Abstract
Aim
Quadripolar lead technology and multi‐point pacing (MPP) are important clinical adjuncts in cardiac resynchronization therapy (CRT) pacing aimed at reducing the rate of non‐response to therapy. Mixed results have been achieved using MPP and it is critical to identify which patients require this approach and how to configure their MPP stimulation, in order to achieve optimal electrical resynchronization.
Methods & Results
We sought to investigate whether electrocardiographic imaging (ECGi), using the CARDIOINSIGHT ™ inverse ECG mapping system, could identify alterations in electrical resynchronization during different methods of device optimization. In no patient did a single form of programming optimization provide the best electrical response. The effects of utilizing MPP were idiosyncratic and highly patient specific. ECGi activation maps were clearly able to discern changes in bulk LV activation during differing MPP programming. In two of the five subjects, MPP resulted in more rapid activation of the left ventricle compared to standard CRT; however, in the remaining three patients, the use of MPP did not appear to acutely improve electrical resynchronization. Crucially, this cohort showed evidence of extensive LV scarring which was well visualized using both CMR and ECGi voltage mapping.
Conclusions
Our work suggests a potential role for ECGi in the optimization of non‐responders to CRT, as it allows the fusion of activation maps and scar analysis above and beyond interrogation of the 12 lead ECG.
Keywords: CRT, electrocardiographic mapping, multi‐point pacing, multi‐site pacing, non‐responders
1. BACKGROUND
Cardiac resynchronization therapy (CRT) aims to restore regional activation synchrony and enhance cardiac contractility and the mechano‐energetic efficiency of the heart.1 Multi‐point pacing (MPP) has been developed as a tool to reduce the rate of non‐response.2, 3, 4 Intuitively, activating the heart from multiple locations could achieve more effective resynchronization; bypassing scarred myocardium and enabling the recruitment of a greater proportion of the left ventricle, resulting in increased conduction velocities and a reduction in the total activation time.5 Whilst some authors have shown improvements in acute hemodynamics6 and chronic echocardiographic remodeling,2, 4 recent data have suggested that its efficacy may be confined to a small proportion of patients and that its effect is conditional on specific electrical and anatomical7, 8 parameters and programming.3 We sought to investigate how myocardial activation varied during programming optimization and whether non‐invasive body surface mapping technology might be capable of identifying patients who may derive the most benefit from MPP.
2. METHODS
Patients on optimal medical therapy (OMT) meeting European Society of Cardiology (ESC)9 and/or Heart Rhythm Society (HRS)10 criteria for CRT implantation were enrolled into the study (Clinical Trails Number; NCT01831518, date approved 4 April 2013). The underlying aetiology of heart failure was determined using clinical history and cardiac MRI (CMR). Patients were implanted with a St Jude CRT Device (St. Jude Medical Inc., St. Paul, MN, USA) capable of MPP programmability and a Quartet™ quadripolar LV lead (St. Jude Medical Inc.). Initially devices were programmed according to the default manufacturer settings. The following day after implantation, each patient underwent an iterative CRT optimization procedure, guided by non‐invasive body surface mapping, looking to identify the optimal pacing settings.
2.1. Non‐invasive body surface mapping
A non‐invasive electrophysiological mapping study was performed using a high resolution electrocardiographic mapping system (ECVUE, CardioInsight Technologies Inc. Medtronic), as previously described.11 The patient's baseline presenting rhythm––either intrinsic sinus rhythm or RV paced rhythm––was first analysed using directional activation maps.12 Further mapping was undertaken using nominal CRT programming, before echo guided device optimisation was attempted. Finally, both local and extended bipolar MPP was used to further optimize the delivery of biventricular pacing. The ECSYNC software calculates four parameters assessing electrical activation:
Global Right/Left Ventricular Electrical Synchrony (VVsync): the mean activation time in the right ventricle minus the mean activation time in the left ventricle. Previously described as ventricular electrical uncoupling.
Global Biventricular Total Activation Time (VVtat): a measurement of the total time required for both ventricles to activate. Previously described as VVTAT.
Global Left Ventricular Total Activation Time (LVtat): a measurement of the total time required for all portions of the left ventricle to activate. Previously described as LVTAT.
Global Left Ventricular Dispersion of Activation (LVdisp): a measure of the dispersion of the activation times in the left ventricular region of interest.
Given the primary objective of CRT is to restore regional activation synchrony, we defined the optimal activation pattern as that which achieved the most effective degree biventricular resynchronization whilst simultaneously minimizing both biventricular and LV activation times. Electrical synchrony is specifically assessed by VVsync, where a figure of 0 represents identical LV and RV activation time.13 As such, the optimal pacing figuration was that which achieved a VVsync approaching 0, whilst also minimizing LV and BiV activation times.
