Abstract
Increased understanding of fructose metabolism, which begins with uptake via the intestine, is important because fructose now constitutes a physiologically significant portion of human diets and is associated with increased incidence of certain cancers and metabolic diseases. New insights in our knowledge of intestinal fructose absorption mediated by the facilitative glucose transporter GLUT5 in the apical membrane and by GLUT2 in the basolateral membrane are reviewed. We begin with studies related to structure as well as ligand binding, then revisit the controversial proposition that apical GLUT2 is the main mediator of intestinal fructose absorption. The review then describes how dietary fructose may be sensed by intestinal cells to affect the expression and activity of transporters and fructolytic enzymes, to interact with the transport of certain minerals and electrolytes, and to regulate portal and peripheral fructosemia and glycemia. Finally, it discusses the potential contributions of dietary fructose to gastrointestinal diseases and to the gut microbiome.
Keywords: carbohydrates, GLUT, ligand recognition, metabolism, nutrition, sugars
1. INTRODUCTION
Increases in our understanding of fructose metabolism, beginning with the initial step of fructose uptake from the lumen via the intestine (Figure 1), are important because fructose, after 50% increases in table sugar consumption in the last 60 years, constitutes a significant portion of the daily caloric intake in the United States and in many other parts of the world (27). Between the mid-1970s and the early 2000s, consumption rates of total (added plus naturally occurring) fructose in the United States increased by 33%, peaking at ~50 g/day in 2004 (83), with the top 10% of the population consuming 75 g/day of fructose. Average daily consumption of added sugars (of which half is likely fructose) has since decreased gradually but remains at 17% (300 kcal or 75 g/day) of total energy intake, still well above the recommended limit of 10% (104). Intake of added sugars in developed countries other than the United States has also decreased or stabilized in recent years but still represents~13% of daily energy intake (95, 142).In the 1990s, consumption of total sugars was lower in these other countries (~110 g/day) compared to the United States’ ~140 g/day rate. Children and teenagers tend to consume the greatest amount of fructose per kilogram body weight. In some developing nations, the daily consumption of total sugar is less, but amounts consumed still represent ~10% of total calorie intake (1). Moreover, high-fructose corn syrup (HFCS) exports from the United States to developing countries like Mexico, Vietnam, the Philippines, and Indonesia increased by 10-to 100-fold between 2005 and 2012, indicating a greater demand for added sugars in these emerging markets (https://www.fas.usda.gov/). In summary, worldwide consumption of dietary fructose, either as added table sugar or as free monosaccharide, is no longer increasing rapidly but still is at historic highs. Due to limited space, this review emphasizes papers published after our 2013 review (27) and does not discuss the large number of studies linking fructose to metabolic syndrome, including nonalcoholic fatty liver disease (NAFLD), hypertension, obesity, diabetes, and hyperinsulinemia.
Figure 1.
Intestinal fructose transport across the small intestinal epithelia. ❶ Dietary fructose (F) is transported across the apical membrane in monosaccharide form by a member of the facilitative GLUT family, GLUT5. Most fructose (solid arrow) exits the cytosol and enters the portal vein via basolateral GLUT2, which is also capable of transporting glucose and galactose (G) absorbed across the apical membrane by SGLT1 via Na+-coupled cotransport. Deletion of GLUT5 completely eliminates transepithelial fructose transport, while total or intestine-specific deletion of GLUT2 only modestly reduces glucose transport, for as yet unclear reasons. ❷ Some fructose is phosphorylated by KHK, thus keeping the lumen-to-cytosol gradient favorable for fructose uptake. Deletion of KHK reduces the transapical fructose transport rate. ❸ When luminal fructose concentrations are high, a product(s) of fructose metabolism (M) stimulate(s) transcription and translation of GLUT5 as well as fructolytic enzymes. Basolateral delivery of fructose via GLUT2 in GLUT5−/− mice cannot induce increases in GLUT5 mRNA, suggesting that GLUT5 may also function as a transceptor. ❹ New GLUT5 is delivered to the apical membrane via Rab11a-mediated (R) endosomes, enhancing transapical fructose transport when luminal fructose levels are high. Intestine-specific deletion of Rab11a also inhibits SGLT1-mediated glucose transport, suggesting that this GTPase participates in the trafficking of transporters bound for the apical membrane. ❺ Portal fructose concentrations increase markedly with fructose intake. Figure modified from Reference 100. Abbreviations: ER, endoplasmic reticulum; GLUT, facilitative glucose transporter; KHK, ketohexokinase; Rab11a, Ras-related protein-in-brain 11a; SGLT1, Na+-dependent glucose transporter 1.
2. STRUCTURE, LIGAND RECOGNITION, AND PHARMACOLOGICAL INHIBITION
Fructose is specifically and passively transported by the facilitative glucose transporter 5 (GLUT5, Slc2a5) across the intestinal apical membrane and then is transported from the cytosol to the blood by GLUT2 (Slc2a2). In humans, the GLUT family (gene family Slc2a) has 14 members that vary in tissue expression as well as substrate specificity and affinity. GLUT2 transports not only fructose but also glucose and galactose, with a Km for fructose more than fivefold higher than that of GLUT5. Normally, GLUT5 is mainly expressed in the small intestine and kidney while GLUT2’s major sites of expression are the liver, pancreas, intestine, kidney, and brain (92). Other GLUTs capable of fructose and glucose transport include GLUT7, GLUT8, GLUT9, and GLUT11, although a recent study showed that neither GLUT7 nor GLUT9 transport fructose (33). GLUT2 is most closely related to GLUT1 (expressed ubiquitously), GLUT3 (mainly in neuronal cells) and GLUT4 (an insulin-dependent transporter in skeletal muscle and adipocytes), which do not transport fructose but have as substrates glucose, dehydroascorbate, glucosamine, galactose, and mannose. Generally, given the sequence homology among GLUTs, a big challenge in pharmaceutically targeting a certain GLUT is that several other GLUTs may be affected, i.e., there is a paucity of specific GLUT inhibitors. This makes understanding ligand recognition in the GLUT family imperative. In particular, when considering decreasing the absorption of dietary fructose in intestines, specific inhibition of GLUT5 seems to be the option with the fewest potential side effects; GLUT2 inhibition in intestines is desirable but in pancreatic beta cells can lead to insulin insensitivity and ultimately diabetes.
2.1. Structure of GLUT5 and Putative Mechanism of Fructose Transport
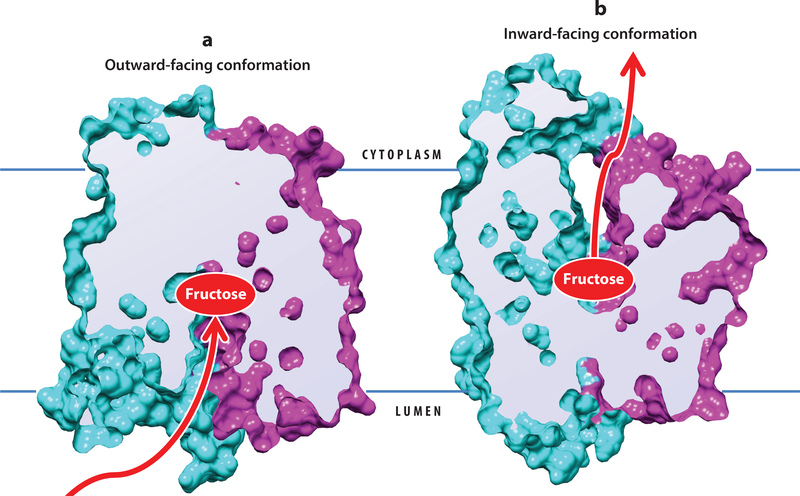
The GLUT family belongs to the major facilitator superfamily (MFS) of transporters. GLUTs share the MFS fold, which consists of 12 transmembrane helices organized into two six-helix domains, the N- and C-halves, related by a pseudo twofold axis of symmetry perpendicular to the cell membrane. The substrate binding site is housed in the central polar cavity between the N- and C-domains (Figure 2). The transport mechanism proposed for MFS proteins postulates that the substrate cavity is alternately exposed to each side of the membrane through four major conformations of the transporter: outward-facing (Figure 2a, substrate cavity opened to lumen or extracellular space) and inward-facing (Figure 2b, substrate cavity opened to cytoplasm), each in turn with two states: open (no substrate) and occluded (bound substrate). Thus, a cycle of transport presumably involves the transporter in the outward-facing open conformation binding the substrate, then going into the outward-facing occluded conformation, which transitions into the inward-facing occluded conformation before finally the substrate is released with the transporter in the inward-facing open conformation. The mechanistic details of what facilitates the transitions among the different conformational states are still being worked out (98), and the proposed models based on GLUT crystal structures ignore the possibility that at least some GLUT members may function as oligomers (17). For mammalian GLUT5, both the inward- and outward-facing conformations are known, and the mechanism of fructose transport proposed by Nomura et al. (98) postulates a central role for two bent helices in the C-domain: transmembrane helix (TM) 7 and TM10 in coupling fructose binding to conformational transitions between open and occluded states. GLUT2 has eluded structural determination so far, but it shares 52–55% amino acid sequence identity with GLUT1 and GLUT3, whose crystal structures capture each of the outward- and inward-facing conformations, respectively, allowing for the GLUT2 structure to be readily modeled. Additionally, GLUT1–4 share conserved amino acid residues in the substrate binding sites. Availability of GLUT crystal structures provides a solid basis for better understanding substrate variability among GLUTs and has allowed in silico high-throughput ligand screening to identify novel inhibitors of GLUTs (41, 89, 135).
Figure 2.
Major conformational states of GLUT5. Surface filling model of GLUT5, sectioned in the middle, in (a) outward-facing conformation (PDB ID 4YBQ) and (b) inward-facing conformation (PDB ID 4YB9). The N-terminal-half-six-helix domain is shown in cyan and the C-terminal-half-six-helix domain in magenta. The fructose binding site is in the central cavity. Binding of fructose in the outward-facing conformation promotes conformational change to the inward-facing conformation, and fructose is released into the cytoplasm. Abbreviations: GLUT, facilitative glucose transporter; PDB, Protein Data Bank.
2.2. Molecular Basis of Ligand Recognition and Inhibition of GLUT5
So how does the substrate binding site of GLUT2 recognize both glucose and fructose while GLUT5 discriminates so well between the two hexoses? Are there substrate binding site differences between GLUT2 and GLUT5 that can be exploited in drug discovery? We first look at the structural diversity of glucose and fructose in solution and when bound to proteins (Figure 3). Both hexoses adopt the pyranose or furanose conformations in water, with the pyranose form being dominant for glucose (99% pyranose, 1% furanose, and 0.002% open-chain) and fructose (67.5% pyranose, 31.5% furanose, and 0.8% open-chain), and the furanose form being much more prevalent for fructose than glucose (2). When surveying the Protein Data Bank (http://www.rcsb.org) for protein crystal structures liganded with glucose or fructose, we found that, as in solution, glucopyranose is the dominant conformation, but for fructose the most frequent conformation is the furanose form (by September 2017, the Protein Data Bank had 759 protein structures with β-glucopyranose, 56 with β-fructofuranose, and 9 with β-fructopyranose). Comparison of glucopyranose with fructofuranose and fructopyranose shows more significant differences in the positions for hydroxyl groups in the fructofuranose-glucopyranose pair than in the pyranose forms of the hexoses (Figure 3a). Studies on GLUT1 and GLUT2 homologues indicate that the transporter binds fructose in the furanose conformation and glucose in the pyranose form (143), although GLUT1 can transport epimers of C3, C4, and C5 fructofuranose moiety (74). It is possible that some GLUTs that transport fructose can bind both the furanose and pyranose forms, while others have a preference for just one of the conformers.
Figure 3.
