Abstract
Objective:
Omentin is a recently identified novel adipocytokine mainly expressed in the epicardial adipose tissue. Although it has favorable effects on cardiovascular disease, the impact of omentin on the hearts is still an understudied issue. The aim of the present study was to investigate the possible effects of omentin on isolated rat heart.
Methods:
Using the Langendorff method, 28 adult male Sprague–Dawley rat hearts were isolated and perfused with modified Krebs–Henseleit solution (mK–Hs). Concentrations of 100, 200, and 400 ng/mL omentin were given to the hearts for 30 min. The control group (n=7) was perfused with mK–Hs alone. Gene expressions in the left ventricle tissue were determined by real-time polymerase chain reaction. Left ventricular cyclic adenosine monophosphate and cyclic guanosine monophosphate (cGMP) concentrations were determined by using enzyme-linked immunosorbent assay.
Results:
All concentrations of omentin significantly decreased left ventricular developed pressure and maximal rate of pressure development that are the indexes of cardiac contractility. At the same time, omentin decreased both phosphoinositide 3-kinase γ (PI3Kγ) and sarcolemmal L-type Ca2+ channel (CaV1.2) mRNA levels. Moreover, this peptide at concentrations of 200 and 400 ng/mL increased endothelial nitric oxide synthase (eNOS) mRNA. Furthermore, concentrations of 200 and 400 ng/mL omentin increased the amount of cGMP.
Conclusion:
We conclude that acute omentin treatment decreases cardiac contractility. Elevated eNOS mRNA and cGMP levels with reduced CaV1.2 mRNA are likely to lead to negative inotropy.
Keywords: cyclic GMP, endothelial nitric oxide synthase, heart contractility, L-type Ca2+ channel, omentin
Introduction
Obesity is a higher risk factor for the development of insulin resistance, type 2 diabetes, cardiovascular disease, and dyslipidemia (1). Adipocytokines are known as proteins secreted from the adipose tissue. Omentin, which was previously named as intelectin, is a new adipocytokine consisting of 313 amino acids (2). It is abundantly expressed in the epicardial and omental adipose tissues (3), but there are limited data on its effects. Previous studies demonstrated that omentin increased insulin-stimulated glucose transport in adipocytes (2) and exerted an anti-inflammatory action in human vascular endothelial cells (4). Omentin enhances the proliferation of human osteoblasts (5) and the growth of neural stem cell (6). Furthermore, it has orexigenic effects (7).
Omentin may affect cardiovascular functions and gene expressions. It has been reported that plasma concentration of omentin as well as its gene expression are reduced in obesity (8), and reduced circulating levels of omentin are associated with cardiovascular diseases, such as atherosclerosis (9), heart failure (10), and acute myocardial infarction (11). The levels of circulating omentin are also decreased in obesity-related diseases, including metabolic syndrome and type 2 diabetes (12). Recently, experimental studies show that omentin has protective effects against myocardial ischemia-reperfusion damage (13). Omentin decreases myocardial hypertrophy and stimulates angiogenesis in ischemia (12). Furthermore, in isolated rat aorta precontracted by noradrenaline, omentin treatment causes vasodilation via endothelium-dependent nitric oxide (NO) and inhibits contractions induced by noradrenaline (14). Omentin acutely decreases agonist-induced hypertension in rats, and it was suggested that NO mediated the action of omentin (15).
Although there are studies (9-11) that omentin affects cardiovascular functions, the role of omentin on cardiovascular functions on isolated perfused rat hearts was not explored. Furthermore, omentin may influence gene expressions together with tissue level of cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP). However, the effects of omentin on the expressions of phosphoinositide 3-kinase α (PI3Kα), phosphoinositide 3-kinase γ (PI3Kγ), beta-adrenergic receptor 1 (β1-AR), beta-adrenergic receptor 2 (β2-AR), endothelial nitric oxide synthase (eNOS), and L-type Ca2+ channel (CaV1.2) genes, as well as the amounts of cAMP and cGMP, have not been studied. The expressions of these genes affect intracellular Ca2+ regulation and cardiac contractility (16-20). Moreover, cAMP and cGMP modulate contractile function of the heart (21, 22). Therefore, we studied the possible effects of omentin on left ventricular developed pressure (LVDP), maximal rate of pressure development (+dP/dtmax), heart rate, coronary flow, monophasic action potential amplitude (MAPamp), MAP duration at 90% repolarization (MAP90), the genes mentioned above, and cAMP and cGMP levels.
