Abstract
Background:
Cord blood adiponectin and leptin concentrations are associated with birth weight and adiposity. Birth size and rate of infant weight gain are associated with future obesity risk. However, it is unclear whether biomarkers reflecting the intrauterine environment are predictive of infant prospective body composition change.
Objectives:
To examine whether cord blood adiponectin and leptin are predictive of neonatal adiposity and fat mass (FM) accrual to 3 months of age.
Methods:
Participants (n=36) were healthy African American infants. Leptin and adiponectin concentrations were measured in umbilical cord blood. At 2 weeks and 3 months, infant body composition was assessed via air displacement plethysmography. Weight-for-length z-scores (WLZ) were calculated using World Health Organization standards. Multiple linear regression was used to examine associations of cord blood adiponectin and leptin with birth WLZ; WLZ, FM, and fat-free mass at 2 weeks, and the conditional change in these variables from 2 weeks to 3 months (body composition at 3 months adjusted for body composition at 2 weeks).
Results:
Adiponectin was positively associated with FM at 2 weeks (r=0.45, P<0.01), but inversely associated with conditional FM change from 2 weeks to 3 months of age (r=−0.38, P<0.05). Leptin was not significantly associated with infant body composition.
Conclusions:
Adiponectin may be a marker for FM accrual in African American infants, a relatively understudied population with a high long-term obesity risk. Mechanistic studies are needed to determine whether adiponectin directly influences infant growth or is simply a marker reflective of other ongoing biological changes after birth.
Keywords: adiponectin, body composition, cord blood, leptin, postnatal growth
Introduction
Adiponectin and leptin are hormones secreted from adipocytes, and their circulating concentrations reflect nutritional status. Adults who are obese have lower concentrations of adiponectin and higher concentrations of leptin (1, 2). Among infants, high leptin concentrations in the umbilical cord blood are associated with greater birth weight (3, 4, 5), but interestingly, the association of cord blood adiponectin with body weight is opposite that of adults. Specifically, heavier infants have higher rather than lower adiponectin in the umbilical cord blood (6, 7). Given the known association of leptin and adiponectin in appetite regulation of adults (8, 9), it would be interesting to know whether concentrations of leptin and adiponectin in umbilical cord blood are predictive of growth and body composition change during infancy.
Several previous studies have examined the associations of cord blood leptin and adiponectin with infant growth, but results have been inconsistent. Some studies (10, 11, 12), but not all (13, 14, 15), have reported that cord blood leptin is inversely associated with longitudinal changes in infant size across infancy and up to 3 years of age, after adjusting for size at birth. The association of umbilical cord blood adiponectin concentration with longitudinal changes in infant weight and adiposity has also been inconsistent (11, 13, 16). The existing literature is limited however, by the use of anthropometric measurements of infant adiposity, and so the association of these adipokines with fat-free mass (FFM) and fat mass (FM), and with prospective change in FFM and FM, is not clear.
There is a dearth of studies assessing associations of cord blood adiponectin and leptin with neonatal FFM and FM, and with the change in FFM and FM across early infancy. Further, previous studies that used anthropometric measures of infant adiposity were largely focused on predominantly Caucasian infants. African American infants, however, are an interesting demographic to examine because as compared to Caucasians, African American infants are lighter at birth with less FFM (17), but gain more weight during infancy and have a greater long-term risk for obesity (18). Thus, the objective of this study is to test the hypothesis that cord blood adiponectin and leptin are positively associated with neonatal adiposity, but inversely associated with body weight gain and absolute FM accrual to 3 months of age in a cohort of African American infants.
Methods
Subject Characteristics
Data from healthy African American infants born to mothers with normal glucose tolerance enrolled in studies examining prenatal health and early childhood growth were used for this study. Women were eligible for the parent studies if they were at least 16 years of age at recruitment and were experiencing a healthy singleton pregnancy. Exclusion criteria were pre-existing type 1 or type 2 diabetes mellitus, gestational diabetes mellitus during the current pregnancy, prior preterm birth (<37 weeks’ gestation) or delivery of a low birth weight infant (<2500 grams), the presence of any medical condition believed to interfere with fetal growth, and the use of tobacco, alcohol, or any illicit drugs during the current pregnancy. Among the N=50 infants from this dataset for whom a cord blood sample was obtained, data from infants who were born preterm (n=3), had a low birth weight (n=1), or who did not return for at least the 2 week follow-up visit (n=10), were excluded. Final analyses were conducted on n=36 infants at birth and 2 weeks, and n=31 infants at 3 months. The Institutional Review Board for Human Use at the University of Alabama at Birmingham approved all study procedures. Informed consent was obtained from the mothers.
