Abstract
Objective:
Intravascular ultrasound (IVUS) has developed as a preferable choice for optimizing the stenting procedures mainly because it will have good access to vessel size, lesion length, or severity accurately. However, it still remains unclear about the benefits of IVUS guidance in drug-eluting stent (DES) implantation for patients with unprotected left main coronary artery (ULMCA) stenosis. The aim of the present study was to evaluate the clinical outcomes with respect to IVUS-guided DES implantation for these patients.
Methods:
A total of 336 consecutive patients from December 2010 to December 2015 were enrolled in the study. The patients were then randomly assigned into two groups: IVUS-guided group (n=167) and control group (n=169). The primary endpoint was the incidence of composite major adverse cardiac events (MACEs), including cardiac death, myocardial infarction (MI), and target vessel revascularization (TVR). The risk of stent thrombosis (ST) was chosen as the safety endpoint.
Results:
After a 1-year follow-up, the occurrence of composite MACE in the IVUS-guided group was significantly lower than that in the control group (13.2% vs. 21.9%, p=0.031), which might mainly result from the significant reduction in the risk of cardiac death (1.8% vs. 5.9%, p=0.048). Dramatically, the risk of MI did not differ significantly between the two groups (11.4% vs. 13.6%, p=0.478), though a tended reduction in TVR was observed under IVUS guidance (4.2% vs. 8.9%, p=0.068). There was no statistical significance between the two groups with respect to the risk of target lesion revascularization (IVUS-guided vs. control: 1.2% vs. 3.0%, p=0.239) and ST (IVUS-guided vs. control: 1.2% vs. 3.0%, p=0.246).
Conclusion:
The possible feasibility of IVUS-guided DES implantation for patients with ULMCA stenosis was supported by the present study. Larger and more powerful randomized trials were still warranted to research the whole benefits of IVUS guidance for these patients.
Keywords: intravascular ultrasound, unprotected left main coronary artery stenosis, drug-eluting stent implantation
Introduction
The successful revascularization for patients with unprotected left main coronary artery (ULMCA) lesions had shown significant benefits in reducing the risk of mortality or morbidity, regardless of whether percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) was selected for them (1, 2). Popularly, the wider usage of drug-eluting stents (DESs) induced by its rapid development, in conjunction with effective pharmacological therapy and advanced equipment, had indicated improved clinical outcomes pertaining to PCI (3, 4). To the best of our knowledge, the full expansion and apposition of implanted stents mean a successful stenting procedure, which could strengthen the relative benefits. On the other hand, intravascular ultrasound (IVUS) has developed as a matured technique, providing accurate evaluation of vessel size, lesion length, or lesion severity, which was considered as a powerful approach for optimizing the stenting procedures (5, 6). Several recent observational clinical trials (3, 7) and one randomized trial (8) with a small sample size had reported the positive effects of IVUS-guided DES implantation for patients with ULMCA stenosis, mainly appearing in decreased risk of mortality. In fact, several meta-analyses published recently had also confirmed the benefits of IVUS guidance in DES implantation, though the mainly analyzed population were patients with complex coronary lesions (9, 10). However, these over-mentioned data were mostly from observational trials or small randomized trial, and it still remains unclear to evaluate the benefits of IVUS guidance in DES implantation for a left main lesion. Thus, this randomized trial was designed and conducted.
Methods
Study design and patient population
This was a randomized, open-label and single-blind clinical trial. From December 2010 to December 2015, a total of 348 consecutive patients with ULMCA lesions (defined as at least 50% stenosis in the left main coronary artery from visual assessment) were considered in the study. Inclusion criteria were as follows: (1) adult patients with ULMCA lesions and planned for receiving DES implantation (age from 18 to 75 years) and (2) good compliance of antiplatelet therapy post-PCI. Patients with (1) acute myocardial infarction (MI) (≤24 h); (2) cardiogenic shock; (3) high-risk factors for bleeding, such as dysfunction of blood coagulation or histories of major hemorrhage (e.g., intracranial or gastrointestinal); and (4) renal or hepatic failure or carcinoma were excluded from the study. Patients with a chronic total occlusion (CTO) in the left anterior descending (LAD) artery or left circumflex (LCx) artery with no access to successful recanalization before randomization or complicated with severe calcification needing rotational atherectomy were also excluded. Opaque envelopes written with different IDs indicating the related groups (1: receiving DES implantation under IVUS guidance, described as the ‘IVUS-guided’ group and 0: receiving DES implantation without IVUS assessment, described as the control group) were used to randomly divide the enrolled patients at a 1:1 ratio. The study was approved by the Ethics Committee. Written informed consent was obtained from all of the included patients.
