Abstract
Bupropion and varenicline are the top two smoking cessation interventions that are marginally successful in increasing abstinence rates when compared to placebo. Although smokers vary in their history and pattern of tobacco use, there is a significant gap in addressing this individual variability with individually targeted treatments. The present study takes the initial step towards a better understanding of individual differences in treatment outcomes by assessing the effect of bupropion or varenicline on nicotine self-administration in rats. Rats were first assessed for their individual economic demand for sucrose and then for self-administered nicotine (0.03 mg/kg/inf; 2 h sessions). We then examined the effect of bupropion (0, 10, 30, 60 mg/kg) or varenicline (0, 0.1, 1.0, 3.0 mg/kg) pretreatment on individual rates of nicotine self-administration using progressive ratio schedule of reinforcement. Thereafter, rats were subjected to four rounds of extinction and reinstatement tests. We found that individual demand for sucrose did not predict individual demand for nicotine. Acute pretreatments with bupropion or varenicline were most effective at decreasing nicotine self-administration in rats that had a higher demand for nicotine. Rats with higher demand for nicotine also showed higher magnitude of responding in extinction and during nicotine-triggered reinstatement tests. Although the acute treatment protocol employed in this study is an important initial step towards a better understanding of individual treatment effects, future research modeling chronic treatment approaches will be needed to further extend our findings.
Keywords: Individualized treatment, smoking cessation, nicotine, self-administration, bupropion, varenicline
1. Introduction
Tobacco use is responsible for more than 7 million deaths each year globally (WHO, 2017). Approximately 68% of U.S. adult smokers report a desire to quit; among those with the desire to quit only about 7.4% are successful in doing so (CDC, 2011). Current FDA-approved treatments for smoking cessation include nicotine replacement therapy (NRT), bupropion, and varenicline (FDA, 2013). With the use of these treatments, there is the only marginal increase in cessation rates (7% for NRT, 8.5% for bupropion, and 17% for varenicline over 10% for placebo; Cahill et al., 2013). Although smokers often vary in their history of tobacco use, there is a significant gap in addressing this individual variability with individually targeted treatments. To develop effective individualized treatment strategies for smoking cessation there is a need to understand how treatment outcomes vary on an individual level and how the previous history with nicotine reinforcement contributes to these treatment outcomes.
Bupropion and varenicline are the top two first-line smoking cessation interventions in the US, European Union, and many other nations. Bupropion is an atypical antidepressant that inhibits dopamine and norepinephrine reuptake while also acting as a non-competitive antagonist at nicotinic acetylcholine receptors (nAChR’s; Santamaría and Arias, 2010). The mechanism by which bupropion improves cessation rates in smokers is not fully understood and preclinical studies show a wide range of effects depending on the model or dose used. In some preclinical self-administration studies, acute or repeated bupropion treatment does not have an effect on responding for nicotine (Paterson et al., 2008; 10–60 mg/k; Shoaib et al., 2003), while others show increased responding at low doses (9 and 15 mg/kg; Rauhut et al., 2003) and decreased responding at higher doses (Hall et al., 2015; 30–75 mg/kg; Liu et al., 2008; Rauhut et al., 2005; Stairs and Dworkin, 2008). Varenicline, on the other hand, is a partial agonist for α4β2 and full agonist for α3β2 and α7 nAChR’s (Rollema et al, 2007, 2009). Acute varenicline pretreatment dose-dependently attenuates intravenous nicotine self-administration in rats while chronic treatment is effective at lower than acute doses (George et al, 2011; O’Connor et al, 2010; Rollema et al, 2007). Importantly, both treatments share similar discriminative stimulus effects with nicotine (for review see Bevins et al, 2012; Charntikov et al, 2014; Glennon and Young, 2011). With this evidence in mind, it is important to note that the effects of these treatments vary based on experimental designs and, more importantly, individual response.
Although there is a significant research effort towards a better understanding of the etiology of nicotine dependence, there is still a significant gap in translating preclinical research into effective smoking cessation treatments. The effectiveness of currently available treatments is difficult to estimate because clinical studies often use different inclusion criteria and duration of observations (Alpert et al., 2013; Le Foll et al., 2014; Le Strat et al., 2011). For example, participants are often excluded from studies for having low motivation to quit or based on their low consumption levels (Alpert et al., 2013; Le Strat et al., 2011). With that said, the effectiveness of current treatments is marginal at best. One of the factors that may be contributing to the lack of “bench to bedside” translation is the qualitatively different approach to subject selections used in clinical studies when compared to grouped preclinical experimental designs. Clinical studies often recruit individuals who have an extensive history of smoking and who have high motivation to quit (e.g., those that responded to the solicitation to participate in the study). Preclinical studies often draw their subjects from a supposedly homogeneous sample, that represents general target population (e.g., outbred rodents), and then randomly assign subjects into experimental conditions. These types of preclinical studies typically treat within group variance as the error. One of the approaches that can improve the external validity of preclinical studies is to study the individual differences along various phases of substance use continuum.
There is a significant gap in understanding how individual variability in nicotine consumption contributes to treatment outcomes. For example, it is unclear whether rats that self-administer large amounts of nicotine are more or less responsive to treatments with bupropion or varenicline when compared to rats that self-administer low amounts of nicotine. Understanding how this individual variability in nicotine consumption may predict treatment outcomes is the initial step towards the development of more efficacious personalized treatment strategies.
One of the ways to assess individual differences in nicotine consumption is with the help of behavioral economics (Hursh, 1993; Hursh et al., n.d.; Hursh and Roma, 2016). The basic framework of behavioral economics derives a value of a reinforcer from a magnitude of consumption relative to the price. According to this framework, increasing the price of the reinforcer would decrease the consumption and the rate of consumption decline is conceptualized as the elasticity of demand. Thus, the consumption data over the range of price values can be fitted into an equation model that can output a number of variables including the elasticity of demand (⍰) and the basal consumption of a reinforcer when the price is 0 or free (Q0). Importantly, the overall strength of a reinforcer, that is termed essential value (EV), can be also derived from that model for each subject in the study (Hursh, 2014; Hursh and Silberberg, 2008). Using this approach, the reinforcing value for self-administered nicotine, that is specific to each rat, can be derived from the amount of nicotine consumed (mg/kg) over a range of different fixed ratio (FR) schedules of reinforcement (prices). In the present study, we used this approach to assess individual demand for nicotine and then used it to predict individual responses to acute treatment with bupropion or varenicline. In addition, we used the same approach to assess individual nicotine seeking in extinction and the magnitude of reinstatement that was triggered by nicotine, non-contingent cues, bupropion, or varenicline.
2. Materials and Methods
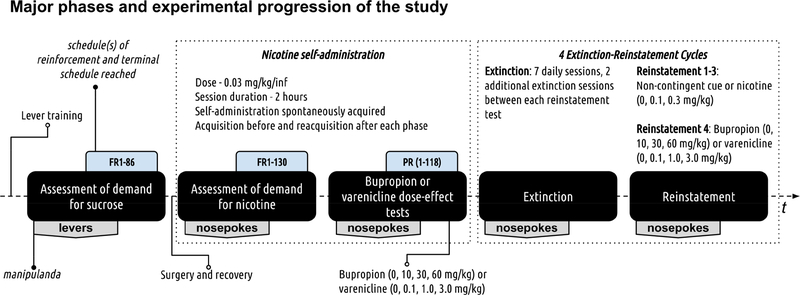
Major phases of experimental progression and pertinent information about manipulanda, schedules of reinforcement, and extinction-reinstatement cycles are outlined in Figure 1.
Figure 1.
Experimental progression. The study used a within-subjects design (N=24) with 5 distinct experimental phases as outlined in the above figure (refer to black filled rounded rectangles). The first phase assessed individual demand for sucrose. The second phase modeled drug taking and assessed individual demand for nicotine (doses for all treatments can be found in the figure). The third phase assessed the effects of acute bupropion or varenicline pretreatment on responding for nicotine. The fourth extinction phase modeled abstinence. The fifth phase modeled relapse and in this phase resurgence of active lever responding in extinction was triggered by non-contingent presentation of cues that were previously paired with nicotine infusions, nicotine, bupropion, or varenicline.
