Abstract
Objective:
The aim of the present study was to assess the predictive value of the CHADS2, CHA2DS2-VASc, R2CHADS2, and APPLE scores for rhythm outcome in patients with atrial fibrillation (AF) after catheter ablation.
Methods:
The cohort of the present study consisted of 192 patients with AF who underwent a total of 265 ablations. Rhythm outcome was documented between 3 and 24 month after ablation. The mentioned scores were calculated for every patient.
Results:
Of the patients, 139 (72%) were successfully treated having freedom of any atrial tachyarrhythmia, whereas 21 (11%) had partial success, and 32 (17%) had failure. For univariate analysis, the APPLE score was the only significant predictor of outcome after ablation with an odds ratio (OR) of 1.485 [95% confidence interval (CI) 1.075–2.052, p-value 0.017]. A multivariate binary regression corrected for possible confounders showed that the APPLE score (OR 1.527, 95% CI 1.082–2.153, p-value 0.016) along with the number of previous ablations (OR 5.831, 95% CI 1.356–25.066, p-value 0.018) is a significant predictor of outcome. A novel score (SUCCESS) was created by adding one point to the APPLE score for each previously performed ablation. This novel score demonstrated an improvement in receiver operating characteristic curve analysis (area under the curve 0.657 vs. 0.620). However, these findings were not significant in our study (p-value 0.219).
Conclusion:
Both the APPLE and the novel SUCCESS scores are superior to the CHADS2, CHA2DS2-VASc, and R2CHADS2 scores in predicting AF recurrence after catheter ablation. The SUCCESS score appears to have a higher predictive value than the APPLE score and might be a valuable tool to estimate the risk of AF recurrence in patients eligible for catheter ablation.
Keywords: catheter ablation, predictor, CHA2DS2-VASc, recurrence
Introduction
Atrial fibrillation (AF) is the most common type of arrhythmia, affecting >1% of all adults worldwide and causing a significant impact on public health (1, 2). Over the last decades, catheter ablation has become an established form of treatment, especially in patients where medical therapy is not sufficient for rhythm stabilization or not tolerated due to side effects (3). Treatment often includes anticoagulation therapy owing to an increased risk of stroke or systemic embolic events in patients with AF (4, 5). Well-established score systems (CHADS2, CHA2DS2-VASc, and R2CHADS2) are commonly used to estimate the risk of cardioembolic events (6-8).
Few studies have aimed to assess these score systems and/or other risk factors, such as enlargement of the left atrium (LA), to predict rhythm outcome after catheter ablation (9–12). However, these studies showed inconsistent results when it came to reproducing a similar significant predictive value of the studied scores. Hence, there are currently no strong recommendations suggesting the use of CHADS2 or CHA2DS2-VASc score rather than other score systems (13, 14).
The aim of the present study was to assess which risk factors and score systems have a predictive value for the rhythm outcome after catheter ablation in our patient cohort of a single tertiary care center in Switzerland. The study focuses primarily on four different score systems (CHADS2, CHA2DS2-VASc, R2CHADS2, and APPLE score) and secondarily on the specific components of those scores independently.
Methods
Study population
All patients suffering from symptomatic AF (either paroxysmal or persistent) undergoing one or multiple catheter ablations at our institution between June 2009 and February 2014 were included in the study. In accordance with the current guidelines, paroxysmal AF was defined as episodes terminating within 7 days, whereas persistent AF was defined as lasting >7 days (15). Data, including sex, age, type of AF, number of previous ablations, history of congestive heart failure, hypertension, diabetes mellitus, history of stroke or transient ischemic attack (TIA), coronary artery disease (CAD), size of the LA, ejection fraction (EF) of the left ventricle, structural heart disease, creatinine blood level, height, and weight, on comorbidities and risk factors were collected in all patients.
Scores
The following data were used to calculate different scores: CHADS2 score (1 point for congestive heart failure, hypertension, age ≥75 years, and diabetes mellitus and 2 points for history of stroke or TIA; range from 0 to 6) (6), CHA2DS2-VASc score (1 point for congestive heart failure, hypertension, age 65–74 years, diabetes mellitus, vascular disease, and female sex and 2 points for age ≥75 years and history of stroke or TIA; range from 0 to 10) (7), R2CHADS2 score (CHADS2 score plus an additional 2 points for creatinine clearance [estimated glomerular filtration rate (eGFR)] <60 mL/min; range from 0 to 8) (8), APPLE score (1 point for age >65 years, persistent AF, eGFR <60 mL/min/1.73 m2, LA diameter ≥43 mm, and left ventricular EF <50%; range from 0 to 5) (13), and eGFR was estimated using the MDRD-Study-Formula: eGFR=175*serum creatinine−1.154*Age−0.203*[1.210 if black]*[0.742 if female]. However, the factor 1.210 was not applied because the race of the patients was not part of the registered data in the present study (16).
