Abstract
Tissue regeneration is a process by which the remaining cells of an injured organ regrow to offset the missed cells. This field is relatively a new discipline that has been a focus of intense research by clinicians, surgeons, and scientists for decades. It constitutes the cornerstone of tissue engineering, creation of artificial organs, and generation and utilization of therapeutic stem cells to undergo transformation to different types of mature cells. Many medical experts, scientists, biologists, and bioengineers have dedicated their efforts to deeply comprehend the process of liver regeneration, striving for harnessing it to invent new therapies for liver failure. Liver regeneration after partial hepatectomy in rodents has been extensively studied by researchers for many years. It is divided into three important distinctive phases including (a) Initiation or priming phase which includes an overexpression of specific genes to prepare the liver cells for replication, (b) Proliferation phase in which the liver cells undergo a series of cycles of cell division and expansion and finally, (c) termination phase which acts as brake to stop the regenerative process and prevent the liver tissue overgrowth. These events are well controlled by cytokines, growth factors, and signaling pathways. In this review, we describe the function, embryology, and anatomy of human liver, discuss the molecular basis of liver regeneration, elucidate the hepatocyte and cholangiocyte lineages mediating this process, explain the role of hepatic progenitor cells and elaborate the developmental signaling pathways and regulatory molecules required to procure a complete restoration of hepatic lobule.
This article is categorized under:
Adult Stem Cells, Tissue Renewal, and Regeneration > Regeneration Signaling Pathways > Global Signaling Mechanisms, Gene Expression and Transcriptional Hierarchies > Cellular Differentiation
Keywords: hepatic progenitor cell, liver regeneration, partial hepatectomy
1 ∣. INTRODUCTION
Liver regeneration is one of the most captivating phenomena in medicine that has fascinated clinicians, surgeons, and scientists who have observed this apparently supernatural process and studied its mechanisms for many years. The liver is the largest internal organ and possesses multiple substantial functions in the human body. It plays an important role in the homeostasis of carbohydrate, protein, and lipid metabolism. It is responsible for synthesis and storage of glycogen from glucose through glycogenesis. This glycogen is utilized by the liver, when needed, to secrete glucose into the blood via a process called glycogenolysis. Also, the liver can convert amino acids, lactate, fatty acids, and glycerol into glucose via the gluconeogenesis pathway. As regards the protein metabolism, the liver produces a large number of proteins, especially, albumin which maintains fluid in the circulation, iron-binding plasma glycoprotein known as transferrin, copper-carrying protein called ceruloplasmin, acute phase proteins that indicate inflammation, blood coagulation factors including I (fibrinogen), II (prothrombin), V, VII, VIII, IX, X, XI, XIII, as well as protein C, protein S, and antithrombin. It also exhibits endocrinal function by secreting insulin-like growth factor that mediates growth-promoting effects of growth hormone, hepcidin which regulates the hemoglobin production, and thrombopoietin that stimulates the platelet production, and exocrine features through the formation of bile acid required for the emulsification of fats particularly the fat-soluble vitamins (A, K, E, and D) to facilitate their digestion and absorption in the gut. In addition to that, the liver is essential in lipid metabolism because it performs cholesterol synthesis, lipogenesis to produce triglycerides, and formation of lipoproteins that act as transport carriers for fatty acids and steroid hormones. Importantly, the liver is a fundamental detoxifying organ in the body as it gets rid of toxic wastes coming from internal sources such as metabolism of nutrients and hormones, and external sources like medications, alcohol, air pollution, and other factors, by neutralizing them into nontoxic metabolites via cytochrome p450 enzymes and then converting them into water-soluble products that can be excreted in the bile, urine, and stool. Moreover, the liver carries out other vital tasks such as immunological clearance of the blood from pathogens by the mononuclear phagocytic system represented by Kupffer cells (KCs) which are specialized macrophages lining the walls of liver sinusoids, regulation of the blood pressure by angiotensinogen production, storage of copper, vitamins like vitamin A for vision, vitamin D for calcium homeostasis, vitamin K for proper blood clotting, and other substances needed for erythropoiesis such as iron, folic acid, and vitamin B12 (Elaine & Marieb, 2012; Jelkmann, 2001; Kmiec, 2001).
The aim of this comprehensive review is to describe the embryology and anatomy of the liver, discuss the molecular basis of liver regeneration, elucidate the hepatocyte and cholangiocyte lineages mediating this process, and elaborate the developmental signaling pathways and regulatory substances required to procure a complete restoration of hepatic lobules following liver injury.
2 ∣. LIVER MICROSCOPIC ANATOMY
As the largest internal organ in the human body, the adult liver weighs about 1.5 kg and is located in the right upper abdomen. The upper surface of the liver is bulging, facing the diaphragm, so it is called the facies diaphragmatica. The liver is divided grossly into left and right lobes by the falciform ligament; the right lobe is large and thick, and the left lobe is small and thin. Surgically, the liver is divided into eight segments by divisions of the right and left portal vein and three (left/middle/right) outflow hepatic veins. The lower surface of the liver faces the lower left side, adjacent to some important organs in the abdominal cavity, so it is called the visceral surface, and the gallbladder is also attached to the visceral surface of the liver. In the center of the visceral surface, there is a transverse sulcus called the hilus hepatis, which is an important site for the hepatic artery, portal vein, common hepatic duct, lymphatic vessels, and nerves to enter the liver.
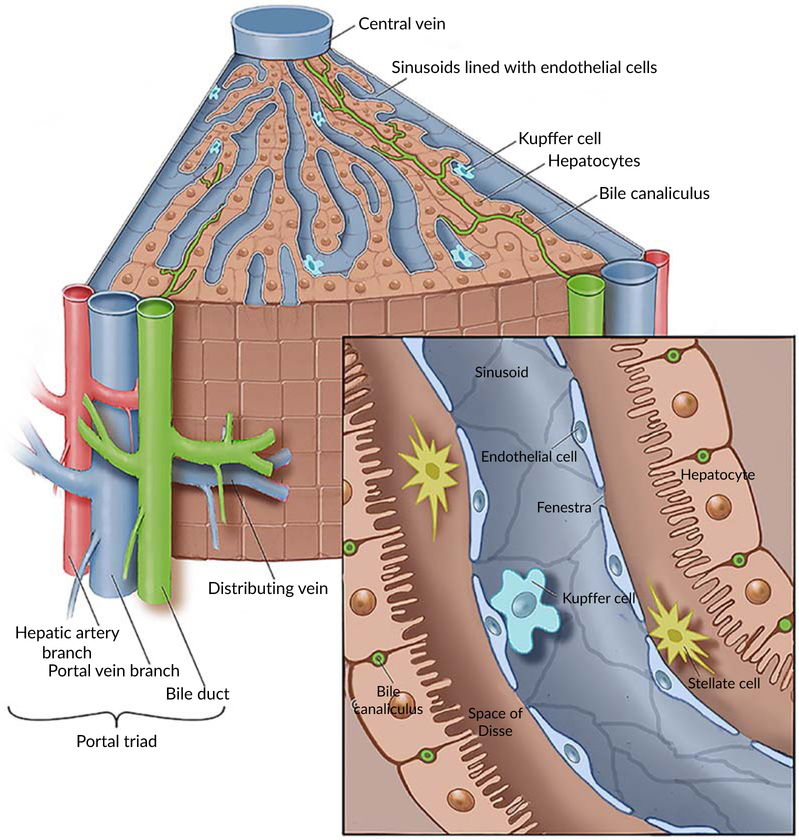
As shown in Figure 1, the liver is made up of 1–1.5 million basic structural units—hepatic lobules (Malarkey, Johnson, Ryan, Boorman, & Maronpot, 2005). The hepatic lobule is hexagonal columnar structure, with a central vein of lobule in the center and portal triad at the periphery. The portal triad (also called portal tract) is comprised of three important structures: a branch of the proper hepatic artery, hepatic portal venule, and intrahepatic bile duct (IHBD). The central vein is surrounded by hepatocyte cords (hepatic cords) arranged radially. The hepatic sinusoids are located in between the hepatic cords. The inner surface of the hepatic sinusoid is colonized with endothelial cells and KCs. The latter is a specific liver macrophages and part of the mononuclear phagocyte system. They can remove foreign particles and senescent erythrocytes in the blood by phagocytosis. In between the sinusoid and hepatocyte, there is a space called per-sinusoidal space which contains another type of cells known as hepatic stellate cells (HSCs). There are bile canaliculi between adjacent hepatocytes which are merged into the canals of Hering, where hepatic progenitor stem cells (HPSCs) are settled, and then merged into interlobular biliary canals (Saxena & Theise, 2004). The interlobular biliary canals pool as step by step into left and right hepatic ducts, and finally assembled into the common hepatic duct. The common hepatic duct and the cystic duct are merged into the common bile duct, and bile flows from the common bile duct into the duodenum. The portal vein and the hepatic artery are branched step by step after entering the liver, and finally connected with the hepatic sinusoids. The hepatic sinusoid is the place where the exchange of substance happens between hepatocytes and blood. The blood in the hepatic sinusoid flows into the hepatic vein through the central vein of the lobule, and finally into the inferior vena cava by the left, middle, and right hepatic veins at the second hepatic portal (Elaine & Marieb, 2012; Malarkey et al., 2005).
FIGURE 1.
Microscopic anatomy of human liver
3 ∣. DEVELOPMENTAL EMBRYOLOGY OF THE HUMAN LIVER
3.1 ∣. Trilaminar germ disc formation
Liver regeneration would be incompletely understood without describing the developmental embryology of the human liver. By the end of the first week after fertilization, the zygote undergoes mitotic cell divisions forming a blastocyst which contains an outer cell mass called trophoblast and an inner cell mass called embryoblast. By the end of the second week, the inner mass differentiates into the bilaminar germ disc which in turn undergoes a specific transformation into trilaminar germ disc consisting of three primordial germ cell layers: ectoderm, mesoderm, and endoderm by the end of the third week.
3.2 ∣. Foregut development
The endodermal lining of the yolk sac gives rise to the primitive gut tube which is further subdivided into three main structures: foregut, midgut, and hindgut (Zorn, 2008). The development of these derivatives is regulated through Hedgehog pathway signals that are transmitted by endodermal cells to stimulate the expression of Homeotic (Hox) genes in the mesoderm producing Hox proteins that control the primitive gut differentiation. These derivatives can also be recognized by the genetic expression of specific transcription factors such as Hematopoietically expressed homeobox protein (HHex) in the foregut, Pancreatic, and duodenal homeobox 1 (Pdx1) in the midgut and Caudal genes (Cdx) in the hindgut (Zorn, 2008). Furthermore, it has been revealed that foregut hepatic endodermal cells must express additional transcription factors such as GATA-4 and Forkhead box protein A1 and 2 (FoxA1 and 2) (previously known as HNF3a and β) for the initiation of hepatogenesis (Zorn, 2008).
3.3 ∣. Establishment of the liver bud
During the fourth week of human gestation, the mesodermal layer results in the formation of the mesenchyme which is an embryonal connective tissue containing mesenchymal cells and reticular fibers. The cranial part of the mesenchyme develops into septum transversum which arises at the site of the embryonic junction between the endoderm of the yolk sac and the ectoderm of the amnion externally, and foregut and midgut internally (Hill, 2018).
Establishment of foregut liver progenitor cells is essential for liver bud development and occurs through a process called hepatic specification. In this stage, foregut hepatic endodermal cells that express FoxA and GATA genes are triggered by specific inductive signals including fibroblast growth factor (FGF) family signals from the developing heart and bone morphogenetic protein bone morphogenetic protein (BMP) signals from the septum transversum, leading to the production of FoxA and GATA transcription factors that stimulate the expression of liver-specific genes such as albumin, alpha-fetoprotein (AFP), hepatocyte nuclear factor 4 alpha (Hnf4α), cytokeratin-19 (CK-19), and eventually the differentiation into hepatoblasts (Bossard & Zaret, 1998, 2000; Zorn, 2008). Shortly after hepatic specification, the differentiating cells thicken as they transform from a simple columnar to a pseudostratified columnar epithelium, thus forming the liver diverticulum which appears as an out-pocket thickening of the ventral floor of the distal foregut endoderm, embedded in the septum transversum and surrounded by a basement membrane and endothelial cell precursors. It is known as the first morphological sign of the embryonic liver development (Bort, Signore, Tremblay, Martinez Barbera, & Zaret, 2006; Margagliotti et al., 2007; Medlock & Haar, 1983).
3.4 ∣. Hepatic bud expansion
During the fifth week of human gestation, the hepatic bud undergoes cellular expansion and becomes the main site of fetal hematopoiesis. Multiple transcription and growth factors have been implicated in the hepatoblast proliferation such as mesenchymal factors like FGF (Berg et al., 2007), hepatocyte growth factor (HGF) (Defrances, Wolf, Michalopoulos, & Zarnegar, 1992), transforming growth factor-beta (TGF-β) (Pelton, Saxena, Jones, Moses, & Gold, 1991), BMP (Berg et al., 2007; Calmont et al., 2006), Wilms tumor protein 1 (Ijpenberg et al., 2007), and hepatoblast factors including c-jun (Eferl et al., 1999), Phosphoinositide 3-kinase (Pi3k) (Fruman et al., 2000), Raf1 (Mikula et al., 2001), Smad2/3 (Weinstein et al., 2001), β-catenin (McLin, Rankin, & Zorn, n.d.; Micsenyi et al., 2004), ETS domain transcription factor (Tang et al., 2003), ADP-ribosylation factor 6 (Arf6) (Suzuki et al., 2006).
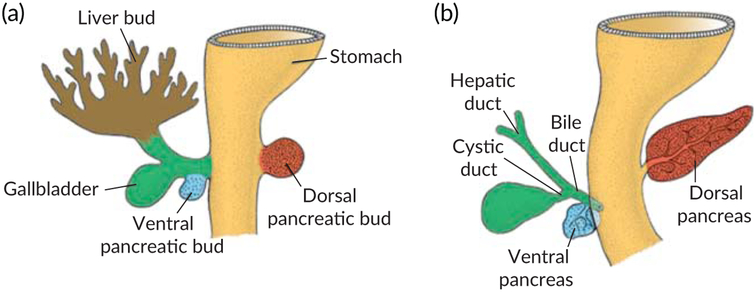
During the growth phase, the hepatoblasts in the hepatic bud invade the caudal part of the septum transversum forming interlacing cords within it. As a result, the bud is split into two main divisions: the larger cranial part known as the liver primordium or pars hepatica which is responsible for the development of liver parenchyma, intrahepatic biliary ducts, hepatic sinusoids, and the smaller caudal part termed as pars cystica which forms the gallbladder and the cystic duct (Bossard & Zaret, 1998; Zorn, 2008) (Figures 2 and 3).
FIGURE 2.
(a) The primitive gut differentiation and hepatic bud development by the endodermal part of foregut. (b) The penetration of hepatic cords into the septum transversum
FIGURE 3.
(a) The development of pars hepatica and pars cystica. (b) Pars hepatica gives rise to the hepatic duct and liver parenchyma whereas pars cystica gives rise to cystic duct and gall bladder
This invasion process is characterized by breaking down of the basal lamina, delamination, and migration of hepatoblasts into the septum transversum. It is regulated by several transcription factors expressed in the hepatoblasts such as HHex, GATA, Prospero homeobox protein 1 (Burke & Oliver, 2002; Sosa-Pineda, Wigle, & Oliver, 2000), One cut homeobox 1 (Onecut-1, also known as Hnf6), and Onecut-2 (OC-2) (Margagliotti et al., 2007). Additionally, some studies have shown that this process is also induced by the expression of other factors including vascular endothelial growth factor receptor gene (VEGFR-2, also known as Flk-1) in the endothelial precursors (Matsumoto, Yoshitomi, Rossant, & Zaret, 2001), and extracellular matrix (ECM) remodeling enzymes such as matrix metalloproteinases (MMPs) (Margagliotti et al., 2008).
3.5 ∣. Differentiation of hepatoblasts
The epithelial cellular structure consists of biliary epithelial cells (cholangiocytes) and hepatocytes. The hepatocytes are responsible for metabolic, synthetic, detoxifying, and excretory function. While cholangiocytes account for establishment of bile ducts which transport the bile into the small intestine.
Differentiation of hepatoblasts into hepatocytes and cholangiocytes starts at ninth week of human gestation and persists through 6 months of age approximately (Suchy, Sokol, & Balistreri, 2014). Initially, the hepatoblasts express multiple liver-specific genes which are paramount for liver development. These include hepatocyte-associated genes such as Hnf4 α, Hnf1 α, Albumin, C/EBPα, FoxA and GATA, and cholangiocyte-specific factors like cytokeratin-19, OC1, OC2, and HNF1β (Suchy et al., 2014; Zorn, 2008).
The differentiation process starts at the hilum and continues toward the periphery. It has been shown that this phase is comprised of two steps.
The first step refers to hepato-biliary lineage segregation in which the hepatoblasts are subdivided into immature hepatocytes and immature cholangiocytes. This process is completely regulated by extracellular signals especially those that are derived from the periportal mesenchyme including TGFβ and Wnt/β-catenin (Clotman & Lemaigre, 2006; Decaens et al., 2008; Hussain et al., 2004; Tan et al., 2008; Weinstein et al., 2001). These factors promote the cholangiocyte differentiation by upregulating cholangiocyte-specific genes and suppressing the expression of hepatogenic factors in the hepatoblasts. There is evidence that adjacent periportal heptoblasts are more exposed to these periportal mesenchyme signals than distant parenchymal hepatoblasts. Therefore, the periportal hepatoblasts will be differentiated into cholangiocytes while parenchymal hepatoblasts will upregulate the expression of pro-hepatic factors along with inhibition of cholangiocyte-specific factors leading to their differentiation into hepatocytes (Shiojiri, Takeshita, Yamasaki, & Iwata, 2004; Suzuki, Sekiya, Buscher, Izpisua Belmonte, & Taniguchi, 2008; Tanimizu & Miyajima, 2004; Yamasaki et al., 2006).