Epicardial voltage maps were also collected, in order to identify any areas of low voltage which may indicate areas of myocardial scar and fibrosis. Where possible, these were correlated against CMR data, see Figure 1.
Figure 1.
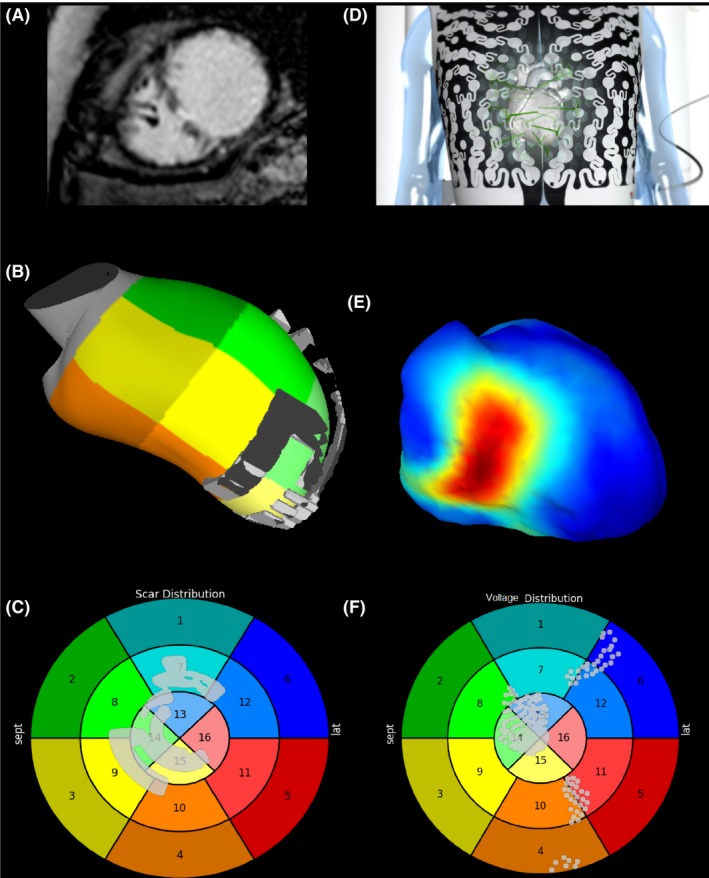
Multi‐panel plot showing a comparison between late gadolinium enhancement (LGE) derived scar from cardiac magnetic resonance (CMR) and areas of low voltage indicated scarred myocardium. A, LGE CMR in short axis showing areas of transmural hyperenhancement in the mid‐septum. B, Areas of LGE derived scar rendered onto on a 3‐D shell of the LV in RAO angulation. C, Areas of LGE displayed on a 16 segment bulls‐eye plot of the LV. D, The CARDIOINSIGHT ™ electrocardiographic mapping vest is applied to the thorax. E, Voltage thresholded CARDIOINSIGHT ™ electrocardiographic map in RAO angulation. Areas of <2mv are displayed in red. F, Areas of low voltage displayed on a 16 segment bulls‐eye plot of the LV
2.2. Device optimisation
The patient's baseline presenting rhythm––either intrinsic sinus rhythm or RV paced rhythm––was first analyzed using directional activation maps.12 Subsequently, mapping was undertaken using nominal biventricular pacing settings (sensed atrioventricular [AV] delay 150 ms/paced AV delay 200 ms/LV & RV [VV] offset of 0 ms). Next, an echo guided iterative approach to device optimization was employed. The AV interval was optimized according to the maximal improvement in LV diastolic filling. An AV interval of 200 ms was first programmed followed by decrements of 20 ms until 60 ms.
The VV offset was optimized according to the maximal improvement in aortic pulsed‐wave Doppler velocity time integral, as previously described.14, 15 Pacing with the LV 60 ms ahead of the RV (+LV60) was initially programmed followed by +LV40, +LV30, +LV20, +LV15, simultaneous LV and RV pacing (sim), RV ahead by 20 ms (+RV20), +RV40, RV only pacing, and LV only pacing.
