Abstract
Background
Left atrial appendage (LAA) closure device is an alternative to anticoagulants for stroke prevention in selected atrial fibrillation (AF) patients. The LAA device implantation is safe with short period of learning curve. The standard implantation technique warrants a transesophageal echocardiography (TEE) guided and general anesthesia. In region of Asia Pacific as well as Indonesia, both TEE and general anesthesia are not always available in district hospital. We studied the safety and efficacy of Amplatzer Cardiac Plug (ACP) implantation guided by fluoroscopy only and without general anesthesia.
Methods
Consecutive nonvalvular AF patients with CHA2DS2VASc score of ≥2 and HASBLED score of ≥3 are participated. Patients requiring long‐life anticoagulant for any other indication are excluded. The choice of implanted first or second‐generation ACP is that with excess size of 2‐4 mm of measured landing zone diameter.
Results
Twenty‐five subjects were implanted ACP by means fluoroscopy only (Group A) and 28 subjects using standard technique group (Group B). The median AF duration was 36 months (6‐276 months) and majority of patients (49%) are having permanent AF. The mean CHA2DS2VASc score is 3.9 ± 1.63. Successful implantation of ACPs was 96% in both groups. Nonfatal pericardial effusion occurred in three patients. During 75 weeks of follow‐up period, there were no significant differences of stroke event and death between groups.
Conclusion
ACP implantation guided with fluoroscopy only is feasible and safe.
Keywords: atrial fibrillation, left atrial appendage, stroke prevention
1. INTRODUCTION
Atrial fibrillation (AF) accounts for more than 15% of all strokes which are more likely to be chronically disabled, bedridden, and require constant nursing care, particularly in older patients.1 Oral anticoagulant is a drug of choice in stroke and systemic embolism prevention in nonvalvular AF patients.2, 3, 4, 5 However, in certain patient's situation anticoagulant is not applicable. Ninety percent of thrombus formation in nonvalvular AF is located at left atrial appendage (LAA).6 Therefore, LAA closure devices are available as an alternative to anticoagulant therapy especially for those who are contraindicated to long‐term anticoagulant therapy or higher bleeding risk. A 5 year outcome of the PREVAIL trial, combined with the 5 year outcomes of the PROTECT AF trial, demonstrate that LAA closure provides stroke prevention in nonvalvular AF comparable to warfarin, with additional reductions in major bleeding, particularly hemorrhagic stroke, and mortality.7 Current standard procedure of LAA closure device implantation is guided by intraprocedure transesophageal echocardiography (TEE) and under general anesthesia. In some district hospital of Indonesia, TEE probe is not available and numbers of anesthesiologist are limited. Therefore, we prospectively study the feasibility of LAA closure device implantation guided by fluoroscopy only and without general anesthesia.
2. METHODS
2.1. Patients
A consecutive of 53 patients (aged 67.5 ± 8.70 years, 37% are women) with nonvalvular AF participated in this study. Written informed consents are obtained from all participants. In 25 patients, ACPs implantation guided by fluoroscopy only and without general anesthesia (Group A). In another group of 28 patients, ACPs implantation was using standard protocol (Group B). To be eligible for this study, patients must have a CHA2DS2VASc score8 of at least 2 and HASBLED score9 of at least 3, and unsuitable for long‐term anticoagulant therapy. Exclusion criteria are the presence of thrombus in the left atrium, acute decompensated heart failure, contraindications for transseptal puncture and require long‐term anticoagulant therapy due to any other indication. Extracardiac source of stroke was excluded in both groups.
2.2. LAA sizing
Preprocedural TEE10 and/or cardiac computed tomography (CCT)11 are used to exclude the presence of thrombus in left and right atrium as well as to determine LAA diameters and morphology.
A 5‐F marked pigtail catheter (Cook Medical Inc., Bloomington, IN, USA) is inserted into the sinus of Valsalva through the left femoral artery. The edge to edge distance between two consecutive markers of the pigtail catheter is 10 mm. Following transseptal puncture, angiography of the LAA is performed by placing an SL‐0 long sheath (St Jude Medical, Minnetonka, MN, USA) in front of the LAA ostium and a 15‐cc of hand injected contrast media are given during RAO 20‐30° with cranial angulation of 20° view. No magnification cine view is used. Careful angiography sizing of LAA is performed using available computerized quantitative coronary analysis (QCA) with 20 mm distance on the marked pigtail as a calibration reference (Figure 1). The Group A subjects used this angiographic method and preprocedural TEE measurement to determine LAA ostial and landing zone diameters. In Group B patient, both angiography and intraprocedural TEE are used to measure ostial and landing zone diameters. The TEE measurements are performed using 45° and 90° to 110° views. The largest diameter from any TEE view is used as final TEE results. If angiographic LAA measurements results are differed from that of TEE results, then we classified it according to its differences. In case of less than 10 mm difference, then the largest diameter is used as the final LAA diameter. However, if the difference between angiographic and TEE results is more than 10 mm, then the average of those diameters is concluded as the final diameters. In both groups, the LAA sizing is performed upon appropriate volume status as confirmed by LA pressure of about 10 mm Hg. Colloid fluid loading is given if necessary. The ACP size selection is 2‐4 mm above final LAA landing zone diameter.