The second step is related to hepatocyte and cholangiocyte maturation. For hepatocyte maturation, the hematopoietic cells in the liver produce the cytokine oncostatin M (OSM), alongside with parenchymal mesenchyme factors (HGF and Wnt), continue the activation of hepatocyte-specific transcription factors leading to the development of full mature hepatocytes (Cheng et al., 2006; Kamiya, Kinoshita, & Miyajima, 2001; Kyrmizi et al., 2006; Matsui et al., 2002; Michalopoulos, Bowen, Mule, & Luo, 2003).
In differentiating cholangiocytes, recent analyses have stated that the JAGGED/NOTCH signaling pathway plays a major role in cholangiocyte maturation as well as a proper bile duct morphogenesis (Kodama, Hijikata, Kageyama, Shimotohno, & Chiba, 2004; Loomes et al., 2007; Lozier, McCright, & Gridley, 2008).
This signaling pathway involves the expression of JAGGED ligands in the periportal mesenchyme, and then these interact with their NOTCH receptors on periportal cholangiocytes resulting in NOTCH activation and cholangiocyte maturation. Unlike other secreted factors in hepatogenesis, the proteins of this pathway are integral membrane proteins which necessitate direct cell to cell contact for their action (Suchy et al., 2014; Zorn, 2008).
3.6 ∣. IHBD development
IHBD development is mainly controlled by JAGGED/NOTCH signaling pathway in addition to SOX9 transcription factor. Their importance in biliary differentiation is supported by multiple mouse studies revealing that these factors are involved in a process known as ductal plate remodeling which includes tubulogenesis and apoptosis (Lemaigre, 2003; Sergi, Kahl, & Otto, 2000). This process starts after converting periportal hepatoblasts into immature cholangiocytes which will form around the portal mesenchyme a single-layered structure called the ductal plate. It contains cuboidal cells with increased immunoreactivity for cytokeratin-19 compared to liver parenchymal cells. After that, these cells will proliferate to make a double-layered structure where focal dilations start to appear. These dilations become surrounded by the portal mesenchyme to develop IHBD. The ductal plate cells forming these dilations will give rise to cholangiocytes lining the IHBD while the remaining bilayer cells undergo degeneration (Hassan, Sliem, & Ellethy, 2013; Suchy et al., 2014).
4 ∣. HISTORY AND DEFINITION OF LIVER REGENERATION
The regenerative capacity of the liver dates back to the ancient Greek myth of Prometheus, a titan culture hero, who stole the fire from Zeus, the king of Olympian gods, to give it to humanity. The titan was punished by Zeus who chained him to the rock and sent his eagle everyday to feed on his liver. Amazingly, the liver regrew to its original volume and so it can be eaten again the next day (Michalopoulos & DeFrances, 1997; Rychtrmoc, Libra, Buncek, Garnol, & Cervinkova, 2009). Furthermore, in the 19th century, liver regeneration is defined and described for the first time when a restoration of the liver tissue was noticed after removing a small part of the liver (Milne, 1909).
In the biological field, the liver is the only visceral organ that shows the ability to regenerate after partial resection and chemical injury. This beautiful feature is technically described as a compensatory hypertrophy (increase in cell size) followed by hyperplasia (increase in cell number) of remaining hepatocytes to compensate for the lost tissue, restore the full liver size, and meet the metabolic needs of the organism without regaining its normal anatomical gross shape. It is also important to state that this process is neither a new creation of the missed tissue nor a true anatomical regeneration because it does not follow the steps of the true regenerative process particularly the blastema formation. Therefore, this phenomenon should be termed as compensatory liver hyperplasia rather than liver regeneration. However, we use the term “liver regeneration” in this review article as it is significantly mentioned in the scholarly literature (Fausto, Campbell, & Riehle, 2006; Mao, Glorioso, & Nyberg, 2014).
5 ∣. LIVER REGENERATION MODELS
Multiple animal models have been studied to describe and investigate the liver regeneration process. These proposed models are:
5.1 ∣. Surgical Partial hepatectomy model:
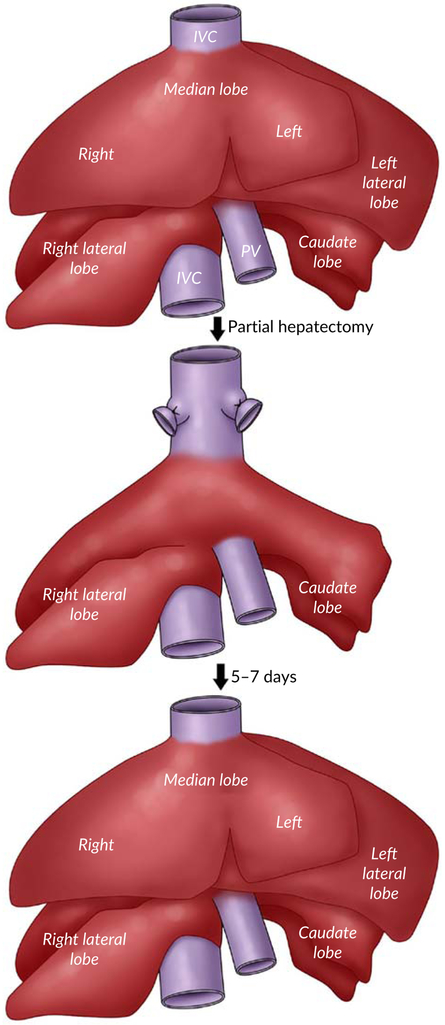
Induction of liver regeneration by two-third hepatectomy is the most common experimental rodent model used to study this outstanding phenomenon. It has been reported for the first time by (Higgins et al in 1931) (Higgins & Anderson, 1931) and studied for many decades, providing us with most of the knowledge about liver regeneration. As illustrated in Figure 4, the rodent partial hepatectomy (PH) model involves a surgical removal of the left lateral, left medial and right medial lobes leaving the right lateral and caudate lobes, which is equal to 66% decrease in the liver size. Following resection, the remaining hepatic tissue proliferates and expands in size to retain the original mass of five lobes within 5–7 days. The peak proliferation time is after 24 hrs in rat whereas, in mice, it is between 36 and 48 hrs (Mao et al., 2014; Michalopoulos, 2007).
FIGURE 4.
Partial hepatectomy in rodent liver
This model is the most preferred and classic model due to the following reasons: (a) The ability of the surgical operator to resect with the high level of accuracy because of the uniformity of the rodent’s liver anatomy. (b) This procedure is a simple operation that does not require an advanced surgical technique. (c) It is well tolerated in rodents without any significant perioperative mortality. (d) It is not associated with any histological damage or injury to the residual liver tissue. (e) The accuracy of timing of the sequence of subsequent events that can be observed from the first 5 min to 5–7 days. This evidence allows PH model to be the best choice for many researchers (Mao et al., 2014; Michalopoulos, 2007; Palmes & Spiegel, 2004; Pritchard & Apte, 2015).
5.2 ∣. Chemical-induced hepatotoxic injury or pharmacological models
Beside the surgical PH model, the liver has the capability to regenerate after a chemical-induced injury. In contrast to PH model, the pharmacological model is easier to be executed with a greater clinical relevance as it induces a necrotic injury that simulates certain liver diseases. These properties made this model an acceptable option to study liver regeneration. The main drawback of this model is the poor liver reproducibility and standardizability, in addition to the difficulty to expect the degree of liver injury and regeneration and avoid substantial differences between experiments; namely, raising the toxin concentration can induce acute liver injury while repeated administration of the toxin can lead to liver cirrhosis. Moreover, the systemic and local effects of the toxin depend on modes of administration, doses, animal species including their age and nutritional status (Mao et al., 2014; Michalopoulos, 2007; Palmes & Spiegel, 2004; Pritchard & Apte, 2015).
Herein, we briefly highlight the most widely employed hepatotoxins in liver regeneration models.
5.2.1 ∣. Carbon tetrachloride
It is a volatile organic and common hepatotoxic compound. It induces an acute liver injury after its breakdown by cytochrome P450 2E1 (CYP2E1) leading to the formation of highly reactive and toxic radicals: trichloromethyl (CCl3*) and trichloromethylperoxy (CCl3OO*) radicals. These metabolites trigger an oxidative damage to DNA, proteins, lipids, and carbohydrates in hepatocytes causing their necrosis. It is also accompanied by stimulation of KCs that produce more oxygen free radicals and cytokines, thus contributing more to the cell damage. These events induce an acute inflammatory response represented by polymorphonuclear leukocytes and macrophages to remove necrotic debris of hepatocytes. This type of injury is a reversible acute hepatic injury, followed by liver regeneration because it was observed that an extreme elevation in plasma ALT activity in mice takes place within 36 hrs, and then it reduces to more than 90% of 36 hr level by 72 hrs after Carbon tetrachloride (CCl4) exposure. It is characterized by centrilobular necrosis that occurs mainly in the pericentral area (Zone 3) where the CYP2E1 is highly concentrated (Mao et al., 2014; Michalopoulos, 2007; Palmes & Spiegel, 2004; Pritchard & Apte, 2015).
5.2.2 ∣. d-galactosamine
It is considered as a strong hepatotoxic compound. It causes an acute liver failure by disturbing the metabolic system in the liver leading to depletion of uridine triphosphate and thus inhibition of RNA and protein synthesis. Moreover, d-galactosamine contributes to the inflammation and necrosis of liver cells via promoting the intestinal mast cell degranulation that represses the intestinal protection barrier, hence allowing more endotoxins to reach the portal circulation to the liver. The sequence of regenerative events occurs at the same time as that for CCL4, however, the liver regenerative capacity of this model is weaker than that of CCL4 model (Mao et al., 2014; Palmes & Spiegel, 2004; Pritchard & Apte, 2015).
5.2.3 ∣. Acetaminophen or paracetamol
It is a well-known antipyretic and analgesic medication. Overdose of acetaminophen has a toxic effect that is clinically manifested as fulminant acute hepatic failure. This model is heavily used in mice to study the mechanisms of acute liver injury. Administration of supra pharmacological toxic dose of acetaminophen tends to overwhelm the physiological metabolizing reactions in the liver leading to the accumulation of toxic metabolite called N-acetyl-benzoquinone imine. Subsequently, the formation of radicals and cytokines along with KC activation are the main pathological issues that ensue an acute inflammatory reaction and cell necrosis. The processes underlying the liver regeneration of this toxic model are relatively less comprehended than other models. Therefore, additional experiments should be performed to study the liver regeneration in the context of acetaminophen overdose (Mao et al., 2014; Palmes & Spiegel, 2004; Pritchard & Apte, 2015).
Additional hepatotoxic substances like thioacetamide and ethanol have also been involved in investigating the liver regeneration phenomenon.
Recently, genetically modified animal models have been extensively employed in the research field of liver regeneration. Several models have been established such as a mouse model produced by Grompe et al. in 2007 (Azuma et al., 2007). This outstanding model is a triple-knockout model that is immunodeficient due to the silencing of Rag2 and II2rg genes, and has hereditary tyrosinemia because of fumarylacetoacetate hydrolase (FAH) deficiency. In large animals such as pigs (Hickey et al., 2014), have also developed FAH single-knockout swine model. Silencing of FAH gene prevents the production of FAH protein which plays an essential part in the tyrosine metabolism by catalyzing the conversion of fumarylacetoacetate into fumarate and acetoacetate, the last step of tyrosine metabolism. As a result the tyrosine will accumulate in the liver of mouse or pig model causing acute liver failure which can be slavaged by administration of NTBC that inhibits the earlier step in the tyrosine pathway and bans the accumulation of toxic metabolites. Therefore, this gives an opportunity for the population of injected human hepatocytes by cycling those FAH single-knockout models on NTBC drug. Namely, these models can serve as incubators for the expansion and population of human hepatocytes after xenogeneic transplantation. Double and triple knockout models seem to act better than FAH single knockout because they have additional knockouts that make them immunodeficient, thus abolishing the possibility of rejection to human hepatocytes. (Mao et al., 2014; Palmes & Spiegel, 2004; Pritchard & Apte, 2015). Lately, Chen et al. 2019 published an interesting article about the effects of the spheroid reservoir bio-artificial liver (SRBAL) treatment using 200 g of primary porcine hepatocyte on survival, serum chemistry, and liver regeneration in ALF pigs after 85% hepatectomy. They found that SRBAL improved the survival, reduced ammonia, and accelerated liver regeneration in posthepatectomy ALF. Five of 6 animals in the SRBAL survived to 90 hours. AT 48 hours following hepatectomy, CT volumetrics demonstrated increased volume regeneration and Ki-67 staining showed increased positive staining. These favorable results emphasize the necessity for further clinical testing of the SRBAL.
6 ∣. DEVELOPMENTAL PATHWAYS OF LIVER REGENERATION
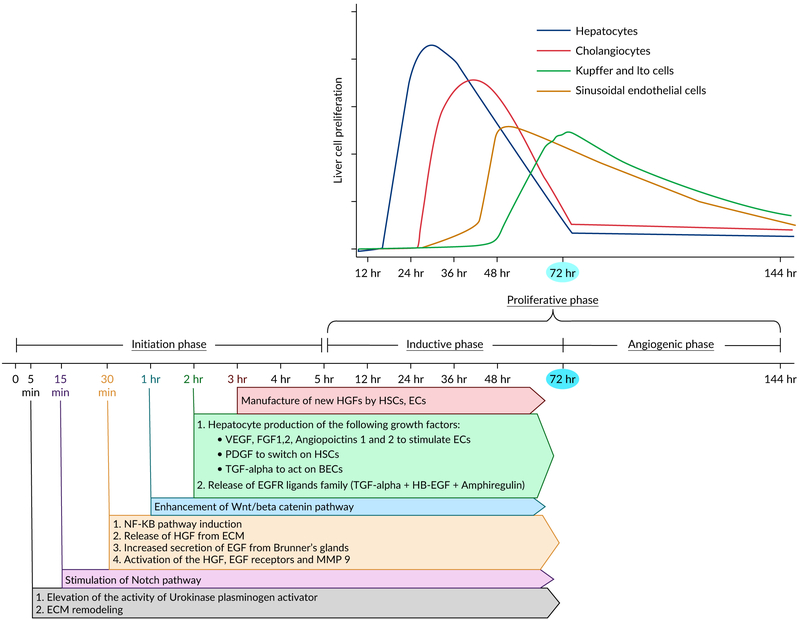
During liver regeneration, hepatocytes pass through three important distinctive phases: (a) Initiation or priming phase, (b) proliferation phase, (c) and termination phase. These phases are regulated and linked together by multiple signaling pathways. These events are highly studied in rodent models following PH compared to other models. Therefore, in this section, we are going to shed light on these phases explaining the network of signaling pathways that secure an optimal liver regeneration following partial hepatectomy. Figure 5 displays the temporal sequence of events of liver regeneration after partial hepatectomy. Figure 6 elaborates the molecular pathways underlying liver regeneration after partial hepatectomy.
FIGURE 5.
Temporal sequence of events of liver regeneration after partial hepatectomy
FIGURE 6.
Molecular pathways underlying liver regeneration process
6.1 ∣. Initiation or priming phase
It refers to the actions that occur in the early period of 0–5 hrs after PH (Michalopoulos, 2007). These events, which are mediated simultaneously by cytokines and growth factors, modify the expression of multiple genes to prepare the entry of liver cells into the cell cycle.
6.2 ∣. Hemodynamic changes and increased activity of urokinase plasminogen activator uPA: The earliest events
Liver is an amazing organ that has a dual blood supply from two routes. About 75% of the blood supply comes from the portal vein that carries deoxygenated blood rich in nutrients drained from the intestine and spleen. The remaining 25% of the blood is delivered via hepatic arteries that provide the liver with the oxygenated blood (Michalopoulos, 2007).
Hemodynamic changes are the least discussed and studied part of liver regeneration. Few literature studies have endorsed that early hemodynamic alterations after PH are necessary for launching the liver regeneration process (Michalopoulos, 2007). It has been observed that these changes are restricted to the portal blood flow without affecting the arterial part. PH causes an increase in the portal contribution per unit liver tissue resulting an increased in the availability per hepatocyte of growth factors and cytokines coming from the intestine and pancreas (Michalopoulos, 2007). Furthermore, the whole portal blood flow must pass through capillary beds with cross-sectional area about one-third of the normal liver. Because of this, a rise of the portal blood pressure is ensued resulting in turbulent blood flow that exerts a mechanical stress on the endothelial cells. Sokabe et al. (2004) have documented that endothelial cells expressed an increased activity of urokinase plasminogen activator uPA following a mechanical stress.
