Abstract
Background
Remote magnetic navigation (RMN) is often used in combination with a 3‐dimensional mapping system to perform catheter ablations. This study aim to investigate the feasibility and effectiveness of a novel 3D‐mapping system, EnSite Precision, combined with RMN for catheter ablation of premature ventricular contractions (PVCs), and compared it to the procedures performed by CARTO3 with RMN.
Methods
Forty‐three consecutive PVC patients were either ablated with the guidance of EnSite Precision (n = 22) or CARTO (n = 21) navigated by RMN. Procedure‐related details, acute and long‐term success were assessed.
Results
Patient characteristics between both the groups were similar (age: 47.1 ± 19.8 vs 47.1 ± 12.7, female: 63.6% vs 57.1%). No significant difference was found in the procedure time (99.5 ± 30.4 vs 92.9 ± 24.8 min, P = 0.436), mapping time (18.6 ± 12.8 vs 15.5 ± 10.2 min, P = 0.390), radiofrequency ablation time (333.4 ± 267.0 vs 469.3 ± 343.1 s, P = 0.154), fluoroscopy time (4.0 ± 1.9 vs 3.8 ± 2.0 min, P = 0.635), and X‐ray dose (1.8 ± 1.4 vs 2.0 ± 1.2 Gycm2, P = 0.649) between the two groups. No significant procedural complication occurred in either group. In addition, there was no significant differences regarding the acute success rate (90.9% vs 90.5%, P = 0.961) and long‐term success rate (86.4% vs 81.0%, P = 0.631) after 16.2 ± 6.2 months of follow‐up between the two groups.
Conclusions
RMN combined with EnSite Precision mapping system is effective and safe for catheter ablation of PVCs.
Keywords: CARTO, EnSite Precision, premature ventricular contractions, remote magnetic navigation
1. INTRODUCTION
Frequent premature ventricular contractions (PVCs) may be highly symptomatic leading to a significantly reduced quality‐of‐life, potentially contributing to congestive heart failure and increased mortality.1, 2 PVCs occur commonly in patients without structural heart disease, but can also be seen in patients with structural heart disease.3, 4 Several studies demonstrated a significant improvement in quality‐of‐life and left ventricular function after successful ablation of PVCs compared to patients without ablation.5, 6 The use of a three‐dimensional (3D) mapping system is needed for a successful ablation of PVCs, with the CARTO (Biosense Webster, Inc., CA, USA) mapping system being the most used system with excellent performance.7, 8 The downside, however, is that only the diagnostic and ablation catheters from the same manufacturer can be used with the CARTO system. The EnSite cardiac mapping system (St Jude Medical, Inc., Minnesota, USA), another 3D mapping system with an open‐platform, allows for visualization of multiple catheters from different manufacturers while simultaneously collecting anatomical and electrophysiological data from all electrodes of any catheter.9 Recent reports utilizing Ensite for PVC ablation demonstrated high success with very limited fluoroscopy.7, 10 However, reports of dislocation of the reference catheter may lead to uncorrectable map shifts using this mapping system, which then manually needs to be adjusted.9 The next generation of this system, EnSite Precision, was recently released, which combined both impedance and magnetic technologies with much more precision and improved stability.11 However, the performance of this new system on ventricular arrhythmia ablation is still unknown.
Remote magnetic navigation (RMN) has emerged as a modern technology for treating most arrhythmias because of its precise control of catheter movement with improved catheter stability and safety.12, 13 It particularly adapts to the treatment of ventricular arrhythmias and exhibits better efficacy compared to the manual technique.14, 15 In order for the RMN system to perform ablation procedures, it needs to be integrated with a 3D mapping.12 Currently, only CARTO mapping system is integrated with RMN, but not the EnSite system.13 The EnSite 3D mapping system has demonstrated safe and effective ablations of ventricular arrhythmias with low fluoroscopy exposure when performed with manual techniques.16 Furthermore, the new features of EnSite Precision offer convenience to the operator by allowing them to map and locate the origins of even the most difficult ventricular arrhythmias. However, the collaboration of EnSite Precision with RMN on ventricular arrhythmia ablation has not been investigated.
The purpose of this study was to determine the acute procedural efficacy, safety and long‐term outcome utilizing the Ensite Precision Mapping system with RMN during PVC ablation procedures.