Figure 1.
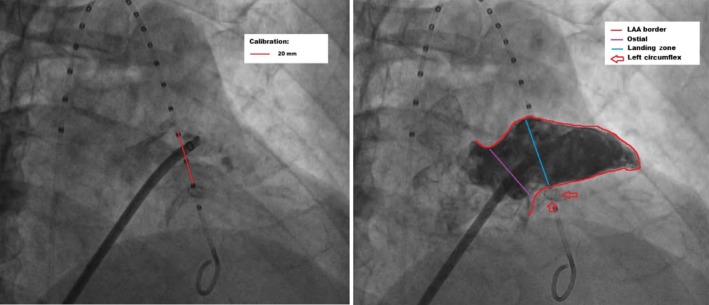
Fluoroscopic‐based measurement of left atrial appendage (LAA). Left panel: Calibration of measurement using marked pigtail. Distance between three markers is 20 mm. Right panel: Red line shows border of LAA, purple line is ostial diameter, blue line is landing zone diameter, and red arrows point stented left circumflex
2.3. Procedure of ACP implantation
In Group A, ACP implantation procedure is performed on conscious condition with local anesthesia of bilateral inguinal area. A 5‐F marked pigtail catheter is inserted into the sinus of Valsava through left femoral artery puncture. A transseptal puncture is performed using BRK Transseptal Needle (St. Jude Medical) delivered inside the SL‐0 Schwartz long sheath through right femoral vein. The pigtail catheter is used as a marker of safe septal puncture. Following angiographic LAA sizing, an appropriate ACP size is delivered using 13‐F Torqvue‐45 sheath into the LAA. The ACP is step by step released in LAA. Of note, that device release should be started at distal part LAA with slowly unsheath the device till the ball shape is formed, then keep unsheath but hold the proximal part of delivery system softly to let the device move slightly proximal inside LAA when the strawberry shape formed. Then hold the delivery system tightly while continue unsheath and keep the device in the landing zone when the lobe completely opens. Once the lobe is positioned at landing zone, release the disk by means of simultaneous unsheath and light push of the device.
In Group B, ACP implantation is performed using standard protocol.12 Procedure is performed under general anesthesia. In brief, the procedure comprised of (a) TEE‐guided transseptal puncture which targets more inferior part of the fossa ovalis, (b) LAA angiography in order to measure landing zone and ostial diameter which is then confirmed by TEE, (c) Step‐by‐step ACP placement, and (d) Finally appropriate position of ACP and absence of residual flow jet is confirmed by TEE before the device is unscrewed from the delivery system.
Appropriate ACP position determined by five criteria including (a) tire shape of the lobe, (b) the lobe position is distal to left circumflex artery, (c) present of distance between lobe and disk, (d) concave shape of the disk, and (e) stable device position during tug test (Figure 2).
Figure 2.
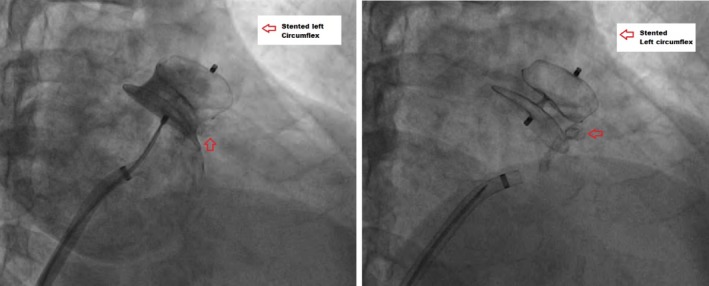
Amplatzer Cardiac Plug (ACP) placement at the appropriate position. Lobe position is distal to left circumflex (red arrow), and the disk is nicely covering the whole ostial of LAA without protrusion to pulmonary vein nor mitral valve. Left panel shows contrast injection confirm complete LAA closure. The figure is taken from the same patient as Figure 1
2.4. Follow‐up
The mean follow‐up period was 75 weeks. Transthoracic echocardiography studies are performed immediate, and TEE at 3 months after ACP implantation. Outpatient‐based clinical observations are performed every month. Dual antiplatelet (clopidogrel 75 mg OD and aspirin 80 mg OD) were administered for 3 months then aspirin 80 mg OD lifelong.12, 13 Anticoagulant is administered only if evidence of left atrial thrombus appears in echocardiography study during the follow‐up period.