Procedures and medications
Five experienced primary interventionists who were in charge of performing all the interventional procedures following the current standards were involved in the study. The selection of different two-stent techniques (Culotte stenting, T/provisional T-stenting, V/simultaneous kissing stents, or double kissing crush (DK crush) stenting) for patients with distal LM bifurcation lesions was decided by these interventionists. Use of glycoprotein IIb/IIIa inhibitors, intra-aortic balloon pump, types of DES, or pre-dilation was their discretion. Post-dilation with noncompliant balloons (≥18 atm pressure) of all stents was recommended (balloon/stent 1:1 ratio), especially for these with suboptimal expansion or stent malapposition confirmed by IVUS or angiography. A successful PCI procedure was considered if thrombolysis in myocardial infarction (TIMI) grade 3 and residual stenosis <10% were achieved. Before the stenting procedures, the New Risk Stratification (NERS) and Synergy between PCI with Taxus and Cardiac Surgery (SYNTAX) scores were estimated (11, 12). In addition, 100 mg aspirin and 300 mg loading dose of clopidogrel were applied for all included patients before the PCI procedures. Unfractionated heparin was used for procedural anticoagulation. After the PCI procedures, all patients were administered aspirin (100 mg/day) for a lifetime and clopidogrel (75 mg/day) for at least 12 months. The additional usage of statins, β-blockers, aldosterone antagonists, or angiotensin-converting enzyme inhibitors was recommended for secondary prevention according to the current guidelines if necessary.
Intravascular ultrasound
After positioning at >10 mm far away from the distal end of the lesion, the IVUS catheter was then pulled back automatically (0.5 mm/s) to the opening of the LM. Simultaneously, an imaging system carrying a 40 MHz mechanical transducer (Boston Scientific Corporation, Natick, MA, USA) was used to collect the images. Several projects were measured to assess the related lesions, including minimal lumen diameter, minimal lumen area, reference lumen area, and burden of lipid plaque, in order to guide the decision-making of stent placements. Repeat IVUS was performed post-PCI to evaluate the optimal results of implanted stents. A successful PCI procedure proven by IVUS was defined as minimum stent lumen cross-sectional area >6.9 mm2, full apposition, and expansion of stents with no observed dissection (13).
Study endpoints and related definitions
The primary study endpoint was the incidence of composite major adverse cardiac events (MACEs) after a 1-year follow-up, including cardiac death, MI, and target vessel revascularization (TVR). The risk of stent thrombosis (ST) was chosen as the safety endpoint. Death from cardiac causes was considered after eliminating a clear non-cardiac cause as confirmed in the clinic or autopsy. Periprocedural MI was confirmed if creatine kinase–myocardial band (CK-MB) increased >10× the upper reference limit (URL) or presenting with any of the following symptoms: (1) newly appeared pathological Q waves in ≥2 contiguous leads or left bundle branch block, (2) imaging evidence indicating new loss of viable myocardium, or (3) CK-MB increased >5× the URL only but presented with new occlusion or severe stenosis proven by angiograph. A repeat revascularization (regardless of PCI or CABG) of the treated lesion or vessel was considered as the target lesion revascularization (TLR) or TVR. ST was defined and classified following the Academic Research Consortium (early: 0–30 days post-PCI, late: 31–360 days and/or very late: >360 days) (14).
Clinical follow-up
Telephone contact or clinical office visit was used for clinical follow-up at 1, 6, and 12 months. Coronary angiography would repeat 12 months later or earlier induced by clinical indications. An independent cardiologist blinded to the study was in charge of assessing all events.
Statistical analysis
The findings from a previous cohort indicated that there were approximately 14.8% of the patients in the IVUS-guided group and 27.7% of the patients in the control group appeared MACEs after a 1-year follow-up (7). As a result, the sample size was subsequently estimated, intending to provide 80% power with a two-sided level of 0.05. Moreover, 10% of the patients lost to follow-up should be assumed. Overall, 348 patients were finally planned for enrollment, among which 174 patients were randomly assigned to each group to demonstrate the superiority of IVUS guidance in DES implantation.
Statistical analyses were made using SPSS version 22.0 (SPSS Institute, Chicago, IL, USA), following the intention-to-treat principle, no matter what treatment was applied. Baseline characteristics and clinical outcomes were recorded using counts, percentages, or mean±standard deviation, as appropriate. The Student’s t-test was used for comparison of normally distributed continuous variables, whereas the Mann–Whitney U test was used for comparison of non-normally distributed data. Comparisons between categorical variables were assessed using the chi-square test or Fisher’s exact test. On the other hand, the independent predictors for the primary endpoint were examined using the multiple Cox proportional hazards regression analysis. When a p value ≤0.1 appeared in the univariate analysis, the present variables were considered as candidates for the multiple models. The Kaplan–Meier analysis was performed to generate the time-to-first event curves, and comparisons between the two groups were assessed using the log-rank test. All P values were two-tailed. A p value <0.05 was considered statistically significant.
Results
Figure 1 shows the patient selection and study design. From December 2010 to December 2015, a total of 348 patients with ULMCA lesions who were then randomly divided into the IVUS-guided group and the control group at a 1:1 ratio met the inclusion criteria. Among these randomly assigned patients, seven patients from the IVUS-guided group and five patients from the control group were excluded from the final analysis because of protocol violation, refusal of enrollment, or withdrawn by the clinician. An acceptable dropout rate (2.7%) was observed in all enrolled patients.
Figure 1.