2. METHODS
2.1. Study population
In this retrospective study, forty‐three consecutive symptomatic patients with ectopic ventricular arrhythmias targeted for ablation were included between January 2016 and January 2018. Twenty‐two patients underwent ablation using EnSite Precision mapping system (Inquiry, St Jude Medical, Inc.; Precision group) and another 21 patients used CARTO®3 mapping system (Biosense Webster, Inc.; CARTO group). All the patients signed informed consent prior to the ablation procedure. No patient had previously undergone an ablation and patients with polymorphic PVCs, coronary artery disease, valvular heart disease, dilated cardiomyopathy or congenital heart disease were excluded from this study. All antiarrhythmic agents were withheld for at least five drug half‐lives before the procedure. Preprocedural examination included a baseline 12‐lead surface ECG and transthoracic echocardiography. In Denmark (and many other countries), a study comparing two routine methods used in the clinic, as two 3D mapping systems with identical ablation strategies in this study, does not require approval from the local ethical committee.
2.2. Procedural preparation
After femoral vein puncture, a 6F steerable catheter (Inquiry, St Jude Medical, Inc.) and a 5F quadripolar catheter (Medtronic, Inc., Minnesota, USA) were placed in the coronary sinus and in the apex of the right ventricle, respectively. Transseptal puncture was performed under hemodynamic pressure and fluoroscopic monitoring when PVC locations were considered in the left ventricle. Surface ECG and endocardial electrograms were continuously monitored and recorded with EP tracer (Schwarzer Cardiotek, Inc., Heilbronn, Germany). If there were no spontaneous or few PVCs, isoproterenol infusion (1‐4 μg/min) was administered in an attempt to induce the PVCs. A single bolus of 50‐100 IU/kg body weight of heparin was administrated if mapping and ablation were necessary during the procedure.
2.3. Use of mapping systems with RMN
RMN was applied to all the procedures in this study, including first‐time procedures and redo procedures. Cardiac mapping system, X‐Ray, electrophysiological recording system and RMN (Stereotaxis, Inc., MO, USA) were gathered on one monitor by Odyssey Vision™ system (Stereotaxis, Inc.). As the CARTO mapping system is integrated with the RMN system, the magnetic vector could be changed in CARTO interface to navigate the directional orientation of catheter (Figure 1A). While EnSite Precision mapping system was not integrated with the RMN system, the magnetic vector could only be operated in RMN system (Figure 1B). A catheter‐advancing system (Cardiodrive®, Stereotaxis Inc) was used to control remote catheter advancement and retraction in the control room.
Figure 1.
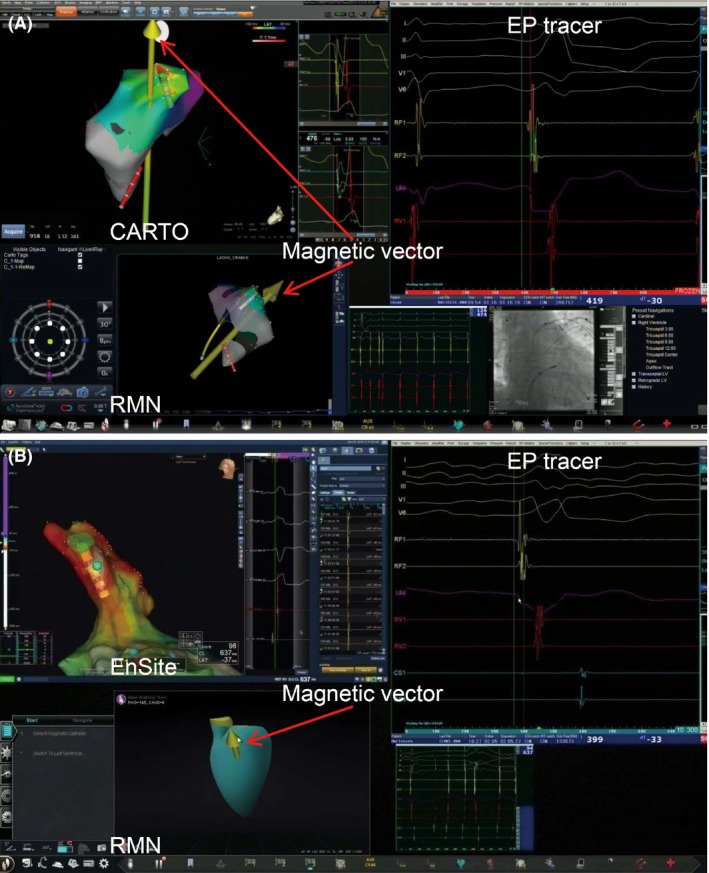
The interface of Odyssey system. (A) CARTO mapping system and electrophysiological recording system were integrated with RMN by Odyssey system. The magnetic vector could be seen and moved in both CARTO and RMN system. (B) EnSite Precision mapping system, electrophysiological recording system and RMN were shown on the interface of Odyssey system. The magnetic vector could be only seen and moved in RMN system.