Ablation
Radiofrequency (RF) energy was used for catheter ablation in most cases. Only in a few cases was an alternative source of energy used (cryoenergy, n=6 and laser light, n=2). The technique used in all patients consisted of a wide-area circumferential point-by-point RF ablation of the ipsilateral pulmonary veins ostia. The acute success was confirmed by the achievement of the procedural endpoint that consisted in electrical isolation of all pulmonary veins from the LA. This was demonstrated by circular mapping of each pulmonary vein showing the entrance and exit block. Additional linear lesions or substrate modifications were performed at the discretion of the operator in patients suffering from persistent AF (17–21).
Definition of success
After the last follow-up, patients were divided into three groups based on their outcome. “Success” was defined as lack of AF lasting >30 s in Holter electrocardiographies (ECGs) and absence of arrhythmia symptoms. “Partial success” was defined as reduction of AF duration >90% in patients without clinically symptomatic AF. This definition is based mainly on Holter ECGs and was included because even though these patients do not meet the criteria of the “success” category, they do not qualify for another ablation. “Failure” was defined as any result not meeting the criteria of the previous two groups, representing recurrence of AF. Antiarrhythmic drugs were used after the intervention if required at the discretion of the treating physician. However, the use of antiarrhythmic drugs was not taken into consideration for the definition of success.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation (SD) or median with interquartile ranges and were compared using the Student’s t-test or Mann-Whitney U test as appropriate. Categorical data are presented as frequency (percentages) and were compared using the Fisher’s exact or chi-square test. Variables with a significant odds ratio (OR) (p<0.05) in a univariate analysis model for the prediction of the primary outcome were included in an Enter-Method multivariate logistic regression model to determine independent predictors of the studied outcome. Calibration was determined by the Hosmer–Lemeshow goodness-of-fit test. For discrimination, the C statistics and receiver operating characteristic (ROC) curves were constructed to assess and compare the ability of the CHADS2, CHA2DS2-VASc, R2CHADS2, APPLE, and SUCCESS scores for the prediction of the recurrence of AF. All probability values and confidence intervals (CIs) were two-sided. A p-value of <0.05 was considered significant, a p-value of <0.1 and >0.05 was considered a trend, and all tests were two-tailed. All statistical analyses were performed using SPSS version 23.0 software (SPSS Inc., Chicago, IL, USA).
Ethical standards
The Local Ethics Committee approved the study in accordance with the ethical standards of the Declaration of Helsinki.
Results
Patient characteristics
The cohort of the present study consisted of 192 patients undergoing a total of 265 catheter ablations. A single procedure was performed in 128 (67%) patients, and multiple procedures were performed in 64 (33%) patients, with 9 (5%) patients requiring a total of three ablations. The mean number of procedures per patient was 1.38±0.57. Of the patients, 146 (76%) were men, and 46 (24%) were women. Out of the 192 patients, 116 (60%) were diagnosed with paroxysmal AF, whereas 76 (40%) were diagnosed with persistent AF. The mean age at the time of the procedure was 61.8±9.2 years, and the mean time between first diagnosis and ablation was 5.8±4.3 years. All ablations were performed between 2009 and 2014. Table 1 summarizes the patient characteristics and risk factors. Table 2 shows the distribution within the different score systems.
Table 1.
Patient characteristics
| Characteristics | ||
|---|---|---|
| No. of patients | 192 | |
| No. of ablations | 265 | |
| Ablations per patient | 1.37±0.58 | |
| Patients with one ablation | 128 | 66.67% |
| Patients with two ablations | 55 | 28.65% |
| Patients with three ablations | 9 | 4.69% |
| Time to procedure (years) | 5.81±4.33 | |
| Male | 146 | 76.04% |
| Female | 46 | 23.96% |
| Age (years) | 61.8±9.19 | |
| Paroxysmal AF | 116 | 60.42% |
| Persistent AF | 76 | 39.58% |
| Risk factors | ||
| Previous procedures | 64 | 33.33% |
| Heart failure | 10 | 5.21% |
| Hypertension | 92 | 47.92% |
| Age 65–74 years | 64 | 33.33% |
| Age >74 years | 16 | 8.33% |
| Diabetes mellitus | 16 | 8.33% |
| History of stroke/TIA | 20 | 10.42% |
| CAD | 20 | 10.42% |
| LA size (mm) | 43.7±6.86 | |
| LA size >42 mm | 103 | 53.65% |
| EF (%) | 58.5±8.06 | |
| EF <50% | 22 | 11.46% |
| Structural heart disease | 13 | 6.77% |
| Creatinine (µmol/L) | 91.7±18.20 | |
| eGFR (mL/min/1.73 m2) | 71.3±16.84 | |
| Renal dysfunction | 52 | 27.08% |
| Weight (kg) | 85.9±15.97 | |
| Height (cm) | 176.0±9.14 | |
| BMI (kg/m2) | 27.7±4.23 |
AF – atrial fibrillation; BMI – body mass index; CAD – coronary artery disease; eGFR – estimated glomerular filtration rate; EF – ejection fraction; LA – left atrium; TIA – transient ischemic attack
Table 2.
