Abstract
Objective:
Low free triiodothyronine (fT3) is common in elderly patients with cardiovascular disease. The purpose of this study was to evaluate the relationship between low fT3 and contrast-induced acute kidney injury (CI-AKI), including the long-term outcomes, in elderly patients after a percutaneous coronary intervention (PCI).
Methods:
A total of 350 patients aged ≥75 years who underwent PCI between January 2012 and December 2015 were consecutively enrolled. The perioperative thyroid function, including fT3, was measured before PCI. A low fT3 was defined as fT3 <3.1 pmol/L with normal thyrotropin and free thyroxine. CI-AKI was defined as an absolute serum creatinine (SCr) increase ≥0.30 mg/dL or a relative increase in SCr ≥50% from the baseline value within 48 hours after contrast media exposure. A multivariate logistic regression analysis was applied to analyze whether low fT3 was an independent risk factor for CI-AKI. The Cox regression analysis was used to evaluate the relationship between low fT3 and long-term prognosis.
Results:
A total of 46 (13.1%) patients developed CI-AKI. The incidence of CI-AKI was significantly higher in the low fT3 group than in the normal group (26.5% vs. 9.9%, p<0.01). A multivariable logistic analysis demonstrated that a low fT3 level was significantly related to CI-AKI [odds ratio (OR)=2.41; 95% confidence interval (CI), 1.11–5.27; p=0.027]. The Cox regression analysis showed that a low fT3 was associated with long-term mortality [adjusted hazard ratio (HR)=2.00; 95% CI, 1.04–3.83; p=0.037] during the follow-up of mean 1.67 years.
Conclusion:
A low fT3 concentration was independently associated with CI-AKI and poor prognosis in elderly patients who had undergone PCI.
Keywords: percutaneous coronary intervention, free triiodothyronine, contrast-induced acute kidney injury, long-term prognosis, elderly
Introduction
Percutaneous coronary intervention (PCI) is one of the most effective strategies in treating coronary artery diseases. Advanced health care has increased the life expectancy among elderly patients, thereby providing the opportunity to undergo angiography and PCI. Although significant achievements have been made in the treatment in recent years, contrast-induced acute kidney injury (CI-AKI) remains a frequent and severe complication following PCI, especially among elderly patients older than 75 years (1, 2). CI-AKI is usually irreversible and associated with short-term and long-term adverse effects (3, 4); therefore, identifying high-risk patients and providing early prophylactic measures are critical in the elderly.
Alterations in plasma concentrations of thyroid hormones during acute and chronic illnesses have been long recognized (5, 6). Non-thyroidal illness syndrome (NTIS) has been used to describe the patients who had alterations in the concentration of the thyroid hormone without being previously diagnosed with intrinsic thyroid disease (5, 7). A decrease in total serum triiodothyronine (T3) and free triiodothyronine (fT3) with normal levels of thyroxine (T4) and thyrotropin (TSH), known as low T3 syndrome, is the most common form of NTIS (5). The thyroid hormone has a great impact on the cardiovascular system, and low T3 is common in patients with cardiac disease and may lead to a poor long-term prognosis (8-10). The interaction between thyroid and kidney functions has been well established for many years. Thyroid hormone levels may affect the renal blood flow (RBF), glomerular filtration rate, tubular function, and electrolyte homeostasis (11). Low T3 syndrome has been common in patients with chronic kidney disease (CKD) and has been confirmed to be a strong predictor of adverse clinical outcomes in the elderly (12, 13). Although the number of elderly patients after PCI who are at high risk for CI-AKI is growing, few studies have analyzed the correlation between a low fT3 state and CI-AKI. Therefore, the current study focuses on the relationship between low circulating fT3 and CI-AKI in patients 75 years or older who had undergone PCI, and it discusses the impact of low fT3 on the short- and long-term prognosis.
Methods
Study population
This study was performed between January 2012 and December 2015, and it retrospectively evaluated consecutive 746 elderly patients aged 75 years and older, with coronary artery disease undergoing PCI. Patients with malignant tumors (n=31), patients with a thyroid dysfunction defined as TSH<0.27 mIU/L (n=50) or TSH>4.20 mIU/L (n=6), or talking L-thyroxine (n=0), patients without pre-procedural or post-procedural serum creatinine (SCr) (n=67) or the fT3 level (n=242) data were excluded from the study. Finally, a total of 350 patients were evaluated in the analysis.
Study protocol
This was a retrospective cohort study. Demographic data and clinical history including age, gender, height, weight, diabetes mellitus, hypertension, smoking, prior myocardial infarction (MI), etc., were collected. The blood pressure was measured at admission. Serum concentrations of fT3, free thyroxine (fT4), and TSH were measured before or 24 hours within PCI by electro-chemiluminescence (Roche, COBAS E601). The SCr was measured at admission and for 2 consecutive days after the contrast medium exposure. Other laboratory data including the lipid profile, hemoglobin level, uric acid, and other standard clinical parameters were measured in the morning of the first or next day after admission. Left ventricular ejection fraction (LVEF) was also measured by echocardiography during hospitalization. PCI was performed by qualified interventional cardiologists in accordance to a standard clinical procedure. Nephrotoxic drugs such as metformin and aminoglycoside were suspended before PCI according to the guidelines (14). All patients received nonionic, low-osmolar contrast media (either Iopamiron or Ultravist, 370 mgI/mL). In addition, all patients received intravenous isotonic saline (0.9%) at a rate of 1 mL/kg/h for 12 hours before and continued for 24 hours after the procedure (or 0.5 mL/kg/h for 12 hours if patients were in overt heart failure) according to the guidelines (14). The usage of medications such as antiplatelet agents (aspirin or clopidogrel), statins, angiotensin receptor blocker, and angiotensin-converting enzyme inhibitors were based on interventional guidelines.