The rise of uPA activity is observed 5 min after PH. Within 10 min, it prompts the conversion of plasminogen into plasmin which breaks down the fibrinogen into fibrinogen degradation products FDPs. In addition to that, an evidence reported by Kim et al (Kim, Mars, Stolz, & Michalopoulos, 2000) revealed that the transformation of pro-matrix metalloproteinases (pro-MMPs) into active MMPs is augmented by plasmin at 30 min and lasts for 24–48 hours after PH. Both MMP and plasmin are involved in matrix remodeling and turnover of many proteins of ECM. Swindle et al. (2001) stated that the remodeling of ECM can initiate signaling impulses through integrin leading to the release of locally bound growth factors that participate in the liver proliferation. During matrix remodeling, an inactive hepatic growth factor HGF attached to ECM is activated to its active form by uPA and excreted locally and systemically causing an activation of hepatic growth factor receptor (HGFR or cMet) within 30 min to 1 hr after PH. At the same time, an epidermal growth factor receptor (EGFR) is also stimulated by epidermal growth factor (EGF) produced by Brunner’s glands of the duodenum and constantly goes to the liver via the portal route (Olsen, Poulsen, & Kirkegaard, 1985; Stolz, Mars, Petersen, Kim, & Michalopoulos, 1999). Several studies have shown that there is a cross interaction between cMet and EGFR in which cMet activation augments the activity of EGFR (Jo et al., 2000).
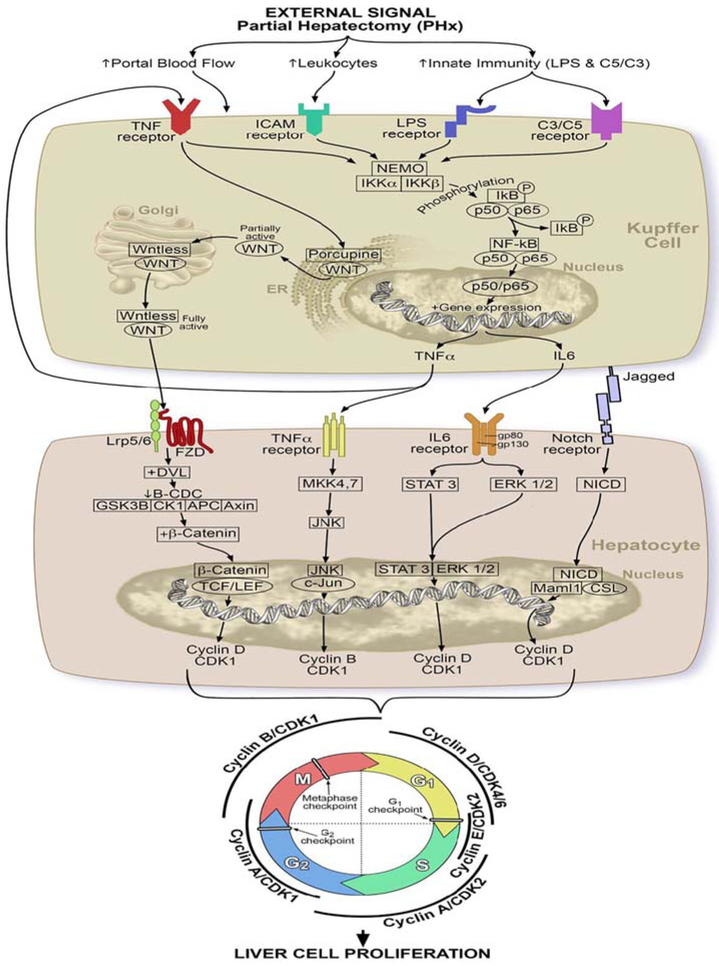
6.3 ∣. NF-KB signaling pathway
6.3.1 ∣. Role of innate immunity components
It is thought that innate immunity is the main stimulator for the initiation of liver regeneration (Fausto et al., 2006; Mao et al., 2014). Following PH, activation of innate immunity takes place leading to the production of its components such as lipopoly-saccharide (LPS) and complements (C3a and C5a). LPS, which is released from the enteric bacteria into the portal circulation, and complement proteins (C3a and C5a) bind to their respective receptors, Toll-like receptor 4 and complement receptors, on the surface of KCs (Fausto et al., 2006; Mao et al., 2014). These interactions lead to the stimulation of an important signaling pathway called nuclear factor KB (NF-KB) pathway. NF-KB is a dimeric transcription factor that consists of five members: P50, P52, P65, ReIB, and c-ReI (Nejak-Bowen & Monga, 2015). The most common NF-KB is made up of the combination of P50 and P65. Under normal conditions, NF-KB is kept inside the cellular cytoplasm of KCs by inhibitory KB protein (IKB) (Nejak-Bowen & Monga, 2015). When extracellular signals like LPS, C3a, and C5a stimulate KCs, IKB undergoes phosphorylation and degradation by IKB kinase, thereby allowing the NF-KB to become free and move into the nucleus where it excites the transcription of tumor necrosis factor (TNF), interleukin 6 (IL6) and cyclin D1 (Nejak-Bowen & Monga, 2015). The whole process occurs within 30 mins to 1 hr after PH (Nejak-Bowen & Monga, 2015).
6.3.2 ∣. Role of tumor necrosis factor alpha (TNF a)
TNF a plays a vital role in the initiation phase through performing two important functions: (a) activation of NF-KB signaling pathway, directly via binding to TNF receptor 1 on KCs creating a vicious cycle, and indirectly through induction of the inhibitory KB kinase (IKK) (Fausto et al., 2006; Hata, Namae, & Nishina, 2007; Zimmermann, 2004). (b) Stimulation of a stress-activated protein kinase (SAPK), also called c-Jun N-terminal kinase (JNK), in hepatocytes.
SAPKs or JNKs are special kinase proteins that have been observed to act as enhancers of liver regeneration. They are mainly activated by SAPK/extracellular signal-regulated kinase kinases or mitogen-activated protein kinase kinases, SEK1/ MKK4, and SEK2/MKK7 (Hata et al., 2007). These kinases are triggered in response to a cellular injury such as PH leading to JNK activation which in turn phosphorylates c Jun transcription factor in the nucleus to induce the transcription of target genes such as cell division cycle protein 2 homolog (CDC2), also known as cyclin-dependent kinase 1 (CDK-1), resulting in hepatocyte proliferation (Fausto et al., 2006; Hata et al., 2007; Zimmermann, 2004).
6.3.3 ∣. Role of interleukin 6
IL 6 is also considered as a critical component in priming the hepatocytes for proliferation. IL 6 binds to its receptor, a complex of gp 80 and gp 130 subunits, on hepatocytes. Activation of gp130 subunit results in tyrosine kinase activity that phosphorylates a signal transducer and activator of transcription (STAT 3) and an extracellular signal-regulated protein kinase 1 and 2 (ERK1/2) causing an expression of multiple target genes important for hepatocyte proliferation such as Cyclin D1 (Fausto et al., 2006; Hata et al., 2007; Zimmermann, 2004).
During this phase, more than 100 different genes, not expressed in normal liver, are immediately triggered and expressed in the remaining liver tissue after PH. It is important to note that IL-6 is responsible for activating approximately 40% of these genes (Li et al., 2001; Mao et al., 2014).
6.4 ∣. Wnt/beta cantenin signaling pathway
Wnt/β-catenin signaling is an important driver of liver regeneration that starts to operate within 1–3 hrs after PH (Nejak-Bowen & Monga, 2015; Russell & Monga, 2018). The secreted TNF a launches this pathway by binding to its specific receptors on KCs, sinusoidal endothelial cells, and HSCs leading to the release of specific glycoproteins called Wnt ligands (Nejak-Bowen & Monga, 2015; Russell & Monga, 2018). Additionally, It has been shown that neither biliary epithelial cells nor hepatocytes secrete Wnt ligands after PH (Nejak-Bowen & Monga, 2015; Russell & Monga, 2018). To be biologically active, Wnt proteins must undergo glycosylation and palmitoylation by the enzyme porcupine which localizes to the endoplasmic reticulum (Nejak-Bowen & Monga, 2015; Russell & Monga, 2018). After that, a cargo receptor proteins called Wntless (WIs) transfer Wnt proteins from Golgi apparatus to the cell membrane for extracellular secretion (Bartscherer, Pelte, Ingelfinger, & Boutros, 2006; Nejak-Bowen & Monga, 2015). The released biologically active Wnt protein binds to its Frizzled receptor which is associated with a co-receptor, low-density lipoprotein receptor-related protein 5 or 6, on hepatocytes to mediate the activation of the Wnt/β-catenin signaling pathway via sending an inactivating signal through disheveled proteins to β-catenin degradation complex (B-CDC) consisting of glycogen synthase kinase 3β, casein kinase 1 (CK1), Axin, and adenomatous polyposis coli gene product to stop the phosphorylation of Wnt proteins (Bhanot et al., 1996; Nejak-Bowen & Monga, 2015). This interaction allows Wnt proteins to liberate from B-CDC and translocate into the nucleus to combine with T cell and lymphoid enhancer transcription factors that induce the transcription of target genes such as Cyclin D1 that play a role in hepatocyte proliferation (Cadigan & Nusse, 1997; Nejak-Bowen & Monga, 2015; Russell & Monga, 2018).
6.5 ∣. Notch signaling pathway
Notch pathway is one of the earliest pathways that is turned on within 15–30 min after PH. Several observational studies on rats have discussed the crucial role of Notch signaling pathway in regulating the liver regeneration after PH. It is generally dependent on two main proteins known as NOTCH-1 receptor and JAGGED-1 (NOTCH-1 ligand) proteins which are markedly upregulated 1–5 days following PH (Morell, Fiorotto, Fabris, & Strazzabosco, 2013; Nejak-Bowen & Monga, 2015; Stanger & Greenbaum, 2012). In normal adult liver, there are four different types of NOTCH transmembrane receptors (Notch-1, 2, 3, and 4) expressed on hepatic progenitor cells (HPCs), hepatocytes, cholangiocytes, and endothelial cells lining the portal tract vessels and sinusoids. They are stimulated by interacting with their specific membrane-bound ligand proteins that fall into two classes:(a) Jagged-1 and -2, and (b) Delta-like 1, 3, and 4 (DLL 1,3,4) (Böhm, Kohler, Speicher, & Werner, 2010; Nijjar, Crosby, Wallace, Hubscher, & Strain, 2001). Jag-1 and DLL 4 are the only NOTCH ligands present in the human liver. Jag-1 ligand is normally expressed in biliary cells, HPCs, and smooth muscle cells in portal vein mesenchyme (Morell et al., 2013). Following a liver injury, a cell-cell interaction between signaling cells that express JAG ligands and receiving cells that carry NOTCH receptors takes place causing an activation of NOTCH receptors by JAG ligands. This leads to the formation of Notch intracellular domain in the cytoplasm as a result of cleavage of Notch receptor by a disintegrin and metalloproteinase (ADAM)/TNF-α-converting enzyme (TACE) and γ-secretase. NCID is then translocated into the nucleus where it forms a complex with the transcription factor CBF1/Su (H)/Lag-1 (CSL), and its coactivator, mastermind-like 1 (Maml1), triggering the transcription of hairy and enhancer of split-1,5 (Hes 1, 5), myc, cyclin D1 and other Notch target genes that boost the proliferation and differentiation stage (Böhm et al., 2010; Nejak-Bowen & Monga, 2015). Furthermore, Notch signaling pathway appears to be important for the regeneration of the liver vasculature through the association of the CSL component-recombination signal binding protein-jκ (RBP-J) with NCID, mediating the upregulation of specific genes important for endothelial cell proliferation and revascularization (Böhm et al., 2010; Nejak-Bowen & Monga, 2015; Wang et al., 2009). This was evidenced by Wang et al (Wang et al., 2009). who have deleted the RBP-J gene in hepatocytes and endothelial cells of mice and then observed the liver regeneration following PH. They reported that such deletion causes a disrupted liver regeneration characterized by abnormal structural obstruction of liver sinusoids along with decreased hepatocyte proliferation.
6.6 ∣. Hippo signaling pathway
Hippo signaling pathway is another important pathway that participates in the liver regeneration. It begins when the liver injury inhibits the activity of mammalian Ste20-like kinases (Mst1/2) and their cofactor Salvador (Sav1) from making a complex and inducing the large tumor suppressor (Lats1/2). As a result, the Yes-associated protein (YAP) will be protected from the phosphorylation, degradation and inactivation process by Lats half. Thereby, YAP moves into the nucleus together with the transcriptional co-activator with PDZ-binding motif (TAZ) switch on the transcription factor, transcriptional enhancer-associated domain (TEAD) factor resulting in hepatocyte proliferation and regeneration. Furthermore, YAP/TAZ activity is also upregulated by growth factors, ERK/MAPK pathway and Wnt/β-catenin pathway (Böhm et al., 2010; Nejak-Bowen & Monga, 2015).
6.7 ∣. Proliferation phase
The proliferative response to PH is divided into two main periods:
The period of induction refers to the proliferation of hepatocytes and cholangiocytes. Notably, the regenerative response of hepatocytes precedes and triggers the expansion of cholangiocytes. This step commences at the end of the priming phase and lasts for 72 hrs reaching the peak at 24–36 hrs after PH (Michalopoulos, 2007; Sadri, Jeschke, & Amini-Nik, 2015).
The angiogenic phase in which the nonparenchymal cells such as HSCs, KCs and hepatic endothelial cells undergo proliferation in response to signals derived from proliferating hepatocytes. It occurs immediately after the inductive period and continues for 2–3 days (Michalopoulos, 2007; Sadri et al., 2015).
Based on previous observations, all the cells firstly undergo cellular hypertrophy followed by cellular proliferation. Since the cell cycle is the key proliferative mechanism, it is useful to give a brief description of the cell cycle in this section.
The cell cycle encompasses five organized phases; G1, S, G2, M, and G0. In G1, the size of the cell increases, and mRNA and proteins are synthesized for DNA synthesis. In S phase, DNA replication takes place. During G2 the cell continues to grow rapidly and manufacture new essential proteins needed for the cell division. M phase is defined as a mitotic cell division and separation of chromosomes to give two separate identical cells. The last phase is G0 when the cells exit the cycle and are now recognized as quiescent cells (Nalesnik, Federle, Buck, Fontes, & Carr, 2009; Vente et al., 2009).
Regulation of the cell cycle is carried out by important proteins called cyclin dependent kinases (CDKs). There are multiple types of CDKs involved in the cell cycle and they are activated by binding to other proteins called cyclins including cyclin D1, cyclin E, cyclin A, and cyclin B. The activity of CDKs is also controlled by cell-division-cycle 25 homolog A (CDC25A), M-phase inducer phosphatase 1, and protein kinase-Wee1. Binding of cyclin and CDK activates CDC25A and suppresses Wee1 ending in dephosphorylating of CDK, and creating a positive feedback loop that produces large amounts of active CDKs. In addition to that, external signals that stimulate the cell entry into the cell cycle also trigger CdC25. These cyclin-CDK complexes control the progression and passage of cells through checkpoints to move to the next phase. There are three different checkpoints: (a) G1-S checkpoint, (b) G2-M checkpoint, and (c) and M checkpoint. These checkpoints prevent the transition into the next phase after making sure the current phase is successfully completed without any errors (Nalesnik et al., 2009; Shen & Huang, 2012; Vente et al., 2009).
During liver regeneration, the cytokine network depicted above in signaling pathways mediate the entry of quiescent hepatic cells into the cell cycle transferring them from G0 to G1 phase (Collin, Gilgenkrantz, & Guidotti, 2012; Fausto et al., 2006).
6.7.1 ∣. The passage from G1 to S phase
Growth factors and signaling pathways induce the activity of CDC25 which activates CDK and upregulates the expression of CCND1 gene that encodes Cyclin D1 protein. These events enable Cyclin D1 to form a complex with CDK4/6, producing Cyclin D1-CDK4/6 complex.
Two pathways have been reported to clarify how Cyclin D1-CDK4/6 complex triggers the progression of hepatic cells through G1 phase and traversing the G1-S checkpoint to enter the S phase.
Cyclin D1-CDK4/6 interacts with pRb-E2F complex causing the phosphorylation of tumor suppressor protein, retinoblastoma protein (pRb). This leads to the inhibition of pRB and dissociation of E2F transcription factor, triggering the transcription of important cell cycle-related genes including cyclin A and D, cdc25, and CDK 2 genes (Diehl, 2002).
Cyclin D1-CDK4 allows the sequestration of CDK interacting protein/Kinase inhibitory protein (Cip/Kip) family, p21 and p27, and thus activating CDK 1 and CDK2. Consequently, CDK 2 combines with cyclin E to form Cyclin E-CDK 2 complex that mediates the G1-S transition (Diehl, 2002). Furthermore, CDK1 or CDC2 is overexpressed by TNF a in the NF-KB pathway as well as by CDC25.
6.7.2 ∣. The transition from S to G2 phase
When the hepatic cell passes from G1 to S phase, cyclin A replaces cyclin E and associate with CDK2 producing Cyclin A-CDK 2 complex which controls the events occurring during S phase. In the late S phase, CDK1 combines with cyclin A making Cyclin A—CDK1 complex which helps in conveying the cell into G2 phase (Bendris, Lemmers, Blanchard, & Arsic, 2011; Pagano, Pepperkok, Verde, Ansorge, & Draetta, 1992).
6.7.3 ∣. The progression from G2 to M phase
After cell transition from S to G2 phase, the cyclin A remains in association with CDK1 controlling the growth and genetic transcription during G2. In late G2 phase, Cyclin A is substituted by Cyclin B to form Cyclin B-CDK1 complex. Some studies have stated that Cyclin A-CDK 1 is proposed to be involved in the stimulation and formation of Cyclin b-CDK1 complex. Moreover, Cyclin b-CDK1 complex is also activated by the dephosphorylating effect of CDC25 (Caldas et al., 2005; De Boer et al., 2008; Yam, Fung, & Poon, 2002).