Scores
| CHADS2 score | ||
|---|---|---|
| 0 point | 77 | 40.10% |
| 1 point | 75 | 39.06% |
| 2 points | 23 | 11.98% |
| 3 points | 15 | 7.81% |
| 4 points | 2 | 1.04% |
| Mean±SD | 0.91±0.96 | |
| CHA2DS2-VASc score | ||
| 0 point | 51 | 26.56% |
| 1 point | 46 | 23.96% |
| 2 points | 43 | 22.40% |
| 3 points | 28 | 14.58% |
| 4 points | 17 | 8.85% |
| 5 points | 6 | 3.13% |
| 6 points | 1 | 0.52% |
| Mean±SD | 1.67±1.44 | |
| R2CHADS2 score | ||
| 0 point | 64 | 33.33% |
| 1 point | 46 | 23.96% |
| 2 points | 30 | 15.63% |
| 3 points | 41 | 21.35% |
| 4 points | 7 | 3.65% |
| 5 points | 3 | 1.56% |
| 6 points | 1 | 0.52% |
| Mean±SD | 1.45±1.36 | |
| APPLE score | ||
| 0 point | 32 | 16.67% |
| 1 point | 54 | 28.13% |
| 2 points | 59 | 30.73% |
| 3 points | 30 | 15.63% |
| 4 points | 14 | 7.29% |
| 5 points | 3 | 1.56% |
| Mean±SD | 1.73±1.21 | |
| SUCCESS score | ||
| 0 point | 27 | 14.06% |
| 1 point | 40 | 20.83% |
| 2 points | 54 | 28.13% |
| 3 points | 39 | 20.31% |
| 4 points | 19 | 9.90% |
| 5 points | 13 | 6.77% |
| Mean±SD | 2.11±1.41 |
Follow-up
Follow-up examinations were performed at 3, 6, 12, and 24 months, which included symptom assessment and ECG monitoring. These findings were used for evaluating the success of the treatment of each patient.
In 83% of the patients, Holter ECGs were available during follow-up. For the remaining patients, outcome was evaluated by ECG recordings and symptom assessment. The mean follow-up duration was 19 (SD±12; range 3–55) months, with 142 (74%) patients being assessed at least 12 months and 70 (36%) patients at least 24 months after the procedure.
Rhythm outcome
Out of all 192 cases, 139 (72%) were classified as “success”, 21 (11%) as “partial success”, and 32 (17%) as “failure”, leading to a total of 160 (83%) patients being treated “successfully” or at least “partial successfully”. A subgroup analysis was performed for the 70 (36%) patients who were followed up for at least 24 months after the procedure. In this subgroup, 41 (59%) patients were in paroxysmal AF, and 29 (41%) patients were in persistent AF. Of the cases, 43 (61%) were classified as “success”, 16 (23%) as “partial success”, and 11 (16%) as “failure”. In conclusion, 59 (84%) out of the 70 cases were considered a “success” or “partial success” after a follow-up of 2 years.
During the duration of our study, 3 (1.6%) patients showed left atrial flutter. All three patients returned to sinus rhythm either spontaneously (one case) or after electrical cardioversion (two cases). All other arrhythmia recurrences were AF. Early recurrence AF within the 3-month blanking period was not considered.
Predictors for recurrence of AF after catheter ablation
During the follow-up period, 32 (17%) patients showed recurrence of AF. The primary results showed that there was no significantly higher incidence or prevalence of heart failure, hypertension, diabetes mellitus, or CAD in patients with AF recurrence than in those with normal sinus rhythm. However, a trend was observable for persistent AF (p-value 0.068), LA size (0.068), time to procedure (0.066), and previous ablations (0.098) (Table 3).
Table 3.
