Abstract
Polychlorinated biphenyls (PCBs) pose significant risk to the developing human brain; however, mechanisms of PCB developmental neurotoxicity (DNT) remain controversial. Two widely posited mechanisms are tested here using PCBs identified in pregnant women in the MARBLES cohort who are at increased risk for having a child with a neurodevelopmental disorder (NDD). As determined by gas chromatography-triple quadruple mass spectrometry, the mean PCB levels in maternal serum was 2.22 ng/mL. The 12 most abundant PCBs were tested singly and as a mixture mimicking the congener profile in maternal serum for activity at the thyroid hormone receptor (THR) and ryanodine receptor (RyR). Neither the mixture nor the individual congeners (2 fM to 2 µM) exhibited agonistic or antagonistic activity in a THR reporter cell line. However, as determined by equilibrium binding of [3H]ryanodine to RyR1-enriched microsomes, the mixture and the individual congeners (50 nM to 50 μM) increased RyR activity by 2.4-19.2-fold. 4-Hydroxyl (OH) and 4-sulfate metabolites of PCBs 11 and 52 had no TH activity; but 4-OH PCB 52 had higher potency than the parent congener towards RyR. These data support evidence implicating RyRs as targets in environmentally-triggered NDDs and suggest that PCB effects on the THR are not a predominant mechanism driving PCB DNT. These findings provide scientific rationale regarding a point of departure for quantitative risk assessment of PCB DNT, and identify in vitro assays for screening other environmental pollutants for DNT potential.
Keywords: In vitro toxicology, MARBLES, neurotoxicology, PCBs, thyroid hormone receptor, risk assessment, ryanodine receptor
Graphical Abstract
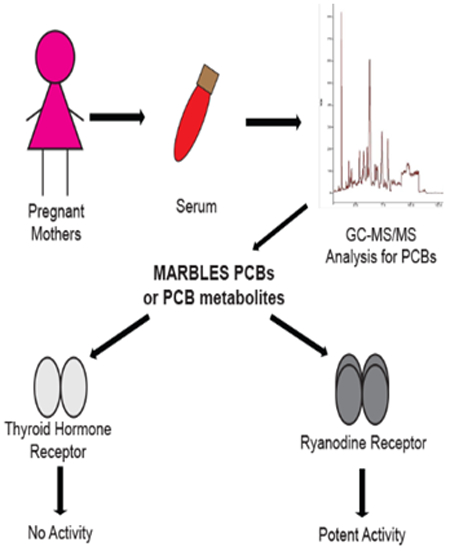
Introduction
Polychlorinated biphenyls (PCBs) are a class of persistent organic pollutants that were synthesized for multiple industrial and commercial applications. Despite the worldwide ban on their production in the early 2000’s, there continues to be widespread human exposure to PCBs,1, 2 including women of child-bearing age and children.3, 4 An important health concern for human PCB exposure is developmental neurotoxicity (DNT). The evidence from epidemiological studies and animal studies strongly supports the contention that PCBs are developmental neurotoxicants.5–7 Emerging epidemiological evidence suggests that PCBs increase risk for neurodevelopmental disorders (NDDs), including autism spectrum disorder (ASD) and attention deficit hyperactivity disorder (ADHD).8–10 The mechanism(s) by which PCBs disrupt neurodevelopment remain controversial. Two prevailing hypotheses include disruption of thyroid hormone (TH) signaling11–13 and modulation of calcium signaling in developing neurons.14–16
The scientific premise underlying the hypothesis that PCBs cause developmental neurotoxicity by interfering with the thyroid system is that TH signaling is critical for normal neurodevelopment. Congenital hypothyroidism, if untreated, causes severe adverse neurodevelopmental outcomes, 17–19 and while conflicting, there is some evidence that TH insufficiency may increase the risk of ASD20, 21 and is related to ADHD.22, 23 Thus, there is apparent overlap of neurodevelopmental deficits related to TH insufficiency and developmental PCB exposure. In addition, multiple studies have demonstrated that developmental PCB exposures decrease serum T4 levels in preclinical models,24–28 although these relationships are less consistent in humans.29, 30 Collectively, these observations have led many to posit that disruption of TH signaling contributes to neuropsychological deficits associated with developmental PCB exposures.11–13 However, this hypothesis is not supported by all studies. Outcomes of studies exploring relationships between PCB exposures and serum thyroid hormone levels are inconsistent and reviews of these studies generally do not support the concept that PCBs affect neurodevelopment by reducing serum thyroid levels.26 An alternative hypothesis is that individual PCB congeners or their metabolites interfere with thyroid hormone receptors, but these data are also inconsistent.31, 32
Calcium signaling is also critical to normal neurodevelopment and synaptic plasticity.33–35 Of the various mechanisms implicated in PCB effects on calcium signaling, the most sensitive is ryanodine receptor (RyR) sensitization.16, 36 The RyR is a Ca2+-regulated Ca2+ ion channel localized to the endoplasmic reticulum, and RyR sensitization by a subset of non-dioxin-like (NDL) PCBs stabilizes the ion channel in its open configuration, which increases release of Ca2+ from intracellular stores.16, 37 PCB effects on RyR are causally linked to enhanced dendritic arborization in vitro via activation of Ca2+-dependent signaling pathways that normally function to link neuronal activity to dendritic growth.38, 39 Effects of RyR-active PCBs on dendritic growth and plasticity have been confirmed in vivo,25, 38 and shown to coincide with deficits in cognitive behaviors.25
The relative contribution of THR vs. RyR-dependent mechanisms to PCB DNT remains an outstanding question in the field, and one that has been a point of discussion in terms of risk assessment. Multiple groups have argued for the need for risk assessment guidelines other than dioxin toxic equivalents or total sum PCBs, and the proposal to integrate either changes in TH signaling and/or RyR activity is a recurring theme.16, 40, 41 This debate is driven in part by the fact that only a small subset of individual PCB congeners have been tested for THR and RyR activity, and the relevance of the congeners that have been tested to current human exposures is uncertain. To address this controversy, we sought to identify PCBs relevant to contemporary human exposures, and to examine the influence of not only the individual congeners but also a mixture on THR and RyR activity. To this end, we leveraged samples from the Markers of Autism Risk in Babies-Learning Early Signs (MARBLES) study. MARBLES is a prospective study of pregnant women in northern California at increased risk for having a child with a NDD.42 The congener profile of PCBs in serum from pregnant women in the MARBLES cohort was determined, and these data were used to develop a mixture, referred to as the MARBLES mix, comprised of the 12 most abundant congeners in maternal serum. This mixture, as well as the individual congeners comprising the mixture and metabolites of two of the congeners in the MARBLES mix, PCB 11 and PCB 52, were tested for agonistic and antagonistic activity at the THR using a luciferase reporter cell line,43, 44 and for activity at the RyR1, as determined by radiolabeled ryanodine (Ry) binding studies.45, 46
Materials and Methods
Materials
Triiodo-L-thryonine (T3) and L-thyroxine (T4) were purchased from Sigma-Aldrich (≥ 95%; St. Louis, MO). PCB 11, 28, 52, 84, 95, 101, 118, 135, 138, 149, 153 and 180, as well as the hydroxylated and sulfated metabolites of PCB 11 and 52, were synthesized and authenticated by the Synthesis Core of the University of Iowa Superfund Research Program (The University of Iowa, Iowa City, IA). Synthesis methods for all congeners are detailed in the supplemental material except for PCB 11 and its metabolites, which have been reported previously.47–49 All synthesized PCBs were > 99% pure as determined by 1H-NMR, 13C-NMR, and GC-MS (Supplemental Material). THR antagonist, NH-3, was synthesized by Dr. Heike Wulff (University of California Davis, Davis, CA) as previously described.50 All stock solutions for in vitro experiments were made in dry sterile dimethylsulfoxide (DMSO, Sigma-Aldrich). PCB standards (PCB 11, 28, 52, 77, 84, 91, 95, 101, 118, 131, 132, 135, 136, 138, 149, 153, 174, 175, 176, 180, and 196) for the analysis of PCBs in maternal serum were purchased from AccuStandard, Inc. (New Haven, CT, USA). Stock solutions for analytical techniques were made in isooctane (Thermo Scientific, Waltham, MA).
Analysis of PCBs in maternal serum
Human maternal plasma samples (n = 241) were obtained from the MARBLES study at the University of California, Davis.42 Women recruited into the MARBLES study lived within a 2.5 hour drive of Sacramento, CA, were currently pregnant, and had a biological child diagnosed with ASD, which significantly increased their risk for having a second child with a NDD. All maternal blood samples were collected into sodium citrate Vacutainer® tubes post venipuncture. Whole blood samples were processed within 12 h of collection to separate plasma, which was stored at −80 °C until thawed on ice for PCB analysis.