Methods
Isolated heart preparation
Adult male Sprague–Dawley rats (250–350 g) were fed with a standard diet and housed in cages with a 12-hour light/dark cycle at 20–25 °C. The procedures in the present study were conducted in accordance to the “Guide to the Care and Use of Experimental Animals” by the Canadian Council of Animal Care (23). The Institutional Animal Care and Use Committee (IACUC) of the university approved the experimental protocols (IACUC approval no.: 385/2014). Twenty-eight adult male Sprague–Dawley rat hearts were divided into four groups, and each group was seven. Concentrations of 100, 200, and 400 ng/ml omentin were given to the hearts for 30 min and perfused with Krebs–Henseleit (mK–Hs) solution. The control group was perfused with mK–Hs alone for 30 min. Rats were anesthetized by sodium thiopental (50 mg/kg) after the intraperitoneal administration of 1.000 IU heparin. The rat’s chest was opened after ensuring the depth of anesthesia. The heart was rapidly isolated and placed into ice cold mK–Hs until contractions stopped. The tissues around the heart were removed, and the heart was quickly transferred to the Langendorff apparatus. The aorta was immediately attached to a stainless-steel aortic cannula. Thereafter, retrograde perfusion was done under a constant pressure (60 mm Hg) using a noncirculating Langendorff method. The pulmonary artery was incised to provide complete coronary drainage in the ventricles. For perfusion, mK–Hs solution containing (mM) NaCl (118), KCl (4.7), CaCl2 (2.5), MgSO4 (1.2), KH2PO4 (1.2), NaHCO3 (25), and glucose (11) was used. The solution was prepared daily and continuously oxygenated with 95% O2 and 5% CO2. The pH and temperature of the solution were maintained at 7.4±0.1 and 37°C, respectively.
Measurement of cardiovascular parameters and experimental protocol
Cardiac contractile force was measured by using the technique previously described by He and Downey (24). A liquid-filled latex balloon was connected to a pressure transducer (Isotec; Hugo Sachs Elektronik, March-Hugstetten, Germany) and inserted to the left ventricle through the mitral valve. The balloon was inflated with the aid of a glass syringe to achieve a diastolic pressure of 8 mm Hg, and the balloon pressure was kept at this value. Peak systolic and end-diastolic pressures were measured. LVDP was calculated as the difference between the systolic and diastolic pressures and used as a contractility index. The left ventricular pressure was processed by a data acquisition software (Isoheart Software, version 1.5 for Microsoft Windows NT/2000/XP; Hugo Sachs Elektronik). +dP/dtmax was determined and was used as the other contractility index. Heart rate was calculated from the signals of the left ventricular pressure. The coronary flow, an index of the coronary vascular tone, was measured from the collection of the coronary effluent during 1 min in a graduated cylinder. All of the cardiovascular parameters, except coronary flow, were analyzed by a data acquisition and analysis system (Isoheart Software). Contact electrode technique (25) was applied, and MAP electrodes (Ag/AgCl2) were used for MAP recordings. MAPamp and MAP90 recordings were measured by pressing an electrode to the epicardium of the left ventricle while the other electrode touched the epicardium. A constant contact pressure between MAP electrode and epicardium was provided. The hearts were allowed to equilibrate for 30 min to obtain a stable baseline. In our study, the inclusion criteria of the hearts were LVDP >60 mm Hg, +dP/dtmax >2800 mm Hg s−1, heart rate >200 beats/min, and normal sinus rhythm. Rat omentin (ProSpec, Brunswick, USA) was dissolved in distilled water, stored at −20°C, and administered to the hearts for 30 min after the stabilization period. Four experimental groups were studied. In group 1, the hearts were perfused with only mK–Hs for 30 min (control group). In groups 2, 3, and 4, the hearts were perfused with mK–Hs containing 100, 200, and 400 ng/mL omentin, respectively. All cardiovascular values were recorded at 10 min, 20 min, and 30 min of the 30 min observation period in the control and experimental groups. After the cardiovascular parameters were studied, the left ventricular tissue was stored at −80°C for subsequent gene expressions and enzyme-linked immunosorbent assay (ELISA) studies.
mRNA preparation and real-time quantitative polymerase chain reaction
All tissues were stored 1 day in the RNA later reagent (Qiagen, Germany) for RNA stabilization and then frozen at −80°C until further molecular analysis. Total RNA was extracted from the left ventricular tissue samples using a TriPure reagent (Roche Life Science, Mannheim, Germany) according to the manufacturer’s instruction (Roche Life Science). The concentration and purity of RNA were measured by reading the absorbance at 260 and 280 nm using a spectrophotometer (NanoDrop 1000 Spectrophotometer; Thermo Fisher Scientific, Wilmington, USA). The 260 nm/280 nm fluorescence ratio of all samples was 1.8. The integrity of RNA was also assessed by electrophoresis using a stained ethidium bromide (Fisher BioReagents, USA) with 1.2% agarose gel (Lonza, Rockland, USA). Intact RNA was reflected by the 18S and 28S ribosomal bands. Five ng of RNA was reverse transcribed by a Transcriptor High Fidelity cDNA Synthesis Kit (Roche Life Science). The mRNA levels of all genes were measured by real-time (RT) quantitative polymerase chain reaction (PCR) using the LightCycler 480 I (Roche Applied Science, Mannheim, Germany). cDNA (50 ng) was quantified using the FastStart Probes Master Kit (Roche Applied Science) and TaqMan Probe/Primer Sets. TaqMan Probe/Primer Sets were PI3Kα, NM_013005.1; PI3Kγ, NM_022213.1; β1-AR, NM_012701.1; β2-AR, NM_012492.2; eNOS, NM_021838.2; CaV1.2, NM_012517.2; and Beta actin, NM_031144.3 (TIB Molbiol, Berlin, Germany). The Beta Actin gene was used as a housekeeping gene, and each sample was run as a duplicate together with negative control. Relative gene expression was normalized and calculated by using the 2−ΔΔCT method (26).
