Abstract
Background
Catheter ablation has become a popular interventional treatment for cardiac tachyarrhythmias and the number has been on the rise year by year. However, little is known about its efficacy and safety in the real‐world settings.
Method
Japanese Catheter Ablation (J‐AB) Registry is a nationwide, multicenter, observational registry, performed by Japanese Heart Rhythm Society (JHRS), collaborated with National Cerebral and Cardiovascular Center. This study is a voluntary nationwide registry and data are collected prospectively using a Research Electronic Data Capture (REDCap) system. Detailed data collection including antiarrhythmic medication is also performed every September. The acute success rate at discharge and the complications associated with ablation procedure will be collected in all cases. Major bleeding events are defined according to Bleeding Academic Research Consortium criteria. Based on the provided information, the annual incidence and predictive factors for outcome will be investigated by the Event Assessment Committee. This registry started in August 2017 and the number of participating medical instructions will be more than 250 hospitals and the target procedure number will be 70 000 per year. We will also compare the results with other registries in foreign countries.
Result
The results of this study are currently under investigation.
Conclusion
The J‐AB registry will provide a real‐world data regarding the acute success and complications in Japan, focusing on various types of catheter ablation for cardiac arrhythmias.
Keywords: catheter ablation, complication, J‐AB, REDCap, registry
1. INTRODUCTION
Catheter ablation is now a standard therapy for patients with cardiac arrhythmias. In recent years, catheter ablation system has significantly developed, including temperature monitoring, irrigated‐tip catheter, new mapping technologies, balloon ablation, chemical ablation, nonfluoroscopy ablation, and remote catheter ablation. Energy source is also developed using cryoablation, microwave, and laser. These noticeable advances can result in the increased success rate, and the total number of catheter ablation cases in Japan exceeded 60 000 per year and is still increasing. However, little is known about the details of the procedures, such as the number of each targeted arrhythmia, complications, and the outcome of the procedures in the real‐world settings. In such situations, it is important to evaluate the safety and the appropriateness of this treatment in the real‐world setting, and it is also a social requirement.
There are several preceding registries of catheter ablation, the majority of which collected data from selected (mostly teaching) centers to reveal the current status of ablations.1, 2, 3 However, such data from selected institutes do not necessarily reflect clinical practice in the real world. Accordingly, the Japanese Heart Rhythm Society (JHRS) and the National Cardiovascular Research Center (NCVC) conducted nationwide, multicenter registry in Japan, named Japanese Catheter Ablation (J‐AB) Registry in August 2017. In contrast to the preceding registries, this is a prospective nationwide multicenter registry designed to collect clinical variables and short‐term outcome data, aiming to register all catheter ablation cases performed in Japan.
2. METHODS
2.1. Objectives
This study is a voluntary nationwide registry, performed by the JHRS, collaborated with the NCVC. The objectives of this registry are to observe and describe developments in the catheter ablation treatment of arrhythmia in Japan and to provide reliable information on the type of activity performed and the facilities available in Japanese arrhythmia units.
2.2. Study population
Total 60 000 patients per year from more than 200 hospitals will be investigated. The inclusion criteria are patients who are treated with any type of catheter ablation for any types of cardiac arrhythmias (atrial fibrillation (AF), atrial flutter, atrial tachycardia, atrioventricular nodal reentrant tachycardia, accessory pathway syndrome, atrial premature contraction, ventricular premature contraction, ventricular tachycardia, ventricular fibrillation, sinus node related tachycardia, congenital heart disease related arrhythmia, etc). Patients who received surgical management for arrhythmias are excluded in this registry.
2.3. Data acquisition and analysis
Data are collected prospectively using a Research Electronic Data Capture (REDcap) system. Detailed data collection including medication and outcome of the procedure is also performed every September. After obtaining the consent and approval of the Local Institutional Review Board (IRB) Committee of the participating instructions, the physician will send a registration form to the J‐AB secretariat at NCVC. All of the compiled data remains anonymous, even to the registry coordinators, without any identifying information for the patients.
An interim analysis is planned every year. All results of the J‐AB, including numbers of operated cases, strategies, outcomes, and complications, will be published and/or reported at respective scientific meetings.
2.4. Study schedule
This study started in August 2017 and has been recruiting the participating medical instructions now.
2.5. Ethics
This study is registered at the Umin Clinical Trial Registry (UMIN000028288) and ClinicalTrials.gov (NCT03729232). This study is being conducted in accordance with the Declaration of Helsinki and the Ethical Guidelines for Clinical Studies issued by the Ministry of Health, Labour and Welfare, Japan, and received approval from the IRB of the National Cerebral and Cardiovascular Center, Japan, along with the IRBs of all participating instructions. All participants will provide informed consent either by a written paper or by an optout fashion and may withdraw their consent at any time.