2.4. Mapping acquisition
In the Precision group, the target chamber anatomic mapping was first acquired with a circular magnetic sensor mapping catheter (Advisor FL, Sensor Enabled; St. Jude Medical, Inc.) manually for nine cases, then the catheter was replaced by the open‐irrigated magnetic catheter (Celsius® THERMOCOOL® RMT, Biosense Webster, Inc.) for precise mapping and ablation navigated by Niobe ES system (Stereotaxis, Inc.). For the remaining 13 cases, both anatomic mapping and activation mapping of PVCs were performed with open‐irrigated magnetic catheter directly. To acquire precise activation mapping, Automap, the new feature of EnSite Precision was applied. A Clinical PVC template was created before the placement of catheters. After Automap initiation, the system compared every beat with the template using 12‐lead ECG morphology and a score was calculated. The point was automatically acquired if the score was >93%. When navigating the RMT catheter using RMN, an activation mapping was obtained automatically. If the activation map was inaccurate, TurboMap was performed to acquire a more precise activation map using new AutoMap settings (Figure 2A‐C).
Figure 2.
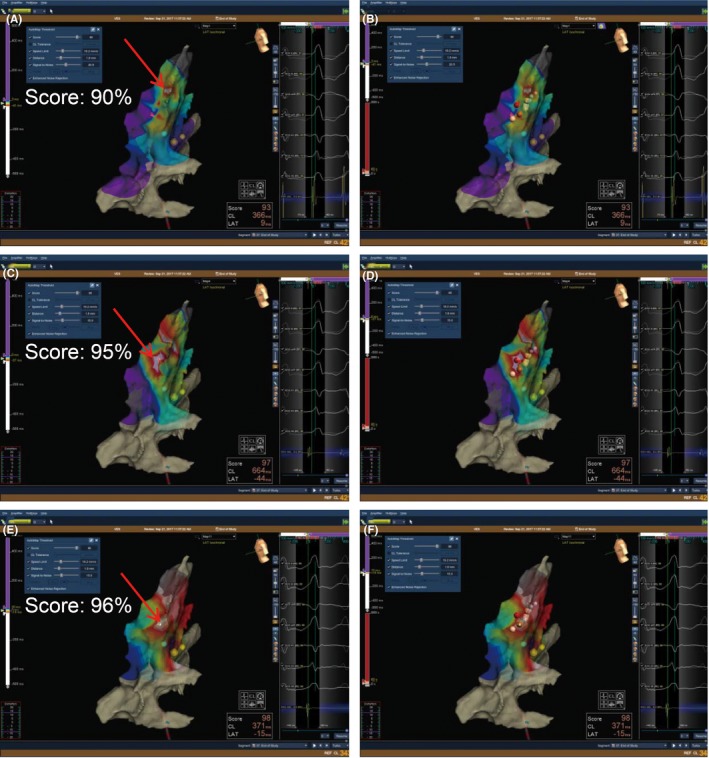
The acquisition of new activation map with TurboMap. (A) In the procedure, clinical PVC was set as template and AutoMap was performed to acquire an activation map with morphological score of 90%. This activation map was merged with final ablation points (B). (C) During the procedure, the activation map was precisely correlated with electrophysiological recording system, a new activation map was performed by AutoMap using those existed points with morphological score of 95% in a 10‐time fast speed (TurboMap).(D) The ablation points correlated with the activation map with morphological score of 95%. (E) After ablation, TurboMap was performed to acquire another activation map using those existed points with morphological score of 96%. (F) The ablation points correlated with the activation map more precisely with morphological score of 96%
In CARTO group, a 3.5‐mm open‐irrigated magnetic catheter (Navistar® THERMOCOOL® RMT, Biosense Webster, Inc.) was introduced into the target ventricular cavity for both mapping and ablation. By navigating the RMT catheter using RMN, anatomic and activation map was acquired simultaneously with point‐by‐point pattern. Clinical PVCs were identified manually to obtain precise activation map. If limited PVCs were noted, Paso mapping was applied to approximate location of PVCs, followed by precise localization of PVC through detailed mapping of this area. A complete detailed map of the entire target ventricle was not necessary.
2.5. Ablation procedure
Ablation was initiated at the earliest activation sites if the following criteria were met: local activation of clinical PVC was at least 25 ms pre‐QRS, the first deflection of bipolar electrograms was negative and virtual unipolar electrograms with QS morphology. Radiofrequency (RF) energy was delivered in the temperature control mode with a target tip temperature of less than 43°. During the ablation, power output was limited between 30‐40 W and an irrigation rate of 10‐30 mL/min. If the PVCs were not affected within the first 15 s, energy delivery was discontinued; otherwise, 60‐180 s of RF energy was delivered. Acute procedural success was defined as no spontaneous PVC and noninducible PVCs with infusion of isoproterenol 15 min after ablation. The whole procedure was recorded by Odyssey Cinema™ system (Stereotaxis, Inc.) to allow for review after the procedure.