Definition and follow-up
The primary end point was CI-AKI, which was defined as an absolute SCr increase ≥0.30 mg/dL or a relative increase in SCr ≥50% from the baseline value within 48 hours after the contrast media administration (15, 16). The additional end point were short-term outcomes, including in-hospital mortality and required renal replacement therapy, long-term outcomes including all-cause mortality, and major adverse clinical events (MACEs) including mortality, stent restenosis, non-fatal MI, and target vessel revascularization. The normal reference ranges of thyroid hormones in our laboratory were as follows: fT3, 3.1–6.8 pmol/L; fT4, 12.0–22.0 pmol/L; TSH, 0.27–4.20 mIU/L; low fT3 was defined as fT3<3.1 pmol/L with normal TSH and fT4 levels. Anemia was defined as hematocrit <0.39 (male) or <0.36 (female). Heart failure at admission was defined as a New York Heart Association class >2 or Killip class >1 at hospital admission (1). Perioperative hypotension was described as systolic blood pressure <80 mm Hg lasting at least 1 hour and requiring inotropic support with medications or intra-aortic balloon pump 24 hours peri-procedure (1).
The participants were followed up by outpatient clinical visits or by telephone after discharge by trained medical workers. The mean follow-up duration after discharge was 1.67 years.
Statistical analysis
The Statistical Program for Social Sciences (SPSS) software 22.0 (SPSS, Inc., Chicago, Illinois, USA) was used for statistical analysis. Continuous variables were expressed as the mean±standard deviation. Categorical variables were described as absolute values (percentages). The continuous variables were evaluated by Student’s t-test or the Wilcoxon rank-sum test, and categorical variables by chi-squared or Fisher’s exact test. A p-value<0.05 indicated statistical significance. The baseline characteristics were compared between the patients with and without CI-AKI. Univariate logistic regression was adopted to identify the variables associated with the development of CI-AKI. Variables found in the univariate analysis with significance and other variables that were confirmed to be significant in previous studies will be included in the multivariate logistic regression analysis. The association of low fT3 level with long-term mortality was investigated by the Cox regression analysis. The Kaplan–Meier curve was used to compare the survival time between the CI-AKI and non-CI-AKI groups, and also between the two groups divided by the lower reference limit of fT3.
Results
This study involved a total of 350 consecutive elderly patients who underwent PCI. The mean age was 79.23±3.65 years, 93 (26.6%) patients were female, 144 (41.1%) had diabetes mellitus, and 271 (77.4%) had hypertension. Sixty-eight (19.4%) patients had a low fT3, and 133 (38.0%) patients were diagnosed with acute MI (AMI). Overall, 46 (13.1%) patients developed CI-AKI. The demographical, medical history, laboratory parameters, perioperative medications, and procedural characteristics are shown in Table 1. Patients who developed CI-AKI had a higher prevalence of heart failure at admission, AMI, perioperative hypotension, and low fT3 (all, p<0.05). However, the LVEF was significantly lower among patients with CI-AKI. The age, baseline SCr, contrast volume, and the incidence of anemia were similar between the two groups (all, p>0.05).
Table 1.
Baseline clinical features in patients with or without CI-AKI
| Variables | Total (n=350) | CI-AKI (+) (n=46) | CI-AKI (–) (n=304) | P |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 79.23±3.65 | 79.85±4.38 | 79.13±3.53 | 0.22 |
| Females, n, % | 93 (26.6) | 14 (30.4) | 79 (26.0) | 0.52 |
| BMI, kg/m2 | 23.54±3.26 | 23.84±4.15 | 23.51±3.16 | 0.64 |
| Heart failure at admission, n, % | 102 (29.1) | 25 (54.3) | 77 (25.4) | <0.001 |
| MI, n, % | 133 (38.0) | 33 (71.7) | 100 (32.9) | <0.001 |
| Medical history | ||||
| Hypertension, n, % | 271 (77.4) | 36 (78.3) | 235 (77.3) | 0.88 |
| Diabetes mellitus, n, % | 144 (41.1) | 22 (47.8) | 122 (40.1) | 0.32 |
| Smoking, n, % | 133 (38.0) | 19 (41.3) | 114 (38.1) | 0.69 |