Once the optimal AV and VV intervals had been established and programmed, differing MPP settings were then acutely programmed. During each configuration, a non‐invasive electro‐anatomical mapping was obtained. The SJM CRT toolkit™ (St. Jude Medical Inc.) was used to identify the RV‐paced to LV‐sensed timings for each of the poles on the quadripolar lead. MPP was then programmed to pace the pole with the longest delay first and the pole with the shortest delay second. The right ventricular lead was always paced last. We delivered MPP with 5, 10, and 20 ms delays between each stimulus using both a local bipole configuration‐distal (D1) to mid 2 (M2) and proximal (P4), and an extended bipole configuration‐ D1 to RV coil and P4 to RV coil. This resulted in our testing 6 MPP settings per patient; 3 local and 3 extended bipole. In order to test the different MPP vectors for capture threshold and phrenic nerve stimulation, a number of standard biventricular recordings were also performed which served as comparators for individual patients.
3. RESULTS
3.1. Patient characteristics
A total of five patients were enrolled in the study.
3.2. Body surface mapping
The effect of changing AV delays, VV delays, the LV pacing vector, and finally the addition of MPP are shown in Table 1. In no patient did a single form of optimization provide the best electrical response. The mean electrical response using each strategy is shown in Table 1.
Table 1.
Mean electrical response of each optimization strategy
| Electrical response | ||||
|---|---|---|---|---|
| VVsync ms (range) | VVTAT ms (range) | LVTAT ms (range) | LV disp (range) | |
| Optimization strategy | ||||
| AV optimization | −15.67 (−59 to 17) | 89.03 (57‐129) | 86.97 (57‐129) | 28.33 (18‐43) |
| VV optimization | −4.9 (−28 to 42) | 86.4 (51‐125) | 81.3 (51‐125) | 25.45 (15‐41) |
| Change in LV vector | −4.26 (−30 to 23) | 84.62 (55‐144) | 78.59 (55‐144) | 24.72 (17‐48) |
| MPP on | −1.17 (−20 to 32) | 87.6 (58‐141) | 81.17 (54‐141) | 25.03 (15‐48) |
Epicardial voltage mapping showed the presence of scar in all of the ischemic patients which corresponded to LGE on MRI in the 3 cases where MRI was performed, see Figure 1. MPP had divergent effects on electrical activation in different patients that are described below.
3.3. Case 1
| Age | 62 |
| Sex | M |
| Aetiology | ICM |
| LVEF | 28% |
| Rhythm | SR |
| QRS morphology | LBBB |
| QRS width | 170 |
| PR interval | 210 |
Echocardiography demonstrated severe biventricular impairment and CMR demonstrated delayed sub‐endocardial late gadolinium enhancement consistent with prior infarction of the apex, antero/infero‐septum, and inferolateral wall. The epicardial voltage map was consistent with extensive scarring in the same distribution as the LGE visualized during CMR.
3.3.1. Electrical effect of MPP
Intrinsic conduction exhibited the activation pattern typically observed in LBBB; ventricular activation is initiated at the distal branching of the right bundle, with activation of the left ventricular endocardium occurring after a significant delay, as a result of slow conduction through the interventricular septum (see Figure 2). This was associated with a broad QRS on the surface ECG. Conventional BiV pacing using nominal pacing settings yielded an improvement in activation pattern, with a significant reduction in both VVtat & LVtat as well as an improvement in VVsync. Further improvements in activation were observed following echo guided optimization of the AV and VV intervals. Activation maps undertaken during iterative programming optimization display advancement of the line of activation in comparison to nominal BiV activation. During extended bipolar pacing, with the RV coil as the anode, the activation maps appeared similar to conventional CRT, with apical to basal activation. Local bipolar activation achieved lateral to septal activation.
Figure 2.
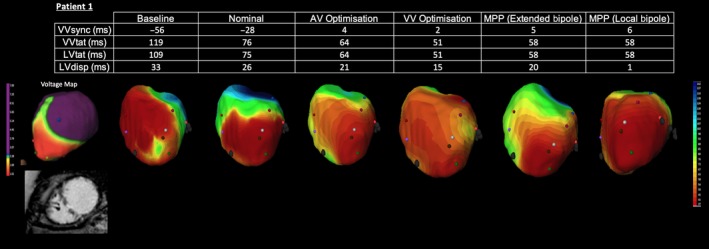
Electrocardiographic activation metrics and directional activation maps during device optimization of Patient 1. A voltage map thresholded to 2mv is shown with a still from the CMR short axis stack (SAX)
Whilst extended bipolar MPP programming was capable of reducing VVtat compared to nominal CRT, the use of local bipolar MPP did not lead to any further improvements in ventricular activation.
3.4. Case 2
| Age | 50 |
| Sex | M |
| Aetiology | ICM |
| LVEF | 33% |
| Rhythm | SR |
| QRS morphology | LBBB |
| QRS width | 176 |
| PR interval | 218 |
MRI demonstrated extensive thinning and scarring of the lateral wall. This was also displayed on the epicardial voltage map. CRT was performed with the LV lead inserted out of scar in an apical position.