Ligand recognition in GLUT2 and GLUT5. (a) In solution, hexoses adopt the pyranose, furanose, and open-chain forms. In crystal structures, three major conformers of glucose and fructose are observed (here are shown the conformers from PDB IDs 3KF3, 5GNY, and 2FA1). The bottom row shows a pairwise superposition among fructofuranose, glucopyranose, and fructopyranose. (b) Modeled glucose binding site of GLUT2 (on the basis of PDB IDs 4GBZ and 4ZW9) with β-glucopyranose. H-XI, -VII, -X, -IV are the transmembrane helices –11, –7, –10, and –4, respectively. The amino acid residues for the substrate binding site are conserved among GLUT1–4. (c) Modeled fructose (F) binding site of GLUT5 (PDB ID 4YBQ). Available crystal structures of GLUT5 do not include fructose, and the conformation of fructose is uncertain. (d) Surface filling model (sectioned in the middle) of GLUT5 liganded with MSNBA. (e) Putative binding site of MSNBA in GLUT5 model. Helices 4 and 5 are omitted for clarity. Abbreviations: GLUT, facilitative glucose transporter; MSNBA, N-[4-(methylsulfonyl)-2-nitrophenyl]-1,3-benzodioxol-5-amine; PDB, Protein Data Bank.
Examining the substrate binding sites of GLUT2 (Figure 3b) and GLUT5 (Figure 3c) reveals three differences: H387GLUT5 versus F411GLUT2, A396GLUT5 versus W420GLUT2, and H419GLUT5 versus N443GLUT2. Mutations of rat GLUT5 H386A (387 in human) and H418A (419 in human) reduced fructose binding affinity (98). H387GLUT5 is also a key residue in recognizing a GLUT5-specific inhibitor, N-[4-(methylsulfonyl)-2-nitrophenyl]-1,3-benzodioxol-5-amine (MSNBA), shown in Figure 3e (41). A396WGLUT5 changes the substrate specificity of the mutant transporter so that it takes up both glucose and fructose (40). Therefore, H387, A396, and H419 are key residues in ligand recognition in GLUT5, and inhibitor design specific for GLUT5 can take advantage of chemical interactions with H387 and H419. A remaining question is how GLUT2 takes up fructose even though the substrate binding site residues of GLUT2 and GLUT1 are identical. The overall substrate binding affinity of GLUT2 (Km for glucose = 17 mM) is lower than that of GLUT1 (Km for glucose = 3 mM) (6). A difference between GLUT1 and GLUT2 near the substrate binding site is I322 (GLUT2) versus V290 (GLUT1), and the I322V mutant of GLUT2 is unable to transport fructose (33, 82). This residue (I322GLUT2) is located two amino acids after N320GLUT2 (or N288GLUT1), which is a substrate binding, conserved amino acid in the same helix (TM7). Thus, the side chain in position 322 of GLUT2 may influence the active site by finetuning the helix movement. Mutants of rat GLUT5 I295V (equivalent of I322GLUT2), I295A, Y296A, and Y297A all showed decreased fructose binding (98). Nevertheless, the answer may lie far away from the substrate binding site, as exemplified by HXT15 and HXT16 (yeast polyol transporters related to GLUTs), which differ in only two amino acids located in a cytoplasmic loop but have very different substrate affinity and specificity (64).
Inhibitors of GLUT5 have the potential to be developed into drugs for prevention of fructose-induced diseases, but until recently, no potent inhibitors of GLUT5 were known. Natural products (–)-epicatechin-gallate and rubusoside are nonspecific inhibitors affecting both GLUT1 and GLUT5. (–)-epicatechin-gallate is extracted from green tea (IC50GLUT5 = 0.1–0.2 mM) (118) and rubusoside from the Chinese blackberry tea plant Rubus suavissimus (IC50GLUT5 = 6.7 mM) (40). Another natural compound, Astragalin-6-glucoside, inhibits GLUT5 but not GLUT1. It is a glycosylated derivative of Astragalin, from the American pokeweed Phytolacca americana (IC50GLUT5 = 6.8 mM) (40). However, the above compounds have low potency. In silico screening of a library of six million small compounds against an inward-facing GLUT5 model, validated by in vitro transport assay in proteoliposomes and MCF7 cells, identified MSNBA (Figure 3d,e) as a potent (Ki ~ 3 μM) and specific GLUT5 inhibitor (it did not affect the transport activities of GLUT1–4) (41). Additionally, a whole-cell-based GLUT5 assay system amenable to high-throughput ligand screening has recently become available, enabling accelerated discovery of future GLUT5 inhibitors (132).
3. A DECADE OF CONTROVERSY: HYPOTHESES DISPLACING GLUT5 AS MAIN TRANSPORTER
3.1. The Apical GLUT2 Hypothesis
This hypothesis has attracted much attention since 2000 because it contradicts the longstanding belief that the active, Na+-dependent glucose transporter 1 (SGLT1, Slc5a1) and GLUT5 are primarily responsible for sugar transport across the apical membrane (68). According to this hypothesis, glucose initially transported by SGLT1 rapidly recruits GLUT2 into the apical membrane via protein kinase C (PKC) βII signaling, a process that provides a sizable facilitated component of sugar absorption to supplement SGLT1-mediated glucose and GLUT5-mediated fructose uptake (70). Complicated crosstalk among three signaling pathways is thought to acutely regulate intestinal fructose transport in perfused isolated loops of rat jejunum, such that GLUT2 levels increase by up to fourfold and eventually mediate most fructose transport across the apical membrane (51). Chronic consumption of sugar-rich diets increases intestinal fructose uptake fivefold in wild-type mice but increases uptake only twofold in GLUT2−/− mice, indicating that upregulation of GLUT5 by dietary fructose (see Section 4) is less substantive than that of GLUT2 (45). Recent models depict GLUT2 becoming a permanent feature of apical membranes in diabetes and during chronic consumption of high-fructose meals (69). The apical GLUT2 model, reviewed in major journals, is based on the initial observation that glucose absorption increased via a phloretin-sensitive transporter in brush-border membrane vesicles and in isolated loops from diabetic rats, and it assumes that high concentrations of phloretin inhibited only GLUT2, not GLUT5 and SGLT1. However, Wright and colleagues (52) argued that phloretin can inhibit SGLT1 in a noncompetitive manner with a dissociation constant (Ki) of 50 μM; thus, the phloretin-dependent component of glucose transport across the apical membrane cannot be attributed solely to GLUT2, an interpretation that grossly overestimated the contribution of apical GLUT2 as well as underestimated the importance of SGLT1 (144). Moreover, they observed that humans with glucose-galactose malabsorption (GGM; absent or defective SGLT1) were completely unable to absorb glucose (144).
The apical GLUT2 hypothesis underwent further scrutiny when gene-knockout animal models lacking GLUT2, GLUT5, and SGLT1 became available. Glucose malabsorption in GGM patients was thought to be compatible with the apical GLUT2 hypothesis, which required at least some SGLT1 to initiate glucose absorption that would stimulate PKC βII signaling (70). However, SGLT1−/− mice were, in a variety of experimental conditions, unable to absorb glucose and to secrete gut hormones normally released during glucose absorption by enterocytes, suggesting little contribution of GLUT2 to apical glucose uptake and to luminal glucose sensing (44). Transcellular glucose transport and glucose tolerance test results were normal in GLUT2−/− mice (global deletion; a model of human Fanconi Bickel Syndrome caused by mutations in GLUT2) (123). Recently, targeted GLUT2 deletion from mouse small intestinal epithelia (GLUT2ΔIEC) only modestly (~20%) and transiently reduced blood glucose levels after bolus feeding (113). Thus, GLUT2 may not be necessary for transepithelial glucose transport—in its absence, transepithelial transport is thought to be mediated by glucose-6-phosphate translocase and membrane vesicles that eventually fuse with the basolateral membrane (123). In SGLT1−/− mice, tracer glucose failed to enter the intestinal mucosa in vivo, suggesting that glucose was unable to cross the apical membrane without SGLT1. In contrast, in GLUT2−/− mice, tracer glucose was mostly retained in the mucosa, suggesting that glucose entered the intestinal cell but was unable to cross the basolateral membrane without GLUT2 (108). Thus, SGLT1 is the primary glucose transporter, and GLUT2 is likely not involved in apical glucose transport irrespective of luminal glucose concentration (108).
What about the apical GLUT2 model as applied to intestinal fructose absorption? Consumption of a high-fructose diet that also contains glucose by GLUT5−/− (global deletion) mice results in malabsorption (4, 100), suggesting that GLUT5 is required for fructose transport and that PKC βII-mediated GLUT2 insertion to the apical membrane cannot compensate for the absence of GLUT5. These findings challenge the suggestion that sufficient amounts of GLUT2 can be recruited to the apical membrane by SGLT1 (69). Moreover, only GLUT5 expression increased with perfusion of the small intestine with high-fructose solutions (13, 14), suggesting that luminal fructose specifically upregulates GLUT5 and not GLUT2.
Taking these findings into account, quantitative models of glucose transport propose that, irrespective of dietary concentration, SGLT1-mediated active transport of glucose across the apical membrane is the major mechanism of glucose absorption (47). Models that simulated increased GLUT2 in the apical membrane predicted that such a scenario would stimulate downhill glucose reflux back to the lumen from the cytosol, thereby reducing, rather than enhancing, net glucose absorption across the luminal surface (93).
3.2. Other Proposed Intestinal Fructose Transporters
What are the roles in intestinal fructose transport of GLUT7, GLUT8, GLUT9, and GLUT12, each of which is reportedly expressed in the small intestine and proposed to have dual specificity for glucose and fructose (33, 82)? Crosstalk between GLUT8 and GLUT12 has been proposed to regulate intestinal fructose transport in Caco2 cells and in mice (18). Recent findings, however, indicate a small to nonexistent contribution by these GLUTs in transintestinal fructose absorption. First, small intestinal expression of GLUT7, GLUT8, and GLUT12 in mice, relative to that of GLUT5, was low (100). Second, unlike GLUT5, which increased in abundance, expression of these other GLUTs remained low in mice fed high fructose, suggesting that their expression is not regulated by dietary fructose. Finally, there was no fructose transport and there were no compensatory increases in intestinal expression of GLUT7, GLUT8, and GLUT12 in GLUT5−/− mice (100), indicating that GLUTs other than GLUT5 cannot transport fructose in the small intestine. Fructose may not even be a substrate of GLUT7 and GLUT9 (33).
In summary, recent evidence derived from mouse genetic models suggests that transapical transport of fructose and glucose in the mammalian small intestine is mediated primarily by GLUT5 and SGLT1, respectively.
3.3. Potential Exceptions to Transcellular Sugar Transport
Work by Karasov and colleagues (87), mainly in vivo, on intestinal glucose transport in several species of small birds and flying mammals that have high metabolic demand indicates that transcellular glucose (and likely fructose) transport is supplemented substantially by a passive paracellular component. Deletion of GLUT5 and SGLT1 from these animal models would confirm the role of the paracellular pathway.
4. DIETARY REGULATION OF INTESTINAL FRUCTOSE ABSORPTION
The rate of appearance of fructose in the blood depends on the number of GLUT5 transporters and on the site of fructose absorption; hence, regulation of levels of GLUT5 by its own substrate is important physiologically. This topic has been covered in earlier reviews (26, 27), and only more recent studies are discussed here. Briefly, chronic feeding, acute gavage-feeding, or in vivo intestinal perfusion of fructose increases GLUT5 expression and activity that occur primarily in the proximal regions of human and rodent small intestine (23, 28–30, 90, 100, 107).