Clinical and Anthropometric Assessments
Infant gestational age at delivery and birth weight and length were retrieved from medical records. Infant race was based on the race of the mother, which was retrieved from medical records. At 2 weeks (18 ± 5 days) and 3 months (93 ± 4 days), mothers self-reported whether they were exclusively, partially or not breastfeeding their infants. Infant length was measured to the nearest 0.1 cm using an Ayrton® M-200 infantometer (Ayrton Corporation, USA) and infant weight was measured to the nearest 0.1 g with infants wearing only a stocking cap using standard clinical procedures. Infant length was measured in triplicate. World Health Organization standards were used to calculate sex-specific weight-for-length z-scores (WLZ) at birth, 2 weeks, and 3 months of age.
Air Displacement Plethysmography (ADP)
At 2 weeks and 3 months of age, infant body FM and FFM were measured by ADP using PEAPOD® (COSMED USA, Concord, CA). Infants were undressed and wore only a stocking cap to cover their hair for the assessments.
Cord Blood Measurements
Umbilical cord blood samples were obtained using venipuncture at birth, processed for serum, and stored at −80°C until assay. Serum leptin and adiponectin were measured by radioimmunoassay (Millipore human leptin and human adiponectin RIA kits, respectively; Millipore Corporation, Billerica, MA, USA), with minimum sensitivities of 0.92 ng/mL and 2.55 μg/mL, respectively. Intra- and inter-assay coefficients of variation were 4.81% and 4.76%, respectively, for leptin and 5.93% and 2.67%, respectively, for adiponectin.
Statistics
The primary outcome variables were infant FM, measured at 2 weeks of age and adjusted for total FFM, and the conditional change in FM from 2 weeks to 3 months of age. Secondary outcomes included WLZ at birth and 2 weeks, FFM at 2 weeks, and the conditional change in these variables from 2 weeks to 3 months of age.
Prior to analyses, all continuous variables were assessed for normality of distribution using the Shapiro-Wilk Test. Skewed variables (cord blood leptin and adiponectin) were log-transformed into normal distributions. Separate simple linear regression models and multiple linear regression (MLR) models were constructed to examine the association of leptin or adiponectin (as the main predictor variable) with each body composition measure as the dependent variable. The model predicting birth WLZ was adjusted for the gestational age at delivery. MLR models predicting WLZ at 2 weeks were adjusted for infant age (in days), the models predicting FM at 2 weeks were adjusted for age at testing and FFM at 2 weeks to account for overall body size and the models predicting FFM at 2 weeks were adjusted for age at testing and infant length at 2 weeks. The association of cord blood leptin and adiponectin with conditional change in infant WLZ, FM, and FFM from 2 weeks to 3 months, was examined in separate MLR models with the infant body measurement at 3 months as the dependent variable, and the corresponding measurement at 2 weeks as a covariate, along with the time between measurements. Mothers’ early pregnancy BMI and gestational weight gain were explored as potential covariates in all models, and infant feeding mode (breast fed, mixed fed, or formula fed) was explored as a potential covariate in models predicting infant body measurements at 2 weeks and the conditional change in these measurements from 2 weeks to 3 months. Mothers’ early pregnancy BMI, gestational weight gain and feeding mode were not associated with the infant outcomes of interest and so they were excluded from final models. All assumptions for multiple linear regression were verified to be met. Data are presented as mean ± SD, unless indicated otherwise.
Data analysis was performed using the Statistical Package for the Social Sciences (SPSS) software version 22.0 (SPSS Inc., Chicago, IL, USA), and statistical significance was accepted at alpha less than or equal to 0.05.