ELISA
Frozen left ventricular tissue (50–100 mg) was treated with phosphate-buffered saline at 0°C and centrifuged at 2000–3000 rpm for 20 min. The supernatant was extracted, and then the cAMP and cGMP concentrations of aqueous phase were measured by a commercial enzyme immunoassay (YH Biosearch, Shanghai, China). The absorbance of samples was read at 450 nm by using an automated ELISA reader (Awareness Technology, Inc., Palm City, USA) according to the manufacturer’s instruction.
Statistical analysis
Statistical analysis was performed by using SPSS for Windows (version 13.0; SPSS Inc., Chicago, USA). The normality of data distribution was analyzed by Shapiro–Wilk test and Kolmogorov–Smirnov test with Lilliefor’s correction. One-way analysis of variance and Tukey HSD multiple comparisons post hoc test were used for data analysis. Values were expressed as mean±SEM. A p value <0.05 was accepted as statistically significant.
Results
Effects of omentin on cardiovascular variables
All of the groups had a stabilization period. The effect of omentin in three different concentrations and the control group was compared after this period. Compared with the control group, administration of 100 ng/mL omentin to the hearts significantly decreased LVDP at 30 min (p<0.01). Concentrations of 200 and 400 ng/mL omentin significantly reduced LVDP at 5 min, 10 min, 20 min, and 30 min (p<0.001). The negative inotropic effect of omentin was concentration-dependent, and maximal decreases in LVDP were found at 30 min (Fig. 1a). As shown in Figure 1b, 100 ng/mL omentin decreased +dP/dtmax value at 30 min (p<0.05). Concentrations of 200 and 400 ng/mL omentin also decreased +dP/dtmax values at 5 min, 10 min, 20 min, and 30 min of the observation period (p<0.001). The time course of the impact of +dP/dtmax was similar to those of LVDP. Omentin-induced reductions in +dP/dtmax values were concentration-dependent, and maximal decreases in +dP/dtmax values were observed at 30 min (Fig. 1b). Furthermore, none of the omentin concentration studied changed heart rate, coronary flow, MAPamp, and MAP90 values throughout a 30-minute observation period (Fig. 2a, 2b and 3a, 3b).
Figure 1.
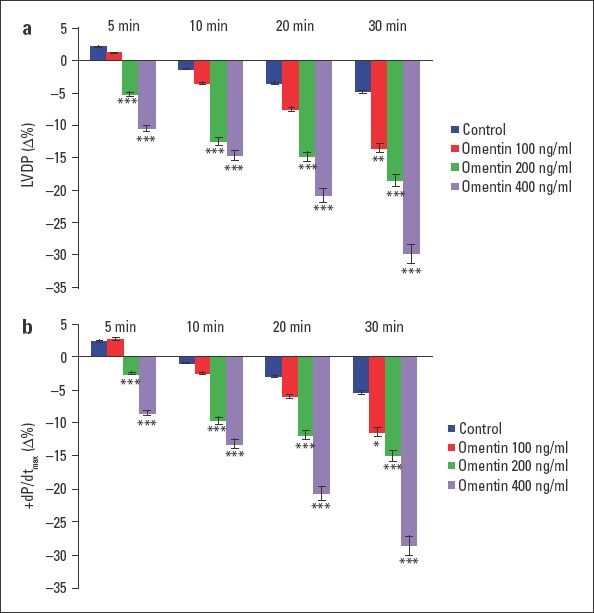
Time-dependent effect of omentin on LVDP (a) and +dP/dtmax (b). Δ% is the change as percentage of the 0 min value that is the value obtained prior to the administration of omentin in the omentin groups and the change as percentage of the 0 min value that is the value obtained after a 30-minute stabilization period in the control groups. −Δ% shows decrease. *P<0.05, **P<0.01, and ***P<0.001 significantly different from the respective control (n=7)
Figure 2.
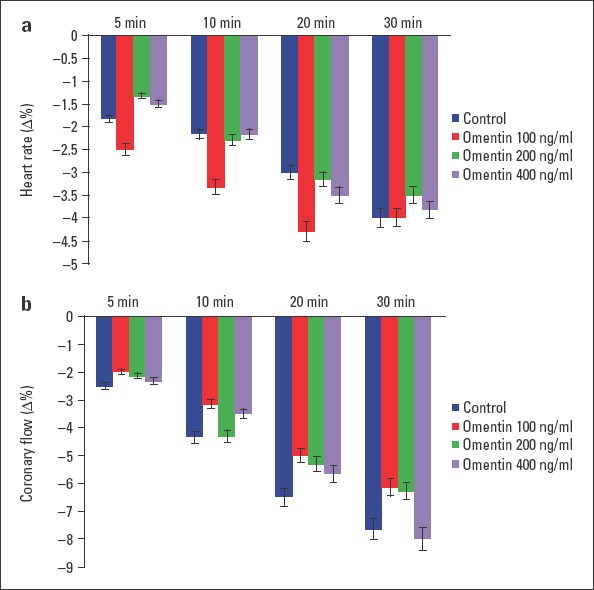
Time-dependent effect of omentin on heart rate (a) and coronary flow (b). Δ% is the change as percentage of the 0 min value that is the value obtained prior to the administration of omentin in the omentin groups and the change as percentage of the 0 min value that is the value obtained after a 30-minute stabilization period in the control groups. −Δ% shows decrease (n=7)
Figure 3.