4.1. Is GLUT5 a Transceptor?
GLUT5 may be acting as a transceptor—a transporter that, upon substrate binding, activates intracellular signaling that initiates a response (129)—able to “sense” luminal levels of fructose, thereby triggering an adaptive response that links expression levels of transporters and enzymes to prevailing nutrient concentrations (35). Fructose sensing by GLUT5 is robust, and even in vitro exposure of primary cell cultures to fructose increases GLUT5 expression (72). This induction of GLUT5 by its own substrate involves transcription and translational components, since it can be blocked by actinomycin-D and cycloheximide, respectively (24, 60). Newly synthesized GLUT5 protein is then trafficked to the luminal membrane via a Ras-related protein-in-brain 11 (Rabil), a small guanosine 5′-triphosphatase that is associated with recycling endosomes and plays a crucial role in the trafficking of various proteins to the apical membrane (119). Rab11aΔIEC (targeted deletion from the small intestinal mucosa) mice failed to induce GLUT5 expression and activity in the small intestine when gavage-fed with fructose solutions, indicating that Rab11a is an essential component of GLUT5-containing endosomes destined for the apical membrane (Figure 1) (100).
The potential role of GLUT5 as transceptor for fructose has been indirectly demonstrated ex vivo in intestinal organoids (72). Since organoids develop with the lumen inside, and only the basolateral membrane is exposed to the incubation medium, cells can utilize GLUT2 to take up fructose from the medium in order to induce an adaptive response by fructolytic enzymes and GLUT5. However, fructose via the basolateral membrane failed to stimulate fructose-responsive genes in intestinal organoids derived from GLUT5−/− mice, suggesting a role for GLUT5 in fructose sensing even though it is not required for basolateral fructose transport into the cytosol.
4.2. Role of KHK
Ketohexokinase (KHK, Khk), also called fructokinase, is highly expressed in human and rodent proximal small intestine (49). Fructose entering the enterocyte cytosol may be phosphorylated by KHK (Figure 1), particularly the KHK-C isoform (21), to fructose-1-phosphate that is broken down to glyceraldehyde and dihydroxyacetone phosphate by aldolase-B. Fructose metabolism bypasses major regulatory steps; thus, its catabolism results in KHK-mediated rapid depletion of ATP and accumulation of metabolic intermediates including pyruvate and acetyl-CoA (130). Deletion of KHK reduces the rate of in vitro transapical transport of fructose from the intestinal lumen, suggesting that KHK-mediated metabolism helps maintain the downhill fructose gradient into the cytosol, favoring facilitative fructose entry (100).
Metabolism of fructose is essential in fructose-induced upregulation of expression not only of GLUT5 but also of KHK, aldolase-B, triokinase, fructose-1,6-bisphosphatase, and glucose-6-phosphatase in the intestinal mucosa (101). Thus, KHK participates along with GLUT5 in optimizing intestinal fructose transport and metabolism to correlate with dietary fructose levels. Intestinal perfusion with the nonmetabolizable fructose analog 3-O-methyl-fructose failed to induce GLUT5 expression and activity in the rat small intestine (59). The signal(s) for induction of GLUT5 and key fructose-metabolizing enzymes may be a metabolite of fructose. Glyceraldehyde is a unique product of fructose metabolism, but its perfusion did not induce GLUT5 expression (100, 101). Thus, KHK optimizes absorption by helping to maintain a steep lumen-to-cytosol fructose gradient and to regulate increases in the synthesis of GLUT5.
4.3. Role of TXNIP
Thioredoxin-interacting protein (TXNIP, Txnip) binds to thioredoxin and regulates cellular metabolism as well as redox state (115). Although its expression is known to be correlated with extracellular glucose concentration and to be inversely correlated with glucose uptake, TXNIP has also been shown to bind to GLUT2 and GLUT5, increasing fructose absorption in human-derived Caco2 cells (22). In turn, TXNIP expression increases with chronic fructose consumption. Global TXNIP deletion decreased fructose levels in the liver and peripheral blood, reduced severity of fructose-induced metabolic diseases, and prevented diabetes-induced increases in intestinal fructose absorption in mice (22). Thus, TXNIP may play a role in regulating intestinal fructose absorption, particularly in diabetes, but the mechanism is not known. The interaction between TXNIP and fructose transport may be associated with fructose-induced changes in AMP kinase (AMPK), carbohydrate response element binding protein (ChREBP), and glucocorticoid metabolism, discussed below in this section and in Section 5.
4.4. Other Signaling Mechanisms
The signaling mechanisms involved in the regulation of GLUT5 and fructolytic enzymes by fructose are still mostly unknown. In adult rats, cAMP and phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) systems mediate fructose-induced increases in GLUT5 activity without affecting GLUT5 mRNA expression (13, 14). This may be due to PI3K regulation of Rab11 (36) required for recruitment of GLUT5 to the apical membrane.
Regulation of GLUT5 during early development of mammals is complex since it involves an interplay among age-, hormone-, and diet-related signals. Fructose can induce intestinal GLUT5 expression and activity when levels of systemic glucocorticoid, but not thyroxine (91), increase during weaning (25, 127). This ontogenetic induction of fructose transport involves activation and translocation of glucocorticoid receptor (GR) into the enterocyte nucleus (24); binding of the GR and polymerase Pol II to the Slc2a5 promoter region; and increased acetylation of histone H3, an established marker of transactivation of many genes (127). Precocious systemic treatment with glucocorticoid analogs can prematurely allow luminal fructose to induce GLUT5 expression and activity in rat small intestine (25).
ChREBP is an important regulator of intestinal and hepatic genes involved in lipogenesis, glycolysis, and fructolysis (56). Deletion of ChREBP reduces expression of GLUT5 (56). Thus, inhibition of ChREBP can potentially block the signal required to induce intestinal GLUT5 expression and activity by luminal fructose. The link between fructose transport and ChREBP may be glucose-6-phosphatase or AMPK, which regulate ChREBP (56) but are also activated by uptake of fructose into the cytosol (71).
4.5. Fructose Sensing by Intestinal Cell Types
The intestinal epithelium is in a constant state of turnover, with stem cells differentiating at the crypts and their progenies migrating toward the tips of villi. Kishida et al. (72) used specialized organoid cultures, each enriched in a single intestinal cell type, to demonstrate that absorptive enterocytes as well as secretory goblet and Paneth cells, but not stem cells, can sense fructose. Since organoids have a sealed lumen and can only be exposed to fructose in the bathing medium via the basolateral membrane, this suggests that fructose sensing from the blood is acquired after differentiation but before divergence into absorptive and secretory lineages.
There are about seven subtypes of enteroendocrine cells (EECs) in the small intestine, and they secrete different hormones targeting a variety of organ systems (see 80 for review). Luminal fructose stimulates the release of glucagon-like peptide 1 (GLP1) from L-subtype EECs in humans and animal models (73, 114). The secretion of gastric inhibitory peptide from K-subtype EECs is induced by fructose in mice (114) but is unaffected (73) or reduced (146) in rats and humans. Fructose also seems to increase secretion of human PYY, cholecystokinin, neurotensin (73), and serotonin (84), suggesting that it stimulates, respectively, cells of subtypes L, I, N, and enterochromaffin. Thus, fructose seems to stimulate secretion in all EEC subtypes. These observations supplement and support the hypothesis of Kishida et al. (72) that both absorptive and secretory progenies of intestinal stem cells can sense fructose. Fructose even affects secretion of leptin and ghrelin, whose EEC subtypes are not located in the small intestine (76, 80, 146). Although fructose effects on secretion are sometimes more modest than glucose effects, findings suggest that many functions modulated by EECs are affected by fructose consumption.
5. POTENTIAL HEALTH IMPLICATIONS OF INTERACTIONS BETWEEN INTESTINAL FRUCTOSE AND ION TRANSPORT
Intestinal fructose absorption reduces the expression of the intestinal Na+-dependent phosphate (Pi) transporter (NaPi2b, Slc34a2), which mediates the apical transport of luminal Pi into the cytosol (14); of the intestinal calcium transporter 1 (CaT1 or ECAC2, Trpv6) (23, 28–30), which mediates passive Ca2+ entry across the apical membrane); and of calcium-binding protein 9k (CaBP9k, Cabp9k), which promotes cytosolic transport of Ca2+. Moreover, intestinal transport of fructose was observed to potentially affect the expression of the intestinal Na+ and H+ exchanger NHE3 (Slc9a3), which is partly responsible for net Na+ absorption in the gastrointestinal (GI) tract, and of the intestinal Cl− transporters PAT1 (Slc26a6) and DRA (also known as CLD, Slc26a3) (4, 117). Thus, intake and transport of fructose somehow interacts with the absorption of certain minerals essential for bone health and of important electrolytes that can impact blood pressure.
5.1. Fructose and Bone Health
Consumption of high levels of caloric sweeteners composed mainly of fructose may be a risk factor for bone loss. Numerous epidemiological studies in the last 25 years, when fructose consumption has increased dramatically, observed that an excessive intake of sweeteners tends to increase the incidence of bone fracture and osteoarthritis, decrease bone mineral density, or reduce the rate of bone mineral accrual (39, 53, 78, 79, 86, 134, 145). One study failed to find a clear correlation between sweetener consumption and bone quality in humans (138). Nonetheless, in rats, chronic consumption of high levels of dietary sucrose and fructose also reduces bone mechanical strength and mineral content (23, 30, 133). The bone loss associated with increased soft drink and fructose consumption has been ascribed to lack of exercise or to displacement of essential nutrients, but statistical adjustments for milk consumption and physical activity fail to reduce the significant correlation between sweetener consumption and low bone quality (53). The mechanism underlying the association between dietary fructose and bone health is still unclear (39), and it makes the link between fructose transport and Pi as well as Ca2+ absorption all the more interesting.
5.2. Potential Mechanisms Underlying the Effect of Fructose on Pi and Ca2+ Transport
Intestinal perfusion of fructose acutely reduces in vitro Pi uptake across the apical membrane and expression of NaPi2b within hours (14). In contrast, while there seems to be no immediate effect of fructose on Ca2+ transport, there are remarkable reductions in CaT1 and CaBP9k expression after chronic consumption of fructose-containing diets in animal models with low Ca2+ status or facing Ca2+ deficits (23, 29, 30). Thus, two potential mechanisms have been proposed to explain the apparent effects of fructose on bone.
First, the interaction between dietary fructose and intestinal Ca2+ transport may be mediated by the active form of vitamin D, 1,25(OH)2D3. When Ca2+ status is sufficient, chronic fructose intake has no effect on intestinal Ca2+ transport. However, when Ca2+ status is deficient because dietary Ca2+ supply is limiting or physiological Ca2+ demand is high, levels of the biologically active 1,25(OH)2D3 typically increase dramatically to compensate and restore Ca2+ sufficiency by inducing transepithelial intestinal absorption and renal reabsorption of Ca2+ (10). High fructose intake prevents compensatory increases in 1,25(OH)2D3 levels, thereby reducing intestinal active Ca2+ absorption mediated by decreased expression of CaT1 and CaBP9k, in rat models of chronic kidney disease (23) as well as in lactating rats, growing rats, and mice fed Ca2+-deficient diets (29, 30). Chronic high fructose intake increased the expression in the kidney of 24-hydroxylase (CYP24A1) and decreased that of 1α-hydroxylase (CYP27B1). The elevated CYP24A1 enhanced the renal catabolism of 1,25(OH)2D3, which regulates intestinal Ca2+ transport, while the decrease in CYP27B1 impaired 1,25(OH)2D3 synthesis. In fact, 1,25(OH)2D3 treatments prevent the deleterious effects of excessive fructose intakes on transepithelial Ca2+ transport, growth rate, and bone quality. Serum FGF23, which is secreted by osteocytes and inhibits CYP27B1 expression, was upregulated by dietary fructose, suggesting a potential role of bone osteocytes in mediating the deleterious fructose effects on 1,25(OH)2D3 synthesis (29, 30). Thus, one mechanism currently hypothesized to underlie the association between bone health and excessive fructose intake entails a marked reduction in 1,25(OH)2D3, potentially mediated by alterations in FGF23 levels. In humans, frequent consumption of sugar-sweetened beverages has recently been significantly associated with lower 25(OH)D3 levels (61).