2.1. Animals
24 Male Sprague Dawley rats (250–300 g) were purchased from Envigo (Indianapolis, IN, USA). Four rats were eliminated from the study (3 due to surgery loss and 1 lost patency during dose-response testing phase). Rats were postnatal days 70–90 at the start of experimental procedures. Upon arrival at the vivarium, rats were singly housed and acclimated to a colony for at least 1 week prior to experimentation. The vivarium was maintained on a 12 h light/dark cycle with lights on at 0700. For all rats, food and water were available ad libitum until day 7 of recovery from self-administration surgeries; thereafter, rats were food deprived to 85 % of their free-feeding weight and this free-feeding weight was increased by 2 g every 30 days. All procedures were in accordance with the Guide for the Care and Use of Laboratory Animals, Eighth Edition (Institute for Laboratory Animal Research, The National Academies Press, Washington, DC, 2011) and were reviewed and approved by the University of New Hampshire Institutional Animal Care and Use Committee.
2.2. Apparatus
Conditioning chambers (ENV-018MD; Med Associates, Inc.; St. Albans, VT, USA; 30.5 × 24.1 × 21.0 cm), were enclosed in a sound- and light-attenuating cubicle equipped with an exhaust fan. Each chamber had aluminum side walls, metal rod floors with polycarbonate front, back, and ceiling. A recessed receptacle (5.2 × 5.2 × 3.8 cm; l × w × d) was centered on one sidewall. A dipper arm, when raised, provided access to 100 μL of 5 % (w/v) sucrose solution in the receptacle. Access to the dipper was monitored by an infrared beam mounted 1.2 cm into the receptacle and 3 cm above the floor. Two additional infrared beams that monitored gross chamber locomotion were located 4 cm above the floor and 5.4 cm from each aluminum side wall. Two retractable levers (147 nN required for micro-switch closure) were mounted on each side of the receptacle. When in use, two nosepokes1 were mounted across from the dipper receptacle on the opposite side wall. A white cue-light (2.54 cm diameter; 28V, 100 mA) was mounted 7 cm above each lever. A house light (two white 28V, 100 mA lamps) was located 10 cm above the conditioning chamber. The infusion pump (PMH-100VS; Med Associates; St. Albans, VT, USA) for each chamber was located outside the sound-attenuating cubicle. A 5-mL syringe mounted on the infusion pump was connected to Tygon® tubing (AAQ04103; VWR; West Chester, PA, USA). The tubing was then attached to a swivel coupled with a spring leash (C313C; Plastics One; Roanoke, VA, USA) which were suspended over the ceiling of the chamber on a balanced metal arm. Med Associates interface and software (Med-PC for Windows, version IV) were used to record data and all programmed events.
2.3. Drugs
Nicotine bitartrate, varenicline tartrate, and bupropion hydrochloride were dissolved in 0.9% sterile saline. Intravenous nicotine dose (0.03 mg/kg/inf; MP Biomedicals; Solon, OH, USA) and nicotine subcutaneous injection dose (0.4 mg/kg) were chosen based on previous research (Charntikov et al., 2014; Liu et al., 2008). Bupropion hydrochloride and varenicline tartrate were obtained from Toronto Research Chemicals (Toronto, ON, Canada). Doses and administration protocols were adopted from previous research (Bruijnzeel and Markou, 2003; George et al., 2011; Liu et al., 2008; Shoaib et al., 2003; Wouda et al., 2011). Nicotine doses are reported as base, whereas bupropion and varenicline doses are reported as salt.
2.4. Preliminary lever training
Rats were first trained to retrieve liquid sucrose (5 % w/v; 100 μL) from a dipper receptacle until reaching 80 % retrieval criterion (3–5 days). These 50 min dipper training sessions consisted of non-contingent sucrose presentations delivered on a variable time interval (~ 3 rewards per minute). Rats then were trained to lever press for liquid sucrose (5 % w/v; 100 μL). At the start of each session, the house-light was turned on and a randomly selected lever (right or left) was inserted. A lever press or lapse of 15 s resulted in sucrose delivery (4-s access), lever retraction, and commencement of a timeout (average=60 s; range=30 to 89 s). Following the timeout, a randomly selected lever was inserted with the condition that the same lever could not be presented more than twice in a row. This protocol was repeated for 60 sucrose deliveries. Sessions lasted 65 to 80 min depending on individual performance. Training continued until a lever press was made on at least 80 % of the lever insertions for two consecutive days (i.e., 3 to 6 sessions).
2.5. Assessment of economic demand for sucrose
Immediately after reaching criterion for lever training, rats were allowed to earn liquid sucrose (5 % w/v; 100 μL) on a fixed-ratio (FR) schedule of reinforcement that was escalating daily (FR sequence: 1, 3, 5, 8, 12, 18, 26, 38, 58, 86, 130, and 195). Active levers were pseudorandomly assigned so that half of the rats would have right and the other half left as an active lever. Each session began with the termination of house light and extension of both levers into the chamber. Once response requirement on the active lever was met, both levers were retracted and the dipper arm was raised to deliver 100 μL of liquid sucrose; 10 s later, levers and dipper arm were reset. These sessions lasted 30 min.
2.6. Catheter implantation surgery
Rats were anesthetized with ketamine/xylazine mixture (90/7 mg/kg respectively; IM) administered 15-min prior to surgery. Polyurethane catheter (RJVR-23; Strategic Applications Inc.; Lake Villa, IL, USA) with a rounded tip and double suture beads, one secured internally and other externally, was implanted into the right external jugular vein. The other end of the catheter was subcutaneously placed around the shoulder and exited below the scapula via subcutaneously implanted polycarbonate back-mount access port (313–000BM; Plastics One Inc.; Roanoke, VA, USA). Immediately following the surgery, catheters were flushed with 0.2 mL of cefazolin (50 mg/mL) diluted in sterile heparinized saline (30 U/mL). Thereafter, these catheter flushes occurred daily until the end of self-administration phase of the experiment to ensure patency. Atipamezole hydrochloride (0.5 mg/kg; IM; Sigma; St. Louis, MO, USA) diluted in saline was used to terminate anesthesia (Charntikov et al., 2018). To manage post-surgical pain, buprenorphine hydrochloride (0.1 mg/kg; SC) was administered immediately after the surgery and daily for the next two recovery days. Catheter patency was assessed when patency loss was suspected or upon completion of the self-administration phase of the study using an infusion of 0.05 mL xylazine (20 mg/mL; IV). This xylazine concentration produces clear motor ataxia within 5–10 s (Charntikov et al., 2018, 2013). Rats that did not exhibit noticeable motor ataxia within 5–10 s following xylazine infusion were considered non-patent.
2.7. Nicotine self-administration and assessment of individual demand for nicotine
Prior to nicotine self-administration phase of the study, rats were pretreated with nicotine (0.4 mg/kg; SC) for 3 consecutive days. These nicotine injections were used to alleviate initial aversive effects of self-administering nicotine. Because it is likely that levers acquired conditioned reinforcing properties through pairings with sucrose, the manipulandum for nicotine self-administration was changed to nosepokes. Nosepokes were installed on the opposite from levers side of the chamber and levers were removed from the chamber. All rats spontaneously acquired nicotine self-administration using nosepokes as manipulandum. Each session began with a termination of the house light, illumination of both nosepoke inlets, and a 0.9 s infusion to flush approximately 90 % of catheter volume. Completion of the required response resulted in the termination of both nosepoke lights for 3 s and a ~1 s infusion of 0.03 mg/kg nicotine. All rats self-administered the exact dose of nicotine using a variation in infusion duration that was automatically controlled by the program based on their pre-session weight (infusion time range: 0.91–1.12 s). Rats self-administer nicotine (0.03 mg/kg/inf; 2 h sessions) for 3 days on a variable-ratio (VR) 1.5 and for additional 3 days on VR3 schedules of reinforcement. Rats then were allowed to earn nicotine on the daily escalated FR schedules of reinforcement (1, 3, 5, 8, 12, 18, 26, 38, 58, 86, 130, and 195). Each rat progressed through the range of FR schedules until failing to earn at least 1 infusion; thereafter rats were allowed to self-administer nicotine for an additional 2 days on FR1 schedule. Following reacquisition of nicotine self-administration, rats self-administered nicotine on a progressive ratio (PR) schedule of reinforcement for the next 5 days (sequence: 1, 3, 6, 10, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118). Thereafter, rats were tested with bupropion (0, 10, 30, 60 mg/kg) or varenicline (0, 0.1, 1.0, 3.0 mg/kg) doses that were assigned to each rat using latin square design and were administered intraperitoneally 30 min prior to nicotine self-administration sessions. Between each treatment, rats were retrained to at least 80% of their baseline responding for nicotine on PR schedule of reinforcement. Baseline responding was determined by the average responding on the last two sessions prior to initiation of dose-effect testing. Following this dose-effect assessment, rats were allowed to self-administer nicotine on VR3 schedule for additional 5 days.