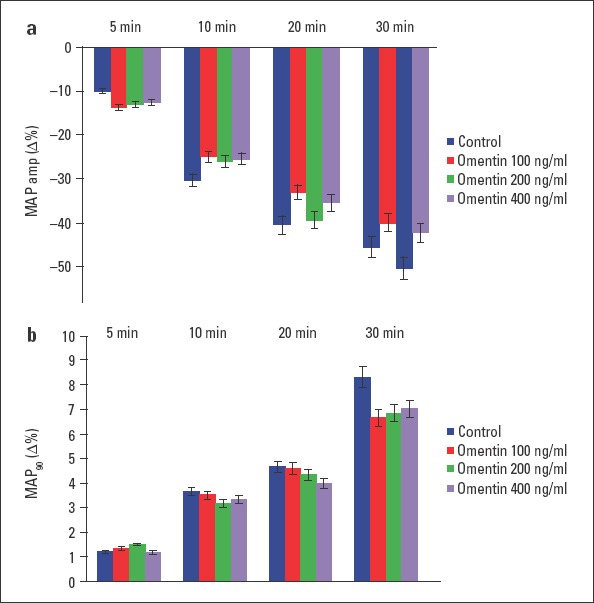
Time-dependent effect of omentin on MAPamp (a) and MAP90 (b). Δ% is the change as percentage of the 0 min value that is the value obtained prior to the administration of omentin in the omentin groups and the change as percentage of the 0 min value that is the value obtained after a 30-minute stabilization period in the control groups. −Δ% and +Δ% show decrease and increase, respectively (n=7)
Impact of omentin on gene expressions and cAMP and cGMP production
Compared with the control, the PI3Kα mRNA levels for each group were not significantly different. However, the PI3Kγ mRNA levels for all concentrations of omentin were significantly lower (p<0.01 for 200 and p<0.001 for 100 and 400 ng/mL). The expression of PI3Kγ mRNA was 1.86-, 1.83-, and 2.07-fold lower for 100, 200, and 400 ng/mL, respectively (Table 1). Omentin treatment did not alter the β1-AR and β2-AR gene expressions. Omentin (100 ng/mL) did not result in significant alterations in eNOS mRNA; however, 200 and 400 ng/mL omentin caused marked increases in eNOS mRNA (p<0.01 for 200 and p<0.001 for 400 ng/mL). The expression of the eNOS gene was increased 4.2-fold for 200 and 4.23-fold for 400 ng/mL omentin concentration groups. Furthermore, omentin significantly decreased the mRNA levels of CaV1.2 in a concentration-dependent manner (p<0.01 for 100 and p<0.001 for 200 and 400 ng/mL). CaV1.2 gene expression was reduced 1.58-, 1.98-, and 2.26-fold for 100, 200, and 400 ng/mL, respectively (Table 1). Although there were concentration-dependent increases in cAMP values measured from omentin-treated hearts, these changes did not reach a significance level (Table 2). Treatment with 100 ng/mL omentin did not change cGMP amount significantly. However, 200 and 400 ng/mL concentration groups had significantly higher cGMP amounts than the control group (p<0.001, Table 2).
Table 1.
The relative expression of PI3Kα, PI3Kγ, β1-AR, β2-AR, eNOS, and CaV1.2 in the control and omentin-treated groups
| Experimental group | Gene name PI3Kα mRNA fold changes | PI3Kγ mRNA fold changes | β1-AR mRNA fold changes | β2-AR mRNA fold changes | eNOS mRNA fold changes | CaV1.2 mRNA fold changes |
|---|---|---|---|---|---|---|
| Control | 1.99±0.22 | 1.08±0.11 | 1.56±0.13 | 0.76±0.07 | 0.59±0.09 | 1.27±0.1 |
| 100 ng/mL omentin | 2.69±0.76 | 0.58±0.05*** | 1.92±0.26 | 0.65±0.07 | 1.59±0.33 | 0.8±0.09** |
| 200 ng/mL omentin | 1.69±0.74 | 0.6±0.09** | 1.66±0.27 | 0.81±0.17 | 2.48±0.65** | 0.65±0.05*** |
| 400 ng/mL omentin | 1.28±0.06 | 0.52±0.04*** | 1.58±0.27 | 0.88±0.28 | 2.6±0.52*** | 0.56±0.07*** |
| ANOVA P values | P=0.34 | P<0.001 | P=0.71 | P=0.82 | P<0.001 | P<0.001 |
| Tukey post hoc test P values | - | 0-1 | - | - | 0-2 | 0-1 |
| P<0.001 | P<0.01 | P<0.01 | ||||
| 0-2 | 0-3 | 0-2 | ||||
| P<0.01 | P<0.001 | P<0.001 | ||||
| 0-3 | 0-3 | |||||
| P<0.001 | P<0.001 |
Data show mRNA fold changes (n=7).