| Prior MI, n, % | 63 (18.0) | 10 (21.7) | 53 (17.4) | 0.48 |
| Prior PCI, n, % | 84 (24.0) | 7 (15.2) | 77 (25.4) | 0.13 |
| Prior CABG, n, % | 2 (0.6) | 0 (0) | 2 (0.7) | 0.58 |
| Laboratory parameters | ||||
| SCr, mg/dL | 1.00±0.33 | 0.96±0.32 | 1.01±0.33 | 0.32 |
| SCr>1.5 mg/dL, n, % | 25 (7.1) | 3 (6.5) | 22 (7.2) | 1.0 |
| Baseline RBC, 1012/L | 4.17±0.57 | 4.2±0.69 | 4.17±0.55 | 0.78 |
| Hemoglobin, g/L | 127.79±16.98 | 125.61±22.3 | 128.12±16.04 | 0.35 |
| Anemia, n, % | 179 (51.1) | 24 (52.2) | 155 (51.0) | 0.88 |
| LDL, mmol/L | 2.58±0.94 | 2.59±0.82 | 2.59±0.96 | 1.00 |
| TC, mmol/L | 4.10±1.09 | 4.01±0.92 | 4.11±1.11 | 0.55 |
| TG, mmol/L | 1.39±0.99 | 1.32±0.67 | 1.41±1.03 | 0.57 |
| HDL, mmol/L | 1.09±0.32 | 1.03±0.33 | 1.1±0.32 | 0.18 |
| Uric acid, µmol/L | 369.17±112.17 | 395.3±107.66 | 365.16±112.48 | 0.09 |
| LVEF, % | 56.42±7.71 | 49.83±9.41 | 57.5±6.84 | <.001 |
| fT3, pmol/L | 3.84±0.93 | 3.39±0.96 | 3.91±0.9 | <.001 |
| fT4, pmol/L | 16.76±3.44 | 17.12±3.31 | 16.7±3.46 | 0.45 |
| S-TSH, mIU/L | 1.90±0.87 | 1.65±0.95 | 1.93±0.86 | 0.09 |
| Low fT3, n, % | 68 (19.4) | 18 (39.1) | 50 (16.4) | <0.001 |
| Perioperative medications | ||||
| Antiplatelet, n, % | 342 (97.7) | 45 (97.8) | 297 (97.7) | 0.72 |
| ACEI/ARB, n, % | 282 (80.6) | 33 (71.7) | 249 (81.9) | 0.10 |
| Statin, n, % | 342 (97.7) | 45 (97.8) | 297 (97.7) | 0.96 |
| Procedural characteristics | ||||
| Number of diseased vessels, n | 2.42±0.82 | 2.63±0.64 | 2.39±0.84 | 0.06 |
| LM, n, % | 38 (10.8) | 5 (10.9) | 33 (10.8) | 1.00 |
| LAD, n, % | 267 (76.3) | 28 (93.3) | 239 (89.2) | 0.75 |
| LCX, n, % | 196 (56.0) | 22 (73.3) | 174 (64.9) | 0.36 |
| RCA, n, % | 213 (60.8) | 24 (80.0) | 189 (70.5) | 0.28 |
| Stent length, mm | 40.10±23.82 | 43.42±22.48 | 39.57±24.02 | 0.31 |
| Number of stents, n | 1.53±0.79 | 1.64±0.77 | 1.52±0.79 | 0.32 |
| Perioperative hypotension, n, % | 24 (6.9) | 11 (23.9) | 13 (4.3) | <0.001 |
| Contrast volume, mL | 215.37±58.96 | 214.09±59.19 | 215.57±59.03 | 0.88 |
| Contrast volume>150 mL, n, % | 309 (88.3) | 40 (86.9) | 269 (88.5) | 0.76 |
Data are presented as the mean±standard deviations or as numbers and percentages.
CI-AKI - contrast-induced acute kidney injury; BMI - body mass index; MI - myocardial infarction; PCI - percutaneous coronary intervention; CABG - coronary artery bypass grafting; SCr - serum creatinine; RBC - red blood cell; LDL - low-density lipoprotein; TC - total cholesterol; TG - triglyceride; HDL - high-density lipoprotein; LVEF - left ventricular ejection fraction; fT3 - free triiodothyronine; fT4 - free thyroxine; S-TSH - sensitive thyroid-stimulating hormone; ACEI/ARB - angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; LM - left main; LAD - left anterior descending branch; LCX - left circumflex branch; RCA - right coronary artery
Compared to patients with a normal fT3 concentration, the low fT3 group was more likely to develop CI-AKI (26.5% vs. 9.9%, p<0.001), had a higher rate of in-hospital mortality (11.8% vs. 2.1%, p=0.002), and required renal replacement therapy (7.4% vs. 0.0%, p<0.001) (Fig. 1). A univariate logistic regression analysis showed that low fT3 was significantly associated with CI-AKI (OR=3.27; 95% CI, 1.70–6.35; p<0.001). The odd ratios of heart failure on admission (OR=3.49; 95% CI, 1.85–6.60; p<0.001), perioperative hypotension (OR=7.04, 95% CI, 2.93–16.90, p<0.001), and MI (OR=5.18; 95% CI, 2.61–10.27; p<0.001) are also statistically significant. All of the above variables, as well as other variables that were confirmed to be significant in previous studies such as age, anemia, diabetes mellitus, SCr >1.5 mg/dL, and contrast volume >150 mL, were conducted in a multivariate analysis. And we found that after adjusting for age, SCr, anemia, diabetes mellitus, contrast >150 mL, and heart failure on admission, low fT3 (OR=2.41; 95% CI, 1.11–5.27; p=0.027), perioperative hypotension (OR=3.40; 95% CI, 1.29–8.99; p=0.013), and MI (OR=3.60; 95% CI, 1.56–8.34; p=0.003) remained significant predictors of the development of CI-AKI in elderly patients after PCI (Table 2).