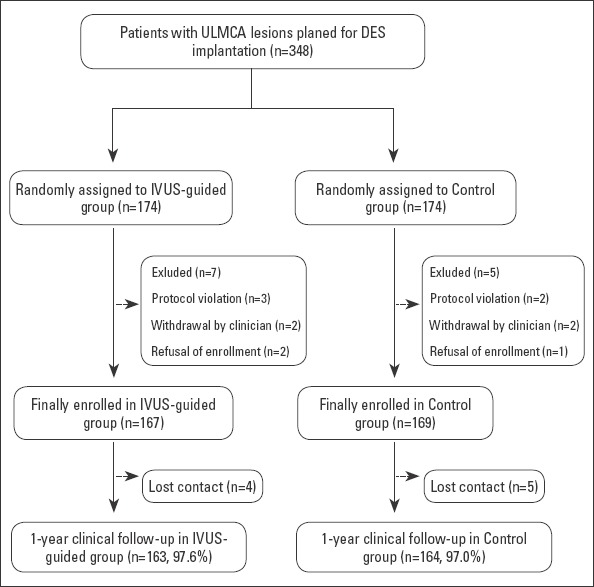
A flowchart depicting the selection of patients included in the study
Baseline clinical characteristics
As shown in Table 1, the baseline characteristics of the involved patients were well matched between the two groups. Approximately 32.1% of patients suffered from diabetes, whereas most of the included patients (75.3%) presented with unstable angina.
Table 1.
Baseline characteristics of the randomized groups
| IVUS-guided (n=167) | Control (n=169) | P value | |
|---|---|---|---|
| Demographics | |||
| Age, years | 65.3±10.6 | 64.9±11.2 | 0.452 |
| Males, n (%) | 106 (63.5) | 108 (63.9) | 0.821 |
| BMI, kg/m2 | 23.8±3.8 | 24.1±2.9 | 0.405 |
| SBP, mm Hg | 133.5±12.5 | 134.7±11.4 | 0.241 |
| DBP, mm Hg | 75.9±9.9 | 76.8±10.7 | 0.669 |
| Heart rate, beats/min | 72.1±10.9 | 71.3±11.7 | 0.707 |
| Risk factors | |||
| Hypertension, n (%) | 116 (69.5) | 122 (72.2) | 0.411 |
| Diabetes, n (%) | 56 (33.5) | 52 (30.8) | 0.169 |
| Hyperlipidemia, n (%) | 63 (37.7) | 64 (37.9) | 0.891 |
| Ischemic stroke, n (%) | 5 (3.0) | 4 (2.4) | 0.523 |
| Current smoker, n (%) | 62 (37.1) | 60 (35.5) | 0.668 |
| PAD, n (%) | 15 (9.0) | 17 (10.1) | 0.717 |
| Creatinine, µmol/L | 77.2±22.9 | 79.3±24.8 | 0.735 |
| eGFR, mL/min/1.73 m2 | 81.9±23.4 | 79.8±22.9 | 0.617 |
| Medical history | |||
| LVEF, % | 55.6±11.7 | 58.4±10.5 | 0.413 |
| CHF, n (%) | 31 (18.6) | 33 (19.2) | 0.798 |
| Previous MI, n (%) | 29 (17.4) | 24 (14.2) | 0.319 |
| Previous PCI, n (%) | 33 (19.8) | 28 (16.6) | 0.388 |
| Previous CABG, n (%) | 2 (1.2) | 2 (1.2) | 0.999 |
| Clinical presentation | |||
| Silent ischemia | 3 (1.8) | 4 (2.4) | 0.812 |
| Stable angina | 20 (12.0) | 18 (10.7) | 0.554 |
| Unstable angina | 127 (76.0) | 126 (74.6) | 0.603 |
| Recent MI (>24 h) | 17 (10.2) | 21 (12.4) | 0.451 |
Values are presented as mean±SD.
BMI - body mass index; CABG - coronary artery bypass grafting; CHF - congestive heart failure; DBP - diastolic blood pressure; eGFR - estimated glomerular filtration rate; IVUS - intravascular ultrasound; LVEF - left ventricular ejection fraction; MI - myocardial infarction; n - number; PAD - peripheral artery disease; PCI - percutaneous coronary intervention; SBP - systolic blood pressure
Angiographic and procedural characteristics
Table 2 shows the angiographic characteristics. A total of 281 patients (IVUS-guided vs. control: 82.6% vs. 84.6%, p=0.782) were complicated with multivessel disease, and most of whom were located with distal LM bifurcation, showing no significant difference when comparing the IVUS-guided group to the control group (58.7% vs. 61.5%, p=0.558). On the other hand, the incidence of CTO or calcification also showed no statistical significance between the two groups, indicating that the complexity of coronary lesions in the two groups was well matched. In addition, risk stratification evaluated by either the SYNTAX or the NERS score showed similar risk scores.
Table 2.