3.4.1. Electrical effect of MPP
In this patient with extensive lateral scar, intrinsic activation was again characterized by typical LBBB propagation with delayed lateral LV wall activation (see Figure 3). Despite this patient having a broad QRS, nominal BiV pacing resulted in prolongation of the LVtat and VVtat, although improvements in V‐V synchronicity were observed. Attempts at optimizing CRT delivery though the use of both local and extended bipolar MPP proved equally ineffective and were associated with no significant change in activation pattern on the ECGi. Instead, iterative AV optimization proved the most effective optimization strategy, yielding a significant reduction in both LV and BiV activation times while also improving VVsync. This case demonstrates despite the deliberate avoidance of scar with an apical LV lead position, the use of MPP was unable to further optimize the pattern of activation.
Figure 3.
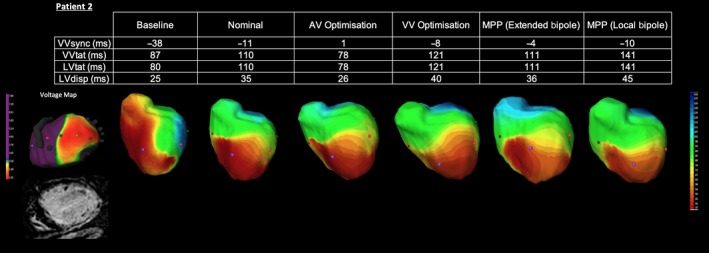
Electrocardiographic activation metrics and directional activation maps during device optimization of Patient 2. A voltage map thresholded to 2mv is shown with a still from the CMR short axis stack (SAX)
3.5. Case 3
| Age | 55 |
| Sex | M |
| Aetiology | ICM |
| LVEF | 13% |
| Rhythm | AF |
| QRS morphology | LBBB |
| QRS width | 160 |
| PR interval | N/A |
This patient was unable to undergo CMR; however, the ECGi revealed an area of low voltage in the apical region in keeping with myocardial scar/fibrosis.
3.5.1. Electrical effect of MPP
The pattern of LBBB activation with delayed activation in the lateral LV wall can again be observed on the baseline ECGi maps (see Figure 4). Interestingly this patient also had the longest LV and BiV activation time of the entire cohort but the shortest VVsync, suggesting activation in both ventricles was retarded. Activation was clearly delayed in the apical region, denoted by the blue isochrones on the activation map. This area corresponded to the previously identified area of low voltage tissue and likely represents delayed activation occurring in a region of scar tissue. Both BiV and LV activation times were significantly reduced during nominal BiV CRT. Electrical resynchronization also improved dramatically. Given this patient's underlying atrial fibrillation, AV optimization was not attempted; however, a small improvement in resynchronization and activation parameters was observed following VV optimization. Again, the addition of both extended bipolar and local bipolar MPP was unable to achieve a superior degree of electrical resynchronization, with the directional activation maps revealing a near identical pattern.
Figure 4.
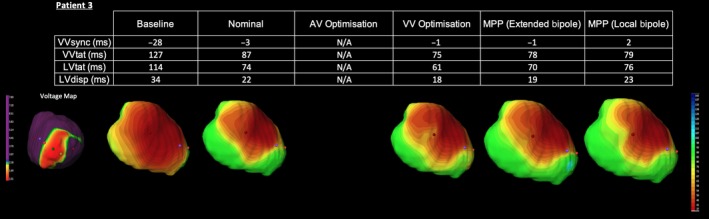
Electrocardiographic activation metrics and directional activation maps during device optimization of Patient 3. A voltage map thresholded to 2mv is shown
3.6. Case 4
| Age | 59 |
| Sex | M |
| Aetiology | ICM |
| LVEF | 30% |
| Rhythm | SR |
| QRS morphology | LBBB |
| QRS width | 160 |
| PR interval | 172 |
MRI demonstrated full thickness infarct in the mid anterior wall and anterio/infero‐septum and apex, which corresponded to areas of low voltage on the ECGi voltage map, see Figure 1.
3.6.1. Electrical effect of MPP
ECGi analysis of the intrinsic rhythm confirms activation is again delayed in the lateral LV wall (see Figure 5). The apical region also shows persistent delayed LV activation, which is in keeping with the CMR and voltage maps findings suggestive of apical scarring. Nominal BiV CRT achieved an improvement in electrical activation with reductions in both LVtat and VVtat and greater electrical resynchronization. In this case, AV optimization, VV optimization, and local bipolar MPP programming do not appear to confer any benefit over nominal BiV CRT. However, optimal vector selection using extended bipolar MPP results in more rapid activation of the left ventricle as demonstrated by the larger area of depolarized myocardium and reduction in delayed activation (blue) at the apex on the ECGi maps. These changes were associated with significant reductions in both LV and BiV activation time. In this example, Extended Bipolar MPP appears to offer a superior degree of resynchronization to conventional CRT.