3. RESULTS
The median AF duration was 36 months (6‐276 months) and majority of patients (49%) are having permanent AF. Based on CHA2DS2VASc score, subjects are at high risk of stroke with mean score of 3.9 ± 1.63. The clinical characteristics of both patient groups are presented in Table 1.
Table 1.
Clinical characteristics
| Characteristics | Fluoroscopic guided (Group A) | TEE guided (Group B) |
|---|---|---|
| Age (y) | 66.16 ± 8.79 | 67.42 ± 9.8 |
| Type of atrial fibrillation, n (%) | ||
| Paroxysmal | 0 | 0 |
| Persistent | 8 | 4 |
| Long‐standing persistent | 2 | 13 |
| Permanent | 15 | 11 |
| CHA2DS2VASc score | 4.29 ± 1.51 | 3.54 ± 1.67 |
| HASBLED score | 3.13 ± 0.99 | 2.64 ± 1.06 |
| Ejection fraction (%) | 64 ± 10.5 | 61 ± 16 |
| Left atrial dimension (mm) | 48 ± 9.67 | 43.9 ± 7.82 |
Successful implantation of ACPs was 96% in both groups. One patient in Group A failed ACP implantation due to chicken wing morphology with very shallow wingette part. One patient in Group B failed ACP implantation due to huge LAA size.
In Group A, mean diameter of LAA landing zone and ostium are 22.8 ± 4.76 and 22.9 ± 4.79 mm, respectively, by means of combine TEE and angiography measurement methods. LAA landing zone and ostial diameters measured with angiography are similar to that measured with TEE. The mean implanted ACP size was 26.96 ± 3.296 mm. There was no device replacement due to under‐ or oversizing. Two patients in Group A implanted second generation of ACP.
In Group B, average size of LAA landing zone and ostial are 22.7 ± 4.87 and 24.3 ± 4.53, respectively. There were no significant differences of ostial diameter measured by TEE vs angiography. However, landing zone diameter is bigger during angiography measurement compared to that measured by TEE (mean difference 3.4 mm, P = 0.008).
Of combine group data, there were no difference of LAA ostium diameter measured by TEE vs angiography. However, LAA landing zone diameter measured with angiography is bigger compared to that measured with TEE (mean difference 2.8 mm, P = 0.002) (Table 2).
Table 2.
Left atrial appendage (LAA) measurements
| Diameter | TEE | Angiography | P value |
|---|---|---|---|
| Group A | |||
| Ostium | 22.2 ± 4.64 | 23.4 ± 4.94 | 0.395 |
| Landing zone | 21.9 ± 5.39 | 23.6 ± 4.06 | 0.226 |
| Group B | |||
| Ostium | 24.5 ± 4.60 | 24.1 ± 4.53 | 0.728 |
| Landing zone | 20.9 ± 4.39 | 24.3 ± 4.78 | 0.008 |
| Combined group | |||
| Ostium | 23.4 ± 4.68 | 23.7 ± 4.69 | 0.699 |
| Landing zone | 21.1 ± 4.67 | 23.9 ± 4.43 | 0.002 |
TEE, transesophageal echocardiography.
Pericardial effusion complicated two subjects of Group B and one subject of Group A. Two of them need pericardiocentesis. In three subjects of Group B, residual gap (less than 5 mm) is found during TEE follow‐up 3 months following implantation.
During the follow‐up of almost 19 months, one of the three patients with residual gap experienced stroke at 23 weeks following implantation and another one experienced device‐related thrombus. Transesophageal echocardiography reexamination 3 months after dabigatran 150 mg bid therapy revealed no residual thrombus. The stroke patient completely recovered without sequalae. Ventricle rate is well controlled by beta blocker, nondihydropyridine calcium channel blocker, and digoxin as a single drug or in combination. One patient of both groups died suddenly at the first month. Both patients had significant coronary artery disease and low ejection fraction. Unfortunately, no autopsy was performed. The Kaplan‐Meier survival analysis of both groups is shown in Figure 3. There is no survival different between groups (P = 0.467).