Lesion characteristics of the randomized groups
| IVUS-guided (n=167) | Control (n=169) | P value | |
|---|---|---|---|
| Multivessel stenting, n (%) | 138 (82.6) | 143 (84.6) | 0.782 |
| LAD | 93 (55.7) | 89 (52.7) | 0.431 |
| LCx | 74 (44.3) | 84 (49.7) | 0.045 |
| RCA | 104 (62.3) | 98 (58.0) | 0.109 |
| LM lesion location | |||
| Ostial | 13 (7.8) | 16 (9.5) | 0.865 |
| Body shaft | 26 (15.6) | 23 (13.6) | 0.677 |
| Distal LM bifurcation | 98 (58.7) | 104 (61.5) | 0.558 |
| Lesion characteristics in LM | |||
| Calcification, n (%) | 64 (38.3) | 65 (38.5) | 0.958 |
| Medina classification | |||
| 1, 1, 1 | 65(38.9) | 67 (40.0) | 0.774 |
| 1, 1, 0 | 16 (9.6) | 18 (10.7) | 0.832 |
| 1, 0, 1 | 8 (4.8) | 6 (3.6) | 0.471 |
| 0, 1, 1 | 22 (13.2) | 19 (11.2) | 0.535 |
| 1, 0, 0 | 7 (4.2) | 6 (3.6) | 0.896 |
| 0, 1, 0 | 16 (9.6) | 21 (12.4) | 0.510 |
| 0, 0, 1 | 3 (1.8) | 6 (3.6) | 0.601 |
| TIMI flow grade <3, n (%) | 42 (25.1) | 47 (28.1) | 0.375 |
| Chronic total occlusion, n (%) | 20 (12.0) | 22 (13.0) | 0.875 |
| SYNTAX score, points | 28.1±7.5 | 30.2±10.1 | 0.345 |
| 0-22 | 21 (12.6) | 18 (10.7) | - |
| 23-32 | 74 (44.3) | 78 (46.2) | - |
| >32 | 72 (43.1) | 73 (43.2) | - |
| NERS score, points | 25.2±7.1 | 26.8±9.7 | 0.618 |
Values are presented as mean±SD.
IVUS - intravascular ultrasound; LAD - left anterior descending artery; LCx - left circumflex artery; LM - left main; n - number; NERS - New Risk Stratification; RCA - right coronary artery; SYNTAX - Synergy between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery; TIMI - thrombolysis in myocardial infarction
Most of the included patients underwent PCI using the transradial approach. Table 3 shows the procedural characteristics. Pre-dilation was performed in 55.0% of the patients in the control group, which was much more frequently than that in the IVUS-guided group (p<0.001). When two-stent technique should be chosen for bifurcation, the DK crush stenting was more preferred in the IVUS-guided group (27.6% vs. 18.3%, p<0.001), whereas the Culotte stenting was used more frequently in the control group (41.4% vs. 48.4%, p<0.001). The larger stents were implanted in the IVUS-guided group than those in the control group (3.46±0.51 mm vs. 3.29±0.33 mm, p=0.023). Alternately, there was no significant difference in stent numbers or lengths between the two groups. Final TIMI flow grade 3 in the main vessel was observed in all the included patients, suggesting a successful PCI procedure.
Table 3.
Procedural characteristics of the randomized groups
| IVUS-guided (n=167) | Control (n=169) | P value | |
|---|---|---|---|
| Transradial approach | 117 (70.1) | 114 (67.5) | 0.238 |
| Pre-dilation before stenting, n (%) | 71 (42.5) | 93 (55.0) | <0.001 |
| Stents in LM | |||
| Total stent number, n (%) | 2.2±0.9 | 2.4±0.7 | 0.872 |
| Total stent length, mm | 32.6±16.9 | 33.3±18.3 | 0.183 |
| Diameter, mm | 3.46±0.51 | 3.29±0.33 | 0.023 |
| Two-stent techniques for bifurcation | 87 (52.1) | 93 (55.0) | 0.282 |
| Culotte | 36 (41.4) | 45 (48.4) | <0.001 |
| T/provisional T-stenting | 16 (18.4) | 18 (19.4) | 0.893 |
| V/SKS stenting | 11 (12.6) | 13 (14.0) | 0.420 |
| Double kissing crush | 24 (27.6) | 17 (18.3) | <0.001 |
| Post-dilation | |||
| Balloon diameter, mm | 3.53±0.37 | 3.45±0.29 | 0.108 |
| Pressure, atm | 15.3±2.9 | 13.9±3.3 | 0.090 |
| Use of IABP, n (%) | 9 (5.4) | 11 (6.5) | 0.820 |
| Use of IIb/IIIa inhibitor, n (%) | 13 (7.8) | 17 (10.1) | 0.597 |
| Final TIMI flow grade 3, n (%) | |||
| Main vessel | 167 (100) | 169 (100) | 1.000 |
| Side branch | 165 (98.8) | 164 (97.0) | 0.226 |
Values are presented as mean±SD.