Figure 5.
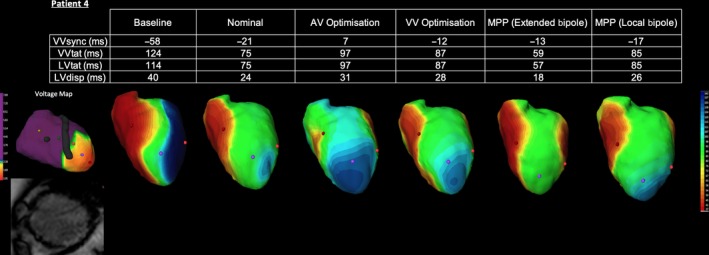
Electrocardiographic activation metrics and directional activation maps during device optimization of Patient 4. A voltage map thresholded to 2mv is shown with a still from the CMR short axis stack (SAX)
3.7. Case 5
| Age | 83 |
| Sex | F |
| Aetiology | NICM |
| LVEF | 35% |
| Rhythm | AF |
| QRS morphology | RV paced |
| QRS width | 174 |
| PR interval | N/A |
The patient had long‐standing AF and had undergone implantation of a VVI pacing system in conjunction with an AV junction ablation.
3.7.1. Electrical effect of MPP
Baseline activation in this case demonstrates the pattern of activation typically associated with RV apical pacing (see Figure 6). The dark blue isochrones on the directional activation map denote an area of late activation in the posterolateral wall. Conventional CRT pacing with nominal settings achieves a dramatic improvement. Biventricular electrical resynchronization is almost entirely restored and both LV and BiV activation times are shorted. ECGi mapping now shows a widespread area of early activation occurring in the previously delayed posterolateral area. This is consistent with LV activation from a LV lead placed in a posterolateral tributary of the coronary sinus. Due to the patients underlying AF, no AV optimization has been attempted; however, VV optimization in this patient confers no obvious advantage. Both extended Bipolar MPP and Local Bipolar MPP result in a much larger area of early myocardial activation and an ensuing reduction in the LV and BiV activation times is apparent. In this case, the ability of MPP to more rapidly capture a greater area of the ventricle could potentially lead to further improvement and greater remodeling.
Figure 6.
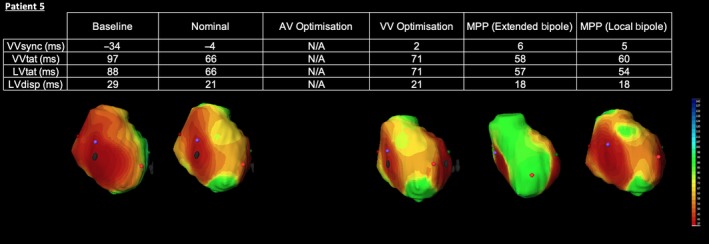
Electrocardiographic activation metrics and directional activation maps during device optimization of Patient 5
4. DISCUSSION
Our series demonstrates that non‐invasive mapping technology is able to accurately delineate the divergent electrical effects of programming optimization. Evaluation of the 12 lead ECG alone during biventricular pacing is frequently challenging due to the merging wave fronts16 and provides only a general overview of ventricular electrical activation.11 ECGi activation maps were clearly able to discern changes in bulk LV activation during differing MPP programming. In addition, voltage mapping was able to delineate areas of fibrotic tissue with showed good correlation with areas of scar defined using LGE CMR. Some degree of discrepancy between these modalities was expected as CMR can struggle to detect areas of homogenous microscopic diffuse fibrosis due to the low resolution of the image. In addition, ECGi is more sensitive at detecting zones of epicardial and transmural fibrosis but may not always be able to detect sub‐endocardial scar.
CRT delivered with nominal settings always proved superior to baseline activation and this improvement may explain why the majority of patients who receive CRT improve, without undergoing programming optimization. In three of our cohorts, the degree of biventricular resynchronization was further enhanced with iterative echo guided optimization of the AV and VV intervals. Whilst there is evidence to support this strategy,14, 15, 17 larger studies have failed to consistently prove it's efficacy.18, 19 Our results would suggest that optimization of the AV and VV intervals may indeed result in more effective resynchronization than can be achieved using nominal settings in a proportion of patients, but not all. This may go some way to explaining the equivocal data surrounding AV/VV programming optimization. Crucially, ECGi was able to detect subtle changes in activation during AV & VV programming optimization.