Second, intestinal fructose transport directly affects Pi transport, likely by perturbing ATP/AMP ratios in the enterocyte. After absorption, some fructose is phosphorylated (Figure 1), reducing ATP/AMP ratios in the intestinal cell (130) and stimulating the energy sensor AMPK, which may downregulate NaPi2b. The renal Pi transporter NaPi2a (Slc34a1) is down-regulated directly by activation of AMPK (19). Activating AMPK by AICAR (5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside) enhanced the inhibition of NaPi-2b gene expression by fructose, whereas inhibiting AMPK with C75 (3-carboxy-4-octyl-2-methylene-butyrolactone trans-4-carboxy-5-octyl-3-methylenebutyrolactone) prevented fructose-induced repression of intestinal NaPi2b expression and Pi uptake (71). Likewise, AMPK inhibition increases renal NaPi2a-mediated Pi transport. Thus, the interaction between intestinal fructose and Pi transport is likely due to fructose-induced perturbations in the ATP/AMP ratio. In contrast, fructose uptake and subsequent decrease in ATP concentration due to fructose metabolism do not directly modulate active Ca2+ transport across the duodenum (130).
5.3. Hypertension and the Effect of Fructose on Na+ and Cl− Transport
Excessive fructose intake has been associated with hypertension (see 81 for review). HFCS-sweetened but not sucrose-sweetened beverages specifically increase systolic blood pressure in humans (77). Potential cardiovascular and metabolic mechanisms (32, 46) have been proposed to explain this intriguing link, but in this review we only describe studies linking fructose uptake to electrolyte transport in the intestine, which may eventually result in electrolyte imbalances that lead to hypertension. In humans, salt intake has been associated with increases in fructose consumption (50). A link between blood pressure and fructose transport was suspected when intestinal GLUT5 expression and activity were observed to be reduced in spontaneously hypertensive rats (85). In fructose-fed mice, blood pressure increases in parallel with increased intestinal fluid absorption as well as enhanced small intestinal expression of GLUT5, the Na+-proton exchanger NHE3, and the apical anion exchanger PAT1, which absorbs Cl− in exchange for a variety of intracellular anions (117). Deletion of PAT1 in mice, and removing Cl− from the high-fructose diet in rats, abolished the fructose-induced increase in blood pressure. Subsequent work showed that the fructose-induced increases in systolic blood pressure were prevented in GLUT5−/− mice, thereby linking fructose transport as a potential mechanism (4), but expression of electrolyte transporters in the small intestine was not shown. Although there were marked increases in expression of NHE3 and of the chloride anion exchanger DRA in the colon of the fructose-fed GLUT5−/− mice, it was possible that fructose malabsorption, luminal fluid accumulation, and intestinal hypertrophy may have confounded these findings.
In summary, a high fructose intake has been linked to reductions in Ca2+ and Pi transport and to changes in expression of Na+ and Cl− exchangers. Fructose reduces expression of Ca2+ transporters and binding proteins by decreasing blood levels of 1,25(OH)2D3, perhaps by perturbing renal function, and of NaPi2b, likely by activating AMPK. Reduced Ca2+ and Pi transport would then compromise Ca2+ and Pi, affecting bone health. However, it is unclear how intestinal GLUT5 expression and fructose transport would directly affect jejunal PAT1 and colonic NHE3 as well as DRA, and thus how intestinal fructose absorption impacts blood pressure.
6. REGULATION OF BLOOD FRUCTOSE LEVELS
Fructose concentration in the peripheral circulation is expected to be consistently low as the liver clears the portal blood of most of its fructose after a single pass (75, 102). Instead, surprisingly, published estimates of normal peripheral serum fructose concentrations vary by more than four orders of magnitude, from 0.008 (65) to 16 mM (54), in humans and laboratory animal models! While blood glucose level is tightly regulated by insulin and glucagon, the dramatic variation in blood fructose levels suggests not only a lack of hormonal regulation but also differences in methods of analysis (26, 29). Gas chromatography/mass spectrometry (GCMS) and high-performance liquid chromatography (HPLC) methods are now recommended for more accurate analysis of blood fructose (125, 136). Some commercial, enzyme-based kits may not be appropriate for estimating blood fructose, because the ~6-mM glucose level in the blood may be too high a background for a subtraction-dependent assay, considering that blood fructose concentration may be only 0.005 to 0.1 mM (27, 125). The level of fructose concentrations in the urine may even be lower (121). Although their specificity decreases with decreasing fructose levels, enzyme-based kits may be useful in estimating serum fructose concentrations that are expected to be >0.1 mM in sugar-fed animal models (23, 106, 125).
6.1. Concentrations in the Portal Vein
There are few estimates of portal fructose concentrations (102). This is unfortunate because NAFLD, now estimated to affect almost one billion individuals worldwide, has been associated with high intakes of dietary fructose (120), increasing concentrations in the portal vein. Intestinal absorption seems to be the primary regulator of portal fructose concentrations, which increase only in animals actively consuming high-fructose diets. In rats fed a bolus of 2-g/kg sucrose (equivalent to about three cans of regular soda in humans), portal fructose concentrations increased rapidly from ~0.1 to 1 mM (124). In baboons gavaged with 2-g/kg sucrose, fructose levels in the portal blood increased to 1.9 mM (12). Compared to those fed 20% glucose, wild-type mice fed an isocaloric diet containing 20% fructose maintained a 2.5-fold higher (~0.13 mM) portal fructose concentration (102). Portal fructose levels drop within hours when wild-type mice cease feeding. In actively feeding, global KHK−/− mice, portal fructose concentrations were already high (~0.8mM) with the 20% glucose diet, then increased twofold with the 20% fructose diet. Since portal fructose levels are fivefold lower in wild-type mice fed the same 20% fructose diet, intestinal KHK in wild-type mice may catabolize fructose in enterocytes prior to release in portal circulation, lowering portal fructose levels.
Peripheral levels of fructose were higher than portal levels in both fed and fasted KHK−/− mice. In fact, while peripheral fructose concentrations were poorly correlated with portal fructose levels in wild-type mice, peripheral fructose levels in KHK−/− mice increased by 2 mM for every 1-mM increase in portal fructose, suggesting that organs other than the liver likely contributed to peripheral fructose concentrations. Fructose is synthesized in the body (55). Low levels (≪0.1 mM by HPLC) of fructose can always be detected in the portal and peripheral blood of rats and of wild-type and GLUT5−/− mice that have no access to dietary fructose (102). Fructose is synthesized in the kidney and other organ systems from glucose by the polyol pathway, which then exports endogenous fructose into the blood. Systemically inhibiting the polyol pathway with the aldose reductase inhibitor lidorestat reduces plasma fructose concentrations (97). In humans, the cerebrospinal fluid has almost 20-fold greater fructose concentrations (~0.18 mM by GCMS) than those in plasma and cord venous blood, suggesting that endogenous production is likely responsible for virtually all fructose found in the brain (55).
Recent work using isotope tracing and mass spectrometry found that small doses of fructose are almost entirely catabolized by KHK in the small intestine, so that only trace concentrations of fructose but high levels of fructose-derived metabolites reach the portal circulation (58). High doses of fructose saturate absorptive and catabolic processes in the small intestine, resulting in fructose reaching both the liver and colonic microbiota.
6.2. Concentrations in the Peripheral Circulation
Earlier estimates of peripheral blood fructose concentrations have been summarized previously (26, 27, 75); we add the caveat that many estimates were derived using enzymatic methods. In rats and mice, peripheral blood concentrations increase when fructose is consumed, and the increase is abolished if GLUT5 is deleted (4, 102). The magnitude of this increase is dependent on dietary fructose levels in both animal models (4, 102) and healthy human volunteers (77, 110, 111, 131). Urine fructose concentrations in mice also increased markedly with dietary fructose (38; M. Fukazawa, personal communication). Peripheral blood fructose concentrations typically increase from <0.05 mM during fasting to ~0.5 mM in those consuming high-fructose diets, with concentrations rarely exceeding 0.5 mM in most species. Regardless of dietary fructose intake, blood fructose concentrations in the peripheral circulation of wild-type mice remain much lower, relative to concentrations of glucose.
Peripheral blood fructose concentrations of fasting type 1 diabetic, type 2 diabetic, and healthy humans (55, 66, 67, 105, 131) are very low (<0.02 mM) when determined by GCMS or HPLC. Postprandial fructose concentrations increase by approximately fivefold, regardless of glycemic condition, in these subjects, suggesting that feeding affects blood fructose levels more than diabetes. Other studies found diabetic patients to exhibit hyperfructosemia and fructosuria (65).
Global deletion of the KHK enzyme in mice prevented systemic metabolism of fructose. This resulted in marked (~ 10-fold) increases in concentration of fructose in the peripheral circulation, reaching up to ~6 mM (57, 102). Regardless of fructose intake, serum fructose concentrations in KHK−/− mice did not seem to increase beyond 8 mM (102), probably because at high concentrations, hexokinase I can phosphorylate fructose taken up by skeletal muscle and adipose tissue (37, 42). In humans, essential or hereditary fructosuria caused by KHK mutation is asymptomatic and may remain undiagnosed in affected individuals, whose systemic serum fructose concentrations can reach ~3 mM, two orders of magnitude greater than normal (42). The prevalence of essential fructosuria, and its consequent fructosemia, is thus unknown.
6.3. Fructosemia, Glycemia, and Glycation
Excessive fructose intake is associated with hyperglycemia and diabetes (62), and hyperglycemia is associated with increased serum and urinary fructose concentrations in patients with diabetes (65). Even though blood fructose, relative to glucose, levels are very low in humans and animal models, there is surprisingly a strong correlation between fructosemia and glycemia (102). A small (0.01 mM) change in peripheral serum fructose concentration was linked in wild-type mice to a 0.4-mM change in glucose levels (Figure 4) and thus to higher HbA1c levels. This link was clearly absent in KHK−/− mice, even though serum fructose was much greater in these mice. The KHK-dependent, fructose-induced hyperglycemia in wild-type mice may be due in part to excess fructose consumption inducing gluconeogenesis (75, 126), and perhaps explains why HFCS consumption results in greater dose-normalized glycemia than equivalent doses of sucrose in humans (77).
Figure 4.
Effect of peripheral blood fructose on blood glucose concentrations. Mice were fed isocaloric 20% glucose + 10% sucrose or 20% fructose + 10% sucrose diets under conditions of a reversed light cycle. Diets were removed at 2001 h (lights on) and returned at 0801 h (lights off) (data obtained from 102). After one week, mice were killed at 0800 h before feeding, and at 0900, 1030, 1200, and 1530 h during the dark phase; then blood sugar levels were analyzed. Peripheral blood glucose concentrations are plotted against blood fructose levels in wild-type mice (blue squares) and in KHK−/− mice (red circles). Note that there are two abscissa lines because of the order-of-magnitude difference in range of blood fructose concentrations between mouse groups. Thus, for wild-type mice, peripheral glucose (5–25 mM) is plotted against peripheral fructose levels ranging from 0 to 0.4 mM (blue numbers), while, for KHK−/− mice, peripheral glucose is plotted against fructose concentrations ranging from 0 to 6 mM (red number). It is quite clear that in wild-type mice, but not in KHK−/− mice, large changes in blood glucose concentrations can be caused by small changes in blood fructose levels (102). Thus, chronic increases in blood fructose levels may eventually lead to hyperglycemia in humans and in animal models, each consuming high amounts of dietary fructose.
Fructose and its metabolite glyceraldehyde are ~ 10-fold more reactive than glucose when forming advanced glycation products (112). However, under chronic conditions, the glycating effect of glucose is still expected to be much greater physiologically than that of fructose, whose normal concentrations are ~500-fold lower than glucose’s. The elevated HbA1c levels in fructose-fed wild-type mice likely reflect fructose-induced, KHK-dependent hyperglycemia, because HbA1c was lower and independent of dietary fructose in KHK−/− mice exhibiting marked hyperfruc- tosemia (102).