Cyclin b-CDK1 complex plays a crucial role in moving the hepatocyte through the G2-M checkpoint into the M phase. It induces the expression of survival gene called survivin which regulates different stages of mitosis and cytokinesis (Caldas et al., 2005).
During metaphase-anaphase transition, CDK 1 phosphorylates a class of ubiquitin ligase called anaphase-promoting complex (APC) which then binds to a cell-division-cycle protein 20 (CDC20) leading to the formation and activation of APC/CDC20 complex. This brings out a proteolytic degradation of mitotic cyclins (Clb2) and CDK1, and inhibition of separase, which in turn deactivates cohesion, a protein that holds sister chromatids together by expressing a protein called Securin. These interactions facilitate the mitotic exit of the liver cell and termination of the cell cycle. APC/CDC20 activation acts as a sign that indicates the alignment of chromosomes at the mitotic plate under a bipolar tension in the metaphase, thus allowing the hepatocyte to cross M checkpoint and progress into the anaphase, dividing eventually into two identical cells (Keaton, 2007; Thornton & Toczyski, 2003).
6.8 ∣. Growth factors in liver regeneration
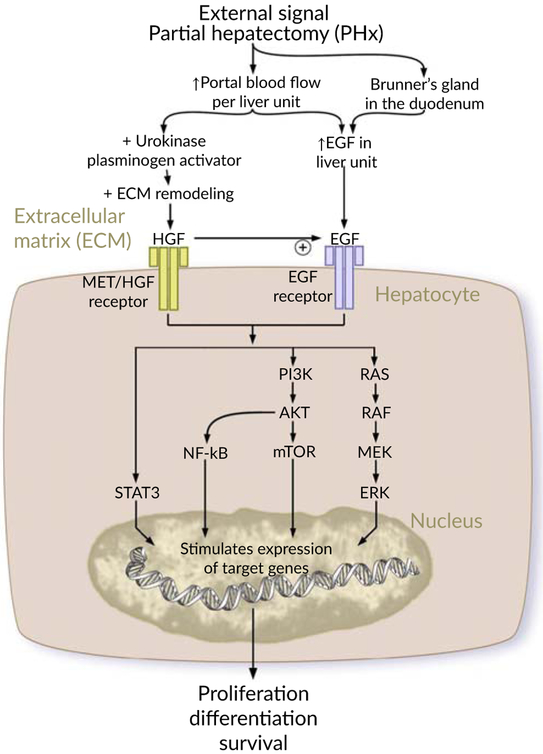
Augmentation of liver regeneration is governed by numerous growth factors that are in charge of priming the parenchymal and nonparenchymal liver cells and boosting their access into the cell cycle to proliferate robustly for the restoration of the original liver size after PH. HGF and EGF are, by far, the most important factors that are considered as initiators and augmenters of the proliferative phase during liver regeneration. Figure 7 explains the contribution of growth factors in liver regeneration.
FIGURE 7.
Contribution of growth factors in liver regeneration
6.8.1 ∣. Hepatocyte growth factor
HGF is a paracrine morphogenic and angiogenesis inducer factor. HGFR or C-met is a HGF-specific receptor which is plentifully expressed on liver parenchymal and nonparenchymal cells. HGF is a matrix-derived growth factor that is attached in its inactive form to the extracellular matrix of several organs. Surprisingly, Appasamy et al. (1993) have proved the abundance of HGF in the liver compared to other organs by finding the highest sequestration in the liver upon parenteral injection of HGF.
Multiple studies have elaborated that HGF plays a key role in the liver regeneration particularly in the proliferative phase. For instance, Liu, Mars, Zarnegar, and Michalopoulos (1994)) and Patijn, Lieber, Schowalter, Schwall, and Kay (1998) have stated that the liver expansion in normal rodents occurs as a result of HGF injection in the portal vein. Also, Block et al. (1996) and Monga et al. (2002)) have reported that the activation of C-met in hepatocyte cultures results in a strong mitogenic response and clonal expansion.
In the early hours of the priming phase and during the matrix remodeling, uPA provokes the activation and release of HGFs within 30 min to 1 hr after PH. The released active HGFs bind to its specific receptors, HGFRs or C-met, on the surface of hepatocytes leading to the enhancement of the cyclins and CDKs and hence a powerful hepatocyte response is initiated. Furthermore, HGFs are newly synthesized by proliferating endothelial and HSCs in response to hepatocyte activation and proliferation leading to a significant rise in the level of HGFs thus bringing about an immense speedy cellular expansion.
6.8.2 ∣. Family of EGFR ligands
Another essential factor that highly participates in the regenerative process is EGF which is produced by Brunner’s glands of the duodenum and continually goes to the liver via the portal route. Within 30 min to 1 h after PH, a synthesis of EGF by Brunner’s gland increases as a result of several reasons: (a) Elevation in the concentration of EGF per unit liver weight after PH, (b) HGF activation, and (c) PH-induced escalation of catecholamine release from adrenal glands. On top of that, a family of ligands including TGF a, heparin-binding EGF-like growth factor (HB-EGF), and amphiregulin (AR) is triggered during liver regeneration and couple with the same receptors for EGF, thereby, increasing the activity of EGFR which acts as a strong mitogen for hepatocytes (Fausto et al., 2006; Michalopoulos, 2007; Michalopoulos & DeFrances, 1997).
TGF a is an important EGFR ligand that is generated by active hepatocytes at 2 hrs after PH and stays at a high level for 48 hrs. TGF a also acts as an inducer of the mitotic reaction of endothelial cells and bile duct epithelial cells (Fausto et al., 2006; Michalopoulos, 2007; Michalopoulos & DeFrances, 1997). HB-EGF is another EGFR ligand that is expressed by KCs and endothelial cells within 1.5 hrs after PH. Mice with HB-EGF knockout KO showed a delayed liver regeneration while HB EGF transgenic mice with liver-targeted production have an enhanced regeneration (Fausto et al., 2006; Michalopoulos, 2007; Michalopoulos & DeFrances, 1997). AR is also a member of the EGFR Ligands family that clearly contributes to the liver regeneration (Fausto et al., 2006; Michalopoulos, 2007; Michalopoulos & DeFrances, 1997). It was evidenced by Berasain et al. (2005) who disclosed that the liver regeneration interference has occurred in mice deficient in AR after PH.
Previous data from existing articles have demonstrated that EGFR and HGF/c-met are tyrosine kinase receptors whose phosphorylation by their specific ligands elicits several signaling cascades including:
Ras-Raf-MEK cascade which induces the phosphorylation and activation of intracellular signaling protein kinase molecules such as mitogen-activated protein kinase 3 (MAPK3) (known as “extracellular signal-regulated kinase 1” [ERK1]) and mitogen-activated protein kinase 1 (MAPK1) (known as “extracellular signal-regulated kinase 2” [ERK2]) that are implicated in the regulation of cell growth and mitosis. This ensues a genetic overexpression of several transcription factors as C-myc, C-fos, and C-jun that are highly specialized in liver regeneration (Fausto et al., 2006; Michalopoulos, 2007, 2010a; Michalopoulos & DeFrances, 1997).
- PI3K/AKT/mTOR cascade which encompasses a stimulating phosphorylation of PI3K Phosphatidylinositol-4,5-bisphosphate 3-kinase and AKT Protein kinase B (PKB) leading to the upregulation of the followings:
- Inhibitor of nuclear factor kappa-B kinase subunit alpha (IKK-α) which inhibits the IκB protein kinase allowing NF-κB transcription factors to enter the nucleus and stimulate NF-KB pathway system (Fausto et al., 2006; Michalopoulos, 2007, 2010a; Michalopoulos & DeFrances, 1997).
- Mammalian target of rapamycin (mTOR) that promotes the eukaryotic initiation factor 4E (eIF4E) involved in directing the ribosomes toward mRNA to start the translation process, and enhances S6 ribosomal protein by S6 kinase leading to increase in protein synthesis and cell division (Fausto et al., 2006; Michalopoulos, 2007, 2010a; Michalopoulos & DeFrances, 1997).
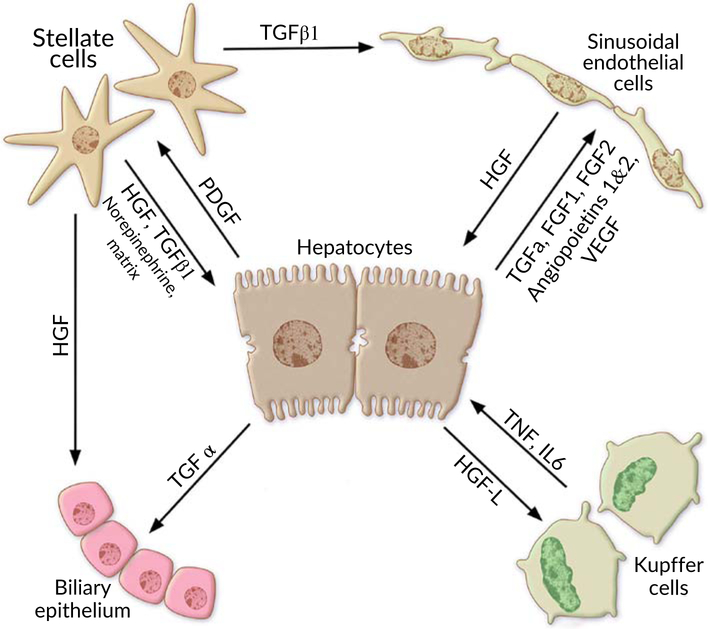
6.9 ∣. Intercellular interactions and contributions of different liver cells
Parenchymal and nonparenchymal liver cells have complex and innumerable intercellular interactions that take place to obtain an optimal liver regenerative response after injury. Figure 8 demostrates the interaction between different liver cells during liver regeneration after partial hepatectomy.
FIGURE 8.
Interaction between different liver cells during liver regeneration after partial hepatectomy
6.9.1 ∣. Hepatocytes
According to transplantation studies, hepatocytes are special living cells that possess intrinsic proliferative mechanisms which act automatically following liver insults. As discussed above, the proliferation of hepatocytes is stimulated by HGFs released and activated by uPA during matrix remodeling in the early hours of the priming phase. Consequently, dividing hepatocytes begin to produce a large number of different growth factors that establish signaling interactions between different liver cells and create a vicious cycle that gives rise to abundant parenchymal and nonparenchymal liver cells (Michalopoulos, 2007; Michalopoulos & DeFrances, 1997).
The emanated mitogenic growth factors that mediate these interactions are:
TGF a that stimulates the multiplication of biliary epithelial cells
HGF-L provokes KCs to expand and generate more TNFs a and ILs.
FGF 1, 2, VEGF, PDEF and angiopoietins 1, 2 are factors that are responsible for the process of vascular angiogenesis and restoration of sinusoidal networks of the regenerative liver via duplicating hepatic endothelial cells.
Platelet-derived growth factor (PDGF) which interacts with HSCs boosting them to undergo strong growth and give out signals like TGF beta 1 which amplifies the number of endothelial cells.
Multiple observations have revealed that hepatocytes are the first cells that enter the cell cycle and undergo proliferation followed by biliary epithelial cells in the inductive phase. The other liver nonparenchymal cells duplicate during the angiogenic phase starting at 3 days and ending 4–5 days after PH (Michalopoulos, 2007; Michalopoulos & DeFrances, 1997).
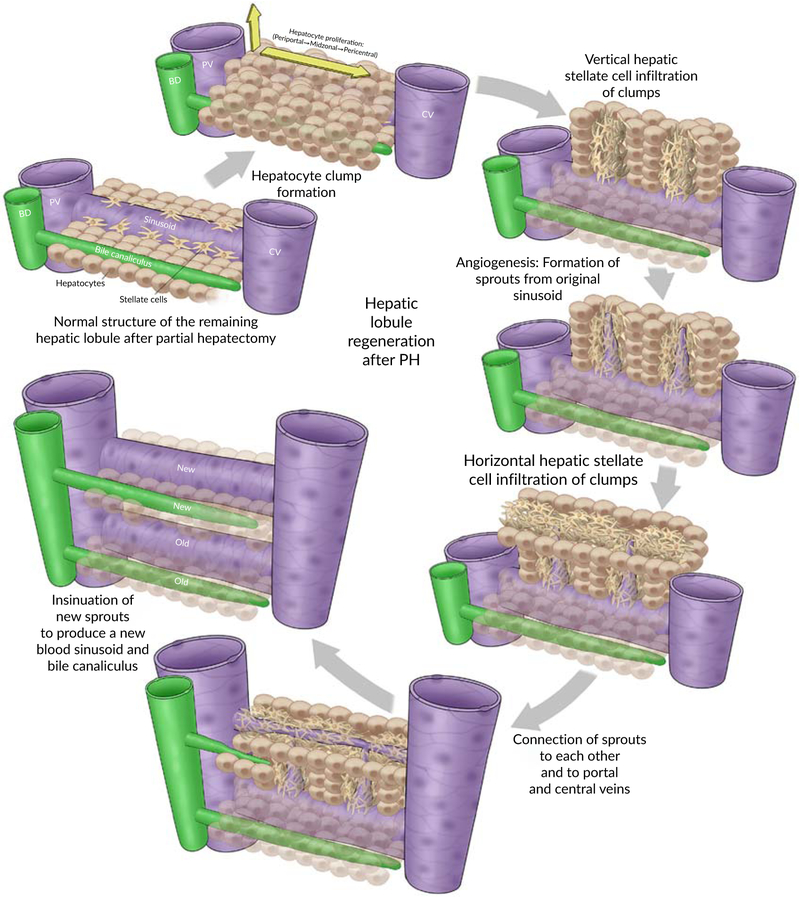
During liver regeneration, a compensatory hypertrophy followed by hyperplasia of hepatocytes proceed perpendicular to the line of hepatic plates which become several cells thick focally, producing clusters or clumps of hepatocytes that resemble the appearance of an embryonal blastemal (Michalopoulos & DeFrances, 1997; Schaffner, 1991). Apparently, the mitotic response starts in zone 1 of liver acinus at portal areas and then streams toward zone 2 and eventually reaches hepatocytes surrounding the central vein in zone 3 by 36–48 hrs after PH. This indicates that the timely order of duplication is as follows: periportal hepatocytes then mid-zonal hepatocytes and finally pericentral hepatocytes (Fausto & Campbell, 2003; Gebhardt, Baldysiak-Figiel, Krugel, Ueberham, & Gaunitz, 2007; Rozga, 2002; Schaffner, 1991).
The remaining hepatocytes after two-third PH undergo a single cycle of cell division replacing 60% of the lost hepatocytes. Afterward, a small portion of hepatocytes goes through additional times of replication to restore the original number of hepatocytes (Michalopoulos, 2007). Therefore, it involves 1.6 divisions of each hepatocyte to rebuild the normal liver size after two-third partial liver resection (Abdellatif & Shiha, 2017; Kholodenko & Yarygin, 2017).
The first periportal hepatocytes duplicate after a latent period of about 12–16 hrs following PH (Abdellatif & Shiha, 2017; Kholodenko & Yarygin, 2017; Michalopoulos & DeFrances, 1997). Notably, it is a circadian rhythm regulated response that peaks at 24 hr for the rat and 36 hr for the mouse and ends around 3 days after PH. Essentially, the HPSCs do not mediate the hepatocyte mitotic response because the mature adult hepatocytes proliferate automatically and independently of (HPSCs) after PH (Abdellatif & Shiha, 2017; Kholodenko & Yarygin, 2017; Michalopoulos & DeFrances, 1997).
6.9.2 ∣. HCSs or Ito cells
HSCs, also called Ito cells, are liver specialized cells located underneath the blood sinusoids and possess long processes that surround the hepatocytes. They synthesize ECM, growth factors, and store vitamin A.
HSC is an essential element that engages in multiple ways in liver regeneration. Its activity begins when the activated hepatocytes secrete PDGFs which in turn promote HSCs to enter the cellular division process. HSCs appear to be extensively involved in regulating the two stages of the proliferative phase in addition to the termination phase. Obviously, HSC switches on the NOTCH signaling pathway and secretes HGFs, thereby initiating a replicating response in hepatocytes and cholangiocytes (Abdellatif & Shiha, 2017; Kholodenko & Yarygin, 2017; Michalopoulos & DeFrances, 1997). Over and above that, Ito cells give out processes that permeate the clusters of hepatocytes and begin to release laminins which are extracellular matrix proteins that form a major component of the basal lamina (Michalopoulos & DeFrances, 1997). These processes establish the structural basis of the normal sinusoidal network of hepatic plates. Together with proliferating endothelial cells and KCs, these infiltrations eventually develop sinusoidal capillaries lined by fenestrated endothelial cells and KCs with scant matrix around them (Michalopoulos & DeFrances, 1997). As regards the termination phase, HSCs secrete TGFs beta which put on brakes and terminate the regenerative response once the normal weight and function are accomplished (Michalopoulos, 2007; Michalopoulos & DeFrances, 1997; Zimmermann, 2004).
Further studies have shown that other contributing factors such as nerve growth factor (NGF), brain-derived neurotrophic factor, neurotrophin 3, neurotrophin 4/5 (NT-4/5), low-affinity NGF receptor, and high-affinity tyrosine kinase receptors (Trk) B and C have been found to share in HSC regeneration (Michalopoulos, 2007). Mice deficient in neurotrophin receptors were found to have an incomplete liver regeneration (Michalopoulos, 2007).
