Hope is the thing with feathers
That perches in the soul
And sings the tune without the words
And never stops at all
- Emily Dickinson
The 4th International Lafora Disease Workshop was held in La Jolla, California, USA at the Sanford Consortium for Regenerative Medicine on September 6–8, 2018. The meeting was organized and hosted by Dr. Matthew Gentry, Director of the Lafora Epilepsy Cure Initiative (LECI) and Professor at the University of Kentucky College of Medicine, Drs. Kim and Jim Rice of Chelsea’s Hope Lafora Children’s Research Fund, and Drs. Jack Dixon and Carolyn Worby of University of California, San Diego (UC-San Diego). The workshop was sponsored by Chelsea’s Hope, the National Institute of Neurological Disease and Stroke (NIH NINDS P01 NS097197), Valerion Therapeutics, Ionis Pharmaceuticals, Third Rock Ventures, and the Dixon laboratory. The workshop was attended by nearly 100 students, postdoctoral scholars, clinicians, academic and company scientists, and National Institutes of Health (NIH) representation from the United States, Spain, Canada, Turkey, India, Italy, France, and Australia as well as approximately 25 friends and family members of patients with Lafora from the US, the Netherlands, United Kingdom, and the Bahamas (Figure 1).
Figure 1.
Over 125 scientists, clinicians, and family and friends of Lafora patients attended the 4th International Lafora Disease Workshop.
It was the fourth Lafora disease workshop since 2014 to bring together scientists, clinicians and families with the goal of accelerating research towards a cure for Lafora disease (LD), a rare and fatal childhood epilepsy. The event was first catalyzed by the parents of Lafora patients and members of Chelsea’s Hope, Jim and Kim Rice and Linda Gerber, who invited scientists from around the world studying Lafora disease to present at the inaugural workshop and pleaded for a collaborative effort towards therapies and a cure [1]. Attending laboratories are now funded by an international $9.1 million P01 program project grant from the National Institutes of Health entitled “Lafora epilepsy: basic mechanisms to therapies” directed by Dr. Gentry that established the LECI in 2016 [2]. The LECI is comprised of researchers from four American universities: University of Kentucky, Indiana University, University of Texas (UT) Southwestern Medical Center, and UC-San Diego; and three Spanish institutions: Autonomous University of Madrid, Institute for Research in Biomedicine (IRB Barcelona), Institute of Biomedicine of Valencia, and the Spanish Research Council (CSIC). With each workshop, researchers continue to present novel work, and hope for a treatment or cure has become increasingly tangible. At this year’s workshop, every scientific talk consisted of a revolutionary mechanistic discovery and/or translational work with direct application to the clinic. Importantly, Valerion Therapeutics and Ionis Pharmaceuticals had a major presence at this workshop, encouraging families to enroll in the upcoming natural history study and providing information on how and when therapies may become available to their children.
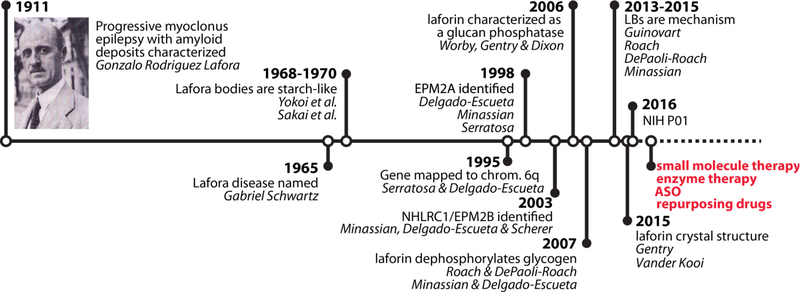
The workshop began on Thursday evening, September 6, with a welcome from Dr. Gentry. He gave a brief history of LD research (Figure 2) and reminded the group of how much progress has been made since the P01 was awarded in 2016. He described four potential lines of therapy that would be discussed throughout the rest of the workshop: 1) small molecule inhibitors, 2) enzyme therapy, 3) antisense oligonucleotides (ASOs), and 4) repurposed drugs. Dr. Frank Harris, president of Chelsea’s Hope and the father of a Lafora patient, spoke about the history and mission of the organization, providing a personal perspective to LD. He described the devastating disease course, highlighted by the recent and tragic loss of a well-loved Lafora patient, Adela Richer. Special honor is given to an individual patient at each Lafora workshop, and Adela was to receive that spotlight at this year’s meeting.