In summary, postprandial blood fructose concentrations are very low in GLUT5−/− mice force-fed fructose and match concentrations observed in fasted wild-type mice, suggesting that GLUT5 is responsible for increased blood fructose levels (102). Despite some KHK-mediated catabolism by enterocytes, most dietary fructose appears in the portal vein at high concentrations, is likely metabolized in the liver, and eventually reaches the peripheral circulation at micromolar concentrations best analyzed using GCMS or HPLC methods. Humans with essential fructosuria and mouse models without KHK exhibit marked hyperfructosemia but appear normal (42, 102). Chronically high fructose intakes increase postprandial peripheral blood fructose concentrations that also seem to increase, in a KHK-dependent manner, blood glucose and HbA1c levels in wild-type mice.
7. GLUT5 AND FRUCTOSE-RELATED GASTROINTESTINAL DISEASES AND SYNDROMES
The numerous studies associating dietary fructose with metabolic diseases and those linking GLUT5 overexpression with various nongastrointestinal (non-GI) cancers are frequently reviewed and thus are not discussed here.
7.1. Fructose Malabsorption
The prevalence and severity of fructose malabsorption are directly proportional to dietary fructose levels and inversely proportional to age (43, 63). There is a greater incidence of fructose malabsorption, as indicated by positive breath hydrogen tests, in infants (up to 90%) and young children (up to 67%) with high fructose intakes (15, 31). Intestinal GLUT5 expression and activity are typically much lower in suckling rodents and in infants, potentially explaining fructose malabsorption in young children (16, 27). Despite this intriguing association between age-associated fructose malabsorption in humans and the developmentally related underexpression of intestinal GLUT5, no GLUT5 mutation could be found in eight children with isolated fructose malabsorption (137).
The prevalence of fructose malabsorption is relatively high in healthy adults (~34%) and is even greater in patients with functional GI disorders (5, 140). Intestinal fructose malabsorption, however, was not associated with levels of intestinal GLUT5 and GLUT2 mRNA and protein (139). Thus, although fructose consumption rates already are at levels known to cause mild GI distress in healthy subjects (7), the mechanism underlying human fructose malabsorption is largely unknown.
Fructose and fermentable oligo-, di-, and monosaccharides or polyols (FODMAPs) in the diet have been associated with irritable bowel syndrome (IBS). These free or short-chain carbohydrates are often poorly digested or absorbed and are fermented in the colon, leading to bloating, borborygmi, diarrhea, and other IBS symptoms. A 25-g fructose load (equivalent to about one 350-mL soda) caused malabsorption and intolerance in 22% and 28%, respectively, of IBS patients (88); conversely, a low-fructose diet resulted in significant relief of IBS symptoms (9, 116) while a low-FODMAP diet resulted in global symptomatic relief in patients with fructose intolerance (141). The low-FODMAP diet is now considered a front-line therapy for IBS worldwide because of its efficacy (50–80%) in relief of IBS symptoms (122). The composition of FODMAP diets and their mechanisms of action in IBS have been intensively studied in the past decade, but since this link is not specific to fructose, readers are directed to a recent review (122).
7.2. Fructose and Gastrointestinal Cancers
GLUTs are overexpressed in many cancer cells that take up glucose at elevated rates, and GLUT5 is specifically overexpressed in many epithelial cancers (6, 27). In this review, we focus on GI cancers that have been positively associated with fructose intake and thus intestinal fructose transport. In the NIH-AARP Diet and Health Study, which included 0.44 million participants aged 50–71 years, a high intake of added sugars was positively associated with esophageal cancer, while added fructose was specifically associated with an increased risk of small intestinal cancer (128). Two epidemiological studies each with >0.1 million participants had contrasting findings: One found a link between added fructose and pancreatic cancer (99), but the other did not (3). A metabolomics study of stool and tissue from colorectal cancer patients revealed significant perturbations in fructose metabolism (8), although an epidemiological study found no link between added fructose in the diet and colorectal cancer (128). Thus, the association between excess fructose intake and GI cancers remains to be established.
7.3. Absorption of Fructose and Transport of Chylomicrons
Chronic fructose feeding in the hamster enhances both intestinal de novo lipogenesis and production of ApoB48 (48), an apolipoprotein specific to the small intestine (94), suggesting that increased intestinal absorption of fructose stimulates lipid packaging into chylomicrons. In healthy volunteers, consumption of a high-fructose diet increases blood levels not only of glucose, triglycerides, and triglyceride-rich lipoproteins but also of ApoB48 (34, 131). In healthy women consuming 0.5-g/kg fructose (equivalent to about one can of regular soda) plus fat, similar results were observed in that fructose plus fat, but not fat alone, sharply raised postprandial levels of ApoB48 and of remnant-like particle triglycerides (110, 111). These fructose-induced increases in ApoB48 persisted even after fasting. Thus, a high fructose intake enhances intestinal export of chylomicrons that may contribute to fructose-induced dyslipidemia.
8. FRUCTOSE AND GUT MICROBIOTA
Changes in levels of dietary fructose profoundly affect the gut microbiota, resulting in the acquisition of a microbiome with altered metabolic capacity (103). This implies that changes in intestinal fructose absorption and luminal concentrations affect the microbiome. For example, GLUT2ΔIEC (targeted GLUT2 deletion from the intestinal mucosa) mice that overexpress GLUT5 have greater levels of Clostridium and Enterococcus spp. belonging to the phylum Firmicutes (113). Ahigh fructose intake increases intestinal bacterial load (147). Changes in dietary fructose levels may alter not only the size but also the composition of the human gut microbiome (122, 141). The types of intestinal bacteria altered by fructose feeding, however, differ among studies. Fructose ingestion increased the numbers of Gram-negative, anaerobic Bacteroides fragilis of the phylum Bacteroidetes, in mice (147), and of Gram-positive anaerobic bacteria Coprococcus, Ruminococcus, and Clostridium, which are Firmicutes, in rats (11, 20). Interestingly, these fructose-induced increases in numbers of specific bacterial genera can be reversed by fecal transplantation from rats fed fructose-free diets. Chronic consumption of a diet high in fructose and fat increased Lactobacillus reuteri (Firmicutes) and B. fragilis in rats (109). In contrast, the phyla Proteobacteria and Actinobacteria were elevated in rats consuming drinks containing 11% sugars (96). Thus, changes in intestinal luminal fructose levels arising from changes in fructose intake and absorption will likely affect bacterial load and composition.
HFCS: high-fructose corn syrup
NAFLD: nonalcoholic fatty liver disease
GLUT: facilitative glucose transporter (Slc2a)
Major facilitator superfamily (MFS): a group of proteins mediating transport of small solutes across cell membranes
TM: transmembrane helix
MSNBA: N-[4-(methylsulfonyl)-2-nitrophenyl]-1,3-benzodioxol-5-amine
SGLT1: Na+-dependent glucose transporter 1 (Slc5a1)
Rab11: Ras-related protein-in-brain 11
KHK: ketohexokinase (fructokinase, Khk)
TXNIP: Thioredoxin-interacting protein (Txnip)
AMPK: AMP (adenosine monophosphate)-activated protein kinase
ChREBP: carbohydrate response element binding protein
PI3K: phosphatidylinositol 3-kinase
EEC: enteroendocrine cell
NaPi2b: Na+ -dependent phosphate (Pi) transporter (Slc34a2)
CaT1: intestinal calcium transporter 1; also known as ECAC2 (Trpv6)
CaBP9k: calcium binding protein 9k
NHE3: Na+-proton exchanger (Slc9a3)
GI: gastrointestinal
FODMAPs: fermentable oligo-, di- and monosaccharides or polyols
IBS: irritable bowel syndrome
SUMMARY POINTS.
The small intestine is being challenged to absorb dietary fructose that is being consumed worldwide at historic highs. Marked increases in fructose intake have led to the development of hypotheses proposing substantive contributions by other transporters, notably GLUT2, to supplement GLUT5-mediated transapical membrane fructose transport in the small intestine. Recent evidence derived mainly from mouse genetic models indicates that GLUT5 is primarily responsible for fructose uptake across the apical membrane.
Models of the fructose binding site in GLUT5 have identified three key amino acid residues that play important roles in ligand recognition. These models can facilitate inhibitor design based on chemical interactions between the substrate fructose and these amino acids. MSNBA has been identified as a potent and specific GLUT5 inhibitor, opening the window for the discovery of related, more potent drugs for the prevention and treatment of fructose-induced diseases.
Intestinal GLUT5 expression and rate of fructose transport are specifically regulated by dietary fructose levels, and regulation is dependent on KHK-mediated metabolism as well as ChREBP, TXNIP, and, during development, corticosterone. All cell types except stem cells in the intestinal mucosa seem able to sense luminal fructose.
High rates of fructose intake and fructose uptake reduce intestinal Ca2+ and Pi transport, lowering Ca2+ and Pi status and potentially compromising bone health. Bone health is known to be compromised in humans consuming large amounts of added sugars.
GLUT5 activity regulates fructose levels in the portal blood. Most fructose seems to be metabolized by the liver, thereby reducing fructose concentrations in the peripheral circulation to micromolar concentrations best analyzed using GCMS or HPLC methods. Chronically high fructose intakes increase, in a KHK-dependent manner, blood glucose and HbA1c levels, even as blood fructose concentrations remain low. Humans with essential fructosuria and mouse models without KHK exhibit marked hyperfructosemia but appear normal, with normal glucose and HbA1c levels.
Prevalence of fructose malabsorption decreases with age and seems inversely correlated with increasing GLUT5 expression in the small intestine. Paradoxically, fructose malabsorption in human adults is not associated with intestinal GLUT5 and GLUT2 expression.
Changes in intestinal fructose absorption that alter luminal fructose concentrations affect bacterial load and modify the composition of the gut microbiome.
FUTURE ISSUES.
The availability of GLUT crystal structures is essential for understanding the molecular basis of the transporters’ function and for drug discovery efforts targeting GLUTs for treating cancer and metabolic syndrome. Crystal structures capture thermodynamically stable conformations; thus, known GLUT structures are at most three snapshots of the conformational variability that the transporter adopts in a cycle of transport. Therefore, it is necessary to determine more structural states of the transporters, including fructose- or inhibitor-liganded GLUT5 and GLUT2 structures, not only by X-ray crystallography, but also with cryo-electron microscopy, nuclear magnetic resonance, and other methods.
Infants frequently malabsorb fructose as measured by breath hydrogen, but the underlying mechanism is not known, though animal experiments indicate developmental limitations in expression of relevant transporters and enzymes. Additional studies analyzing levels of GLUT5 and KHK in infants malabsorbing fructose are warranted. In contrast, fructose malabsorption in adults may not be related to GLUT5 levels. However, the role of KHK, whose deletion in mice inhibits fructose absorption, has not yet been fully investigated in human fructose malabsorption. Metabolomic or genomic studies that provide clues to underlying potential mechanisms are warranted.
While there are numerous studies associating high fructose intakes with NAFLD and dyslipidemia, the effect of fructose on lipogenesis in the small intestine and the role of intestinal lipogenesis in systemic dyslipidemia are not clear. Enterocytes already can catabolize a significant amount of absorbed fructose and can export lipids via chylomicrons.
Differentiation seems to confer on intestinal cells, including various subtypes of EECs, the ability to sense fructose, but the mechanism(s) is not yet well understood. Likewise, GLUT5 expression in intestinal epithelia is specifically modulated by fructose, with potential mediation from AMPK, glucose-6-phosphatase, and ChREBP, but the mechanism is still unclear.
A clue to increasing our understanding of mechanisms underlying the link between excessive fructose intake and metabolic syndrome including glycation may be present in the relationship between fructosemia and glycemia, and how fractional changes in blood fructose and fructose absorption can cause marked alterations in blood glucose.
While it is not surprising to find dietary fructose affecting gut microbiota, the fructose effect needs clarification. It can best be elucidated using mouse models incapable of fructose transport and metabolism and doses of fructose that saturate small intestinal absorptive and catabolic processes. The link between dietary fructose and the microbiome may be relevant to issues related to FODMAPs and IBS.
More epidemiological studies should examine the suspected link among fructose intake, GLUT5 expression, and bone health, and should determine vitamin D levels in the blood.