Figure 1.
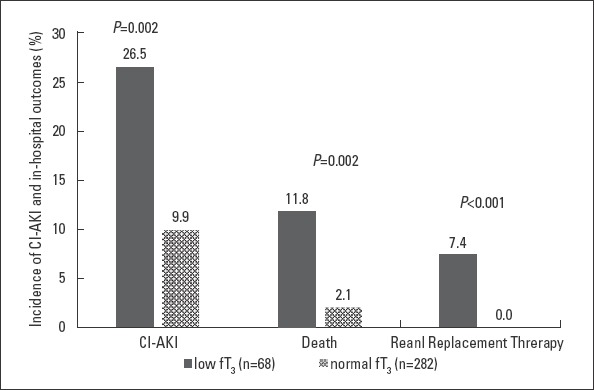
Incidence of CI-AKI, in-hospital death, and renal replacement therapy between a low fT3 and normal fT3.
fT3 - free triiodothyronine; CI-AKI - contrast-induced acute kidney injury
Table 2.
Multivariate logistic analysis of CI-AKI risk indicators
| Risk factors | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Age | 1.05 | 0.97-1.14 | 0.219 | 1.02 | 0.93-1.11 | 0.688 |
| Low fT3 | 3.27 | 1.68-6.35 | <0.001 | 2.41 | 1.11-5.27 | 0.027 |
| Heart failure in admission | 3.49 | 1.85-6.60 | <0.001 | 1.47 | 0.66-3.25 | 0.348 |
| Anemia | 1.05 | 0.56-1.95 | 0.881 | 0.63 | 0.30-1.32 | 0.634 |
| Perioperative hypotension | 7.04 | 2.93-16.90 | <0.001 | 3.32 | 1.25-8.83 | 0.016 |
| Myocardial infarction | 5.18 | 2.61-10.27 | <0.001 | 3.56 | 1.54-8.24 | 0.003 |
| Diabetes mellitus | 1.37 | 0.73-2.55 | 0.324 | 1.30 | 0.64-2.61 | 0.468 |
| SCr>1.5 mg/dL | 0.89 | 0.26-3.12 | 0.894 | 0.44 | 0.11-1.79 | 0.255 |
| Contrast volume>150 mL | 0.82 | 0.29-2.50 | 0.724 | 1.33 | 0.39-4.48 | 0.647 |
CI-AKI - contrast-induced acute kidney injury; OR - odds ratio; CI - confidence interval; fT3 - free triiodothyronine; SCr - serum creatinine
The mean follow-up duration was 1.67 years. After adjusting for variables that were found in univariate analysis with statistical significance such as age, heart failure at admission, anemia, SCr >1.5 mg/dL, perioperative hypotension, and MI, low fT3 remained an independent risk factor for long-term mortality in elderly patients undergoing PCI (HR=2.00; 95% CI, 1.04–3.83; p=0.037; Table 3).
Table 3.
Cox regression analysis for independent risk factors of long-term mortality
| Risk factors | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age | 1.10 | 1.02-1.18 | 0.012 | 1.05 | 0.98-1.13 | 0.153 |
| SCr>1.5 mg/dL | 2.60 | 1.16-5.81 | 0.02 | 1.29 | 0.53-3.11 | 0.578 |
| Heart failure at admission | 3.79 | 2.11-6.82 | <0.001 | 1.97 | 0.96-4.04 | 0.065 |
| Anemia | 3.69 | 1.78-7.64 | <0.001 | 2.58 | 1.22-5.48 | 0.014 |
| Perioperative hypotension | 4.13 | 1.99-8.56 | <0.001 | 1.69 | 0.75-3.84 | 0.208 |
| Myocardial infarction | 3.46 | 1.88-6.34 | <0.001 | 1.52 | 0.72-3.19 | 0.268 |
| Low fT3 | 3.73 | 2.09-6.67 | <0.001 | 2.00 | 1.04-3.83 | 0.037 |
HR - hazard ratio; CI - confidence interval; SCr - serum creatinine; fT3 - free triiodothyronine
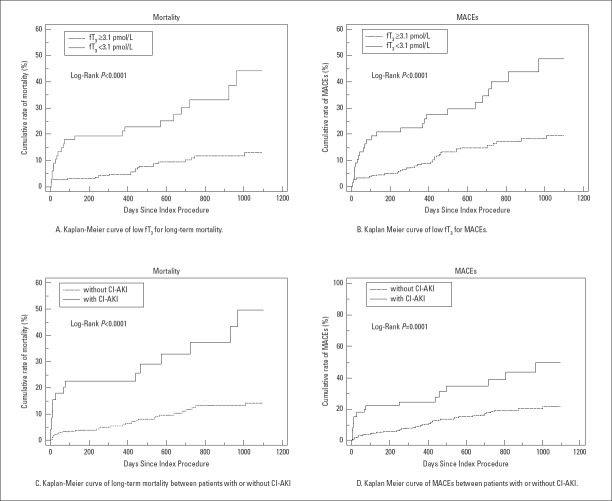
The Kaplan–Meier curve indicated that low fT3 concentration had a higher all-cause mortality and MACEs as compared to normal fT3 (p<0.001), and patients who developed CI-AKI displayed a higher rate of all-cause mortality and MACEs as compared to those without CI-AKI (p<0.001) (Fig. 2).