All PCB analyses were conducted using a validated standard operating procedure to extract, separate, and detect PCBs by gas chromatography coupled with triple quadruple mass spectrometry (GC/MS/MS, Scion TQ triple quadruple mass spectrometer, Bruker, Fremont, CA, USA). The detailed description for sample preparation and GC-MS/MS parameters is reported elsewhere.51 In summary, 500 µL of plasma were extracted by solid phase extraction, further purified by silica cartridges and analyzed for PCB 11, 28, 52, 77, 84, 91, 95, 101, 118, 131, 132, 135, 136, 138, 149, 153, 174, 175, 176, 180, and 196. 13C12 labeled PCB 97 was used as surrogate internal standard throughout the extraction and analytical procedures (Cambridge Isotope Laboratories, Inc, Tewksbury, MA, USA). Another internal standard, Mirex, was added after extraction to monitor any shifts in instrument performance during analysis. Standard reference material (SRM1957) was purchased from the National Institute of Standards and Technology (NIST, Gaithersburg, MD, USA). Human control serum (DDC Mass Spect Gold®, MSG 3000, Golden West Biologicals, InC. Temecula, CA, USA) was fortified with all PCB standard solutions (AccuStandard, Inc., New Haven, CT, USA) at concentrations of 0.1, 0.8 and 4 ng/mL for quality control (QC) purposes. The analytical laboratory participates in the Arctic Monitoring and Assessment Program (also known as AMAP Ring Test of Persistent Organic Pollutants in Human Serum) 52 for PCB analyses with z-scores between −2< Z’-score < 2. Limit of quantification (LOQ) and limit of detection (LOD) were determined by the concentrations where the signal to noise ratios were 10: 1 and 3:1, correspondingly. For concentrations below LOQ, but above LOD, we used the reported values provided by the GC-MS/MS analysis software, Bruker MSWS 8.0.1 (Bruker, CA, U.S.A.). For statistical analysis, a non-detected congener was assigned a value of the corresponding LOD divided by . PCBs were then ranked for abundance in maternal serum and the top 12 most abundant congeners were selected to comprise the MARBLES mix (Table 2).
Table 2.
The 12 most abundant congeners detected in serum of MARBLES subjects
| PCB Congener | Proportion (%) of the sum of the 12 most abundant PCB congeners in maternal serum |
|---|---|
| PCB 28 | 48.2 |
| PCB 11 | 24.3 |
| PCB 118 | 4.9 |
| PCB 101 | 4.5 |
| PCB 52 | 4.5 |
| PCB 153 | 3.1 |
| PCB 180 | 2.8 |
| PCB 149 | 2.0 |
| PCB 138 | 1.7 |
| PCB 84 | 1.5 |
| PCB 135 | 1.3 |
| PCB 95 | 1.2 |
Analyses of TH activity
GH3.TRE-Luc43 cells, which were generously provided by Dr. J. David Furlow (University of California, Davis, CA) were derived from a pituitary tumor of a 7-month-old female rat. These cells were grown in DMEM/F12 (Thermo Scientific) supplemented with 10% fetal bovine serum (GIBCO/Thermo Scientific) in T75 flasks (Thermo Scientific) at 37 ºC in a 5% CO2 humidified incubator. GH3.TRE-Luc cells tested negative for mycoplasma at an early and late passage as determined using the MycoAlert™ Plus Mycoplasma Detection Kit (Lonza, Basel, Switzerland). GH3.TRE-Luc cells were passaged every 4–5 days at a 1/8 split. All experiments were performed using cells between passage numbers 5–12. To assess the activity of PCBs at the THR, a 24-well plate format assay was used.44 GH3.TRE-Luc cells were trypsinized and plated in 24-well cell culture plates (Thermo Scientific) at a density of 150,000 cells/well in DMEM/F12 supplemented with 10% FBS. Cells were rinsed with phosphate buffered saline (PBS) 24 h after plating, and media was changed to serum-free PCM medium.53 At 24 h after the PCM medium change, medium was exchanged to either PCM + vehicle (0.1% DMSO), or PCM + PCB (1:1000 dilution from stocks) in the absence or presence of T3 (0.2 nM) or T4 (2 nM). We included T4 in this experiment because it is converted to T3 by the type 1 deiodinase (Dio1) in these cells prior to its action. Therefore, if PCB congeners interfered with Dio1, it would be revealed if a PCB congener suppressed THR activation in response to T4 but not T3. The MARBLES mix was prepared by creating 10 millimolar (mM) stocks of each individual congener and then mixing the appropriate volume of each individual congener to approximate proportions found in the maternal serum resulting in a 10 mM stock of the MARBLES mix that was then serially diluted for experimentation. The MARBLES mix was tested at concentrations ranging from 2 femtomolar (fM) to 2 micromolar (µM). Individual PCB congeners were tested at 10 nanomolar (nM), 100 nM and 1 µM. After a 24 h exposure, cells were washed with PBS and lysed with 100 µL of Reporter Lysis Buffer (Promega, Madison, WI). Plates containing the lysed cells were immediately frozen at −80 ºC. Lysate was then thawed to room temperature, and 5 µl of lysate was combined with 20 µl of Luciferase assay reagent (Promega). Luciferase activity was measured in a Synergy H1 hybrid microplate reader (BioTek Instruments, Winooski, VT), and normalized to lysate protein concentrations determined using a BCA assay (Thermo Scientific). To account for plate-to-plate variability, each sample was normalized to the vehicle control wells within that plate. Each duplicate assay was independently repeated 4–6 times.
Analyses of RyR activity
RyR1-enriched microsomal membrane fractions were prepared from rabbit skeletal muscle by differential centrifugation.54 Equilibrium binding of [3H]ryanodine ([3H]Ry; 56.6 Ci/mmol; Perkin Elmer Life, Bellerica, MA) to microsomes (0.05 mg/mL) was measured in tightly sealed test tubes at 37 °C after 3 h with constant shaking in binding buffer consisting of 2nM [3H]Ry (in mM), 250 KCl, 14 NaCl, 20 HEPES, pH 7.4, and 2 μM free Ca2+ (obtained by the addition of EGTA calculated according to the software Bound-and-Determined 6).55 DMSO was used as a vehicle at final concentrations ≤1% and had no influence on basal RyR1 activity.56, 57 Non-specific [3H]Ry binding was measured as the residual binding measured in the presence of a 1,000-fold excess of unlabeled ryanodine.54 Each radioligand–receptor binding experiment was performed in triplicate and repeated 2–5 times on separate days. The EC50 and maximal activation parameters were determined by nonlinear regression equations using Origin 9.1 (OriginLab). The equations used for the best fit are as follows:
Where y is the variable corresponds to the bound [3H]Ry (fold increase); A1= initial value (Bound); A2 = final value (Bound); X = the independent variable (total concentration of PCB congener(s)); Xo = center; p = power; ΔX = slope at Xo.
Results and Discussion
Concentration of PCBs in serum from MARBLES Mothers
Mass spectrometry was used to analyze PCB content in plasma samples collected from women enrolled in the MARBLES study at UC Davis.42 MARBLES is a longitudinal study of pregnant women who have a biological child diagnosed with ASD, and thus are at increased risk for having a second child diagnosed with a NDD. PCBs were detected in all of the 241 samples that were analyzed. The sum total PCBs present in human maternal serum samples ranged from 0.74 ng/mL to 12.58 ng/mL with a mean value of 2.22 ng/mL (Table 1). The top 12 congeners in order from most abundant to least abundant was: PCB 28, 11, 118, 101, 52, 153, 180, 149, 138, 84, 135, and 95. The relative proportion of the top 12 congeners that comprised the MARBLES mix is listed in Table 2 (% of Σ12PCBs).
Table 1.
Total sum PCBs detected in serum from women enrolled in the MARBLES study
| Sample Type | Minimum | 25th Percentile | Mean | 75th Percentile | Maximum |
|---|---|---|---|---|---|
| Maternal Plasma | 0.74 ng/mL | 1.66 ng/mL | 2.22 ng/mL | 2.37 ng/mL | 12.58 ng/mL |
N = 241 serum samples from 126 women at varying stages of pregnancy ranging from 1st to 3rd trimester
A simplified mixture comprised of the 12 most abundant PCB congeners identified in the MARBLES samples was chosen for THR and RyR activity assays in part to avoid confounding by non-PCB contaminants present in Aroclors, and because the two most abundant congeners, PCB 28 and PCB 11, which together comprised almost 75% of the PCB burden in the maternal serum samples, are not present in the Aroclors.43 The caveats of this approach include the lack of consideration of enantiomeric enrichment and the possibility that congeners important in PCB DNT may have been overlooked. Moreover, the MARBLES mixture is representative of a specific at-risk population in northern California, and other PCB profiles may be present in other populations, or even of individuals within any given population. However, an independent study that assessed all 209 PCB congeners in samples obtained from mothers and their children living in Midwestern United States detected the 12 congeners included in the MARBLES mix at relatively high frequencies,4, 58 thus, the 12 congeners included in the MARBLES mix are not unique to the MARBLES cohort.