2.6. Procedural characteristics measurements
The procedure time was calculated from the vein puncture to catheter withdrawal. Mapping time was calculated from the start of mapping to the start of the first RF ablation. RF ablation time and fluoroscopy time were acquired from the EP tracer and X‐Ray machine records, respectively.
2.7. Follow‐up
All patients were routinely evaluated in our outpatient clinic at 3rd months after discharge, then every 6 month by their home doctor or referred hospitals. Patients were instructed to go to an outpatient clinic if they had any symptomatic palpitations where twelve‐lead ECGs or Holter recordings could be performed. Recurrence was defined as symptomatic and/or asymptomatic PVCs confirmed by ECG or Holter recordings. Long‐term success was defined as no PVC recurrence. A second procedure was recommended if symptomatic recurrences were documented.
2.8. Complications
Complications were divided into two categories: major and minor. Major complications consisted of acute myocardial infarction, stroke, major bleeding, and cardiac tamponade. Minor complications included pericarditis and inguinal hematoma.
2.9. Statistical analysis
Variables are expressed as mean ± SD. Comparisons were performed between the two groups. Chi‐square was used for nominal variables and Student's t test was used for comparison of continuous variables, where appropriate. Levene's test was used to check the homogeneity of variance. Equivalent nonparametric tests were used when the Kolmogorov‐Smirnov test was in favor of absence of normal distribution. All tests were performed with a two‐tailed significance level of 0.05. SPSS 17.0 was used for data analysis.
3. RESULTS
3.1. Baseline characteristics of patients
Population characteristics are detailed in Table 1. The mean age of patients was 47.1 ± 16.5 years. The proportion of female was 60.5%. There were four patients in the Precision group and five patients in the CARTO group with hypertension. The left ventricular ejection fraction was normal in all the patients. Three patients in Precision group and five patients in the CARTO group were documented with nonsustained ventricular tachycardia. One patient in the CARTO group was implanted with an ICD before the procedure because of sudden cardiac death with monomorphic PVC‐induced ventricular fibrillation. Overall, no significant differences were observed between the two groups.
Table 1.
Baseline characteristics of patients
| Parameters | Precision (n = 22) | CARTO (n = 21) | Total | P‐value |
|---|---|---|---|---|
| Age, years | 47.1 ± 19.8 | 47.1 ± 12.7 | 47.1 ± 16.5 | 0.421 |
| Gender, female (%) | 14 (63.6) | 12 (57.1) | 26 (60.5) | 0.663 |
| HP (%) | 4 (18.2) | 5 (23.8) | 9 (20.9) | 0.650 |
| LVEF | 62.1 ± 4.7 | 63.6 ± 3.6 | 62.8 ± 4.2 | 0.879 |
| PVC with VT (%) | 3 (13.6) | 5 (23.8) | 8 (18.6) | 0.391 |
| ICD (%) | 0 (0.0) | 1(4.8) | 1 (2.3) | 0.300 |
HP, hypertension; LVEF, left ventricular ejection fraction; PVC, premature ventricular contracts; VT, ventricular tachycardia; ICD, implantable cardioverter‐defibrillator.
3.2. Procedural characteristics
Procedural data are detailed in Table 2. No significant difference was found in the procedure time 99.5 ± 30.4 vs 92.9 ± 24.8 min, P = 0.436), mapping time (18.6 ± 12.8 vs 15.5 ± 10.2 min, P = 0.390), RF ablation time (333.4 ± 267.0 vs 469.3 ± 343.1 s, P = 0.154), fluoroscopy time (4.0 ± 1.9 vs 3.8 ± 2.0 min, P = 0.635), and X‐ray dose (1.8 ± 1.4 vs 2.0 ± 1.2 Gycm2, P = 0.649) when comparing Precision to CARTO. No major procedural complication occurred in either group. One minor groin hematoma complication occurred in the Precision group.
Table 2.