6.9.3 ∣. Liver sinusoidal endothelial cells
Liver sinusoidal endothelial cells (LSECs) respond to hepatocyte-released growth factors by getting replicated and producing more HGFs which, in turn, stimulate the hepatocytes. During the angiogenic phase of hepatic proliferation, a physiological event called angiogenesis commences at 2–3 days and terminates at 5–6 days after PH (Michalopoulos, 2007; Michalopoulos & DeFrances, 1997; Sadri et al., 2015). It is characterized by the development of new blood vessels from preexisting ones. It occurs during liver regeneration in several steps (Karamysheva, 2007; Papetti & Herman, 2002):
Injury: Exposure of the liver to injurious events which could be chemical as acetaminophen or mechanical as PH. These insults drive numerous signaling pathways that result in a hepatocyte production of angiogenic growth factors such as FGF 1,2, PDGF VEGF, and angiopoietins
Protease production: VEGF is considered as the key regulator of angiogenesis. It interacts with its specific receptor VEGFR on the surface of endothelial cells of preexisting blood vessels. This stimulates the cells to release a proteolytic enzyme called MMP (Karamysheva, 2007; Papetti & Herman, 2002; Senger & Davis, 2011).
Migration: MMP then lyses the collagen fibers of the basement membrane and hence giving the opportunity for endothelial cells to escape from the original blood vessels. Importantly, ECM proteins adhere and support the migrating cells for sprouting of the new blood vessels (Karamysheva, 2007; Papetti & Herman, 2002; Senger & Davis, 2011).
Proliferation and sprouting: VEGF along with (FGF) 1, 2 are deemed necessary for the proliferation of all cell types that constitute the vessel wall including endothelial cells, smooth muscle cells, and fibroblast cells. Importantly, the NOTCH signaling pathway contributes to angiogenesis by identifying the endothelial tip and stalk cell during sprouting (Karamysheva, 2007; Papetti & Herman, 2002; Ribatti & Crivellato, 2012; Senger & Davis, 2011). The proliferating cells then penetrate the hepatocyte clusters through the same infiltrations made by HSCs, forming solid sprouts that elongate into cords filled with endothelial cells and connecting neighboring vessels (Michalopoulos, 2007).
Tubulogenesis: This step refers to the insinuation of vascular cords and formation of a central lumen that result from specific structural alterations in endothelial cells. FGF and PDGF are thought to be implicated in this process (Papetti & Herman, 2002). Several theories have been proposed to explain this stage; however, it is not well understood. These proposals are (a) cell hollowing which refers to the formation of intracytoplasmic vacuoles in the central cells. These vesicles coalesce together to form an internal lumen of the vascular tube (Ahmed et al., 2017; Ribatti & Crivellato, 2012; Xu & Cleaver, 2011). (b) Cord hollowing which includes the abolishment of intercellular adhesive junctions at the cord center. This creates slits between central ECs which expand to form the central lumen (Xu & Cleaver, 2011). (c) Apoptosis of endothelial cells at the center of the vascular cord.
Stabilization and maturation: During this phase, PDGF and angiopoietin contribute to angiogenesis by remodeling of newly synthesized blood vessels, organizing the endothelial lining of tubes, stabilizing the intercellular junctions between ECs and recruiting pericytes which surround endothelial cells to form a new basement membrane, thus producing new mature well-stabilized blood vessels (Ahmed et al., 2017; Ribatti & Crivellato, 2012; Xu & Cleaver, 2011).
6.9.4 ∣. Biliary epithelial cells (cholangiocytes)
As explained above, Notch and Wnt signaling systems are equally stimulated very shortly after PH. On top of that, during the inductive phase, TGFs produced by activated hepatocytes and HGFs released by induced HSCs act on biliary epithelial cells rendering them to proliferate after hepatocytes.
These pathways along with the released growth factors are highly conserved and essential elements that govern the mitogenic response of cholangiocytes for optimal morphogenesis, and remodeling of new IHBDs and canaliculi for hepatocyte clumps. Li et al. (1997) and McDaniell et al. (2006) have reported that Notch/Jagged mutations in humans are responsible for the development of alagille syndrome associated with the paucity of IHBDs. Also, Lu et al. (2016) have described how the impairment of biliary regeneration after PH in mice is attributable to the deletion of RBPJ-downstream transcription factor for Notch receptors.
Following PH, a typical ductular reaction (DR) involving the replication of cholangiocytes of the remaining IHBDs within portal triads is established. The proliferating biliary epithelial cells then form tubular structures with a well-defined lumen that serve as new IHBDs of hepatocyte clumps. Out pouches arise from new IHBDS and penetrate the clumps to construct the intercellular bile canaliculi (Alvaro, Gigliozzi, & Attili, 2000). At the end of regeneration, the hepatocyte clumps become reorganized and supplied with new mature branches of the bile duct, hepatic artery, portal vein and hepatic vein in addition to sinusoidal networks and bile duct canaliculi that pass in between the regenerated hepatocytes to produce a new hepatic plates similar to those seen in the normal liver. Apparently, the size of the liver lobules is remarkably larger than before and the thickness of the hepatocyte plates is almost twice the thickness of the normal plates (Michalopoulos, 2007; Michalopoulos & DeFrances, 1997). Figure 9 demostrates the structural regeneration of hepatic lobule after partial hepatectomy.
FIGURE 9.
Structural regeneration of hepatic lobule after partial hepatectomy
6.10 ∣. Endocrinal regulation of liver regeneration
6.10.1 ∣. Norepinephrine
Norepinephrine (also called noradrenaline) is one of the most important chemical compounds that play a vital part in the physiology of the human body. It is related to the catecholamine family and categorized as a hormonal neurotransmitter because it is secreted by the adrenal medulla into the systemic circulation as a stress hormone, and released from the nerve endings as a chemical messenger inducing the sympathetic arm in the central and peripheral nervous system. This bifunctional compound acts on specific receptors known as noradrenergic alpha and beta receptors. The role of norepinephrine was introduced to the field of liver regeneration when Cruise, Houck, and Michalopoulos (1985) have demonstrated that norepinephrine dramatically elevates the influences of EGF and HGF in hepatocyte cultures. Furthermore, Houck, Cruise, and Michalopoulos (1988) have reported that norepinephrine substantially cancels the inhibitory effects of TGFb1 in terminating the regenerative response. Cruise, Knechtle, Bollinger, Kuhn, and Michalopoulos (1987)) have stated that DNA synthesis after PH is inhibited for 72 hr after dosing by prazosin (alpha1 receptor blocker). Besides that, the release of norepinephrine rapidly increases within 1 hr after PH and acts as strong stimulus for Brunner’s glands and portal myofibroblasts to secrete more EGFs, and HGF, respectively (Broten, Michalopoulos, Petersen, & Cruise, 1999; Michalopoulos, 2007; Michalopoulos & DeFrances, 1997).
6.10.2 ∣. Insulin
Insulin is an anabolic peptide hormone synthesized by beta cells of islets of Langerhans in the pancreas. It is an essential hormone that regulates the glucose level in the blood by acting on specific insulin receptors which decrease the blood glucose concentration back to the normal value and upregulate the protein and fat synthesis. It is continually transferred from the pancreas to the liver via the portal venous blood flow. Attention towards the benefits of insulin in the liver regeneration arose when Bucher and Weir (1976) and Starzl, Watanabe, Porter, and Putnam (1976) have published that the liver in rats and dogs shrinks to about one-third of its original size if the insulin delivery to the liver is precluded by shunting the portal blood flow to the vena cava flow and thus decreasing the flow of portal blood contents to the liver. Likewise, Starzl et al. (1976) have proved this observation by infusing insulin in dogs after porto-caval shunt and the result was a reduction of atrophy, preservation of liver ultrastructure, and unlocking the regenerative process. Overall, it seems that insulin is clearly involved in the liver regeneration including the metabolic regulation of homeostatic functions of regenerating hepatocytes (Michalopoulos, 2007; Michalopoulos & DeFrances, 1997).
6.10.3 ∣. Thyroid hormones
Follicular cells of thyroid gland are specifically responsible for the secretion of two fundamental tyrosine-based thyroid hormones, triiodothyronine (T3) and thyroxine (t4). These iodine containing proteins regulate the growth and tissue differentiation in addition to their homeostatic functions in controlling the basal metabolic rate, heat production, oxygen consumption, and cardiac properties. Short, Wedmore, Kibert, and Zemel (1980) have the studied the potential impact of this hormone on liver regeneration and found out that injecting a single dose of triiodothyronine in rats yields a striking enhancement of DNA synthesis and proliferation in the liver lasting for at least 4 days after this single injection. These results indicate that thyroid hormones could be considered as a hepatomitogenic factor for hepatocytes (Michalopoulos, 2007; Michalopoulos & DeFrances, 1997).
6.10.4 ∣. Serotonin
Serotonin is a chemical neurotransmitter that exists in the brain, GIT and platelets. Its secretion contributes to maintaining mood balance and feeling of well-being. Lesurtel et al. (2006) have divulged that decreased platelet level hinders the mitotic response of hepatocytes, and so, treating thrombocytopenic mice with serotonin reversed the attenuating effects of platelet depletion on liver regeneration. The mechanism by which serotonin affects the liver regeneration is still obscure. Therefore, further investigations on hepatocytes in culture should be carried out to illustrate the mechanism of its action.
6.11 ∣. Termination
Following the proliferation phase, the remnant liver tissue expands and retrieves the original size equivalent to the normal liver. The autonomic hepatocyte proliferative capacity represents the primary mechanism of this process. Nevertheless, this feature can keep working and giving more liver cells which may lead to carcinogenesis in severe cases. For that reason, the liver must go through a termination phase controlled by several proliferative inhibiting factors acting as brakes to restrain the liver cell proliferation, correct an overshooting of the regenerative response and prevent the carcinogenic effect of the excess proliferating cells. This denotes that the liver regeneration is a well-orchestrated process that necessitates a balance between mitogenic factors and proliferation-inhibiting factors in order to get the most favorable results (Liu & Chen, 2017).
Obviously, the termination phase is equally important as the initiation and proliferation phases. The mechanisms underlying the termination phase are not well addressed and investigated in the medical literature compared to other phases. Further studies should be done to elaborate and elucidate what happens during this phase.
In this section, we are going to discuss the most important elements and associated pathways that are proposed to be the leader of this stage.
6.12 ∣. Transforming growth factor β
TGF-β is the most popular anti-proliferative factor that stop the process of liver regeneration. Russell, Coffey Jr., Ouellette, and Moses (1988) have demonstrated that the liver regeneration in two-third PH rat model is inhibited subsequent to the administration of platelet-derived TGF-β. TGF-β is mainly secreted by nonparenchymal cells, including HSCs, KCs and platelets (Ikeda et al., 1998; Liu & Chen, 2017; Michalopoulos, 2007).
Pericellular hepatic ECM consists of two important components called glycosaminoglycan which is bound to HGF and decorin which is attached to pro-TGF β. As described above, urokinase is activated shortly after PH leading to ECM degradation and release of certain growth factors through integrin signaling pathway. The level of TGF β elevates, with the same kinetics as that of HGF, at 3 hrs and reaches its peak at 72 hr following PH. HGF is transformed to its active form by urokinase activity, while pro TGF β is found to be neutralized by α−2-macroglobulin in the blood plasma and thus blocking its activity, tilting the net balance toward HGF in the initiation phase (Liu & Chen, 2017; Michalopoulos, 2007; Michalopoulos, 2010b).
In the middle of the proliferation phase, the induction of gene of cation-independent mannose 6-phosphate receptor (CIMPR), an indirect inhibitor of hepatocyte growth, starts to take place causing the conversion of pro TGF β into active TGF β. It also mediates the interaction between active TGF β and its specific receptors on hepatocytes thus terminating the mitotic response after complete expansion of liver cells (Hines et al., 2007; Liu & Chen, 2017; Walesky & Apte, 2015).
Multiple mechanisms have been postulated to describe how TGF β stops liver regeneration.
Firstly, binding of TGF β to TGFR results in the phosphorylation of the receptor-regulated cytoplasmic small mothers against decpentaplegic (R-Smad) proteins, which are then translocated into the nucleus where they stimulate the expression of cell cycle inhibitors like CDK inhibitors and suppress the production of cell cycle inducers such as CDKs 2 and 4, and cyclins D and E leading to the cell cycle arrest at the G1/S transition (Hines et al., 2007; Liu & Chen, 2017; Walesky & Apte, 2015).
Secondly, TGF-β 1initiates the hepatic lobule remodeling and corrects the overshooting of proliferation by inducing an apoptosis of excess hepatocytes through the release of reactive oxygen species (ROS) via c-Jun-independent mechanism (Liu & Chen, 2017; Samson et al., 2002).
Thirdly TGF-β 1 appears to inhibit cdc25A, a CDK-activating tyrosine phosphatase, by raising the attachment of histone deacetylase 1 to p130 repressor complex (Bouzahzah et al., 2000; Liu & Chen, 2017).
6.13 ∣. Activin
Activin is a member of the TGF-β superfamily, and has a chemical structure identical to that of TGF-β. It is an autocrine inhibitor of DNA synthesis in a similar fashion as TGF-B through SMAD signaling pathway.
Schwall et al. (1993) have found that the apoptotic effects of activin and TFG-B are different in which activin is one tenth as potent as TGF-β. Kogure et al. (1996) have studied the impact of IV delivery of follistatin, an activin-binding protein, to rats after PH. They noticed that follistatin accumulates in the liver and promotes the liver regeneration following the procedure. These results suggest the potential role of activin in the termination process.
7 ∣. HEPATIC PROGENITOR CELLS
The reproducibility of the liver after two-third PH is merely dependent on the proliferative features of the remaining parenchymal and nonparenchymal liver cells. Nearly, all remnant hepatocytes enter one or two cell cycles to restore the original liver mass with very minimal engagement of HPSCs. It has been shown that HPSCs take the lead of the liver regeneration process when the mitotic capacity of liver cells is restricted and overwhelmed as a result of severe liver injury. HPSCs differentiate into mature hepatocytes in circumstances associated with severe hepatocyte damage such as fulminant acute hepatitis (Fujita et al., 2000), chronic viral hepatitis (Libbrecht, Desmet, Van Damme, & Roskams, 2000), alcoholic liver cirrhosis (Ray, Mendenhall, French, & Gartside, 1993), NASH (Machado et al., 2015), and massive liver resection. On the other hand, the bipotential ability of these cells gives them the opportunity to transform into mature cholangiocytes in cholestatic conditions such as primary biliary cirrhosis (PBC) and primary sclerosing cholangitis (PSC) (Roskams et al., 1998).
Investigators have discussed the contributory role of HPSCs in multiple human studies and experimental models including:
Zebrafish models, for example, ethanol (Goessling et al., 2008; Huang et al., 2014), and near-complete hepatocyte ablation models (Choi, Ninov, Stainier, & Shin, 2014; He, Lu, Zou, & Luo, 2014).
Chronic liver injury rodent models established by administering a choline-deficient, ethionine-supplemented (CDE) diet to induce NASH (Machado et al., 2015), chronic injection of CCl4 (Petersen, Zajac, & Michalopoulos, 1998), chronic use of the chemical N-acetylaminofluorene (AAF) (Ohlson, Koroxenidou, & Hallstrom, 1998).
Chronic biliary injury rodent models prepared by 5-diethoxycarbonyl-1, 4-dihydrocollidine (DDC) diet and bile duct ligation (Espanol-Suner et al., 2012).
HPSCs were firstly identified in rodents by Farber (1956)) who depicted them as heterogeneous small oval-shaped cells containing a pale blue-staining oval nuclei and slightly basophilic cytoplasm with a large nuclear-to-cytoplasm ratio.
HPSCs normally reside in the most distal parts of the intrahepatic biliary tree known as canals of Hering (CoH). These canals form a normal connection between biliary ductules in the portal triads and the bile canalicular system in between hepatocytes.
7.1 ∣. HPSC-dependent liver regeneration mechanism
During the past decades, an increasing number of research articles have addressed the HPSC-dependent liver regeneration mechanism. HPSCs are considered as a second line of repair after a severe liver injury which exhausts and precludes the classical hepatocyte-mediated liver regeneration (Mao et al., 2014; Michalopoulos, 2007).
The HPSC niche is a unique microenvironment comprised of variable cell types (hepatocytes, cholangiocytes, HSCs, endothelial cells, macrophages and other inflammatory cells), growth factors and cytokines, and pro-fibrogenic ECM components.
Anatomically, it is thought that canals of Hering, bile ductules in the portal triads, and peri-portal parenchyma collectively constitute the HPSC niche. This niche is necessary to regulate the characteristics of HPSCs and the balance of their activation, proliferation, and differentiation (Roskams et al., 2004).
Basically, HPSCs are heterogeneous bi-potent stem cells that are labeled by different panel of markers including adult biliary markers (OV 6, CK7, and CK19), adult hepatocyte markers (CK8, CK18, C-met, and albumin), fetal hepatocyte markers (α-fetoprotein, epithelial cell adhesion molecule [EPCAM], CD34, and Sca-1), adult hematopoietic markers (c-Kit, CXCR4, prominin1 [CD133], and Ska-1 Thy1/CD90), and adult neural cell marker (neural cell adhesion molecule [NCAM]) (Gilgenkrantz, 2018; Kaur, Siddiqui, & Bhat, 2015; Michalopoulos, 2007).