A key observation from the analyses of the MARBLES samples was the abundance of the lightly chlorinated congeners, PCB 11 and PCB 28. This observation raises questions regarding the common practice of using PCB 153 as a general marker for overall PCB exposure and for comparison of PCB burdens amongst various populations.59 PCB 153 was among the 12 most abundant congeners detected in the pregnant women assessed in our study; however, PCB 28 and PCB 11 were present at quantities 16 and 8 times greater than that of PCB 153, respectively (Table 2). One other study has similarly demonstrated an abundance of lower chlorinated PCBs in serum samples from mothers and their children.3 In light of recent reports indicating the potential for PCB 11 to interfere with neuronal morphogenesis,60, 61 these observations warrant rethinking of traditional exposure assessment strategies to quantify lower chlorinated congeners in addition to PCB 153 in human biomonitoring studies.
The MARBLES mix, its individual congeners and metabolites lack activity at the THR
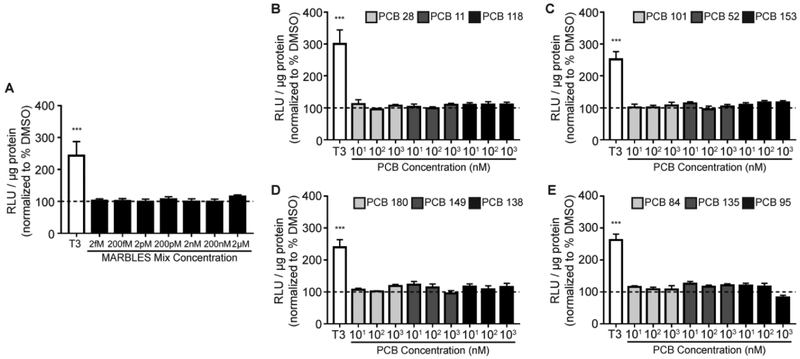
We first tested whether the MARBLES mix possesses agonistic activity at the THR using a sensitive THR reporter cell line43 that expresses a luciferase reporter gene under the regulation of thyroid hormone response elements. Exposure to T3 (0.2 nM) significantly increased luciferase activity as expected; however, the MARBLES mix had no effect on luciferase activity at concentrations ranging from 2 fM to 2 µM (Figure 1A). Since TH effects can vary depending on the PCB congener,62 we next tested each of the 12 PCB congeners making up the MARBLES mix individually. None of the 12 individual congeners displayed agonistic activity at the THR at any concentration tested (10 nM, 100 nM and 1 µM) (Figure 1B-E).
Figure 1.
The MARBLES mix and its individual components do not activate the THR. Luciferase activity was measured in GH3.TRE-Luc cells treated with either T3 (0.2 nM) or (A) MARBLES mix, (B) PCB 28, 11, 118, (C) PCB 101, 52, 153, (D) PCB 180, 149, 138, or (E) PCB 84, 135, 95. Luciferase activity is expressed as relative light units (RLU) normalized to total protein concentration in the same sample. Data presented as the mean ± SE (n = 5-6 independent experiments). *Significantly different from vehicle (0.1% DMSO) control at p < 0.05, ** p < 0.01, *** p < 0.001 as determined using a one-way ANOVA (p < 0.05) followed by a Holm-Sidak’s multiple comparisons test.
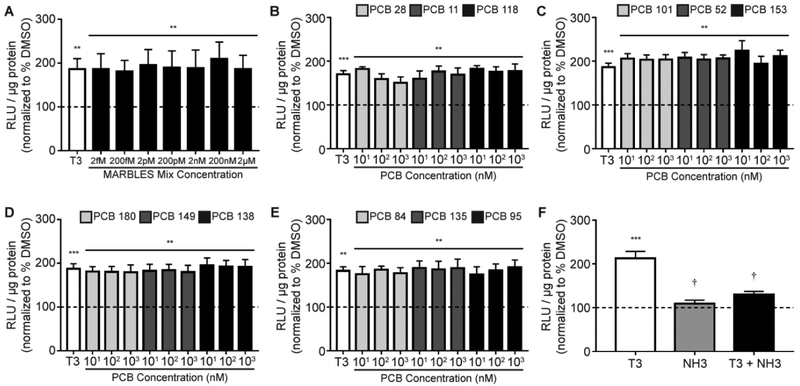
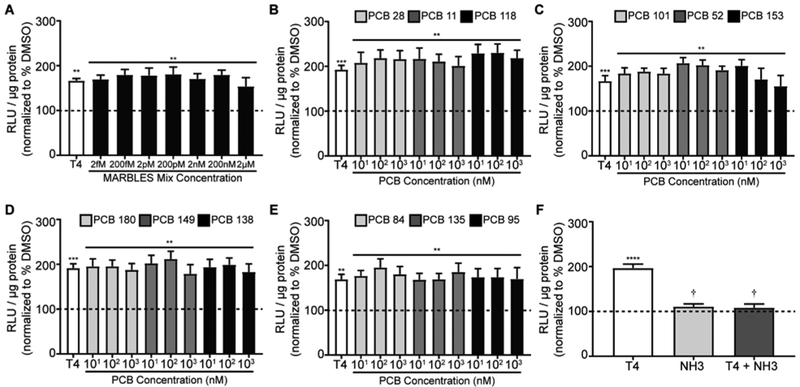
PCBs have also been reported to block THR signaling,11 so GH3.TRE cells were exposed to the MARBLES mix in the presence of T3 (0.2 nM) or T4 (2 nM) to test for antagonism of THR signaling. Co-exposure to the MARBLES mix had no effect on T3- (Figure 2A) or T4- (Figure 3A) induced luciferase activity. Similarly, none of the 12 congeners of the MARBLES mix blocked the luciferase activity induced by T3 (Figure 2B-E) or T4 (Figure 3B-E) when tested individually. Co-treatment with NH-3, a THR antagonist,50 decreased the T3 and T4 responses to levels that were not significantly different from vehicle controls (Figures 2F and 3F).
Figure 2.
The MARBLES mix and its individual congeners do not block T3 activation of the THR. Luciferase activity was measured in GH3.TRE-Luc cells treated with T3 (0.2 nM) in the absence or presence of the MARBLES mix (A) or the individual congeners in the mix (B-E). (F) Luciferase activity in cells treated with T3 (0.2 nM) in the absence or presence of the THR blocker, NH-3 (100 pM). Luciferase activity is expressed as relative light units (RLU) normalized to total protein concentration. Data presented as the mean ± SE (n = 6 independent experiments). *Significantly different from vehicle (0.1% DMSO) at p < 0.05, ** p < 0.01, *** p < 0.001, †Significantly different from T3 at p < 0.01 as determined using a one-way ANOVA (p < 0.05) and post hoc Holm-Sidak’s multiple comparisons test.
Figure 3.
The MARBLES mix and its individual components do not antagonize T4-induced THR signaling. Luciferase activity was measured in GH3.TRE-Luc cells treated with T4 (2 nM) in the absence or presence of the MARBLES mix (A) or the individual congeners in the mix (B-E). (F) Luciferase activity in cells treated with T4 (2 nM) in the absence or presence of the THR blocker, NH-3 (100 nM). Luciferase activity is expressed as relative light units (RLU) normalized to total protein concentration of each sample. Data presented as the mean ± SE (n = 5-6 independent experiments). *Significantly different from vehicle (0.1% DMSO) at p < 0.05, ** p < 0.01, *** p < 0.001, †Significantly different from T4 at p < 0.0001 as determined using a one-way ANOVA (p < 0.05) and post hoc Holm-Sidak’s multiple comparisons test.