Results
Subject characteristics are presented in Table 1. The gestational age at birth ranged from 37 weeks and 4 days to 41 weeks and 4 days, and neonatal birth weight ranged from 2670 to 4140 g. Maternal early pregnancy BMI ranged from 18.0 to 52.9 kg/m2. Cord blood leptin and adiponectin concentrations ranged from 2.4 to 36.9 ng/mL and from 19.2 to 59.3 μg/mL, respectively. Cord blood leptin concentration was significantly higher among female infants compared to male infants (15.2 ± 12.6 vs. 9.2 ± 10.0 ng/mL, respectively; P<0.05; not shown), even after controlling for gestational age at delivery and size at birth. Cord blood adiponectin concentration did not differ significantly by infant sex. Median (IQR) infant weight gain from 2 weeks to 3 months postpartum was 2302.9 (479.7) g. The mean change in FM and FFM from 2 weeks to 3 months was 796.0 ± 318.4 g and 1608.6 ± 281.0 g, respectively.
Table 1.
Maternal and infant characteristics
| Mean ± SD or median (IQR) | |
|---|---|
| Maternal characteristics (n=36) | |
| Age at enrollment (years) | 22.5 ± 3.7 |
| Education (% completed high school) | 86.1% (n=31) |
| Nulliparous (%) | 47.2% (n=17) |
| Early pregnancy BMI (kg/m2) | 29.1 ± 8.2 |
| Gestational weight gain (kg) | 13.9 ± 8.2 |
| Cesarean Section (%) | 19.4% (n=7) |
| Infant outcomes at birth (n=36) | |
| Gestational age at delivery (weeks) | 39.8 ± 1.0 |
| Male sex (%) | 52.8% (n=19) |
| Cord leptin (ng/mL) | 12.3 (12.7) |
| Cord adiponectin (μg/mL) | 37.2 (13.7) |
| Weight (g) | 3347.4 ± 380.3 |
| WLz | −0.6 ± 1.1 |
| Infant outcomes at 2 weeks (n=36) | |
| Age at 2 week visit (days) | 17.5 ± 5.3 |
| Weight (g) | 3702.7 ± 413.4 |
| WLz | −0.1 ± 1.4 |
| Fat mass (g) | 573.5 ± 196.3 |
| Fat-free mass (g) | 3129.4 ± 312.8 |
| %Breastfed; %Formula-fed; %Mixed | 8.3% (n=3); 75.0% (n=27); 16.7% (n=6) |
| Infant outcomes at 3 months (n=31) | |
| Age at 3 month visit (days) | 93.0 ± 3.7 |
| Weight (g) | 6086.2 ± 639.4 |
| WLz | 0.1 ± 1.0 |
| Fat mass (g) | 1362.2 ± 373.3 |
| Fat-free mass (g) | 4724.1 ± 460.0 |
| %Breastfed; %Formula-fed; %Mixed | 0% (n=0); 93.5% (n=29); 6.5% (n=2) |
BMI, body mass index; WLz, weight-for-length z-score.
The results of simple linear regression models and the fully adjusted MLR models analyzing the associations of umbilical cord leptin and adiponectin with the outcomes of interest are reported in Table 2. In the crude models, birth WLZ was positively correlated with cord blood leptin and cord blood adiponectin (r=0.39, P=0.02 and r=0.41, P=0.01, respectively). When adjusted for gestational age at delivery, cord blood leptin remained significantly associated with birth WLZ (r=0.39, P=0.02), however the overall model was no longer significant (R2=0.16, P=0.06; not shown). The association of adiponectin with birth WLZ remained significant after adjustment for gestational age at delivery (r=0.40, P=0.02), as did the overall model (not shown).
Table 2.
Associations of cord blood adipokines with infant growth and body composition
| Cord blood leptin | Cord blood adiponectin | |||
|---|---|---|---|---|
|
Partial Correlation |
Partial Correlation |
|||
| Crude model | Adjusted model | Crude model | Adjusted model | |
| Birth1 (n=36) | ||||
| WLz | 0.39* | 0.39* | 0.41* | 0.40* |
| 2 weeks2 (n=36) | ||||
| WLz | 0.35* | 0.35* | 0.16 | 0.17 |
| Fat mass3 | 0.24 | 0.27 | 0.46** | 0.45** |
| Fat-free mass4 | −0.01 | 0.18 | 0.07 | 0.04 |
| 3 months5 (n=31) | ||||
| WLz | 0.20 | 0.03 | 0.15 | 0.12 |
| Fat mass | −0.01 | −0.19 | −0.02 | −0.38* |
| Fat-free mass | −0.07 | 0.08 | −0.06 | −0.1 |
WLz, weight-for-length z-score
Adjusted for gestational age at delivery.