0: control, 1: 100 ng/mL omentin, 2: 200 ng/mL omentin, 3: 400 ng/mL omentin
Table 2.
The impact of omentin on cAMP and cGMP amount in the left ventricular tissue
| Experimental group | cAMP nmol/g | cGMP nmol/g |
|---|---|---|
| Control | 5.25±0.87 | 7.5±1.14 |
| 100 ng/mL omentin | 7.2±2.03 | 6.9±1.18 |
| 200 ng/mL omentin | 9.19±1.5 | 14.5±0.7*** |
| 400 ng/mL omentin | 11.29±1.8 | 15.04±1.43*** |
| ANOVA P values | P=0.08 | P<0.001 |
| Tukey post hoc test P values 0-3 P<0.001 | 0-2 P<0.001 |
0: control, 1: 100 ng/mL omentin, 2: 200 ng/mL omentin, 3: 400 ng/mL omentin.
cAMP - cyclic adenosine monophosphate; cGMP - cyclic guanosine monophosphate
Discussion
In the present study, omentin significantly reduced LVDP and +dP/dtmax values in isolated perfused rat hearts. Application of isolated hearts with omentin protein increased eNOS mRNA, and cGMP levels reduced the expression of the CaV1.2 gene in the cardiac tissue. These results indicate that NO and CaV1.2 may mediate the decrease in cardiac contractility.
It has been reported that adipocytokines activate several signaling pathways and omentin activated PI3K/Akt signal pathway in human osteoblast (5). Activation of this signaling pathway results in phosphorylation of Akt, and phosphorylated Akt enhances NO production by eNOS phosphorylation. NO activates soluble guanyl cyclase, leading to the production of cGMP (27) and protein kinase G (PKG) activation (28). The stimulation of PKG inhibits L-type Ca2+ channel currents that cause the negative inotropic effect. The activation of PKG also desensitizes cardiac myofilaments to Ca2+ (29), and desensitization produces negative inotropy. Furthermore, cGMP is involved in the regulation of phosphodiesterases (PDEs) by stimulating PDE2 and inhibiting PDE3. cGMP-dependent inhibition of PDE3 at low levels of NO and cGMP elevates L-type Ca2+ channel currents by cAMP–protein kinase A (PKA)-dependent mechanism (27). Thus, a positive inotropic effect occurs. On the other hand, high NO levels decrease contractions via the activation of PKG (30).
A decrease in PI3Kα, which is a PI3K isoform, reduces the number of L-type Ca2+ channels present in cardiomyocytes, and decreased L-type Ca2+ current results in a reduction of cardiac contractility (31). On the contrary, the PI3Kα overexpression causes increased cardiac contractions in transgenic mice (32). In the present study, omentin did change the PI3Kα gene expression. That is why the PI3Kα gene probably did not contribute to the omentin-induced negative inotropic effect.
Another isoform of PI3K is PI3Kγ. Both PI3Kα and PI3Kγ are expressed by mammalian cardiomyocytes. PI3Kγ inhibits cardiac contractility and cAMP formation (27). The catalytic subunit of PI3Kγ, p110γ, binds to PKA that increases the activation of PDE3, and a decrease in cAMP levels occurs (18). In contrast, the loss of PI3Kγ enhances cardiac contractions and cAMP amounts (16). There is a relationship between cAMP levels and cardiac contractility. For example, stimulation of β-AR elevates cAMP levels and induces positive inotropic and chronotropic effects in the myocardium (33, 34). The literature shows that intracellular cAMP levels increase when the PI3Kγ gene expression levels decrease (18, 16). The amounts of cAMP that is required to be evaluated together with decreased PI3Kγ gene expression did not increase statistically, but demonstrated two-fold increase in comparison to the control values. Omentin did not influence β1-AR and β2-AR gene expressions, suggesting that β1-AR and β2-AR genes play no role in the negative inotropy. Further studies are needed to fully explain the mechanisms underlying omentin-induced decrease in myocardial contractility.
It has been also observed that heart rate was not changed after omentin administration to normotensive rats (34). Similarly, we observed that the administration of omentin did not change heart rate. Our result suggests that omentin does not play a role in the regulation of heart rate. Additionally, we found that omentin did not affect coronary flow. Yamawaki et al. (14) reported that omentin (300 ng/mL) inhibits noradrenaline-induced contraction responses in the endothelium-intact isolated rat aorta and mesenteric artery. Thus, they demonstrated that omentin produces a vasodilating action mediated by NO. It is known that vasodilation increases coronary flow. The concentrations of omentin in both studies are similar, but Yamawaki et al. (14) examined the effect of omentin in the aorta precontracted by noradrenaline, and their vessel preparation had a high tone. However, we investigated omentin action in preparations that were not precontracted, and our isolated heart preparation had no high vessel tone. Therefore, different results may depend on the difference in methods used.