The lack of effect of the MARBLES mix and its individual congeners on THR-dependent signaling is consistent with an earlier study that also failed to detect a direct interaction between PCBs and the THR.31 More recent screening studies using reporter cell lines that express only the THR alpha isoform, including one study of 25 individual PCBs that included 5 of the PCBs we examined here,63 and another study of lower chlorinated PCBs,64 also reported no agonistic effects of PCBs. These findings from THR reporter assays are at odds with the observation that PCB 118 mimics TH in an oligodendrocyte differentiation assay.62 These discrepancies may reflect cell-context differences; however, the possibility that the effects of PCB 118 on oligodendrocyte differentiation are mediated by THR-independent mechanisms cannot be ruled out because early in development, there is a THR-independent pathway to oligodendrocyte differentiation.65
Our observations that neither the MARBLES mix nor the individual congeners block T3 or T4 induced THR activity are inconsistent with previous reports that PCBs interfere with THR-mediated signaling.32, 66 This may reflect the fact that these prior results were obtained using cell lines that expressed only the THR beta 1 isoform, in contrast to the GH3.TRE-Luc cell line that expresses THR alpha1, THR beta1 and THR beta 2, as well as their heterodimer partners and respective cofactors.67 Interestingly, in the studies that used the THR beta 1-expressing cell lines, THR activity was significantly suppressed not by the parent PCBs, but by their corresponding hydroxyl metabolites (OH-PCBs).32, 66 In contrast, other studies using the GH3 cell line detected agonistic activity of OH-PCBs at the THR.68, 69 While the reason for the lack of consistent outcomes in tests of OH-PCB activity at the THR remains unknown, these observations suggest that metabolism may contribute to the influences of PCBs on TH signaling. Consistent with this suggestion, more pronounced effects of PCBs on TH signaling during development were observed in animals exposed to a PCB congener that upregulated the cytochrome P450 enzymes that metabolize PCBs.70
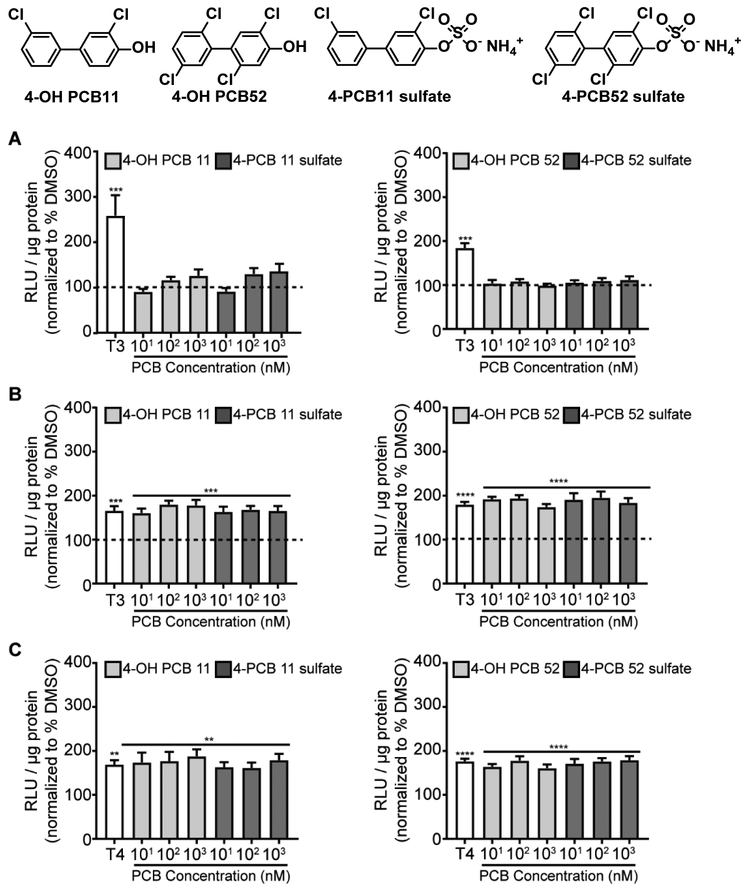
To determine whether human-relevant metabolites of the PCB congeners in the MARBLES mixture had activity at the THR, we tested hydroxylated and sulfated metabolites of PCB 11 and 52 using the GH3.TRE-Luc cell line. While metabolites of eight (PCB 11, 28, 52, 101, 118, 138, 153, 180)3, 71–73 of the twelve PCB congeners in the MARBLES mix have been detected in human serum, we were only able to obtain purified metabolites for PCB 11 and 52. Neither the 4-hydroxy nor 4-sulfate metabolites of PCB 11 or PCB 52 exhibited agonistic (Figure 4A) or antagonistic (Figure 4B-C) activity at the THR. In a separate set of studies we screened the parent, 4-OH and 4-sulfate metabolites of PCBs 3, 8, 11 and 52 at concentrations ranging from 10 pM – 10 µM using a different model: GH3 cells transiently transfected with a THR reporter construct (see Supplemental materials for detailed description). Consistent with findings generated using the GH3.TRE-Luc cell line, at concentrations ≤ 1 µM, none of these PCB congeners (parents or metabolites) had agonistic or antagonistic effects on THR activity (Supplemental materials, Figures S26-S29). At 10 µM, PCB 11, 4-OH PCB 3, PCB 3 sulfate and PCB 8 sulfate significantly altered THR activity, with PCB 11 inhibiting T3 activation of THR, 4-OH PCB 3 increasing THR activity in the absence or presence of T3 or T4, and the 4-sulfate metabolites of both PCB 3 and PCB 8 increasing THR activity in the presence of T4 (Supplemental materials, Figures S26-S28). The relevance of these observations is not clear since 10 µM is significantly higher than PCB concentrations documented in human tissues.
Figure 4.
PCB 11 and 52 metabolites exhibit no agonistic or antagonistic activity at the THR. (A) To test for THR agonism, luciferase activity was measured in GH3.TRE-Luc cells treated with either T3 (0.2 nM) or metabolites of PCB 11 or 52. (B-C) To test for THR antagonism, luciferase activity was measured in cells treated with 0.2 nM T3 (B) or 2 nM T4 (C) in the absence or presence of PCB 11 or 52 metabolites. Luciferase activity is expressed as relative light units (RLU) normalized to total protein concentration. Data presented as the mean ± SE (n = 4-6 independent experiments). *Significantly different from vehicle (0.1% DMSO) at p < 0.05, ** p < 0.01, *** p < 0.001 as determined using one-way ANOVA (p < 0.05) with post hoc Holm-Sidak’s multiple comparisons test.
Our current data do not support the hypothesis that PCBs in combination or individually interact with the THR in a manner that could explain PCB developmental neurotoxicity. Although GH3 cells cannot be considered a model of all neuronal cell types, there is sufficient evidence in the literature at large to indicate that THRs are not obvious targets of PCB congeners of relevance to contemporary human exposures. However, these data do not preclude the possibility that PCBs or their metabolites interfere with the delivery of T3 to target cells during critical windows of neurodevelopment.74
MARBLES mix and each of its individual congeners stabilize the open state of RyR1
As previously reported, RyR channels are a direct and highly sensitive target of PCBs,16, 36 and RyR sensitization by PCBs has been linked to adverse effects on neuronal Ca2+ dynamics that alter dendritic arborization.25, 38, 54 Here, we report the structure-activity relationship of the 12 PCB congeners comprising the MARBLES mix using high affinity [3H]Ry binding to RyR1-enriched microsomal preparations as a quantitative biochemical indicator of PCB-induced modification of channel conformations previously demonstrated in muscle and brain.46, 75 Figure 5 shows that in the presence of the MARBLES mix (Fig 5A) or individual PCB congeners (Fig 5 B-E), specific [3H]Ry binding increased in a concentration-dependent manner. The MARBLES mix had an EC50 = 4.46 ± 0.88 μM, reaching a maximal efficacy of 3.96 ± 0.25-fold increase from the baseline of control (Figure 5A). Among the 12 individual congeners, PCB 95, PCB 135, and PCB 149 were the most efficacious of the parent congeners, enhancing [3H]Ry binding ~12–19 fold over baseline (Figure 5D, E). Although PCB 135 and PCB 149 showed slightly higher efficacy than PCB 95 (54% and 15% more efficacious; p<0.001 and 0.05, respectively; using one-way ANOVA followed by Tukey means comparison test) under the experimental conditions used, they are 5- and 3-fold less potent than PCB 95, respectively (Figure 5E; Table 3).
Figure 5. The MARBLES mix and its 12 individual congeners allosterically enhance the binding of [3H]Ry to RyR1 in its high affinity open conformational state.
