− mice although Hdac5 and Mef2c expression did not significantly change in the Hdac4ob−/− mice with and without PTH. Notably, Runx2 expression was decreased in the Hdac4ob−/− mice after continuous PTH treatment suggesting that HDAC4 is required for maintaining Runx2 expression under these conditions. One reason for the decrease in Sost/sclerostin expression, and the lesser increase in Mmp13, in PTH-treated Hdac4ob−/− mice may be due to the reduction in Runx2.
Previous reports showed that Sost/sclerostin expression is suppressed by PTH.(13, 14, 17, 39) Our results demonstrate no suppression of Sost/sclerostin expression after continuous PTH treatment in wild-type mice. Perhaps this difference is due to the dose and duration of PTH. Many studies have observed this effect with anabolic PTH injections or in vitro. Others have shown suppression of Sost/sclerostin expression in rodent models with catabolic treatment with PTH (continuous treatment)(13, 14), but they examined acute effects of PTH. We used 80µg/kg/d for 14 days, whereas these studies used 1.5–7.5 times higher doses of PTH and Sost/sclerostin expression was examined 90 min, 4 hours or 24–96 hours after PTH treatment, while we examined expression at the end of the experiment after 14 days. Actually, it has been reported that intermittent PTH decreased Sost mRNA levels but the effect was transient.(13, 14) Intermittent PTH did not significantly affect Sost expression or sclerostin protein after 4 days. Continuous PTH decreased Sost expression around 40 h and after that, it tended to return to original levels.(14)
We found an interesting observation that mice lacking Hdac4 in mature osteoblasts had a shorter tail due to shorter caudal vertebrae (without affecting the caudal vertebrae numbers), with almost no growth plate, and caudal vertebral cortical bone mass and strength were decreased in Hdac4ob−/− mice. Interestingly, the phenotype of the tail takes place after 7 postnatal days (data not shown), suggesting it is due to postnatal, not embryologic development. This is the period when caudal vertebrae undergo endochondral ossification(40), so the transition to bone may be responsible for the aberrant intervertebral discs. Our data indicate that HDAC4 in the mature osteoblast lineage is necessary for the correct proliferation and differentiation of chondrocytes in the caudal vertebrae, and the effect is more severe than in lumbar vertebrae or long bones (data not shown). Further studies are needed to better understand the function of HDAC4 in the mature osteoblast lineage for caudal vertebrae and development of the intervertebral disc.
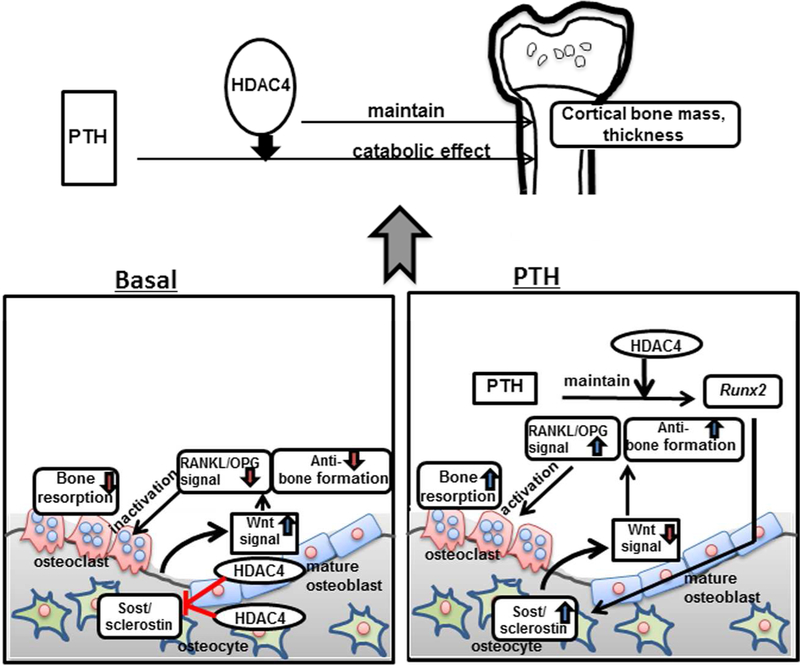
In conclusion, the results of the present report demonstrate that HDAC4 in the mature osteoblast lineage influences cortical bone mass and thickness and is necessary for the catabolic effect of PTH in cortical bone. HDAC4 inhibits bone resorption and also may have an anabolic effect via inhibiting Sost/sclerostin expression (Fig. 9).