The effects of utilizing MPP were idiosyncratic and highly patient specific. In 2 of the 5 subjects (patients 4 and 5) Extended Bipolar and Local Bipolar MPP resulted in more rapid activation of the left ventricle compared to optimized echo optimized CRT. It is possible accelerating LV activation has the potential to improve the degree of cardiac resynchronization and as such, may explain the greater hemodynamic improvements20 and enhanced response rate21 observed during early studies of MPP stimulation.
The remaining 3 patients showed evidence of extensive LV scarring which was well visualized using both CMR & ECGi voltage mapping. The use of MPP in this cohort did not appear to acutely improve electrical resynchronization. In part, this may be explained by the focal nature of the scar burden,22 which can disrupt the efficacy of resynchronization pacing, especially when concentrated lateral or posterolateral walls.23 Our preliminary findings appear to confirm the hypothesis that MPP may be able to acutely improve electrical resynchronization in selected patients but the presence of extensive scar may preclude response irrespective of the stimulation strategy. Nevertheless, the Cardioinsight ™ ECGi system represents a non‐invasive technique capable of assessing the acute response to MPP and may be of use in identifying patients likely to gain the most from MPP as well as how best to configure this multi‐polar pacing.
5. LIMITATIONS
This is a small study and the results are hypothesis generating rather than conclusive. Our primary objective was to analyze how myocardial activation varied during device programming optimization and whether non‐invasive body surface mapping technology might be capable of identifying these changes in activation. Our hypothesis did not extend to evaluating rates of response to CRT and given the small number of patients, it would be impossible to draw reliable conclusions. Instead, non‐invasive electrical measurements were analyzed acutely and it is unclear whether these results can be extrapolated to the chronic delivery of CRT. Biventricular pacing which improves the degree of biventricular electrical resynchronization has been associated greater response.11, 24, 25, 26 However, non‐response to CRT is a multifactorial issue requiring a comprehensive assessment of the various pre‐implant, peri‐implant, and post implant factors.27 As such, improvement in clinical status is not a direct corollary of programming optimization even when this yields a superior degree of electrical resynchronization.
6. CONCLUSIONS
Iterative echo guided AV & VV optimization & quadripolar lead technology in conjunction with MPP are important clinical adjuncts to CRT pacing. The range of programming options provide greater cost efficiency by reducing the need for reintervention after implantation for technical issues including high capture thresholds, lead displacement, and phrenic nerve stimulation.28 Our analysis with ECGi mapping confirms that this tool is capable of detecting subtle changes in activation pattern achieved using different device optimization strategies. Furthermore, our findings suggest that traditional CRT with nominal settings is able to largely restore biventricular electrical synchronicity in selected patients and may explain the consistent response rate of 50%‐70% to conventional CRT.
Amongst carefully selected patients; however, the use of optimal device programming can achieve a superior degree of electrical resynchronization when compared to conventional CRT with nominal settings. However, these strategies are not without cost. Echo guided device optimization can be expensive and time consuming29 whilst MPP is associated with a reduction in battery longevity.30 Neither strategy has been consistently shown to be superior to conventional CRT with nominal settings in large multicenter studies.7, 8, 18, 19
Our analysis suggests that judicious use of device reprogramming optimization may be a useful strategy; however, the main issue remains identifying which patients may require this approach and then successfully optimizing their programming to achieve optimal electrical resynchronization. ECGi is a non‐invasive technique capable of accurately delineating the electrical effects of CRT pacing as well as the presence and distribution of myocardial scar. Our work suggests a potential a role for this tool in the optimization of non‐responders to CRT, as it allows the fusion of activation maps and scar analysis above and beyond interrogation of the 12 lead ECG.
CONFLICTS OF INTERESTS
Authors declare no conflict of interests for this article.
ACKNOWLEDGEMENTS
Dr Benjamin Sieniewicz & Dr Thomas Jackson jointly share first authorship of this manuscript. The authors would like to acknowledge the contribution of Dr Maria Panayiotou, Dr Rashed Karim, Mr Daniel Toth, Dr Joshua Blower, and Dr Peter Mountney who contributed to preparation and analysis of CMR and ECGi data allowing the comparison of these two modalities.