They are regarded as dynamic stem cells that persistently switch their differentiation patterns and the expression of their markers according to the type of cell injury and which epithelial cells are subjected to most of the damage. For instance, in fulminant hepatitis, the primary damaged cells are hepatocytes; therefore, HPSCs will be activated to enter the pathways that produce hepatocytes. In contrast, if the cholangiocytes are the main injured cells as in diseases like PBC and PSC, differentiation of HPSCs into biliary epithelial cells will take place (Katoonizadeh & Poustchi, 2014).
Hepatocyte and cholangiocyte lineage generation from HPSCs is highly organized cellular process that involves signaling pathways and regulator molecules under the control of signals elicited by KCs, HSCs, and endothelial cells. In this part, we are going to delineate the events underlying the HPSC-based liver regeneration process.
7.2 ∣. Activation and proliferation of HPSCs
During an acute liver injury, the liver cells (Hepatocytes and cholangiocytes) undergo acute episodes of cell necrosis. The necrotic debris from damaged hepatocytes are engulfed and removed by the KCs. This phagocytosis stimulates KCs to secrete an essential cytokine called TNF-like weak inducer of Apoptosis (TWEAK) which binds to its specific highly expressed receptor, FGF-inducible 14 (Fn14), on the surface of HPSCs. The TWEAK/Fn14 complex then excites an important intracellular signaling pathway, NK-KB, leading to HPSC proliferation. E. Tirnitz-Parker et al. (2010) have published that TWEAK injection in unharmed mouse liver can result in a robust expansion of HPSCs. They also found that Fn14 is also overexpressed in many chronic human liver diseases.
Besides that, a multitude of growth factors released by activated HSCs (TGF-α, HGF, FGF, and TNFs,), and endothelial cells (VEGFs) play a crucial role in the expansion of HPSCs (Bria et al., 2017; Katoonizadeh & Poustchi, 2014; Kaur et al., 2015).
In human liver, activation and proliferation of quiescent resident HPSCs progress from the peri-portal to the peri-central zone of the hepatic lobule. This results in the initiation of reactive ductules or DRs which represent the regenerative response. These reactions are characterized by an increased number of HPSCs-containing ductules that infiltrate the liver plates and undergo cellular differentiation into hepatocytes as well as cholangiocytes (Best et al., 2015; Bria et al., 2017; Katoonizadeh & Poustchi, 2014; Kaur et al., 2015).
7.3 ∣. HPSC specification
The first step is the cell specification which means that HPSCs will undergo certain phenotypic changes and transform into more specific cells which are capable of differentiating into particular single cell line.
The commitment of HPSCs to specific cell fates involves their conversion into committed immature intermediate hepatocyte and cholangiocyte precursors. These partially differentiated cells have features such as size, phenotypes and expressed markers that are intermediate between mature cells and HPSCs. This phase is largely governed by Notch and Wnt pathways (Best et al., 2015; Bria et al., 2017; Gaudio et al., 2009; Katoonizadeh & Poustchi, 2014; Kaur et al., 2015; Turner et al., 2011).
During hepatocyte regeneration, Wnt signaling pathway is highly activated in HPSCs leading to increased transcription of Numb (a Notch pathway inhibitor). The overexpressed NUMB suppresses the expression of biliary-specific markers (CK7, CK19, CK14, and 0V 6) through blocking the activity of Notch signaling pathway. Therefore, the committed intermediate hepatocyte precursors become more immunogenic to hepatocyte markers with faint immunoreactivity to CK 7 and OV6, and absent expression of CK19. Furthermore, The outcome of the differentiation process is also represented by the upregulation of hepatocyte transcription factors such as HNF1,4 and C/EBP-B, and gaining the morphological and functional features of hepatocytes (Best et al., 2015; Bria et al., 2017; Gaudio et al., 2009; Katoonizadeh & Poustchi, 2014; Kaur et al., 2015; Turner etal., 2011).
On the contrary, biliary regeneration involves the stimulation of HSCs. Induced HSCs are converted into myofibroblasts which express Jagged ligand proteins. HPSCs-HSCs interaction allows the coordination of Jagged with its specific receptor, Notch, on the surface of HPSCs leading to the initiation of Notch pathway- the main signaling pathway of the biliary differentiation (Best et al., 2015; Bria et al., 2017; Katoonizadeh & Poustchi, 2014; Kaur et al., 2015). Geisler and Strazzabosco (2015) have comprehensively described the emerging role of Notch signaling pathway. They found that the impairment of Notch signaling in male rats by γ-secretase inhibitor after 2-AAF treatment prior to 70% PH has an abolishing effect on the liver regenerative process. As a result, the committed intermediate cholangiocyte precursors are now characterized with preserved and upregulated biliary markers with minimal expression of hepatocyte markers (Best et al., 2015; Bria et al., 2017; Gaudio et al., 2009; Katoonizadeh & Poustchi, 2014; Kaur et al., 2015; Turner et al., 2011).
7.4 ∣. Maturation of intermediate precursors
Partially differentiated cell precursors are cells that possess characteristics that overlap those of HPSCs and mature liver cells. For that reason it is important that these undergo further cellular modifications through process called maturation in order to become fully differentiated hepatocytes and cholangiocytes.
Hepatocyte maturation is mainly driven by Wnt pathway, growth factors (HGF) and some cytokines especially OSM released by activated KCs (Gaudio et al., 2009; Miyajima, Tanaka, & Itoh, 2014; Roskams, Katoonizadeh, & Komuta, 2010; Tanaka, Itoh, Tanimizu, & Miyajima, 2011; Turner et al., 2011). This process occurs rapidly and involves the following amendments to obtain the mature phenotype:
Completion of the expression of hepatocyte-specific transcription factors
Loss of the remaining biliary markers particularly CK 7 and OV 6
Cellular possession of aminotransferase enzymes and cytochrome p 450 species.
Notch pathway and TGF-beta are considered the prime regulators of cholangiocyte maturation. They complete the upregulation of the biliary specific markers, transcription factors (HNF 6, HNF1 B, FOXA1,3, Hex), and GGT enzyme in addition to the loss of hepatocyte markers, and acquisition of the phenotypic properties of mature biliary epithelial cell (Gaudio et al., 2009; Miyajima et al., 2014; Roskams et al., 2010; Tanaka et al., 2011; Turner et al., 2011).
7.5 ∣. Facultative stem cells: Hepatocytes and cholangiocytes replace each other
As explained above, HPSC is regarded as the prime inducer of the liver regeneration when the classical pathway is inhibited. Under these circumstances, hepatocytes and cholangiocytes also work as facultative stem cells to enrich their population. It was observed that major hepatocyte damage, biliary epithelial cells can convert into HPSCs which then differentiate into hepatocytes. In the same way, hepatocytes have shown the ability to transform into mature cholangiocytes when the latter is severely injured (Alison, Poulsom, & Forbes, 2001; Crosby et al., 1998; Michalopoulos, 2007). Michalopoulos, Barua, and Bowen (2005) have proven this phenomenon by establishing chimeric rats using dipeptidyl peptidase IV (DPPIV) and then exposing them to bile duct ligation, with or without pretreatment with the biliary toxin methylene dianiline (DAPM). Their results have demonstrated the formation of bile ductules harboring DPPIV 30 days after ligation. Another evidence supporting this feature is published by Crosby et al. (1998) who stated that histological findings in cases of PBC/PSC revealed that hepatocytes may act as facultative stem cells for biliary cholangiocytes.
These facultative stem cells can rescue each other during liver regeneration when their replicative activity is being macerated, thus acting as an adjuvant pathway to HPSC-mediated liver regeneration.
7.6 ∣. Homeostatic liver regeneration in uninjured liver
Beside the liver regeneration after acute injury, another surprising feature, described as homeostatic liver regeneration in uninjured liver, is discussed in medical literature. Wnt^-catenin signaling pathway is one of the important pathways which take part in the liver regeneration process after PHx via inducing certain transcription factors including Axin2 (axis inhibition protein 2) (Clevers, Loh, & Nusse, 2014; Lustig et al., 2002). In the adult liver, Axin2 is highly expressed in pericentral hepatocytes located around the central vein. Wang, Zhao, Fish, Logan, and Nusse (2015) were one of the first who reported homeostatic liver regeneration in mice. They used the tamoxifen-inducible Axin2-CreERT2; Rosa26-mTmG mouse model in which Axin2 positive cells were labeled with membrane GFP after tamoxifen administration. They found that GFP+ cells (Axin2 positive cells) located around the central vein were positive for glutamine synthetase (GS) which is a Wnt target gene and marks pericentral hepatocytes. They were also negative for carbamoyl-phosphate synthase 1 (CPS), which is a marker for midlobular and periportal hepatocytes. After giving the tamoxifen for a period of time, they detected that the population of GFP positive cells expanded and spread from the central vein toward the portal vein. The labeled pericentral cells were mostly diploid cells and maintained their genetic profile, exhibiting Axin2 and GS but not CPS. On the contrary, the descendants of the labeled cells in the midlobular and periportal areas were polyploid cells, lost Axin2 and GS expression and gained CPS expression. This indicates that as Axin2 positive cells migrate away from the pericentral region they lose their genetic profile and acquire the expression of new genes, thus preventing them from receiving Wnt signals and renewing to produce new hepatocytes. Furthermore, it has been shown that descendants of Axin2 positive cells replaced 30% of the area of the entire liver which is equivalent to approximately 40% of hepatocytes after 1 year of tamoxifen administration. They also quantified the DNA synthesis rates of Axin2 positive and negative cells. It has been revealed that Axin2+ cells undergo DNA replication twice as frequently as Axin2− hepatocytes. Based on that, we believe that Axin2 positive pericentral hepatocytes are unique cells that act as stem cells and have the capability to self-renew and replace other hepatocytes along the hepatic lobule in uninjured liver to maintain normal liver homeostasis. This unlimited replicative potential of pericentral cells is maintained by Wnt signals produced by central vein endothelial cells (Wang et al., 2015).
8 ∣. CONCLUSION
Liver regeneration following PH is complicated and well-orchestrated process. Many cytokines, growth factors, and signaling pathways work together in a perfect harmony to ensure an optimal growth of the remaining hepatic lobules. After an injurious event, hemodynamic changes and increased activity of urokinase plasminogen activator uPA take place leading to upregulation of specific signaling pathways including NF-KB, Wnt/beta cantenin, Notch, and Hippo in the remaining hepatocytes and production of growth factor, HGF and EGF. This in turn stimulates the proliferation of hepatocytes that begin to release several types of growth factors (TGF alpha, HGF-L, FGF 1, 2, VEGF, PDEF) that induce the proliferation of hepatocytes, cholangiocytes and KCs. This means that liver regeneration following acute injury like PHX arises from the remaining hepatocytes and not from stem cells. On the other hand, during embryonic development of the liver, the pluripotent embryonic stem cells derived from the inner cell mass of the blastocyst differentiates into trilaminar germ disc consisting of three primordial germ cell layers: ectoderm, mesoderm, and endoderm. Then the anterior part of the endoderm will form the foregut under the effect of Hedgehog signaling pathway. Following hepatic specification of foregut endoderm, foregut liver progenitor cells are established and then differentiate into hepatoblasts that bud into the septum transversum and undergo a series of proliferation, differentiation and finally maturation into hepatocytes and bile epithelial cells. Liver bud formation is tightly controlled by a network of transcription factors, including Hex, GATA-6, (HNF)-6, OC-2, Prox-1. Proliferation of hepatoblasts occurs under the influence of a variety of cytokines and growth factors secreted by mesenchymal cells in the septum transversum, such as FGF, EGF, HGF, TGF-β, TNF-α, and IL-6. Additionally, Maturation of hepatoblasts into hepatocytes is carried out by cytokine OSM, mesenchyme factors (HGF and Wnt), whereas JAGGED/NOTCH signaling pathway plays a major role in cholangiocyte maturation as well as a proper bile duct morphogenesis.
Further studies are needed to elaborate the hemodynamic events, the hormonal contribution to liver regeneration and the molecules that control the termination phase. Indeed, studying all the processes underlying liver regeneration has a huge impact in the clinical practice and can lead to the invention of new technologies and drugs that treat different types of liver failure. Current works are focusing on populating human hepatocytes in large animals like pigs and use them as an incubator to produce human liver. Furthermore, other research efforts involve the use of a drug to change the activity of a specific molecule involved in the liver regeneration leading to a robust hepatocyte regeneration. These researches are going to be a turning point in the liver field and solve all the problems pertained to the liver transplantation process including pretransplant waiting, shortage of organ, rejection, and long-term immunosuppression after the transplantation. Therefore, the process of converting the concept of liver regeneration from the bench work to the bedside practice should be high considered and investigated in the future studies.
Footnotes
CONFLICT OF INTEREST
The authors have declared no conflicts of interest for this article.