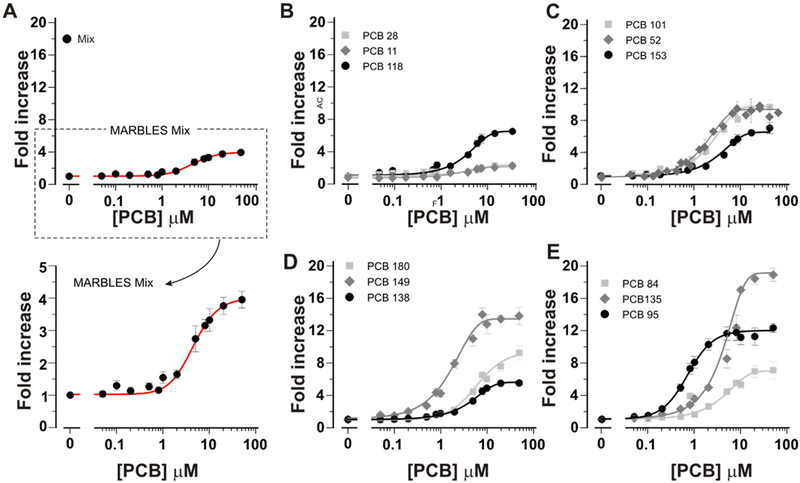
Specific [3H]Ry binding in the presence of the MARBLES mix (A), individual PCB congeners (B-E) or vehicle (DMSO, 1%). DMSO concentrations in PCB samples varied from 1% to 0.2%. Data was plotted as fold increase from respective baseline binding. Baseline binding in the presence of DMSO (1%) averaged 0.16±0.06 pmol/mg protein across experimental trials. Each data point represents the mean ± SE of triplicate determinations repeated 2-5 times under identical experiment conditions. Concentration-effect data were fitted using nonlinear regression Sigmoidal Boltzmann or Logistic with Origin 9.1, and the latter was used to obtain EC50 values and their maximal activation levels (a measure of efficacy), which is summarized in Table 3.
Table 3.
Summary of EC50 values and maximal activation of [3H]ryanodine ([3H]Ry) binding to the RyR1 as fold increase from the baseline by the MARBLES mix and its 12 individual congeners.
| PCB Congener(s) | [3H]Ry Binding | |
|---|---|---|
| EC50 (µM) | Activitymax (Fold Control) | |
| MARBLES mix | 4.5 ± 0.9 | 4.0 ± 0.2 |
| PCB 28 | 7.1 ± 5.2 | 2.4 ± 0.8 |
| PCB 11 | 5.1 ± 6.1 | 2.1 ± 0.2 |
| 4-OH PCB 11 | ND | ND |
| 4-PCB 11 sulfate | ND | ND |
| PCB 118 | 5.2 ± 1.8 | 6.8 ± 0.1 |
| PCB 101 | 4.7 ± 3.3 | 6.5 ± 0.6 |
| PCB 52 | 1.9 ± 0.6 | 9.4 ± 0.3 |
| 4-OH PCB 52 | 0.6 ± 0.2 | 9.4 ± 0.2 |
| 4-PCB 52 sulfate | 17.3 ± 6.6 | 2.4 ± 0.3 |
| PCB 153 | 3.0 ± 1.3 | 9.7 ± 0.7 |
| PCB 180 | 6.0 ± 1.4 | 9.4 ± 1.1 |
| PCB 149 | 2.2 ± 2.5 | 13.8 ± 2.0 |
| PCB 138 | 4.9 ± 1.4 | 5.6 ± 2.2 |
| PCB 84 | 5.3 ± 0.9 | 7.0 ± 2.4 |
| PCB 135 | 3.6 ± 0.7 | 19.2 ± 0.8 |
| PCB 95 | 0.7 ± 0.2 | 12.0 ± 3.8 |
ND, Not significantly different from DMSO control up to 50 µM.
In our previous studies, we found negligible RyR activity of highly purified PCB 1137, 76. However, due to recent appreciation of the relatively high volatility of lightly chlorinated PCBs, including PCB 11,77–79 we reassessed the activity of PCB 11 and tested its 4-hydroxy and 4-sulfated metabolites of PCB 11 using tightly sealed test tubes to minimize loss through air-water repartitioning. Using this experimental approach, PCB 11 showed consistent activity toward RyR1 with an EC50 of 5.1 µM and efficacy 2-fold that of the DMSO baseline (Figs 5B; Fig 6A; Table 3). Moreover, neither 4-OH nor PCB 11 sulfate had any detectable activity at the maximum concentration tested (50 µM) (Fig 6A; Table 3). We believe the divergent results from earlier studies were caused by the loss of PCB 11 from the aqueous assay buffer over the 3 hr incubation at 37ºC, as predicted by its high Henry’s Law Constant compared to higher chlorinated PCBs.80 Likewise, lower chlorinated PCB 28 was also active towards RyR1, reaching 2.4-fold increase over baseline and an EC50 = 7.1 ± 5.2 μM. Importantly, as determined using a previously described model,37 the activity of the MARBLES mix at the RyR is consistent with a purely additive model in which the sum of the relative RyR activity of the individual congeners predicts the RyR activity of the mixture.
Figure 6:
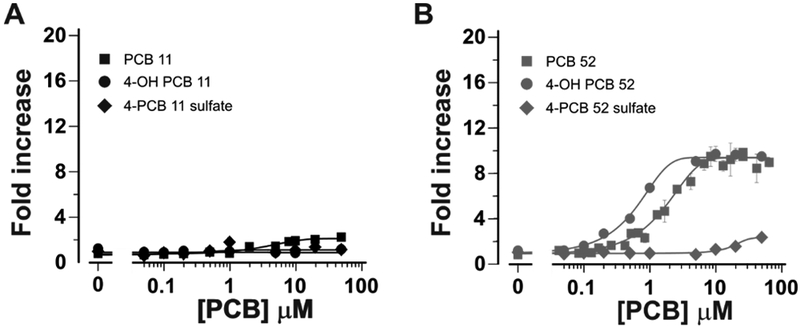
The parent congener and 4-OH and sulfate metabolites of PCB 11 (A) and PCB 52 (B) were tested using [3H]Ry binding analysis as described in Figure 5. Data represent 12 replicates performed on at least 4 independent measurements and are plotted as fold-increase from DMSO control. Baseline binding average is 0.14±0.01pmol/mg protein).
In contrast, 4-OH PCB 52 exceeded the potency of PCB 52 by ~3-fold (EC50 0.6 µM; p<0.01) without change in efficacy (Fig 6B; Table 3). PCB 52 sulfate showed much lower potency and efficacy than its parent PCB (EC50 17.3 µM; and Activitymax 2.4-fold of DMSO baseline; p<0.001; Fig 6B and Table 3). These results reaffirm the activity of lightly chlorinated PCBs and the importance of the bulk of para-substitutions in determining both potency and efficacy of more highly chlorinated PCBs.37, 81 We acknowledge that EC50 values reported from [3H]Ry binding analysis are higher than the highest concentration tested in the THR cell reporter assay. Based on alternative electrophysiological methods we have previously used to investigate direct modulation of RyR1 single channel gating kinetics by PCBs, we concluded that [3H]Ry binding assays likely underestimate the true potency of active PCBs due to the high lipid content of the microsomes used to measure equilibrium receptor binding.37 Partitioning of PCBs with the lipid phase during the 3 h incubation undoubtedly reduces the free PCB concentration available to interact with binding sites on the RyR.
The two major genetic RyR isoforms, RyR1 and RyR2, have been established as among the most sensitive targets of NDL-PCBs both in vivo25, 82, 83 and in vitro.37, 38, 84 Samso and coworkers provided direct evidence that PCB 95 interacts with RyR1 using two complementary approaches: (1) single channel voltage clamp, and (2) cyroEM reconstruction of open and closed conformations of RyR1 at ~10 angstrom resolution in the presence and absence of PCB 95.85 Interactions of NDL-PCBs with RyRs meet several criteria for specificity, including a stringent structure-activity relationship where potency and efficacy vary with the position and degree of chlorination.16 Atropisomers of chiral PCB 136 and PCB 95 have clearly shown consistent stereoselectivity with higher activity for the (−)-atropisomer.54, 86 Predictions from biochemical and biophysical studies of stereoselectivity of PCB 136 were largely validated by their stereoselective influences on dendritic arborization in primary neuronal cell cultures.39 Moreover, the RyR is necessary for the effects of PCBs on neuronal connectivity in vitro.25, 38, 84 Changes in either expression and/or activity of RyRs in vivo have also been associated with deficits in the development of primary auditory cortex82 and deficits in spatial memory the Morris water maze, the latter indicative of cognitive deficits.25, 87 Interestingly, PCB-associated deficits in the Morris water maze correlated more closely with PCB effects on RyR expression and activity in the brain than with changes in circulating TH levels.25 A novel finding in this study was the high efficacies of PCB 135 and PCB 149 towards RyR1, as well as the relative potency of PCB 52 compared to its known metabolite 4-OH-PCB 52, which not only maintained high efficacy but ~3-fold greater potency. The fact that the MARBLES mix is dominated by lightly chlorinated PCBs from contemporary sources, exemplified by PCB 11 and PCB 28, and that this complex mixture maintains significant RyR1 activity warrant the further study of how complex environmentally relevant mixtures and their constituent congeners modify neurodevelopmental profiles and behavioral outcomes.