2.8. Extinction and Reinstatement
Extinction sessions were identical to self-administration sessions, except that nosepoke entries had no programmed consequences. Following 7 days of extinction, the first cycle of reinstatement tests commenced with nicotine (0, 0.1, and 0.3 mg/kg; SC; 5 min prior) or non-contingent cue presentations, that consisted of nosepoke lights off for 3 s every 5 min from the start of the session, as triggers. All rats had 2 additional intervening extinction sessions between each reinstatement test. Following the first round of reinstatements, three additional reinstatement rounds were carried out with 7 days of extinction in between each round; subsequent two rounds were identical to the initial one and in the last round triggers were switched to bupropion or varenicline. These additional extinction-reinstatements rounds were carried out to assess individual differences in drug seeking over extended period of time. The last round of reinstatement tests with bupropion or varenicline were carried out to assess individual reactivity to these treatments after a prolonged extinction that included extinction with non-contingent nicotine stimulus during previous reinstatement tests (see Discussion for additional rationale).
2.9. Dependent Measures and Statistical Analyses
Responding on the active manipulanda (levers or nosepokes) and gross chamber locomotion (total number of times two photocell beams were interrupted during a session) were used as primary dependent measures. Economic demand parameters were assessed using the demand model by Hursh and Silberberg (Hursh, 2014; 2008). The essential value, conceptualized as a strength of the reinforcer to maintain operant behavior, was derived from the economic demand model. Analyses were performed in R 3.4.0 (R Core Team, 2017) using {stats} package for t-tests and {nlme} package for all analyses associated with linear mixed-effects modeling (Pinheiro et al., 2017). Linear regression analysis and least squares nonlinear fit for the assessment of economic demand parameters were performed using GraphPad Prism version 7.04 (GraphPad Software, Inc., La Jolla, CA).
2.9.1. Analytical approach
To assess the effects of the bupropion or varenicline on the responding for nicotine and to assess responding during reinstatement tests triggered by either nicotine, bupropion, or varenicline we used two levels of assessments - grouped and individual. These grouped and individual effects were analyzed using linear mixed-effect modeling approach (Charntikov et al., 2018; S. Charntikov et al., 2017). Linear mixed-effects modeling approach provides a number of advantages when compared to ANOVAs. For example, this analysis does not require the assumption that the relation between the covariate and the outcome is the same across the groups and thus does not require meeting the assumption of homogeneity. Furthermore, unlike ANOVA, linear mixed-effects modeling does not assume that the different cases of data were independent and hence can model relations between different outcomes which may be interrelated. Linear mixed-effects modeling is also more robust in dealing with missing data or unequal group sizes which is often the case in preclinical animal models. Finally, this approach is especially effective at analyzing individual effects - like those investigated in this study. For these reasons, most of the effects in this study were analyzed using linear mixed-effects modeling.
2.9.2. Essential value assessment
The essential value was derived from the economic demand model that was calculated from the nonlinear least squares regression model fit to the individual consumption data from each schedule of reinforcement using the following formula: . In this formula Q represents reinforcer consumption, Q0 is a consumption when price is zero or free, κ is a constant for the range of demand, e is the base of the natural logarithm, C is the varying cost of each reinforcer, and α is the rate of decline in relative log consumption with increases in price. The essential value was calculated from the demand model using the following formula .
2.9.3. Exponential demand comparison
To assess overall differences in the magnitude of responding when reinforcer price was simulated by the model as “free” (Q0), we used fold analysis (sucrose Q0∕nicotine Q0). To compare overall demand for nicotine with demand for sucrose we used linear mixed-effects modeling approach as described above with a number of reinforcers earned as a dependent measure and Price (FR) or Reward (sucrose or nicotine) as predictors.
2.9.4. Grouped effects
Grouped effects of the bupropion or varenicline on the responding for nicotine and the effects of nicotine, bupropion, or varenicline during reinstatement tests were analyzed by building a model with a maximum likelihood fit from a baseline that does not include any predictors other than an intercept. The model was then built by first adding predictor 1 (Dose), then predictor 2 (Locomotion), and finally an interaction between predictor 1 and 2 (Dose × Locomotion). The predictor or an interaction was declared significant when its addition improved the model by accounting for significantly more variance (the fit was examined using the Likelihood Ratio test of fixed effects; p<0.05). Pairwise comparisons were performed using estimates from the model.
2.9.5. Individual effects
Individual effects were analyzed using linear mixed-effects modeling approach as described above with Dose as a predictor 1, Essential Value as predictor 2, and an interaction between Dose × Essential Value as a final predictor. Significance was declared as described above. Pairwise comparisons and assessment of contrasts were performed using estimates from the model.
2.9.6. Locomotion effects
Locomotion effects were analyzed as described above but with Locomotion (total beam brakes during the session) as a dependent measure and Dose, Essential Value, and Dose × Essential Value as predictors.
2.9.7. Cue-triggered reinstatement
The effect of non-contingent cue presentations on the responding during extinction tests was analyzed using a pairwise t-test by comparing responding during reinstatement test to the average of responding on the last two extinction sessions preceding that cycle of reinstatement tests.
3. Results
Detailed statistical outputs for major tests are presented in Tables S1–6. Acquisition and reacquisition of nicotine self-administration are visualized in Figure S1.
3.1. Exponential Demand Comparison
Responding for sucrose was 2.04-fold higher than responding for nicotine when the price for each of these reinforcers was simulated as “free” (FR1) using the demand model (see Q0 values at the bottom of Figure 2A). Likewise, the essential value for sucrose was 2.13-fold higher than for nicotine (compare essential values at the bottom of Figure 2A; additional analyses provided in the Supplemental Results). The degree of variance in demand for sucrose or nicotine is visualized by plotting the demand curves from subjects with highest or lowest demands on the left panel and total variance on the right panel of Figures 2C and 2D.
Figure 2.
Exponential demand curves for nicotine, sucrose, and their comparison (N=21). Critical demand values are found below each panel showing demand curves. SD - standard deviation. (A) A group-level comparison of demand curves for nicotine and sucrose. (B) A scatter plot and a linear fit of individual demand for nicotine and sucrose. Individual demand is presented as individual “essential values” that were derived from the nonlinear least squares regression model fit to the individual consumption data. (C) Exponential demand curves for subject with highest and lowest essential values for sucrose. (D) Exponential demand curves for subject with highest and lowest essential values for nicotine.
3.2. Relationship between the individual demand for nicotine and sucrose
Individual demand for sucrose did not predict individual demand for nicotine (see Table S1 for statistical output; p=0.16; Figure 2B).