IABP - intra-aortic balloon pump; IVUS - intravascular ultrasound; LM - left main; n - number; SKS - simultaneous kissing stents; TIMI - thrombolysis in myocardial infarction
Clinical outcomes
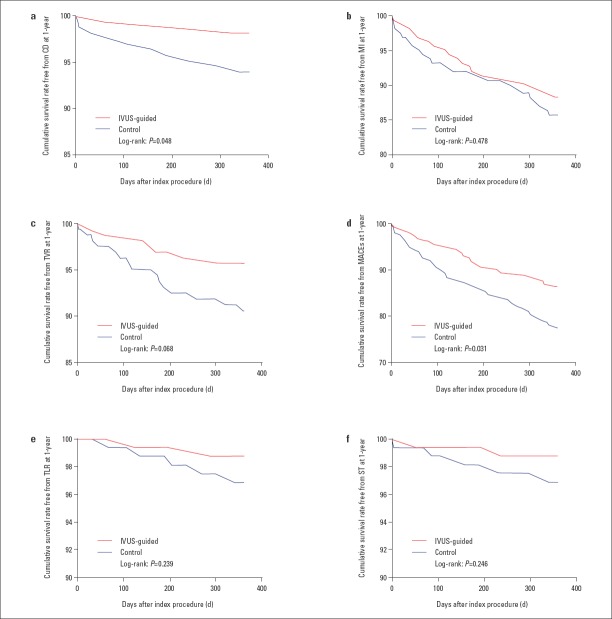
There were approximately 97.3% of the patients who finished the 1-year clinical follow-up, and related outcomes were listed in Table 4. After a 1-year follow-up, the incidence of composite MACE in the IVUS-guided group was significantly lower than that in the control group (13.2% vs. 21.9%, p=0.031) Figure 2, which might mainly be derived from the significant reduction in the risk of cardiac death (1.8% vs. 5.9%, p=0.048). Dramatically, the risk of MI did not differ significantly between the two groups (11.4% vs. 13.6%, p=0.478), whereas a tended reduction in the risk of TVR was observed under the IVUS guidance (4.2% vs. 8.9%, p=0.068). There was no statistical significance between the two groups with respect to the risk of TLR (IVUS-guided vs. control: 1.2% vs. 3.0%, p=0.239) and ST (IVUS-guided vs. control: 1.2% vs. 3.0%, p=0.246). In addition, based on the Cox regression multiple analysis, IVUS guidance [hazard ratio (HR) 0.51, 95% confidence interval (CI) 0.34–0.84, p=0.038] and distal LM bifurcation (HR 1.66, 95% CI 1.28–2.26, p=0.045) appeared as independent predictors of MACE.
Table 4.
Clinical outcomes in the randomized groups
| IVUS-guided (n=167) | Control (n=169) | P value | |
|---|---|---|---|
| In-hospital, n (%) | |||
| Cardiac death | 0 | 2 (1.2) | 0.159 |
| MI | 1 (0.6) | 3 (1.8) | 0.320 |
| STEMI | 0 | 1 (0.6) | 0.320 |
| NSTEMI | 1 (0.6) | 2 (1.2) | 0.567 |
| TVR | 0 | 1 (0.6) | 0.320 |
| TLR | 0 | 0 | 1.000 |
| CABG | 0 | 0 | 1.000 |
| MACE | 1 (0.6) | 3 (1.8) | 0.320 |
| Stent thrombosis | 0 | 1 (0.6) | 0.320 |
| Definite | 0 | 0 | 1.000 |
| Probable | 0 | 1 (0.6) | 0.320 |
| 12-month follow-up, n (%) | |||
| Cardiac death | 3 (1.8) | 10 (5.9) | 0.048 |
| MI | 19 (11.4) | 23 (13.6) | 0.478 |
| STEMI | 2 (1.2) | 4 (2.4) | 0.403 |
| NSTEMI | 17 (10.2) | 19 (11.2) | 0.690 |
| TVR | 7 (4.2) | 15 (8.9) | 0.068 |
| TLR | 2 (1.2) | 5 (3.0) | 0.239 |
| CABG | 0 | 0 | 1.000 |
| MACE | 22 (13.2) | 37 (21.9%) | 0.031 |
| Stent thrombosis | 2 (1.2) | 5 (3.0) | 0.246 |
| Definite | 0 | 1 (0.6) | 0.313 |
| Probable | 2 (1.2) | 3 (2.4) | 0.643 |
| Late | 0 | 1 (0.6) | 0.313 |
CABG - coronary artery bypass grafting; IVUS - intravascular ultrasound; MACE - major adverse cardiac event; MI - myocardial infarction; NSTEMI - non-ST segment elevation myocardial infarction; STEMI - ST segment elevation myocardial infarction; TLR - target lesion revascularization; TVR - target vessel revascularization
Figure 2.
Freedom from adverse events in the IVUS-guided group versus the control group. Freedom from cardiac death (CD) (a), myocardial infarction (MI) (b), target vessel revascularization (TVR) (c), major adverse cardiac events (MACEs) (d), target lesion revascularization (TLR) (e), and stent thrombosis (ST) (f) in the IVUS-guided group (red line) versus the control group (blue line) at a 1-year follow-up
Discussion
In this randomized study, the major finding was that IVUS-guided DES implantation significantly reduced the incidence of composite MACE among patients with ULMCA lesions, particularly for decreasing the risk of cardiac death. Nonetheless, there were no beneficial effects with respect to IVUS guidance in preventing ST, as well as MI, though the relative risk of TVR tended to be decreased.