To our knowledge, this study is the first to test for direct effects on THR and RyR1 activities of a PCB mixture representative of PCBs in pregnant women at increased risk for NDDs. The relevance of these findings to human health is underscored by recent epidemiologic studies linking PCBs to increased risk for NDDs, including ADHD and ASD.8–10 While altered TH signaling has been hypothesized to play a role in ADHD and ASD, the human data are conflicting.20–23 In contrast, heritable mutations in Ca2+ signaling are strongly associated with increased risk of NDDs.16, 36, 88 While a better understanding of the PCB exposure profile, including metabolites, within the developing brain is needed to truly understand the relevance of outcomes from THR and RyR assays, collectively, these observations, together with the findings reported herein, argue that the RyR activity of at least some PCB congeners is more important than their THR activity as a point of departure for risk assessments of the developmental neurotoxicity of human relevant PCB mixtures.
Supplementary Material
Acknowledgements:
This research was supported by the National Institutes of Health (R01 ES014901, R01 ES030318, P30 ES023513, P01 ES011269, T32 ES007059 [predoctoral fellowship to SS] and by the United States Environmental Protection Agency (RD 83543201). The synthesis of the MARBLES mix was supported by the Superfund Research Center at The University of Iowa (P42 ES013661). We gratefully acknowledge Dr. David J. Furlow (University of California-Davis, Davis, CA) who provided the TRE-Luc cell line. The contents of this work do not necessarily represent the official views of the funding agencies, and the funding agencies do not endorse the purchase of any commercial products or services mentioned in the publication.
Footnotes
Supporting Information:
Detailed methods of PCB synthesis and thyroid hormone receptor (THR) activity assays using transiently transfected GH3 cells, NMR spectra of individual PCB congeners (24 figures), supplemental THR activity data (5 figures), and references.
CFI Statement: The authors declare they have no actual or potential competing financial interests.
References
- 1.Hopf NB; Ruder AM; Succop P, Background levels of polychlorinated biphenyls in the U.S. population. Sci Total Environ 2009, 407, (24), 6109–19. [DOI] [PubMed] [Google Scholar]
- 2.Whitehead TP; Crispo Smith S; Park JS; Petreas MX; Rappaport SM; Metayer C, Concentrations of persistent organic pollutants in California women’s serum and residential dust. Environ Res 2015, 136, 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koh WX; Hornbuckle KC; Thorne PS, Human Serum from Urban and Rural Adolescents and Their Mothers Shows Exposure to Polychlorinated Biphenyls Not Found in Commercial Mixtures. Environ Sci Technol 2015, 49, (13), 8105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marek RF; Thorne PS; DeWall J; Hornbuckle KC, Variability in PCB and OH-PCB serum levels in children and their mothers in urban and rural U.S. communities. Environ Sci Technol 2014, 48, (22), 13459–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berghuis SA; Bos AF; Sauer PJ; Roze E, Developmental neurotoxicity of persistent organic pollutants: an update on childhood outcome. Arch Toxicol 2015, 89, (5), 687–709. [DOI] [PubMed] [Google Scholar]
- 6.Schantz SL; Widholm JJ; Rice DC, Effects of PCB exposure on neuropsychological function in children. Environ Health Perspect 2003, 111, (3), 357–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boucher O; Muckle G; Bastien CH, Prenatal exposure to polychlorinated biphenyls: a neuropsychologic analysis. Environ Health Perspect 2009, 117, (1), 7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheslack-Postava K; Rantakokko PV; Hinkka-Yli-Salomaki S; Surcel HM; McKeague IW; Kiviranta HA; Sourander A; Brown AS, Maternal serum persistent organic pollutants in the Finnish Prenatal Study of Autism: A pilot study. Neurotoxicol Teratol 2013, 38, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyall K; Croen LA; Sjodin A; Yoshida CK; Zerbo O; Kharrazi M; Windham GC, Polychlorinated Biphenyl and Organochlorine Pesticide Concentrations in Maternal Mid-Pregnancy Serum Samples: Association with Autism Spectrum Disorder and Intellectual Disability. Environ Health Perspect 2017, 125, (3), 474–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sagiv SK; Thurston SW; Bellinger DC; Tolbert PE; Altshul LM; Korrick SA, Prenatal organochlorine exposure and behaviors associated with attention deficit hyperactivity disorder in school-aged children. Am J Epidemiol 2010, 171, (5), 593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crofton KM, Thyroid disrupting chemicals: mechanisms and mixtures. Int J Androl 2008, 31, (2), 209–23. [DOI] [PubMed] [Google Scholar]
- 12.Gilbert ME; Rovet J; Chen Z; Koibuchi N, Developmental thyroid hormone disruption: prevalence, environmental contaminants and neurodevelopmental consequences. Neurotoxicology 2012, 33, (4), 842–52. [DOI] [PubMed] [Google Scholar]
- 13.Zoeller RT, Environmental chemicals impacting the thyroid: targets and consequences. Thyroid 2007, 17, (9), 811–7. [DOI] [PubMed] [Google Scholar]
- 14.Tilson HA; Kodavanti PR, The neurotoxicity of polychlorinated biphenyls. Neurotoxicology 1998, 19, (4–5), 517–25. [PubMed] [Google Scholar]
- 15.Bal-Price A; Lein PJ; Keil KP; Sethi S; Shafer T; Barenys M; Fritsche E; Sachana M; Meek MEB, Developing and applying the adverse outcome pathway concept for understanding and predicting neurotoxicity. Neurotoxicology 2017, 59, 240–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pessah IN; Cherednichenko G; Lein PJ, Minding the calcium store: Ryanodine receptor activation as a convergent mechanism of PCB toxicity. Pharmacol Ther 2010, 125, (2), 260–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rovet JF, The role of thyroid hormones for brain development and cognitive function. Endocr Dev 2014, 26, 26–43. [DOI] [PubMed] [Google Scholar]
- 18.Oppenheimer JH; Schwartz HL, Molecular basis of thyroid hormone-dependent brain development. Endocr Rev 1997, 18, (4), 462–75. [DOI] [PubMed] [Google Scholar]
- 19.Williams GR, Neurodevelopmental and neurophysiological actions of thyroid hormone. J Neuroendocrinol 2008, 20, (6), 784–94. [DOI] [PubMed] [Google Scholar]
- 20.Kalikiri MK; Mamidala MP; Rao AN; Rajesh V, Analysis and functional characterization of sequence variations in ligand binding domain of thyroid hormone receptors in autism spectrum disorder (ASD) patients. Autism Res 2017, 10, (12), 1919–1928. [DOI] [PubMed] [Google Scholar]
- 21.Lyall K; Anderson M; Kharrazi M; Windham GC, Neonatal thyroid hormone levels in association with autism spectrum disorder. Autism Res 2017, 10, (4), 585–592. [DOI] [PubMed] [Google Scholar]
- 22.Drover SSM; Villanger GD; Aase H; Skogheim TS; Longnecker MP; Zoeller RT; Reichborn-Kjennerud T; Knudsen GP; Zeiner P; Engel SM, Maternal Thyroid Function During Pregnancy or Neonatal Thyroid Function and Attention Deficit Hyperactivity Disorder: A Systematic Review. Epidemiology 2019, 30, (1), 130–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Modesto T; Tiemeier H; Peeters RP; Jaddoe VW; Hofman A; Verhulst FC; Ghassabian A, Maternal Mild Thyroid Hormone Insufficiency in Early Pregnancy and Attention-Deficit/Hyperactivity Disorder Symptoms in Children. JAMA Pediatr 2015, 169, (9), 838–45. [DOI] [PubMed] [Google Scholar]
- 24.Kato Y; Haraguchi K; Yamazaki T; Ito Y; Miyajima S; Nemoto K; Koga N; Kimura R; Degawa M, Effects of polychlorinated biphenyls, kanechlor-500, on serum thyroid hormone levels in rats and mice. Toxicol Sci 2003, 72, (2), 235–41. [DOI] [PubMed] [Google Scholar]
- 25.Yang D; Kim KH; Phimister A; Bachstetter AD; Ward TR; Stackman RW; Mervis RF; Wisniewski AB; Klein SL; Kodavanti PR; Anderson KA; Wayman G; Pessah IN; Lein PJ, Developmental exposure to polychlorinated biphenyls interferes with experience-dependent dendritic plasticity and ryanodine receptor expression in weanling rats. Environ Health Perspect 2009, 117, (3), 426–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hagmar L, Polychlorinated biphenyls and thyroid status in humans: a review. Thyroid 2003, 13, (11), 1021–8. [DOI] [PubMed] [Google Scholar]