Sieniewicz BJ, Jackson T, Claridge S, et al. Optimization of CRT programming using non‐invasive electrocardiographic imaging to assess the acute electrical effects of multipoint pacing. J Arrhythmia. 2019;35:267–275. 10.1002/joa3.12153
Funding information
JG and BP have received fellowship funding from Abbott outside of the submitted work. BSS has received fellowship funding from Medtronic outside of the submitted work. CAR receives research funding and/or consultation fees from Abbott, Medtronic, Boston Scientific, Spectranetics and LivaNova outside of the submitted work. CY is employed by CARDIOINSIGHT Technologies. BS is supported by a BHF Project Grant.
REFERENCES
- 1. Nelson GS, Berger RD, Fetics BJ, et al. Left ventricular or biventricular pacing improves cardiac function at diminished energy cost in patients with dilated cardiomyopathy and left bundle‐branch block. Circulation. 2000;102(25):3053–3059. [DOI] [PubMed] [Google Scholar]
- 2. Pappone C, Ćalović Ž, Vicedomini G, et al. Multipoint left ventricular pacing improves acute hemodynamic response assessed with pressure‐volume loops in cardiac resynchronization therapy patients. Hear Rhythm. 2014;11(3):394–401. [DOI] [PubMed] [Google Scholar]
- 3. Rinaldi CA, Leclercq C, Kranig W, et al. Improvement in acute contractility and hemodynamics with multipoint pacing via a left ventricular quadripolar pacing lead. J Interv Card Electrophysiol. 2014;40(1):75–80. [DOI] [PubMed] [Google Scholar]
- 4. Forleo GB, Santini L, Giammaria M, et al. Multipoint pacing via a quadripolar left‐ventricular lead: preliminary results from the Italian registry on multipoint left‐ventricular pacing in cardiac resynchronization therapy (IRON‐MPP). Europace. 2017;19(7):1170–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Menardi E, Ballari GP, Goletto C, Rossetti G, Vado A. Characterization of ventricular activation pattern and acute hemodynamics during multipoint left ventricular pacing. Hear Rhythm. 2015;12(8):1762–1769. [DOI] [PubMed] [Google Scholar]
- 6. Umar F, Taylor RJ, Stegemann B, et al. Haemodynamic effects of cardiac resynchronization therapy using single‐vein, three‐pole, multipoint left ventricular pacing in patients with ischaemic cardiomyopathy and a left ventricular free wall scar: the MAESTRO study. Europace. 2016;18(8):1227–1234. [DOI] [PubMed] [Google Scholar]
- 7. van Gelder BM, Bracke FA. Acute hemodynamic effects of single‐ and dual‐site left ventricular pacing employing a dual cathodal coronary sinus lead. Pacing Clin Electrophysiol. 2015;38(5):558–564. [DOI] [PubMed] [Google Scholar]
- 8. Padeletti L, Colella A, Michelucci A, et al. Dual‐site left ventricular cardiac resynchronization therapy. Am J Cardiol. 2008;102(12):1687–1692. [DOI] [PubMed] [Google Scholar]
- 9. European Society of Cardiology (ESC) , European Heart Rhythm Association (EHRA) , Brignole M, et al. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association. Europace. 2013;15(8):1070–1118. [DOI] [PubMed] [Google Scholar]
- 10. Russo AM, Stainback RF, Bailey SR, et al. ACCF/HRS/AHA/ASE/HFSA/SCAI/SCCT/SCMR 2013 appropriate use criteria for implantable cardioverter‐defibrillators and cardiac resynchronization therapy. J Am Coll Cardiol. 2013;61(12):1318–1368. 10.1016/j.jacc.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 11. Ploux S, Lumens J, Whinnett Z, et al. Noninvasive electrocardiographic mapping to improve patient selection for cardiac resynchronization therapy: beyond QRS duration and left bundle branch block morphology. J Am Coll Cardiol. 2013;61(24):2435–2443. [DOI] [PubMed] [Google Scholar]
- 12. Dubois R, Labarthe S, Coudière Y, Hocini M, Haïssaguerre M. Global and directional activation maps for cardiac mapping in electrophysiology. Comput Cardiol. 2012;39:349–352. [Google Scholar]
- 13. Jia P, Ramanathan C, Ghanem RN, Ryu K, Varma N, Rudy Y. Electrocardiographic imaging of cardiac resynchronization therapy in heart failure: observation of variable electrophysiologic responses. Hear Rhythm. 2006;3(3):296–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brabham WW, Gold MR. The role of AV and VV optimization for CRT. J Arrhythmia. 2013;29(3):153–161. 10.1016/j.joa.2013.02.001. [DOI] [Google Scholar]