ACKNOWLEDGMENTS
R.P.F. acknowledges current and recent support from the National Science Foundation (IOS-722365, 1121049, 1456673) and J.C. from the National Institutes of Health (R01-DK091754, R01-GM123103). We are grateful to V. Douard (INRA, France) for manuscript suggestions.
Footnotes
DISCLOSURE STATEMENT
J.C. and C.R.P. are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review. R.P.F.’s laboratory received support from Matsutani Chemical Co., Ltd. (Japan), in 2016.
LITERATURE CITED
- 1.Amarra MS, Khor GL, Chan P. 2016. Intake of added sugar in Malaysia: a review. Asia Pac. J. Clin. Nutr 25:227–40 [DOI] [PubMed] [Google Scholar]
- 2.Angyal SJ. 1984. The composition of reducing sugars in solution. Adv. Carbohydrate Chem. Biochem 42:15–68 [Google Scholar]
- 3.Bao Y, Stolzenberg-Solomon R, Jiao L, Silverman DT, Subar AF, et al. 2008. Added sugar and sugar-sweetened foods and beverages and the risk of pancreatic cancer in the National Institutes of Health-AARP Diet and Health Study. Am. J. Clin. Nutr 88:431–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barone S, Fussell SL, Singh AK, Lucas F, Xu J, et al. 2009. Slc2a5 (Glut5) is essential for the absorption of fructose in the intestine and generation of fructose-induced hypertension. J. Biol. Chem 284:5056–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrett JS, Irving PM, Shepherd SJ, Muir JG, Gibson PR. 2009. Comparison of the prevalence of fructose and lactose malabsorption across chronic intestinal disorders. Aliment. Pharmacol. Ther 30:165–74 [DOI] [PubMed] [Google Scholar]
- 6.Barron CC, Bilan PJ, Tsakiridis T, Tsiani E. 2016. Facilitative glucose transporters: implications for cancer detection, prognosis and treatment. Metabolism 65:124–39 [DOI] [PubMed] [Google Scholar]
- 7.Beyer PL, Caviar EM, McCallum RW. 2005. Fructose intake at current levels in the United States may cause gastrointestinal distress in normal adults. J. Am. Diet. Assoc 105:1559–66 [DOI] [PubMed] [Google Scholar]
- 8.Brown DG, Rao S, Weir TL, O’Malia J, Bazan M, et al. 2016. Metabolomics and metabolic pathway networks from human colorectal cancers, adjacent mucosa, and stool. Cancer Metab. 4:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi YK, Kraft N, Zimmerman B, Jackson M, Rao SS. 2008. Fructose intolerance in IBS and utility of fructose-restricted diet. J. Clin. Gastroenterol 42:233–38 [DOI] [PubMed] [Google Scholar]
- 10.Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G. 2016. Vitamin D: metabolism, molecular mechanism of action, and pleiotropic effects. Physiol. Rev 96:365–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crescenzo R, Mazzoli A, Di Luccia B, Bianco F, Cancelliere R, et al. 2017. Dietary fructose causes defective insulin signalling and ceramide accumulation in the liver that can be reversed by gut microbiota modulation. Food Nutr. Res 61:1331657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crossley JN, Macdonald I. 1970. The influence in male baboons, of a high sucrose diet on the portal and arterial levels of glucose and fructose following a sucrose meal. Nutr. Metab 12:171–78 [DOI] [PubMed] [Google Scholar]
- 13.Cui XL, Schlesier AM, Fisher EL, Cerqueira C, Ferraris RP. 2005. Fructose-induced increases in neonatal rat intestinal fructose transport involve the PI3-kinase/Akt signaling pathway. Am. J. Physiol. Gastrointest. Liver Physiol 288:G1310–20 [DOI] [PubMed] [Google Scholar]
- 14.Cui XL, Soteropoulos P, Tolias P, Ferraris RP. 2004. Fructose-responsive genes in the small intestine of neonatal rats. Physiol. Genom 18:206–17 [DOI] [PubMed] [Google Scholar]
- 15.Dabritz J, Muhlbauer M, Domagk D, Voos N, Hennebohl G, et al. 2014. Significance of hydrogen breath tests in children with suspected carbohydrate malabsorption. BMC Pediatr. 14:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davidson NO, Hausman AM, Ifkovits CA, Buse JB, Gould GW, et al. 1992. Human intestinal glucose transporter expression and localization of GLUT5. Am. J. Physiol 262:C795–800 [DOI] [PubMed] [Google Scholar]
- 17.De Zutter JK, Levine KB, Deng D, Carruthers A. 2013. Sequence determinants of GLUT1 oligomerization: analysis by homology-scanning mutagenesis. J. Biol. Chem 288:20734–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeBosch BJ, Chi M, Moley KH. 2012. Glucose transporter 8 (GLUT8) regulates enterocyte fructose transport and global mammalian fructose utilization. Endocrinology 153:4181–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dermaku-Sopjani M, Almilaji A, Pakladok T, Munoz C, Hosseinzadeh Z, et al. 2013. Down-regulation of the Na+-coupled phosphate transporter NaPi-IIa by AMP-activated protein kinase. Kidney Blood Press Res. 37:547–56 [DOI] [PubMed] [Google Scholar]
- 20.Di Luccia B, Crescenzo R, Mazzoli A, Cigliano L, Venditti P, et al. 2015. Rescue of fructose-induced metabolic syndrome by antibiotics or faecal transplantation in a rat model of obesity. PLOS ONE 10:e0134893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diggle CP, Shires M, Leitch D, Brooke D, Carr IM, et al. 2009. Ketohexokinase: expression and localization of the principal fructose-metabolizing enzyme. J. Histochem. Cytochem 57:763–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dotimas JR, Lee AW, Schmider AB, Carroll SH, Shah A, et al. 2016. Diabetes regulates fructose absorption through thioredoxin-interacting protein. eLife 5:e18313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Douard V, Asgerally A, Sabbagh Y, Sugiura S, Shapses SA, et al. 2010. Dietary fructose inhibits intestinal calcium absorption and induces vitamin D insufficiency in CKD. J. Am. Soc. Nephrol 21:261–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Douard V, Choi HI, Elshenawy S, Lagunoff D, Ferraris RP. 2008. Developmental reprogramming of rat GLUT5 requires glucocorticoid receptor translocation to the nucleus. J. Physiol 586:3657–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Douard V, Cui XL, Soteropoulos P, Ferraris RP. 2008. Dexamethasone sensitizes the neonatal intestine to fructose induction of intestinal fructose transporter (Slc2A5) function. Endocrinology 149:409–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Douard V, Ferraris RP. 2008. Regulation of the fructose transporter GLUT5 in health and disease. Am. J. Physiol. Endocrinol. Metab 295:E227–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Douard V, Ferraris RP. 2013. The role of fructose transporters in diseases linked to excessive fructose intake. J. Physiol 591:401–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Douard V, Patel C, Lee J, Tharabenjasin P, Williams E,et al. 2014. Chronic high fructose intake reduces serum 1,25-(OH)2D3 levels in calcium-sufficient rodents. PLOS ONE 9:e93611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Douard V, Sabbagh Y, Lee J, Patel C, Kemp FW, et al. 2013. Excessive fructose intake causes 1,25- (OH)2D3-dependent inhibition of intestinal and renal calcium transport in growing rats. Am. J. Physiol. Endocrinol. Metab 304:E1303–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Douard V, Suzuki T, Sabbagh Y, Lee J, Shapses S, et al. 2012. Dietary fructose inhibits lactation-induced adaptations in rat 1,25-(OH)2D3 synthesis and calcium transport. FASEBJ. 26:707–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duro D, Rising R, Cedillo M, Lifshitz F. 2002. Association between infantile colic and carbohydrate malabsorption from fruit juices in infancy. Pediatrics 109:797–805 [DOI] [PubMed] [Google Scholar]
- 32.Dushay JR, Toschi E, Mitten EK, Fisher FM, Herman MA, Maratos-Flier E. 2015. Fructose ingestion acutely stimulates circulating FGF21 levels in humans. Mol. Metab 4:51–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ebert K, Ludwig M, Geillinger KE, Schoberth GC, Essenwanger J, et al. 2017. Reassessment of GLUT7 and GLUT9 as putative fructose and glucose transporters. J. Membr. Biol 250:171–82 [DOI] [PubMed] [Google Scholar]
- 34.Egli L, Lecoultre V, Theytaz F, Campos V, Hodson L, et al. 2013. Exercise prevents fructose-induced hypertriglyceridemia in healthy young subjects. Diabetes 62:2259–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferraris RP, Diamond J. 1997. Regulation of intestinal sugar transport. Physiol. Rev 77:257–302 [DOI] [PubMed] [Google Scholar]
- 36.Franco I, Gulluni F, Campa CC, Costa C, Margaría JP, et al. 2014. PI3K class II alpha controls spatially restricted endosomal PtdIns3P and Rab11 activation to promote primary cilium function. Dev. Cell 28:647–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Froesch ER, Ginsberg JL. 1962. Fructose metabolism of adipose tissue. I. Comparison of fructose and glucose metabolism in epididymal adipose tissue of normal rats. J. Biol. Chem 237:3317–24 [PubMed] [Google Scholar]
- 38.Fukuzawa T, Fukazawa M, Ueda O, Shimada H, Kito A, et al. 2013. SGLT5 reabsorbs fructose in the kidney but its deficiency paradoxically exacerbates hepatic steatosis induced by fructose. PLOS ONE 8:e56681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fung TT, Arasaratnam MH, Grodstein F, Katz JN, Rosner B, et al. 2014. Soda consumption and risk of hip fractures in postmenopausal women in the Nurses’ Health Study. Am. J. Clin. Nutr. 100:953–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.George Thompson AM, Iancu CV, Nguyen TT, Kim D, Choe JY. 2015. Inhibition of human GLUT1 and GLUT5 by plant carbohydrate products; insights into transport specificity. Sci. Rep 5:12804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.George Thompson AM, Ursu O, Babkin P, Iancu CV, Whang A, et al. 2016. Discovery of a specific inhibitor of human GLUT5 by virtual screening and in vitro transport evaluation. Sci. Rep 6:24240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gitzelmann R, Steinmann B, Van den Bergh G. 1989. Disorders of fructose metabolism In The Metabolic Basis of Inherited Disease, ed. Stanbury JB, Wyngaarden JB, Fredrickson DS, pp. 399–424. New York: McGraw-Hill [Google Scholar]
- 43.Gomara RE, Halata MS, Newman LJ, Bostwick HE, Berezin SH, et al. 2008. Fructose intolerance in children presenting with abdominal pain. J. Pediatr. Gastroenterol. Nutr 47:303–8 [DOI] [PubMed] [Google Scholar]
- 44.Gorboulev V, Schurmann A, Vallon V, Kipp H, Jaschke A, et al. 2012. Na+-D-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes 61:187–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gouyon F, Caillaud L, Carriere V, Klein C, Dalet V, et al. 2003. Simple-sugar meals target GLUT2 at enterocyte apical membranes to improve sugar absorption: a study in GLUT2-null mice. J. Physiol 552:823–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grasser EK, Dulloo A, Montani JP. 2014. Cardiovascular responses to the ingestion of sugary drinks using a randomised cross-over study design: Does glucose attenuate the blood pressure-elevating effect of fructose? Br. J. Nutr 112:183–92 [DOI] [PubMed] [Google Scholar]
- 47.Gruzdkov AA, Gromova LV. 2013. [Glucose absorption in the rat small intestine in vivo after various levels of local substrate load]. Ross Fiziol. Zh. Im. I. M. Sechenova 99:630–41 [PubMed] [Google Scholar]