3. RESULTS
Figure 1 demonstrates the numbers of monthly accumulated participating centers and the registered ablation cases during the first 12 months since the beginning of this registry. The detailed analysis of this study is currently under investigation and will be published elsewhere in the near future.
Figure 1.
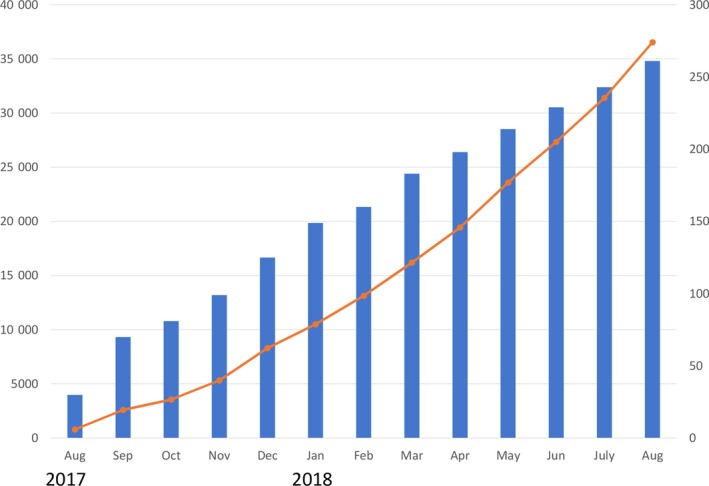
Number of patients and participating instructions enrolled as of August 2018. The left and right vertical axes indicate the total number of patients enrolled (orange line) and the total number of participating instructions (blue bars), respectively
4. DISCUSSION
This revolutionary therapy, catheter ablation for arrhythmia, had been first introduced using direct current (DC) energy in the 1980s to manage refractory atrial fibrillation (AF) with rapid ventricular response by creating complete atrioventricular block and spread to treat the cases of the Wolff‐Parkinson‐White (WPW) syndrome, ventricular tachycardia, and atrial tachycardia. DC ablation had been replaced by radiofrequency (RF) ablation in the 1990s because of high incidence of serious complications such as impaired left ventricular dysfunction and cardiac rupture by high‐energy discharge. RF ablation is applied first to paroxysmal supraventricular tachycardia and spread to other all atrial and ventricular arrhythmias. After the 2000s, RF ablation for AF under the three‐dimensional (3D) mapping technique introduced and after that, the number of catheter ablation procedures has been on the big rise year by year.
During the past two decades, the number of cases, who underwent catheter ablation for cardiac arrhythmias, has dramatically increased and exceeded 70 000 per year as reported by The Japanese Registry Of All Cardiac and Vascular Diseases(JROAD) in 2017.4 In spite of these marked developments, the current status of catheter ablation, including the strategy, outcome, and complication, remains to be elucidated.
Japanese catheter ablation is designed as a prospective Japanese nationwide multicenter registry designed to collect clinical variables and short‐term outcome data. The amount of data and variety of aspects covered by this registry will give new insights into the catheter ablation field of Japan as well as in the world.
When looking over the previous ablation registries, there have been accumulated reports from German, Spanish, and Japanese Ablation Registries. In the Spanish Catheter Ablation Registry, which started in 2000, they have continuously reported the annual features of the catheter ablation for all kinds of arrhythmias every year, demonstrating the annual change of the target arrhythmias, success rate, complication rate, etc. In the German Ablation Registry, they succeeded in revealing the special features of the catheter ablation for selected arrhythmias, for example, ablation for atrioventricular nodal reentrant tachycardia, comparison of radiofrequency and cryoballoon ablation for AF, etc. In both of these large‐scale ablation registries, they consisted of 50‐90 selected centers of each country. The Japanese Catheter Ablation Registry for Atrial Fibrillation (J‐CARAF) was conducted by the JHRS and AF ablation cases performed in September of 2011‐2017, retrospectively. This registry included cases performed not only in high‐volume centers but also low‐volume ones and published reports continuously.5, 6, 7, 8, 9, 10, 11, 12 However, this registry included exclusively AF ablation cases performed in September and features of other arrhythmias were not investigated. In contrast to these preceding registries, the current J‐AB registry is taking a step forward since it is aiming to collect all catheter ablation cases performed in one country, from various types of centers (small‐ and large‐volume centers), to reveal the current real‐world status of catheter ablation in Japan.
The registry items in each case of J‐AB are not complicated but rather simple since the basic concept of this registry is to gather all ablation cases in Japan; however, we are collecting detailed ablation data for all cases operated during every September as a detailed investigation. We believe that this unique data collection (simple and detailed data registries) will work well to reveal the current status of catheter ablation in Japan.
5. CONCLUSION
The J‐AB registry will provide a real‐world data regarding the acute success and complications in Japan, focusing on variety type of catheter ablation for cardiac arrhythmias.