REFERENCES
- Abdellatif H, & Shiha G (2017). Liver Regeneration: Summary Of Involved Cell Types. Journal of Stem Cell and Regenerative Biology, 3(1), 122–125. [Google Scholar]
- Ahmed O, Patel MV, Masrani A, Chong B, Osman M, Tasse J, … Arslan B (2017). Assessing intra-arterial complications of planning and treatment angiograms for Y-90 Radioembolization. CardioVascular and Interventional Radiology, 40(5), 704–711. [DOI] [PubMed] [Google Scholar]
- Alison MR, Poulsom R, & Forbes SJ (2001). Update on hepatic stem cells. Liver, 21(6), 367–373. [DOI] [PubMed] [Google Scholar]
- Alvaro D, Gigliozzi A, & Attili AF (2000). Regulation and deregulation of cholangiocyte proliferation. Journal of Hepatology, 33(2), 333–340. [DOI] [PubMed] [Google Scholar]
- Appasamy R, Tanabe M, Murase N, Zarnegar R, Venkataramanan R, Van Thiel DH, & Michalopoulosm GK (1993). Hepatocyte growth factor, blood clearance, organ uptake, and biliary excretion in normal and partially hepatectomized rats. Laboratory Investigation, 68(3), 270–276. [PubMed] [Google Scholar]
- Azuma H, Paulk N, Ranade A, Dorrell C, Al-Dhalimy M, Ellis E, … Grompe M (2007). Robust expansion of human hepatocytes in Fah(−/−)/Rag2(−/−)/Il2rg (−/−) mice. Nature Biotechnology, 25(8), 903–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartscherer K, Pelte N, Ingelfinger D, & Boutros M (2006). Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell, 125(3), 523–533. [DOI] [PubMed] [Google Scholar]
- Bendris N, Lemmers B, Blanchard JM, & Arsic N (2011). Cyclin A2 mutagenesis analysis: A new insight into CDK activation and cellular localization requirements. PLoS One, 6(7), e22879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berasain C, Garcia-Trevijano ER, Castillo J, Erroba E, Lee DC, Prieto J, & Avila MA (2005). Amphiregulin: An early trigger of liver regeneration in mice. Gastroenterology, 128(2), 424–432. [DOI] [PubMed] [Google Scholar]
- Berg T, Rountree CB, Lee L, Estrada J, Sala FG, Choe A, … Wang KS (2007). Fibroblast growth factor 10 is critical for liver growth during embryogenesis and controls hepatoblast survival via beta-catenin activation. Hepatology, 46(4), 1187–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best J, Manka P, Syn W-K, Dollé L, van Grunsven LA, & Canbay A (2015). Role of liver progenitors in liver regeneration. Hepatobiliary Surgery and Nutrition, 4(1), 48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhanot P, Brink M, Samos CH, Hsieh JC, Wang Y, Macke JP, … Nusse R (1996). A new member of the frizzled family from drosophila functions as a wingless receptor. Nature, 382(6588), 225–230. [DOI] [PubMed] [Google Scholar]
- Block GD, Locker J., Bowen WC, Petersen BE, Katyal S, Strom SC, … Michalopoulos GK (1996). Population expansion, clonal growth, and specific differentiation patterns in primary cultures of hepatocytes induced by HGF/SF, EGF and TGF alpha in a chemically defined (HGM) medium. The Journal of Cell Biology, 132(6), 1133–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhm F, Köhler UA, Speicher T, & Werner S (2010). Regulation of liver regeneration by growth factors and cytokines. EMBO Molecular Medicine, 2(8), 294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bort R, Signore M, Tremblay K, Martinez Barbera JP, & Zaret KS (2006). Hex homeobox gene controls the transition of the endoderm to a pseudostratified, cell emergent epithelium for liver bud development. Developmental Biology, 290(1), 44–56. [DOI] [PubMed] [Google Scholar]
- Bossard P, & Zaret KS (1998). GATA transcription factors as potentiators of gut endoderm differentiation. Development, 125(24), 4909–917. [DOI] [PubMed] [Google Scholar]
- Bossard P, & Zaret KS (2000). Repressive and restrictive mesodermal interactions with gut endoderm: Possible relation to Meckel's diverticulum. Development (Cambridge, England), 127(22), 4915–4923. [DOI] [PubMed] [Google Scholar]
- Bouzahzah B, Fu M, Iavarone A, Factor VM, Thorgeirsson SS, & Pestell RG (2000). Transforming growth factor-beta1 recruits histone deacetylase 1 to a p130 repressor complex in transgenic mice in vivo. Cancer Research, 60(16), 4531–4537. [PubMed] [Google Scholar]
- Bria A, Marda J, Zhou J, Sun X, Cao Q, Petersen BE, & Pi L (2017). Hepatic progenitor cell activation in liver repair. Liver Research, 1(2), 81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broten J, Michalopoulos G, Petersen B, & Cruise J (1999). Adrenergic stimulation of hepatocyte growth factor expression. Biochemical and Biophysical Research Communications, 262(1), 76–79. [DOI] [PubMed] [Google Scholar]
- Bucher NLR, & Weir GC (1976). Insulin, glucagon, liver regeneration, and DNA synthesis. Metabolism, Clinical and Experimental, 25(11), 1423–1425. [DOI] [PubMed] [Google Scholar]
- Burke Z, & Oliver G (2002). Prox1 is an early specific marker for the developing liver and pancreas in the mammalian foregut endoderm. Mechanisms of Development, 118(1–2), 147–155. [DOI] [PubMed] [Google Scholar]
- Cadigan KM, & Nusse R (1997). Wnt signaling: A common theme in animal development. Genes & Development, 11(24), 3286–3305. [DOI] [PubMed] [Google Scholar]
- Caldas H, Jiang Y, Holloway MP, Fangusaro J, Mahotka C, Conway EM, & Altura RA (2005). Survivin splice variants regulate the balance between proliferation and cell death. Oncogene, 24(12), 1994–2007. [DOI] [PubMed] [Google Scholar]
- Calmont A, Wandzioch E, Tremblay KD, Minowada G, Kaestner KH, Martin GR, & Zaret KS (2006). An FGF response pathway that mediates hepatic gene induction in embryonic endoderm cells. Developmental Cell, 11(3), 339–348. [DOI] [PubMed] [Google Scholar]
- Chen HS, Joo DJ, Shaheen M, Li Y, Wang Y, Yang J, … Mounajjed T (2019). Randomized trial of spheroid reservoir bioartificial liver in porcine model of posthepatectomy liver failure. Hepatology, 69(1), 329–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng W, Guo L, Zhang Z, Soo HM, Wen C, Wu W, & Peng J (2006). HNF factors form a network to regulate liver-enriched genes in zebrafish. Developmental Biology, 294(2), 482–496. [DOI] [PubMed] [Google Scholar]
- Choi TY, Ninov N, Stainier DY, & Shin D (2014). Extensive conversion of hepatic biliary epithelial cells to hepatocytes after near total loss of hepatocytes in zebrafish. Gastroenterology, 146(3), 776–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H, Loh KM, & Nusse R (2014). Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science, 346(6205), 1248012. [DOI] [PubMed] [Google Scholar]
- Clotman F, & Lemaigre FP (2006). Control of hepatic differentiation by activin/TGFbeta signaling. Cell Cycle, 5(2), 168–171. [DOI] [PubMed] [Google Scholar]
- Collin de l'Hortet A, Gilgenkrantz H, & Guidotti J-E (2012). EGFR: A master piece in G1/S phase transition of liver regeneration. International Journal of Hepatology, 2012, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosby HA, Hubscher S, Fabris L, Joplin R, Sell S, Kelly D, & Strain AJ (1998). Immunolocalization of putative human liver progenitor cells in livers from patients with end-stage primary biliary cirrhosis and sclerosing cholangitis using the monoclonal antibody OV-6. The American Journal of Pathology, 152(3), 771–779. [PMC free article] [PubMed] [Google Scholar]
- Cruise JL, Houck KA, & Michalopoulos GK (1985). Induction of DNA synthesis in cultured rat hepatocytes through stimulation of alpha 1 adrenoreceptor by norepinephrine. Science, 227(4688), 749–751. [DOI] [PubMed] [Google Scholar]
- Cruise JL, Knechtle SJ, Bollinger RR, Kuhn C, & Michalopoulos G (1987). α1-Adrenergic effects and liver regeneration. Hepatology, 7(6), 1189–1194. [DOI] [PubMed] [Google Scholar]
- De Boer L, Oakes V, Beamish H, Giles N, Stevens F, Somodevilla-Torres M, … Gabrielli B (2008). Cyclin a/cdk2 coordinates centrosomal and nuclear mitotic events. Oncogene, 27, 4261–4268. [DOI] [PubMed] [Google Scholar]
- Decaens T, Godard C, de Reynies A, Rickman DS, Tronche F, Couty JP, … Colnot S (2008). Stabilization of beta-catenin affects mouse embryonic liver growth and hepatoblast fate. Hepatology, 47(1), 247–258. [DOI] [PubMed] [Google Scholar]
- Defrances MC, Wolf HK, Michalopoulos GK, & Zarnegar R (1992). The presence of hepatocyte growth factor in the developing rat. Development, 116(2), 387–395. [DOI] [PubMed] [Google Scholar]
- Diehl JA (2002). Cycling to cancer with cyclin D1. Cancer Biology & Therapy, 1(3), 226–231. [DOI] [PubMed] [Google Scholar]
- Eferl R, Sibilia M, Hilberg F, Fuchsbichler A, Kufferath I, Guertl B, … Zatloukal K (1999). Functions of c-Jun in liver and heart development. The Journal of Cell Biology, 145(5), 1049–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elaine N, & Marieb KNH (2012). Human Anatomy & Physiology + new mastering a&p with Pearson Etext (9th ed.). London, England: Benjamin-Cummings Pub Co. [Google Scholar]
- Espanol-Suner R, Carpentier R, Van Hul N, Legry V, Achouri Y, Cordi S, …. Leclercq IA (2012). Liver progenitor cells yield functional hepatocytes in response to chronic liver injury in mice. Gastroenterology, 143(6), 1564–1575. [DOI] [PubMed] [Google Scholar]
- Farber E (1956). Similarities in the sequence of early histological changes induced in the liver of the rat by ethionine, 2-acetylamino-fluorene, and 3′-methyl-4-dimethylaminoazobenzene. Cancer Research, 16(2), 142–148. [PubMed] [Google Scholar]
- Fausto N, & Campbell JS (2003). The role of hepatocytes and oval cells in liver regeneration and repopulation. Mechanisms of Development, 120(1), 117–130. [DOI] [PubMed] [Google Scholar]
- Fausto N, Campbell JS, & Riehle KJ (2006). Liver regeneration. Hepatology, 43(2 Suppl 1), S45–S53. [DOI] [PubMed] [Google Scholar]
- Fruman DA, Mauvais-Jarvis F, Pollard DA, Yballe CM, Brazil D, Bronson RT, … Cantley LC (2000). Hypoglycaemia, liver necrosis and perinatal death in mice lacking all isoforms of phosphoinositide 3-kinase p85 alpha. Nature Genetics, 26(3), 379–382. [DOI] [PubMed] [Google Scholar]
- Fujita M, Furukawa H, Hattori M, Todo S, Ishida Y, & Nagashima K (2000). Sequential observation of liver cell regeneration after massive hepatic necrosis in auxiliary partial orthotopic liver transplantation. Modern Pathology, 13(2), 152–157. [DOI] [PubMed] [Google Scholar]
- Gaudio E, Carpino G, Cardinale V, Franchitto A, Onori P, & Alvaro D (2009). New insights into liver stem cells. Digestive and Liver Disease, 41(7), 455–462. [DOI] [PubMed] [Google Scholar]
- Gebhardt R, Baldysiak-Figiel A, Krugel V, Ueberham E, & Gaunitz F (2007). Hepatocellular expression of glutamine synthetase: An indicator of morphogen actions as master regulators of zonation in adult liver. Progress in Histochemistry and Cytochemistry, 41(4), 201–266. [DOI] [PubMed] [Google Scholar]
- Geisler F, & Strazzabosco M (2015). Emerging roles of Notch signaling in liver disease. Hepatology, 61(1), 382–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilgenkrantz H (2018). Collin de l’Hortet a. Understanding liver regeneration: From mechanisms to regenerative medicine. The American Journal of Pathology, 188 (6), 1316–1327. [DOI] [PubMed] [Google Scholar]
- Goessling W, North TE, Lord AM, Ceol C, Lee S, Weidinger G, … Zon LI (2008). APC mutant zebrafish uncover a changing temporal requirement for wnt signaling in liver development. Developmental Biology, 320(1), 161–174. [DOI] [PubMed] [Google Scholar]
- Hassan GM, Sliem HA, & Ellethy AT (2013). Hepatic embryonic development and anomalies of the liver. Journal of Gastroenterology and Hepatology Research, 2(4), 489–493. [Google Scholar]
- Hata S, Namae M, & Nishina H (2007). Liver development and regeneration: From laboratory study to clinical therapy. Development, Growth & Differentiation, 49 (2), 163–170. [DOI] [PubMed] [Google Scholar]
- He J, Lu H, Zou Q, & Luo L (2014). Regeneration of liver after extreme hepatocyte loss occurs mainly via biliary transdifferentiation in zebrafish. Gastroenterology, 146(3), 789–800. [DOI] [PubMed] [Google Scholar]
- Hickey RD, Mao SA, Glorioso J, Lillegard JB, Fisher JE, Amiot B, … Nyberg SL (2014). Fumarylacetoacetate hydrolase deficient pigs are a novel large animal model of metabolic liver disease. Stem Cell Research, 13(1), 144–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins GM, & Anderson RM (1931). Experimental pathology of the liver. I. Restoration of the liver of the white rat following partial surgical removal. Archives of Pathology, 12, 186–202. [Google Scholar]
- Hines IN, Kremer M, Isayama F, Perry AW, Milton RJ, Black AL, … Wheeler MD (2007). Impaired liver regeneration and increased oval cell numbers following T cell-mediated hepatitis. Hepatology, 46(1), 229–241. [DOI] [PubMed] [Google Scholar]
- Houck KA, Cruise JL, & Michalopoulos G (1988). Norepinephrine modulates the growth-inhibitory effect of transforming growth factor-beta in primary rat hepatocyte cultures. Journal of Cellular Physiology, 135(3), 551–555. [DOI] [PubMed] [Google Scholar]
- Huang M, Chang A, Choi M, Zhou D, Anania FA, & Shin CH (2014). Antagonistic interaction between Wnt and Notch activity modulates the regenerative capacity of a zebrafish fibrotic liver model. Hepatology, 60(5), 1753–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain SZ, Sneddon T, Tan X, Micsenyi A, Michalopoulos GK, & Monga SP (2004). Wnt impacts growth and differentiation in ex vivo liver development. Experimental Cell Research, 292(1), 157–169. [DOI] [PubMed] [Google Scholar]
- Ijpenberg A, Perez-Pomares JM, Guadix JA, Carmona R, Portillo-Sanchez V, Macias D, … Muñoz-Chápuli R (2007). Wt1 and retinoic acid signaling are essential for stellate cell development and liver morphogenesis. Developmental Biology, 312(1), 157–170. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Nagoshi S, Ohno A, Yanase M, Maekawa H, & Fujiwara K (1998). Activated rat stellate cells express c-met and respond to hepatocyte growth factor to enhance transforming growth factor beta1 expression and DNA synthesis. Biochemical and Biophysical Research Communications, 250(3), 769–775. [DOI] [PubMed] [Google Scholar]
- Jelkmann W (2001). The role of the liver in the production of thrombopoietin compared with erythropoietin. European Journal of Gastroenterology & Hepatology, 13 (7), 791–801. [DOI] [PubMed] [Google Scholar]
- Jo M, Stolz DB, Esplen JE, Dorko K, Michalopoulos GK, & Strom SC (2000). Cross-talk between epidermal growth factor receptor and c-Met signal pathways in transformed cells. The Journal of Biological Chemistry, 275(12), 8806–8811. [DOI] [PubMed] [Google Scholar]
- Kamiya A, Kinoshita T, & Miyajima A (2001). Oncostatin M and hepatocyte growth factor induce hepatic maturation via distinct signaling pathways. FEBS Letters, 492(1–2), 90–94. [DOI] [PubMed] [Google Scholar]
- Karamysheva AF (2007). Review: Mechanisms of angiogenesis. Biochemistry (Moscow), 73, 751. [DOI] [PubMed] [Google Scholar]
- Katoonizadeh A, & Poustchi H (2014). Adult hepatic progenitor cell niche: How it affects the progenitor cell fate. Middle East Journal of Digestive Diseases, 6(2), 57–64. [PMC free article] [PubMed] [Google Scholar]
- Kaur S, Siddiqui H, & Bhat MH (2015). Hepatic progenitor cells in action: Liver regeneration or fibrosis? The American Journal of Pathology, 185(9), 2342–2350. [DOI] [PubMed] [Google Scholar]
- Keaton MA (2007). Review of “The Cell Cycle: Principles of Control” by David O. Morgan. Cell Division, 2, 27. [Google Scholar]
- Kholodenko IV, & Yarygin KN (2017). Cellular mechanisms of liver regeneration and cell-based therapies of liver diseases. BioMed Research International, 2017, 8910821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TH, Mars WM, Stolz DB, & Michalopoulos GK (2000). Expression and activation of pro-MMP-2 and pro-MMP-9 during rat liver regeneration. Hepatology, 31(1), 75–82. [DOI] [PubMed] [Google Scholar]
- Kmiec Z (2001). Cooperation of liver cells in health and disease. Advances in Anatomy, Embryology, and Cell Biology, 161:III–XIII, 1–151. [DOI] [PubMed] [Google Scholar]
- Kodama Y, Hijikata M, Kageyama R, Shimotohno K, & Chiba T (2004). The role of notch signaling in the development of intrahepatic bile ducts. Gastroenterology, 127(6), 1775–1786. [DOI] [PubMed] [Google Scholar]
- Kogure K, Zhang YQ, Kanzaki M, Omata W, Mine T, & Kojima I (1996). Intravenous administration of follistatin: Delivery to the liver and effect on liver regeneration after partial hepatectomy. Hepatology, 24(2), 361–366. [DOI] [PubMed] [Google Scholar]
- Kyrmizi I, Hatzis P, Katrakili N, Tronche F, Gonzalez FJ, & Talianidis I (2006). Plasticity and expanding complexity of the hepatic transcription factor network during liver development. Genes & Development, 20(16), 2293–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaigre FP (2003). Development of the biliary tract. Mechanisms of Development, 120(1), 81–87. [DOI] [PubMed] [Google Scholar]
- Lesurtel M, Graf R, Aleil B, Walther DJ, Tian Y, Jochum W, … Clavien PA (2006). Platelet-derived serotonin mediates liver regeneration. Science, 312 (5770), 104–107. [DOI] [PubMed] [Google Scholar]
- Li L, Krantz ID, Deng Y, Genin A, Banta AB, Collins CC, … Spinner NB (1997). Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nature Genetics, 16(3), 243–251. [DOI] [PubMed] [Google Scholar]
- Li W, Liang X, Leu JI, Kovalovich K, Ciliberto G, & Taub R (2001). Global changes in interleukin-6-dependent gene expression patterns in mouse livers after partial hepatectomy. Hepatology, 33(6), 1377–1386. [DOI] [PubMed] [Google Scholar]
- Libbrecht L, Desmet V, Van Damme B, & Roskams T (2000). Deep intralobular extension of human hepatic 'progenitor cells' correlates with parenchymal inflammation in chronic viral hepatitis: Can'progenitor cells' migrate? The Journal of Pathology, 192(3), 373–378. [DOI] [PubMed] [Google Scholar]
- Liu M, & Chen P (2017). Proliferation-inhibiting pathways in liver regeneration (review). Molecular Medicine Reports, 16(1), 23–35. [DOI] [PubMed] [Google Scholar]
- Liu ML, Mars WM, Zarnegar R, & Michalopoulos GK (1994). Collagenase pretreatment and the mitogenic effects of hepatocyte growth factor and transforming growth factor-alpha in adult rat liver. Hepatology, 19(6), 1521–1527. [PubMed] [Google Scholar]