Figure 9. Proposed model depicting the functions of HDAC4 in the mature osteoblast and osteocyte.
Under basal conditions, HDAC4 in mature osteoblasts and osteocytes inhibits bone resorption and also has an anabolic effect via inhibiting Sost gene and sclerostin protein expression. After continuous PTH treatment, HDAC4 is necessary for maintaining Runx2 expression. Since Runx2 contributes to Sost gene transcription, continuous PTH decreases Sost and sclerostin in the absence of HDAC4. This leads to a combined increase in bone formation and no resorptive effect of PTH in the absence of HDAC4. These actions indicate the function of HDAC4 in maintaining cortical bone mass and its role in the catabolic effects of PTH on cortical bone.
Supplementary Material
Acknowledgements:
We thank Dr. Eric Olson for kindly giving us the HDAC4 floxed mice (Hdac4fl/fl). We thank Dr. Malvin Janal for advice on statistical analyses.
Financial support:
This work was supported by NIH grant R01DK4720 to Dr. Nicola C. Partridge.
Footnotes
Disclosure Statement: The authors state that they have no conflicts of interest pertaining to the work described.
REFERENCES
- 1.Silva BC, Costa AG, Cusano NE, Kousteni S, Bilezikian JP. Catabolic and anabolic actions of parathyroid hormone on the skeleton. J Endocrinol Invest 2011;34:801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao W, Byrne MH, Boyce BF, Krane SM. Bone resorption induced by parathyroid hormone is strikingly diminished in collagenase-resistant mutant mice. J Clin Invest 1999;103:517–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGee-Lawrence ME, Westendorf JJ. Histone deacetylases in skeletal development and bone mass maintenance. Gene 2011;474:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vega RB, Matsuda K, Oh J, Barbosa AC, Yang X, Meadows E, McAnally J, Pomajzl C, Shelton J, Richardson JA, Karsenty G, and Olson EN. Histone deacetylase 4 controls chondrocyte hypertrophy during skeletogenesis. Cell 2004;119: 555–66. [DOI] [PubMed] [Google Scholar]
- 5.Arnold MA,Yim K, Czubryt MP, Phan D, McAnally J, Qi X, Shelton JM, Richardson JA,, Bassel-Duby R, and Olson EN. MEF2C transcription factor controls chondrocyte hypertrophy and bone development. Dev Cell 2007;12:377–89. [DOI] [PubMed] [Google Scholar]
- 6.Jeon EJ, Lee KY, Coi NS, Jin YH, Ryoo HM, Choi JY, Yoshida M, Nishino N, Oh BC, Lee KS, LeeYH, Bae SC. Bone morphogenetic protein-2 stimulates Runx2 acetylation. J Biol Chem 2006;281:16502–11. [DOI] [PubMed] [Google Scholar]
- 7.Nakatani T, Chen T, Partridge NC. MMP-13 is one of the critical mediators of the effect of HDAC4 deletion on the skeleton. Bone 2016;90:142–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimizu E, Selvamurugan N, Westendorf JJ, Olson EN, Partridge NC. HDAC4 represses matrix metalloproteinase-13 transcription in osteoblastic cells, and parathyroid hormone controls this repression. J Biol Chem 2010;285:9616–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakatani T, Partridge NC. MEF2C interacts with c-FOS in PTH-stimulated Mmp13 gene expression in osteoblastic cells. Endocrinology 2017;158:3778–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Obri A, Makinistoglu MP, Zhang H, Karsenty G. HDAC4 integrates PTH and sympathetic signaling in osteoblasts. J Cell Biol 2014; 205:771–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winkler DG, Sutherland MK, Geoghegan JC, Yu C, Hayes T, Skonier JE, Shpektor D, M Jonas M, Kovacevich BR, Staehling‐Hampton K, Appleby M, Brunkow ME, Latham JA. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J 2003;22:6267–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Bezooijen RL, Roelen BA, Visser A, van der Wee-Pals L, de Wilt E, Karperien M, Hamersma H, Papapoulos SE, ten Dijke P, Löwik CW. Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J Exp Med 2004;199:805–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keller H, Kneissel M. SOST is a target gene for PTH in bone. Bone 2005;37:148–58. [DOI] [PubMed] [Google Scholar]