3.3. The effect of varenicline on nicotine self-administration
3.3.1. Grouped effects
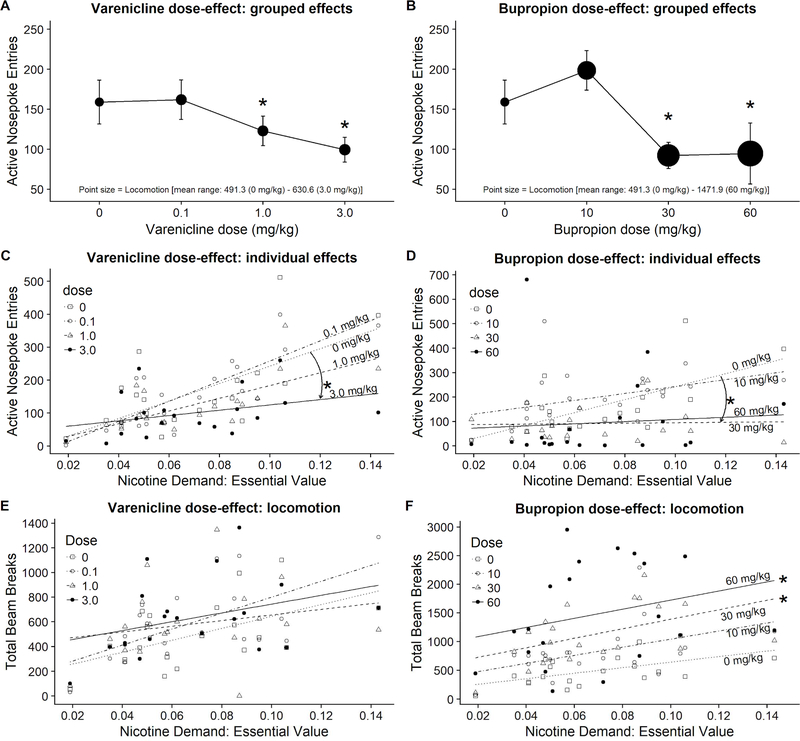
Active nosepoke entries significantly differed depending on varenicline Dose (p < 0.01), general chamber Locomotion (p < 0.001), and there was significant Dose × Locomotion interaction (p < 0.01; see Table S2 for full statistical output). Responding following pretreatment with 1 or 3 mg/kg varenicline was significantly lower than responding after pretreatment with 0 mg/kg (Figure 3A; for locomotion compare point size).
Figure 3.
Grouped (A-B) and individual (C-F) effects of bupropion or varenicline on active nosepoke entries or general chamber locomotion (total beam breaks; N=20). *Indicates significant difference in responding from 0 mg/kg. Chamber locomotion on panels A and B is visualized using a point size with all point sizes normalized across these panels for accuracy and ease of visual data assessment between these panels.
3.3.2. Individual effects
To test whether responding after pretreatment with varenicline varied based on individual demand for nicotine, we performed additional tests with Essential Value, derived from Hursh’s demand model for each subject, as an additional predictor. These tests showed that entries into active nosepokes significantly differed depending on varenicline Dose (p < 0.01), Demand for nicotine (Essential Value; p < 0.0001), and there was significant Dose × Demand interaction (p < 0.01). Assessment of contrasts indicated that responding across nicotine demand spectrum was significantly different after pretreatment with 3.0 mg/kg of varenicline when comparing to responding after 0 mg/kg (b = −1847.02, t(54) = −2.93, p < 0.01; see downward arrow in Figure 3C highlighting this effect). The outcome of these tests suggests that a) responding on the progressive ratio of reinforcement positively related to the Essential Value derived from the demand mode (supported by the effect of Demand) and b) the decrease in responding after pretreatment with 3.0 mg/kg was largely driven by rats with higher demand for nicotine.
3.3.3. Locomotion effects
To test whether chamber locomotion varied across varenicline doses and over the spectrum of demand for nicotine, we performed additional separate tests with Locomotion as a dependent measure and Dose with individual Essential Value as predictors. These tests show that Locomotion did not significantly differ depending on the varenicline Dose (p = 0.19). On the other hand, Locomotion significantly differed depending on the Nicotine Demand (Essential Value; p < 0.01). There was no significant Dose × Demand interaction (p < 0.27). Rats with higher demand for nicotine were more active independent of the varenicline treatment (Figure 3E).
3.4. The effect of bupropion on nicotine self-administration
3.4.1. Grouped effects
Entries into active nosepokes significantly differed depending on the bupropion Dose (p <0.01), general chamber Locomotion (p < 0.05), and there was a significant Dose × Locomotion interaction (p < 0.05). Responding following pretreatment with 30 or 60 mg/kg was significantly lower than responding after pretreatment with 0 mg/kg (Figure 3B; for locomotion compare point size).
3.4.2. Individual effects
To test whether responding after pretreatment with bupropion varied based on individual demand for nicotine, we performed additional tests with Essential Value as an additional predictor. These tests show that entries into active nosepokes significantly differed depending on the bupropion Dose (p < 0.01) and Demand for nicotine (p < 0.05). There was no Dose × Demand interaction (p = 0.14). Assessment of contrasts indicated that responding across nicotine demand spectrum was significantly different after pretreatment with 30 mg/kg of bupropion when comparing to responding after 0 mg/kg (b = −2559.97, t(54) = −2.06, p < 0.05; see downward arrow in Figure 3D highlighting this effect). The outcome of these tests suggests that higher overall responding was driven by the rats with higher demand for nicotine and that rats with higher demand for nicotine showed a more pronounced decrease in responding following pretreatment with 30 mg/kg than after pretreatment with 0 mg/kg.
3.4.3. Locomotion effects
To test whether chamber locomotion varied after pretreatment with bupropion and over the demand spectrum for nicotine, we performed additional tests with Locomotion as a dependent measure and Dose with Essential Value as predictors. These tests show that Locomotion significantly differed depending on the bupropion Dose (p < 0.0001) and Nicotine Demand (p < 0.01). There was no significant Dose × Demand interaction (p = 0.92). Locomotion was significantly higher following pretreatment with 30 and 60 mg/kg. Rats with higher demand for nicotine were consistently more active across the range of bupropion doses (Figure 3F).
3.5. Extinction
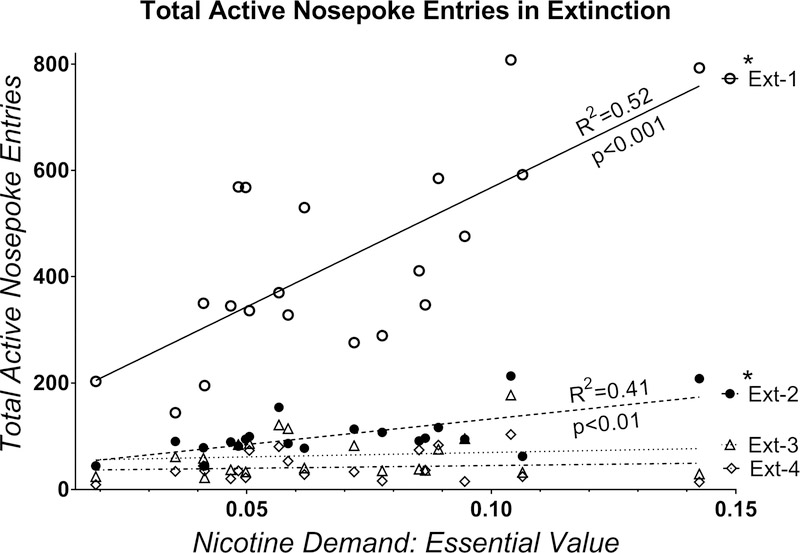
To assess whether persistence of responding in extinction varied according to individual demand for nicotine we performed additional linear-regression assessment of these effects for each extinction cycle (Figure 4). Recall that this study design consists of 4 total extinction-reinstatement cycles with 7 days of extinction in each cycle. Individual demand for nicotine (Essential Value) significantly predicted total active nosepoke entries in extinction cycles 1 and 2 but not 3 or 4 (Figure 4; p values and R2 shown in figure). Rats with higher demand for nicotine during self-administration phase had higher nicotine seeking in extinction for approximately 24 days after the access to nicotine was terminated (7 days of each extinction cycle plus 10 days of reinstatement tests and intervening extinction sessions in between).
Figure 4.
Total active nosepoke entries over each 7-day extinction cycle (N=20). *Indicates significant deviation from zero slope (flat horizontal line) based on linear regression analysis.