Predictors of atrial fibrillation
| Variables | Study population n=192 | Arrhythmia recurrences | P-value | |
|---|---|---|---|---|
| No (n=160) | Yes (n=32) | |||
| Age (years) | 61.77±9.188 | 61.7±9.1 | 62.1±9.8 | 0.848 |
| Males (%) | 76 | 76.7 | 72.4 | 0.640 |
| Heart failure (%) | 5.2 | 4.9 | 6.9 | 0.649 |
| Persistent AF (%) | 39.6 | 36.8 | 55.2 | 0.068 |
| Hypertension (%) | 47.9 | 47.2 | 51.7 | 0.691 |
| Diabetes (%) | 8.3 | 9.2 | 3.4 | 0.474 |
| History of stroke/TIA (%) | 10.4 | 10.4 | 10.3 | 1.000 |
| CAD (%) | 10.4 | 9.8 | 13.8 | 0.513 |
| LA size >42 mm (%) | 53.6 | 50.9 | 69 | 0.105 |
| LA size (mm) | 43.67±6.8 | 43.3±6.8 | 45.9±6.8 | 0.068 |
| EF <50% (%) | 11.5 | 10.4 | 17.2 | 0.339 |
| EF (%) | 58.52±8.06 | 58.7±7.5 | 57.3±10.9 | 0.420 |
| SHD (%) | 7.3 | 6.6 | 11.1 | 0.419 |
| Creatinine (µmol/L) | 91.74±18 | 91.1±17.2 | 95.1±22.8 | 0.279 |
| eGFR <60 mL/min/1.73 m2 (%) | 27.1 | 25.2 | 37.9 | 0.175 |
| eGFR (mL/min/1.73 m2) | 71.25±16.8 | 71.8±16.9 | 68.3±16.8 | 0.306 |
| Previous ablation (%) | 33.3 | 31.3 | 44.8 | 0.199 |
| BMI (kg/m2) | 27.67±4.23 | 27.6±4.4 | 27.8±3.5 | 0.844 |
| Time to procedure (years) | 5.81±4.3 | 5.6±4.3 | 7.2±4.5 | 0.066 |
| Total ablations | 1.38±0.57 | 1.3±0.5 | 1.6±0.7 | 0.098 |
| CHADS2 score | 0.91±0.96 | 0.9±1.0 | 0.9±0.8 | 0.881 |
| CHA2D2-VASc score | 1.67±1.43 | 1.6±1.5 | 1.8±1.3 | 0.608 |
| R2CHADS2 score | 1.45±1.86 | 1.4±1.4 | 1.7±1.3 | 0.302 |
| APPLE score | 1.73±1.2 | 1.6±1.7 | 2.2±1.4 | 0.014 |
AF – atrial fibrillation; BMI – body mass index; CAD – coronary artery disease; eGFR – estimated glomerular filtration rate; EF – ejection fraction; LA – left atrium; TIA – transient ischemic attack; SHD - structural heart disease
Of the different scores, only the APPLE score demonstrated a significant predictive value for recurrence of AF in the univariate logistic regression analysis (OR 1.485, 95% CI 1.075–2.052, p-value 0.017), whereas the CHADS2, CHA2D2-VASc, and R2CHADS2 scores were all not significant predictors for rhythm outcome (Table 4).
Table 4.
Odds ratio
| Scores | OR | 95% CI | P-value |
|---|---|---|---|
| LA size (mm) | 1.056 | 0.995-1.120 | 0.071 |
| EF (%) | 0.980 | 0.934-1.029 | 0.419 |
| Persistent AF | 2.113 | 0.951-4.693 | 0.066 |
| eGFR (mL/min/1.73 m2) | 0.987 | 0.963-1.012 | 0.305 |
| Time to procedure (years) | 1.082 | 0.994-1.179 | 0.070 |
| Previous ablations | 1.917 | 1.030-3.569 | 0.040 |
| CHADS2 score | 1.032 | 0.686-1.552 | 0.880 |
| CHA2D2-VASc score | 1.074 | 0.819-1.407 | 0.607 |
| R2CHADS2 score | 1.160 | 0.876-1.536 | 0.301 |
| APPLE score | 1.485 | 1.075-2.052 | 0.017 |
AF – atrial fibrillation; eGFR – estimated glomerular filtration rate; EF – ejection fraction; LA – left atrium; OR - odds ratio
The APPLE score also showed a better predictive value using the ROC curve analysis [area under the curve (AUC) 0.620, p-value 0.040] than CHADS2 (AUC 0.535, p-value 0.548), CHA2D2-VASc (AUC 0.542, p-value 0.472) and R2CHADS2 (AUC 0.570, p-value 0.228). Nevertheless, the difference in AUC did not reach statistical significance (p-value >0.05) (Fig. 1).
Figure 1.