Figure 2.
Kaplan–Meier curve of long-term outcomes
fT3 - free triiodothyronine; MACEs - major adverse clinical events, which include all-cause mortality, stent restenosis, non-fatal myocardial infarction, and target vessel revascularization; CI-AKI - contrast-induced acute kidney injury
Discussion
This is the first study, to the best of our knowledge, to demonstrate that a low serum fT3 concentration is positively associated with an increased risk of CI-AKI and is negatively correlated with short- and long-term outcomes in elderly patients, 75 years or older, who underwent PCI.
CI-AKI is an important complication of the intravascular administration of contrast media, which is compulsory in many diagnostic and therapeutic procedures and is strongly correlated with prolonged hospitalization, late renal and cardiovascular adverse events, mortality, and higher costs (3, 16, 17). As the number of catheterizations in patients with coronary disease increases, the incidence of CI-AKI will continually increase as well. Previous studies have identified several patient-related risk factors for CI-AKI, including pre-existing CKD, diabetes mellitus, old age, reduced left ventricular systolic function, simultaneous use of nephrotoxic drugs, anemia, and hemodynamic instability (1, 18, 19). An advanced age is considered to be an important risk factor for CI-AKI. A meta-analysis by Song et al. (20) showed that the estimated incidence of patients older than 75 years is 16.5%, which is higher than the previously reported incidence in a non-segregated population (21). Then, we focused on the very old patients (≥75 years) and found that the incidence of CI-AKI in our study was 13.1%, which is similar to the previous studies. There are no effective therapies to cure CI-AKI (15), so timely preventive measures are crucial for the patients who are at high risk for CI-AKI. Therefore, identifying the potential risk factors for CI-AKI prior to contrast exposure and adopting preventive measures for elderly patients with risk factors is important.
Low T3 syndrome is common in patients with acute and chronic heart disease, especially among elderly patients. The reduction in the T3 level during illness has been generally considered to be a diminution in the hepatic/renal type I iodothyronine deiodinase activity and an increase in hepatic type III iodothyronine deiodinase activity, resulting in the reduction of T4 to T3 and an increase in the rT3 level (22). Its pathophysiological role is not well understood, and the prevailing view is that it is an adaptive mechanism to conserve energy (23, 24). Numerous studies have shown that serum fT3 levels are related to cardiovascular risk factors or events. Wang et al. (25) showed that fT3 was significantly and negatively correlated with lg-CKMB and lg-TnI, indicating that a lower fT3 level is correlated with a more severe cardiac injury in the ST segment elevation MI (STEMI) patients. In another study, Jankauskienė et al. (26) demonstrated that low fT3 levels were significantly associated with worse left ventricular (LV) mechanics and important for the prediction of the LV structure and function after MI. Furthermore, low T3 is known to affect the short-term and long-term prognosis of patients with cardiovascular disease, including STEMI (27), heart failure (28), and coronary artery bypass grafting (29). On the other hand, low fT3 is also associated with renal disease. A retrospective study of 2284 cases with a normal TSH level showed that low T3 syndrome was highly prevalent in CKD and was a remarkable finding in early CKD, and serum T3 levels were associated with CKD severity (30). Low T3 is not only closely associated with CKD, but also significantly increases the risk of cardiovascular events and all-cause mortality in CKD patients (31). However, to the best of our knowledge, the relationship between low fT3 and CI-AKI in patients who underwent PCI has not been reported previously. Therefore, we examined fT3 as a variable to explore whether a low fT3 is a CI-AKI risk factor. The present study demonstrated that the incidence of CI-AKI in elderly patients with a low fT3 who underwent PCI was significantly higher than those with a normal fT3, after adjusting for other confounding variables, such as anemia, diabetes mellitus, SCr >1.5 mg/dL, and contrast volume >150 mL, low fT3 remained an independent risk factor of CI-AKI. Meanwhile, we found that perioperative hypotension and MI were also the independent predictors of CI-AKI.