It should be noted that a large amount of jeopardized myocardium would occur in patients with ULMCA stenosis, in which no graft to the LAD artery and LCx artery, leading to higher risk of mortality (15). Based on several randomized trials, the 2014 USA guidelines recommended CABG for most of these patients mainly because of its superiority in reducing the risk of TLR when compared with PCI with bare-metal or first-generation DES (1, 16, 17). Recently, the improved clinical outcomes had been indicated resulting from the wider usage of DES since it was rapidly developed, as well as in conjunction with effective pharmacological therapy and advanced equipment (3, 4). In fact, the potential interfering effects of aortic cusp opacification would limit angiography in assessing ULMCA lesion characteristics and subsequently interfered the decisions of stenting strategies, leading to adverse stenting outcomes (18). As a result, IVUS was widely applied before the PCI procedures because this imaging equipment had been reported to make it easier to achieve more accurate details of target vessels, including lesion morphology and true luminal size, and then provided better approach for selecting the appropriate diameter and length of the implanted stents (19). Furthermore, IVUS guidance can be helpful to determine these complications during the PCI procedure earlier, leading to better clinical outcomes. However, it still remains unclear if IVUS guidance in DES implantation would have positive effects in patients with ULMCA stenosis. Two recent meta-analyses (9, 10) had indicated the benefits of IVUS-guided DES implantation but in which the mainly analyzed population were these patients with composite of complex coronary lesions. Several previous observational clinical trials indicated similar results. Gao et al. (7) analyzed the data of 582 patients after propensity score matching and showed that IVUS-guided treatment of ULMCA using a DES is associated with less frequent 1-year MACE, mainly resulting from a significant reduction of cardiac death and TVR. On the other hand, the results from the Revascularization for ULMCA Stenosis: Comparison of Percutaneous Coronary Angioplasty Versus Surgical Revascularization trial indicated that IVUS guidance in DES implantation for these patients might significantly lower the 3-year mortality than the angiography-guided group (4.7% vs. 16.0%, p<0.048). In addition, a recent randomized trial reported the benefits of IVUS-guided DES implantation for patients with ULMCA stenosis, mainly in terms of reduced risk of TLR, though only 123 elderly patients (≥70 years) were involved (8). Therefore, the current supporting data for IVUS-guided DES implantation in such patients were mostly from observational trials or small randomized trials, making the benefits of IVUS guidance unconvincing.
Indeed, better clinical outcomes would be achieved if the risk of adverse events related to the PCI procedures was decreased, which mainly relied on the full expansion and apposition of implanted stents. In our study, the mean diameter of implanted stents in the IVUS-guided group was larger than that in the control group, without any complications following post-dilation. These benefits were mostly due to the accurate details of the true luminal size, lumen area, reference lumen area, and lesion morphology provided by IVUS guidance. It had been reported that distal LM bifurcation lesions might involve a wider bifurcation angle, larger diameters, and more frequent occurrence of three vessel segments (trifurcations), increasing the risk of under expansion and malapposition of implanted stents significantly (20, 21). Therefore, applying IVUS before the stenting procedures could also be helpful to evaluate the lipid plaque distribution in distal LM and the approach for side branch, which would make it easier to judge the true angle of the distal LM bifurcation and subsequently have very positive effects in deciding the selection of different PCI strategies, especially for the two-stent techniques. On the other hand, IVUS guidance allowed these patients with good access to appropriate stent diameter and length to optimize the stenting procedure, resulting in better clinical outcomes.
In recent years, the improved clinical outcomes had been observed with respect to the technique of DK crush stenting for bifurcation lesions. In the DKCRUSH-III trial, the superiority of DK crush stenting was proven mainly because of the higher incidence of TVR pertaining to the Culotte stenting for ULMCA bifurcation lesions, leading to a significantly increased MACE (22). The possible reasons for such results explained by themselves were mainly due to the selected population in the Culotte stenting group located with wide bifurcation lesions (angle ≥70°), for whom a T-stent technique should have been selected (23, 24). Then, the DKCRUSH-V randomized trial was published and found that DK crush stenting is superior to provisional stenting (PS) mainly appearing as lower incidence of target vessel MI (PS vs. DK crush: 2.9% vs. 0.4%, p=0.03) and definite or probable ST (PS vs. DK crush: 3.3% vs. 0.4%, p=0.02), causing a significantly decreased rate of target lesion failure (PS vs. DK crush: 10.7% vs. 5.0%, p=0.02) (25). In our study, most included patients were located with distal LM bifurcation (60.1%), which was confirmed as another independent predictor of MACE via the Cox regression multivariable analysis. In addition, the DK crush stenting was more preferred in the IVUS-guided group (27.6% vs. 18.3%, p<0.001), whereas the Culotte technique was used more frequently in the control group (41.4% vs. 48.4%, p<0.001), which might also result in better clinical outcomes in the IVUS-guided group. However, in the IVUS-guided group involving a total of 87 cases who underwent two-stent techniques for bifurcation lesions, there were only 24 (27.6%) cases who received DK crush stenting, whereas 36 cases were selected for Culotte stenting. These might be explained for why a significantly reduced incidence of cardiac death was observed, whereas no beneficial effects of IVUS guidance on preventing ST, as well as MI, were observed, though the relative risk of TVR tended to be reduced in the IVUS-guided group.