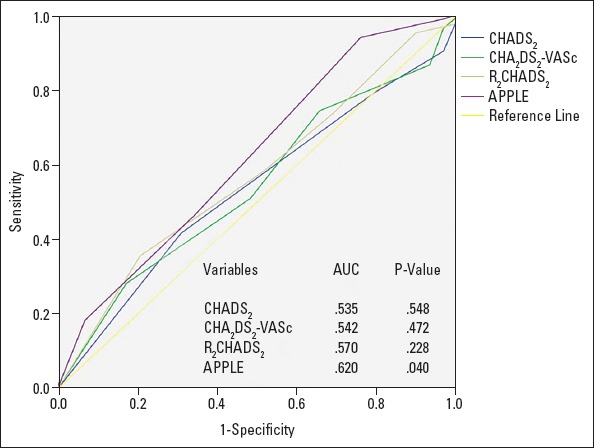
ROC curve 1. Prediction of outcome for CHADS2, CHA2DS2-VASc, R2CHADS2, and APPLE scores
The distribution of the cohort within the APPLE score for 0, 1, 2, and ≥3 points was 17%, 28%, 31%, and 24%, respectively. The rates for AF recurrence for these subgroups were 6% (APPLE score 0), 15% (1), 14% (2), and 31% (≥3) (p=0.227) (Fig. 2). The risks (OR) for recurrence of AF were 2.609 (95% CI 0.518–13.133, p=0.245) (APPLE score 1), 2.353 (95% CI 0.469–11.816, p=0.299) (APPLE score 2), and 4.583 (95% CI 0.942–22.310, p=0.059) (APPLE score ≥3) compared with a score of 0.
Figure 2.
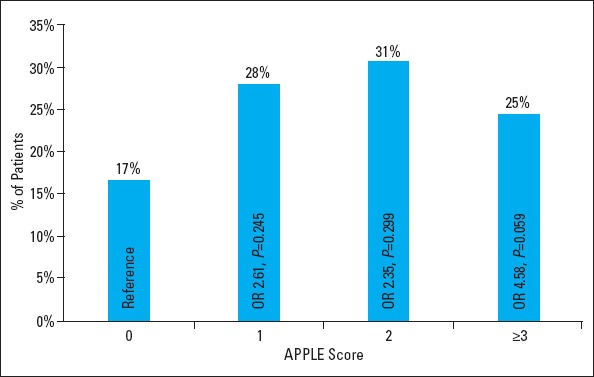
APPLE score distribution. Distribution and risk for atrial fibrillation recurrence of study cohort.
OR - odds ratio
After this initial analysis, we performed a multivariate analysis, including the APPLE score and the two risk factors with the highest significance from the logistic regression analysis that were previous ablations (OR 1.917, 95% CI 1.030–3.569, p-value 0.040) and time to procedure (OR 1.082, 95% CI 0.994–1.179, p-value 0.070). In this analysis, both the APPLE score (OR 1.527, 95% CI 1.082–2.153, p-value 0.016) and previous ablations ≥2 (OR 5.831, 95% CI 1.356–25.066, p-value 0.018) remained significant. We also generated a multivariate binary regression model corrected for three significant confounding variables with an appropriate fit (H and L test: chi-square 4.039, p-value 0.854).
These findings showed that the number of previous ablations appears to have a significant impact on the rhythm outcome. In order to confirm this observation, we created a new score system based on the APPLE score by adding a point for every previous ablation. We called this novel score SUCCESS [Severity of AF type (persistent AF), Unsuccessful previous ablations (1 point per ablation), Creatinine Clearance (eGFR <60 mL/min/1.73 m2), Elderly (>65 years), Size of LA (≥43 mm), Systolic left ventricular EF (<50%)].
We compared those two score systems using the ROC curve analysis. The newly formed SUCCESS score demonstrated an improvement (AUC 0.657) compared with the APPLE score (AUC 0.620), which was not significant however (p-value 0.219) (Fig. 3). Furthermore, the SUCCESS score remained a significant predictor of recurrence despite the addition of partial success to recurrence [outcome of recurrence including partial success: OR 1.453 (95% CI 1.146–1.843), p-value 0.002 and outcome of recurrence excluding partial success: OR 1.539 (95% CI 1.145–2.051), p-value 0.003].
Figure 3.
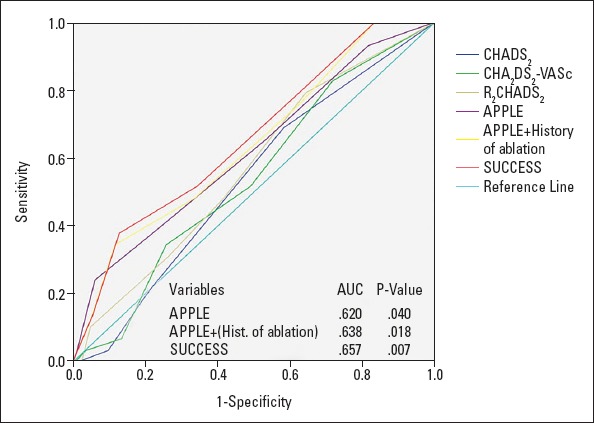
ROC curve 2. Comparison between all scores including “APPLE+ (history of ablation)” (max. 1 additional point) and “SUCCESS” (1 additional point for each previous ablation)
Discussion
To the best of our knowledge, this is the first study comparing the predictive value for rhythm outcome after catheter ablation in patients with AF of all four scores CHADS2, CHA2DS2-VASc, R2CHADS2, and APPLE. Among these score systems, only the APPLE score was a significant predictor for rhythm outcome in our patient cohort. The newly introduced SUCCESS score might predict outcome even better.