Adjusted for age at measurement.
Also adjusted for fat-free mass.
Also adjusted for length.
Adjusted for baseline (2 week) measure and time between measurements.
P<0.1.
P<0.05.
P<0.01.
At 2 weeks of age, cord blood leptin was positively associated with WLZ (r=0.35, P=0.04). This association remained significant after adjustment for the infant age at the measurement (r=0.35, P=0.04), however, the overall model was no longer significant (R2=0.13, P=0.11). Cord blood adiponectin was positively associated with FM at 2 weeks of age (r=0.46, P<0.01), and this association remained in the fully adjusted models (r=0.45, P<0.01; Fig. 1a). Including infant sex as a covariate in the models did not change the results (not shown).
Figure 1.
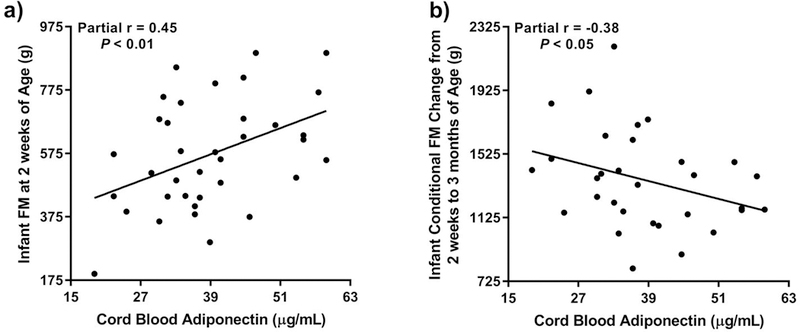
Relationship of cord blood adipokines with infant body composition: (a) Cord blood adiponectin is positively associated with infant fat mass (FM) at 2 weeks of age after adjustment for FFM at 2 weeks and age at measurement (overall MLR model: F=6.09, P<0.005); and (b) Cord blood adiponectin is inversely associated with conditional FM change from 2 weeks to 3 months of age after adjustment for FM at 2 weeks and days between measurements (overall MLR model: F=6.28, P<0.005). Partial r and P values are from partial correlations obtained as part of the multiple linear regression (MLR) analyses.
With respect to the conditional change in infant growth, cord blood leptin concentration was not associated with change in WLZ, FM or FFM from 2 weeks to 3 months. However, cord blood adiponectin was significantly inversely associated with the conditional change in FM from 2 weeks to 3 months only after adjustment for FM at 2 weeks and the time between the measurements (r=−0.38, P=0.04, Fig. 1b). Results remained unchanged when infant sex was included as an additional covariate in the models (not shown).
Discussion
The overall objective of this study was to examine whether umbilical cord blood leptin and adiponectin are predictive of neonatal adiposity and change in body composition up to 3 months of age. These analyses were conducted in a cohort of infants born to African American mothers, which is a relatively understudied group despite their lower birth weights but greater risk for childhood obesity and type 2 diabetes mellitus (19, 20). Results extend the literature by showing that higher cord blood adiponectin was associated with greater FM at 2 weeks of age, but reduced FM accrual to 3 months of age. In contrast, cord blood leptin was not associated with FM or FM accrual to 3 months of age in this cohort. Together, these findings suggest that concentrations of adiponectin, but not leptin, in the umbilical cord blood may be predictive of prospective change in adiposity in infants born to African American mothers. If this relationship is verified in future, larger studies, cord blood adiponectin may be a useful marker for obesity risk.