Procedural results
| Parameters | Precision (n = 22) | CARTO (n = 21) | Total (n = 43) | P‐value |
|---|---|---|---|---|
| Procedure time (min) | 99.5 ± 30.4 | 92.9 ± 24.8 | 96.3 ± 27.7 | 0.436 |
| Mapping time (min) | 18.6 ± 12.8 | 15.5 ± 10.2 | 17.1 ± 11.5 | 0.390 |
| RF ablation time (s) | 333.4 ± 267.0 | 469.3 ± 343.1 | 399.8 ± 310.5 | 0.154 |
| Fluoroscopy time (min) | 4.0 ± 1.9 | 3.8 ± 2.0 | 3.9 ± 1.9 | 0.635 |
| Radiation dose (Gycm2) | 1.8 ± 1.4 | 2.0 ± 1.2 | 1.9 ± 1.3 | 0.649 |
| Complication (Major) | 0 | 0 | 0 | – |
| Complication (Minor) | 1 | 0 | 1 | 0.323 |
3.3. Location of ablation target
The earliest activation location of PVCs was identified in all the cases and the origins of PVCs are listed in Table 3. In Precision group, 12 foci originated from right ventricular outflow tract (RVOT), including eight foci in the RVOT septum and four foci in the RVOT free wall. Six foci originated from the left ventricular outflow tract (LVOT) with four from the LVOT septum and two from aortomitral continuity. Other four total foci originated from the LV mitral annulus, the LV anterior papillary muscle, the left anterior fascicular and the LV free wall, respectively. In CARTO group, 12 foci were localized at RVOT, including 10 foci in the RVOT septum and two foci in the RVOT free wall. Five foci originated from the LVOT with four from the LVOT septum and one from aortomitral continuity. One focus originated from RV fascicular, two foci were from the left posterior fascicular and one focus was from the left coronary artery. Distributions of PVCs origins were not significantly different between the two groups (P > 0.05).
Table 3.
Characteristics of target location
| Location | Precision (n = 22) | CARTO (n = 21) | Total (n = 43) | P‐value | |
|---|---|---|---|---|---|
| VOT | RVOT | 12 (54.5) | 12 (57.1) | 24 (55.8) | 0.864 |
| LVOT | 6 (27.3) | 5 (23.8) | 11 (25.6) | 0.795 | |
| RV | RVOT septum | 8 (36.4) | 10 (47.6) | 18 (41.9) | 0.455 |
| RVOT free wall | 4 (18.2) | 2 (9.5) | 6 (14.0) | 0.413 | |
| RV fascicular | 0 (0.0) | 1 (4.8) | 1 (2.3) | 0.300 | |
| RV MA | 1 (4.5) | 0 (0.0) | 1 (2.3) | 0.323 | |
| LV | LVOT septum | 4 (18.2) | 4 (19.0) | 8 (18.6) | 0.942 |
| LVOT AMC | 2 (9.1) | 1 (4.8) | 3 (7.0) | 0.578 | |
| Anterior papillary muscle | 1 (4.5) | 0 (0.0) | 1 (2.3) | 0.323 | |
| LAF | 1 (4.5) | 0 (0.0) | 1 (2.3) | 0.323 | |
| LPF | 0 (0.0) | 2 (9.5) | 2 (4.7) | 0.138 | |
| Left main trunk | 0 (0.0) | 1 (4.8) | 1 (2.3) | 0.300 | |
| Free wall | 1 (4.5) | 0 (0.0) | 1 (2.3) | 0.323 |
VOT, ventricular outflow tract; RV, right ventricle; LV, left ventricle; MA, mitral annulus; AMC, aortomitral continuity; LAF, left anterior fascicular; LPF, left posterior fascicular.
3.4. Acute results and long‐term follow‐up
Acute results and long‐term follow‐up data are presented in Table 4. Acute ablation success was achieved in 20/22 (90.9%) patients in the Precision group and in 19/21 (90.5%) patients in the CARTO group, no statistically significance difference was found between the two groups. For the acute failed cases in the Precision group, the target in the first patient likely originated from the RVOT lateral epicardium, and was 53 ms earlier than the surface QRS, but RF ablation could not abolish the PVCs. The target in the second patient came from LV lateral wall, which might have also come from the epicardium source. Both of these patients refused the epicardial approach procedure. For the acute failed cases in the CARTO group, the target in the first patient originated from the RVOT septum with no effect by RF ablation, further investigation of the LVOT was warranted; however, the patient refused to continue the procedure. And this patient was re‐ablated later in the LVOT septum and obtained both acute and long‐term success with CARTO. In the second case, the earliest activation was found in the RVOT septum first, and an RF ablation was performed without effect. The LVOT was mapped and it was earlier than the RVOT but ablation still could not abolish the PVCs. After precise mapping, the focus was found in the main trunk of left coronary artery and ablation was not performed.
Table 4.