The most recently reported APPLE scoring system by Kornej et al. (13) showed to be capable of predicting rhythm outcome in patients after first and repeated ablation procedures and has proven to be superior to the previously reported scoring systems (22). In our study, we obtained very similar results to those by Kornej et al. (13) who introduced the novel APPLE score. Using the ROC curve analysis, we received an AUC of 0.620 [Kornej et al. (13): 0.634]. The distribution of patients within the APPLE score of the two cohorts was comparable (APPLE scores of 0, 1, 2, and ≥3: 17%, 28%, 31%, and 25%, respectively) [Kornej et al. (13): 21%, 34%, 31%, and 25%, respectively], as was the risk (OR) for arrhythmia recurrence [APPLE scores of 1, 2, or ≥3: 2.61 (95% CI 0.52–3.13), 2.35 (95% CI 0.47–11.81), and 5.58 (95% CI 0.94–22.31), respectively] [Kornej et al. (13): 1.73 (95% CI 1.17–2.55), 2.79 (95% CI 1.90–4.12), and 4.70 (95% CI 3.03–7.30), respectively]. While Kornej et al. (13) demonstrated the predictive value of the APPLE score in patients undergoing their first ablation and also for repeated ablations (22), they did not include previous interventions as a factor in their score. We sought to further improve the predictive value of this score by awarding an additional point for every previously performed ablation in the patient’s medical history, which was the most significant specific risk factor in our study. This newly created SUCCESS score performed slightly better in the ROC curve analysis than the APPLE score (AUC 0.657 vs. 0.620) (Fig. 3). However, this improvement did not reach statistical significance (p-value 0.219). This might be due to the low number of AF recurrences [32] in our cohort of 192 patients. The predictive value of the SUCCESS score proposed in the present study certainly needs to be tested in a larger cohort.
Previously, several studies evaluated predictors for rhythm outcome after catheter ablation in patients with AF. For the specific risk factors, a meta-analysis found that the most significant variables were persistent AF, valvular AF, size of LA >50 mm, and recurrence of AF within 30 days (11). However, the two most significant specific risk factors of our results (previous ablations and time to procedure) were not analyzed in this meta-analysis. A recent study reported that a shorter period between diagnosis and ablation of AF increases the rate of success of the procedure (23). The predictive value of the number of previous ablations for prediction rhythm outcome in patients with AF has not been evaluated yet. However, there are data available comparing the success rates of first-time ablations and repeated ablations. In contrast to our findings, data suggest that these success rates remain unchanged, independently of the total count of ablations (14), or that they increase with each additional procedure (24, 25).
CHADS2 and CHA2DS2-VASc scores are the two most extensively studied scores. Both are primarily used to determine the usefulness of anticoagulation in patients with AF. Although they appear to be associated with recurrence after ablations as proposed by multiple studies, their predictive value is, however, modest (9, 12, 14, 25, 26). Fewer studies have evaluated the role of novel scores, such as the R2CHADS2 score, which was created to assess the risk of stroke and systemic embolism in patients with AF (8). R2CHADS2 appeared to have a better predictive value than the CHADS2 and CHA2DS2-VASc score systems (9).
We did not include other scoring systems because they either had no predictive value for rhythm outcome [HATCH (27, 28)], included early recurrence and therefore were unpractical for baseline prediction [BASE-AF (29) and MB-Later (30)] or were used for patients who underwent repeated ablations [ALARMEc (31)].
Since the SUCCESS score is mainly based on the existing APPLE score, it also shares its advantages (13). Its composition is based on the results of a multivariate analysis of a cohort of 2067 patients (9). Combining the significant, independent predictors of AF recurrence of that study (persistent AF, renal insufficiency, age, size of LA, and reduced EF) with our own results (previous number of ablation procedure) results in a score system, which is easy to use and consists of parameters routinely assessed in patients, making it convenient for clinical practice. Further studies with larger cohorts should be conducted to confirm our findings.
Study limitations
This was a single-center cohort. The main limitation of the present study is the small number of patients, which is not sufficient to establish a novel scoring system on its own. However, we suggest that by adding an additional point for previously performed ablations to the APPLE score might improve its predictive value and should be tested in larger cohorts. Furthermore, as arrhythmia recurrences might be underdetected, further studies with continuous rhythm monitoring are needed to confirm these findings.