- Loomes KM, Russo P, Ryan M, Nelson A, Underkoffler L, Glover C, …. Oakey RJ (2007). Bile duct proliferation in liver-specific Jag1 conditional knockout mice: Effects of gene dosage. Hepatology, 45(2), 323–330. [DOI] [PubMed] [Google Scholar]
- Lozier J, McCright B, & Gridley T (2008). Notch signaling regulates bile duct morphogenesis in mice. PLoS One, 3(3), e1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Zhou Y, Hu T, Zhang H, Shen M, Cheng P, … Guo C (2016). Notch signaling coordinates progenitor cell-mediated biliary regeneration following partial hepatectomy. Scientific Reports, 6, 22754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, … Behrens J (2002). Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Molecular and Cellular Biology, 22(4), 1184–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado MV, Michelotti GA, Xie G, Almeida Pereira T, Boursier J, Bohnic B, … Diehl AM (2015). Mouse models of diet-induced nonalcoholic steatohepatitis reproduce the heterogeneity of the human disease. PLoS One, 10(5), e0127991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malarkey DE, Johnson K, Ryan L, Boorman G, & Maronpot RR (2005). New insights into functional aspects of liver morphology. Toxicologic Pathology, 33 (1), 27–34. [DOI] [PubMed] [Google Scholar]
- Mao SA, Glorioso JM, & Nyberg SL (2014). Liver regeneration. Translational Research, 163(4), 352–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margagliotti S, Clotman F, Pierreux CE, Beaudry JB, Jacquemin P, Rousseau GG, & Lemaigre FP (2007). The Onecut transcription factors HNF-6/OC-1 and OC-2 regulate early liver expansion by controlling hepatoblast migration. Developmental Biology, 311(2), 579–589. [DOI] [PubMed] [Google Scholar]
- Margagliotti S, Clotman F, Pierreux CE, Lemoine P, Rousseau GG, Henriet P, & Lemaigre FP (2008). Role of metalloproteinases at the onset of liver development. Development, Growth & Differentiation, 50(5), 331–338. [DOI] [PubMed] [Google Scholar]
- Matsui T, Kinoshita T, Morikawa Y, Tohya K, Katsuki M, Ito Y, … Miyajima A (2002). K-Ras mediates cytokine-induced formation of E-cadherin-based adherens junctions during liver development. The EMBO Journal, 21(5), 1021–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K, Yoshitomi H, Rossant J, & Zaret KS (2001). Liver organogenesis promoted by endothelial cells prior to vascular function. Science, 294(5542), 559–563. [DOI] [PubMed] [Google Scholar]
- McDaniell R, Warthen DM, Sanchez-Lara PA, Pai A, Krantz ID, Piccoli DA, & Spinner NB (2006). NOTCH2 mutations cause Alagille syndrome, a heterogeneous disorder of the NOTCH signaling pathway. American Journal of Human Genetics, 79(1), 169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLin VA, Rankin SA, & Zorn AM (2007). Repression of Wnt/beta-catenin signaling in the anterior endoderm is essential for liver and pancreas development. Development (Cambridge, England), 134(12), 2207–2217. [DOI] [PubMed] [Google Scholar]
- Medlock ES, & Haar JL (1983). The liver hemopoietic environment: I. Developing hepatocytes and their role in fetal hemopoiesis. The Anatomical Record, 207 (1), 31–41. [DOI] [PubMed] [Google Scholar]
- Michalopoulos GK (2007). Liver regeneration. Journal of Cellular Physiology, 213(2), 286–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalopoulos GK (2010a). Liver regeneration after partial hepatectomy: Critical analysis of mechanistic dilemmas. The American Journal of Pathology, 176 (1), 2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalopoulos GK (2010b). Liver regeneration after partial hepatectomy. The American Journal of Pathology, 176(1), 2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalopoulos GK, Barua L, & Bowen WC (2005). Transdifferentiation of rat hepatocytes into biliary cells after bile duct ligation and toxic biliary injury. Hepatology, 41(3), 535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalopoulos GK, Bowen WC, Mule K, & Luo J (2003). HGF-, EGF-, and dexamethasone-induced gene expression patterns during formation of tissue in hepatic organoid cultures. Gene Expression, 11(2), 55–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalopoulos GK, & DeFrances MC (1997). Liver regeneration. Science, 276(5309), 60. [DOI] [PubMed] [Google Scholar]
- Micsenyi A, Tan X, Sneddon T, Luo JH, Michalopoulos GK, & Monga SP (2004). Beta-catenin is temporally regulated during normal liver development. Gastroenterology, 126(4), 1134–1146. [DOI] [PubMed] [Google Scholar]
- Mikula M, Schreiber M, Husak Z, Kucerova L, Ruth J, Wieser R, … Baccarini M (2001). Embryonic lethality and fetal liver apoptosis in mice lacking the c-raf-1 gene. The EMBO Journal, 20(8), 1952–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne LS (1909). The histology of liver tissue regeneration. The Journal of Pathology and Bacteriology, 13(1), 127–160. [Google Scholar]
- Miyajima A, Tanaka M, & Itoh T (2014). Stem/progenitor cells in liver development, homeostasis, regeneration, and reprogramming. Cell Stem Cell, 14(5), 561–574. [DOI] [PubMed] [Google Scholar]
- Monga SP, Mars WM, Pediaditakis P, Bell A, Mule K, Bowen WC, … Michalopoulos GK (2002). Hepatocyte growth factor induces Wnt-independent nuclear translocation of beta-catenin after met-beta-catenin dissociation in hepatocytes. Cancer Research, 62(7), 2064–2071. [PubMed] [Google Scholar]
- Morell CM, Fiorotto R, Fabris L, & Strazzabosco M (2013). Notch signalling beyond liver development: Emerging concepts in liver repair and oncogenesis. Clinics and Research in Hepatology and Gastroenterology, 37(5), 447–454. [DOI] [PubMed] [Google Scholar]
- Nalesnik MA, Federle M, Buck D, Fontes P, & Carr BI (2009). Hepatobiliary effects of 90yttrium microsphere therapy for unresectable hepatocellular carcinoma. Human Pathology, 40(1), 125–134. [DOI] [PubMed] [Google Scholar]
- Nejak-Bowen KN, & Monga SPS (2015). Chapter 6 - Developmental pathways in liver regeneration-I In Apte U (Ed.), Liver regeneration (pp. 77–101). Boston, MA: Academic Press. [Google Scholar]
- Nijjar SS, Crosby HA, Wallace L, Hubscher SG, & Strain AJ (2001). Notch receptor expression in adult human liver: a possible role in bile duct formation and hepatic neovascularization. Hepatology, 34(6), 1184–1192. [DOI] [PubMed] [Google Scholar]
- Ohlson LC, Koroxenidou L, & Hallstrom IP (1998). Inhibition of in vivo rat liver regeneration by 2-acetylaminofluorene affects the regulation of cell cycle-related proteins. Hepatology, 27(3), 691–696. [DOI] [PubMed] [Google Scholar]
- Olsen PS, Poulsen SS, & Kirkegaard P (1985). Adrenergic effects on secretion of epidermal growth factor from Brunner's glands. Gut, 26, 920–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano M, Pepperkok R, Verde F, Ansorge W, & Draetta G (1992). Cyclin a is required at two points in the human cell cycle. The EMBO Journal, 11(3), 961–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmes D, & Spiegel HU (2004). Animal models of liver regeneration. Biomaterials, 25(9), 1601–1611. [DOI] [PubMed] [Google Scholar]
- Papetti M, & Herman IM (2002). Mechanisms of normal and tumor-derived angiogenesis. American Journal of Physiology: Cell Physiology, 282(5), C947–C970. [DOI] [PubMed] [Google Scholar]
- Patijn GA, Lieber A, Schowalter DB, Schwall R, & Kay MA (1998). Hepatocyte growth factor induces hepatocyte proliferation in vivo and allows for efficient retroviral-mediated gene transfer in mice. Hepatology, 28(3), 707–716. [DOI] [PubMed] [Google Scholar]
- Pelton RW, Saxena B, Jones M, Moses HL, & Gold LI (1991). Immunohistochemical localization of TGF beta 1, TGF beta 2, and TGF beta 3 in the mouse embryo: Expression patterns suggest multiple roles during embryonic development. The Journal of Cell Biology, 115(4), 1091–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen BE, Zajac VF, & Michalopoulos GK (1998). Hepatic oval cell activation in response to injury following chemically induced periportal or pericentral damage in rats. Hepatology, 27(4), 1030–1038. [DOI] [PubMed] [Google Scholar]
- Pritchard MT, & Apte U (2015). Models to study liver regeneration In Apte U (Ed.), Liver regeneration: Basic mechanisms, relevant models and clinical applications (pp. 15–40). Cambridge, MA: Academic press. [Google Scholar]
- Ray MB, Mendenhall CL, French SW, & Gartside PS (1993). Bile duct changes in alcoholic liver disease. The veterans administration cooperative study group. Liver, 13(1), 36–45. [DOI] [PubMed] [Google Scholar]
- Ribatti D, & Crivellato E (2012). “Sprouting angiogenesis”, a reappraisal. Developmental Biology, 372(2), 157–165. [DOI] [PubMed] [Google Scholar]
- Roskams T, De Vos R, Van Eyken P, Myazaki H, Van Damme B, & Desmet V (1998). Hepatic OV-6 expression in human liver disease and rat experiments: Evidence for hepatic progenitor cells in man. Journal of Hepatology, 29(3), 455–463. [DOI] [PubMed] [Google Scholar]
- Roskams T, Katoonizadeh A, & Komuta M (2010). Hepatic progenitor cells: An update. Clinics in Liver Disease, 14(4), 705–718. [DOI] [PubMed] [Google Scholar]
- Roskams TA, Theise ND, Balabaud C, Bhagat G, Bhathal PS, Bioulac-Sage P, … West AB (2004). Nomenclature of the finer branches of the biliary tree: Canals, ductules, and ductular reactions in human livers. Hepatology, 39(6), 1739–1745. [DOI] [PubMed] [Google Scholar]
- Rozga J (2002). Hepatocyte proliferation in health and in liver failure. Medical Science Monitor, 8(2), RA32–8. [PubMed] [Google Scholar]
- Russell JO, & Monga SP (2018). Wnt/β-catenin signaling in liver development, homeostasis, and pathobiology. Annual Review of Pathology, 13, 351–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell WE, Coffey RJ Jr., Ouellette AJ, & Moses HL (1988). Type beta transforming growth factor reversibly inhibits the early proliferative response to partial hepatectomy in the rat. Proceedings of the National Academy of Sciences of the United States of America, 85(14), 5126–5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rychtrmoc D, Libra A, Buncek M, Garnol T, & Cervinkova Z (2009). Studying liver regeneration by means of molecular biology: How far we are in interpreting the findings? Acta Medica (Hradec Královè), 52(3), 91–99. [DOI] [PubMed] [Google Scholar]
- Sadri A-R, Jeschke MG, & Amini-Nik S (2015). Cellular and molecular cascades during liver regeneration. Surgical Research Open Journal, 2(2), 53–61. [Google Scholar]
- Samson CM, Schrum LW, Bird MA, Lange PA, Brenner DA, Rippe RA, & Behrns KE (2002). Transforming growth factor-beta1 induces hepatocyte apoptosis by a c-Jun independent mechanism. Surgery, 132(3), 441–449. [DOI] [PubMed] [Google Scholar]
- Saxena R, & Theise N (2004). Canals of Hering: Recent insights and current knowledge. Seminars in Liver Disease, 24(1), 43–48. [DOI] [PubMed] [Google Scholar]
- Schaffner F (1991). Structural and functional aspects of regeneration of human liver. Digestive Diseases and Sciences, 36(9), 1282–1286. [DOI] [PubMed] [Google Scholar]
- Schwall RH, Robbins K, Jardieu P, Chang L, Lai C, & Terrell TG (1993). Activin induces cell death in hepatocytes in vivo and in vitro. Hepatology, 18(2), 347–356. [DOI] [PubMed] [Google Scholar]
- Senger DR, & Davis GE (2011). Angiogenesis. Cold Spring Harbor Perspectives in Biology, 3(8), a005090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergi C, Kahl P, & Otto HF (2000). Contribution of apoptosis and apoptosis-related proteins to the malformation of the primitive intrahepatic biliary system in Meckel syndrome. The American Journal of Pathology, 156(5), 1589–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen T, & Huang S (2012). The role of Cdc25A in the regulation of cell proliferation and apoptosis. Anti-Cancer Agents in Medicinal Chemistry, 12(6), 631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiojiri N, Takeshita K, Yamasaki H, & Iwata T (2004). Suppression of C/EBP alpha expression in biliary cell differentiation from hepatoblasts during mouse liver development. Journal of Hepatology, 41(5), 790–798. [DOI] [PubMed] [Google Scholar]
- Short J, Wedmore R, Kibert L, & Zemel R (1980). Triidothyronine: On its role as a specific hepatomitogen. Cytobios, 28(111–112), 165–177. [PubMed] [Google Scholar]
- Sokabe T, Yamamoto K, Ohura N, Nakatsuka H, Qin K, Obi S, … Ando J (2004). Differential regulation of urokinase-type plasminogen activator expression by fluid shear stress in human coronary artery endothelial cells. American Journal of Physiology. Heart and Circulatory Physiology, 287(5), H2027–H2034. [DOI] [PubMed] [Google Scholar]
- Sosa-Pineda B, Wigle JT, & Oliver G (2000). Hepatocyte migration during liver development requires Prox1. Nature Genetics, 25(3), 254–255. [DOI] [PubMed] [Google Scholar]
- Stanger BZ, & Greenbaum L (2012). The role of paracrine signals during liver regeneration. Hepatology, 56(4), 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starzl TE, Watanabe K, Porter KA, & Putnam CW (1976). Effects of insulin, glucagon, and insuling/glucagon infusions on liver morphology and cell division after complete portacaval shunt in dogs. Lancet, 1(7964), 821–825. [DOI] [PubMed] [Google Scholar]
- Stolz DB, Mars WM, Petersen BE, Kim TH, & Michalopoulos GK (1999). Growth factor signal transduction immediately after two-thirds partial hepatectomy in the rat. Cancer Research, 59(16), 3954–3960. [PubMed] [Google Scholar]
- Suchy FJ, Sokol RJ, & Balistreri WF (2014). Liver disease in children. Cambridge: Cambridge University Press. [Google Scholar]
- Suzuki A, Sekiya S, Buscher D, Izpisua Belmonte JC, & Taniguchi H (2008). Tbx3 controls the fate of hepatic progenitor cells in liver development by suppressing p19ARF expression. Development (Cambridge, England), 135(9), 1589–1595. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Kanai Y, Hara T, Sasaki J, Sasaki T, Kohara M, …Kanaho Y (2006). Crucial role of the small GTPase ARF6 in hepatic cord formation during liver development. Molecular and Cellular Biology, 26(16), 6149–6156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swindle CS, Tran KT, Johnson TD, Banerjee P, Mayes AM, Griffith L, & Wells A (2001). Epidermal growth factor (EGF)-like repeats of human tenascin-C as ligands for EGF receptor. The Journal of Cell Biology, 154(2), 459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X, Yuan Y, Zeng G, Apte U, Thompson MD, Cieply B, … Monga SP (2008). Beta-catenin deletion in hepatoblasts disrupts hepatic morphogenesis and survival during mouse development. Hepatology, 47(5), 1667–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Itoh T, Tanimizu N, & Miyajima A (2011). Liver stem/progenitor cells: Their characteristics and regulatory mechanisms. The Journal of Biochemistry, 149(3), 231–239. [DOI] [PubMed] [Google Scholar]
- Tang Y, Katuri V, Dillner A, Mishra B, Deng CX, & Mishra L (2003). Disruption of transforming growth factor-beta signaling in ELF beta-spectrin-deficient mice. Science, 299(5606), 574–577. [DOI] [PubMed] [Google Scholar]
- Tanimizu N, & Miyajima A (2004). Notch signaling controls hepatoblast differentiation by altering the expression of liver-enriched transcription factors. Journal of Cell Science, 117(Pt 15), 3165–3174. [DOI] [PubMed] [Google Scholar]
- Thornton BR, & Toczyski DP (2003). Securin and B-cyclin/CDK are the only essential targets of the APC. Nature Cell Biology, 5(12), 1090–1094. [DOI] [PubMed] [Google Scholar]
- Tirnitz-Parker JE, Viebahn CS, Jakubowski A, Klopcic BR, Olynyk JK, Yeoh GC, & Knight B (2010). Tumor necrosis factor-like weak inducer of apoptosis is a mitogen for liver progenitor cells. Hepatology, 52(1), 291–302. [DOI] [PubMed] [Google Scholar]
- Turner R, Lozoya O, Wang Y, Cardinale V, Gaudio E, Alpini G, … Reid LM (2011). Human hepatic stem cell and maturational liver lineage biology. Hepatology, 53(3), 1035–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vente MA, Wondergem M, van der Tweel I, van den Bosch MA, Zonnenberg BA, Lam MG, … Nijsen JF (2009). Yttrium-90 microsphere radioembolization for the treatment of liver malignancies: A structured meta-analysis. European Radiology, 19(4), 951–959. [DOI] [PubMed] [Google Scholar]
- Walesky C, & Apte U (2015). Chapter 7 - mechanisms of termination of liver regeneration In Apte U (Ed.), Liver regeneration (pp. 103–111). Boston, MA: Academic Press. [Google Scholar]
- Wang B, Zhao L, Fish M, Logan CY, & Nusse R (2015). Self-renewing diploid Axin2(+) cells fuel homeostatic renewal of the liver. Nature, 524(7564), 180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Wang C-M, Hou L-H, Dou G-R, Wang Y-C, Hu X-B, … Han H (2009). Disruption of the transcription factor recombination signal-binding protein-J kappa (RBP-J) leads to veno-occlusive disease and interfered liver regeneration in mice. Hepatology, 49, 268–277. [DOI] [PubMed] [Google Scholar]
- Weinstein M, Monga SP, Liu Y, Brodie SG, Tang Y, Li C, … Deng CX (2001). Smad proteins and hepatocyte growth factor control parallel regulatory pathways that converge on beta1-integrin to promote normal liver development. Molecular and Cellular Biology, 21(15), 5122–5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, & Cleaver O (2011). Tubulogenesis during blood vessel formation. Seminars in Cell & Developmental Biology, 22(9), 993–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam CH, Fung TK, & Poon RY (2002). Cyclin a in cell cycle control and cancer. Cellular and Molecular Life Sciences: CMLS, 59(8), 1317–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki H, Sada A, Iwata T, Niwa T, Tomizawa M, Xanthopoulos KG, … Shiojiri N (2006). Suppression of C/EBPalpha expression in periportal hepatoblasts may stimulate biliary cell differentiation through increased Hnf6 and Hnf1b expression. Development, 133(21), 4233–4243. [DOI] [PubMed] [Google Scholar]
- Zimmermann A (2004). Regulation of liver regeneration. Nephrology, Dialysis, Transplantation, 19(Suppl 4), iv6–iv10. [DOI] [PubMed] [Google Scholar]
- Zorn AM (2008). Liver development. StemBook [Internet]. Cambridge, MA: Harvard Stem Cell Institute. [PubMed] [Google Scholar]