MAP recording is an important tool for physiological and pathophysiological studies in cardiology, and MAP is very similar to the transmembrane MAP recorded by an electrode placed outside of the cell (35, 36). MAP produces accurate information for repolarization abnormalities causing arrhythmia (37) and is used to understand the actions and mechanisms of anti-arrhythmic drugs (38). It has been shown that MAPamp is formed due to the diffusion of Na+ ions into the cell via voltage-gated Na+ channels during the first phase of MAP (39). MAP90 is inversely proportional to heart beats, and MAP duration is affected by the balance between inward depolarizing and outward repolarizing currents in the myocytes (40, 41). Our findings suggest that omentin does not influence these currents. Moreover, in the present study, omentin treatment did not change heart rate. Therefore, we did not observe a significant change in MAP90.
Study limitations
The Langendorff method, despite being widely employed, suffers from certain restrictions and shortcomings. Coronary perfusion pressure is recognised as a useful index of coronary vascular tone. Rather than coronary perfusion pressure, in this study, however, we measured coronary effluent amounts through the collection of the coronary effluent during one minute in a graduated cylinder. The findings suggest that there was no increase in coronary flow despite the rise in eNOS mRNA quantities, which is why counterintuitive outcomes may be also the result of the different methods used.
Conclusion
We, for the first time, observed that acute omentin treatment exerts a negative inotropic effect, whereas it does not affect heart rate, coronary flow, MAPamp, and MAP90. Our results also demonstrated that application of omentin to isolated rat hearts reduces the PI3Kγ and CaV1.2 gene expressions. However, this peptide enhances eNOS mRNA and tissue cGMP levels. In addition, omentin does not influence β1-AR, β2-AR, and PI3Kα mRNA expressions and tissue cAMP levels. Our findings also suggest that increased eNOS mRNA and cGMP levels as well as reduced gene expression of CaV1.2 might be involved in the negative inotropy. Moreover, additional studies are necessary to explain the effect of omentin on cardiovascular parameters and gene expressions.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – Ö.K., Z.K., B.K.; Design – Ö.K., Z.K., B.K.; Supervision – Ö.K., Z.K., B.K.; Fundings – Ö.K., Z.K., B.K.; Materials – Ö.K., Z.K., B.K.; Data collection &/or processing – Ö.K., Z.K., B.K.; Analysis &/or interpretation – Ö.K., Z.K., B.K.; Literature search – Ö.K., Z.K., B.K.; Writing – Ö.K., Z.K., B.K.; Critical review – Ö.K., Z.K., B.K.
References
- 1.Yang RZ, Lee MJ, Hu H, Pray J, Wu HB, Hansen BC, et al. Identification of omentin as a novel depot-specific adipokine in human adipose tissue:possible role in modulating insulin action. Am J Physiol Endocrinol Metab. 2006;290:E1253–61. doi: 10.1152/ajpendo.00572.2004. [DOI] [PubMed] [Google Scholar]
- 2.Schäffler A, Neumeier M, Herfarth H, Fürst A, Schölmerich J, Büchler C. Genomic structure of human omentin, a new adipocytokine expressed in omental adipose tissue. Biochim Biophys Acta. 2005;1732:96–102. doi: 10.1016/j.bbaexp.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Fain JN, Sacks HS, Buehrer B, Bahouth SW, Garrett E, Wolf RY, et al. Identification of omentin mRNA in human epicardial adipose tissue:comparison to omentin in subcutaneous, internal mammary artery periadventitial and visceral abdominal depots. Int J Obes (Lond) 2008;32:810–5. doi: 10.1038/sj.ijo.0803790. [DOI] [PubMed] [Google Scholar]
- 4.Yamawaki H, Kuramoto J, Kameshima S, Usui T, Okada M, Hara Y. Omentin, a novel adipocytokine inhibits TNF-induced vascular inflammation human endothelial cells. Biochem Biophys Res Commun. 2011;408:339–43. doi: 10.1016/j.bbrc.2011.04.039. [DOI] [PubMed] [Google Scholar]
- 5.Wu SS, Liang QH, Liu Y, Cui RR, Yuan LQ, Liao EY. Omentin-1stimulates human osteoblast proliferation through PI3K/Akt signal pathway. Int J Endocrinol. 2013;2013:368970. doi: 10.1155/2013/368970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao LR, Du YJ, Chen L, Liu ZG, Jia XY, Pan YH, et al. Ometin-1 promotes the growth of neural stem cells via activation of Akt signaling. Mol Med Rep. 2015;11:1859–64. doi: 10.3892/mmr.2014.2937. [DOI] [PubMed] [Google Scholar]