The exact pathophysiological mechanism of CI-AKI is unclear and is considered to be complex and multifactorial. The potential mechanisms underlying the relationship between a low fT3 and CI-AKI may include the following. First, a low fT3 may affect the RBF. The medullary hypoxia due to medullary vasoconstriction was an important pathophysiological mechanism of CI-AKI. In an animal experiment conducted by Sendeski et al. (32), single specimens of descending vasa recta was isolated from rats and perfused with a buffered solution containing iodixanol, the authors found that the contrast medium reduced bioavailability of nitric oxide (NO) and intensified angiotensin II-induced vasoconstriction. In another study, 24 rats were divided into 3 groups, sham operated control group (n=8), 5/6 nephrectomized group (Nx, n=8), and 5/6 nephrectomized group treated with T3 for 2 weeks (T3-Nx, n=8), the author showed that the endothelial NO synthase expression was increased in the remnant kidney of the T3-Nx group, and the serum levels of urea and creatinine were significantly decreased compared to the Nx group (33). Therefore, a low fT3 might enhance the effect of vasoconstriction by contrast media and reduce RBF, resulting in the development of CI-AKI, which was probably a mechanism that linked low fT3 and CI-AKI. Second, both renal endothelial cells and proximal tubular epithelial cells damaged by a contrast medium produced cytokines and chemokines that result in inflammation, which may be another important mechanism that contributes to CI-AKI (34, 35), the association between a low T3 and different inflammatory markers has been well established. Fan et al. (13) showed that serum T3 was negatively related to interleukin-6 (IL-6) and C-reactive protein (CRP), which reflect the inflammatory status in non-dialysis patients with CKD. Zoccali et al. (36) demonstrated a strong and inverse associations between fT3 and IL-6, CRP, intercellular adhesion molecule-1 (ICAM-1), and vascular cellular adhesion molecule-1 (VCAM-1). Meanwhile, a low fT3 might represent an active inflammation and lead to an increased risk of CI-AKI. Third, a low T3 is associated with the traditional risk factors for CI-AKI, such as CKD, advanced age, atherosclerotic disease, and myocardial injury, which may indirectly increase the risk of CI-AKI. Another mechanism underlying the inverse link between a low fT3 and CI-AKI may be anemia and malnutrition. Fan et al. (13) found that the serum T3 was positively correlated to protein metabolism (serum total protein, albumin) and anemia indicators (hemoglobin and red blood cells), while lower serum albumin and anemia were associated with a higher CI-AKI risk (37, 38).
Furthermore, this study showed that a low fT3 was not only related to the occurrence of CI-AKI, but also to adverse events during hospitalization and long-term adverse outcomes after discharge. Therefore, a low fT3 may be a prognostic indicator and should be closely monitored by clinicians.
Study limitations
The results of the current study should be evaluated keeping in mind some limitations. 1) We only analyzed fT3 without analyzing T3, T4, and rT3, because these indexes were not regularly checked in our routine practice. 2) We did not follow up the postoperative fT3 and renal function, so we could not further clarify the relationship between a low fT3 and CI-AKI. 3) This is an observational study that was conducted at a single center, and it only included a small population. 4) Numerous patients were excluded because of the lack of data on thyroid function, which may lead to selection confounding. 5) As the deregulation of fT3 often occurs in patients with AMI, including patients with MI seems to complicate the analysis. However, our sample size was too small to conduct a subgroup analysis. In our future studies, we may expand the sample size and focus on the elective and stable ischemic heart disease patients. 6) Measurements of the peak SCr levels might have been missed due to the variations in the measurement times. Consequently, it caused an underestimation of the true incidence of CI-AKI in this population. Future multi-center studies with larger sample sizes are needed to confirm these findings.
Conclusion
This study demonstrated that a low serum fT3 concentration may be associated with the development of CI-AKI and poor prognosis in patients older than 75 years who are undergoing PCI. Therefore, given the importance of fT3 and its cost-effective measurement, it should be routinely measured before PCI.
Acknowledgments
We thank the staff of the medical records office at Fujian Provincial Hospital for compiling the medical records of the selected patients in this study.
Footnotes
Ethical approval: This study was approved by the Ethics Committee of the Fujian Provincial Hospital, China (ethics approval number: K2012-001-01). All procedures performed in studies involving human participants were in accordance with the Ethical Standards of the Institutional Research Committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all participants included in the study.
Funding: The study was supported by a grant from the Startup Fund for Scientific research, Fujian Medical University (grant number: 2017XQ1134).
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – P.Z., Y.G.; Design – K.L., C.L.; Supervision – K.L., F.L.; Fundings – Z.Y.; Materials – Z.Y., T.G.; Data collection &/or processing – W.Z.; Analysis &/or interpretation – K.L., C.L.; Literature search – Z.Y.; Writing – C.L.; Critical review – P.Z., Y.G.