Study limitations
Our study has several limitations with respect to the design and conduct of the current study. First, though 336 patients were finally analyzed in this trial, it still appears as a single-blind randomized study with 80% power only. A larger, multicenter and more powerful randomized trial was still warranted. Second, withdrawal of consent or contact lost during the follow-up was another limitation, though the whole results would not be influenced obviously. Third, an extended follow-up was aimed, which may be critical to assess the long-term clinical benefits of IVUS-guided DES implantation for such patients. Fourth, the absence of quantitative IVUS and angiographic analysis should also represent limitations. In addition, subgroup analysis for confirming the effects of IVUS guidance in distal left main bifurcation lesions was not performed either. Additionally, usage of different implanted DES types or sheaths with different sizes limited us to explore the true effects of IVUS guidance for these patients with LM lesions. Finally, several other risk factors related with pharmacological therapy post-PCI were not considered, including dual antiplatelet therapy (DAPT) regimen, treatment platelet reactivity, and compliance for DAPT, which might also influence the final results.
Conclusion
IVUS-guided DES implantation was related with a significantly reduced 1-year incidence of composite MACE among patients with ULMCA lesions, particularly for decreasing the risk of cardiac death, which would support the advantages of IVUS guidance. Larger and more powerful randomized trials were still warranted to identify the overall benefits with respect to IVUS guidance for these patients.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – X.M.L., Z.M.Y.; Design – X.M.L., Z.M.Y., X.K.L.; Supervision – X.M.L., X.K.L.; Fundings – C.Q.L., Q.L.H.; Materials – C.Q.L., J.H.S.; Data collection &/or processing – Q.Z., C.Q.L.; Analysis &/or interpretation – X.M.L., X.K.L.; Literature search – Q.Z., J.H.S.; Writing – X.M.L., Z.M.Y.; Critical review – J.H.S.
References
- 1.Morice MC, Serruys PW, Kappetein AP, Feldman TE, Ståhle E, Colombo A, et al. Five-year outcomes in patients with left main disease treated with either percutaneous coronary intervention or coronary artery bypass grafting in the synergy between percutaneous coronary intervention with taxus and cardiac surgery trial. Circulation. 2014;129:2388–94. doi: 10.1161/CIRCULATIONAHA.113.006689. [DOI] [PubMed] [Google Scholar]
- 2.Cavalcante R, Sotomi Y, Lee CW, Ahn JM, Farooq V, Tateishi H, et al. Outcomes After Percutaneous Coronary Intervention or Bypass Surgery in Patients With Unprotected Left Main Disease. J Am Coll Cardiol. 2016;68:999–1009. doi: 10.1016/j.jacc.2016.06.024. [DOI] [PubMed] [Google Scholar]
- 3.Park SJ, Kim YH, Park DW, Lee SW, Kim WJ, Suh J, et al. Impact of intravascular ultrasound guidance on long-term mortality in stenting for unprotected left main coronary artery stenosis. Circ Cardiovasc Interv. 2009;2:167–77. doi: 10.1161/CIRCINTERVENTIONS.108.799494. [DOI] [PubMed] [Google Scholar]
- 4.de la Torre Hernandez JM, Baz Alonso JA, Gómez Hospital JA, Alfonso Manterola F, Garcia Camarero T, Gimeno de Carlos F, et al. Clinical impact of intravascular ultrasound guidance in drug-eluting stent implantation for unprotected left main coronary disease:pooled analysis at the patient-level of 4 registries. JACC Cardiovasc Interv. 2014;7:244–54. doi: 10.1016/j.jcin.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 5.Mintz GS. Features and parameters of drug-eluting stent deployment discoverable by intravascular ultrasound. Am J Cardiol. 2007;100:26M–35M. doi: 10.1016/j.amjcard.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 6.Roy P, Steinberg DH, Sushinsky SJ, Okabe T, Pinto Slottow TL, Kaneshige K, et al. The potential clinical utility of intravascular ultrasound guidance in patients undergoing percutaneous coronary intervention with drug-eluting stents. Eur Heart J. 2008;29:1851–7. doi: 10.1093/eurheartj/ehn249. [DOI] [PubMed] [Google Scholar]
- 7.Gao XF, Kan J, Zhang YJ, Zhang JJ, Tian NL, Ye F, et al. Comparison of one-year clinical outcomes between intravascular ultrasound-guided versus angiography-guided implantation of drug-eluting stents for left main lesions:a single-center analysis of a 1,016-patient cohort. Patient Prefer Adherence. 2014;8:1299–309. doi: 10.2147/PPA.S65768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan Q, Wang Q, Liu D, Zhang S, Zhang Y, Li Y. Intravascular ultrasound-guided unprotected left main coronary artery stenting in the elderly. Saudi Med J. 2015;36:549–53. doi: 10.15537/smj.2015.5.11251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan ZG, Gao XF, Li XB, Shao MX, Gao YL, Chen SL, et al. The outcomes of intravascular ultrasound-guided drug-eluting stent implantation among patients with complex coronary lesions:a comprehensive meta-analysis of 15 clinical trials and 8,084 patients. Anatol J Cardiol. 2017;17:258–68. doi: 10.14744/AnatolJCardiol.2016.7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bavishi C, Sardar P, Chatterjee S, Khan AR, Shah A, Ather S, et al. Intravascular ultrasound-guided vs angiography-guided drug-eluting stent implantation in complex coronary lesions:Meta-analysis of randomized trials. Am Heart J. 2017;185:26–34. doi: 10.1016/j.ahj.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 11.Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961–72. doi: 10.1056/NEJMoa0804626. [DOI] [PubMed] [Google Scholar]