Figure 2. A timeline of LD-related research.
In red are the four platforms that LECI scientists are pursuing as LD treatments. ASO – antisense oligonucleotides.
Dr. Jose Serratosa, Chief of Neurology and Professor at the Autonomous University of Madrid, gave the keynote lecture. Dr. Serratosa takes a unique, genetic approach to study epilepsies. He described the clinical characteristics and evolution of LD, noting that LD produces a unique electroencephalophagram (EEG) signature that is distinct from the EEG recordings observed in juvenile myoclonic epilepsy (JME). Since LD is indistinguishable from JME in early stages, often leading to misdiagnosis, EEG could be used as an early biomarker for LD. Additionally, Dr. Serratosa described the clinical features and genetics of the various progressive forms of LD: early-onset, classic, and mild/slow-course LD. Distinct progressive forms of LD break the current disease paradigm, since for decades, neurologists believed LD produced a homogenous disease course. Finally, Dr. Serratosa spoke about possible new therapies for LD, focusing on drug re-purposing for the treatment of genetic epilepsies. He showed that prenatal and postnatal treatment of LD mouse models with metformin, a drug commonly used to treat diabetes, slowed the neurological defects and seizure progression in mice. Following the keynote address, Dr. Kim Rice introduced all of the Lafora family members and friends present at the workshop. Dr. Rice also paid tribute to Adela Richer and her family with a slideshow of photos and diary entries from before and after her diagnosis.
A somber atmosphere followed, and Dr. Paul Goldberg, Vice President of Clinical Development at Ionis Pharmaceuticals, addressed the audience regarding the upcoming LD natural history study that is due to enroll the first patient in 2018. Dr. Goldberg described this study as a potential “historical control” to define the natural course of the disease, establish a primary endpoint, and identify biomarkers, all of which will be done while potential therapeutics are still in preclinical development. He explained how this study could take the place of a control group once a therapeutic is available to conduct the clinical trials. This approach is necessary and appropriate in situations of severe and ultra-rare diseases such as LD. Dr. Goldberg invited all patients with LD to enroll in the study, irrespective of their current medications. The study will follow approximately 30 patients over a 2-year time period, with detailed evaluations at approximately every 6 months including clinical assessments, seizure monitoring, neurocognitive testing, video EEGs, as well as motor and disability assessments. Dr. Goldberg further indicated the importance for patients to contribute blood and cerebrospinal fluid samples (optional) that would allow for the assessment of key neuronal biomarker that may provide significant insights into LD. Lafora family members and friends were then given the opportunity to ask questions of clinicians, pharmaceutical representatives, and scientists. Dr. Hal Landy, Chief Medical Officer of Valerion Therapeutics, joined Dr. Goldberg in fielding questions. The evening concluded with dinner at The Village at Torrey Pines, to which all attendees were invited.
The second day of the workshop consisted of exciting scientific sessions. Dr. Anna DePaoli-Roach, Professor at Indiana University, described the biogenesis of LD as “the perfect storm.” Using gene-editing technology in collaboration with Dr. Peter Roach, she generated mice harboring the C265S mutation in laforin, which renders the protein catalytically inactive. In humans, mutations in the genes encoding laforin cause LD, but the relevance of laforin’s catalytic activity to LD has been questioned. Dr. DePaoli-Roach showed that this mutation does not lead to the formation of Lafora bodies (LBs), the abnormal carbohydrate accumulations that drive disease progression, and confirmed that elevated glycogen phosphate alone does not drive LB formation. Dr. Pascual Sanz from the Institute of Biomedicine of Valencia (Spanish Research Council, CSIC) then presented his findings of LBs in astrocytes and microglia. He reported that in mouse models of LD, glutamate levels are elevated, glutamate transport and microglial morphology are altered, and pro-inflammatory mediators are upregulated. His results clearly demonstrate a metabolic defect in LD and suggest that glial cells contribute to LD etiology. Dr. Gentry also identified novel defects in brain metabolism in LD mouse models using RNAseq and metabolomics. His group discovered that while most metabolic pathways are upregulated in the LD mouse brain, there is a significant defect in glycosylation, and both glycogen and LBs contain glucosamine, a metabolite required for glycosylation. These results indicate glycogen provides substrates for glycosylation pathways in the brain, and suggests that defective glycosylation could contribute to LD pathophysiology.