3.6. Reinstatement 1
3.6.1. Cue-triggered reinstatement: grouped effects
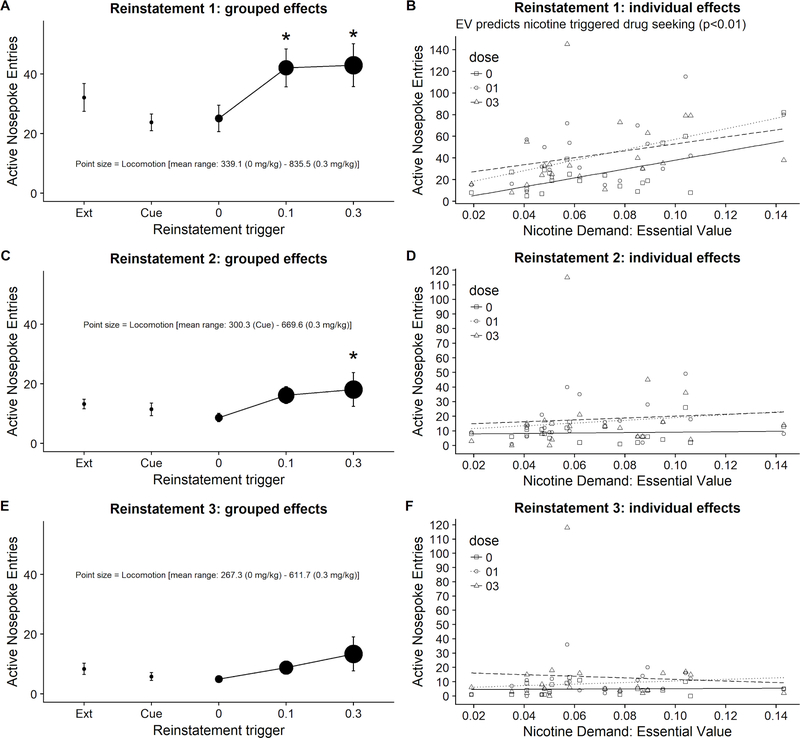
Non-contingent cue presentation did not reinstate entries into active nosepokes (p = 0.13; Figure 5A).
Figure 5.
Grouped and individual active nosepoke entries during reinstatement rounds 1–3 (N=20). *Indicates significant difference from average responding during two extinction sessions immediately preceding a round of reinstatement tests (see “Ext” on the x axis of panels A, C, and E). EV- Essential Value. Chamber locomotion on panels A, C, and E is visualized using a point size with all point sizes normalized across these panels for accuracy and ease of visual data assessment between these panels.
3.6.2. Nicotine-triggered reinstatement: grouped effects
Responding on the active nosepokes during nicotine-triggered reinstatement tests significantly differed depending on the nicotine Dose (p < 0.05) and Locomotion (p < 0.05). There was no significant Dose × Locomotion interaction (p = 0.91). Responding following 0.1 and 0.3 mg/kg nicotine pretreatment was significantly higher than after pretreatment with 0 mg/kg (Figure 5A).
3.6.3. Nicotine-triggered reinstatement: individual effects
Assessment of nicotine-triggered responding over a spectrum of individual demand for nicotine showed that responding on the active nosepokes significantly differed depending on the nicotine Dose (p < 0.05) and Demand for nicotine (p < 0.01). There was no Dose × Demand interaction (p = 0.65). Importantly, rats with higher demand for nicotine had higher responding in extinction (0 mg/kg; Figure 5B) and higher responding following nicotine pretreatment (0.1, 0.3 mg/kg; Figure 5B).
3.6.4. Nicotine-triggered reinstatement: locomotion
Locomotion significantly differed depending on the nicotine Dose (p < 0.0001) but not Demand for nicotine (p = 0.14). There was no Dose × Demand interaction (p = 0.22). Locomotion following pretreatment with 0.1 and 0.3 mg/kg was significantly higher than after pretreatment with 0 mg/kg (Figure 5A, compare point size of each dose).
3.7. Reinstatement 2
3.7.1. Cue-triggered reinstatement: grouped effects
Non-contingent cue presentation did not reinstate entries into active nosepokes (p = 0.50; Figure 5C).
3.7.2. Nicotine-triggered reinstatement: grouped effects
Although effects were trending towards significance, responding on the active nosepokes did not differ depending on the Dose (p = 0.07) or Locomotion (p = 0.08). There was no Dose × Locomotion interaction (p = 0.40; Figure 5C). Trending effect of Dose was followed by pairwise comparisons. Active nosepoke entries following 0.3 mg/kg pretreatment were significantly higher than following 0 mg/kg (p=0.03; Figure 5C).
3.7.3. Nicotine-triggered reinstatement: individual effects
Responding on the active nosepokes did not significantly differed depending on the nicotine Dose (p = 0.07) or Demand for nicotine (Essential Value; p = 0.53). There was no Dose × Demand interaction (p = 0.85; Figure 5D).
3.8. Reinstatement 3
3.8.1. Cue-triggered reinstatement: grouped effects
Non-contingent cue presentation did not reinstate entries into active nosepokes (p = 0.26; Figure 5E).
3.8.2. Nicotine-triggered reinstatement: grouped effects
Responding on the active nosepokes did not differ depending on the Dose (p = 0.12) or Locomotion (p = 0.58). There was no Dose × Locomotion interaction (p = 57; Figure 5E).
3.8.3. Nicotine-triggered reinstatement: individual effects
Responding on the active nosepokes did not significantly differed depending on the nicotine Dose (p = 0.12) or Demand for nicotine (Essential Value; p = 0.98); there was no Dose × Demand interaction (p = 0.73; Figure 5F).
3.9. Varenicline-triggered reinstatement
3.9.1. Grouped effects
Although the effect of Dose was trending towards significance, responding on the active nosepokes did not differ depending on the Dose (p = 0.06), Locomotion (p = 0.63), and there was no Dose × Locomotion interaction (p = 0.61; Figure 6A). Trending effect of Dose was followed by pairwise comparisons and revealed that responding after pretreatment with 1 mg/kg of varenicline was significantly higher than responding following pretreatment with saline (0 mg/kg; Figure 6A)
Figure 6.
Grouped and individual active nosepoke entries during the final reinstatement round (N=20). *Indicates significant difference from 0 mg/kg. Chamber locomotion on panels A and B is visualized using a point size with all point sizes normalized across these panels for accuracy and ease of visual data assessment between these panels.
3.9.2. Individual effects
Responding on the active nosepokes did not significantly differed depending on the varenicline Dose (p = 0.06), Demand for nicotine (Essential Value; p = 0.93), and there was no Dose × Demand interaction (p = 0.87; Figure 6C).
3.10. Bupropion-triggered reinstatement
3.10.1. Grouped effects
Responding on the active nosepokes did not differ depending on the Dose (p = 0.26), Locomotion (p = 0.51), and there was no Dose × Locomotion interaction (p = 0.91; Figure 6B).
3.10.2. Individual effects
Responding on the active nosepokes did not significantly differed depending on the nicotine Dose (p = 0.26) but did depending on the Demand for nicotine (p < 0.01) and there was significant Dose × Demand interaction (p < 0.0001; Figure 6D). Assessment of contrasts indicated that responding across nicotine demand spectrum was significantly different after pretreatment with 60 mg/kg of bupropion when compared to responding after 0 mg/kg (b = 2560.60, t(54) = 4.74, p < 0.0001; see upward arrow in Figure 6D highlighting this effect). Although it appears that this increase in responding following pretreatment with 60 mg/kg was driven by few rats with high demand for nicotine, this remains a critical finding because this experiment, and the statistical approach that was chosen for this analysis, were specifically designed to investigate these types of individual effects.