- 48.Haidari M, Leung N, Mahbub F, Uffelman KD, Kohen-Avramoglu R, et al. 2002. Fasting and postprandial overproduction of intestinally derived lipoproteins in an animal model of insulin resistance. Evidence that chronic fructose feeding in the hamster is accompanied by enhanced intestinal de novo lipogenesis and ApoB48-containing lipoprotein overproduction. J. Biol. Chem 277:31646–55 [DOI] [PubMed] [Google Scholar]
- 49.Hayward BE, Bonthron DT. 1998. Structure and alternative splicing of the ketohexokinase gene. Eur. J. Biochem 257:85–91 [DOI] [PubMed] [Google Scholar]
- 50.He FJ, Marrero NM, MacGregor GA. 2008Salt intake is related to soft drink consumption in children and adolescents: a link to obesity? Hypertension 51:629–34 [DOI] [PubMed] [Google Scholar]
- 51.Helliwell PA, Richardson M, Affleck J, Kellett GL. 2000. Regulation of GLUT5, GLUT2 and intestinal brush-border fructose absorption by the extracellular signal-regulated kinase, p38 mitogen-activated kinase and phosphatidylinositol 3-kinase intracellular signalling pathways: implications for adaptation to diabetes. Biochem.J 350(Pt. 1):163–69 [PMC free article] [PubMed] [Google Scholar]
- 52.Hirayama BA, Diez-Sampedro A, Wright EM. 2001. Common mechanisms of inhibition for the Na+/glucose (hSGLT1) and Na+/Cl-/GABA (hGAT1) cotransporters. Br. J. Pharmacol 134:484–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hostmark AT, Sogaard AJ, Alvaer K, Meyer HE. 2011. The Oslo Health Study: a dietary index estimating frequent intake of soft drinks and rare intake of fruit and vegetables is negatively associated with bone mineral density. J. Osteoporos 2011:102686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hui H, Huang D, McArthur D, Nissen N, Boros LG, Heaney AP. 2009. Direct spectrophotometric determination of serum fructose in pancreatic cancer patients. Pancreas 38:706–12 [DOI] [PubMed] [Google Scholar]
- 55.Hwang JJ, Johnson A, Cline G, Belfort-DeAguiar R, Snegovskikh D, et al. 2015. Fructose levels are markedly elevated in cerebrospinal fluid compared to plasma in pregnant women. PLOS ONE 10:e0128582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iizuka K 2017. The role of carbohydrate response element binding protein in intestinal and hepatic fructose metabolism. Nutrients 9:E181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ishimoto T, Lanaspa MA, Le MT, Garcia GE, Diggle CP, et al. 2012. Opposing effects of fructokinase C and A isoforms on fructose-induced metabolic syndrome in mice. PNAS 109:4320–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jang C, Hui S, Lu W, Cowan AJ, Morscher RJ, et al. 2018. The small intestine converts dietary fructose into glucose and organic acids. Cell Metab. 27(2):351–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jiang L, David ES, Espina N, Ferraris RP. 2001. GLUT-5 expression in neonatal rats: crypt-villus location and age-dependent regulation. Am.J. Physiol. Gastrointest. Liver Physiol 281:G666–74 [DOI] [PubMed] [Google Scholar]
- 60.Jiang L, Ferraris RP. 2001. Developmental reprogramming of rat GLUT5 requires de novo mRNA and protein synthesis. Am. J. Physiol. Gastrointest. Liver Physiol 280:G113–20 [DOI] [PubMed] [Google Scholar]
- 61.Joh HK, Lim CS, Cho B. 2015. Lifestyle and dietary factors associated with serum 25-hydroxyvitamin D levels in Korean young adults. J. Korean Med. Sci 30:1110–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Johnson RJ, Nakagawa T, Sanchez-Lozada LG, Shafiu M, Sundaram S, et al. 2013. Sugar, uric acid, and the etiology of diabetes and obesity. Diabetes 62:3307–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jones HF, Burt E, Dowling K, Davidson G, Brooks DA, Butler RN. 2011. Effect of age on fructose malabsorption in children presenting with gastrointestinal symptoms. J. Pediatr. Gastroenterol. Nutr 52:581–84 [DOI] [PubMed] [Google Scholar]
- 64.Jordan P, Choe JY, Boles E, Oreb M. 2016. Hxt13, Hxt15, Hxt16 and Hxt17 from Saccharomyces cerevisiae represent a novel type of polyol transporters. Sci. Rep 6:23502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kawasaki T, Akanuma H, Yamanouchi T. 2002. Increased fructose concentrations in blood and urine in patients with diabetes. Diabetes Care 25:353–57 [DOI] [PubMed] [Google Scholar]
- 66.Kawasaki T, Igarashi K, Ogata N, Oka Y, Ichiyanagi K, Yamanouchi T. 2012. Markedly increased serum and urinary fructose concentrations in diabetic patients with ketoacidosis or ketosis. Acta Diabetol. 49:119–23 [DOI] [PubMed] [Google Scholar]
- 67.Kawasaki T, Ogata N, Akanuma H, Sakai T, Watanabe H, et al. 2004. Postprandial plasma fructose level is associated with retinopathy in patients with type 2 diabetes. Metabolism 53:583–88 [DOI] [PubMed] [Google Scholar]
- 68.Kellett GL. 2001. The facilitated component of intestinal glucose absorption. J. Physiol 531:585–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kellett GL, Brot-Laroche E, Mace OJ, Leturque A. 2008. Sugar absorption in the intestine: the role of GLUT2. Annu. Rev. Nutr 28:35–54 [DOI] [PubMed] [Google Scholar]
- 70.Kellett GL, Helliwell PA. 2000. The diffusive component of intestinal glucose absorption is mediated by the glucose-induced recruitment of GLUT2 to the brush-border membrane. Biochem. J 350(Pt. 1):155–62 [PMC free article] [PubMed] [Google Scholar]
- 71.Kirchner S, Muduli A, Casirola D, Prum K, Douard V, Ferraris RP. 2008. Luminal fructose inhibits rat intestinal sodium-phosphate cotransporter gene expression and phosphate uptake. Am. J. Clin. Nutr 87:1028–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kishida K, Pearce SC, Yu S, Gao N, Ferraris RP. 2017. Nutrient sensing by absorptive and secretory progenies of small intestinal stem cells. Am. J. Physiol. Gastrointest. Liver Physiol 312:G592–G605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kuhre RE, Gribble FM, Hartmann B, Reimann F, Windelov JA, et al. 2014. Fructose stimulates GLP-1 but not GIP secretion in mice, rats, and humans. Am. J. Physiol. Gastrointest. Liver Physiol 306:G622–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kumar Kondapi VP, Soueidan OM, Cheeseman CI, West FG. 2017. Tunable GLUT-hexose binding and transport via modulation of hexose C-3 hydrogen-bonding capabilities. Chemistry 23:8073–81 [DOI] [PubMed] [Google Scholar]
- 75.Laughlin MR. 2014. Normal roles for dietary fructose in carbohydrate metabolism. Nutrients 6:3117–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Le KA, Faeh D, Stettler R, Ith M, Kreis R, et al. 2006. A 4-wk high-fructose diet alters lipid metabolism without affecting insulin sensitivity or ectopic lipids in healthy humans. Am. J. Clin. Nutr 84:1374–79 [DOI] [PubMed] [Google Scholar]
- 77.Le MT, Frye RF, Rivard CJ, Cheng J, McFann KK, et al. 2012. Effects of high-fructose corn syrup and sucrose on the pharmacokinetics of fructose and acute metabolic and hemodynamic responses in healthy subjects. Metabolism 61:641–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lu B, Ahmad O, Zhang FF, Driban JB, Duryea J, et al. 2013. Soft drink intake and progression of radiographic knee osteoarthritis: data from the osteoarthritis initiative. BMJ Open 3:e002993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ma D, Jones G. 2004. Soft drink and milk consumption, physical activity, bone mass, and upper limb fractures in children: a population-based case-control study. Calcif. Tissue Int 75:286–91 [DOI] [PubMed] [Google Scholar]
- 80.Mace OJ,Tehan B, Marshall F. 2015. Pharmacology and physiology of gastrointestinal enteroendocrine cells. Pharmacol. Res. Perspect 3:e00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Malik AH, Akram Y, Shetty S, Malik SS, Yanchou Njike V. 2014. Impact of sugar-sweetened beverages on blood pressure. Am. J. Cardiol 113:1574–80 [DOI] [PubMed] [Google Scholar]
- 82.Manolescu AR, Witkowska K, Kinnaird A, Cessford T, Cheeseman C. 2007. Facilitated hexose transporters: new perspectives on form and function. Physiology 22:234–40 [DOI] [PubMed] [Google Scholar]
- 83.Marriott BP, Cole N, Lee E. 2009. National estimates of dietary fructose intake increased from 1977 to 2004 in the United States. J. Nutr 139:1228S–35S [DOI] [PubMed] [Google Scholar]
- 84.Martin AM, Lumsden AL, Young RL, Jessup CF, Spencer NJ, Keating DJ. 2017. Regional differences in nutrient-induced secretion of gut serotonin. Physiol. Rep 5(6):e13199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mate A, Barfull A, Hermosa AM, Planas JM, Vazquez CM. 2004. Regulation of D-fructose transporter GLUT5 in the ileum of spontaneously hypertensive rats. J. Membr. Biol 199:173–79 [DOI] [PubMed] [Google Scholar]
- 86.McGartland C, Robson PJ, Murray L, Cran G, Savage MJ, et al. 2003Carbonated soft drink consumption and bone mineral density in adolescence: the Northern Ireland Young Hearts project. J. Bone Miner Res 18:1563–69 [DOI] [PubMed] [Google Scholar]
- 87.McWhorter TJ, Bakken BH, Karasov WH, del Rio CM. 2006. Hummingbirds rely on both paracellular and carrier-mediated intestinal glucose absorption to fuel high metabolism. Biol. Lett 2:131–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Melchior C, Gourcerol G, Dechelotte P, Leroi AM, Ducrotte P. 2014. Symptomatic fructose malabsorption in irritable bowel syndrome: a prospective study. United Eur. Gastroenterol. J 2:131–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mishra RK, Wei C, Hresko RC, Bajpai R, Heitmeier M, et al. 2015In silico modeling-based identification of glucose transporter 4 (GLUT4)-selective inhibitors for cancer therapy. J. Biol. Chem 290:14441–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Miyauchi E, Tachikawa M, Decleves X, Uchida Y, Bouillot JL, et al. 2016. Quantitative atlas of cytochrome P450, UDP-glucuronosyltransferase, and transporter proteins in jejunum of morbidly obese subjects. Mol. Pharm. 13:2631–40 [DOI] [PubMed] [Google Scholar]
- 91.Monteiro IM, Ferraris RP. 1997. Precocious enhancement of intestinal fructose uptake by diet in adrenalectomized rat pups. Pediatr. Res 41:353–58 [DOI] [PubMed] [Google Scholar]
- 92.Mueckler M, Thorens B.2013. The SLC2 (GLUT) family of membrane transporters. Mol. Aspects Med 34:121–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Naftalin RJ. 2014. Does apical membrane GLUT2 have a role in intestinal glucose uptake? F1000Res 3:304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nakajima K, Nagamine T, Fujita MQ, Ai M, Tanaka A, Schaefer E.2014. Apolipoprotein B-48: a unique marker of chylomicron metabolism. Adv. Clin. Chem 64:117–77 [PubMed] [Google Scholar]
- 95.Newens KJ, Walton J. 2016. A review of sugar consumption from nationally representative dietary surveys across the world. J. Hum. Nutr. Diet 29:225–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Noble EE, Hsu TM, Jones RB, Fodor AA, Goran MI, Kanoski SE. 2017. Early-life sugar consumption affects the rat microbiome independently of obesity. J. Nutr 147:20–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Noh HL, Hu Y, Park TS, DiCioccio T, Nichols AJ, et al. 2009. Regulation of plasma fructose and mortality in mice by the aldose reductase inhibitor lidorestat. J. Pharmacol. Exp. Ther 328:496–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nomura N, Verdon G, Kang HJ, Shimamura T, Nomura Y, et al. 2015. Structure and mechanism of the mammalian fructose transporter GLUT5. Nature 526:397–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nothlings U, Murphy SP, Wilkens LR, Henderson BE, Kolonel LN. 2007. Dietary glycemic load, added sugars, and carbohydrates as risk factors for pancreatic cancer: the Multiethnic Cohort Study. Am. J. Clin. Nutr 86:1495–501 [DOI] [PubMed] [Google Scholar]