Acute results and long‐term follow‐up
| Parameters | Precision (n = 22) | CARTO (n = 21) | Total (n = 43) | P‐value |
|---|---|---|---|---|
| Acute success rate | 20 (90.9) | 19 (90.5) | 39 (90.7) | 0.961 |
| Follow‐up (month) | 16.0 ± 5.1 | 16.4 ± 7.4 | 16.2 ± 6.2 | 0.706 |
| Long‐term success | 19 (86.4) | 17 (81.0) | 36 (83.7) | 0.631 |
| Recurrences | 1 (4.5) | 2 (9.5) | 3 (7.0) | 0.522 |
After a mean follow‐up of 16.2 ± 6.2 months, the long‐term success rate demonstrated no significant difference between the two groups (Figure 3, P = 0.650). One patient in the Precision group and two patients in the CARTO group had recurrence of PVCs with no statistical significance (P = 0.522). In the Precision group, the patient had recurrence of PVCs from the same site and a repeat ablation had both acute and long‐term success. In the CARTO group, one patient with recurrence of PVCs scheduled for new ablation. Another patient with mono‐morphological PVCs induced idiopathic ventricular fibrillation had a PVC recurrence, was re‐ablated using EnSite Precision and achieved both acute and long‐term success, and an ICD was implanted before discharge.
Figure 3.
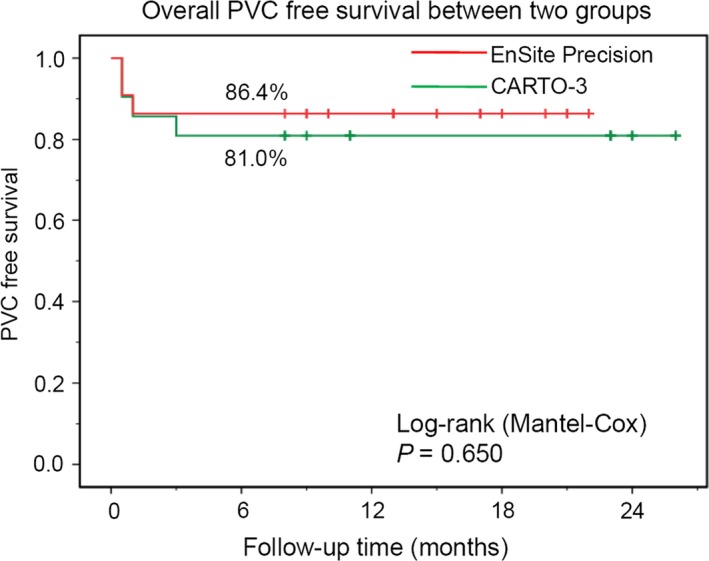
The overall PVCs free survival curve. Kaplan‐Meier estimates overall PVCs free survival rate between EnSite Precision mapping system and CARTO mapping system
4. DISCUSSION
To the best of our knowledge, this is the first study evaluating the feasibility, safety, acute efficacy, and long‐term outcome using the combination of EnSite Precision with RMN in PVC ablation. Our main finding is that even without direct integration between Precision and RMN, it was feasible to perform PVC ablation using these two systems with similar acute and long‐term success as using CARTO and RMN.
4.1. Generation of maps with EnSite Precision
The new‐generation mapping system, EnSite Precision System uniquely combines impedance and magnetic field hybrid technology, to enable navigation and tracking of mapping or ablation catheters during the procedure. When a catheter with magnetic sensor is introduced, the system dynamically optimizes the model by adjusting the dimensions of the navigation field between the position and orientation of magnetic sensors and electrodes. The combination of impedance field and magnetic field improved the stability and accuracy of location and navigation.11 In this study, for nine cases with PVCs from the outflow tract, the anatomical model of target cardiac chamber was performed manually with a circular mapping catheter and an anatomical model could be acquired in just a few minutes, suggesting that circular mapping catheter could be applied for outflow tract mapping. After acquisition of the anatomical model, activation map of the PVCs was performed with the ablation catheter navigated by RMN. The acute outcome and long‐term results between the circular catheter plus ablation catheter and ablation catheter only were investigated. As shown in Table S1, there was no significant differences regarding the acute success rate (88.9% vs 92.3%, P = 0.784) and long‐term success rate (88.9% vs 84.6%, P = 0.774) between the two groups.
By using the Automap module, clinical PVC was set as the template and the system correlated every beat with the template. When the score meets the criterion of the clinical PVC, the system collected the points automatically with no need of manual recognition. If the activation map was not correlated with the electrogram of local activation, a new activation map could be generated with the existing points by adjusting the collection criterion in a 10‐time fast speed, which is called TurboMap (Figure 2). For the cases with multimorphological PVCs, TurboMap could also be applied to locate the origin of PVC one by one without manually collecting new points. Therefore, this novel mapping system facilitates catheter ablation of PVCs, especially multimorphological PVCs.