- 12.Chen SL, Chen JP, Mintz G, Xu B, Kan J, Ye F, et al. Comparison between the NERS (New Risk Stratification) score and the SYNTAX (Synergy between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery) score in outcome prediction for unprotected left main stenting. JACC Cardiovasc Interv. 2010;3:632–41. doi: 10.1016/j.jcin.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Hong MK, Mintz GS, Lee CW, Park DW, Choi BR, Park KH, et al. Intravascular ultrasound predictors of angiographic restenosis after sirolimus-eluting stent implantation. Eur Heart J. 2006;27:1305–10. doi: 10.1093/eurheartj/ehi882. [DOI] [PubMed] [Google Scholar]
- 14.Mauri L, Hsieh WH, Massaro JM, Ho KK, D'Agostino R, Cutlip DE. Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med. 2007;356:1020–9. doi: 10.1056/NEJMoa067731. [DOI] [PubMed] [Google Scholar]
- 15.Ragosta M, Dee S, Sarembock IJ, Lipson LC, Gimple LW, Powers ER. Prevalence of unfavorable angiographic characteristics for percutaneous intervention in patients with unprotected left main coronary artery disease. Catheter Cardiovasc Interv. 2006;68:357–62. doi: 10.1002/ccd.20709. [DOI] [PubMed] [Google Scholar]
- 16.Fihn SD, Blankenship JC, Alexander KP, Bittl JA, Byrne JG, Fletcher BJ, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease:a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2014;64:1929–49. doi: 10.1016/j.jacc.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 17.Morice MC, Feldman TE, Mack MJ, Ståhle E, Holmes DR, Colombo A, et al. Angiographic outcomes following stenting or coronary artery bypass surgery of the left main coronary artery:fifteen-month outcomes from the synergy between PCI with TAXUS express and cardiac surgery left main angiographic substudy (SYNTAX-LE MANS) EuroIntervention. 2011;7:670–9. doi: 10.4244/EIJV7I6A109. [DOI] [PubMed] [Google Scholar]
- 18.Sano K, Mintz GS, Carlier SG, de Ribamar Costa J, Jr, Qian J, Missel E, et al. Assessing intermediate left main coronary lesions using intravascular ultrasound. Am Heart J. 2007;154:983–8. doi: 10.1016/j.ahj.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Nissen SE, Yock P. Intravascular ultrasound:novel pathophysiological insights and current clinical applications. Circulation. 2001;103:604–16. doi: 10.1161/01.cir.103.4.604. [DOI] [PubMed] [Google Scholar]
- 20.Carrié D, Eltchaninoff H, Lefèvre T, Silvestri M, Brunel P, Fajadet J, et al. Early and long-term results of unprotected left main coronary artery stenosis with paclitaxel-eluting stents:the FRIEND (French multicentre RegIstry for stenting of uNprotecteD LMCA stenosis) registry. EuroIntervention. 2011;7:680–8. doi: 10.4244/EIJV7I6A110. [DOI] [PubMed] [Google Scholar]
- 21.Lee PH, Ahn JM, Chang M, Baek S, Yoon SH, Kang SJ, et al. Left Main Coronary Artery Disease:Secular Trends in Patient Characteristics, Treatments, and Outcomes. J Am Coll Cardiol. 2016;68:1233–46. doi: 10.1016/j.jacc.2016.05.089. [DOI] [PubMed] [Google Scholar]
- 22.Chen SL, Xu B, Han YL, Sheiban I, Zhang JJ, Ye F, et al. Comparison of double kissing crush versus Culotte stenting for unprotected distal left main bifurcation lesions:results from a multicenter, randomized, prospective DKCRUSH-III study. J Am Coll Cardiol. 2013;61:1482–8. doi: 10.1016/j.jacc.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 23.Lee CW, Ahn JM, Cavalcante R, Sotomi Y, Onuma Y, Suwannasom P, et al. Coronary Artery Bypass Surgery Versus Drug-Eluting Stent Implantation for Left Main or Multivessel Coronary Artery Disease:A Meta-Analysis of Individual Patient Data. JACC Cardiovasc Interv. 2016;9:2481–9. doi: 10.1016/j.jcin.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 24.Behan MW, Holm NR, de Belder AJ, Cockburn J, Erglis A, Curzen NP, et al. Coronary bifurcation lesions treated with simple or complex stenting:5-year survival from patient-level pooled analysis of the Nordic Bifurcation Study and the British Bifurcation Coronary Study. Eur Heart J. 2016;37:1923–8. doi: 10.1093/eurheartj/ehw170. [DOI] [PubMed] [Google Scholar]
- 25.Chen SL, Zhang JJ, Han Y, Kan J, Chen L, Qiu C, et al. Double Kissing Crush Versus Provisional Stenting for Left Main Distal Bifurcation Lesions:DKCRUSH-V Randomized Trial. J Am Coll Cardiol. 2017;70:2605–17. doi: 10.1016/j.jacc.2017.09.1066. [DOI] [PubMed] [Google Scholar]