Figure 3.
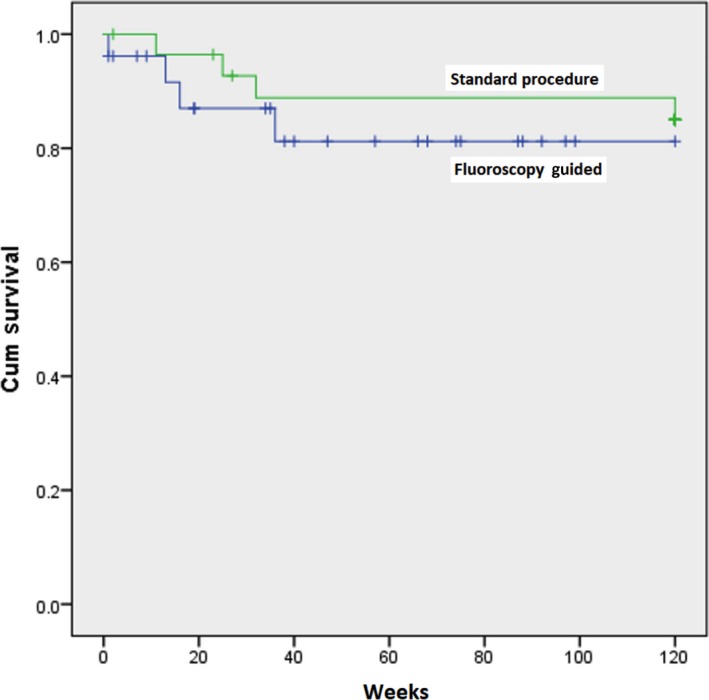
Kaplan‐Meier Survival Graphic. There is no different of cumulative survival between standard procedure and fluoroscopy‐guided groups (P = 0.467)
4. DISCUSSION
This long‐term observational study showed that ACP implantation guided by fluoroscopy only is safe and effective. The successful implantation is 96% which is similar to standard protocol. Nonfatal serious complication occurred in three patients.
Similar results were achieved by Streb et al14 in Poland who had more than 95% success in 22 nonvalvular AF patients with CHA2DS2VASc score of 2 and HASBLED score of 3 that implanted ACP using standard protocol. Serious complication occurred in one patient in their series. Other series with 52 patients aged 74 ± 8 years and median CHADS2 score of 3 achieved success rate of 98.1%. Procedure‐related complications were device embolization (1.9%) and pericardial effusion (1.9%), with no cases of peri‐procedural stroke.15
Complication and mortality rate during implantation and follow‐up period were similar to previous study. Urena et al, reported that during mean follow‐up of 20 ± 5 months, the rates of death, stroke, systemic embolism, pericardial effusion, and major bleeding were 5.8%, 1.9%, 0%, 1.9%, and 1.9%, respectively. The presence of mild peri‐device leak was observed in 16.2% of patients at 6 months follow‐up as evaluated by TEE. There were no cases of device thrombosis.14 Two sudden cardiac deaths happened in the first month of ACP implantation in our series, unfortunately autopsy was not done to determine the cause of death. Both patients had significant coronary artery disease and low ejection fraction which were known as risk factors for sudden death. However, device embolization is still possible to occur a year following implantation and might cause fatal situation.16
The accuracy of fluoroscopic‐based measurement of LAA landing zone diameter is crucial for the proposed implantation technique. Interestingly, in our data, the landing zone diameter measured with angiography was larger by 2.8 mm as compared to that measured with TEE. Despite statistically significant, the 2.8 mm difference is clinically not importance. The implanted device size is usually 4 mm larger from the measured landing zone. Therefore, the particular difference in landing zone measurement will not change the choice of implanted device. Various imaging modalities such as cardiac computed tomography angiography (CCTA), TEE, and fluoroscopy are correlated well to each other for assessment of LAA ostium and landing zone measurement. For ACP landing zone, mean maximal measurements were 24.1 ± 4.7 mm with CCTA, 22.3 ± 4.9 mm TEE, and 19.9 ± 5.6 mm fluoroscopy (P < 0.001); with R value 0.81 fluoroscopy/CCTA, 0.67 fluoroscopy/TEE, and 0.80 CCTA/TEE. The values from CCTA were consistently higher than the other two modalities. This is presumably because of the superior multiplanar imaging with CCTA, compared with TEE and fluoroscopy, allowing CT interpreters to manipulate the oblique angles appropriately to select the maximal dimensions.17
4.1. Limitation
Patients assignment is not randomized; however, important clinical characteristics that might influence the outcome were not different between two groups.