References
- 1.Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention:development and initial validation. J Am Coll Cardiol. 2004;44:1393–9. doi: 10.1016/j.jacc.2004.06.068. [DOI] [PubMed] [Google Scholar]
- 2.Maioli M, Toso A, Leoncini M, Gallopin M, Musilli N, Bellandi F. Persistent renal damage after contrast-induced acute kidney injury:incidence, evolution, risk factors, and prognosis. Circulation. 2012;125:3099–107. doi: 10.1161/CIRCULATIONAHA.111.085290. [DOI] [PubMed] [Google Scholar]
- 3.Uzunhasan I, Yildiz A, Arslan S, Abaci O, Kocas C, Kocas BB, et al. Contrast-Induced Acute Kidney Injury Is Associated With Long-Term Adverse Events in Patients With Acute Coronary syndrome. Angiology. 2017;68:621–6. doi: 10.1177/0003319716676173. [DOI] [PubMed] [Google Scholar]
- 4.Giacoppo D, Madhavan MV, Baber U, Warren J, Bansilal S, Witzenbichler B, et al. Impact of Contrast-Induced Acute Kidney Injury After Percutaneous Coronary Intervention on Short- and Long-Term Outcomes:Pooled Analysis From the HORIZONS-AMI and ACUITY Trials. Circ Cardiovasc Interv. 2015;8:e002475. doi: 10.1161/CIRCINTERVENTIONS.114.002475. [DOI] [PubMed] [Google Scholar]
- 5.Farwell AP. Nonthyroidal illness syndrome. Curr Opin Endocrinol Diabetes Obes. 2013;20:478–84. doi: 10.1097/01.med.0000433069.09294.e8. [DOI] [PubMed] [Google Scholar]
- 6.Bermudez F, Surks MI, Oppenheimer JH. High incidence of decreased serum triiodothyronine concentration in patients with nonthyroidal disease. J Clin Endocrinol Metab. 1975;41:27–40. doi: 10.1210/jcem-41-1-27. [DOI] [PubMed] [Google Scholar]
- 7.Warner MH, Beckett GJ. Mechanisms behind the non-thyroidal illness syndrome:an update. J Endocrinol. 2010;205:1–13. doi: 10.1677/JOE-09-0412. [DOI] [PubMed] [Google Scholar]
- 8.Iervasi G, Pingitore A, Landi P, Raciti M, Ripoli A, Scarlattini M, et al. Low-T3 syndrome:a strong prognostic predictor of death in patients with heart disease. Circulation. 2003;107:708–13. doi: 10.1161/01.cir.0000048124.64204.3f. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt-Ott UM, Ascheim DD. Thyroid hormone and heart failure. Curr Heart Fail Rep. 2006;3:114–9. doi: 10.1007/s11897-006-0010-1. [DOI] [PubMed] [Google Scholar]
- 10.Kim DH, Choi DH, Kim HW, Choi SW, Kim BB, Chung JW, et al. Prediction of infarct severity from triiodothyronine levels in patients with ST-elevation myocardial infarction. Korean J Intern Med. 2014;29:454–65. doi: 10.3904/kjim.2014.29.4.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basu G, Mohapatra A. Interactions between thyroid disorders and kidney disease. Indian J Endocrinol Metab. 2012;16:204–13. doi: 10.4103/2230-8210.93737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Chang Y, Ryu S, Cho J, Lee WY, Rhee EJ, et al. Thyroid hormone levels and incident chronic kidney disease in euthyroid individuals:the Kangbuk Samsung Health Study. Int J Epidemiol. 2014;43:1624–32. doi: 10.1093/ije/dyu126. [DOI] [PubMed] [Google Scholar]
- 13.Fan J, Yan P, Wang Y, Shen B, Ding F, Liu Y. Prevalence and Clinical Significance of Low T3 Syndrome in Non-Dialysis Patients with Chronic Kidney Disease. Med Sci Monit. 2016;22:1171–9. doi: 10.12659/MSM.895953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolh P, Windecker S, Alfonso F, Collet JP, Cremer J, Falk V, et al. Task Force on Myocardial Revascularization of the European Society of Cardiology and the European Association for Cardio-Thoracic Surgery;European Association of Percutaneous Cardiovascular Interventions. 2014 ESC/EACTS Guidelines on myocardial revascularization:the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI) Eur J Cardiothorac Surg. 2014;46:517–92. doi: 10.1093/ejcts/ezu366. [DOI] [PubMed] [Google Scholar]
- 15.Ozkok S, Ozkok A. Contrast-induced acute kidney injury:A review of practical points. World J Nephrol. 2017;6:86–99. doi: 10.5527/wjn.v6.i3.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raje V, Feldman G, Jovin IS. Diagnosing and treating contrast-induced acute kidney injury in 2017. J Thorac Dis. 2017;9:1443–5. doi: 10.21037/jtd.2017.05.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wi J, Ko YG, Kim JS, Kim BK, Choi D, Ha JW, et al. Impact of contrast-induced acute kidney injury with transient or persistent renal dysfunction on long-term outcomes of patients with acute myocardial infarction undergoing percutaneous coronary intervention. Heart. 2011;97:1753–7. doi: 10.1136/hrt.2010.218677. [DOI] [PubMed] [Google Scholar]
- 18.Rihal CS, Textor SC, Grill DE, Berger PB, Ting HH, Best PJ, et al. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation. 2002;105:2259–64. doi: 10.1161/01.cir.0000016043.87291.33. [DOI] [PubMed] [Google Scholar]
- 19.Dangas G, Iakovou I, Nikolsky E, Aymong ED, Mintz GS, Kipshidze NN, et al. Contrast-induced nephropathy after percutaneous coronary interventions in relation to chronic kidney disease and hemodynamic variables. Am J Cardiol. 2005;95:13–9. doi: 10.1016/j.amjcard.2004.08.056. [DOI] [PubMed] [Google Scholar]