- 7.Brunetti L, Orlando G, Ferrante C, Recinella L, Leone S, Chiavaroli A, et al. Orexigenic effects of omentin-1related to decreased CARTand CRH gene expression and increased norepinephrine synthesis and release in the hypothalamus. Peptides. 2013;44:66–74. doi: 10.1016/j.peptides.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 8.de Souza Batista CM, Yang RZ, Lee MJ, Glynn NM, Yu DZ, Pray J, et al. Omentin plasma levels and gene expression are decreased in obesity. Diabetes. 2007;56:1655–61. doi: 10.2337/db06-1506. [DOI] [PubMed] [Google Scholar]
- 9.Yoo HJ, Hwang SY, Hong HC, Choi HY, Yang SJ, Seo JA, et al. Association of circulating omentin-1 level with arterial stiffness and carotid plaque in type 2 diabetes. Cardiovasc Diabetol. 2011;10:103. doi: 10.1186/1475-2840-10-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Narumi T, Watanabe T, Kadowaki S, Kinoshita D, Yokoyama M, Honda Y, et al. Impact of serum omentin-1 levels on cardiac prognosis in patients with heart failure. Cardiovasc Diabetol. 2014;13:84. doi: 10.1186/1475-2840-13-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kadoglou NP, Tahmatzidis DK, Giannakoulas C, Kapelouzou A, Gkontopoulos A, Parissis J, et al. Serum levels of novel adipokines, omentin-1 and chemerin, in patients with acute myocardial infarction:KOZANI STUDY. J Cardiovasc Med (Hagerstown) 2015;16:341–6. doi: 10.2459/JCM.0000000000000053. [DOI] [PubMed] [Google Scholar]
- 12.Matsuo K, Shibata R, Ohashi K, Kambara T, Uemura Y, Hiramatsu-Ito M, et al. Omentin functions to attenuate cardiac hypertrophic response. J Mol Cell Cardiol. 2015;79:195–202. doi: 10.1016/j.yjmcc.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 13.Kataoka Y, Shibata R, Ohashi K, Kambara T, Enomoto T, Uemura Y, et al. Omentin prevents myocardial ischemic injury through AMP-activated protein kinase- and Akt-dependent mechanisms. J Am Coll Cardiol. 2014;63:2722–33. doi: 10.1016/j.jacc.2014.03.032. [DOI] [PubMed] [Google Scholar]
- 14.Yamawaki H, Tsubaki N, Mukohda M, Okada M, Hara Y. Omentin, a novel adipokine, induces vasodilation in rat isolated blood vessels. Biochem Biophys Res Commun. 2010;393:668–72. doi: 10.1016/j.bbrc.2010.02.053. [DOI] [PubMed] [Google Scholar]
- 15.Kazama K, Okada M, Hara Y, Yamawaki H. Novel adipocytokine, omentin, inhibits agonists-induced increases of blood pressure in rats. J Vet Med Sci. 2013;75:1029–34. doi: 10.1292/jvms.12-0537. [DOI] [PubMed] [Google Scholar]
- 16.Kerfant BG, Gidrewicz D, Sun H, Oudit GY, Penninger JM, Backx PH. Cardiac sarcoplasmic reticulum calcium release and load are enhanced by subcellular cAMP elevations in PI3Kgamma-deficient mice. Circ Res. 2005;96:1079–86. doi: 10.1161/01.RES.0000168066.06333.df. [DOI] [PubMed] [Google Scholar]
- 17.Brodde OE, Michel MC. Adrenergic and muscarinic receptors in the human heart. Pharmacol Rev. 1999;51:651–90. [PubMed] [Google Scholar]
- 18.Ghigo A, Morello F, Perino A, Damilano F, Hirsch E. Specific PI3K isoform modulation in heart failure:Lessons from transgenic mice. Curr Heart Fail Rep. 2011;8:168–75. doi: 10.1007/s11897-011-0059-3. [DOI] [PubMed] [Google Scholar]
- 19.Garnicelli V, Frascarelli S, Ghelardoni S, Ronca-Testoni S, Zucchi R. Short-term effects of pressure overload on the expression of genes involved in calcium homeostasis. Mol Cell Biochem. 2008;313:29–36. doi: 10.1007/s11010-008-9738-0. [DOI] [PubMed] [Google Scholar]
- 20.Peng T, Lu X, Lei M, Feng Q. Endothelial nitric-oxide synthase enhances lipopolysaccharide-stimulated tumor necrosis factor-αexpression via cAMP-mediated p38 MAPK pathway in cardiomyocytes. J Biol Chem. 2003;278:8099–105. doi: 10.1074/jbc.M207288200. [DOI] [PubMed] [Google Scholar]
- 21.Alloatti G, Marcantoni A, Levi R, Gallo MP, Del Sorbo L, Patrucco E, et al. Phosphoinositide 3-kinase gamma controls autonomic regulation of the mouse heart through Gi-independent downregulation of cAMP level. FEBS Lett. 2005;579:133–40. doi: 10.1016/j.febslet.2004.11.059. [DOI] [PubMed] [Google Scholar]
- 22.Pellegrino D, Shiva S, Angelone Gladwin MT, Tota B. Nitrite exerts potent negative inotropy in the isolated heart via eNOS-independent nitric oxide generation and cGMP-PKG activation. Biochim Biophys Acta. 2009;1787:818–27. doi: 10.1016/j.bbabio.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Canadian Council on Animal Care:Guide to the care and use of experimental animals. Vol. 2. CCAC Ottawa: 1984. pp. 176–87. [Google Scholar]