4. Discussion
Large body of preclinical evidence shows that both bupropion and varenicline decrease nicotine self-administration in rats (Bruijnzeel and Markou, 2003; Funk et al., 2016; George et al., 2011; Hall et al., 2015; Le Foll et al., 2012; Liu et al., 2008; O’Connor et al., 2010; Rauhut et al., 2005, 2003; Stairs and Dworkin, 2008; Wouda et al., 2011). However, there is a significant gap in understanding individual variability in responding to these treatments. The main goals of this study were a) to develop an effective approach that allows assessment of individual differences in responding for reinforcers like sucrose or nicotine, and b) to be able to correlate this individual variability in responding for nicotine to individual treatment outcomes. We found that with the help of behavioral economics we can assess individual demand for nicotine on a continuum from low to high and then use it to predict responding for nicotine on a PR schedule of reinforcement, responding to bupropion or varenicline treatments, drug seeking in extinction, and magnitude of nicotine triggered reinstatement in extinction. We also show that the individual demand for sucrose did not predict individual demand for nicotine. This finding indicates that individual sensitivity to primary reinforcers, like food, does not generalize to nicotine and rules out the general sensitivity of the reward system as one of the explanations for the individual variation in responding for nicotine. Furthermore, our results show that bupropion and varenicline were most effective at decreasing nicotine self-administration in rats with higher demand for nicotine. Overall, our findings demonstrate that the individual variability can be assessed across various phases of nicotine self-administration study and that this variability can be further used as an explanatory or a response variable to answer various questions of interest, including those concerning individual reactivity to various pharmacological treatments.
Nicotine consumption, whether through traditional combustible methods or through growing in popularity electronic delivery systems, varies among individuals and is often conceptualized as a continuum from light to heavy. Light smokers are sometimes categorized based on cigarettes per day (cpd) consumption (e.g., <15 or <5), sometimes based on days per week consumption (e.g., <5 cpd at least 5 days a week), and sometimes based on a number of cigarettes smoked per year or a lifetime (e.g., <100 in a lifetime), to name a few criteria found in a literature (Evans et al., 1992; Husten, 2009; Okuyemi et al., 2002; Shiffman et al., 1994). Like heavy smokers, light smokers have difficulties to quit smoking and have worse health outcomes than non-smokers (DHHS, 1998). As much as 50% of all smokers can be categorized as light smokers (Kandel and Chen, 2000; Owen et al., 1995; Russell, 1990). Light smokers are often excluded from cessation intervention studies due to perceived low health risk associated with light smoking and methodological strategies to focus only on heavy smokers. These reasons contribute to the lack of individualized smoking cessation treatment strategies that incorporate the history of consumption into a treatment plan. Furthermore, at the present, it is not clear what treatment strategy would be most beneficial to heavy smokers versus light smokers. Likewise, there is a significant gap in understanding how the individual pattern of nicotine consumption interacts with currently available and widely used tobacco cessation treatments in the preclinical field. The study presented here takes the initial step towards filling this gap.
For the first time, we are here demonstrating a methodological approach to study individual treatment outcomes in a preclinical model of nicotine use. This approach leverages the power of behavioral economics to assess the individual history of substance consumption and the power of multivariate statistics to predict individual responding to a pharmacological intervention in the later phase of a preclinical study using the individual history of substance consumption as an explanatory variable. Behavioral economics approach allows estimation of individual demand for any given self-administered substance by assessing consumption of that substance over a range of FR schedules. This approach takes into consideration consumption when the price of a reinforcer is low (e.g., FR1), when the price of the reinforcer is high (e.g., higher or terminal FR schedules), and the slope of the demand curve fitted into consumption data over the range of prices (FR values) for that reinforcer - also referred as elasticity of demand (Hursh, 2014; Hursh and Silberberg, 2008). Furthermore, to assess how individual demand for a reinforcer relates to the treatment outcome, a researcher can use a multivariate systems approach that is especially well suited for assessment of individual effects. This multivariate approach also allows to test a research question or a hypothesis by assessing the existence, direction, and a magnitude of a relationship between a predictor and a dependent variable. Importantly, this analytical approach allows assessment of relationships between a dependent variable and multiple predictors. Using this strategy, we are here demonstrating that the acute pretreatment with bupropion or varenicline differentially affected individual nicotine consumption - an effect that varied across a spectrum of previously derived individual demand for nicotine.
Consistent with previous findings, we show that on a group level both bupropion and varenicline decreased nicotine self-administration in rats (Bruijnzeel and Markou, 2003; Funk et al., 2016; George et al., 2011; Hall et al., 2015; Le Foll et al., 2012; Liu et al., 2008; O’Connor et al., 2010; Rauhut et al., 2005, 2003; Stairs and Dworkin, 2008; Wouda et al., 2011). We further extend previous reports by assessing these effects on an individual level using multivariate analysis approach with an individual demand for nicotine and a chamber locomotion as explanatory factors. We show that rats with a higher demand for nicotine had a greater decrease in nicotine self-administration rates following acute pretreatment with bupropion or varenicline in comparison to rats with lower demand for nicotine. There are various factors that may be involved in this effect. Nicotine is a stimulant with discriminative properties and both bupropion and varenicline substitute for the interoceptive effects of nicotine (for review on this topic see Bevins et al., 2012; Charntikov et al., 2014; Sergios Charntikov et al., 2017). Interoceptive effects of bupropion or varenicline may summate with those evoked by the nicotine over the course of the test and then interact with stimulant effects induced by nicotine to affect nicotine self-administration rates during the test. Furthermore, the nature of this interaction may differ on the individual level and may depend on the individual pattern of nicotine consumption in the early phase of the study, the amount of nicotine consumed during the test, and the individual reactivity to the pharmacological effects involved. For example, in some rats pharmacological treatment may substitute for nicotine stimulus or reinforcing effects to a higher degree which could result in decreased demand for nicotine for that subset of rats. Conversely, other rats may be more sensitive to a stimulant effects evoked by the treatment and that could result in a higher demand for nicotine. There is some evidence of this speculation in our study. For example, assessing y-intercepts of linear fit for active nosepoke entries after bupropion or varenicline pretreatment indicates elevated y-intercepts for higher doses of these treatments (Figures 3C and 3D; examine y-intercepts for each dose). This elevation of y-intercepts suggests that, in addition to the decrease in responding among rats with higher demand, rats with lower demand for nicotine increased their responding after pretreatment with bupropion or varenicline. There is one study known to us that tangentially supports this speculation. George et al., (2011) showed that low dose of varenicline (0.3 mg/kg) increased responding for nicotine on a similar PR schedule of reinforcement in rats with low baseline of nicotine intake in comparison to the decreased responding of rats with high baseline of nicotine intake. Another speculative explanation of our individual effects may take into account different starting self-administration baselines for rats with high or low nicotine demand and argue that rats with higher initial rates of responding had proportionally greater degree of response suppression by the treatments when comparing to rats with lower self-administration baseline. This proportionally greater decrease in responding among rats with higher demand for nicotine could then possibly explain the difference in responding detected through our analysis. Although this may be a viable hypothesis, the nature of our design and the statistical analysis employed here do not allow us to unequivocally support or deny this claim. Furthermore, although there may be various unknown factors underlying response to treatments presented in our study, our results show that the effect of bupropion or varenicline on nicotine self-administration varies across subjects and a history of nicotine consumption provides a statistical explanation for a large degree of that variance.
Our study suggests that the individual demand for nicotine may be stable over the long self-administration period of the study. For example, we show that rats that had a higher demand for nicotine in the early phase of experiment also had higher responding on the PR schedule of reinforcement in the later phase of the experiment. This effect is important because it suggests that the individual demand for nicotine is stable over time and PR schedules with moderate escalation steps can serve as a proxy for established economic demand. Furthermore, this type of approach could be especially valuable for the assessment of acute treatment effects in these types of preclinical studies. It is also important to note that our analytical approach does not explicitly differentiate between rats with “high” demand versus rats with “low” demand, rather it conceptualizes demand for nicotine as a continuous variable and all associated effects are treated as a change over the range of this continuous variable. For example, although we refer to some rats having “higher demand” and others having “lower demand” for nicotine, to make our narrative more accessible to a wider audience, our results more specifically show that a) bupropion or varenicline pretreatment effects on nicotine self-administration vary over the range of the demand for nicotine [positive slope of linear fit across all doses] and b) the pattern of responding at higher treatment doses over the range of individual nicotine demand differs from the control [slopes of linear fit at higher doses significantly different from the slope of a line at 0 mg/kg]. Furthermore, to strengthen the assessment of effects in this study we prioritized the use of raw values, values that have not been converted to any indexes or ratios. Use of these unadulterated values is more suitable if the goal is to assess patterns in individual performance (Glass and Mackey, 1988; O’Connor, 1990). With this approach in mind, we show that the effect of bupropion or varenicline on nicotine self-administration varies across the spectrum of previously established demand for nicotine and that the chamber locomotion during these tests is a contributing factor. Because of the complex nature of underlying factors that may be influencing locomotion during these tests, further investigation of this interaction in subsequent studies is warranted.