- 15. Gorcsan J, Abraham T, Agler DA, et al. Echocardiography for cardiac resynchronization therapy: recommendations for performance and reporting‐a report from the American Society of Echocardiography Dyssynchrony Writing Group Endorsed by the Heart Rhythm Society. J Am Soc Echocardiogr. 2008;21(3):191–213. [DOI] [PubMed] [Google Scholar]
- 16. van Stipdonk A, Wijers S, Meine M, Vernooy K. ECG patterns in cardiac resynchronization therapy. J Atr Fibrillation. 2015;7(6):33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Spartalis M, Tzatzaki E, Spartalis E, et al. The role of echocardiography in the optimization of cardiac resynchronization therapy: current evidence and future perspectives. Open Cardiovasc Med J. 2017;11(1):133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ellenbogen KA, Gold MR, Meyer TE, et al. Primary results from the smartdelay determined AV optimization: a comparison to other AV delay methods used in cardiac resynchronization therapy (SMART‐AV) trial: a randomized trial comparing empirical, echocardiography‐ guided, and algorithmic atrioventr. Circulation. 2010;122(25):2660–2668. [DOI] [PubMed] [Google Scholar]
- 19. Kedia N, Ng K, Apperson‐Hansen C, et al. Usefulness of atrioventricular delay optimization using doppler assessment of mitral inflow in patients undergoing cardiac resynchronization therapy. Am J Cardiol. 2006;98(6):780–785. [DOI] [PubMed] [Google Scholar]
- 20. Zanon F, Baracca E, Pastore G, et al. Multipoint pacing by a left ventricular quadripolar lead improves the acute hemodynamic response to CRT compared with conventional biventricular pacing at any site. Hear Rhythm. 2015;12(5):975–981. 10.1016/j.hrthm.2015.01.034. [DOI] [PubMed] [Google Scholar]
- 21. Pappone C, Ćalović Ž, Vicedomini G, et al. Improving cardiac resynchronization therapy response with multipoint left ventricular pacing: twelve‐month follow‐up study. Hear Rhythm. 2015;12(6):1250–1258. [DOI] [PubMed] [Google Scholar]
- 22. Chen Z, Sohal M, Sammut E, et al. Focal but not diffuse myocardial fibrosis burden quantification using cardiac magnetic resonance imaging predicts left ventricular reverse modeling following cardiac resynchronization therapy. J Cardiovasc Electrophysiol. 2016;27(2):203–209. 10.1111/jce.12855. [DOI] [PubMed] [Google Scholar]
- 23. Birnie D, DeKemp RA, Ruddy TD, et al. Effect of lateral wall scar on reverse remodeling with cardiac resynchronization therapy. Hear Rhythm. 2009;6(12):1721–1726. [DOI] [PubMed] [Google Scholar]
- 24. Hsing JM, Selzman KA, Leclercq C, et al. Paced left ventricular QRS width and ECG parameters predict outcomes after cardiac resynchronization therapy PROSPECT‐ECG substudy. Circ Arrhythmia Electrophysiol. 2011;4(6):851–857. [DOI] [PubMed] [Google Scholar]
- 25. Bonakdar HR, Jorat MV, Fazelifar AF, et al. Prediction of response to cardiac resynchronization therapy using simple electrocardiographic and echocardiographic tools. Europace. 2009;11(10):1330–1337. [DOI] [PubMed] [Google Scholar]
- 26. Lecoq G, Leclercq C, Leray E, et al. Clinical and electrocardiographic predictors of a positive response to cardiac resynchronization therapy in advanced heart failure. Eur Heart J. 2005;26(11):1094–1100. [DOI] [PubMed] [Google Scholar]
- 27. Sieniewicz BJ, Gould J, Porter B, et al. Understanding non‐response to cardiac resynchronisation therapy: common problems and potential solutions. Heart Fail Rev. 2018. [Epub ahead of print]. 10.1007/s10741-018-9734-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Behar JM, Bostock J, Zhu Li AP, et al. Cardiac resynchronization therapy delivered via a multipolar left ventricular lead is associated with reduced mortality and elimination of phrenic nerve stimulation: long‐term follow‐up from a multicenter registry. J Cardiovasc Electrophysiol. 2015;26(5):540–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stanton T, Hawkins NM, Hogg KJ, Goodfield NER, Petrie MC, McMurray JJV. How should we optimize cardiac resynchronization therapy? Eur Heart J. 2008;29:2458–2472. [DOI] [PubMed] [Google Scholar]
- 30. Akerström F, Narváez I, Puchol A, et al. Estimation of the effects of multipoint pacing on battery longevity in routine clinical practice. Europace. 2018;20(7):1161–1167. [DOI] [PubMed] [Google Scholar]