- 14.Bellido T, Ali AA, Gubrij I, Plotkin LI, Fu Q, O’Brien CA, Manolagas SC, Jilka RL. Chronic elevation of parathyroid hormone in mice reduces expression of sclerostin by osteocytes: a novel mechanism for hormonal control of osteoblastogenesis. Endocrinology 2005;146:4577–83. [DOI] [PubMed] [Google Scholar]
- 15.Wein MN, Spatz J, Nishimori S, Doench J, Root D, Babij P, Nagano K, Baron R, Brooks D, Bouxsein M, Pajevic PD, Kronenberg HM. HDAC5 controls MEF2C-driven sclerostin expression in osteocytes. J Bone Miner Res 2015;30:400–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baertschi S, Baur N, Lueders-Lefevre V, Voshol J, Keller H. Class I and IIa histone deacetylases have opposite effects on sclerostin gene regulation. J Biol Chem 2014;289:24995–5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leupin O, Kramer I, Collette NM, Loots GG, Natt F, Kneissel M, Keller H. Control of the SOST bone enhancer by PTH using MEF2 transcription factors. J Bone Miner Res. 2007;22:1957–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kern B, Shen J, Sterbuck M, and Karsenty G. Cbfa1 contributes to the osteoblast-specific expression of type I collagen genes. J. Biol. Chem 2001;276:7101–07. [DOI] [PubMed] [Google Scholar]
- 19.Selvamurugan N, Jefcoat SC, Kwok S, Kowalewski R, Tamasi JA, Partridge NC. Overexpression of Runx2 directed by the matrix metalloproteinase-13 promoter containing the AP-1 and Runx/RD/Cbfa sites alters bone remodeling in vivo. J. Cell Biochem 2006;99:545–57. [DOI] [PubMed] [Google Scholar]
- 20.Ortuño MJ, Susperregui A, Artigas N, Rosa JL, Ventura F. Osterix induces Col1a1 gene expression through binding to Sp1 sites in the bone enhancer and proximal promoter regions. Bone 2013;52:548–56. [DOI] [PubMed] [Google Scholar]
- 21.Pérez-Campo FM, Santurtún A, García-Ibarbia C, Pascual MA, Valero C, Garcés C, Sañudo C, Zarrabeitia MT, Riancho JA. Osterix and RUNX2 are transcriptional regulators of sclerostin in human bone. Calcif Tissue Int 2016;99:302–9. [DOI] [PubMed] [Google Scholar]
- 22.Poole KE, Reeve J. Parathyroid hormone - a bone anabolic and catabolic agent. Curr Opin Pharmacol 2005;5:612–7. [DOI] [PubMed] [Google Scholar]
- 23.Lotinun S, Evans GL, Bronk JT, Bolander ME, Wronski TJ, Ritman EL, Turner RT. Continuous parathyroid hormone induces cortical porosity in the rat: effects on bone turnover and mechanical properties. J Bone Miner Res 2004;19:1165–71. [DOI] [PubMed] [Google Scholar]
- 24.Iida-Klein A, Lu SS, Kapadia R, Burkhart M, Moreno A, Dempster DW, Lindsay R. Short-term continuous infusion of human parathyroid hormone 1–34 fragment is catabolic with decreased trabecular connectivity density accompanied by hypercalcemia in C57BL/J6 mice. J Endocrinol 2005;186:549–57. [DOI] [PubMed] [Google Scholar]
- 25.Dempster DW, Parisien M, Silverberg SJ, Liang XG, Schnitzer M, Shen V, Shane E, Kimmel DB, Recker R, Lindsay R, Bilezikian JP. On the mechanism of cancellous bone preservation in postmenopausal women with mild primary hyperparathyroidism. J Clin Endocrinol Metab 1999;84:1562–66. [DOI] [PubMed] [Google Scholar]
- 26.Qin L, Raggatt LJ, Partridge NC. Parathyroid hormone: a double-edged sword for bone metabolism. Trends Endocrinol Metab 2004;15:60–5. [DOI] [PubMed] [Google Scholar]