- 100.Patel C, Douard V, Yu S, Gao N, Ferraris RP. 2015. Transport, metabolism, and endosomal trafficking-dependent regulation of intestinal fructose absorption. FASEB J. 29:4046–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Patel C, Douard V, Yu S, Tharabenjasin P, Gao N, Ferraris RP. 2015. Fructose-induced increases in expression of intestinal fructolytic and gluconeogenic genes are regulated by GLUT5 and KHK. Am. J. Physiol. Regul. Integr. Comp. Physiol 309:R499–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Patel C, Sugimoto K, Douard V, Shah A, Inui H, et al. 2015. Effect of dietary fructose on portal and systemic serum fructose levels in rats and in KHK−/− and GLUT5−/− mice. Am. J. Physiol. Gastrointest. Liver Physiol 309:G779–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Payne AN, Chassard C, Lacroix C. 2012. Gut microbial adaptation to dietary consumption of fructose, artificial sweeteners and sugar alcohols: implications for host-microbe interactions contributing to obesity. Obes. Rev 13:799–809 [DOI] [PubMed] [Google Scholar]
- 104.Powell ES, Smith-Taillie LP, Popkin BM. 2016. Added sugars intake across the distribution of US children and adult consumers: 1977–2012. J. Acad. Nutr. Diet 116:1543–50.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Preston GM, Calle RA. 2010. Elevated serum sorbitol and not fructose in type 2 diabetic patients. Biomark Insights 5:33–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Prieto PG, Cancelas J, Villanueva-Penacarrillo ML, Valverde I, Malaisse WJ 2004. Plasma D-glucose, D-fructose and insulin responses after oral administration of D-glucose, D-fructose and sucrose to normal rats. J. Am. Coll. Nutr 23:414–19 [DOI] [PubMed] [Google Scholar]
- 107.Ritze Y, Bardos G, D’Haese JG, Ernst B, Thurnheer M, et al. 2014. Effect of high sugar intake on glucose transporter and weight regulating hormones in mice and humans. PLOS ONE 9:e101702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Roder PV, Geillinger KE, Zietek TS, Thorens B, Koepsell H, Daniel H. 2014. The role of SGLT1 and GLUT2 in intestinal glucose transport and sensing. PLOS ONE 9:e89977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rosas-Villegas A, Sanchez-Tapia M, Avila-Nava A, Ramirez V, Tovar AR, Torres N. 2017. Differential effect of sucrose and fructose in combination with a high fat diet on intestinal microbiota and kidney oxidative stress. Nutrients 9:E393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Saito H, Kagaya M, Suzuki M, Yoshida A, Naito M. 2013. Simultaneous ingestion of fructose and fat exacerbates postprandial exogenous lipidemia in young healthy Japanese women. J. Atheroscler Thromb. 20:591–600 [DOI] [PubMed] [Google Scholar]
- 111.Saito H, Kato M, Yoshida A, Naito M. 2015. The ingestion of a fructose-containing beverage combined with fat cream exacerbates postprandial lipidemia in young healthy women. J. Atheroscler. Thromb 22:85–94 [DOI] [PubMed] [Google Scholar]
- 112.Schalkwijk CG, Stehouwer CD, van Hinsbergh VW. 2004. Fructose-mediated non-enzymatic glycation: sweet coupling or bad modification. Diabetes Metab. Res. Rev 20:369–82 [DOI] [PubMed] [Google Scholar]
- 113.Schmitt CC, Aranias T, Viel T, Chateau D, Le Gall M, et al. 2017. Intestinal invalidation of the glucose transporter GLUT2 delays tissue distribution of glucose and reveals an unexpected role in gut homeostasis. Mol. Metab 6:61–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Seino Y, Ogata H, Maekawa R, Izumoto T, Iida A, et al. 2015. Fructose induces glucose-dependent insulinotropic polypeptide, glucagon-like peptide-1 and insulin secretion: role of adenosine triphosphate-sensitive K+ channels. J. Diabetes Investig 6:522–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shalev A 2014. Minireview: Thioredoxin-interacting protein: regulation and function in the pancreatic beta-cell. Mol. Endocrinol 28:1211–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shepherd SJ, Parker FC, Muir JG, Gibson PR.2008. Dietary triggers of abdominal symptoms in patients with irritable bowel syndrome: randomized placebo-controlled evidence. Clin. Gastroenterol. Hepatol 6:765–71 [DOI] [PubMed] [Google Scholar]
- 117.Singh AK, Amlal H, Haas PJ, Dringenberg U, Fussell S, et al. 2008. Fructose-induced hypertension: essential role of chloride and fructose absorbing transporters PAT1 and Glut5. Kidney Int. 74:438–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Slavic K, Derbyshire ET, Naftalin RJ, Krishna S, Staines HM. 2009. Comparison of effects of green tea catechins on apicomplexan hexose transporters and mammalian orthologues. Mol. Biochem. Parasitol 168:113–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sobajima T, Yoshimura S, Iwano T, Kunii M, Watanabe M, et al. 2014. Rab11a is required for apical protein localisation in the intestine. Biol. Open 4:86–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Softic S, Cohen DE, Kahn CR. 2016. Role of dietary fructose and hepatic de novo lipogenesis in fatty liver disease. Dig. Dis. Sci 61:1282–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Song X, Navarro SL, Diep P, Thomas WK, Razmpoosh EC, et al. 2013. Comparison and validation of 2 analytical methods for measurement of urinary sucrose and fructose excretion. Nutr. Res 33:696–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Staudacher HM, Whelan K. 2017The low FODMAP diet: recent advances in understanding its mechanisms and efficacy in IBS. Gut 66:1517–27 [DOI] [PubMed] [Google Scholar]
- 123.Stumpel F, Burcelin R, Jungermann K, Thorens B. 2001. Normal kinetics of intestinal glucose absorption in the absence of GLUT2: evidence for a transport pathway requiring glucose phosphorylation and transfer into the endoplasmic reticulum. PNAS 98:11330–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sugimoto K, Hosotani T, Kawasaki T, Nakagawa K, Hayashi S, et al. 2010. Eucalyptus leaf extract suppresses the postprandial elevation of portal, cardiac and peripheral fructose concentrations after sucrose ingestion in rats. J. Clin. Biochem. Nutr 46:205–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sugimoto K, Inui H, Yamanouchi T. 2012. Assays of fructose in experimental nutrition In Dietary Sugars: Chemistry, Analysis, Function and Effects, ed. Preedy VR, pp. 466–85. London: R. Soc. Chem. [Google Scholar]
- 126.Sun SZ, Empie MW. 2012. Fructose metabolism in humans–what isotopic tracer studies tell us. Nutr. Metab 9:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Suzuki T, Douard V, Mochizuki K, Goda T, Ferraris RP. 2011. Diet-induced epigenetic regulation in vivo of the intestinal fructose transporter Glut5 during development of rat small intestine. Biochem. J 435:43–53 [DOI] [PubMed] [Google Scholar]
- 128.Tasevska N, Jiao L, Cross AJ, Kipnis V, Subar AF, et al. 2012. Sugars in diet and risk of cancer in the NIH-AARP Diet and Health Study. Int.J. Cancer 130:159–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Taylor PM. 2014. Role of amino acid transporters in amino acid sensing. Am. J. Clin. Nutr 99:223S–30S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tharabenjasin P, Douard V, Patel C, Krishnamra N, Johnson RJ, et al. 2014. Acute interactions between intestinal sugar and calcium transport in vitro. Am. J. Physiol. Gastrointest. Liver Physiol 306:G1–12 [DOI] [PubMed] [Google Scholar]
- 131.Theytaz F, de Giorgi S, Hodson L, Stefanoni N, Rey V, et al. 2014. Metabolic fate of fructose ingested with and without glucose in a mixed meal. Nutrients 6:2632–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Thomik T, Wittig I, Choe JY, Boles E, Oreb M. 2017. An artificial transport metabolon facilitates improved substrate utilization in yeast. Nat. Chem. Biol 13(11):1158–63 [DOI] [PubMed] [Google Scholar]
- 133.Tjaderhane L, Larmas M. 1998. A high sucrose diet decreases the mechanical strength of bones in growing rats. J. Nutr 128:1807–10 [DOI] [PubMed] [Google Scholar]
- 134.Tucker KL, Morita K, Qiao N, Hannan MT, Cupples LA, Kiel DP. 2006. Colas, but not other carbonated beverages, are associated with low bone mineral density in older women: the Framingham Osteoporosis Study. Am.J. Clin. Nutr 84:936–42 [DOI] [PubMed] [Google Scholar]
- 135.Ung PM, Song W, Cheng L, Zhao X, Hu H, et al. 2016. Inhibitor discovery for the human GLUT1 from homology modeling and virtual screening. ACS Chem. Biol 11:1908–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wahjudi PN, Patterson ME, Lim S, Yee JK, Mao CS, Lee WN. 2010. Measurement of glucose and fructose in clinical samples using gas chromatography/mass spectrometry. Clin. Biochem 43:198–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wasserman D, Hoekstra JH, Tolia V, Taylor CJ, Kirschner BS, et al. 1996. Molecular analysis of the fructose transporter gene (GLUT5) in isolated fructose malabsorption. J. Clin. Investig 98:2398–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Whiting SJ, Vatanparast H, Baxter-Jones A, Faulkner RA, Mirwald R, Bailey DA. 2004. Factors that affect bone mineral accrual in the adolescent growth spurt. J. Nutr 134:696S–700S [DOI] [PubMed] [Google Scholar]
- 139.Wilder-Smith CH, Li X, Ho SS, Leong SM, Wong RK, et al. 2014. Fructose transporters GLUT5 and GLUT2 expression in adult patients with fructose intolerance. United Eur. Gastroenterol. J 2:14–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wilder-Smith CH, Materna A, Wermelinger C, Schuler J. 2013. Fructose and lactose intolerance and malabsorption testing: the relationship with symptoms in functional gastrointestinal disorders. Aliment. Pharmacol. Ther 37:1074–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wilder-Smith CH, Olesen SS, Materna A, Drewes AM. 2017. Predictors of response to a low-FODMAP diet in patients with functional gastrointestinal disorders and lactose or fructose intolerance. Aliment. Pharmacol. Ther 45:1094–106 [DOI] [PubMed] [Google Scholar]
- 142.Wittekind A, Walton J. 2014. Worldwide trends in dietary sugars intake. Nutr. Res. Rev. 27:330–45 [DOI] [PubMed] [Google Scholar]
- 143.Woodrow CJ, Burchmore RJ, Krishna S. 2000. Hexose permeation pathways in Plasmodium falciparum-infected erythrocytes. PNAS 97:9931–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wright EM, Loo DD, Hirayama BA. 2011. Biology of human sodium glucose transporters. Physiol. Rev 91:733–94 [DOI] [PubMed] [Google Scholar]
- 145.Wyshak G 2000. Teenaged girls, carbonated beverage consumption, and bone fractures. Arch. Pediatr. Adolesc. Med 154:610–13 [DOI] [PubMed] [Google Scholar]
- 146.Yau AM, McLaughlin J, Gilmore W, Maughan RJ, Evans GH. 2017. The acute effects of simple sugar ingestion on appetite, gut-derived hormone response, and metabolic markers in men. Nutrients 9:E135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zubiria MG, Gambaro SE, Rey MA, Carasi P, Serradell MLA, Giovambattista A. 2017. Deleterious metabolic effects of high fructose intake: the preventive effect of Lactobacillus kefiri administration. Nutrients 9:E470. [DOI] [PMC free article] [PubMed] [Google Scholar]