4.2. Concurrent use of RMN and EnSite Precision
The Odyssey system combines the cardiac mapping system, X‐Ray, electrophysiological recording system and RMN on one monitor, which makes it possible to operate these four systems in this unique monitor. There are three differences between CARTO and EnSite Precision when used with RMN. First, as CARTO mapping system was integrated with RMN, the vector direction of magnetic field could be changed on the 3D model in CARTO system, which made the operation of vector direction very convenient. While EnSite Precision system is not integrated with RMN, the vector direction could only be moved in the RMN system but not in the EnSite Precision system. Second, the settings for CARTO could be done in the RMN system but not within the EnSite Precision system. Third, the 3Dmodels created by CARTO could be integrated with the fluoroscopy reference, which made it feasible for the physicians to know the anatomical location of the model in the fluoroscopy reference. However, the models obtained by EnSite Precision system could not be merged with the fluoroscopy reference.
Despite this, the mapping and ablation of PVCs using EnSite Precision and RMN were feasible and effective with the same procedural characteristics, acute and long‐term outcome. When Automap module was applied to perform an activation map with the preset template, it could recognize and map clinical PVCs automatically. The operator only need to click the mouse and move the magnetic vector, and then the activation map of the clinical PVCs could be acquired. Furthermore, TurboMap has significantly reduced the mapping time by automatically fast editing acquired maps.
4.3. Comparison of procedural data to previous studies
In this study, the procedural time and fluoroscopy time did not show a significant difference between the two groups. Parreira et al reported an average procedural time of more 200 min and mean fluoroscopy time of 10 ± 7.8 min using CARTO and RMN for PVC ablation.17 Zhang et al compared remote magnetic catheter control to manual catheter control for ablation of RVOT ventricular arrhythmias, with a reported mean procedural duration of 131.8 ± 19.4 min using RMN and 115.1 ± 27.4 min by manual (data not significantly different).18 Their mean fluoroscopy time for RMN was 5.2 ± 2.6 min and 10.5 ± 5.0 min for manual. The mean procedure duration of this current study was 96.3 ± 27.7 min, which might be shorter than previous studies using RMN but similar to manual studies.6, 17, 18 The fluoroscopy time of the current study was 3.9 ± 1.9 min, which was shorter or similar to other studies using RMN, but was significantly lower than manual studies.17, 18, 19 This suggests that RMN could reduce the fluoroscopy time to guide ablation of PVCs.
It was reported that the acute success rate varies between 66% and 100% and the long‐term success rate varies between 60% and 87% for PVC ablation, while in the current study the acute success rate was 90.7% and the long‐term success rate was 83.7% with no significant difference between the two groups, which suggests that the collaboration of EnSite Precision with Niobe system was as effective as the collaboration of CARTO3 with Niobe system.5, 6, 18, 19, 20
4.4. Limitations
Although this is currently the first study reporting data from PVC ablation using EnSite Precision combined with RMN, the patient numbers were relatively low hampering more complex analyses, and larger studies are needed. In addition, this study is limited by the inherent nature of a retrospective study and prospective studies are needed to confirm.
5. CONCLUSIONS
The current study demonstrates the first clinical experience reporting the utility of EnSite Precision and RMN on ventricular arrhythmia ablation. Our initial data demonstrate that the combination of EnSite Precision and RMN is safe and effective for PVC ablation with both high acute success rates and reasonable long‐term outcome. Further large‐scale prospective studies with focus on ventricular arrhythmia ablation procedures are warranted to determine a potential benefit for patients’ treatment.
CONFLICT OF INTEREST
The authors declare no conflict of interests for this article.
Supporting information
ACKNOWLEDGEMENTS
The authors wish to thank the technical staff of the Cardiac Catheterization Laboratory for their help. This work was supported by National Natural Science Foundation of China (81400297) and Jiangsu province Young Medical Talents (QNRC2016185).