Conclusion
Both the APPLE and the novel SUCCESS scores are superior to the CHADS2, CHA2DS2-VASc, and R2CHADS2 scores in predicting the recurrence of atrial tachyarrhythmia after catheter ablation in patients with AF. The SUCCESS score appears to have a higher predictive value than the APPLE score. Further studies with larger number of patients should be performed to confirm our findings.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – F.N.J., L.M.H.; Design – F.N.J., S.O., F.D., L.M.H.; Supervision – F.D., L.M.H.; Fundings – None; Materials – L.M.H.; Data collection &/or processing – F.N.J., L.M.H.; Analysis &/or interpretation – F.N.J., S.O., F.D., L.M.H.; Literature search – F.N.J., L.M.H.; Writing – F.N.J.; Critical review – F.N.J., S.O., F.D., L.M.H.
References
- 1.Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, et al. Worldwide epidemiology of atrial fibrillation:a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–47. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–25. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 3.Lubitz SA, Fischer A, Fuster V. Catheter ablation for atrial fibrillation. BMJ. 2008;336:819–26. doi: 10.1136/bmj.39513.555150.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, et al. Management of Patients With Atrial Fibrillation (Compilation of 2006 ACCF/AHA/ESC and 2011 ACCF/AHA/HRS Recommendations) A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:1935–44. doi: 10.1016/j.jacc.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Lip GY, Frison L, Halperin JL, Lane DA. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation:the HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) score. J Am Coll Cardiol. 2011;57:173–80. doi: 10.1016/j.jacc.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 6.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke - Results from the national registry of Atrial Fibrillation. JAMA. 2001;285:2864–70. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 7.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach:the euro heart survey on atrial fibrillation. Chest. 2010;137:263–72. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 8.Piccini JP, Stevens SR, Chang Y, Singer DE, Lokhnygina Y, Go AS, et al. ROCKET AF Steering Committee and Investigators. Renal dysfunction as a predictor of stroke and systemic embolism in patients with nonvalvular atrial fibrillation:validation of the R(2)CHADS(2) index in the ROCKET AF (Rivaroxaban Once-daily, oral, direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation) and ATRIA (AnTicoagulation and Risk factors In Atrial fibrillation) study cohorts. Circulation. 2013;127:224–32. doi: 10.1161/CIRCULATIONAHA.112.107128. [DOI] [PubMed] [Google Scholar]
- 9.Kornej J, Hindricks G, Kosiuk J, Arya A, Sommer P, Husser D, et al. Comparison of CHADS2R2CHADS2and CHA2DS2-VASc scores for the prediction of rhythm outcomes after catheter ablation of atrial fibrillation:the Leipzig Heart Center AF Ablation Registry. Circ Arrhythm Electrophysiol. 2014;7:281–7. doi: 10.1161/CIRCEP.113.001182. [DOI] [PubMed] [Google Scholar]
- 10.Saad EB, d'Avila A, Costa IP, Aryana A, Slater C, Costa RE, et al. Very low risk of thromboembolic events in patients undergoing successful catheter ablation of atrial fibrillation with a CHADS2score ≤3:a long-term outcome study. Circ Arrhythm Electrophysiol. 2011;4:615–21. doi: 10.1161/CIRCEP.111.963231. [DOI] [PubMed] [Google Scholar]
- 11.D'Ascenzo F, Corleto A, Biondi-Zoccai G, Anselmino M, Ferraris F, di Biase L, et al. Which are the most reliable predictors of recurrence of atrial fibrillation after transcatheter ablation?:a meta-analysis. Int J Cardiol. 2013;167:1984–9. doi: 10.1016/j.ijcard.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Letsas KP, Efremidis M, Giannopoulos G, Deftereos S, Lioni L, Korantzopoulos P, et al. CHADS2and CHA2DS2-VASc scores as predictors of left atrial ablation outcomes for paroxysmal atrial fibrillation. Europace. 2014;16:202–7. doi: 10.1093/europace/eut210. [DOI] [PubMed] [Google Scholar]