- 20.Song W, Zhang T, Pu J, Shen L, He B. Incidence and risk of developing contrast-induced acute kidney injury following intravascular contrast administration in elderly patients. Clin Interv Aging. 2014;9:85–93. doi: 10.2147/CIA.S55157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCullough PA. Contrast-induced acute kidney injury. J Am Coll Cardiol. 2008;51:1419–28. doi: 10.1016/j.jacc.2007.12.035. [DOI] [PubMed] [Google Scholar]
- 22.Peeters RP, Wouters PJ, van Toor H, Kaptein E, Visser TJ, Van den Berghe G. Serum 3,3',5'-triiodothyronine (rT3) and 3,5,3'-triiodothyronine/rT3 are prognostic markers in critically ill patients and are associated with postmortem tissue deiodinase activities. J Clin Endocrinol Metab. 2005;90:4559–65. doi: 10.1210/jc.2005-0535. [DOI] [PubMed] [Google Scholar]
- 23.Polikar R, Burger AG, Scherrer U, Nicod P. The thyroid and the heart. Circulation. 1993;87:1435–41. doi: 10.1161/01.cir.87.5.1435. [DOI] [PubMed] [Google Scholar]
- 24.Utiger RD. Altered thyroid function in nonthyroidal illness and surgery. To treat or not to treat? N Engl J Med. 1995;333:1562–3. doi: 10.1056/NEJM199512073332310. [DOI] [PubMed] [Google Scholar]
- 25.Wang WY, Tang YD, Yang M, Cui C, Mu M, Qian J, et al. Free triiodothyronine level indicates the degree of myocardial injury in patients with acute ST-elevation myocardial infarction. Chin Med J (Engl) 2013;126:3926–9. [PubMed] [Google Scholar]
- 26.Jankauskienė E, Orda P, Barauskienė G, Mickuvienė N, Brožaitienė J, Vaškelytė JJ, et al. Relationship between left ventricular mechanics and low free triiodothyronine levels after myocardial infarction:a prospective study. Intern Emerg Med. 2016;11:391–8. doi: 10.1007/s11739-015-1370-x. [DOI] [PubMed] [Google Scholar]
- 27.Özcan KS, Osmonov D, Toprak E, Güngör B, Tatlısu A, Ekmekçi A, et al. Sick euthyroid syndrome is associated with poor prognosis in patients with ST segment elevation myocardial infarction undergoing primary percutaneous intervention. Cardiol J. 2014;21:238–44. doi: 10.5603/CJ.a2013.0108. [DOI] [PubMed] [Google Scholar]
- 28.Rays J, Wajngarten M, Gebara OC, Nussbacher A, Telles RM, Pierri H, et al. Long-term prognostic value of triiodothyronine concentration in elderly patients with heart failure. Am J Geriatr Cardiol. 2003;12:293–7. doi: 10.1111/j.1076-7460.2003.01737.x. [DOI] [PubMed] [Google Scholar]
- 29.Cerillo AG, Storti S, Kallushi E, Haxhiademi D, Miceli A, Murzi M, et al. The low triiodothyronine syndrome:a strong predictor of low cardiac output and death in patients undergoing coronary artery bypass grafting. Ann Thorac Surg. 2014;97:2089–95. doi: 10.1016/j.athoracsur.2014.01.049. [DOI] [PubMed] [Google Scholar]
- 30.Song SH, Kwak IS, Lee DW, Kang YH, Seong EY, Park JS. The prevalence of low triiodothyronine according to the stage of chronic kidney disease in subjects with a normal thyroid-stimulating hormone. Nephrol Dial Transplant. 2009;24:1534–8. doi: 10.1093/ndt/gfn682. [DOI] [PubMed] [Google Scholar]
- 31.Afsar B, Yilmaz MI, Siriopol D, Unal HU, Saglam M, Karaman M, et al. Thyroid function and cardiovascular events in chronic kidney disease patients. J Nephrol. 2017;30:235–42. doi: 10.1007/s40620-016-0300-y. [DOI] [PubMed] [Google Scholar]
- 32.Sendeski M, Patzak A, Pallone TL, Cao C, Persson AE, Persson PB. Iodixanol, constriction of medullary descending vasa recta, and risk for contrast medium-induced nephropathy. Radiology. 2009;251:697–704. doi: 10.1148/radiol.2513081732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.El Agaty SM. Triiodothyronine attenuates the progression of renal injury in a rat model of chronic kidney disease. Can J Physiol Pharmacol. 2018;96:603–10. doi: 10.1139/cjpp-2017-0252. [DOI] [PubMed] [Google Scholar]
- 34.Akcay A, Nguyen Q, Edelstein CL. Mediators of inflammation in acute kidney injury. Mediators Inflamm. 2009;2009:137072. doi: 10.1155/2009/137072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuan Y, Qiu H, Hu X, Luo T, Gao X, Zhao X, et al. Predictive value of inflammatory factors on contrast-induced acute kidney injury in patients who underwent an emergency percutaneous coronary intervention. Clin Cardiol. 2017;40:719–25. doi: 10.1002/clc.22722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zoccali C, Tripepi G, Cutrupi S, Pizzini P, Mallamaci F. Low triiodothyronine:a new facet of inflammation in end-stage renal disease. J Am Soc Nephrol. 2005;16:2789–95. doi: 10.1681/ASN.2005040356. [DOI] [PubMed] [Google Scholar]
- 37.Murat SN, Kurtul A, Yarlioglues M. Impact of Serum Albumin Levels on Contrast-Induced Acute Kidney Injury in Patients With Acute Coronary Syndromes Treated With Percutaneous Coronary Intervention. Angiology. 2015;66:732–7. doi: 10.1177/0003319714551979. [DOI] [PubMed] [Google Scholar]
- 38.Nikolsky E, Mehran R, Lasic Z, Mintz GS, Lansky AJ, Na Y, et al. Low hematocrit predicts contrast-induced nephropathy after percutaneous coronary interventions. Kidney Int. 2005;67:706–13. doi: 10.1111/j.1523-1755.2005.67131.x. [DOI] [PubMed] [Google Scholar]