Dr. Jordi Duran from Dr. Joan Guinovart’s laboratory at IRB Barcelona gave a talk posing the question, “Is LD an astrocytic disease?” challenging the long-held belief that LBs are exclusively found in neurons. Dr. Duran showed that astrocytic and neuronal LBs are morphologically and immunologically distinct, and that the majority of LBs in LD mice could be eliminated by the astrocyte-specific genetic deletion of glycogen synthase. After his talk, Dr. Berge Minassian, Chair of Pediatric Neurology at UT-Southwestern and a prominent neurologist in the LD field, affirmed that after a historical review of the literature, he had concluded that LBs in fine processes which were assumed to be neuronal are indeed likely to be astrocytic. Dr. Carolyn Worby of UC-San Diego who oversees the LECI Biological Core presented cellular and murine models of specific LD patient mutations that she has generated. Dr. Worby’s formidable efforts have now established important patient-specific tools that can be used by other LECI scientists for mechanistic and translational studies of LD. Wrapping up the morning session, Dr. Tamar Grossman, Director of Translational Medicine at Ionis Pharmaceuticals, and Dr. Minassian updated the group on their efforts using ASOs to downregulate glycogen synthase for the treatment of LD.
After a lively lunch, poster session and animated discussions, Dr. Belén Molla, a postdoctoral researcher in the Sanz laboratory, presented her behavioral data on LD mouse models, identifying the best tests for detecting the neurological defects found in LD mice. Next, Dr. Tom Hurley, a structural biologist and glycogen synthase expert at Indiana University, gave an update on the collaborative effort between his laboratory with Drs. Peter Roach and Anna DePaoli-Roach to identify small molecule inhibitors of glycogen synthase. Dr. Marina Sánchez from Dr. Serratosa’s laboratory presented her neuroimaging studies of LD mouse models, in which she identified brain abnormalities that were perplexingly different than those found in human patients. Dr. Joan Guinovart of IRB Barcelona then presented his work defining the therapeutic window for LD. Using Cre/lox technology, the Guinovart laboratory generated a LD mouse model with a tamoxifen-inducible deletion of glycogen synthase, and showed that tamoxifen administration in adult LD mice halted LB formation, neurodegeneration, and gliosis. This study was critical for demonstrating the potential efficacy of therapeutics targeting glycogen synthase in adolescent and adult humans. Dr. Felix Nitschke of the Minassian laboratory followed, presenting a rigorous structural characterization of the polyglucosan bodies found in mouse models of LD and adult polyglucosan body disease. Katy Brewer, a Ph.D. student in the Gentry laboratory, and Dr. Tracy McKnight, Director of Translation Research at Valerion Therapeutics, concluded the scientific portion of the day with exciting data demonstrating the reduction of polyglucosan in LD mouse models using antibody-enzyme fusion (AEF) technology. One of these AEF drugs is already in clinical trials for the treatment of Pompe disease, and provides hope for the treatment of LD in the near future. Dr. Antonio Delgado-Escueta, a Lafora neurologist from the University of California, Los Angeles, gave a final discourse highlighting the importance of the natural history study and clinical trial readiness to cure LD. The evening culminated with a rooftop dinner at the Bella Vista Social Club and Cafe.
Saturday, September 8 consisted of a NIH P01 planning meeting, in which LECI investigators met to discuss project details, exchange data and materials, and set benchmarks for the upcoming year.
Acknowledgments
Research reported in this publication is supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under Award Numbers R01NS070899 and P01NS097197. We thank Ms. Cheylene Plummer at the University of Kentucky College of Medicine for logistical support and planning the event.
Footnotes
Disclosure: Drs. Grossman and Goldberg are employees of Ionis Pharmaceuticals. Drs. Landy and McKnight are employed by Valerion Therapeutics. Dr. Gentry has a sponsored project funded by Valerion Therapeutics.
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- [1].Rice K, Gentry MS. Passionate parents catalyze research: Serendipitous meetings and basic biochemistry accelerate the push for a cure for the rare and fatal Lafora disease In: ASBMB Today. Rockville, MD: American Society for Biochemistry and Molecular Biology; 2017. [Google Scholar]
- [2].Brewer MK, Gentry MS. The 3rd International Lafora Epilepsy Workshop: Evidence for a cure. Epilepsy Behav 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]