- 27.Maervoet J; Vermeir G; Covaci A; Van Larebeke N; Koppen G; Schoeters G; Nelen V; Baeyens W; Schepens P; Viaene MK, Association of thyroid hormone concentrations with levels of organochlorine compounds in cord blood of neonates. Environ Health Perspect 2007, 115, (12), 1780–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin L; Klaassen CD, Differential effects of polychlorinated biphenyl congeners on serum thyroid hormone levels in rats. Toxicol Sci 2010, 117, (1), 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Itoh S; Baba T; Yuasa M; Miyashita C; Kobayashi S; Araki A; Sasaki S; Kajiwara J; Hori T; Todaka T; Fujikura K; Nakajima S; Kato S; Kishi R, Association of maternal serum concentration of hydroxylated polychlorinated biphenyls with maternal and neonatal thyroid hormones: The Hokkaido birth cohort study. Environ Res 2018, 167, 583–590. [DOI] [PubMed] [Google Scholar]
- 30.Lam J; Lanphear BP; Bellinger D; Axelrad DA; McPartland J; Sutton P; Davidson L; Daniels N; Sen S; Woodruff TJ, Developmental PBDE Exposure and IQ/ADHD in Childhood: A Systematic Review and Meta-analysis. Environ Health Perspect 2017, 125, (8), 086001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gauger KJ; Kato Y; Haraguchi K; Lehmler HJ; Robertson LW; Bansal R; Zoeller RT, Polychlorinated biphenyls (PCBs) exert thyroid hormone-like effects in the fetal rat brain but do not bind to thyroid hormone receptors. Environ Health Perspect 2004, 112, (5), 516–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iwasaki T; Miyazaki W; Takeshita A; Kuroda Y; Koibuchi N, Polychlorinated biphenyls suppress thyroid hormone-induced transactivation. Biochem Biophys Res Commun 2002, 299, (3), 384–8. [DOI] [PubMed] [Google Scholar]
- 33.Brini M; Cali T; Ottolini D; Carafoli E, Neuronal calcium signaling: function and dysfunction. Cell Mol Life Sci 2014, 71, (15), 2787–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Konur S; Ghosh A, Calcium signaling and the control of dendritic development. Neuron 2005, 46, (3), 401–5. [DOI] [PubMed] [Google Scholar]
- 35.Berridge MJ, Calcium microdomains: organization and function. Cell Calcium 2006, 40, (5–6), 405–12. [DOI] [PubMed] [Google Scholar]
- 36.Stamou M; Streifel KM; Goines PE; Lein PJ, Neuronal connectivity as a convergent target of gene x environment interactions that confer risk for Autism Spectrum Disorders. Neurotoxicol Teratol 2013, 36, 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holland EB; Feng W; Zheng J; Dong Y; Li X; Lehmler HJ; Pessah IN, An Extended Structure-Activity Relationship of Nondioxin-Like PCBs Evaluates and Supports Modeling Predictions and Identifies Picomolar Potency of PCB 202 Towards Ryanodine Receptors. Toxicol Sci 2017, 155, (1), 170–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wayman GA; Yang D; Bose DD; Lesiak A; Ledoux V; Bruun D; Pessah IN; Lein PJ, PCB-95 promotes dendritic growth via ryanodine receptor-dependent mechanisms. Environ Health Perspect 2012, 120, (7), 997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang D; Kania-Korwel I; Ghogha A; Chen H; Stamou M; Bose DD; Pessah IN; Lehmler HJ; Lein PJ, PCB 136 atropselectively alters morphometric and functional parameters of neuronal connectivity in cultured rat hippocampal neurons via ryanodine receptor-dependent mechanisms. Toxicol Sci 2014, 138, (2), 379–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dingemans MM; Kock M; van den Berg M, Mechanisms of Action Point Towards Combined PBDE/NDL-PCB Risk Assessment. Toxicol Sci 2016, 153, (2), 215–24. [DOI] [PubMed] [Google Scholar]
- 41.Yang JM; Salmon AG; Marty MA, Development of TEFs for PCB congeners by using an alternative biomarker--thyroid hormone levels. Regul Toxicol Pharmacol 2010, 56, (2), 225–36. [DOI] [PubMed] [Google Scholar]
- 42.Hertz-Picciotto I; Schmidt RJ; Walker CK; Bennett DH; Oliver M; Shedd-Wise KM; LaSalle JM; Giulivi C; Puschner B; Thomas J; Roa DL; Pessah IN; Van de Water J; Tancredi DJ; Ozonoff S, A Prospective Study of Environmental Exposures and Early Biomarkers in Autism Spectrum Disorder: Design, Protocols, and Preliminary Data from the MARBLES Study. Environ Health Perspect 2018, 126, (11), 117004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Freitas J; Cano P; Craig-Veit C; Goodson ML; Furlow JD; Murk AJ, Detection of thyroid hormone receptor disruptors by a novel stable in vitro reporter gene assay. Toxicol In Vitro 2011, 25, (1), 257–66. [DOI] [PubMed] [Google Scholar]
- 44.Mengeling BJ; Furlow JD, Pituitary specific retinoid-X receptor ligand interactions with thyroid hormone receptor signaling revealed by high throughput reporter and endogenous gene responses. Toxicol In Vitro 2015, 29, (7), 1609–18. [DOI] [PubMed] [Google Scholar]
- 45.Pessah IN; Zimanyi I, Characterization of multiple [3H]ryanodine binding sites on the Ca2+ release channel of sarcoplasmic reticulum from skeletal and cardiac muscle: evidence for a sequential mechanism in ryanodine action. Mol Pharmacol 1991, 39, (5), 679–89. [PubMed] [Google Scholar]
- 46.Wong PW; Brackney WR; Pessah IN, Ortho-substituted polychlorinated biphenyls alter microsomal calcium transport by direct interaction with ryanodine receptors of mammalian brain. J Biol Chem 1997, 272, (24), 15145–53. [DOI] [PubMed] [Google Scholar]
- 47.Alam S; Carter GS; Krager KJ; Li X; Lehmler HJ; Aykin-Burns N, PCB11 Metabolite, 3,3’-Dichlorobiphenyl-4-ol, Exposure Alters the Expression of Genes Governing Fatty Acid Metabolism in the Absence of Functional Sirtuin 3: Examining the Contribution of MnSOD. Antioxidants (Basel) 2018, 7, (9), E121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li X; Parkin S; Duffel MW; Robertson LW; Lehmler HJ, An efficient approach to sulfate metabolites of polychlorinated biphenyls. Environ Int 2010, 36, (8), 843–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodriguez EA; Li X; Lehmler HJ; Robertson LW; Duffel MW, Sulfation of Lower Chlorinated Polychlorinated Biphenyls Increases Their Affinity for the Major Drug-Binding Sites of Human Serum Albumin. Environ Sci Technol 2016, 50, (10), 5320–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singh L; Pressly B; Mengeling BJ; Fettinger JC; Furlow JD; Lein PJ; Wulff H; Singh V, Chasing the Elusive Benzofuran Impurity of the THR Antagonist NH-3: Synthesis, Isotope Labeling, and Biological Activity. J Org Chem 2016, 81, (5), 1870–6. [DOI] [PubMed] [Google Scholar]
- 51.Lin YP; Pessah IN; Puschner B, Simultaneous determination of polybrominated diphenyl ethers and polychlorinated biphenyls by gas chromatography-tandem mass spectrometry in human serum and plasma. Talanta 2013, 113, 41–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.CTQ Artic Monitoring and Assessment Program. https://www.inspq.qc.ca/en/ctq/eqas/amap/description (October/16/2015),
- 53.Sirbasku DA; Pakala R; Sato H; Eby JE, Thyroid hormone dependent pituitary tumor cell growth in serum-free chemically defined culture. A new regulatory role for apotransferrin. Biochemistry 1991, 30, (30), 7466–77. [DOI] [PubMed] [Google Scholar]
- 54.Feng W; Zheng J; Robin G; Dong Y; Ichikawa M; Inoue Y; Mori T; Nakano T; Pessah IN, Enantioselectivity of 2,2’,3,5’,6-Pentachlorobiphenyl (PCB 95) Atropisomers toward Ryanodine Receptors (RyRs) and Their Influences on Hippocampal Neuronal Networks. Environ Sci Technol 2017, 51, (24), 14406–14416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brooks SP; Storey KB, Bound and determined: a computer program for making buffers of defined ion concentrations. Anal Biochem 1992, 201, (1), 119–26. [DOI] [PubMed] [Google Scholar]
- 56.O’Neill ER; Sakowska MM; Laver DR, Regulation of the calcium release channel from skeletal muscle by suramin and the disulfonated stilbene derivatives DIDS, DBDS, and DNDS. Biophys J 2003, 84, (3), 1674–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Abramson JJ; Mullen SP; Koehler S; Mansoor D; Anderson P; Wamser CC; Swan TJ; Favero TG, o-Phthalaldehyde activates the Ca(2+) release mechanism from skeletal muscle sarcoplasmic reticulum. Arch Biochem Biophys 2001, 391, (2), 235–44. [DOI] [PubMed] [Google Scholar]