- 27.Zhou H, Shen V, Dempster DW, Lindsay R. Continuous parathyroid hormone and estrogen administration increases vertebral cancellous bone volume and cortical width in the estrogen-deficient rat. J Bone Miner Res 2001;16:1300–7. [DOI] [PubMed] [Google Scholar]
- 28.Zebaze RM, Ghasem-Zadeh A, Bohte A, Iuliano-Burns S, Mirams M, Price RI, Mackie EJ, Seeman E. Intracortical remodelling and porosity in the distal radius and post-mortem femurs of women: a cross-sectional study. Lancet 2010;375:1729–36. [DOI] [PubMed] [Google Scholar]
- 29.Seeman E Periosteal bone formation — a neglected determinant of bone strength. N Engl J Med 2003;349:320–23. [DOI] [PubMed] [Google Scholar]
- 30.Rauch F Bone growth in length and width: The Yin and Yang of bone stability. J Musculoskelet Neuronal Interact 2005;5:194–201. [PubMed] [Google Scholar]
- 31.Seeman E Structural basis of growth-related gain and age-related loss of bone strength. Rheumatology 2008;47:iv2–iv8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wishart J, Horowitz M, Need A, Nordin BE. Relationship between forearm and vertebral mineral density in postmenopausal women with primary hyperparathyroidism. Arch. Intern. Med 1990;150:1329–31. [PubMed] [Google Scholar]
- 33.Parisien M Silverberg SJ, Shane E, de la Cruz L, Lindsay R, Bilezikian JP, Dempster DW. The histomorphometry of bone in primary hyperparathyroidism: preservation of cancellous bone structure. J. Clin. Endocrinol. Metab 1990;70:930–8. [DOI] [PubMed] [Google Scholar]
- 34.Loots GG, Kneissel M, Keller H, Baptist M, Chang J, Collette NM, Ovcharenko D, Plajzer-Frick I, and Rubin EM. Genomic deletion of a long-range bone enhancer misregulates sclerostin in Van Buchem disease. Genome Res 2005;15:928–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Collette NM, Genetos DC, Economides AN, Xie L, Shahnazari M, Yao W, Lane NE, Harland RM, Loots GG. Targeted deletion of Sost distal enhancer increases bone formation and bone mass. Proc Natl Acad Sci U S A 2012;109:14092–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wein MN, Liang Y, Goransson O, Sunberg TB, Wang J, Williams EA, O’Meara MJ, Govea N, Beqo B, Nishimori S, Nagano K, Brooks DJ, Martins JS, Corbin B, Anselmo A, Sadreyev R, Wu JY, Sakamoto K, Foretz M, Xavier RJ, Baron R, Bouxsein ML, Gardella TJ, Divieti-Pajevic P, Gray NS, Kronenberg HM. SIKs control osteocyte responses to parathyroid hormone. Nat Commun 2016. October 19;7:13176. doi: 10.1038/ncomms13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu F, Woitge HW, Braut A, Kronenberg MS, Lichtler AC, Mina M, Kream BE. Expression and activity of osteoblast-targeted Cre recombinase transgenes in murine skeletal tissues. Int. J. Dev. Biol 2004;48:645–53. [DOI] [PubMed] [Google Scholar]
- 38.Selvamurugan N, Chou WY, Pearman AT, Pulumati MR, Partridge NC. Parathyroid hormone regulates the rat collagenase-3 promoter in osteoblastic cells through the cooperative interaction of the activator protein-1 site and the runt domain binding sequence. J Biol Chem 1998;273:10647–57. [DOI] [PubMed] [Google Scholar]
- 39.Bellido T, Saini V, Pajevic PD. Effects of PTH on osteocyte function. Bone 2013;54:250–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hankenson FC, Garzel LM, Fischer DD, Nolan B, Hankenson KD. Evaluation of tail biopsy collection in laboratory mice (Mus musculus): vertebral ossification, DNA quantity, and acute behavioral responses. J Am Assoc Lab Anim Sci 2008;28:10–18. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.