5. CONCLUSION
ACP implantation guided with fluoroscopy only is feasible and safe.
CONFLICT OF INTEREST
Dr Yoga Yuniadi received honorarium from St Jude Medical as regional clinical proctor for ACP Implantation.
Yuniadi Y, Hanafy DA, Raharjo SB, Yugo D. Left atrial appendage closure device implantation guided with fluoroscopy only: Long‐term results. J Arrhythmia. 2019;35:262–266. 10.1002/joa3.12151
REFERENCES
- 1. Reiffel JA. Atrial fibrillation and stroke: epidemiology. Am J Med. 2014;127:e15–6. [DOI] [PubMed] [Google Scholar]
- 2. SPAF Investigator . Stroke prevention in atrial fibrillation study. Final results. Circulation. 1991;84:527–39. [DOI] [PubMed] [Google Scholar]
- 3. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51. [DOI] [PubMed] [Google Scholar]
- 4. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–91. [DOI] [PubMed] [Google Scholar]
- 5. Granger CB, Alexander JH, McMurray JJ, et al., Committees A and Investigators . Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–92. [DOI] [PubMed] [Google Scholar]
- 6. Watson T, Shantsila E, Lip GY. Mechanisms of thrombogenesis in atrial fibrillation: Virchow's triad revisited. Lancet. 2009;373:155–66. [DOI] [PubMed] [Google Scholar]
- 7. Reddy VY, Doshi SK, Kar S, et al., Prevail and Investigators PA . 5‐year outcomes after left atrial appendage closure: from the PREVAIL and PROTECT AF Trials. J Am Coll Cardiol. 2017;70:2964–75. [DOI] [PubMed] [Google Scholar]
- 8. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–72. [DOI] [PubMed] [Google Scholar]
- 9. Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user‐friendly score (HAS‐BLED) to assess 1‐year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–100. [DOI] [PubMed] [Google Scholar]
- 10. Sallach JA, Puwanant S, Drinko JK, et al. Comprehensive left atrial appendage optimization of thrombus using surface echocardiography: the CLOTS multicenter pilot trial. J Am Soc Echocardiogr. 2009;22:1165–72. [DOI] [PubMed] [Google Scholar]
- 11. Romero J, Husain SA, Kelesidis I, Sanz J, Medina HM, Garcia MJ. Detection of left atrial appendage thrombus by cardiac computed tomography in patients with atrial fibrillation: a meta‐analysis. Circ Cardiovasc Imaging. 2013;6:185–94. [DOI] [PubMed] [Google Scholar]
- 12. Lam YY, Yip GW, Yu CM, et al. Left atrial appendage closure with AMPLATZER cardiac plug for stroke prevention in atrial fibrillation: initial Asia‐Pacific experience. Catheter Cardiovasc Interv. 2012;79:794–800. [DOI] [PubMed] [Google Scholar]
- 13. Schmidt B, Chun KR. Antithrombotic therapy after left atrial appendage closure. Expert Rev Cardiovasc Ther. 2015;13:105–9. [DOI] [PubMed] [Google Scholar]
- 14. Streb W, Szymala M, Kukulski T, et al. Percutaneous closure of the left atrial appendage using the Amplatzer Cardiac Plug in patients with atrial fibrillation: evaluation of safety and feasibility. Kardiol Pol. 2013;71:8–16. [PubMed] [Google Scholar]
- 15. Urena M, Rodes‐Cabau J, Freixa X, et al. Percutaneous left atrial appendage closure with the AMPLATZER cardiac plug device in patients with nonvalvular atrial fibrillation and contraindications to anticoagulation therapy. J Am Coll Cardiol. 2013;62:96–102. [DOI] [PubMed] [Google Scholar]
- 16. Schroeter MR, Danner BC, Hunlich M, Schillinger W. Uncommon delayed and late complications after percutaneous left atrial appendage closure with Amplatzer((R)) Cardiac Plug. Clin Res Cardiol. 2014;103:285–90. [DOI] [PubMed] [Google Scholar]
- 17. Saw J, Fahmy P, Spencer R, et al. Comparing measurements of CT angiography, TEE, and fluoroscopy of the left atrial appendage for percutaneous closure. J Cardiovasc Electrophysiol. 2016;27:414–22. [DOI] [PubMed] [Google Scholar]