CONFLICT OF INTEREST
T.Y.: Daiichi‐Sankyo, Nippon Boehringer Ingelheim, Abbott Japan, Bristol‐Myers Squibb, Bayer Pharmaceutical Company, Medtronic Japan. K.I. receives honorarium and consulting fee from Medtronic Japan, Johnson and Johnson KK, Bayer Pharmaceutical Company, Boehringer Ingelheim, Daiichi‐Sankyo, and Bristol‐Myers Squibb. K.K.: Daiichi‐Sankyo, Bristol‐Myers Squibb, Bayer Pharmaceutical Company, Medtronic Japan, Biotronic, Boston Scientific, Pfizer, EP‐CRSU Co. M.G. Daiichi‐Sankyo, Japan Lifeline, Johnson and Johnson KK. A.N. receives honoraria from Abbott and Daiichi‐Sankyo; and an endowment from Medtronic Japan. None: M.T., Y.M.N, Y.M. (Yoshihiro Miyamoto), K.U., M.S., Y.M.
Yamane T, Inoue K, Kusano K, et al. Study design of nationwide Japanese catheter Ablation registry: Protocol for a prospective, multicenter, open registry. J Arrhythmia. 2019;35:167–170. 10.1002/joa3.12163
REFERENCES
- 1. García‐Fernández FJ, Ibáñez Criado JL, Quesada Dorador A, et al. Spanish catheter ablation registry. 17th official report of the Spanish society of cardiology working group on electrophysiology and arrhythmias (2017). Rev Esp Cardiol 2018;71:941‐51. [DOI] [PubMed] [Google Scholar]
- 2. Schmidt M, Dorwarth U, Andresen D, et al. Cryoballoon versus RF ablation in paroxysmal atrial fibrillation: results from the German Ablation registry. J Cardiovasc Electrophysiol. 2014;25:167–7. [DOI] [PubMed] [Google Scholar]
- 3. Hoffmann BA, Brachmann J, Andresen D, et al. Ablation of atrioventricular nodal reentrant tachycardia in the elderly: results from the German Ablation Registry. Heart Rhythm. 2011;8:981–7. [DOI] [PubMed] [Google Scholar]
- 4. Yasuda S, Nakao K, Nishimura K, et al. The current status of cardiovascular medicine in Japan – analysis of a large number of health records from a nationwide claim‐based database. JROAD‐DPC. Circ J. 2016;80:2327–35. [DOI] [PubMed] [Google Scholar]
- 5. Inoue K, Murakawa Y, Nogami A, et al. Clinical and procedural predictors of early complications of ablation for atrial fibrillation: analysis of the national registry data. Heart Rhythm. 2014;11:2247–53. [DOI] [PubMed] [Google Scholar]
- 6. Murakawa Y, Nogami A, Shoda M, et al. Nationwide survey of catheter ablation for atrial fibrillation: the Japanese Catheter AblationRegistry of Atrial Fibrillation (J‐CARAF)–report of 1‐year follow‐up. Circ J. 2014;78:1091–6. [DOI] [PubMed] [Google Scholar]
- 7. Inoue K, Murakawa Y, Nogami A, et al. Current status of catheter ablation for atrial fibrillation–updated summary of the Japanese Catheter Ablation Registry of Atrial Fibrillation (J‐CARAF). Circ J. 2014;78:1112–20. [DOI] [PubMed] [Google Scholar]
- 8. Murakawa Y, Nogami A, Shoda M, et al. Nationwide survey of catheter ablation for atrial fibrillation: the Japanese catheter ablation registry of atrial fibrillation (J‐CARAF)‐A report on periprocedural oral anticoagulants. J Arrhythm. 2015;31:29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Inoue K, Murakawa Y, Nogami A, et al. Current status of catheter ablation of atrial fibrillation in Japan: summary of the 4th survey of the Japanese Catheter Ablation Registry of Atrial Fibrillation (J‐CARAF). J Cardiol. 2016;68:83–8. [DOI] [PubMed] [Google Scholar]
- 10. Murakawa Y, Yamane T, Goya M, et al. Incidence and predictors of pericardial effusion as an early complication of catheter ablation for atrial fibrillation: the Japanese Catheter Ablation Registry of Atrial Fibrillation (J‐CARAF). J Arrhythm. 2017;33:430–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Murakawa Y, Nogami A, Shoda M, et al. Report of periprocedural oral anticoagulants in catheter ablation for atrial fibrillation: the Japanese Catheter Ablation Registry of Atrial Fibrillation (J‐CARAF). J Arrhythm. 2017;33:172–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Murakawa Y, Yamane T, Goya M, et al. Influence of substrate modification in catheter ablation of atrial fibrillation on the incidence of acute complications: analysis of 10 795 procedures in J‐CARAF Study 2011‐2016. J Arrhythm. 2018;34:435–40. [DOI] [PMC free article] [PubMed] [Google Scholar]