- 13.Kornej J, Hindricks G, Shoemaker MB, Husser D, Arya A, Sommer P, et al. The APPLE score:a novel and simple score for the prediction of rhythm outcomes after catheter ablation of atrial fibrillation. Clin Res Cardiol. 2015;104:871–6. doi: 10.1007/s00392-015-0856-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chao TF, Ambrose K, Tsao HM, Lin YJ, Chang SL, Lo LW, et al. Relationship between the CHADS(2) score and risk of very late recurrences after catheter ablation of paroxysmal atrial fibrillation. Heart Rhythm. 2012;9:1185–91. doi: 10.1016/j.hrthm.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. ESC Scientific Document Group. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 16.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YP, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–54. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 17.Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–66. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 18.Haegeli LM. CardioPulse. Percutaneous radiofrequency catheter ablation of atrial fibrillation. Eur Heart J. 2012;33:2625–7. [PubMed] [Google Scholar]
- 19.Haegeli LM, Kotschet E, Byrne J, Adam DC, Lockwood EE, Leather RA, et al. Cardiac injury after percutaneous catheter ablation for atrial fibrillation. Europace. 2008;10:273–5. doi: 10.1093/europace/eum273. [DOI] [PubMed] [Google Scholar]
- 20.Haegeli LM, Wolber T, Ercin E, Altwegg L, Krasniqi N, Novak PG, et al. Double transseptal puncture for catheter ablation of atrial fibrillation:safety of the technique and its use in the outpatient setting. Cardiol Res Pract. 2010;2010:295297. doi: 10.4061/2010/295297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haegeli LM, Jud F, Jun On C, Gstrein C, Saguner AM, Steffel J, et al. Catheter ablation for atrial fibrillation in a real-world setting. Cardiovascular Medicine. 2015;18:319–23. [Google Scholar]
- 22.De Greef Y, Schwagten B, Chierchia GB, de Asmundis C, Stockman D, Buysschaert I. Diagnosis-to-ablation time as a predictor of success:early choice for pulmonary vein isolation and long-term outcome in atrial fibrillation:results from the Middelheim-PVI Registry. Europace. 2018;20:589–95. doi: 10.1093/europace/euw426. [DOI] [PubMed] [Google Scholar]
- 23.Winkle RA, Mead RH, Engel G, Patrawala RA. Long-term results of atrial fibrillation ablation:the importance of all initial ablation failures undergoing a repeat ablation. Am Heart J. 2011;162:193–200. doi: 10.1016/j.ahj.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 24.Chao TF, Tsao HM, Lin YJ, Tsai CF, Lin WS, Chang SL, et al. Clinical outcome of catheter ablation in patients with nonparoxysmal atrial fibrillation:results of 3-year follow-up. Circ Arrhythm Electrophysiol. 2012;5:514–20. doi: 10.1161/CIRCEP.111.968032. [DOI] [PubMed] [Google Scholar]
- 25.Chao TF, Cheng CC, Lin WS, Tsao HM, Lin YJ, Chang SL, et al. Associations among the CHADS(2) score, atrial substrate properties, and outcome of catheter ablation in patients with paroxysmal atrial fibrillation. Heart Rhythm. 2011;8:1155–9. doi: 10.1016/j.hrthm.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 26.Kornej J, Hindricks G, Arya A, Sommer P, Husser D, Bollmann A. The APPLE Score-A Novel Score for the Prediction of Rhythm Outcomes after Repeat Catheter Ablation of Atrial Fibrillation. PLoS One. 2017;12:e0169933. doi: 10.1371/journal.pone.0169933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Vos CB, Pisters R, Nieuwlaat R, Prins MH, Tieleman RG, Coelen RJ, et al. Progression from paroxysmal to persistent atrial fibrillation clinical correlates and prognosis. J Am Coll Cardiol. 2010;55:725–31. doi: 10.1016/j.jacc.2009.11.040. [DOI] [PubMed] [Google Scholar]
- 28.Tang RB, Dong JZ, Long DY, Yu RH, Ning M, Jiang CX, et al. Efficacy of catheter ablation of atrial fibrillation beyond HATCH score. Chin Med J (Engl) 2012;125:3425–9. [PubMed] [Google Scholar]
- 29.Canpolat U, Aytemir K, Yorgun H, Sahiner L, Kaya EB, Oto A. A proposal for a new scoring system in the prediction of catheter ablation outcomes:Promising results from the Turkish Cryoablation Registry. Int J Cardiol. 2013;169:201–6. doi: 10.1016/j.ijcard.2013.08.097. [DOI] [PubMed] [Google Scholar]
- 30.Wójcik M, Berkowitsch A, Greiss H, Zaltsberg S, Pajitnev D, Deubner N, et al. Repeated catheter ablation of atrial fibrillation:how to predict outcome? Circ J. 2013;77:2271–9. doi: 10.1253/circj.cj-13-0308. [DOI] [PubMed] [Google Scholar]
- 31.Mujović N, Marinković M, Marković N, Shantsila A, Lip GY, Potpara TS. Prediction of very late arrhythmia recurrence after radiofrequency catheter ablation of atrial fibrillation:The MB-LATER clinical score. Sci Rep. 2017;7:40828. doi: 10.1038/srep40828. [DOI] [PMC free article] [PubMed] [Google Scholar]