This is the first study to show an association of higher cord blood adiponectin with neonatal fat mass in an exclusively African American cohort. These findings were consistent with our hypothesis and confirm previous studies which have largely relied on anthropometric measurements as markers of adiposity (10, 16, 21, 22, 23, 24, 25). It is possible that these findings reflect an association of adiponectin with fetal insulin sensitivity, consistent with the known positive association of adiponectin with insulin sensitivity in adults (26). However, future mechanistic studies in animal models are needed to elucidate whether there is a mechanistic relationship between adiponectin and infant body composition or whether it is merely a marker of changes in adiposity or some other ongoing biological mechanism(s) occurring after birth.
In contrast with the association between adiponectin and FM at 2 weeks of age, we reported here that higher cord blood adiponectin was associated with less conditional fat mass gain from 2 weeks to 3 months of age. This is consistent with one other study that reported cord blood adiponectin was inversely correlated with WLZ change across the first year of life (25). However, our finding is in contrast to that of Teague et al. (16), who reported a positive association of cord blood high-molecular weight adiponectin (HMWA) with the change in infant weight and SSF from birth to 1-month of age in a cohort of Hispanic and Native American infants (16). These disparate findings may be attributable to the fact that Teague et al. measured HMWA, which is suggested to be the most active form of adiponectin, whereas we used total adiponectin. Thus, future studies are needed to determine whether there are distinct and direct effects of the different molecular isoforms of adiponectin on fetal fat accrual, and whether these effects differ by infant sex (27). Additionally, Teague et al. (24) and others rely on a change score, rather than conditional change in weight and SSF, to characterize infant growth. By using the conditional change in infant FM, our study accounted for the size of the infants at baseline, which is important as size at baseline may impact the rate of growth moving forward (28, 29).
In the present study, cord blood leptin was positively associated with infant WLZ at birth and at 2 weeks. The positive association of cord blood leptin with neonatal body size is consistent with previously published studies (15, 24, 30). Contrary to our hypothesis, cord blood leptin was not associated with FM at 2 weeks of age. Several other studies have measured the association of cord blood leptin with neonatal fat mass by ADP or skinfolds and reported a positive association (4, 5, 16, 23). It is not clear why no association was found in this study, but it is possible that the sample size limited our ability to detect an association, or that the association of cord blood leptin with adiposity is not as strong in African American infants, who are typically smaller at birth, as compared to other racial groups.
Similarly, we did not find an association of cord blood leptin with change in FM across the first 3 months of life. Most (5, 10, 11, 12, 16, 23, 24), but not all (13, 14, 15), previously published studies have found cord blood leptin to be inversely associated with weight gain in early infancy; however, this association may reflect slower growth among infants who were larger at birth (e.g. catch-down growth) (29). One study among Irish infants with repeat body composition measurements at birth and 2 months of age using ADP reported cord blood leptin was positively associated with both FM and FFM at birth and was inversely associated with the conditional change in FM from 0 to 2 months of age (12). They also did not observe any associations between cord blood leptin and conditional gains in weight at subsequent intervals up to 2 years of age (12). As such, methodological differences, including the duration of time between baseline and follow-up measurements and the techniques used to measure cord blood leptin, may contribute to inconsistencies in the reported associations of cord blood leptin with infant weight and body composition change. However, it is also possible that the association between cord blood leptin and infant growth is obscured by maternal feeding practices or other factors postpartum. Ultimately, more research is needed to better understand associations of leptin with FM and FFM, and how these associations change across infancy and early childhood.
This study is strengthened by the prospective design, the exclusively African American cohort and the use of ADP to measure infant body composition longitudinally. However, this study is not without limitations including the use of total adiponectin rather than HMWA, the small sample size and loss to follow-up which could introduce bias and limit the ability to include other covariates in our models.
To our knowledge, our study is the first to assess infant FM and FFM, specifically, at multiple timepoints and examine the association of cord blood adiponectin and leptin with the conditional change in these outcomes in a cohort of African American infants. Overall, we found that cord blood adiponectin, but not leptin, was positively associated with neonatal FM, but inversely associated with FM change in early infancy in a cohort of African American infants. It is important to understand how the concentration of adipokines in cord blood relate to fetal and infant growth because ultimately, this may affect future obesity risk. As such, these adipokines may serve as potentially useful markers for the early identification of infants at-risk for future obesity, allowing for the targeting of early preventative interventions. However, further research in larger, more diverse cohorts is needed to elucidate whether leptin and adiponectin can be used as markers for future obesity risk and what, if any, roles these adipokines may play in fetal and neonatal development and programming of obesity and future disease risk.