- 58.Marek RF; Thorne PS; Wang K; Dewall J; Hornbuckle KC, PCBs and OH-PCBs in serum from children and mothers in urban and rural U.S. communities. Environ Sci Technol 2013, 47, (7), 3353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Longnecker MP; Wolff MS; Gladen BC; Brock JW; Grandjean P; Jacobson JL; Korrick SA; Rogan WJ; Weisglas-Kuperus N; Hertz-Picciotto I; Ayotte P; Stewart P; Winneke G; Charles MJ; Jacobson SW; Dewailly E; Boersma ER; Altshul LM; Heinzow B; Pagano JJ; Jensen AA, Comparison of polychlorinated biphenyl levels across studies of human neurodevelopment. Environ Health Perspect 2003, 111, (1), 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sethi S; Keil KP; Chen H; Hayakawa K; Li X; Lin Y; Lehmler HJ; Puschner B; Lein PJ, Detection of 3,3’-Dichlorobiphenyl in Human Maternal Plasma and Its Effects on Axonal and Dendritic Growth in Primary Rat Neurons. Toxicol Sci 2017, 158, (2), 401–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sethi S; Keil KP; Lein PJ, Species and Sex Differences in the Morphogenic Response of Primary Rodent Neurons to 3,3’-Dichlorobiphenyl (PCB 11). Toxics 2017, 6, (1), E4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fritsche E; Cline JE; Nguyen NH; Scanlan TS; Abel J, Polychlorinated biphenyls disturb differentiation of normal human neural progenitor cells: clue for involvement of thyroid hormone receptors. Environ Health Perspect 2005, 113, (7), 871–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takeuchi S; Anezaki K; Kojima H, Effects of unintentional PCBs in pigments and chemical products on transcriptional activity via aryl hydrocarbon and nuclear hormone receptors. Environ Pollut 2017, 227, 306–313. [DOI] [PubMed] [Google Scholar]
- 64.Pencikova K; Svrzkova L; Strapacova S; Neca J; Bartonkova I; Dvorak Z; Hyzdalova M; Pivnicka J; Palkova L; Lehmler HJ; Li X; Vondracek J; Machala M, In vitro profiling of toxic effects of prominent environmental lower-chlorinated PCB congeners linked with endocrine disruption and tumor promotion. Environ Pollut 2018, 237, 473–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dugas JC; Ibrahim A; Barres BA, The T3-induced gene KLF9 regulates oligodendrocyte differentiation and myelin regeneration. Mol Cell Neurosci 2012, 50, (1), 45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miyazaki W; Iwasaki T; Takeshita A; Tohyama C; Koibuchi N, Identification of the functional domain of thyroid hormone receptor responsible for polychlorinated biphenyl-mediated suppression of its action in vitro. Environ Health Perspect 2008, 116, (9), 1231–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lazar MA, Sodium butyrate selectively alters thyroid hormone receptor gene expression in GH3 cells. J Biol Chem 1990, 265, (29), 17474–7. [PubMed] [Google Scholar]
- 68.Ghisari M; Bonefeld-Jorgensen EC, Impact of environmental chemicals on the thyroid hormone function in pituitary rat GH3 cells. Mol Cell Endocrinol 2005, 244, (1–2), 31–41. [DOI] [PubMed] [Google Scholar]
- 69.You SH; Gauger KJ; Bansal R; Zoeller RT, 4-Hydroxy-PCB106 acts as a direct thyroid hormone receptor agonist in rat GH3 cells. Mol Cell Endocrinol 2006, 257–258, 26–34. [DOI] [PubMed] [Google Scholar]
- 70.Giera S; Bansal R; Ortiz-Toro TM; Taub DG; Zoeller RT, Individual polychlorinated biphenyl (PCB) congeners produce tissue- and gene-specific effects on thyroid hormone signaling during development. Endocrinology 2011, 152, (7), 2909–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grimm FA; Lehmler HJ; Koh WX; DeWall J; Teesch LM; Hornbuckle KC; Thorne PS; Robertson LW; Duffel MW, Identification of a sulfate metabolite of PCB 11 in human serum. Environ Int 2017, 98, 120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Park JS; Petreas M; Cohn BA; Cirillo PM; Factor-Litvak P, Hydroxylated PCB metabolites (OH-PCBs) in archived serum from 1950–60s California mothers: a pilot study. Environ Int 2009, 35, (6), 937–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Quinete N; Schettgen T; Bertram J; Kraus T, Occurrence and distribution of PCB metabolites in blood and their potential health effects in humans: a review. Environ Sci Pollut Res Int 2014, 21, (20), 11951–72. [DOI] [PubMed] [Google Scholar]
- 74.Grimm FA; Lehmler HJ; He X; Robertson LW; Duffel MW, Sulfated metabolites of polychlorinated biphenyls are high-affinity ligands for the thyroid hormone transport protein transthyretin. Environ Health Perspect 2013, 121, (6), 657–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wong PW; Pessah IN, Ortho-substituted polychlorinated biphenyls alter calcium regulation by a ryanodine receptor-mediated mechanism: structural specificity toward skeletal- and cardiac-type microsomal calcium release channels. Mol Pharmacol 1996, 49, (4), 740–51. [PubMed] [Google Scholar]
- 76.Li X; Holland EB; Feng W; Zheng J; Dong Y; Pessah IN; Duffel MW; Robertson LW; Lehmler HJ, Authentication of synthetic environmental contaminants and their (bio)transformation products in toxicology: polychlorinated biphenyls as an example. Environ Sci Pollut Res Int 2018, 25, (17), 16508–16521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Martinez A; Awad AM; Herkert NJ; Hornbuckle KC, Determination of PCB fluxes from Indiana Harbor and Ship Canal using dual-deployed air and water passive samplers. Environ Pollut 2019, 244, 469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Herkert NJ; Jahnke JC; Hornbuckle KC, Emissions of Tetrachlorobiphenyls (PCBs 47, 51, and 68) from Polymer Resin on Kitchen Cabinets as a Non-Aroclor Source to Residential Air. Environ Sci Technol 2018, 52, (9), 5154–5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shanahan CE; Spak SN; Martinez A; Hornbuckle KC, Inventory of PCBs in Chicago and Opportunities for Reduction in Airborne Emissions and Human Exposure. Environ Sci Technol 2015, 49, (23), 13878–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fang F; Chu S; Hong CS, Air-water Henry’s law constants for PCB congeners: Experimental determination and modeling of structure-property relationship. Anal Chem 2006, 78, (15), 5412–8. [DOI] [PubMed] [Google Scholar]
- 81.Pessah IN; Hansen LG; Albertson TE; Garner CE; Ta TA; Do Z; Kim KH; Wong PW, Structure-activity relationship for noncoplanar polychlorinated biphenyl congeners toward the ryanodine receptor-Ca2+ channel complex type 1 (RyR1). Chem Res Toxicol 2006, 19, (1), 92–101. [DOI] [PubMed] [Google Scholar]
- 82.Kenet T; Froemke RC; Schreiner CE; Pessah IN; Merzenich MM, Perinatal exposure to a noncoplanar polychlorinated biphenyl alters tonotopy, receptive fields, and plasticity in rat primary auditory cortex. Proc Natl Acad Sci U S A 2007, 104, (18), 7646–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schantz SL; Seo BW; Wong PW; Pessah IN, Long-term effects of developmental exposure to 2,2’,3,5’,6-pentachlorobiphenyl (PCB 95) on locomotor activity, spatial learning and memory and brain ryanodine binding. Neurotoxicology 1997, 18, (2), 457–67. [PubMed] [Google Scholar]
- 84.Lesiak A; Zhu M; Chen H; Appleyard SM; Impey S; Lein PJ; Wayman GA, The environmental neurotoxicant PCB 95 promotes synaptogenesis via ryanodine receptor-dependent miR132 upregulation. J Neurosci 2014, 34, (3), 717–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Samso M; Feng W; Pessah IN; Allen PD, Coordinated movement of cytoplasmic and transmembrane domains of RyR1 upon gating. PLoS Biol 2009, 7, (4), e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pessah IN; Lehmler HJ; Robertson LW; Perez CF; Cabrales E; Bose DD; Feng W, Enantiomeric specificity of (−)-2,2’,3,3’,6,6’-hexachlorobiphenyl toward ryanodine receptor types 1 and 2. Chem Res Toxicol 2009, 22, (1), 201–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Adasme T; Haeger P; Paula-Lima AC; Espinoza I; Casas-Alarcon MM; Carrasco MA; Hidalgo C, Involvement of ryanodine receptors in neurotrophin-induced hippocampal synaptic plasticity and spatial memory formation. Proc Natl Acad Sci U S A 2011, 108, (7), 3029–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Krey JF; Dolmetsch RE, Molecular mechanisms of autism: a possible role for Ca2+ signaling. Curr Opin Neurobiol 2007, 17, (1), 112–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.