- 24.He MX, Downey HF. Downregulation of ventricular contractile function during early ischemia is flow but not pressure dependent. Am J Physiol. 1998;275:H1520–3. doi: 10.1152/ajpheart.1998.275.5.H1520. [DOI] [PubMed] [Google Scholar]
- 25.Franz MR. Current status of monophasic action potential recording:theories, measurements and interpretations. Cardiovasc Res. 1999;41:25–40. doi: 10.1016/s0008-6363(98)00268-5. [DOI] [PubMed] [Google Scholar]
- 26.Fleige S, Pfaffl MW. RNA integrity and the effect on the real-time qRT-PCR performance. Mol Aspects Med. 2006;27:126–39. doi: 10.1016/j.mam.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Walther S, Pluteanu F, Renz S, Nikonova Y, Maxwell JT, Yang LZ, et al. Urocortin 2 stimulates nitric oxide production in ventricular myocytes via Akt- and PKA-mediated phosphorylation of eNOS at serine 1177. Am J Physiol Heart Circ Physiol. 2014;307:H689–700. doi: 10.1152/ajpheart.00694.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seddon M, Shah AM, Casadei B. Cardiomyoctes as effectors of nitric oxide signalling. Cardiovasc Res. 2007;75:315–26. doi: 10.1016/j.cardiores.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 29.Manoury B, Montiel V, Balligand JL. Nitric oxide synthase in post ischaemic remodelling:new pathways and mechanisms. Cardiovasc Res. 2012;94:304–15. doi: 10.1093/cvr/cvr360. [DOI] [PubMed] [Google Scholar]
- 30.Kojda G, Kottenberg K, Nix P, Schluter KD, Piper HM, Noack E. Low increase in cGMP induced by organic nitrates and nitrovasodilator improves contractile response of rat ventricular myocytes. Circ Res. 1996;78:91–101. doi: 10.1161/01.res.78.1.91. [DOI] [PubMed] [Google Scholar]
- 31.Lu Z, Ballou LM, Jiang YP, Cohen IS, Lin RZ. Restoration of defective L-type Ca2+ current in cardiac myocytes of type 2 diabetic db/dbmice by Akt and PKC-ι. J Cardiovasc Pharmacol. 2011;58:439–45. doi: 10.1097/FJC.0b013e318228e68c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yano N, Tseng A, Zhao TC, Robbins J, Padbury JF, Tseng YT. Temporally controlled overexpression of cardiac-specific PI3Kalpha induces enhanced myocardial contractility a new transgenic model. Am J Physiol Heart Circ Physiol. 2008;295:H1690–4. doi: 10.1152/ajpheart.00531.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brodde OT, Bruck H, Leineweber K. Cardiac adrenoceptors:Physiological and pathophysiological relevance. J Pharmacol Sci. 2006;100:323–37. doi: 10.1254/jphs.crj06001x. [DOI] [PubMed] [Google Scholar]
- 34.Alloatti G, Montrucchio G, Lembo G, Hirsch E. Phosphoinositide 3-kinase gamma:kinase-dependent and-independet activities in cardiovascular function and disease. Biochem Soc Trans. 2004;32:383–6. doi: 10.1042/bst0320383. [DOI] [PubMed] [Google Scholar]
- 35.Brunetti L, Leone S, Orlando G, Ferrante C, Recinella L, Chiavaroli A, et al. Hypotensive effects of omentin-1 related to increased adiponectin and decreased interleukin-6 in intra-thoracic pericardial adipose tissue. Pharmacol Rep. 2014;66:991–5. doi: 10.1016/j.pharep.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 36.Yang SG, Kittnar O. New insights into application of cardiac monophasic action potential. Physiol Res. 2010;59:645–50. doi: 10.33549/physiolres.931864. [DOI] [PubMed] [Google Scholar]
- 37.Zhou X, Huang J, Ideker RE. Transmural recording of monophasic action potentials. Am J Physiol Heart Circ Physiol. 2002;282:H855–61. doi: 10.1152/ajpheart.01172.2000. [DOI] [PubMed] [Google Scholar]
- 38.Patterson E, Jackman WM, Scherlag BJ, Lazzara R. The monophasic action potential in clinical cardiology. Clin Cardiol. 1991;14:505–10. doi: 10.1002/clc.4960140610. [DOI] [PubMed] [Google Scholar]
- 39.Xia JS, Li Z, Dong JW, Tu H, Zeng FD. Dauricine-induced changes in monophasic action potentials and effective refractory period of rabbit left ventricle in situ. Acta Pharmacol Sin. 2002;23:371–5. [PubMed] [Google Scholar]
- 40.Wang Y, Chen MS, Liu HC, Xiao JH, Wang JL. The relationship between frequency dependence of action potential duration and the expression of TRPC3 in rabbit ventricular myocardium. Cell Physiol Biochem. 2014;33:646–56. doi: 10.1159/000358641. [DOI] [PubMed] [Google Scholar]
- 41.Dorian P, Newman D. Rate dependence of the effect of antiarrhythmic drugs delaying cardiac repolarization:an overview. Europace. 2000;2:277–85. doi: 10.1053/eupc.2000.0114. [DOI] [PubMed] [Google Scholar]