For the first time, we are reporting the effects of bupropion or varenicline on general chamber locomotion during nicotine self-administration tests. We show that both bupropion and varenicline dose-dependently increased chamber locomotion while decreasing active nosepoke entries. For comparison, some previous reports used inactive lever responding as an indirect measure of non-specific effects like general activity, “arousal”, “specificity” of responding, or reported the effects on inactive lever without speculating about the relationship to the responding on the active lever or the amount of drug consumed (Bruijnzeel and Markou, 2003; George et al., 2011; Liu et al., 2008; O’Connor et al., 2010; Rauhut et al., 2005, 2003; Stairs and Dworkin, 2008; Wouda et al., 2011). Importantly, chamber locomotion during self-administration tests involving pharmacological pretreatments may be affected by a host of factors that may be quite diverse in their underlying mechanisms. For example, locomotor activity may be affected by the non-specific effects of test drug on locomotor system, by affecting learned excitation associated with a reinforcer, or by affecting learned excitation associated with the testing environment (Bouton, 2000; Crombag et al., 2008; for reviews see Rescorla and Solomon, 1967). Specifically, decreasing interoceptive effects of a reinforcer may decrease overall conditioned excitation and associated with it chamber locomotion. Furthermore, because the interoceptive effect of a self-administered drug may be a part of a more complex context that is comprised of both interoceptive and exteroceptive elements (e.g., chamber floor, walls, manipulanda, etc.), decreasing the salience of the interoceptive effects of the self-administered drug may decrease context evoked excitation (for review on this topic see Bevins et al., 2012). Additional complexity arises when attempting to interpret individual locomotor effects. For example, we show that bupropion dose-dependently increased locomotion across nicotine demand spectrum, however, rats with higher demand for nicotine, and those that self-administer more during the tests, show higher locomotion across all doses of bupropion in comparison to those with lower nicotine demand (Figure 3F). Increasing overall locomotor activity may increase or interfere with a goal-directed responding depending on the individual tolerance for hyperactivity and/or initial baseline levels of locomotion during these tests. Although these locomotor effects are challenging to interpret, these effects are a part of behavioral profile associated with bupropion or varenicline treatments and they need to be studied further to better understand their role in smoking cessation.
Abstinence and relapse are the two critical parts of substance use cycle that warrants ever-growing focus of the scientific community in the search for novel therapeutic approaches to nicotine dependence. Preclinical self-administration models allow to model these phases of dependence cycle using extinction and reinstatement tests where a self-administered drug is initially withdrawn for a period of time to model abstinence and then reintroduced prior to additional extinction sessions to model drug triggered relapse. Withdrawal of the drug results in extinction of responding and reintroduction of the drug, prior to the extinction session, triggers drug seeking in a form of increased responding on the manipulandum that was previously associated with drug infusions. One of the goals of this study was to assess whether individual demand for nicotine predicts drug seeking in extinction or during pharmacologically triggered reinstatement tests. To improve our understanding of prolonged extinction effects on individual level, we have implemented multiple rounds of extinction followed by the reinstatements tests. This approach allows to assess persistence in extinction when the nicotine is removed but also extinction patterns when the nicotine is administered non-contingently prior to multiple rounds of nicotine triggered reinstatement tests. As a bonus feature, at the end of the three rounds of extinction and nicotine triggered reinstatement tests, we have conducted additional extinction and reinstatement tests with bupropion or varenicline as triggers. This final round of reinstatement tests was designed to inform us whether individual learning history about non-reinforcement with non-contingent nicotine during previous reinstatement tests transfers to bupropion or varenicline stimuli which share discriminative stimulus effects with nicotine (Bevins et al., 2012; Charntikov et al., 2014). In the present study, we show that the higher demand for nicotine in the early phase of the study predicted higher nicotine seeking in extinction. Specifically, rats that had higher demand for nicotine showed higher cumulative nicotine seeking during the first and second rounds of extinction that each lasted for 7 consecutive days. In addition, rats that had higher demand for nicotine also showed a higher magnitude of nicotine triggered drug seeking extinction (Figure 5B; main effect of EV and no interaction with Dose). The ability to predict individual patterns of extinction or nicotine triggered reinstatement with high degree of confidence is a necessary first step if the long-term goal is to assess individualized treatment strategies geared towards diminishing nicotine seeking in extinction and modeled relapse. Findings from our final reinstatement tests, with bupropion or varenicline as triggers, suggests that at least some rats do not show a transfer of non-reinforced learning with nicotine to bupropion or varenicline stimuli as evidenced by the increased nicotine seeking during these tests. Furthermore, we demonstrate that few rats with higher demand for nicotine show striking increase in nicotine seeking when challenged with bupropion more than a month after the last self-administration session (see Figure 6D; compare y-axis values with 6A-C). Overall, our findings suggest that the individual demand for nicotine is a reliable behavioral marker that is associated with various facets of nicotine reinforcement spanning from drug taking, to persistence of drug seeking in extinction, to nicotine triggered seeking in extinction. In addition, this study demonstrates that rats show high degree of individual variation over various phases of nicotine self-administration cycle and that this individual variability in nicotine consumption can be used as a predictor for various behaviors in modeled abstinence, relapse, or responses to treatment outcomes along that continuum. Future studies will be required to confirm these findings and extend translational relevance by including sex as a biological variable and by using long-access self-administration protocols to better model patterns of nicotine consumption over time.
Supplementary Material
Individual demand for sucrose did not predict demand for nicotine
Individual demand for nicotine predicted responding to bupropion or varenicline
Individual demand for nicotine predicted responding in extinction
Individual demand for nicotine predicted responding in reinstatement
5. Acknowledgments
Funding: S. Charntikov was partially supported by GM113131 (CIBBR, P20) while preparing this manuscript for publication. Partial funding was provided by the Undergraduate Research Opportunities Program at the University of New Hampshire awarded to M. Duranko, K. Taylor, and E. Hart.
Footnotes
6. Disclosures
The authors report no conflicts of interest.
Disambiguation: “nosepoke” - a manipulandum used in the operant chambers; “nosepoke entry” - an action of inserting a rat’s snout into a nosepoke hole.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7. References
- Alpert HR, Connolly GN, Biener L, 2013. A prospective cohort study challenging the effectiveness of population-based medical intervention for smoking cessation. Tob. Control 22, 32–37. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Barrett ST, Polewan RJ, Pittenger ST, Swalve N, Charntikov S, 2012. Disentangling the nature of the nicotine stimulus. Behav. Processes 90, 28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, 2000. A Learning Theory Perspective on Lapse, Relapse, and the Maintenance of Behaviour Change. Health Psychol. 19, 57–63. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Markou A, 2003. Characterization of the effects of bupropion on the reinforcing properties of nicotine and food in rats. Synapse 50, 20–28. [DOI] [PubMed] [Google Scholar]
- Cahill K, Stevens S, Perera R, Lancaster T, 2013. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst. Rev CD009329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC), 2011. Quitting smoking among adults--United States, 2001–2010. MMWR Morb. Mortal. Wkly. Rep 60, 1513–1519. [PubMed] [Google Scholar]
- Charntikov S, deWit NR, Bevins RA, 2014. Interoceptive conditioning with nicotine using extinction and re-extinction to assess stimulus similarity with bupropion. Neuropharmacology 86, 181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charntikov S, Falco AM, Fink K, Dwoskin LP, Bevins RA, 2017. The effect of sazetidine-A and other nicotinic ligands on nicotine controlled goal-tracking in female and male rats. Neuropharmacology 113, 354–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charntikov S, Pittenger ST, Pudiak CM, Bevins RA, 2018. The effect of N-acetylcysteine or bupropion on methamphetamine self-administration and methamphetamine-triggered reinstatement of female rats. Neuropharmacology 135, 487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charntikov S, Pittenger ST, Swalve N, Li M, Bevins RA, 2017. Double dissociation of the anterior and posterior dorsomedial caudate-putamen in the acquisition and expression of associative learning with the nicotine stimulus. Neuropharmacology 121, 111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charntikov S, Swalve N, Pittenger S, Fink K, Schepers S, Hadlock GC, Fleckenstein AE, Hu G, Li M, Bevins RA, 2013. Iptakalim attenuates self-administration and acquired goal-tracking behavior controlled by nicotine. Neuropharmacology 75, 138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Bossert JM, Koya E, Shaham Y, 2008. Review. Context-induced relapse to drug seeking: a review. Philos. Trans. R. Soc. Lond. B Biol. Sci 363, 3233–3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Health and Human Services, U.S., 1998. Cigars: Health effects and trends (Smoking and Tobacco Control Monograph 9, NIH Publication No. 98–4302). Bethesda, MD: National Institutes. [Google Scholar]
- Evans NJ, Gilpin E, Pierce JP, Burns DM, Borland R, Johnson M, Bal D, 1992. Occasional smoking among adults: evidence from the California Tobacco Survey. Tob. Control 1, 169. [Google Scholar]
- FDA: Office of the Commissioner, 2013. Consumer Updates - Want to Quit Smoking? FDA-Approved Products Can Help [WWW Document]. URL https://www.fda.gov/forconsumers/consumerupdates/ucm198176.htm (accessed 6.14.18).