Dang S, Jons C, Jacobsen PK, Pehrson S, Chen X. Feasibility of a novel mapping system combined with remote magnetic navigation for catheter ablation of premature ventricular contractions. J Arrhythmia. 2019;35:244–251. 10.1002/joa3.12157
REFERENCES
- 1. Sadron Blaye‐Felice M, Hamon D, Sacher F, et al. Premature ventricular contraction‐induced cardiomyopathy: related clinical and electrophysiologic parameters. Heart Rhythm. 2016;13:103–10. [DOI] [PubMed] [Google Scholar]
- 2. Lerman BB. Mechanism, diagnosis, and treatment of outflow tract tachycardia. Nat Rev Cardiol. 2015;12:597–608. [DOI] [PubMed] [Google Scholar]
- 3. Dukkipati SR, Choudry S, Koruth JS, Miller MA, Whang W, Reddy VY. Catheter ablation of ventricular tachycardia in structurally normal hearts: indications, strategies, and outcomes—Part I. J Am Coll Cardiol. 2017;70:2909–23. [DOI] [PubMed] [Google Scholar]
- 4. Dukkipati SR, Koruth JS, Choudry S, Miller MA, Whang W, Reddy VY. Catheter ablation of ventricular tachycardia in structural heart disease: indications, strategies, and outcomes—Part II. J Am Coll Cardiol. 2017;70:2924–41. [DOI] [PubMed] [Google Scholar]
- 5. Penela D, Fernandez‐Armenta J, Aguinaga L, et al. Clinical recognition of pure premature ventricular complex‐induced cardiomyopathy at presentation. Heart Rhythm. 2017;14:1864–70. [DOI] [PubMed] [Google Scholar]
- 6. Fichtner S, Senges J, Hochadel M, et al. Safety and efficacy in ablation of premature ventricular contraction: data from the German ablation registry. Clin Res Cardiol. 2017;106:49–57. [DOI] [PubMed] [Google Scholar]
- 7. Bharmanee A, Gowda S, Singh HR. Feasibility, accuracy, and safety of 3‐dimensional electroanatomic mapping without fluoroscopy in patients with congenital heart defects. Heart Rhythm. 2016;13:1667–73. [DOI] [PubMed] [Google Scholar]
- 8. Moak JP, Sumihara K, Swink J, Hanumanthaiah S, Berul CI. Ablation of the vanishing PVC, facilitated by quantitative morphology‐matching software. Pacing Clin Electrophysiol. 2017;40:1227–33. [DOI] [PubMed] [Google Scholar]
- 9. Nedios S, Sommer P, Bollmann A, Hindricks G. Advanced mapping systems to guide atrial fibrillation ablation: electrical information that matters. J Atr Fibrillation. 2016;8:1337–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Akdeniz C, Gul EE, Celik N, Karacan M, Tuzcu V. Catheter ablation of idiopathic right ventricular arrhythmias in children with limited fluoroscopy. J Interv Card Electrophysiol. 2016;46:355–60. [DOI] [PubMed] [Google Scholar]
- 11. Lin C, Pehrson S, Jacobsen PK, Chen X. Initial experience of a novel mapping system combined with remote magnetic navigation in the catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2017;35:244–6. [DOI] [PubMed] [Google Scholar]
- 12. Bradfield J, Tung R, Mandapati R, Boyle NG, Shivkumar K. Catheter ablation utilizing remote magnetic navigation: a review of applications and outcomes. Pacing Clin Electrophysiol. 2012;35:1021–34. [DOI] [PubMed] [Google Scholar]
- 13. Da Costa A, Lafond P, Romeyer‐Bouchard C, et al. Remote magnetic navigation and arrhythmia ablation. Arch Cardiovasc Dis. 2012;105:446–53. [DOI] [PubMed] [Google Scholar]
- 14. Aagaard P, Natale A, Di Biase L. Robotic navigation for catheter ablation: benefits and challenges. Expert Rev Med Devices. 2015;12:457–69. [DOI] [PubMed] [Google Scholar]
- 15. Ernst S. Catheter ablation: general principles and advances. Card Electrophysiol Clin. 2017;9:311–7. [DOI] [PubMed] [Google Scholar]
- 16. Ozyilmaz I, Ergul Y, Akdeniz C, Ozturk E, Tanidir IC, Tuzcu V. Catheter ablation of idiopathic ventricular tachycardia in children using the EnSite NavX system with/without fluoroscopy. Cardiol Young. 2014;24:886–92. [DOI] [PubMed] [Google Scholar]
- 17. Parreira L, Cavaco D, Reis‐Santos K, et al. Remote magnetic navigation for mapping and ablation of right and left ventricular outflow tract arrhythmias. Rev Port Cardiol. 2013;32:489–95. [DOI] [PubMed] [Google Scholar]
- 18. Zhang F, Yang B, Chen H, et al. Magnetic versus manual catheter navigation for mapping and ablation of right ventricular outflow tract ventricular arrhythmias: a randomized controlled study. Heart Rhythm. 2013;10:1178–83. [DOI] [PubMed] [Google Scholar]
- 19. Christoph M, Wunderlich C, Moebius S, et al. Fluoroscopy integrated 3D mapping significantly reduces radiation exposure during ablation for a wide spectrum of cardiac arrhythmias. Europace. 2015;17:928–37. [DOI] [PubMed] [Google Scholar]
- 20. Sheldon SH, Latchamsetty R, Morady F, Bogun F. Catheter ablation in patients with pleomorphic, idiopathic, premature ventricular complexes. Heart Rhythm. 2017;14:1623–251. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