Acknowledgements
All authors were involved in the study design, data interpretation, manuscript editing, and had final approval of the submitted version. C.S. conducted data collection, statistical analyses and wrote the manuscript. The authors thank Britney Blackstock for data collection. Research reported in this publication was supported by pilot funding from the University of Alabama at Birmingham (UAB) Diabetes Research Center (P30DK079626), by a career development award to PCC from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (K01DK090126), by laboratory support from the UAB Nutrition Obesity Research Center (P30DK056336), by UAB Center for Clinical and Translational Science (UL1TR00165), and by the National Heart, Lung, and Blood Institute of the National Institutes of Health (T32HL105349) training award to CS. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest Statement
Ms. Schneider and Drs. Gower and Chandler-Laney report grants from NIH, during the conduct of the study. Drs. Catalano and Biggio have nothing to disclose.
References
- 1.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. The New England journal of medicine 1996;334: 292–295. [DOI] [PubMed] [Google Scholar]
- 2.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochemical and biophysical research communications 1999;257: 79–83. [DOI] [PubMed] [Google Scholar]
- 3.Marchini G, Fried G, Ostlund E, Hagenas L. Plasma leptin in infants: relations to birth weight and weight loss. Pediatrics 1998;101: 429–432. [DOI] [PubMed] [Google Scholar]
- 4.Kadakia R, Zheng Y, Zhang Z, Zhang W, Hou L, Josefson JL. Maternal pre-pregnancy BMI downregulates neonatal cord blood LEP methylation. Pediatric obesity 2017;12 Suppl 1: 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunner S, Schmid D, Huttinger K, Much D, Bruderl M, Sedlmeier EM, et al. Effect of reducing the n-6/n-3 fatty acid ratio on the maternal and fetal leptin axis in relation to infant body composition. Obesity (Silver Spring, Md) 2014;22: 217–224. [DOI] [PubMed] [Google Scholar]
- 6.Kamoda T, Saitoh H, Saito M, Sugiura M, Matsui A. Serum adiponectin concentrations in newborn infants in early postnatal life. Pediatric research 2004;56: 690–693. [DOI] [PubMed] [Google Scholar]
- 7.Kotani Y, Yokota I, Kitamura S, Matsuda J, Naito E, Kuroda Y. Plasma adiponectin levels in newborns are higher than those in adults and positively correlated with birth weight. Clinical endocrinology 2004;61: 418–423. [DOI] [PubMed] [Google Scholar]
- 8.Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 2001;411: 480–484. [DOI] [PubMed] [Google Scholar]
- 9.Kubota N, Yano W, Kubota T, Yamauchi T, Itoh S, Kumagai H, et al. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell metabolism 2007;6: 55–68. [DOI] [PubMed] [Google Scholar]
- 10.Mantzoros CS, Rifas-Shiman SL, Williams CJ, Fargnoli JL, Kelesidis T, Gillman MW. Cord blood leptin and adiponectin as predictors of adiposity in children at 3 years of age: a prospective cohort study. Pediatrics 2009;123: 682–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parker M, Rifas-Shiman SL, Belfort MB, Taveras EM, Oken E, Mantzoros C, et al. Gestational glucose tolerance and cord blood leptin levels predict slower weight gain in early infancy. The Journal of pediatrics 2011;158: 227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaoimh CN, Murray DM, Kenny LC, Irvine AD, Hourihane JO, Kiely M. Cord blood leptin and gains in body weight and fat mass during infancy. European journal of endocrinology 2016;175: 403–410. [DOI] [PubMed] [Google Scholar]
- 13.Nakano Y, Itabashi K, Nagahara K, Sakurai M, Aizawa M, Dobashi K, et al. Cord serum adiponectin is positively related to postnatal body mass index gain. Pediatrics international : official journal of the Japan Pediatric Society 2012;54: 76–80. [DOI] [PubMed] [Google Scholar]