- Funk D, Lo S, Coen K, Lê AD, 2016. Effects of varenicline on operant self-administration of alcohol and/or nicotine in a rat model of co-abuse. Behav. Brain Res 296, 157–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Lloyd A, Carroll FI, Damaj MI, Koob GF, 2011. Varenicline blocks nicotine intake in rats with extended access to nicotine self-administration. Psychopharmacology 213, 715–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass L, Mackey MC, 1988. From clocks to chaos: the rhythms of life. Princeton University Press. [Google Scholar]
- Hall BJ, Slade S, Wells C, Rose JE, Levin ED, 2015. Bupropion-varenicline interactions and nicotine self-administration behavior in rats. Pharmacol. Biochem. Behav 130, 84–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR, 2014. Behavioral Economics and the Analysis of Consumption and Choice, in: McSweeney FK, Murphy ES (Eds.), The Wiley Blackwell Handbook of Operant and Classical Conditioning. John Wiley & Sons, Ltd, Oxford, UK, pp. 275–305. [Google Scholar]
- Hursh SR, 1993. Behavioral economics of drug self-administration: an introduction. Drug Alcohol Depend. 33, 165–172. [DOI] [PubMed] [Google Scholar]
- Hursh SR, Galuska CM, Winger G, Woods J.H., n.d. Economics of Drug Abuse : Review Literature And Arts Of The Americas 20–28.
- Hursh SR, Roma PG, 2016. Behavioral Economics and the Analysis of Consumption and Choice: BEHAVIORAL ECONOMICS ANALYSIS. Manage. Decis. Econ 37, 224–238. [Google Scholar]
- Hursh SR, Silberberg A, 2008. Economic demand and essential value. Psychol. Rev 115, 186–198. [DOI] [PubMed] [Google Scholar]
- Husten CG, 2009. How should we define light or intermittent smoking? Does it matter? Nicotine Tob. Res 11, 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel DB, Chen K, 2000. Extent of smoking and nicotine dependence in the United States: 1991–1993. Nicotine Tob. Res 2, 263–274. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Chakraborty-Chatterjee M, Lev-Ran S, Barnes C, Pushparaj A, Gamaleddin I, Yan Y, Khaled M, Goldberg SR, 2012. Varenicline decreases nicotine self-administration and cue-induced reinstatement of nicotine-seeking behaviour in rats when a long pretreatment time is used. Int. J. Neuropsychopharmacol 15, 1265–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Pushparaj A, Pryslawsky Y, Forget B, Vemuri K, Makriyannis A, Trigo JM, 2014. Translational strategies for therapeutic development in nicotine addiction: rethinking the conventional bench to bedside approach. Prog. Neuropsychopharmacol. Biol. Psychiatry 52, 86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Strat Y, Rehm J, Le Foll B, 2011. How generalisable to community samples are clinical trial results for treatment of nicotine dependence: a comparison of common eligibility criteria with respondents of a large representative general population survey. Tob. Control 20, 338–343. [DOI] [PubMed] [Google Scholar]
- Liu X, Caggiula AR, Palmatier MI, Donny EC, Sved AF, 2008. Cue-induced reinstatement of nicotine-seeking behavior in rats: effect of bupropion, persistence over repeated tests, and its dependence on training dose. Psychopharmacology 196, 365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor EC, Parker D, Rollema H, Mead AN, 2010. The alpha4beta2 nicotinic acetylcholine-receptor partial agonist varenicline inhibits both nicotine self-administration following repeated dosing and reinstatement of nicotine seeking in rats. Psychopharmacology 208, 365–376. [DOI] [PubMed] [Google Scholar]
- O’Connor K, 1990. Towards a process paradigm in psychophysiology. Int. J. Psychophysiol 9, 209–223. [DOI] [PubMed] [Google Scholar]
- Okuyemi KS, Harris KJ, Scheibmeir M, Choi WS, Powell J, Ahluwalia JS, 2002. Light smokers: issues and recommendations. Nicotine Tob. Res 4 Suppl 2, S103–12. [DOI] [PubMed] [Google Scholar]
- Owen N, Kent P, Wakefield M, Roberts L, 1995. Low-rate smokers. Prev. Med 24, 80–84. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Balfour DJK, Markou A, 2008. Chronic bupropion differentially alters the reinforcing, reward-enhancing and conditioned motivational properties of nicotine in rats. Nicotine Tob. Res 10, 995–1008. [DOI] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, (R CoreTeam), 2017. {nlme}: Linear and Nonlinear Mixed Effects Models.
- Rauhut AS, Dwoskin LP, Bardo MT, 2005. Tolerance does not develop to the decrease in nicotine self-administration produced by repeated bupropion administration. Nicotine Tob. Res 7, 901–907. [DOI] [PubMed] [Google Scholar]
- Rauhut AS, Neugebauer N, Dwoskin LP, Bardo MT, 2003. Effect of bupropion on nicotine self-administration in rats. Psychopharmacology 169, 1–9. [DOI] [PubMed] [Google Scholar]
- R Core Team, 2017. R: A Language and Environment for Statistical Computing.
- Rescorla RA, Solomon RL, 1967. Two-process learning theory: Relationships between Pavlovian conditioning and instrumental learning. Psychol. Rev 74, 151–182. [DOI] [PubMed] [Google Scholar]
- Russell MA, 1990. The nicotine addiction trap: a 40-year sentence for four cigarettes. Br. J. Addict 85, 293–300. [DOI] [PubMed] [Google Scholar]
- Santamaría A, Arias HR, 2010. Neurochemical and behavioral effects elicited by bupropion and diethylpropion in rats. Behav. Brain Res 211, 132–139. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Kassel JD, Paty J, Gnys M, Zettler-Segal M, 1994. Smoking typology profiles of chippers and regular smokers. J. Subst. Abuse 6, 21–35. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Sidhpura N, Shafait S, 2003. Investigating the actions of bupropion on dependence-related effects of nicotine in rats. Psychopharmacology 165, 405–412. [DOI] [PubMed] [Google Scholar]
- Stairs DJ, Dworkin SI, 2008. Rate-dependent effects of bupropion on nicotine self-administration and food-maintained responding in rats. Pharmacol. Biochem. Behav 90, 701–711. [DOI] [PubMed] [Google Scholar]
- WHO, 2017. WHO Report on the Global Tobacco Epidemic.
- Wouda JA, Riga D, De Vries W, Stegeman M, van Mourik Y, Schetters D, Schoffelmeer ANM, Pattij T, De Vries TJ, 2011. Varenicline attenuates cue-induced relapse to alcohol, but not nicotine seeking, while reducing inhibitory response control. Psychopharmacology 216, 267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.