- 14.Alderete TL, Song AY, Bastain T, Habre R, Toledo-Corral CM, Salam MT, et al. Prenatal traffic-related air pollution exposures, cord blood adipokines and infant weight. Pediatric obesity 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fonseca MJ, Santos AC. Umbilical cord blood adipokines and newborn weight change. Archives of gynecology and obstetrics 2015;291: 1037–1040. [DOI] [PubMed] [Google Scholar]
- 16.Teague AM, Fields DA, Aston CE, Short KR, Lyons TJ, Chernausek SD. Cord blood adipokines, neonatal anthropometrics and postnatal growth in offspring of Hispanic and Native American women with diabetes mellitus. Reproductive biology and endocrinology : RB&E 2015;13: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh KA, Huston-Presley LP, Mencin P, Thomas A, Amini SB, Catalano PM. Birth weight and body composition of neonates born to Caucasian compared with African-American mothers. Obstetrics and gynecology 2010;115: 998–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taveras EM, Gillman MW, Kleinman K, Rich-Edwards JW, Rifas-Shiman SL. Racial/ethnic differences in early-life risk factors for childhood obesity. Pediatrics 2010;125: 686–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prevention CfDCa. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Atlanta, GA: USA, 2014. [Google Scholar]
- 20.Rendall MS, Weden MM, Fernandes M, Vaynman I. Hispanic and black US children’s paths to high adolescent obesity prevalence. Pediatric obesity 2012;7: 423–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan TF, Yuan SS, Chen HS, Guu CF, Wu LC, Yeh YT, et al. Correlations between umbilical and maternal serum adiponectin levels and neonatal birthweights. Acta obstetricia et gynecologica Scandinavica 2004;83: 165–169. [DOI] [PubMed] [Google Scholar]
- 22.Kadowaki K, Waguri M, Nakanishi I, Miyashita Y, Nakayama M, Suehara N, et al. Adiponectin concentration in umbilical cord serum is positively associated with the weight ratio of fetus to placenta. The Journal of clinical endocrinology and metabolism 2006;91: 5090–5094. [DOI] [PubMed] [Google Scholar]
- 23.Tsai PJ, Yu CH, Hsu SP, Lee YH, Chiou CH, Hsu YW, et al. Cord plasma concentrations of adiponectin and leptin in healthy term neonates: positive correlation with birthweight and neonatal adiposity. Clinical endocrinology 2004;61: 88–93. [DOI] [PubMed] [Google Scholar]
- 24.Weyermann M, Beermann C, Brenner H, Rothenbacher D. Adiponectin and leptin in maternal serum, cord blood, and breast milk. Clinical chemistry 2006;52: 2095–2102. [DOI] [PubMed] [Google Scholar]
- 25.Zhang ZQ, Lu QG, Huang J, Jiao CY, Huang SM, Mao LM. Maternal and cord blood adiponectin levels in relation to post-natal body size in infants in the first year of life: a prospective study. BMC pregnancy and childbirth 2016;16: 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berg AH, Combs TP, Scherer PE. ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends in endocrinology and metabolism: TEM 2002;13: 84–89. [DOI] [PubMed] [Google Scholar]
- 27.Simon-Muela I, Naf S, Ballesteros M, Vendrell J, Ceperuelo-Mallafre V, de la Flor M, et al. Gender determines the actions of adiponectin multimers on fetal growth and adiposity. American journal of obstetrics and gynecology 2013;208: 481e481–487. [DOI] [PubMed] [Google Scholar]
- 28.Hediger ML, Overpeck MD, Maurer KR, Kuczmarski RJ, McGlynn A, Davis WW. Growth of infants and young children born small or large for gestational age: findings from the Third National Health and Nutrition Examination Survey. Archives of pediatrics & adolescent medicine 1998;152: 1225–1231. [DOI] [PubMed] [Google Scholar]
- 29.Chiavaroli V, Cutfield WS, Derraik JG, Pan Z, Ngo S, Sheppard A, et al. Infants born large-for-gestational-age display slower growth in early infancy, but no epigenetic changes at birth. Scientific reports 2015;5: 14540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang MJ, Liu RS, Hung JH. Leptin concentrations in the umbilical vein and artery. Relationship to maternal and neonatal anthropometry. The Journal of reproductive medicine 2002;47: 645–650. [PubMed] [Google Scholar]


